Abstract
Introduction
HIV‐associated cryptococcal, TB and pneumococcal meningitis are the leading causes of adult meningitis in sub‐Saharan Africa (SSA). We performed a systematic review and meta‐analysis with the primary aim of estimating mortality from major causes of adult meningitis in routine care settings, and to contrast this with outcomes from clinical trial settings.
Methods
We searched PubMed, EMBASE and the Cochrane Library for published clinical trials (defined as randomized‐controlled trials (RCTs) or investigator‐managed prospective cohorts) and observational studies that evaluated outcomes of adult meningitis in SSA from 1 January 1990 through 15 September 2019. We performed random effects modelling to estimate pooled mortality, both in clinical trial and routine care settings. Outcomes were stratified as short‐term (in‐hospital or two weeks), medium‐term (up to 10 weeks) and long‐term (up to six months).
Results and discussion
Seventy‐nine studies met inclusion criteria. In routine care settings, pooled short‐term mortality from cryptococcal meningitis was 44% (95% confidence interval (95% CI):39% to 49%, 40 studies), which did not differ between amphotericin (either alone or with fluconazole) and fluconazole‐based induction regimens, and was twofold higher than pooled mortality in clinical trials using amphotericin based treatment (21% (95% CI:17% to 25%), 17 studies). Pooled short‐term mortality of TB meningitis was 46% (95% CI: 33% to 59%, 11 studies, all routine care). For pneumococcal meningitis, pooled short‐term mortality was 54% in routine care settings (95% CI:44% to 64%, nine studies), with similar mortality reported in two included randomized‐controlled trials. Few studies evaluated long‐term outcomes.
Conclusions
Mortality rates from HIV‐associated meningitis in SSA are very high under routine care conditions. Better strategies are needed to reduce mortality from HIV‐associated meningitis in the region.
Keywords: cryptococcal meningitis, pneumococcal meningitis, TB meningitis, sub‐Saharan Africa, systematic review
1. Introduction
Approximately 37 million people were living with HIV worldwide in 2017, with over two‐thirds in sub‐Saharan Africa (SSA) 1. Although access to combined antiretroviral therapy (ART) has improved, a large proportion of people living with HIV (PLHIV) still present with advanced immune suppression and are at high risk for HIV‐related infections 2, 3. Central nervous system (CNS) infections are a major cause of mortality in PLHIV in SSA, with cryptococcal, TB and pneumococcal meningitis the most common microbiologically confirmed aetiologies 4, 5. Cryptococcal meningitis alone results in approximately 15% of HIV‐associated deaths worldwide, with almost three‐quarters of these in SSA 6.
Treatment for HIV‐associated meningitis is challenging even in well‐resourced settings. Recommended cryptococcal meningitis management involves initial “induction” therapy with intravenous amphotericin (ideally for one week with flucytosine 7, which remains unavailable throughout most of Africa 8), an effective but highly toxic drug 9, followed by consolidation and maintenance therapy with fluconazole 10. However, less potent fluconazole monotherapy is often used in resource‐constrained settings due to low cost, ease of oral administration and better drug toxicity profile. Additionally, cryptococcal meningitis survival is associated with performance of therapeutic lumbar punctures (LPs) to reduce intracranial pressure (ICP) 11, 12, intravenous fluid (IVF) hydration and electrolyte supplementation and monitoring to prevent life‐threatening amphotericin‐related toxicities 9, 10, 13, and appropriate follow‐up after hospital discharge to initiate antiretroviral therapy (ART) around four to six weeks to reduce the risk of immune‐reconstitution inflammatory syndrome 14. Significant barriers exist to optimal management in most SSA settings, such as lack of more potent antifungal drugs, lack of manometers to measure ICP, hospital understaffing, limited laboratory services and loss to follow‐up after hospital discharge. Similarly, effective management of TB meningitis is complicated in resource‐constrained settings with challenges in management of common antituberculous drug toxicities, long duration of treatment (of a minimum of six months), poor sensitivity of diagnostic studies and delays for culture results, emergence of drug resistance and lack of integration of TB and HIV services 15, 16. Survival from pneumococcal meningitis can be negatively affected by delayed healthcare access and lack of timely initiation of effective antimicrobial therapy 17.
Several systematic reviews have evaluated outcomes of HIV‐associated meningitides in Africa, but each has important limitations. A recent review of long‐term outcomes from HIV‐associated cryptococcal meningitis included only a small subset of studies with long‐term outcomes data which may not be generally representative 18. In a previous systematic review of TB meningitis in Africa, investigators did not differentiate between short‐ and long‐term outcomes and combined outcomes from studies using heterogeneous case definitions (e.g. microbiologically diagnosed TB or TB diagnosed clinically using various criteria), limiting interpretation 19. A prior well‐conducted systematic review of pneumococcal meningitis in Africa focused on paediatric populations 20.
Given the large burden and mortality from HIV‐associated meningitis in SSA and limitations of prior studies, we performed a systematic review and meta‐analysis to evaluate short‐ and long‐term outcomes of common HIV‐associated meningitides (cryptococcal, TB and pneumococcal). We estimated mortality in “routine care” studies (which we defined as studies in which hospital care was provided primarily by the local medical team, for example, retrospective cohort studies), providing realistic global burden estimates important for resource allocation and prevention efforts such as cryptococcal antigen (CrAg) screening 21. We compared outcomes from routine care to “clinical trial” studies (which we defined as randomized‐controlled trials (RCTs) or prospective cohorts with regular day‐to‐day management of patients involving study investigators) to characterize the outcomes gap and provide insights into potential reductions in mortality that might be realized through improved management strategies.
2. Methods
2.1. Types of outcomes measures
Our overall aim was to determine the mortality associated with the most common HIV‐associated meningitides in sub‐Saharan Africa, captured at time points reflecting acute‐ and long‐term outcomes. Our mortality endpoints were defined as: (1) Short‐term (death ≤2 weeks after hospitalization or enrolment); (2) medium‐term (death ≤10 weeks); and long‐term (≤6 months). Study deviations from these pre‐defined timepoints were described in the analysis.
2.2. Types of studies and participants
We included published RCTs and observational studies (cohort studies, case‐control studies, cross‐sectional audits or surveillance studies) of adults (≥18‐years) treated for cryptococcal, TB and/or pneumococcal meningitis. Inclusion criteria included: (1) Study from sub‐Saharan Africa (or restricted to patients from sub‐Saharan Africa in multi‐centre studies with participants also enrolled from outside of the region 22); (2) ≥15 participants treated for cryptococcal, TB or pneumococcal meningitis; and 3) full period of observation after 1990 (to reflect emergence of HIV in SSA and modern antimicrobial therapy regimens). Exclusion criteria included: (1) Paediatric study or with a majority of patients <18‐years old without disaggregated results provided in adults; (2) case report, case series, or <15 participants with cryptococcal, TB or pneumococcal meningitis; (3) antimicrobial treatment not available or treatment with therapies either not used or contraindicated (e.g. acetazolamide for cryptococcal meningitis) 22, 23; (4) non‐representative or biased sampling (e.g. significant RCT recruitment delay causing immortal time bias 14, 24; (5) missing mortality data for >15% of participants or mortality outcomes not provided; (6) diagnostic criteria not clearly defined; (7) repeat data from another study; or 8) not a full article (e.g. conference abstract).
For cryptococcal meningitis, we included studies of cases confirmed by cerebrospinal fluid (CSF) testing (i.e. positive India ink, culture or CrAg), excluding those with a significant number (>10%) of patients diagnosed through peripheral blood CrAg testing alone, as blood CrAg testing is unable to definitively confirm central nervous system involvement. For TB meningitis, we included studies of either microbiologically confirmed cases or with diagnostic criteria that combined CSF findings and other supportive evidence, for example, isolation of Mycobacterium tuberculosis at another site or suggestive imaging 25. For pneumococcal meningitis, we included studies with mortality for microbiologically confirmed cases or, when results were not disaggregated with other cases, if a majority of cases (>50%) were microbiologically confirmed. These definitions were used so that outcomes would reflect mortality from the aetiologies of interest rather than other potential causes.
2.3. Search method and data collection
We searched for studies from 1 January 1990 through 30 April 2018 using PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL). The search was updated through 31 December 2018 in PubMed and EMBASE with a subsequent update through 15 September 2019 in PubMed. Our search strategy combined geographic and cryptococcal, TB and pneumococcal meningitis search terms (Figure 1 and Appendix S1). We excluded conference abstracts and studies that were not peer‐reviewed. No restrictions were placed on age or language. The search strategy and protocol was developed by the authors before the search; the study protocol was not published.
Figure 1.

PubMed search strategy.
Articles obtained in database searches were aggregated and de‐duplicated in Covidence 26. Two reviewers (MWT and AMG) independently performed a primary title and abstract search to identify potentially eligible studies. The authors then reviewed full text articles to assess for inclusion, with data from included studies abstracted using standardized data collection forms. Secondary review was provided by DSL and NW. Discrepancies were decided through consensus or adjudication from JNJ if needed. We contacted authors as needed for clarification. Reference lists of included articles as well as relevant reviews were searched to identify other potential studies. We generated a flow diagram, following PRISMA recommendations, and summary tables of excluded full‐text articles with rationale (in Appendix S1), and reporting was conducted per PRISMA recommendations 27.
2.4. Data synthesis and analysis
Data were pooled using standard random effects meta‐analyses for proportions, with variance of proportions stabilized using Freeman‐Tukey double arcsine transformation before pooling 28. We estimated the pooled proportion of deaths and 95% confidence interval for each pathogen (Cryptococcus, TB and pneumococcus) by study type (clinical trial vs routine care study) and by timepoint (short‐term, medium‐term and long‐term mortality). Patient outcomes data were combined for multiple routine care studies from the same facility if the studies included patients from non‐overlapping periods. Heterogeneity was assessed with I2 values, representing the percentage of total variability due to between‐study heterogeneity. Results were presented using forest plots, along with summary tables of study and patient characteristics (included in Appendix S1). Analyses were conducted in R Studio using the metaprop command 29.
