Abstract
Introduction
Cultured stratified epithelial cell sheets have been clinically utilized as transplantable grafts for the regeneration of epithelial tissues, such as the esophagus, cornea, skin, and intraoral cavity. These cell sheets are expected to gain widespread use as regenerative medicine products and save many patients. For this purpose, establishing and disseminating the stale protocol of fabricating the cell sheet is crucial. The fabrication of cultured stratified epithelial cell sheets consists of many important steps, and since the patients’ epithelial cell conditions vary widely and are sometimes unstable, the qualities of the epithelial cell grafts are likewise potentially unstable. Therefore, in this paper, we report the stable protocol for fabrication of the transplantable cell sheet particularly from patient-derived oral mucosal tissues.
Methods
Serum extracted from blood and buccal mucosal tissue were collected in Nagasaki University and transported to Tokyo Women's Medical University. Oral mucosal epithelial cells were collected by minimum trypsin method, and this treatment was studied whether to be a critical procedure. After 14 days cultivation, cultured cells were examined whether to be transplantable as cell sheets.
Results
We successfully transported buccal mucosal tissue and serum without damage and contamination. Oral mucosal epithelial cells were collected with high viability by minimum trypsin method. Finally, we succeeded to stably fabricate oral mucosal epithelial cell sheets in all 10 patients.
Conclusions
We established a stable protocol for the fabrication of human oral mucosal epithelial cell sheets and their transportation in clinical settings in this study. These methodologies could also be basis for transplantation therapy using cultured cell sheets of various types other than oral mucosal epithelial cell and will contribute largely to the future development of regenerative medicine.
Keywords: Primary cell culture, Minimum trypsinization, Epithelial cell sheet, Clinical application
Abbreviations: Dulbecco's modified Eagle's medium, (DMEM); epithelial growth factor, (EGF); poly(N-isopropyl acrylamide), (PIPAAm); keratinocyte culture medium, (KCM); DMEM with antibiotics, (DMEM-AB); polymerase chain reaction, (PCR)
Highlights
-
•
A stable protocol for fabricating oral mucosal cell sheets.
1. Introduction
Regenerative therapy using cultured cell grafts has recently developed as a novel and promising technology for future therapeutics, with the history of regenerative therapy only tracing back to 1979 [1]. In the early studies of establishing the primary culture, epidermal keratinocytes were cultured using keratinocyte culture media (e.g., DMEM and F-12 media), which were optimized by the addition of growth factors and hormones, such as epithelial growth factor (EGF) [2] and cholera toxin [3]. More than 10 years later, cell culture dish is immobilized with poly(N-isopropyl acrylamide) (PIPAAm), also known as thermo-responsive polymers, was developed, and this technology enabled cell adhesion to be controlled through temperature adjustments [4]. Thereafter, several types of culturewares―dish, plate, and insert―have been also deveolped. With these advances, confluent cell layers can be harvested as a sheet without the use of any proteolytic enzyme [5], and the harvested sheets retaining ECM proteins at a basal side are capable of adhering to human tissues/organs without suturing. Among the various types of cells, the cultured oral mucosal epithelial cell sheets prepared using the temperature responsive cell culture dish or insert have been used to treat esophagus [6,7] and cornea [8] in clinical settings. Although various cell sheets are largely expected to become established as a regenerative product and be used in the treatment of many patients, the precise fabrication procedure is not standardized.
As an example of the clinical applications, we recently successfully achieved transplantations of the oral mucosal epithelial cell sheets for 10 patients with accompanying transportation of these epithelial cell grafts over 7 h by airplane [7]. In these clinical studies, we experienced that the patients’ epithelial cell conditions varied widely and were sometimes unstable. Thus, the quality control of the epithelial cell grafts by stable and standardized protocol is recognized to be crucial. Therefore, based on this previous study, we herein present a step by step stable protocol for the fabrication of oral mucosal epithelial cell sheets. The protocol is comprised of three parts: preparations, primary culture, and quality check. A schematic diagram of the procedures outlined in this study is presented in Fig. 1.
