Abstract
The motion of cerebrospinal fluid (CSF) within the subarachnoid space and ventricles is greatly modulated when propagating synchronously with the cardiac pulse and respiratory cycle and path through the nerves, blood vessels, and arachnoid trabeculae. Water molecule movement that propagates between two spaces via a stoma, foramen, or duct presents increased acceleration when passing through a narrow area and can exhibit “turbulence.” Recently, neurosurgeons have started to perform fenestration procedures using neuroendoscopy to treat hydrocephalus and cystic lesions. As part of the postoperative evaluation, a noninvasive diagnostic technique to visualize the water molecules at the fenestrated site is necessary. Because turbulence is observed at this fenestrated site, an imaging technique appropriate for observing this turbulence is essential. We therefore investigated the usefulness of a dynamic improved motion-sensitized driven-equilibrium steady-state free precession (Dynamic iMSDE SSFP) sequence of magnetic resonance imaging that is superior for ascertaining turbulent motions in healthy volunteers and patients. Images of Dynamic iMSDE SSFP from volunteers revealed that CSF motion at the ventral surface of the brainstem and the third ventricle is augmented and turbulent. Moreover, our findings confirmed that this technique is useful for evaluating treatments that utilize neuroendoscopy. As a result, Dynamic iMSDE SSFP, a simple sequence for visualizing CSF motion, entails a short imaging time, can extensively visualize CSF motion, does not require additional processes such as labeling or trigger setting, and is anticipated to have wide-ranging clinical applications in the future.
Keywords: cerebrospinal fluid, fluid dynamics, magnetic resonance imaging, dynamic improved motion-sensitized driven-equilibrium steady-state free precession, hydrocephalus
Introduction
The cerebrospinal fluid (CSF), which exists in the ventricles and the subarachnoid space, does not simply circulate or disperse in a regular pattern, but rather moves in a complex manner, repeating acceleration and deceleration in association with the respiration and heartbeat cycles.1) Moreover, some areas present increased acceleration, and others show attenuated acceleration, indicating the unevenness of CSF even within the cranial cavity.2) In such areas that present with increased acceleration, the water molecule movement often becomes turbulent due to the relationship with the surrounding structures; this turbulence cannot be properly captured or followed even with tracer infusion into the CSF. In addition to accelerating and becoming turbulent like this in certain spaces, it is also known that when, for example, the movement of water molecules propagates through a narrow stoma between two cavities, water molecules move more aggressively at this time, and when fluid propagates to another cavity, it becomes turbulent.3) In the case of endoscopic third ventriculostomy (ETV), the fenestration of the third ventricle floor corresponds to such stoma, and neurosurgeons equalize the CSF motion between the third ventricle and the subarachnoid space of the ventral surface of the brainstem through this stoma, thereby improving hydrocephalus. To achieve this, neurosurgeons must determine the turbulence at the fenestration after ETV by evaluating images of the stoma patency. We therefore utilized dynamic improved motion-sensitized driven-equilibrium steady-state free precession (Dynamic iMSDE SSFP), which is superior for visualizing CSF turbulence, and investigated whether this method is effective for evaluating lesions that impair CSF motion in the ventricular system or subarachnoid space.
Subjects and Methods
Internal review board
This research was approved by our institution’s Institutional Review Board for Clinical Research, Tokai University Hospital (http://irb.med.u-tokai.ac.jp/) IRB No. 13R-066 (Flow dynamic study of cerebrospinal fluid using MRI). Written, informed consent concerning diagnostic procedures and image analysis was obtained from all volunteers and patients.
Volunteers
We investigated 17 healthy volunteers (ages 23–60 years) to observe CSF motion with the Dynamic iMSDE SSFP method. The imaging selection was the mid-sagittal plane, and the field of view included the entire cranial cavity.