2.5. Sub‐group analyses
We performed additional analyses to stratify estimates by clinically important confounders. For cryptococcal meningitis, we stratified outcomes by induction therapy; amphotericin B with and without flucytosine (for <2 or ≥2 weeks), fluconazole with flucytosine, fluconazole or treatment not specified. Outcomes from induction regimens with amphotericin B alone or amphotericin B with fluconazole were combined, as no mortality difference has been documented between groups 30, 31. For TB meningitis, we stratified analyses by studies with microbiological‐confirmed cases and with a majority of cases defined using combined microbiological and clinical criteria without Mycobacterium tuberculosis confirmed from CSF culture, smear or PCR. For pneumococcal meningitis, we stratified analyses by treatment regimen. We further stratified outcomes by region (Southern, Eastern, Middle (Central) and Western Africa) as defined by the United Nations geoscheme 32, and studies conducted after 2000 or before 2000. We initially planned additional stratified analyses by HIV prevalence (≥50% or <50%) within a population, but most studies included a majority or only HIV‐positive patients and we therefore did not perform sub‐group analyses based on HIV status.
2.6. Dealing with missing data and assessing risk of bias
We described missingness of data, including clinical characteristics (e.g. HIV status) and mortality. We performed intention‐to‐treat analysis when multiple groups were compared. Outcomes were reported for patients with known outcomes at each mortality timepoint. This was done so as not to systematically bias estimates toward higher survival as would have done if those lost to follow‐up were all assumed to have survived. If loss to follow‐up exceeded 15%, we excluded the study from the analysis at that timepoint due to the high likelihood that outcomes were not missing at random (i.e. those who died were more likely to be lost to follow‐up). Given the heterogeneity of study types, we undertook a subjective assessment of study quality based on previous guidance for systematic reviews 33. This incorporated quality of reporting of important covariates, for example, timing of ART in ART‐naïve patients treated for cryptococcal meningitis. Randomized‐controlled trials of HIV‐associated cryptococcal meningitis have previously been assessed for risk of bias using GRADE criteria 30.
3. Results and discussion
3.1. Overall search findings
The initial database search yielded 3661 titles, with two additional articles found that met inclusion criteria. After removing duplicates, 2593 titles and abstracts were reviewed, with 272 selected for full text review. Of these, 193 were excluded (Figure 2 and Appendix S1). Seventy‐nine studies were included from the primary database search and additional references.
Figure 2.
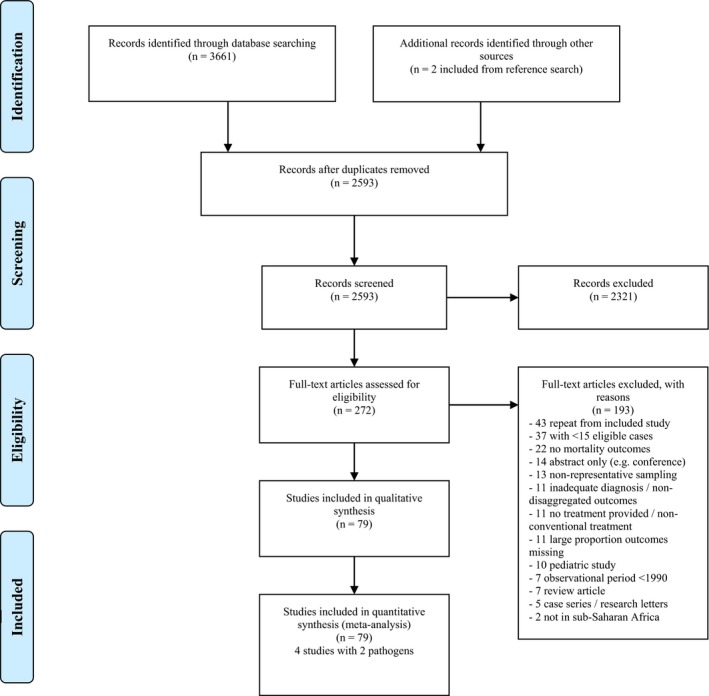
PRISMA diagram.
3.2. Cryptococcal meningitis outcomes from treatment provided under “routine care” settings
Forty‐one observational studies with 10,139 cases of cryptococcal meningitis were included, most in HIV‐positive patients treated at district or referral hospitals (Appendix S1). The majority of studies were judged of low quality, with limited description of clinical management (e.g. performance of therapeutic lumbar punctures, antifungal therapy dose/duration, timing of ART following initiation of antifungal therapy in ART‐naïve patients). Seventeen were prospective cohorts and one was a mixed prospective and retrospective cohort 34, which we classified as routine care studies. Three of these prospective cohorts described more active involvement from a study team [35, 36, 37], but at irregular intervals with day‐to‐day management under local conditions. In 10% (4/41) of included studies, the period of observation began prior to 2000. Seventeen countries were represented, with greatest representation from South Africa (14 studies) 34, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, Ethiopia (three studies) 50, 51, 52 and Uganda (three studies) 53, 54, 55; other countries had two or fewer studies 11, 35, 36, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74. Amphotericin B‐based induction therapy (with or without fluconazole) was the predominant induction regimen in 16 studies, fluconazole in 13 studies, and a mix of treatments (45% fluconazole and 43% amphotericin B) in one study 44, with antifungal regimens not specified in 11 studies. In 28 studies with details on ART status, 26% (1792/6777) had a history of current or previous ART use. Reasons for exclusion are detailed in Appendix S1; these included non‐representative sampling 24, 75, 76, 77, high loss to follow‐up or duration of follow‐up not clear 62, 78, antifungal treatment not available 23, 79, 80 and use of treatment regimens not used or contraindicated in management 81, 82, 83.
Short‐term mortality was reported in 40/41 included studies (35 in‐hospital and five studies with two‐week mortality reported). Pooled short‐term mortality was 44% (95% CI: 39% to 49%, 40 studies), with high between‐study heterogeneity (I2 = 89%) (Figure 3). Mortality was lowest in Southern Africa (37% (95% CI: 31% to 43%), I2 = 86%, 16 studies) and highest in Central Africa (55% (95% CI: 41% to 69%), I2 = 75%, three studies) and West Africa (54% (95% CI: 40% to 67%), I2 = 83%, eight studies) (Figure S1). Restricting to studies with a full observation period after 2000, pooled mortality was 44% (95% CI: 39% to 49%, I2 = 90%, 36 studies) (similar in a post‐hoc analysis restricted to studies with a full period of observation after 2005, with a pooled mortality of 42% (95% CI: 36% to 47%, I2 = 89%, 29 studies)). For these studies, short‐term mortality was 41% (95% CI: 32% to 51%, I2 = 91%, 15 studies) with exclusive or predominant amphotericin B‐based induction therapy (none using flucytosine), 41% (95% CI: 31% to 50%, I2 = 89%, 11 studies) with exclusive or predominant fluconazole, and 50% (95% CI: 39% to 61%, I2 = 78%, nine studies) with treatment not specified. One study was excluded in which the proportion of patients receiving amphotericin B‐based and fluconazole induction therapy was almost equivalent 44.
Figure 3.
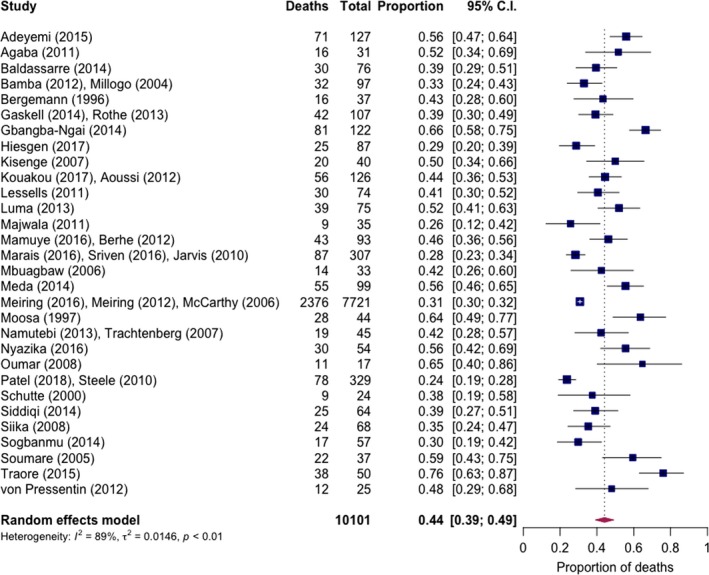
Overall pooled short‐term mortality of cryptococcal meningitis in routine care settings.
Few studies reported medium‐ or long‐term mortality. Medium‐term mortality (defined as death ≤10‐weeks, except ≤3‐months in one study) was described in five studies with 422 patients in Southern Africa (two studies) or East Africa (three studies), with a pooled mortality of 51% (95% CI: 43% to 60%, I2 = 60%) (Figure S2). Long‐term mortality was described in three studies (at nine months in one and 12 months in two 35, 42, 56) with 294 patients in Southern Africa (two studies) and East Africa (one study). Pooled mortality was 63% (95% CI: 46% to 78%, I2 = 79%) (Figure S3). Studies from Botswana 56, with standard amphotericin B and fluconazole induction therapy, and Malawi 35, with high‐dose fluconazole (800 mg/day) induction therapy, reported one‐year mortality of 65% (142/219) and 77% (43/56) respectively among those not lost to follow‐up. The third study, a prospective cohort from South Africa, reported a nine‐month mortality of 37% (7/19) 42. Few participants in this study had baseline altered mental status (11% (2/19) noted to have confusion), an established predictor of mortality 84, and investigators may have recruited less seriously ill patients. Regions in SSA with the highest acute mortality (Central and Western Africa) did not report long‐term mortality data.