Fig. 1.
Schematic diagram of oral mucosal epithelial cell sheet fabrication. (a Primary culture procedure. (b) Media replacement procedure. (c) Quality check procedure.
2. Materials and methods
2.1. Preparations
This study was approved by the Ethical Committees and Internal Review Boards of Nagasaki University and Tokyo Women's Medical University. Approval for this clinical study by the Health, Labor and Welfare Ministry was obtained on March 29, 2013. Moreover, the patients were confirmed to be free from any infections of human immunodeficiency virus, human T-lymphotropic virus, syphilis, and hepatitis B and C virus, prior to blood collection and oral mucosal biopsy. The following procedure was performed in a biological safety cabinet in a GMP-grade cell culture room.
The preparation of the keratinocyte culture medium (KCM) is as follows; Dulbecco's modified Eagle's medium (DMEM; Sigma aldrich, MO, USA) and F-12 medium (Sigma aldrich) were mixed at a 3:1 ratio. The medium was supplemented with 3.3 mM l-glutamine solution (Sigma Aldrich), 84 μM gentamicin (Merck Sharp and Dohme, NJ, USA), 0.3 μM amphotericin B (Bristol-Myers Squibb, NY, USA), 0.25 μM hydrocortisone (Kowa, Nagoya, Japan), 140 mU/mL insulin (Lilly, IN, USA), 2.0 nM 3,3′,5-Triiodo-l-thyronine (MP Biomedicals, CA, USA), 1.0 nM cholera toxin (List Biological Laboratories, CA, USA), 0.2 μM recombinant hEGF (Higeta-Shoyu, Tokyo, Japan), 0.1 mM sulbactam, 0.2 mM ampicillin (Pfizer, NY, USA), and 14 μM streptomycin (Meiji Seika Pharma, Tokyo, Japan). The preparation of the transport medium is prepared from DMEM with antibiotics of 0.1 mM sulbactam, 14 μM streptomycin, and 1.1 μM amphotericin B (DMEM-AB).
Whole blood (100 mL) from each patient was carefully collected with a 21-gauge needle, and incubated at 37 °C for 1–2 h. After formation of blood clots, the sample was centrifuged at 1830×g for 10 min at room temperature. The supernatant as serum was collected and filtered into a new 50-mL centrifuge tube with a 0.2 μm-pore size polyether sulfone filter. The obtained serum was transported from Nagasaki to Tokyo by airplane at 4 °C, then added to KCM at a concentration of 5%.
2.2. Primary culture
The patients’ buccal mucosal tissues of 2.5–4.5 cm2 were biopsied using a scalpel under anesthesia [9] and then washed in DMEM-AB. The tissue samples were then immersed briefly in povidone-iodine and dried for 2 min. The disinfected tissues were transported in DMEM-AB from Nagasaki to Tokyo by airplane at 4 °C. After transportation, the tissues were disinfected again by povidone-iodine twice and then the normality of the tissues were confirmed (Fig. 2a and b). Next, each tissue was cut into 5-mm cubes for effective enzymatic reactions (Fig. 2c); 1000 U/mL dispase (Wako, Osaka, Japan) was reacted with the tissue in a new 35-mm cell culture dish in a CO2-atmosphere controlled incubator at 37 °C for 2 h. After the incubation, the epithelial layers were separated from the connective tissue using two pairs of tweezers and then placed in fresh DMEM-AB (Fig. 2d). The epithelial tissues were transferred to the center of a new empty 35-mm cell culture dish and quickly cut into 1-mm cubes using small scissors (Fig. 2e), and 3 mL of trypsin–EDTA was added.
Fig. 2.
Primary culture procedure. (a) Disinfection and washing of biopsied oral mucosal tissue. (b) Photograph of washed oral mucosal tissue in 35 mm dish. (c) Cutting of oral tissue into 5 mm cubes for dispase treatment. (d) Separation of epithelial layer from connective tissue. (e) Cutting of separated epithelial tissue into 1 mm cubes for trypsin–EDTA treatment. (f) 1000 μL micropipette tip with cutting down the tip at 0-mm long (Normal), 1-mm long, and 2-mm long. (g) Counting suspended cells. Seeding cells into thermo-responsive cell culture inserts at a density of 8 × 104 viable cells/cm2.