Patients
We investigated five patients (ages 3–66 years) who were admitted to our hospital between April and November 2018. The primary diseases were: hydrocephalus caused by an obstruction of the Sylvian aqueduct (n = 1), hydrocephalus caused by an obstruction of the fourth ventricle outlet (n = 1), intraventricular cystic lesion (n = 1), colloid cyst of the foramen of Monro (n = 1), and hydrocephalus caused by a pineal tumor (n = 1). Fenestration was performed for the intraventricular cystic lesion, cyst removal by endoscope for colloid cyst, and ETV was performed in the other cases. For the pineal region tumor, tumor biopsy was performed in addition to ETV. The imaging selection was the mid-sagittal plane, and the field of view included the entire cranial cavity. The imaging conditions for Dynamic iMSDE SSFP are described below. A 1.5-T clinical magnetic resonance scanner (Ingeina R5.3.5, Philips Medical Systems, Best, The Netherlands) with a 15-channel phased array, receive-only head coil was used. For iMSDE preparation, the following parameters were used: preparation delay, 20 ms; velocity encoding, 1 cm/s. The following were used for SSFP acquisition: sequence, balanced turbo field-echo; slice thickness, 7 mm; field of view, 250 × 250 mm; matrix, 208 × 512 (voxel size, 0.49 × 0.49 × 7 mm); repetition time (TR)/echo time (TE), 2.9/1.43 ms; flip angle, 90°; readout bandwidth, 1068.4 Hz/pixel; reduction factor, 2.0; turbo field echo factor, 107; number of acquisitions, 1. These settings led to the temporal resolution of dynamic scans of 835 ms. Such an acquisition was repeated 15 times with iMSDE off and 15 times with iMSDE on as a set of dynamic scans for foot-head (FH) direction. The receiver gain for both iMSDE-on and -off scans was set to remain constant. To visualize CSF motion and accentuate its features, a selected image (tenth image) with iMSDE-off was deduced from that with iMSDE-on. With this subtraction, the brain parenchyma, skull, and other extracranial soft tissues were subtracted, and the image that best accentuated the information of CSF and that was most greatly influenced by dephasing as a fluid was completed. In the subtraction image, CSF with strong dephasing is represented as low signal intensity compared to subtracted tissue, and furthermore, displaying consecutive images as a video enhances the visibility of CSF motion. Please refer to the cited literature for details on the magnetic resonance imaging (MRI) sequence.3)
Results
The common findings among all healthy volunteers were that the Dynamic iMSDE SSFP signal change was strong at the ventral surface of the brainstem, cisterna magna, and third ventricle and that CSF turbulence was observed in these areas (Fig. 1). In three volunteers, turbulence was observed through the foramen of Monro to the anterior horn of the lateral ventricles (Fig. 1).
Fig. 1.
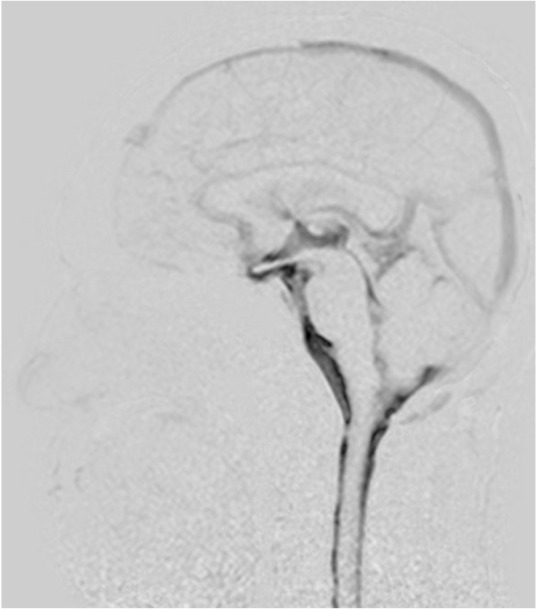
Mid-sagittal image of cerebrospinal fluid (CSF) motion in a 48-year-old healthy male volunteer visualized using dynamic improved motion sensitized driven-equilibrium steady-state free precession (Dynamic iMSDE SSFP). Generally, the dark area on the grayscale images shows vigorous turbulent CSF motion. The dark image contrast is achieved by signal attenuation induced by irregular or turbulent CSF motions in each site compared with the surrounding areas where CSF moves relatively mildly in the Dynamic iMSDE SSFP image. In this healthy individual, increased signal intensity is shown in the area of the chiasmatic cistern, anterior part of the brainstem, cisterna magna, Sylvian aqueduct, and third ventricle. Note increased signal intensity in the anterior horn. This may indicate vigorous CSF motion that is transmitted from the third ventricle to the anterior horn through the foramen of Monro.