3.3. Cryptococcal meningitis outcomes for treatment provided under clinical trial settings
We included 18 studies from clinical trials (randomized trials or investigator‐managed cohorts that compared ≥1 treatment regimen for cryptococcal meningitis) with a total of 2048 cases, almost all HIV positive. These included 10 RCTs and eight prospective cohorts, most at referral centres and all conducted after 2000 (Appendix S1). The quality was judged to be good for most studies (details on therapeutic lumbar puncture details in 12/18, timing of ART initiation in 17/18 and description of antifungal regimen in all studies), and most RCTs had been previously evaluated using GRADE criteria 30. Nine countries were represented (including several multi‐country RCTs); greatest representation was from South Africa (six studies) 85, 86, 87, 88, 89, 90, Uganda (six studies) 22, 91, 92, 93, 94, 95 and Malawi (four studies) 7, 22, 96, 97, with other countries represented in two or fewer studies 98, 99, 100. Ten of the eighteen studies excluded patients on ART at the time of enrolment. Two studies described fluconazole monotherapy as the only regimen and a third RCT evaluated fluconazole as one treatment arm 93, 97, 100, two RCTs evaluated combined flucytosine and fluconazole therapy 7, 97, and the rest described use of amphotericin B with or without flucytosine or fluconazole. Reasons for study exclusion are detailed in Appendix S1: These included use of abnormal treatment regimens 101, 102, 103, 104, studies with non‐representative sampling, for example, delayed enrolment for one week after initiation of antifungal therapy 14, and a study that reported longer‐term outcomes from only a subset of patients from an included study 105. For RCTs that compared standard regimens to experimental regimens not part of clinical practice, for example, adjunctive interferon‐gamma or sertraline 87, 106, or therapies demonstrated to be harmful, for example, adjunctive dexamethasone 22, we restricted our analysis to patients who received standard antifungal regimens. For one RCT that recruited patients both in African and Asian health centres, we restricted our analysis to patients from Africa 22.
Short‐term mortality was reported in 17 studies (all reporting two‐week outcome) with 1991 cases. Pooled two‐week mortality was 21% (95% CI: 17% to 25%, I2 = 75%, 17 studies) (Figure 4). Stratified by treatment regimen, mortality was 12% (95% CI: 7% to 18%, I2 = 40%, 5 studies) for amphotericin and flucytosine induction therapy, 17% (95% CI: 12% to 22%, I2 = 0%, two studies) for flucytosine with fluconazole, 23% (95% CI: 19% to 28%, I2 = 72%, 12 studies) for amphotericin with or without fluconazole and 30% (95% CI: 21% to 40%, I2 = 0%, three studies) for fluconazole alone. Little mortality difference was observed for shorter (<2 weeks) and longer (two‐week) amphotericin‐based induction regimens.
Figure 4.
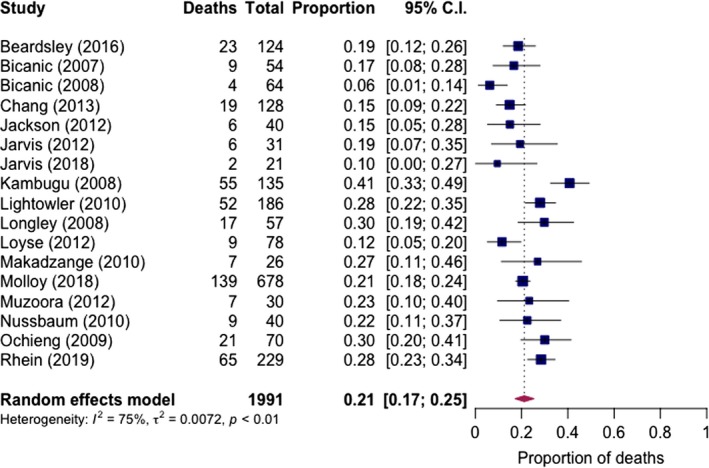
Pooled short‐term mortality of cryptococcal meningitis in clinical trial settings.
Medium‐term mortality (at 10 weeks except four months in one study 92) was reported in 13 studies with 1487 cases, with a pooled mortality of 37% (95% CI: 33% to 41%, I2 = 42%) (Figure S4). Stratified by induction regimen, mortality was 30% (95% CI: 25% to 34%, I2 = 0%, five studies) for amphotericin with flucytosine, 35% (95% CI: 29% to 42%, I2 = 0%, two studies) for flucytosine with fluconazole, 39% (95% CI: 35% to 43%, I2 = 14%, eight studies) for amphotericin with or without fluconazole and 49% (95% CI: 39% to 60%, I2 = 29%, three studies) for fluconazole. Long‐term mortality (within six months except 18 weeks in one) was described in six studies with 614 cases, with a pooled mortality of 44% (95% CI: 36% to 52%, I2 = 71%) (Figure S5). Stratified by regimen, mortality was 33% (95% CI: 21% to 45%, one study) for amphotericin with flucytosine, 46% (95% CI: 36% to 55%, I2 = 78%, four studies) for amphotericin with or without fluconazole and 50% (95% CI: 30% to 70%, one study) in a small study from Zimbabwe of fluconazole monotherapy.
3.4. TB meningitis outcomes
Twelve studies with 1008 TB meningitis cases were included, all routine care studies and all but one with a majority or all HIV positive (median 84%) 107. One recent study in Zambia, with 19% culture‐confirmed adults TB meningitis cases and a 86% HIV prevalence, contributed to over half of these cases 108. Study quality was relatively low, with most studies not including details of ART or antituberculous treatment or average duration of hospitalization. Five included cases before 2000 34, 109, 110, 111, 112, whereas seven had a full period of observation after 2000 53, 60, 67, 107, 108, 113, 114. Eight countries were represented, with greatest representation from South Africa (three studies), Uganda (two studies) and Zambia (two studies). Six studies included exclusively definite cases (with positive CSF AFB smear, culture or PCR) and six included mostly probable/possible cases (range 0% to 21% microbiologically confirmed, with two studies using Marais criteria 25, 108). One study specified use of adjunctive dexamethasone 111. Several potential studies were excluded on full‐text review (Appendix S1): Reasons included TB case series, for example, exclusively multi‐drug resistant cases 115, 116, 117, high risk of selection bias 42, 118, 119, 120, significant missingness of outcomes 121, 122, 123 and unknown or inadequate description of diagnostic criteria 50, 74, 124, 125, 126.
Short‐term mortality for 1008 cases was reported in 11 studies (two from a single hospital with combined patient outcomes in Uganda 53, 113 and two from a single hospital with combined outcomes in Zambia 67, 108; two‐week mortality specified in one study 114, in‐hospital mortality in ten studies with length of hospitalization not specified). Pooled mortality was 46% (95% CI: 33% to 59%, I2 = 92%, 11 studies) (Figure 5), and was higher in studies with only definite cases (51% (95% CI: 37% to 65%), I2 = 78%, six studies) and mostly possible/probable cases (40% (95% CI: 23% to 59%), five studies). Restricting to studies after 2000, pooled short‐term mortality was 42% (95% CI: 25% to 59%, I2 = 93%, seven studies). Ten‐week mortality from a national meningitis audit in Botswana of microbiologically confirmed cases was 46% (95% CI: 31% to 61%) 114. Long‐term mortality was reported in two studies, within one year in the study from Botswana and after completion of six months of antituberculous therapy in a Nigerian study 111, both studies with exclusively definite cases. Reported mortality for these studies was 56% (27/48) in Botswana and 78% (31/40) in Nigeria. Of note, the Botswana study relied on national electronic death registry data, which has been previously shown to accurately capture deaths but might slightly under‐estimate mortality 56. Long‐term outcomes in the large study were excluded because of high degree of data missingness (>20% at one year) 108. These investigators reported a one‐year mortality of 59% among patients who could be tracked, or 67% assuming those lost to follow‐up had died.
Figure 5.
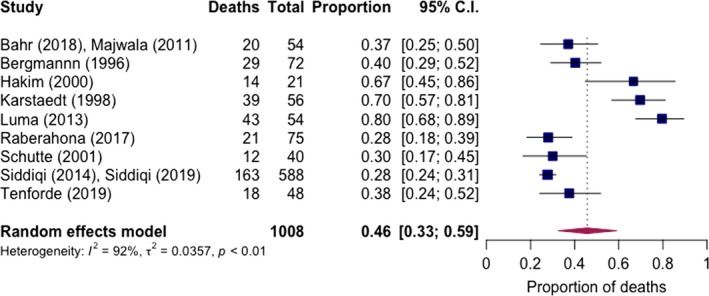
Pooled short‐term mortality of TB meningitis in routine care settings.
3.5. Pneumococcal meningitis outcomes
Twelve studies with 3935 cases of pneumococcal meningitis were included, a majority HIV‐positive in nine studies, <50% HIV positive in one, and with HIV prevalence not specified in two studies. Nine were classified as routine care studies 114, 127, 128, 129, 130, 131, 132, 133, 134 and two were RCTs 135, 136. One study consisted of two sequential cohorts, a lead‐in observational routine care cohort followed by a prospective cohort receiving a bundled Goal Directed Therapy (GDT) intervention that included parenteral ceftriaxone therapy within one hours of registration, airway support, and fluid resuscitation according to sepsis guidelines, among other supportive measures 137. The clinical trial studies were judged to be of high quality, but routine care studies of relatively low quality (including lack of description of ART status and dose and duration of antibiotic therapy). Eight countries were represented, with four studies (two RCTs, one observational cohort and the combined sequential cohort study) from a single referral hospital in Malawi. Nine studies included a full period of observation after 2000. Ten provided outcomes for microbiologically confirmed cases (CSF culture, PCR, and/or antigen testing) and two studies with Streptococcus pneumoniae microbiologically confirmed for a majority of cases (64% to 69%). Treatment varied significantly between studies; four described ceftriaxone as standard antibiotic therapy (one RCT with adjunctive dexamethasone used in a single treatment arm) 114, 135, two included chloramphenicol‐based treatment as the predominant treatment (one combined with intravenous penicillin G) 127, 129, two included aminopenicillins (amoxicillin or ampicillin) with or without gentamycin as the predominant therapy given 130, 132, three studies did not specify therapy 128, 131, 134, and one study included a similar mix of treatment with ceftriaxone and chloramphenicol 133. Several potential studies were excluded on full text review (Appendix S1): Reasons included a minority of cases with Streptococcus pneumoniae isolation 34, 138, 139, 140 and high loss to follow‐up / missing outcomes data 141, 142, 143, 144, 145.