Control of the trypsin–EDTA treatment in this step is quite crucial in fabrication of the stable, viable, and transplantable cell sheet for clinical therapy. Initially, the cubic pieces of tissues were incubated at 37 °C in a CO2-atmosphere controlled incubator for 10 min, and then the tissues were pipetted up and down for more than 10 times using a 1000 μL micropipette tip with cutting down the tip at 2-mm long. After sufficiently suspending the isolated individual cells from the digested tissues, an approximate 1-mL suspension was collected with the 2-mm cut tip and promptly transferred to a 15 mL centrifuge tube containing 1 mL of KCM-HS to stop trypsinization for avoiding unnecessary long exposure. Another 5 min trypsin–EDTA incubation―15 min in total―was conducted to the remaining tissues, and then the isolated cells were collected again after pipetting well with a 1 mm-cut micro pipette tip. This second sample was mixed with the first sample. After further incubating to completely digest the remaining tissues for another 10 min–20 min in total, the cell suspension was mixed with 2 mL of KCM-HS and then with the sample collected before. This stepwise collection with gradually extending the incubation time effectively works to avoid unnecessary long exposure of the isolated cells to trypsin–EDTA, and it is a key point to successfully fabricate a transplantable epithelial cell sheet with highly viable cells.
The whole mixed cell suspension was transferred to a new 15 mL centrifuge tube with a 40-μm cell strainer filtration and centrifuged at 270×g for 5 min at 4 °C (Fig. 2g). The supernatant was removed, KCM was added, and cell numbers and viability were assessed by trypan blue assay. Cells were seeded on each temperature responsive cell culture insert of a 6-well type at a density of 8 × 104 viable cells/cm2.
Media changes were performed on days 5, 8, 10, 12, 13, 14, 15, and 16 post-isolation. The new media for the medium change was pre-warmed at 37 °C, and medium change was performed on a glass thermo-plate (Tokai-hit, Shizuoka, Japan) to avoid unexpected detachment due to fall of temperature.
2.3. Quality check
To check contamination of culture, we performed polymerase chain reaction (PCR) or loop-mediated isothermal amplification (LAMP), and cultivation method for M. pneumoniae; Limulus colorimetric tests for endotoxin; and sterility test using the media used for epithelial cell culture.
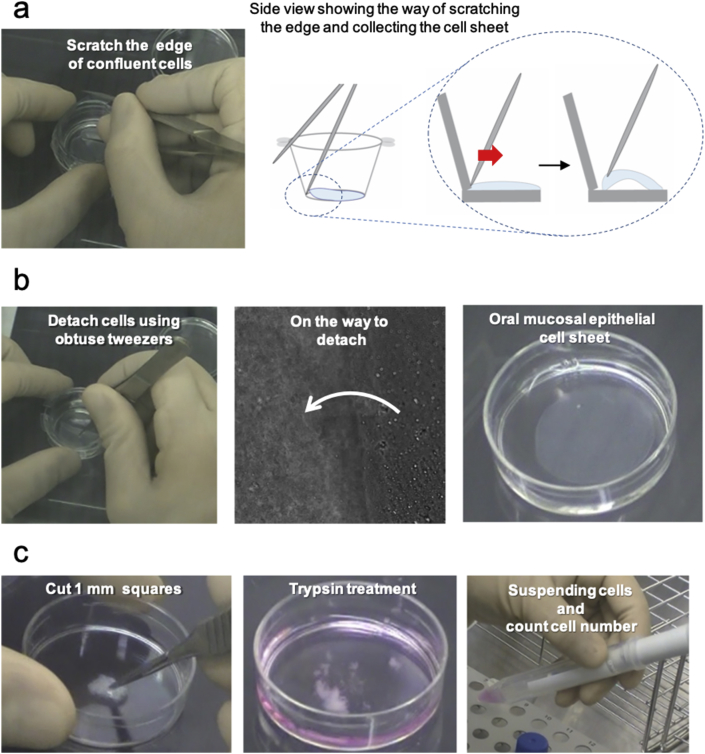
One sample was used to examine three quality check tests on day 14: number, viability, and purity. Others were utilized for transplantation. To harvest these epithelial cells as cell sheets, the inserts were incubated at 20 °C for 30 min. Both surfaces of the insert membrane were washed three times using HBSS (Sigma aldrich), and then, cells were carefully detached from the edge to the center. Because the cells also stuck to the PIPAAm-unimmobilized interspace of insert membrane―frame joints―and the cells could not spontaneously detach, we dragged out by sharp tweezers (Fig. 3a). In this process, we used sharp tweezers only when handling edge of the cell sheet and obtuse tweezers were used for pinching the sheet center (Fig. 3b).