In the patient group, a satisfactory recovery of CSF motion was observed throughout the third ventricle in two patients who underwent ETV for hydrocephalus caused by obstruction of the Sylvian aqueduct and obstruction of the fourth ventricle outlet. Follow-up imaging after 3 months in one patient and after 15 months in the other patient showed an increase in CSF motion from the third ventricle to the foramen of Monro, confirming the patency of fenestration (Fig. 2).
Fig. 2.
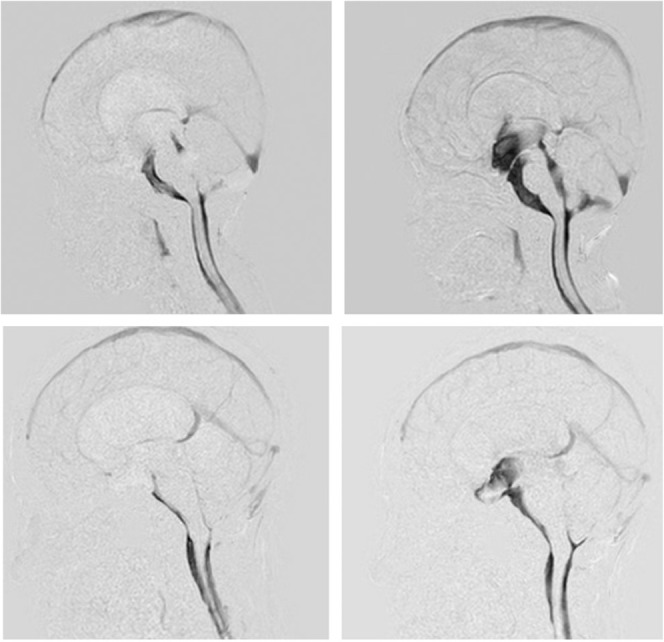
Upper: A 3-year-old boy with obstruction of cerebrospinal fluid (CSF) movement at the outlet of the fourth ventricle. Restricted CSF motion in the third and fourth ventricle was shown in the pre-operative sagittal images of the dynamic improved motion sensitized driven-equilibrium steady-state free precession (Dynamic iMSDE SSFP) (left). CSF motion in the third and fourth ventricles recovered after endoscopic third ventriculostomy (ETV) (right). Lower: A 33-year-old female presented with obstruction of the Sylvian aqueduct. Restricted CSF motion was shown in the ventricular system in the preoperative sagittal image of Dynamic iMSDE SSFP. CSF motion reappeared in the third ventricle in the post-ETV image.
In the patient with impaired CSF motion complicated by a pineal region tumor, ETV to treat impaired CSF motion and a pineal region tumor biopsy resulted in recovery of CSF motion in the third ventricle. However, due to the pineal region tumor compressing the third ventricle, the recovery of CSF motion was in a limited area (Fig. 3).
Fig. 3.