Short‐term mortality was reported in all nine routine care studies with 3480 cases (including 14‐day 114, 30‐day 128, in‐hospital 129, 130, 131, 132, 134 and with length of follow‐up not specified in two surveillance studies but assumed short‐term 127, 133). Over half of these cases (64%, 2210/3480) were from two surveillance studies in Gauteng Province, South Africa 128, 134; the first from 2003 to 2008 reporting mortality up to 30 days and the second with data from 2013 to 2016 with in‐hospital mortality reported, with results pooled together for the meta‐analysis. Pooled mortality was 54% (95% CI: 44% to 64%, I2 = 96%, nine studies) (Figure 6). Medium‐ and long‐term mortality was described in two routine care studies, one from Botswana and the other from Malawi 114, 129. Both found generally favourable long‐term survival in those with short‐term survival. The study from Malawi showed a short‐term (in‐hospital up to nine days), medium‐term (10‐week) and long‐term (six‐month) mortality of 65% (42/65), 69% (45/65) and 69% (45/65) respectively, with one‐year mortality of 75% (49/65). The study from Botswana found short‐term (two‐week), medium‐term (10‐weeks), long‐term (six‐month) and one‐year mortality of 44% (105/238), 47% (112/238), 47% (113/238) and 49% (117/238) respectively.
Figure 6.
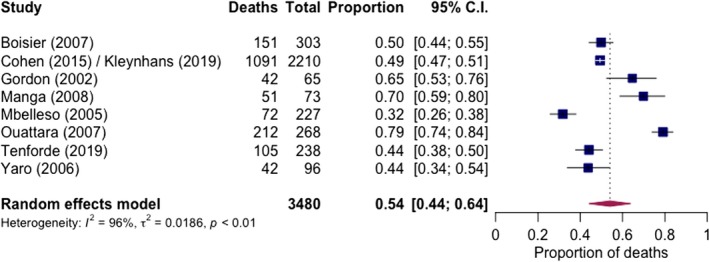
Pooled short‐term mortality of pneumococcal meningitis in routine care settings.
The two RCTs were both conducted at the same referral hospital in Malawi, provided 40‐day outcomes, and reported treatment with ceftriaxone (with or without dexamethasone) 135, 136. We combined data for patients treated with or without adjunctive dexamethasone in one RCT as no difference in mortality was found between arms 136, and patients receiving adjunctive glycerol in one RCT were excluded as glycerol did not improve outcomes and may have been associated with severe adverse events 135. Short‐term (here defined as 40‐day) mortality ranged from 39% (20/51) to 51% (140/272); however, mortality from pneumococcal meningitis was certainly under‐estimated in the smaller trial as 17% of patients with suspected acute bacterial meningitis died before they could be recruited. In the RCT with a 51% (140/272) 40‐day mortality, long‐term mortality was reported at 61% (150/245) in patients not lost to follow‐up.
The study with the combined sequential observational routine care cohort and interventional Goal Directed Therapy cohort was conducted at the same centre as the two RCTs, with ceftriaxone treatment provided as standard treatment 137. At the primary endpoint of 40 days, among patients with known outcomes 49% (28/57) had died in the lead‐in observational cohort and 63% (38/60) in the subsequent clinical trial cohort.
4. Discussion
This systematic review and meta‐analysis provides comprehensive estimates of outcomes from common HIV‐associated meningitides in sub‐Saharan Africa and addresses important limitations of previous systematic reviews, including disaggregation of mortality from clinical trial (prone to selection of non‐representative patients as well as more intensive management) and routine care settings, and evaluation of outcomes at different timepoints. Pooled mortality in routine care settings was high, with approximately half of patients diagnosed with cryptococcal, TB and pneumococcal meningitis dying within two weeks or during hospitalization. Our results highlight an overall poor understanding of long‐term outcomes in routine care settings given the lack of published outcomes. Furthermore, most studies reported outcomes for patients treated at referral centres, where staffing, pharmaceutical and laboratory resources are likely better than in lower‐level health facilities. Our findings also suggest significant heterogeneity in mortality overall and between regions: Pooled short‐term mortality for cryptococcal meningitis in routine care settings within Central and West Africa exceeded 50%, compared to 37% in Southern Africa. The large heterogeneity in outcome could be attributable to differences in resources between regions and individual hospital facilities, intensity of care received and time from symptoms onset to presentation to care for patients, among other factors.
Comparing mortality of patients treated under routine care conditions to clinical trial settings, we observed significant differences for cryptococcal meningitis but similar mortality for pneumococcal meningitis. Short‐term mortality for cryptococcal meningitis managed under routine care conditions was twofold higher compared to mortality in clinical trial settings (44% vs. 21%); which remained similar when stratified by treatment regimen (e.g. 41% vs. 23% for amphotericin B without flucytosine). This difference reflects a degree of differences in patients enrolled in clinical trials compared to routine care studies (e.g. exclusion of more severely ill patients, such as those with severe metabolic abnormalities or unable to provide consent, in randomized‐controlled trials). However, this likely also reflects benefits from therapeutic lumbar punctures, standardized intravenous fluid and electrolyte supplementation, and better management of intravenous catheters to prevent thrombophlebitis and catheter‐associated bacteraemia in clinical trials 146. In contrast, short‐term mortality from pneumococcal meningitis differed little between RCTs and routine care settings, perhaps reflecting less intensive management currently recommended for pneumococcal disease and highlighting a need for better management strategies. In routine care settings, more than two‐thirds of patients died during the course of treatment for TB meningitis from two studies, although data were not available to compare these findings with clinical trials. This contrasts with 39% (68/174) nine‐month mortality in HIV‐infected patients with TB meningitis receiving standard treatment in a recent RCT from Vietnam 147. One prospective cohort from South Africa with investigator management reported a 12% (3/34) nine‐month mortality of patients treated for HIV‐associated TB meningitis 42; but this study excluded patients with severe TB and those who did not initiate ART or were judged to have poor drug adherence.
Our findings suggest that the previously estimated 70% one‐year mortality for HIV‐associated cryptococcal meningitis in sub‐Saharan Africa may be conservative in some regions 6, particularly Central and West Africa where pooled short‐term mortality exceeded 50% and long‐term outcomes were not reported. Our findings also suggest that improved availability of more fungicidal – but more toxic – amphotericin B therapy alone may not have a large impact in reducing mortality. Under routine care settings, short‐term mortality for amphotericin‐based and fluconazole‐based regimens was similar at around 40% and long‐term mortality was reported at 65% even with amphotericin B and high‐dose fluconazole in Botswana 56. Recent evidence from a large RCT suggests that, combined with flucytosine (5FC), a shortened one‐week course of amphotericin B is the most effective antifungal regimen to reduce mortality from HIV‐associated cryptococcal meningitis 7, with similar rates of early fungal clearance compared to longer two‐week courses and less drug toxicity 30. Of a subset of participants from Malawi in this trial 105, mortality remained relatively low at 28% (11/40) at one year in the group treated with a one‐week course of amphotericin B combined with flucytosine. Thus, alongside expanding access to amphotericin B formulations, there is an urgent need for flucytosine access in sub‐Saharan Africa as well as future outcomes data evaluating this regimen in usual care settings. Furthermore, standardized intravenous fluid and electrolyte supplementation and therapeutic lumbar puncture schedules are associated with improved outcomes in HIV‐associated cryptococcal meningitis and care checklists and protocols could be operationalized even in many resource‐limited settings to reduce mortality 11, 13.
Our study has a number of important limitations. First, we applied strict diagnostic criteria. This resulted in the exclusion of a number of TB meningitis studies in setting where microbiological tests (e.g. culture or PCR) were not available or a majority of cases were diagnosed based on clinical suspicion without clear diagnostic standards. This may have over‐sampled cases from clinical settings with better diagnostic capability (e.g. TB PCR) or cases of TB meningitis with a higher CSF bacillary burden. We found higher mortality in studies with only microbiologically confirmed cases than in studies with most cases diagnosed using combined laboratory, clinical, and/or imaging features. Second, in comparing outcomes for cryptococcal meningitis between routine care and clinical trial settings, we were unable to determine the proportion of excess mortality in routine care settings attributable to different factors (e.g. lack of therapeutic lumbar punctures vs. poor laboratory monitoring vs. poor completion of therapy). In reality, the high mortality in routine care settings is likely multi‐factorial and our findings highlight the need for better management strategies such as protocolized care, less toxic short‐course therapy, and better preventive strategies including CrAg screening for individuals with advanced HIV/AIDS 21. Third, the classification of some prospective cohort studies as clinical trials vs. routine care studies required some judgment by the author team. Furthermore, patient enrolment in prospective cohorts considered “routine care” studies may have led to better clinical management from local clinicians as well as some selection bias. Finally, duration of follow‐up varied between studies evaluating short‐term mortality, and observational studies of cryptococcal meningitis typically reported in‐hospital rather than two‐week mortality. This may have biased results slightly toward a higher acute mortality in routine care settings if length of stay was longer on average than two weeks; however, the average length of hospitalization reported from most studies was about two weeks.
5. Conclusions
In conclusion, we found very high mortality from common HIV‐associated meningitides in sub‐Saharan Africa, with pooled short‐term mortality for pneumococcal meningitis around 50% and long‐term mortality for TB and cryptococcal meningitis well in excess of 50% but with an overall lack of long‐term outcomes data. Importantly, we found a significant degree of heterogeneity in outcomes between different regions of SSA, likely reflecting both relative resource‐availability and other differences in management. Improved management strategies are needed and prevention efforts should be prioritized, including CrAg screening for prevention of cryptococcal meningitis and childhood pneumococcal vaccination to provide some population herd immunity against invasive pneumococcal disease 148.
Competing interest
The authors report no relevant conflicts of interest.
Authors’ contributions
MWT, BLG, CF and JNJ designed the research study. MWT, AMG, DSL, NKW and JNJ performed the research. MWT analysed the data and wrote the primary draft of the manuscript. All authors contributed to subsequent drafts and approved the final version of the manuscript.
Supporting information
Appendix S1. Details of included and excluded studies and additional analyses.