Fig. 3.
Harvesting an oral mucosal epithelial cell sheet and pre-treatment for quality check. (a) Scratching the edge of confluent cells using sharp tweezers. (b) Completely detaching confluent cells as a cell sheet using obtuse tweezers. (c) After trypsin treatment, separated cells were investigated for number, viability, and keratin positive ratio.
To check cell number and viability, 1 mL of trypsin–EDTA was added to the harvested cell sheet for dispersing into individual cells (Fig. 3c), and incubated for 10–15 min at 37 °C. After the cell suspension was obtained, trypsin reaction was stopped by 1 mL of KCM-HS. The cell suspension was transferred to a new 15 mL centrifuge tube, filtered using a 40-μm cell strainer, and then centrifuged at 270×g for 5 min at 4 °C. The supernatant was removed, KCM was added to the pellet, and live cell numbers were counted using trypan blue staining. For cell sheet preparation, 1.0 × 105 cells per cell sheet with more than 70% viability were set to be at least required.
Cell purity analysis was performed using the same cell suspension and BD Cytofix/Cytoperm™ Kit. Procedure of washings are described as follows; the cell suspension were treated with the washing buffer, and centrifuged at 270×g for 5 min at 4 °C. Then, the supernatant was removed. This washing procedure was performed twice. Next, 250 μL of fixation solution was added with pipetting gently, and the mixed cell suspension was incubated for 20 min at 4 °C. Thereafter, washing procedure and collection of the cell pellet were performed once again for effective reaction with antibodies as follows. Another 200 μL of washing buffer was added and the cell suspensions were equally divided into two different 1.5 mL centrifuge tubes in order to analyze both cytokeratin and isotype control. Anti-keratin type II FITC mouse monoclonal antibody (Ks pan1-8, Progen, Heidelberg, Germany) or normal mouse IgG1-FITC antibody solution (Progen) was added to the cell suspension at a ratio of 1/20 (5 μL). Samples were incubated at room temperature for 60 min in an opaque container. Cell suspensions were washed and centrifuged twice at 2300×g for 10 min at 4 °C. The cell suspensions were filtered with a 40-μm cell strainer and transferred to a new flow-cytometer tube. The ratio of the keratin positive cells was analyzed using a flow cytometer (Gallios®, Beckman Coulter, CA, USA), and the value more than 70% was set to be at least required.
3. Results
3.1. Preparations for primary culture
Reliability as a stable protocol of this study is evidenced by the successful cell-sheet transplantation therapy on the post-ESD esophagus (n = 10) [7]. For preparing the culture media, the serum separated from the patient's blood is required. At first, we performed transportation of a whole blood sample from Nagasaki to Tokyo by airplane at 4 °C as a pre-clinical study. However, because hemolysis was observed in this pre-clinical study, we changed the procedure, and the serum was collected before transportation for establishing a stable protocol (data not shown). In this modified procedure, the serum was reproducibly collected from approximately 40% of the total blood volume (Table 1). As 3 mL of serum is required for preparing a cell sheet, 10 cell sheets can be fabricated when 100 mL of blood is collected. These serum samples were successfully transported, and we also succeeded in transporting buccal mucosal tissue without damage and contamination.