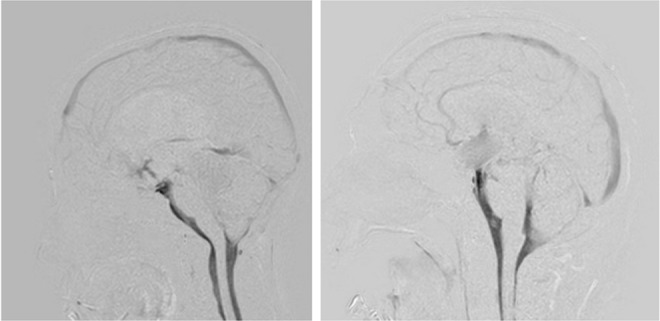
Left: A 13-year-old female who presented with obstruction of the Sylvian aqueduct due to a pineal region tumor. The patient underwent endoscopic third ventriculostomy (ETV) and biopsy of the pineal region tumor. Postoperative dynamic improved motion sensitized driven-equilibrium steady-state free precession (Dynamic iMSDE SSFP) showed vigorous cerebrospinal fluid (CSF) motion that was transmitted from the anterior part of the brainstem to the third ventricle, but this motion was limited in the third ventricle. Right: A 56-year-old male presented with an intraventricular hemorrhage. We performed ETV after external ventricular drainage. During the ETV procedure, the surgeons confirmed patency of fenestration. However, the patient’s neurological condition did not improve, and the ventricular size remained the same after the ETV. Dynamic iMSDE SSFP suggested obstruction of the fenestration site, and thus, we added a ventriculo-peritoneal shunt. After the shunting procedure, the patient recovered well.
In the patient with an intraventricular hemorrhage, ETV was performed. Although CSF motion at the fenestration recovered intraoperatively, CSF motion was not observed on Dynamic iMSDE SSFP at 1 month postoperative in the third ventricle. Neurological symptoms also did not improve, and the patient was therefore diagnosed with dysfunction after ETV and subsequently underwent ventriculoperitoneal shunting (Fig. 3).
In the patient who developed a barrier wall due to an intraventricular cyst, the cyst wall was penetrated under neuroendoscopy, and fenestration and improved CSF motion were confirmed postoperatively (Fig. 4).
Fig. 4.
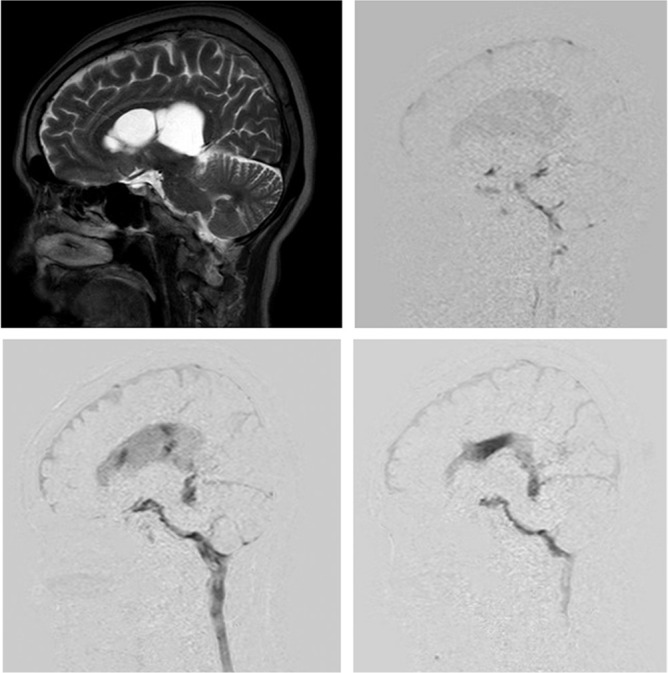
A 66-year-old female presented with multiple cystic compartmentation in the lateral ventricle (upper left). Preoperative dynamic improved motion sensitized driven-equilibrium steady-state free precession (Dynamic iMSDE SSFP) showed restricted cerebrospinal fluid (CSF) motion in the lateral ventricle (upper right). The CSF motion recovered in a limited area after endoscopic fenestration of the cystic wall (lower left), but this recovered CSF motion was transmitted in the lateral ventricle in the other timing of the Dynamic iMSDE SSFP image.
Discussion
Magnetic resonance imaging with Dynamic iMSDE SSFP showed changes in signals at the ventral surface of the brainstem, cisterna magna, and third ventricle caused by turbulence in all volunteers. Moreover, this MRI with Dynamic iMSDE SSFP in patients with impaired CSF motion showed that CSF motion is restricted in the ventricles and cyst in the images before surgical intervention, and that the imaging technique is useful in that (1) the fenestration and cyst wall penetration sites can be confirmed after endoscopic treatment, (2) the spreading of CSF motion that recovered in the re-communicating ventricle can be verified, and (3) dysfunction after ETV can be detected.