Figure S1. Pooled short‐term mortality of cryptococcal meningitis in routine care settings, by region.
Figure S2. Pooled medium‐term mortality of cryptococcal meningitis in routine care settings, by region.
Figure S3. Pooled long‐term mortality of cryptococcal meningitis in routine care settings, by region.
Figure S4. Pooled medium‐term mortality of cryptococcal meningitis in clinical trial settings.
Figure S5. Pooled long‐term mortality of cryptococcal meningitis in clinical trial settings.
Acknowledgements
None declared.
1. Funding
The research was commissioned in part by the National Institute for Health Research using Official Development Assistance (ODA) funding through a Global Health Professorship (RP‐2017‐08‐ST2‐012) and CDC Foundation support to JNJ, MWT received salary support from the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health (NIH) (grant F32AI140511). The views expressed are those of the authors and not necessarily those of the NHS, NIHR, the Department of Health and Social Care, the NIH, or the CDC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Tenforde, M. W. , Gertz, A. M. , Lawrence, D. S. , Wills, N. K. , Guthrie, B. L. , Farquhar, C. and Jarvis, J. N. . Mortality from HIV‐associated meningitis in sub‐Saharan Africa: a systematic review and meta‐analysis. J Intern AIDS Soc. 2020; 23(1):e25416
References
- 1. UNAIDS . Fact sheet ‐ latest statistics on the status of the AIDS epidemic. [cited 2018 Apr 23] 2018. Available from: http://www.unaids.org/en/resources/fact-sheet
- 2. Avila D, Althoff KN, Mugglin C, Wools‐Kaloustian K, Koller M, Dabis F, et al. Immunodeficiency at the start of combination antiretroviral therapy in low‐, middle‐, and high‐income countries. J Acquir Immune Defic Syndr. 2014;65(1):e8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub‐Saharan Africa, 2002–2013: a meta‐analysis. Clin Infect Dis. 2015;60(7):1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis. 2010;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wall EC, Everett DB, Mukaka M, Bar‐Zeev N, Feasey N, Jahn A, et al. Bacterial meningitis in Malawian adults, adolescents, and children during the era of antiretroviral scale‐up and Haemophilus influenzae type B vaccination, 2000–2012. Clin Infect Dis. 2014;58(10):e137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV‐associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17(8):873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Molloy SF, Kanyama C, Heyderman RS, Loyse A, Kouanfack C, Chanda D, et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med. 2018;378(11):1004–17. [DOI] [PubMed] [Google Scholar]
- 8. Tenforde MW, Jarvis JN. HIV‐associated cryptococcal meningitis: ongoing challenges and new opportunities. Lancet Infect Dis. 2019;19(8):793–4. [DOI] [PubMed] [Google Scholar]
- 9. Bicanic T, Bottomley C, Loyse A, Brouwer AE, Muzoora C, Taseera K, et al. Toxicity of Amphotericin B deoxycholate‐based induction therapy in patients with HIV‐associated cryptococcal meningitis. Antimicrob Agents Chemother. 2015;59(12):7224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV‐infected adults, adolescence and children. Geneva: WHO; 2018. [PubMed] [Google Scholar]
- 11. Meda J, Kalluvya S, Downs JA, Chofle AA, Seni J, Kidenya B, et al. Cryptococcal meningitis management in Tanzania with strict schedule of serial lumber punctures using intravenous tubing sets: an operational research study. J Acquir Immune Defic Syndr. 2014;66(2):e31–6. [DOI] [PubMed] [Google Scholar]
- 12. Rolfes MA, Hullsiek KH, Rhein J, Nabeta HW, Taseera K, Schutz C, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis. 2014;59(11):1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bahr NC, Rolfes MA, Musubire A, Nabeta H, Williams DA, Rhein J, et al. Standardized electrolyte supplementation and fluid management improves survival during amphotericin therapy for cryptococcal meningitis in resource‐limited settings. Open Forum. Infect Dis. 2014;1(2):ofu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370(26):2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marra F, Marra CA, Bruchet N, Richardson K, Moadebi S, Elwood RK, et al. Adverse drug reactions associated with first‐line anti‐tuberculosis drug regimens. Int J Tuberc Lung Dis. 2007;11(8):868–75. [PubMed] [Google Scholar]
- 16. Naidoo P, Theron G, Rangaka MX, Chihota VN, Vaughan L, Brey ZO, et al. The South African Tuberculosis Care Cascade: Estimated Losses and Methodological Challenges. J Infect Dis. 2017;216 suppl_7:S702–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Køster‐Rasmussen R, Korshin A, Meyer CN. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008;57(6):449–54. [DOI] [PubMed] [Google Scholar]
- 18. Pasquier E, Kunda J, De Beaudrap P, Loyse A, Temfack E, Molloy SF, et al. Long‐term mortality and disability in cryptococcal meningitis: a systematic literature review. Clin Infect Dis. 2018;66(7):1122–32. [DOI] [PubMed] [Google Scholar]
- 19. Woldeamanuel YW, Girma B. A 43‐year systematic review and meta‐analysis: case‐fatality and risk of death among adults with tuberculous meningitis in Africa. J Neurol. 2014;261(5):851–65. [DOI] [PubMed] [Google Scholar]
- 20. Ramakrishnan M, Ulland AJ, Steinhardt LC, Moïsi JC, Were F, Levine OS. Sequelae due to bacterial meningitis among African children: a systematic literature review. BMC Med. 2009;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, et al. Cryptococcal meningitis screening and community‐based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open‐label, randomised controlled trial. Lancet. 2015;385(9983):2173–82. [DOI] [PubMed] [Google Scholar]
- 22. Beardsley J, Wolbers M, Kibengo FM, Ggayi AB, Kamali A, Cuc NT, et al. Adjunctive dexamethasone in hiv‐associated cryptococcal meningitis. N Engl J Med. 2016;374(6):542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heyderman RS, Gangaidzo IT, Hakim JG, Mielke J, Taziwa A, Musvaire P, et al. Cryptococcal meningitis in human immunodeficiency virus‐infected patients in Harare, Zimbabwe. Clin Infect Dis. 1998;26(2):284–9. [DOI] [PubMed] [Google Scholar]
- 24. Sunpath H, Edwin C, Chelin N, Nadesan S, Maharaj R, Moosa Y, et al. Operationalizing early antiretroviral therapy in HIV‐infected in‐patients with opportunistic infections including tuberculosis. Int J Tuberc Lung Dis. 2012;16(7):917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–12. [DOI] [PubMed] [Google Scholar]
- 26. Covidence systematic review software , Veritas Health Innovation; Melbourne, Australia: [cited 2019 Nov 10]. Available from: https://www.covidence.org [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller J. The inverse of the Freeman‐Tukey double arcsine transformation. Am Stat. 1978;32:138. [Google Scholar]
- 29. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta‐analysis of binomial data. Arch Public Health. 2014;72(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tenforde MW, Shapiro AE, Rouse B, Jarvis JN, Li T, Eshun‐Wilson I, et al. Treatment for HIV‐associated cryptococcal meningitis. Cochrane Database Syst Rev. 2018;7:CD005647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Day JN, Chau TT, Lalloo DG. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med. 2013;368(26):2522–3. [DOI] [PubMed] [Google Scholar]
- 32. United Nations Statistics Division: Africa geoscheme. [cited 2019 Feb21]. Available from: http://millenniumindicators.un.org/unsd/methods/m49/Un
- 33. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bergemann A, Karstaedt AS. The spectrum of meningitis in a population with high prevalence of HIV disease. QJM. 1996;89(7):499–504. [DOI] [PubMed] [Google Scholar]
- 35. Rothe C, Sloan DJ, Goodson P, Chikafa J, Mukaka M, Denis B, et al. A prospective longitudinal study of the clinical outcomes from cryptococcal meningitis following treatment induction with 800 mg oral fluconazole in Blantyre, Malawi. PLoS One. 2013;8:e67311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaskell KM, Rothe C, Gnanadurai R, Goodson P, Jassi C, Heyderman RS, et al. A prospective study of mortality from cryptococcal meningitis following treatment induction with 1200 mg oral fluconazole in Blantyre, Malawi. PLoS One. 2014;9:e110285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scriven JE, Graham LM, Schutz C, Scriba TJ, Wilkinson KA, Wilkinson RJ, et al. A glucuronoxylomannan‐associated immune signature, characterized by monocyte deactivation and an increased interleukin 10 level, is a predictor of death in cryptococcal meningitis. J Infect Dis. 2016;213(11):1725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adeyemi B, Ross A. Profile and mortality outcome of patients admitted with cryptococcal meningitis to an urban district hospital in KwaZulu‐Natal, South Africa. J Int AIDS Soc. 2014;17 4 suppl 3:19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hiesgen J, Schutte C, Olorunju S, Retief J. Cryptococcal meningitis in a tertiary hospital in Pretoria, mortality and risk factors ‐ a retrospective cohort study. Int J Std AIDS. 2017;28(5):480–5. [DOI] [PubMed] [Google Scholar]
- 40. Jarvis JN, Meintjes G, Harrison TS. Outcomes of cryptococcal meningitis in antiretroviral naïve and experienced patients in South Africa. J Infect. 2010;60(6):496–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lessells RJ, Mutevedzi PC, Heller T, Newell ML. Poor long‐term outcomes for cryptococcal meningitis in rural South Africa. S Afr Med J. 2011;101(4):251–2. [DOI] [PubMed] [Google Scholar]