Table 1.
Preparation of serum from human blood.
| Withdrawn blood (mL) | Serum (mL) | |
|---|---|---|
| Case 1 | 100.0 | 43.3 |
| Case 2 | 100.0 | 45.0 |
| Case 3 | 120.0 | 43.5 |
| Case 4 | 120.0 | 42.5 |
| Case 5 | 120.0 | 37.0 |
| Case 6 | 120.0 | 41.2 |
| Case 7 | 100.0 | 37.8 |
| Case 8 | 200.0 | 92.3 |
| Case 9 | 120.0 | 50.0 |
| Case 10 | 120.0 | 46.0 |
3.2. Primary culture
From approximately 140 mm2 of patient oral mucosal tissue, more than 1.0 × 106 oral mucosal epithelial cells with high viability were obtained by trypsinization (Table 2). These cells adhered and proliferated well on a temperature responsive insert and reached approximately 40% confluency at day 5 (Fig. 4a). Limulus colorimetric test revealed that endotoxin levels were kept under 1.0 EU/mL (n = 10). Both PCR and culture experiments for detecting M. pneumoniae showed negative results (n = 10) [7]. In addition, bacteria and fungi were also negative by three types of agar culture. Altogether, we successfully cultivated oral mucosal epithelial cells without contamination. Seeded oral mucosal cells reached confluence after 8 days of culture. Moreover, after the cells became confluent, stratification was observed using phase-contrast microscopy at day 16 (Fig. 4a).
Table 2.
Conditions of primary oral mucosal epithelial cells for cell sheet preparation.
| Viable cell count (cells) | Total cell count (cells) | Viability (%) | Number of cell sheets | |
|---|---|---|---|---|
| Case 1 | 2.27 × 106 cells | 2.42 × 106 cells | 93.6% | 8 inserts |
| Case 2 | 3.04 × 106 cells | 3.15 × 106 cells | 96.3% | 9 inserts |
| Case 3 | 1.91 × 106 cells | 2.14 × 106 cells | 88.8% | 6 inserts |
| Case 4 | 2.95 × 106 cells | 3.00 × 106 cells | 98.1% | 9 inserts |
| Case 5 | 2.81 × 106 cells | 2.92 × 106 cells | 96.3% | 9 inserts |
| Case 6 | 4.99 × 106 cells | 5.54 × 106 cells | 90.1% | 13 inserts |
| Case 7 | 4.04 × 106 cells | 4.10 × 106 cells | 98.7% | 11 inserts |
| Case 8 | 6.90 × 106 cells | 7.56 × 106 cells | 91.2% | 17 inserts |
| Case 9 | 1.85 × 106 cells | 1.92 × 106 cells | 96.0% | 6 inserts |
| Case 10 | 3.24 × 106 cells | 3.56 × 106 cells | 92.0% | 10 inserts |
Fig. 4.
(a) Observation of cell morphology and proliferation using phase-contrast microscopy ( × 100). (b) A whole image of the oral mucosal epithelial cell sheet. (c) Flow cytometry analysis showing that a cell sheet was composed of 99% cytokeratin positive cells.
3.3. Quality check
At 14 days of culture, epithelial cells could be detached as oral mucosal cell sheets with tweezers (Fig. 3b). Fabricated cell sheets (1.0 × 105 cells/sheet) retained many viable cells (over 90%) and almost all these cells were cytokeratin-positive (over 80% based on all cells in a cell sheet, Fig. 4c, and described in our previous article [7]).
We could not find any abnormalities in cell sheets prior to clinical trial usage, even after airplane transportation. In the clinical trial, we performed a complete perimeter or semiperimeter ESD of the esophagus and then transplanted several cell sheets on the ESD wound bed. As a result, no complications were noted in all 10 patients [7]. Moreover, cell sheet transplantation was effective in promoting wound healing and preventing esophageal stricture [6,10].