Horie et al.3) presented the Dynamic iMSDE SSFP method that visualizes random CSF motion in a short duration. This method is excellent for capturing turbulence. When CSF motion propagates from a cavity with augmented CSF motion to an area with relatively decreased CSF motion in a different cavity, turbulence occurs at the time in which CSF motion passes through a narrow space such as a stoma that connects the two cavities, and this is visualized as a signal change. For instance, Dynamic iMSDE SSFP presents typical images before and after ETV and can visualize the change – when CSF motion propagates from the ventral surface of the brainstem where CSF motion is augmented to the third ventricle where CSF motion is relatively low through the ETV fenestration – as a signal change. Next, we will discuss the imaging principles of Dynamic iMSDE SSFP. The iMSDE4,5) is a technique that induces spin dephasing using a magnetic field gradient that is sensitive to motion called the motion-sensitized gradient (MSG). The feature of this technology is that there are no limitations in imaging sections or range, and it can be combined with various other imaging methods. For this reason, its application has rapidly grown in many areas, including not only the head region, but also the body trunk area. Up till now, it has been used for carotid artery walls,6,7) aneurysms,8) blood vessels of the lower limbs,9,10) brain metastasis detection,11,12) and visualization of peripheral nerves (MR neurography).13,14) On the other hand, until the report by Horie et al.,3) there were no reports that applied iMSDE to observe CSF dynamics. However, actively inducing dephasing using MSG is feasible in CSF similar to blood, and we reported this usefulness in this study. In addition, SSFP that is frequently used as constructive interference in steady state in the neurology field15,16) can rapidly take consecutive images of free water at a high signal. Dynamic iMSDE SSFP combines these two advantages. Specifically, from the rapid consecutive imaging combining iMSDE and SSFP, we presented images that were superior for visualizing turbulence emphasizing CSF dynamics through dephasing using subtraction processing with/without MSG.
Rather than moving in a uniform matter, CSF moves in the subarachnoid space and ventricles, randomly in all directions (cranially, caudally, anteroposteriorly, laterally), repeating both acceleration and deceleration.1,17) Moreover, it has been found that CSF motion is augmented and becomes turbulent in the subarachnoid space at the ventral surface of the brainstem and third ventricle.2,18) The reason of increased turbulent CSF motion at the ventral surface of the brainstem was explained that a significant pressure gradient that was transmitted from the spinal canal to the cisterna magna, and rapidly traveled in the upward direction to the ventral surface of the brainstem.1,17) An intense pressure gradient was readily apparent around the vertebro-basilar artery in particular, which is located on the ventral surface of the brainstem.17) This prominent pressure gradient introduced significant CSF motion at the ventral surface of the brainstem. Neurosurgeons therefore need a noninvasive diagnostic imaging method that can be used to repeatedly observe CSF motion in order to check the stoma patency after cyst fenestration or ETV.19) However, administering a contrast agent or a tracer into CSF is invasive to patients, and visualizing the complex behavior of CSF that passes through the fenestration and becomes turbulent is challenging. On T2 that is performed postoperatively with the purpose of observing the ventricles or morphological changes in the cyst, CSF motion through the fenestration is observed as an artifact in addition to morphological information, albeit with mediocre visibility.20,21) Imaging methods such as phase contrast (PC),22–24) the time-spatial inversion