- 42. Marais S, Meintjes G, Lesosky M, Wilkinson KA, Wilkinson RJ. Interleukin‐17 mediated differences in the pathogenesis of HIV‐1‐associated tuberculous and cryptococcal meningitis. AIDS. 2016;30(3):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCarthy KM, Morgan J, Wannemuehler KA, Mirza SA, Gould SM, Mhlongo N, et al. Population‐based surveillance for cryptococcosis in an antiretroviral‐naive South African province with a high HIV seroprevalence. AIDS. 2006;20(17):2199–206. [DOI] [PubMed] [Google Scholar]
- 44. Meiring ST, Quan VC, Cohen C, Dawood H, Karstaedt AS, McCarthy KM, et al. A comparison of cases of paediatric‐onset and adult‐onset cryptococcosis detected through population‐based surveillance, 2005–2007. AIDS. 2012;26(18):2307–14. [DOI] [PubMed] [Google Scholar]
- 45. Meiring S, Fortuin‐de Smidt M, Kularatne R, Dawood H, Govender NP, GERMS‐SA. Prevalence and Hospital Management of Amphotericin B Deoxycholate‐Related Toxicities during Treatment of HIV‐Associated Cryptococcal Meningitis in South Africa. PLoS Negl Trop Dis. 2016;10:e0004865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moosa MY, Coovadia YM. Cryptococcal meningitis in Durban, South Africa: a comparison of clinical features, laboratory findings, and outcome for human immunodeficiency virus (HIV)‐positive and HIV‐negative patients. Clin Infect Dis. 1997;24(2):131–4. [DOI] [PubMed] [Google Scholar]
- 47. Schutte CM, Van der Meyden CH, Magazi DS. The impact of HIV on meningitis as seen at a South African Academic Hospital (1994 to 1998). Infection. 2000;28(1):3–7. [DOI] [PubMed] [Google Scholar]
- 48. Sogbanmu OO, John MA, Lalloo U. Management of cryptococcal meningitis in adults at Mthatha Hospital Complex, Eastern Cape, South Africa. S Afr J HIV Med. 2014;15(3):104–7. [Google Scholar]
- 49. Von Pressentin K, Conradie HH, Mash R. A medical audit of the management of cryptococcal meningitis in HIV‐positive patients in the Cape Winelands (East) district, Western Cape. S Afr Fam Pract. 2012;54(4):339–46. [Google Scholar]
- 50. Berhe T, Melkamu Y, Amare A. The pattern and predictors of mortality of HIV/AIDS patients with neurologic manifestation in Ethiopia: a retrospective study. AIDS Res Ther. 2012;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Beyene T, Zewde AG, Balcha A, Hirpo B, Yitbarik T, Gebissa T, et al. High dose fluconazole monotherapy is inadequate for CSF cryptococcal antigen positive HIV‐infected persons in an Ethiopian CrAg screening program. Clin Infect Dis. 2017;65(12):2126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mamuye AT, Bornstein E, Temesgen O, Blumberg HM, Kempker RR. Point‐of‐care testing for cryptococcal disease among hospitalized human immunodeficiency virus‐infected adults in Ethiopia. Am J Trop Med Hygiene. 2016;95(4):786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Majwala A, Burke R, Patterson W, Pinkerton R, Muzoora C, Wilson LA, et al. Handheld point‐of‐care cerebrospinal fluid lactate testing predicts bacterial meningitis in Uganda. Am J Trop Med Hyg. 2013;88(1):127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Namutebi AM, Kamya MR, Byakika‐Kibwika P. Causes and outcome of hospitalization among HIV‐infected adults receiving antiretroviral therapy in Mulago hospital, Uganda. Afr Health Sci. 2013;13(4):977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trachtenberg JD, Kambugu AD, McKellar M, Semitala F, Mayanja‐Kizza H, Samore MH, et al. The medical management of central nervous system infections in Uganda and the potential impact of an algorithm‐based approach to improve outcomes. Int J Infect Dis. 2007;11(6):524–30. [DOI] [PubMed] [Google Scholar]
- 56. Patel RKK, Leeme T, Azzo C, Tlhako N, Tsholo K, Tawanana EO, et al. High mortality in HIV‐associated cryptococcal meningitis treated with amphotericin B‐based therapy under routine care conditions in Africa. Open Forum. Infect Dis. 2018;5(11):ofy267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Steele KT, Thakur R, Nthobatsang R, Steenhoff AP, Bisson GP. In‐hospital mortality of HIV‐infected cryptococcal meningitis patients with C. gattii and C. neoformans infection in Gaborone, Botswana. Med Mycol. 2010;48(8):1112–5. [DOI] [PubMed] [Google Scholar]
- 58. Bamba S, Barro‐Traoré F, Sawadogo E, Millogo A, Guiguemdé RT. Retrospective study of cases of neuromeningeal cryptococcosis at the University Hospital of Bobo Dioulasso since accessibility to antiretroviral in Burkina Faso. J Mycol Med. 2012;22(1):30–4. [DOI] [PubMed] [Google Scholar]
- 59. Millogo A, Ki‐Zerbo GA, Andonaba JB, Lankoandé D, Sawadogo A, Yaméogo I, et al. Cryptococcal meningitis in HIV‐infected patients at Bobo‐Dioulasso hospital (Burkina Faso). Bull Soc Pathol Exot. 2004;97(2):119–21. [PubMed] [Google Scholar]
- 60. Luma HN, Tchaleu BC, Temfack E, Doualla MS, Ndenga DP, Mapoure YN, et al. HIV‐associated central nervous system disease in patients admitted at the Douala general hospital between 2004 and 2009: a retrospective study. AIDS Res Treat. 2013;2013:709810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mbuagbaw JN, Biholong Njamnshi AK. La cryptococcoses neuro‐meningee et l'infection au VIH dans le service de medicine du Centre Hospitalier et Universitaire de Yaounde, Cameroon. Afr J Neurol Sci. 2006;25(2):13–20. [Google Scholar]
- 62. Yassibanda S, Kamalo CG, Mbolidi CD, Koffi B, Camengo SM, Akelelo N, et al. Les infections neuromeningees de l'adulte en milieu hospitalier a Bangui: aspects étiologiques cliniques et évolutifs. Méd Afrique Noire. 2002;49(6):299–303. [Google Scholar]
- 63. Traore FA, Cissoko Y, Tounkara TM, Sako FB, Mouelle AD, Kpami DO, et al. Étiologies des méningites lymphocytaires chez les personnes vivant avec le VIH suivies dans le service des maladies infectieuses de Conakry. Méd Santé Trop. 2015;25:52–5. [DOI] [PubMed] [Google Scholar]
- 64. Oumar AA, Dao S, Ba M, Poudiougou B, Diallo A. Epidemiological, clinical and prognostic aspects of cryptococcal meningitis in hospital area of Bamako, Mali. Rev Med Brux. 2008;29(3):149–52. [PubMed] [Google Scholar]
- 65. Agaba PA, Digin E, Makai R, Apena L, Agbaji OO, Idoko JA, et al. Clinical characteristics and predictors of mortality in hospitalized HIV‐infected Nigerians. J Infect Develop Count. 2011;5(5):377–82. [DOI] [PubMed] [Google Scholar]
- 66. Kisenge PR, Hawkins AT, Maro VP, McHele JP, Swai NS, Mueller A, et al. Low CD4 count plus coma predicts cryptococcal meningitis in Tanzania. BMC Infect Dis. 2007;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Siddiqi OK, Ghebremichael M, Dang X, Atadzhanov M, Kaonga P, Khoury MN, et al. Molecular diagnosis of central nervous system opportunistic infections in HIV‐infected Zambian adults. Clin Infect Dis. 2014;58(12):1771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nyazika TK, Hagen F, Machiridza T, Kutepa M, Masanganise F, Hendrickx M, et al. Cryptococcus neoformans population diversity and clinical outcomes of HIV‐associated cryptococcal meningitis patients in Zimbabwe. J Med Microbiol. 2016;65(11):1281–8. [DOI] [PubMed] [Google Scholar]
- 69. Gbangba‐Ngai E, Fikouma V, Mossoro‐Kpinde CD, Tekpa G, Ouavene JO, Yangba Mongba DS, et al. Cryptococcal neuromeningitidis in HIV‐infected patients in Bangui, in the era of antiretroviral treatment. Bull Soc Pathol Exot. 2014;107(2):106–9. [DOI] [PubMed] [Google Scholar]
- 70. Kouakou GA, Ello NF, Kassi NA, Keita M, Doumbia A, Mossou C, et al. Fluconazole 1200mg or 800mg for cryptococcal meningitis treatment in Ivory Coast. J Mycol Med. 2017;27(1):72–8. [DOI] [PubMed] [Google Scholar]
- 71. Aoussi EF, Ehui E, Dembélé JP, Kolia‐Diafouka P, Elloh NF, Ouattara SI, et al. Cryptoccocal meningitis and HIV in the era of HAART in Côte d'Ivoire. Med Mal Infect. 2012;42(8):349–54. [DOI] [PubMed] [Google Scholar]
- 72. Soumaré M, Seydi M, Ndour CT, Fall N, Dieng Y, Sow AI, et al. Epidemiological, clinical, etiological features of neuromeningeal diseases at the Fann Hospital Infectious Diseases Clinic, Dakar (Senegal). Med Mal Infect. 2005;35(7–8):383–9. [DOI] [PubMed] [Google Scholar]
- 73. Baldassarre R, Mdodo R, Omonge E, Jaoko W, Baddley J, Pappas P, et al. Mortality after clinical management of aids‐associated cryptococcal meningitis in Kenya. East Afr Med J. 2014;91(5):145–51. [PMC free article] [PubMed] [Google Scholar]
- 74. Siika AM, Ayuo PO, Sidle MJ, Wools‐Kaloustian K, Kimaiyo SN, Tierney WM. Admission characteristics, diagnoses and outcomes of HIV‐infected patients registered in an ambulatory HIV‐care programme in western Kenya. East Afr Med J. 2008;85(11):523–8. [DOI] [PubMed] [Google Scholar]
- 75. Wake RM, Britz E, Sriruttan C, Rukasha I, Omar T, Spencer DC, et al. High cryptococcal antigen titers in blood are predictive of subclinical cryptococcal meningitis among HIV‐infected patients. Clin Infect Dis. 2017;66:686‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jarvis JN, Meintjes G, Williams Z, Rebe K, Harrison TS. Symptomatic relapse of HIV‐associated cryptococcal meningitis in South Africa: the role of inadequate secondary prophylaxis. S Afr Med J. 2010;100(6):378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ousley J, Niyibizi AA, Wanjala S, Vandenbulcke A, Kirubi B, Omwoyo W, et al. High proportions of patients with advanced HIV are antiretroviral therapy experienced: hospitalization outcomes from 2 Sub‐Saharan African sites. Clin Infect Dis. 2018;66 suppl_2:S126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Park BJ, Shetty S, Ahlquist A, Greenbaum A, Miller JL, Motsi A, et al. Long‐term follow‐up and survival of antiretroviral‐naive patients with cryptococcal meningitis in the pre‐antiretroviral therapy era, Gauteng Province. S Afr Int J STD AIDS. 2011;22(4):199–203. [DOI] [PubMed] [Google Scholar]