4. Discussion
Cultured oral mucosal epithelial grafts have been used in clinical settings for treatment of the cornea [[11], [12], [13]], skin [14], and intraoral cavity [[14], [15], [16]], suggesting that the cell sheets can be applied for regenerative therapy for many tissues. In particular, we succeeded to prevent esophageal stricture after ESD by transplanting the oral mucosal epithelial cell sheet to the wound beds [6]. The first-in-human study was successfully conducted in a single institution of Tokyo Women's Medical University, and then we expanded the study as an inter-institutional clinical trials, which included transportation of patients’ tissues as the sources of cells and the cell sheet products between Tokyo and Nagasaki. In the inter-institutional clinical trials, we experienced contamination of Candida albicans, and thus we optimized the amphotericin B concentration of the transport medium [17].
After transportation of the source tissue, epithelial cells must be separated and collected with high viability for preparation of cell sheet. A stable protocol is required to be established for this purpose. There are some methods to collect epithelial cells: the two step enzymatic treatments using dispase and trypsin [18], normal trypsin EDTA treatment [19,20], and the explant culture method [21]. A key point in cell collection is a short-time exposure to enzyme and reagent, because, for example, long time trypsin EDTA treatments induce damage of cell membranes [[22], [23], [24], [25]]. Therefore, we developed a new technique by combining the trypsin–EDTA treatment and the multi-step process with changing the exposure time. Our idea is to isolate cells first from the basal layer of an epithelial tissue, where the cells have a higher proliferation ability [8,26] and the cell–cell junctions are relatively week [27].Therefore, we can isolate and collect the highly active cells with short exposure time of early stage of trypsin EDTA treatment. In the experiment, we conducted three-step treatment. The cells isolated from the tissue were transferred to KCM solution to stop trypsin digestion by three-stage reaction times, 10 min, 15 min, and 20 min. In 10 min, the cells in basal layer could be obtained. However, indeed, it was difficult to collect cells by pipetting because some cell clusters were larger than the diameter of 1000 μL pipette tips. Thus, we used specially cut 1000 μL pipette tips to suspend and collect supernatant where suspended cells existed (Fig. 2f). After this first stage, we collected other suspended cell fractions in the stages of 15-min and 20-min reaction time. These fractions should contain the cells from the upper layer, where the cell–cell junctions are strong, and extended reaction times are inevitably required for isolation. Our new technique allows the collection of isolated epithelial cells from basal layer and upper layer of the tissue respectively with minimum trypsin incubation (Fig. 2), and this process is particularly important for fabricating cell sheets of all types of epithelial tissues. Using this method, we succeeded in stably and reproducibly making oral mucosal epithelial cells confluent for preparing the sheet grafts for transplantation therapy of 10 patients.
Even though human oral mucosal epithelial cell sheets have strong adhesion properties and are not easily harvested as a whole cell sheet from the temperature-responsive cell culture surfaces by just lowing the temperature, a part of cells within the sheet were detached [5]. Therefore, strict temperature control is necessary to avoid partial cell loss before forming and harvesting a complete sheet, and we used pre-warmed new media and performed medium changes on thermo-plate (Fig. 1b). Moreover, because culture medium in the well below the insert membrane is supplied to the cells from the basal side and crucial to maintain the cells rather than the medium in the insert-cup, where the cells were immersed. Therefore, a bottom surface of the membrane must not be dried, and we first prepared another new 6-well plate supplied with media, and then moved the insert to this new plate. After 2 weeks of primary culture, we observed stratification of cells (Fig. 4a), which was a reliable indication of formation of a stratified squamous epithelial cell sheet with sufficient cell–cell interaction. Indeed, cell sheets were successfully peeled off within 10 min without any rupture (Fig. 4b).
There have been published papers on the methods of fabricating oral mucosal cell sheets: temperature responsive cell culture insert without feeder cells [28], temperature responsive cell culture insert with feeder cells [8], and conventional cell culture dish with feeder cells [1]. These studies simply outlined the procedures of cell sheet fabrication―cell isolation, medium change, and harvesting cell sheet . However, more precise protocol is crucial and effective to successfully fabricate the cell sheet with high quality for clinical use. Therefore, in this study, the precise protocol was described: collecting and seeding cells which have high proliferative potential, keeping stable temperature during medium change, the way of harvesting cell sheet, and these sequential procedures We believe that our precise protocol is useful for expanding the clinical therapies using the cell sheets and contribute largely to the future development of regenerative medicine.