pulse (Time-SLIP) method,25) and two-dimensional fast imaging with steady-state precession magnetic resonance sequence26) have been used for post-ETV evaluation as a superior method to visualize CSF motion. The Dynamic iMSDE SSFP method described in this manuscript is superior for visualizing the irregular CSF motions, and our results showed that fenestration of cyst or ventricle with limited CSF motion resulted in clear visualization of recovered CSF motion as a turbulent movement. Table 1 shows the characteristics of PC, Time-SLIP, and Dynamic iMSDE SSFP. Each imaging method has its own features. Because several reports show that CSF motion is strong and turbulent in the subarachnoid space of the ventral surface of the brainstem,1,2,17,27) when ETV is performed in patients with ventricular dilatation with suppressed CSF motion, changes in ETV fenestration can be visualized clearly as turbulence with the Dynamic iMSDE SSFP method. In addition, an extensive improvement in the suppressed CSF motion can be observed through better communication between the subarachnoid space of the ventral surface of the brainstem and third ventricle after ETV, demonstrating its superiority for postoperative imaging evaluation. Furthermore, we could also capture the recovery of CSF motion through fenestration of the intraventricular cyst wall. In addition, focusing on the imaging time, the commonly reported PC method requires 2–3 min (dependent on pulse),3) and the Time-SLIP method requires approximately 4 min,25) whereas the Dynamic iMSDE SSFP only takes 30 s,3) indicating that the short imaging time is an advantage especially for pediatric patients or at outpatient clinics. Moreover, the Dynamic iMSDE SSFP method can achieve asynchronous images, which do not require triggers such as electrocardiogram or peripheral pulse waveform required by the PC method, indicating that the settings are simple and that fluctuation in the imaging time that relies on a trigger is minimal. As another advantage, Dynamic iMSDE SSFP can be used to observe the CSF motion in the whole imaging section. Therefore, the Dynamic iMSDE SSFP method is superior for screening CSF motion in the whole cranial cavity as well as for follow-up investigations of the patency of an established fenestration such as after ETV procedure.
Table 1.
Characteristics of each imaging technique (Reprinted from Matsumae et al.’s1) Table 2)
| Time-SLIP | 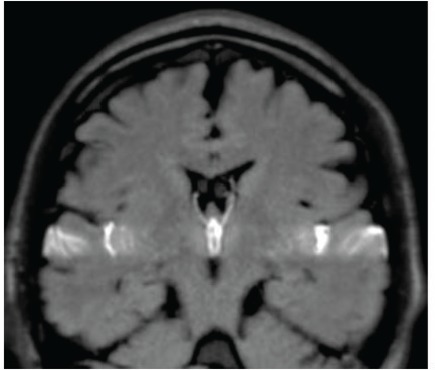 |
Directly observes the signal intensity change due to transference of water protons from certain slab-like regions, in which the proton spins are excited sometime before (about 1–6 s). |
| Dynamic iMSDE SSFP | 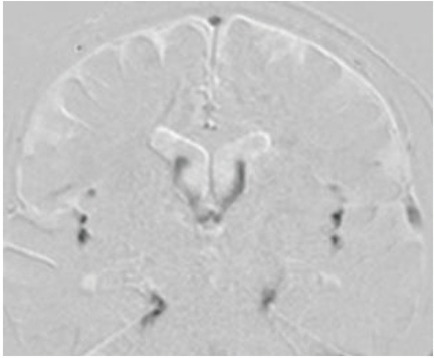 |
Detects and visualizes irregular movement of water protons as signal attenuation induced by phase dispersion in each voxel. |
| 3DPC |  |
Quantifies and visualizes time-resolved CSF velocity in 3D space, and thus enables characterization of CSF motion in a quantitative manner. |
3DPC: time-resolved three-dimensional phase contrast, Dynamic iMSDE SSFP: dynamic improved motion-sensitized driven-equilibrium steady-state free precession, Time-SLIP: time-spatial labeling inversion pulse.
Dynamic iMSDE SSFP is excellent for visualizing random CSF motions; however, it cannot be used to quantify or calculate various physical parameters such as CSF velocity that uses the PC method. Moreover, Dynamic iMSDE SSFP is inferior for visualization of mild and slow CSF motion. Furthermore, Dynamic iMSDE SSFP requires careful attention as it may not be able to capture CSF motion with imaging section settings when the stoma between compartments is small. To supplement these shortcomings of dynamic iMSDE SSFP, it is necessary to perform a comprehensive evaluation of CSF motion combining the PC or Time-SLIP methods. We therefore anticipate the development of a three-dimensional Dynamic iMSDE SSFP as well.