- 79. Mwaba P, Mwansa J, Chintu C, Pobee J, Scarborough M, Portsmouth S, et al. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad Med J. 2001;77(914):769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maher D, Mwandumba H. Cryptococcal meningitis in Lilongwe and Blantyre, Malawi. J Infect. 1994;28(1):59–64. [DOI] [PubMed] [Google Scholar]
- 81. Wang W, Carm AR. A clinical manifestation of AIDS with cryptococcal meningitis in Equatorial Guinea. Trop Doct. 2001;31(4):221–2. [DOI] [PubMed] [Google Scholar]
- 82. Seboxa T, Alemu S, Assefa A, Asefa A, Diro E. Cryptococcal meningitis in patients with acquired immunudeficiency syndrome in prehaart era at Gondar College of Medical Sciences Hospital north‐west Ethiopia. Ethiop Med J. 2010;48(3):237–41. [PubMed] [Google Scholar]
- 83. Eholie SP, N'gbocho L, Bissagnene E, Coulibaly M, Ehui E, Kra O, et al. Mycoses profondes au cours du sida à Abidjan (Côte d'Ivoire). Bull Soc Path Exot. 1997;90(5):307–11. [PubMed] [Google Scholar]
- 84. Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, et al. Determinants of mortality in a combined cohort of 501 patients with HIV‐associated Cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis. 2014;58(5):736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral‐naive or antiretroviral‐experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45(1):76–80. [DOI] [PubMed] [Google Scholar]
- 86. Bicanic T, Wood R, Meintjes G, Rebe K, Brouwer A, Loyse A, et al. High‐dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV‐infected patients: a randomized trial. Clin Infect Dis. 2008;47(1):123–30. [DOI] [PubMed] [Google Scholar]
- 87. Jarvis JN, Meintjes G, Rebe K, Williams GN, Bicanic T, Williams A, et al. Adjunctive interferon‐γ immunotherapy for the treatment of HIV‐associated cryptococcal meningitis: a randomized controlled trial. AIDS. 2012;26(9):1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lightowler JV, Cooke GS, Mutevedzi P, Lessells RJ, Newell ML, Dedicoat M. Treatment of cryptococcal meningitis in KwaZulu‐Natal, South Africa. PLoS One. 2010;5:e8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Loyse A, Wilson D, Meintjes G, Jarvis JN, Bicanic T, Bishop L, et al. Comparison of the early fungicidal activity of high‐dose fluconazole, voriconazole, and flucytosine as second‐line drugs given in combination with amphotericin B for the treatment of HIV‐associated cryptococcal meningitis. Clin Infect Dis. 2012;54(1):121–8. [DOI] [PubMed] [Google Scholar]
- 90. Chang CC, Dorasamy AA, Gosnell BI, Elliott JH, Spelman T, Omarjee S, et al. Clinical and mycological predictors of cryptococcosis‐associated immune reconstitution inflammatory syndrome. AIDS. 2013;27(13):2089–99. [DOI] [PubMed] [Google Scholar]
- 91. Kambugu A, Meya DB, Rhein J, O'Brien M, Janoff EN, Ronald AR, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46(11):1694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Katwere M, Kambugu A, Piloya T, Wong M, Hendel‐Paterson B, Sande MA, et al. Clinical presentation and aetiologies of acute or complicated headache among HIV‐seropositive patients in a Ugandan clinic. J Int AIDS Soc. 2009;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Longley N, Muzoora C, Taseera K, Mwesigye J, Rwebembera J, Chakera A, et al. Dose response effect of high‐dose fluconazole for HIV‐associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. 2008;47(12):1556–61. [DOI] [PubMed] [Google Scholar]
- 94. Muzoora CK, Kabanda T, Ortu G, Ssentamu J, Hearn P, Mwesigye J, et al. Short course amphotericin B with high dose fluconazole for HIV‐associated cryptococcal meningitis. J Infect. 2012;64(1):76–81. [DOI] [PubMed] [Google Scholar]
- 95. Tugume L, Rhein J, Hullsiek KH, Mpoza E, Kiggundu R, Ssebambulidde K, et al. HIV‐associated Cryptococcal Meningitis Occurring at Relatively Higher CD4 counts. J Infect Dis. 2019;219(6):877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jackson AT, Nussbaum JC, Phulusa J, Namarika D, Chikasema M, Kanyemba C, et al. A phase II randomized controlled trial adding oral flucytosine to high‐dose fluconazole, with short‐course amphotericin B, for cryptococcal meningitis. AIDS. 2012;26(11):1363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nussbaum JC, Jackson A, Namarika D, Phulusa J, Kenala J, Kanyemba C, et al. Combination flucytosine and high‐dose fluconazole compared with fluconazole monotherapy for the treatment of cryptococcal meningitis: a randomized trial in Malawi. Clin Infect Dis. 2010;50(3):338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jarvis JN, Leeme TB, Molefi M, Chofle AA, Bidwell G, Tsholo K, et al. Short Course high‐dose liposomal amphotericin b for HIV‐associated cryptococcal meningitis: a phase‐II randomized controlled trial. Clin Infect Dis. 2019;68(3):393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ochieng PO, McLigeyo SO, Amayo EO, Kayima JK, Omonge EO. Nephrotoxicity of amphotericin B in the treatment of cryptococcal meningitis in acquired immunodeficiency syndrome patients. East Afr Med J. 2009;86(9):435–41. [DOI] [PubMed] [Google Scholar]
- 100. Makadzange AT, Ndhlovu CE, Takarinda K, Reid M, Kurangwa M, Gona P, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub‐saharan Africa. Clin Infect Dis. 2010;50(11):1532–8. [DOI] [PubMed] [Google Scholar]
- 101. Joly V, Aubry P, Ndayiragide A, Carrière I, Kawa E, Mlika‐Cabanne N, et al. Randomized comparison of amphotericin B deoxycholate dissolved in dextrose or Intralipid for the treatment of AIDS‐associated cryptococcal meningitis. Clin Infect Dis. 1996;23(3):556–62. [DOI] [PubMed] [Google Scholar]
- 102. Rhein J, Morawski BM, Hullsiek KH, Nabeta HW, Kiggundu R, Tugume L, et al. Efficacy of adjunctive sertraline for the treatment of HIV‐associated cryptococcal meningitis: an open‐label dose‐ranging study. Lancet Infect Dis. 2016;16(7):809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mayanja‐Kizza H, Oishi K, Mitarai S, Yamashita H, Nalongo K, Watanabe K, et al. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clin Infect Dis. 1998;26(6):1362–6. [DOI] [PubMed] [Google Scholar]
- 104. Katende A, Mbwanji G, Faini D, Nyuri A, Kalinjuma AV, Mnzava D, et al. Short course amphotericin B in addition to sertraline and fluconazole for treatment of HIV‐associated cryptococcal meningitis in rural Tanzania. Mycoses. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kanyama C, Molloy SF, Chan AK, Lupiya D, Chawinga C, Adams J, et al. One year mortality outcomes from the ACTA trial of cryptococcal meningitis treatment in Malawi. Clin Infect Dis. 2019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rhein J, Huppler Hullsiek K, Tugume L, Nuwagira E, Mpoza E, Evans EE, et al. Adjunctive sertraline for HIV‐associated cryptococcal meningitis: a randomised, placebo‐controlled, double‐blind phase 3 trial. Lancet Infect Dis. 2019;19(8):843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Raberahona M, Rakotoarivelo RA, Razafinambinintsoa T, Andrianasolo RL, Randria MJ. Clinical features and outcome in adult cases of tuberculous meningitis in tertiary care hospital in Antananarivo, Madagascar. Biomed Res Int. 2017;2017:9316589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Siddiqi OK, Birbeck GL, Ghebremichael M, Mubanga E, Love S, Buback C, et al. Prospective cohort study on performance of cerebrospinal fluid (CSF) xpert MTB/RIF, CSF lipoarabinomannan (LAM) lateral flow assay (LFA), and urine LAM LFA for diagnosis of tuberculous meningitis in Zambia. J Clin Microbiol. 2019;57(8):pii: e00652‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hakim JG, Gangaidzo IT, Heyderman RS, Mielke J, Mushangi E, Taziwa A, et al. Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS. 2000;14(10):1401–7. [DOI] [PubMed] [Google Scholar]
- 110. Karstaedt AS, Valtchanova S, Barriere R, Crewe‐Brown HH. Tuberculous meningitis in South African urban adults. QJM. 1998;91(11):743–7. [DOI] [PubMed] [Google Scholar]
- 111. Ogun SA, Ojini F, Okubadejo N, Danesi M, Kolapo K, Osalusi B. Pattern and outcome of neurological manifestations of HIV/AIDS ‐ a review of 154 cases in a Nigerian University Teaching Hospital ‐ a preliminary report. Afr J Neurol Sci. 2005;24(1):29–36. [Google Scholar]
- 112. Schutte CM. Clinical, cerebrospinal fluid and pathological findings and outcomes in HIV‐positive and HIV‐negative patients with tuberculous meningitis. Infection. 2001;29(4):213–7. [DOI] [PubMed] [Google Scholar]