5. Conclusion
The epithelial cell sheet has significant potential as a transplantation graft in clinical therapy. The stable protocol that we established and standardized in this study is suitable for culturing grafts for clinical usage, and it would contribute to the fabrication of various types of the epithelial cell sheets other than oral mucosal epithelial cell grafts for future advances in regenerative therapy.
Declaration of competing interest
Yamato Masayuki is a stakeholder of CellSeed Inc., and Tokyo Women's Medical University receives research funding from CellSeed Inc. Yamato Masayuki is also an advisor of Helios and Nippi. Teruo Okano is a founder of CellSeed Inc. which has licenses for certain cell sheet-related technologies and patents from Tokyo Women's Medical University.
Acknowledgements
This work was supported by Global Center of Excellence Practical Chemical Wisdom, the Formation of Innovation Center for Fusion of Advanced Technologies in the Special Coordination Funds for Promoting Science and Technology “Cell Sheet Tissue Engineering Center (CSTEC)” and the Global Center of Excellence program, the Multidisciplinary Education and Research Center for the Establishment of Regenerative Medicine (MERCREM), from the Ministry of Education, Culture, Sports, Science and Technology-Japan (MEXT), The Jikei University Strategic Prioritizing Research Fund, and Misao Yanagihara's research fellowship from the Academy of Future Medicine. We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Green H., Kehinde O., Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979;76:5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rheinwald J.G., Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977;265:421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- 3.Green H. Cyclic AMP in relation to proliferation of the epidermal cell: a new view. Cell. 1978;15:801–811. doi: 10.1016/0092-8674(78)90265-9. [DOI] [PubMed] [Google Scholar]
- 4.Okano T., Yamada N., Sakai H., Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J Biomed Mater Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 5.Okano T., Yamada N., Okuhara M., Sakai H., Sakurai Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials. 1995;16:297–330. doi: 10.1016/0142-9612(95)93257-e. [DOI] [PubMed] [Google Scholar]
- 6.Ohki T., Yamato M., Ota M., Takagi R., Murakami D., Kondo M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–588 e2. doi: 10.1053/j.gastro.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi N., Isomoto H., Kobayashi S., Kanai N., Kanetaka K., Sakai Y. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci Rep. 2017;7:17460. doi: 10.1038/s41598-017-17663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki R., Yamato M., Takagi R., Ohki T., Matsumine H., Okano T. Punch and spindle-shaped biopsies for collecting oral mucosal tissue for the fabrication of transplantable autologous epithelial cell sheets. J Biomed Mater Res A. 2012;100:2849–2854. doi: 10.1002/jbm.a.34216. [DOI] [PubMed] [Google Scholar]
- 10.Kanai N., Yamato M., Ohki T., Yamamoto M., Okano T. Fabricated autologous epidermal cell sheets for the prevention of esophageal stricture after circumferential ESD in a porcine model. Gastrointest Endosc. 2012;76:873–881. doi: 10.1016/j.gie.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T., Inatomi T., Sotozono C., Amemiya T., Kanamura N., Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88:1280–1284. doi: 10.1136/bjo.2003.038497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Sheha H., Fu Y., Giegengack M., Tseng S.C. Oral mucosal graft with amniotic membrane transplantation for total limbal stem cell deficiency. Am J Ophthalmol. 2011;152 doi: 10.1016/j.ajo.2011.03.037. 739−747 e1. [DOI] [PubMed] [Google Scholar]