Conclusion
We showed images of various diseases with impaired CSF motion using Dynamic iMSDE SSFP, demonstrating the characteristics of this method. Dynamic iMSDE SSFP is a simple sequence that visualizes CSF motion, entails a short imaging time, can extensively visualize CSF motion, does not require processes such as labeling or trigger setting, and is anticipated for wide-ranging clinical applications in the future.
Footnotes
Conflicts of Interest Disclosure
The authors declare no conflicts of interest.
References
- 1).Matsumae M, Kuroda K, Yatsushiro S, et al. : Changing the currently held concept of cerebrospinal fluid dynamics based on shared findings of cerebrospinal fluid motion in the cranial cavity using various types of magnetic resonance imaging techniques. Neurol Med Chir (Tokyo) 59: 133–146, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Hayashi N, Matsumae M, Yatsushiro S, Hirayama A, Abdullah A, Kuroda K: Quantitative analysis of cerebrospinal fluid pressure gradients in healthy volunteers and patients with normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 55: 657–662, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Horie T, Kajihara N, Matsumae M, et al. : Magnetic resonance imaging technique for visualization of irregular cerebrospinal fluid motion in the ventricular system and subarachnoid space. World Neurosurg 97: 523–531, 2017 [DOI] [PubMed] [Google Scholar]
- 4).Wang J, Yarnykh VL, Yuan C: Enhanced image quality in black-blood MRI using the improved motion-sensitized driven-equilibrium (iMSDE) sequence. J Magn Reson Imaging 31: 1256–1263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Obara M, Kuroda K, Wang J, et al. : Comparison between two types of improved motion-sensitized driven-equilibrium (iMSDE) for intracranial black-blood imaging at 3.0 tesla. J Magn Reson Imaging 40: 824–831, 2014 [DOI] [PubMed] [Google Scholar]
- 6).Koktzoglou I, Li D: Submillimeter isotropic resolution carotid wall MRI with swallowing compensation: imaging results and semiautomated wall morphometry. J Magn Reson Imaging 25: 815–823, 2007 [DOI] [PubMed] [Google Scholar]
- 7).Fan Z, Zhang Z, Chung YC, et al. : Carotid arterial wall MRI at 3T using 3D variable-flip-angle turbo spin-echo (TSE) with flow-sensitive dephasing (FSD). J Magn Reson Imaging 31: 645–654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Nagahata S, Nagahata M, Obara M, et al. : Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three-dimensional turbo spin-echo sequence with motion-sensitized driven-equilibrium: a sign of ruptured aneurysm? Clin Neuroradiol 26: 277–283, 2016 [DOI] [PubMed] [Google Scholar]
- 9).Fan Z, Sheehan J, Bi X, Liu X, Carr J, Li D: 3D noncontrast MR angiography of the distal lower extremities using flow-sensitive dephasing (FSD)-prepared balanced SSFP. Magn Reson Med 62: 1523–1532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Priest AN, Graves MJ, Lomas DJ: Non-contrast-enhanced vascular magnetic resonance imaging using flow-dependent preparation with subtraction. Magn Reson Med 67: 628–637, 2012 [DOI] [PubMed] [Google Scholar]
- 11).Nagao E, Yoshiura T, Hiwatashi A, et al. : 3D turbo spin-echo sequence with motion-sensitized driven-equilibrium preparation for detection of brain metastases on 3T MR imaging. AJNR Am J Neuroradiol 32: 664–670, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Kikuchi K, Hiwatashi A, Togao O, et al. : 3D MR sequence capable of simultaneous image acquisitions with and without blood vessel suppression: utility in diagnosing brain metastases. Eur Radiol 25: 901–910, 2015 [DOI] [PubMed] [Google Scholar]
- 13).Yoneyama M, Takahara T, Kwee TC, Nakamura M, Tabuchi T: Rapid high resolution MR neurography with a diffusion-weighted pre-pulse. Magn Reson Med Sci 12: 111–119, 2013 [DOI] [PubMed] [Google Scholar]