- 113. Bahr NC, Nuwagira E, Evans EE, Cresswell FV, Bystrom PV, Byamukama A, et al. Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV‐infected adults: a prospective cohort study. Lancet Infect Dis. 2018;18(1):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tenforde MW, Mokomane M, Leeme TB, Tlhako N, Tsholo K, Chebani T, et al. Mortality in adult patients with culture‐positive and culture‐negative meningitis in the Botswana national meningitis survey: a prevalent cohort study. Lancet Infect Dis. 2019;19(7):740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pepper DJ, Marais S, Maartens G, Rebe K, Morroni C, Rangaka MX, et al. Neurologic manifestations of paradoxical tuberculosis‐associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis. 2009;48(11):e96–107. [DOI] [PubMed] [Google Scholar]
- 116. Patel VB, Padayatchi N, Bhigjee AI, Allen J, Bhagwan B, Moodley AA, et al. Multidrug‐resistant tuberculous meningitis in KwaZulu‐Natal, South Africa. Clin Infect Dis. 2004;38(6):851–6. [DOI] [PubMed] [Google Scholar]
- 117. Marais S, Roos I, Mitha A, Mabusha SJ, Patel V, Bhigjee AI. Spinal tuberculosis: clinicoradiological findings in 274 patients. Clin Infect Dis. 2018;67(1):89–98. [DOI] [PubMed] [Google Scholar]
- 118. Burton NT, Forson A, Lurie MN, Kudzawu S, Kwarteng E, Kwara A. Factors associated with mortality and default among patients with tuberculosis attending a teaching hospital clinic in Accra, Ghana. Trans R Soc Trop Med Hyg. 2011;105(12):675–82. [DOI] [PubMed] [Google Scholar]
- 119. Jowi JO, Mativo PM, Musoke SS. Clinical and laboratory characteristics of hospitalised patients with neurological manifestations of HIV/AIDS at the Nairobi hospital. East Afr Med J. 2007;84(2):67–76. [DOI] [PubMed] [Google Scholar]
- 120. Patel VB, Burger I, Connolly C. Temporal evolution of cerebrospinal fluid following initiation of treatment for tuberculous meningitis. S Afr Med J. 2008;98(8):610–3. [PubMed] [Google Scholar]
- 121. Cresswell FV, Bangdiwala AS, Bahr NC, Trautner E, Nuwagira E, Ellis J, et al. Can improved diagnostics reduce mortality from Tuberculous meningitis? Findings from a 6.5‐year cohort in Uganda. Wellcome Open Res. 2018;3:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Patel VB, Singh R, Connolly C, Coovadia Y, Peer AK, Parag P, et al. Cerebrospinal T‐cell responses aid in the diagnosis of tuberculous meningitis in a human immunodeficiency virus‐ and tuberculosis‐endemic population. Am J Respir Crit Care Med. 2010;182(4):569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bahr NC, Tugume L, Rajasingham R, Kiggundu R, Williams DA, Morawski B, et al. Improved diagnostic sensitivity for tuberculous meningitis with Xpert(®) MTB/RIF of centrifuged CSF. Int J Tuberc Lung Dis. 2015;19(10):1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chapp‐Jumbo EN. Neurologic infections in a Nigerian university teaching hospital. Afr Health Sci. 2006;6(1):55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gordon SB, Walsh AL, Chaponda M, Gordon MA, Soko D, Mbwvinji M, et al. Bacterial meningitis in Malawian adults: pneumococcal disease is common, severe, and seasonal. Clin Infect Dis. 2000;31(1):53–7. [DOI] [PubMed] [Google Scholar]
- 126. Thinyane KH, Motsemme KM, Cooper VJ. Clinical Presentation, Aetiology, and Outcomes of Meningitis in a Setting of High HIV and TB Prevalence. J Trop Med. 2015;2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Boisier P, Maïnassara HB, Sidikou F, Djibo S, Kairo KK, Chanteau S. Case‐fatality ratio of bacterial meningitis in the African meningitis belt: we can do better. Vaccine. 2007;25 suppl 1:A24–9. [DOI] [PubMed] [Google Scholar]
- 128. Cohen C, Naidoo N, Meiring S, de Gouveia L, von Mollendorf C, Walaza S, et al. Streptococcus pneumoniae serotypes and mortality in adults and adolescents in south africa: analysis of national surveillance data, 2003–2008. PLoS One. 2015;10:e0140185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gordon SB, Chaponda M, Walsh AL, Whitty CJ, Gordon MA, Machili CE, et al. Pneumococcal disease in HIV‐infected Malawian adults: acute mortality and long‐term survival. AIDS. 2002;16(10):1409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Manga NM, Ndour CT, Diop SA, Ka‐Sall R, Dia NM, Seydi M, et al. Adult purulent meningitis caused by Streptococcus pneumoniae in Dakar, Senegal. Med Trop (Mars). 2008;68(6):625–8. [PubMed] [Google Scholar]
- 131. Mbelesso P, Tatangba‐Bakozo A, Fikouma V. Les méningites bactériennes de l'adulte en milieu hospitalier centrafricain. Bull Soc Pathol Exot. 2006;99(4):261–3. [PubMed] [Google Scholar]
- 132. Ouattara BES, Adoubryn KD, Kra O, Tia H, Kouadio‐Yapo CG. Étude retrospective des méningites bactériennes et à cryptocoques chez des sujets adultes infectés par le VIH à Abidjan (Côte d'Ivoire). J Mycol Méd. 2007;17:82–6. [Google Scholar]
- 133. Yaro S, Lourd M, Traoré Y, Njanpop‐Lafourcade BM, Sawadogo A, Sangare L, et al. Epidemiological and molecular characteristics of a highly lethal pneumococcal meningitis epidemic in Burkina Faso. Clin Infect Dis. 2006;43(6):693–700. [DOI] [PubMed] [Google Scholar]
- 134. Kleynhans J, Cohen C, McMorrow M, Tempia S, Crowther‐Gibson P, Quan V, et al. Can pneumococcal meningitis surveillance be used to assess the impact of pneumococcal conjugate vaccine on total invasive pneumococcal disease? A case‐study from South Africa, 2005–2016. Vaccine. 2019;37(38):5724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ajdukiewicz KM, Cartwright KE, Scarborough M, Mwambene JB, Goodson P, Molyneux ME, et al. Glycerol adjuvant therapy in adults with bacterial meningitis in a high HIV seroprevalence setting in Malawi: a double‐blind, randomised controlled trial. Lancet Infect Dis. 2011;11(4):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Scarborough M, Gordon SB, Whitty CJ, French N, Njalale Y, Chitani A, et al. Corticosteroids for bacterial meningitis in adults in sub‐Saharan Africa. N Engl J Med. 2007;357(24):2441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wall EC, Mukaka M, Denis B, Mlozowa VS, Msukwa M, Kasambala K, et al. Goal directed therapy for suspected acute bacterial meningitis in adults and adolescents in sub‐Saharan Africa. PLoS One. 2017;12:e0186687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Békondi C, Bernede C, Passone N, Minssart P, Kamalo C, Mbolidi D, et al. Primary and opportunistic pathogens associated with meningitis in adults in Bangui, Central African Republic, in relation to human immunodeficiency virus serostatus. Int J Infect Dis. 2006;10(5):387–95. [DOI] [PubMed] [Google Scholar]
- 139. Boumandouki P, Oyongo A, Yala F. [Clinical, epidemiological and therapeutic aspects of purulent meningitis in the adult. Apropos of 74 cases treated at CHU of Brazzaville (Congo)]. Bull Soc Pathol Exot. 1993;86(2):141–3. [PubMed] [Google Scholar]
- 140. Letsa T, Noora CL, Kuma GK, Asiedu E, Kye‐Duodu G, Afari E, et al. Pneumococcal meningitis outbreak and associated factors in six districts of Brong Ahafo region, Ghana, 2016. BMC Public Health. 2018;18(1):781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Aku FY, Lessa FC, Asiedu‐Bekoe F, Balagumyetime P, Ofosu W, Farrar J, et al. Meningitis Outbreak Caused by Vaccine‐Preventable Bacterial Pathogens ‐ Northern Ghana, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(30):806–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bozio CH, Abdul‐Karim A, Abenyeri J, Abubakari B, Ofosu W, Zoya J, et al. Continued occurrence of serotype 1 pneumococcal meningitis in two regions located in the meningitis belt in Ghana five years after introduction of 13‐valent pneumococcal conjugate vaccine. PLoS One. 2018;13:e0203205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kambiré D, Soeters HM, Ouédraogo‐Traoré R, Medah I, Sangare L, Yaméogo I, et al. Nationwide trends in bacterial meningitis before the introduction of 13‐valent pneumococcal conjugate vaccine‐Burkina Faso, 2011–2013. PLoS One. 2016;11:e0166384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Okome‐Nkoumou M, Loembe PM. [Bacterial meningitis in the adult. Study of 85 cases observed in the infectious disease unit of the Fondation Jeanne Ebori (F.J.E.), Libreville, Gabon]. Bull Soc Pathol Exot. 1999;92(5):288–91. [PubMed] [Google Scholar]
- 145. Traore Y, Tameklo TA, Njanpop‐Lafourcade BM, Lourd M, Yaro S, Niamba D, et al. Incidence, seasonality, age distribution, and mortality of pneumococcal meningitis in Burkina Faso and Togo. Clin Infect Dis. 2009;48 Suppl 2:S181–9. [DOI] [PubMed] [Google Scholar]
- 146. Rajasingham R, Williams D, Meya DB, Meintjes G, Boulware DR, Scriven J. Nosocomial drug‐resistant bacteremia in 2 cohorts with cryptococcal meningitis,Africa. Emerg Infect Dis. 2014;20(4):722–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Heemskerk AD, Bang ND, Mai NT, Chau TT, Phu NH, Loc PP, et al. Intensified antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med. 2016;374(2):124–34. [DOI] [PubMed] [Google Scholar]
- 148. von Gottberg A, de Gouveia L, Tempia S, Quan V, Meiring S, von Mollendorf C, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371(20):1889–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Details of included and excluded studies and additional analyses.
Figure S1. Pooled short‐term mortality of cryptococcal meningitis in routine care settings, by region.
Figure S2. Pooled medium‐term mortality of cryptococcal meningitis in routine care settings, by region.
Figure S3. Pooled long‐term mortality of cryptococcal meningitis in routine care settings, by region.
Figure S4. Pooled medium‐term mortality of cryptococcal meningitis in clinical trial settings.
Figure S5. Pooled long‐term mortality of cryptococcal meningitis in clinical trial settings.