- 13.Burillon C., Huot L., Justin V., Nataf S., Chapuis F., Decullier E. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Investig Ophthalmol Vis Sci. 2012;53:1325–1331. doi: 10.1167/iovs.11-7744. [DOI] [PubMed] [Google Scholar]
- 14.Hata K., Ueda M. Fabrication of cultured epithelium using oral mucosal cells and its clinical applications. Hum Cell. 1996;9:91–96. [PubMed] [Google Scholar]
- 15.Deluca M., Albanese E., Megna M., Cancedda R., Mangiante P.E., Cadoni A. Evidence that human oral epithelium reconstituted in vitro and transplanted onto patients with defects in the oral-mucosa retains properties of the original donor site. Transplantation. 1990;50:454–459. doi: 10.1097/00007890-199009000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Izumi K., Feinberg S.E., Iida A., Yoshizawa M. Intraoral grafting of an ex vivo produced oral mucosa equivalent: a preliminary report. Int J Oral Maxillofac Surg. 2003;32:188–197. doi: 10.1054/ijom.2002.0365. [DOI] [PubMed] [Google Scholar]
- 17.Takagi R., Kobayashi S., Yamato M., Owaki T., Kasai Y., Hosoi T. How to prevent contamination with Candida albicans during the fabrication of transplantable oral mucosal epithelial cell sheets. Regen Ther. 2015;1:1–4. doi: 10.1016/j.reth.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitano Y., Okada N. Separation of the epidermal sheet by dispase. Br J Dermatol. 1983;108:555–560. doi: 10.1111/j.1365-2133.1983.tb01056.x. [DOI] [PubMed] [Google Scholar]
- 19.Rheinwald J.G. Serial cultivation of normal human epidermal keratinocytes. Methods Cell Biol. 1980;21A:229–254. doi: 10.1016/s0091-679x(08)60769-4. [DOI] [PubMed] [Google Scholar]
- 20.Parkinson E.K., Yeudall W.A. The epidermis. In: Ei Freshney, Freshney M.G., editors. Culture of epithelial cells. 2nd ed. Johon Wiley & Sons, Inc.; NY, USA: 2002. pp. 65–94. [Google Scholar]
- 21.Morino T., Takagi R., Yamamoto K., Kojima H., Yamato M. Explant culture of oral mucosal epithelial cells for fabricating transplantable epithelial cell sheet. Regen Ther. 2018;10:36–45. doi: 10.1016/j.reth.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osunkoya B.O., Mottram F.C., Isoun M.J. Synthesis and fate of immunological surface receptors on cultured Burkitt lymphoma cells. Int J Cancer. 1969;4:159–165. doi: 10.1002/ijc.2910040206. [DOI] [PubMed] [Google Scholar]
- 23.Okano T., Yamada N., Sakai H., Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J Biomed Mater Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 24.Revel J.P., Hoch P., Ho D. Adhesion of culture cells to their substratum. Exp Cell Res. 1974;84:207–218. doi: 10.1016/0014-4827(74)90398-x. [DOI] [PubMed] [Google Scholar]
- 25.Waymouth C. To disaggregate or not to disaggregate injury and cell disaggregation, transient or permanent? In Vitro. 1974;10:97–111. doi: 10.1007/BF02615343. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Moles M.A., Ruiz-Avila I., Rodriguez-Archilla A., Martinez-Lara I. Suprabasal expression of Ki-67 antigen as a marker for the presence and severity of oral epithelial dysplasia. Head Neck. 2000;22:658–661. doi: 10.1002/1097-0347(200010)22:7<658::aid-hed3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 27.Malminen M., Koivukangas V., Peltonen J., Karvonen S.L., Oikarinen A., Peltonen S. Immunohistological distribution of the tight junction components ZO-1 and occludin in regenerating human epidermis. Br J Dermatol. 2003;149:255–260. doi: 10.1046/j.1365-2133.2003.05438.x. [DOI] [PubMed] [Google Scholar]
- 28.Murakami D., Yamato M., Nishida K., Ohki T., Takagi R., Yang J. Fabrication of transplantable human oral mucosal epithelial cell sheets using temperature-responsive culture inserts without feeder layer cells. J Artif Organs. 2006;9:185–191. doi: 10.1007/s10047-006-0342-3. [DOI] [PubMed] [Google Scholar]