- 14).Hiwatashi A, Togao O, Yamashita K, et al. : Evaluation of chronic inflammatory demyelinating polyneuropathy: 3D nerve-sheath signal increased with inked rest-tissue rapid acquisition of relaxation enhancement imaging (3D SHINKEI). Eur Radiol 27: 447–453, 2017 [DOI] [PubMed] [Google Scholar]
- 15).Thiele H, Nagel E, Paetsch I, et al. : Functional cardiac MR imaging with steady-state free precession (SSFP) significantly improves endocardial border delineation without contrast agents. J Magn Reson Imaging 14: 362–367, 2001 [DOI] [PubMed] [Google Scholar]
- 16).Bloomer TN, Plein S, Radjenovic A, et al. : Cine MRI using steady state free precession in the radial long axis orientation is a fast accurate method for obtaining volumetric data of the left ventricle. J Magn Reson Imaging 14: 685–692, 2001 [DOI] [PubMed] [Google Scholar]
- 17).Matsumae M, Hirayama A, Atsumi H, Yatsushiro S, Kuroda K: Velocity and pressure gradients of cerebrospinal fluid assessed with magnetic resonance imaging. J Neurosurg 120: 218–227, 2014 [DOI] [PubMed] [Google Scholar]
- 18).Takizawa K, Matsumae M, Hayashi N, et al. : The choroid plexus of the lateral ventricle as the origin of CSF pulsation is questionable. Neurol Med Chir (Tokyo) 58: 23–31, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Udayakumaran S, Joseph T: Can we predict early endoscopic third ventriculostomy failure? The role of ultra-early postoperative magnetic resonance imaging in predicting early endoscopic third ventriculostomy failure. World Neurosurg X 2: 100013, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Okano A, Ogiwara H: Long-term follow-up for patients with infantile hydrocephalus treated by choroid plexus coagulation. J Neurosurg Pediatr 22: 638–645, 2018 [DOI] [PubMed] [Google Scholar]
- 21).Dinçer A, Yildiz E, Kohan S, Memet Özek M: Analysis of endoscopic third ventriculostomy patency by MRI: value of different pulse sequences, the sequence parameters, and the imaging planes for investigation of flow void. Childs Nerv Syst 27: 127–135, 2011 [DOI] [PubMed] [Google Scholar]
- 22).Fukuhara T, Vorster SJ, Ruggieri P, Luciano MG: Third ventriculostomy patency: comparison of findings at cine phase-contrast MR imaging and at direct exploration. AJNR Am J Neuroradiol 20: 1560–1566, 1999 [PMC free article] [PubMed] [Google Scholar]
- 23).Bargalló N, Olondo L, Garcia AI, Capurro S, Caral L, Rumia J: Functional analysis of third ventriculostomy patency by quantification of CSF stroke volume by using cine phase-contrast MR imaging. AJNR Am J Neuroradiol 26: 2514–2521, 2005 [PMC free article] [PubMed] [Google Scholar]
- 24).Algin O, Ucar M, Ozmen E, et al. : Assessment of third ventriculostomy patency with the 3D-SPACE technique: a preliminary multicenter research study. J Neurosurg 122: 1347–1355, 2015 [DOI] [PubMed] [Google Scholar]
- 25).Abe K, Ono Y, Yoneyama H, et al. : Assessment of cerebrospinal fluid flow patterns using the time-spatial labeling inversion pulse technique with 3T MRI: early clinical experiences. Neuroradiol J 27: 268–279, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Lucic MA, Koprivsek K, Kozic D, Spero M, Spirovski M, Lucic S: Dynamic magnetic resonance imaging of endoscopic third ventriculostomy patency with differently acquired fast imaging with steady-state precession sequences. Bosn J Basic Med Sci 14: 165–170, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Yamada S, Miyazaki M, Kanazawa H, et al. : Visualization of cerebrospinal fluid movement with spin labeling at MR imaging: preliminary results in normal and pathophysiologic conditions. Radiology 249: 644–652, 2008 [DOI] [PubMed] [Google Scholar]


