Abstract
Tisagenlecleucel is a CD19-specific chimeric antigen receptor (CAR)-T cell therapy approved for patients aged ≤25 years with relapsed or refractory B cell precursor acute lymphoblastic leukemia (B-ALL) and adults with relapsed or refractory diffuse large B cell lymphoma (DLBCL). The initial tisagenlecleucel manufacturing process technology was developed at an academic center and was subsequently transferred, optimized, validated, and scaled out to supply large global trials before commercialization. Tisagenlecleucel manufactured in two centralized facilities has been successfully used in global multicenter trials for B-ALL and DLBCL (>50 clinical centers in 12 countries). In this paper, we describe some of the continuous process improvements made to tisagenlecleucel manufacturing over time to meet global demand while maintaining and improving product quality. During early tisagenlecleucel clinical trials, process enhancements were made to address logistical challenges related to manufacturing for multicenter trials and to accommodate the variability observed in patient starting cellular material. These enhancements resulted in improvements in manufacturing capacity, process robustness, manufacturing success rates, and product quality, and reductions in throughput time. In summary, through continuous evaluation and improvements based on experience during global trials, a consistent and robust commercial manufacturing process for tisagenlecleucel has been developed, leading to improvements in manufacturing success when compared to the initial processes.
Keywords: chimeric antigen receptor-T cell therapy, commercial manufacturing, immunotherapy, manufacturing process optimization, global manufacturing
Introduction
Chimeric antigen receptor (CAR)-T cell therapy involves the reprogramming of a patient’s T cells to target and attack tumor cells,1,2 and it has been successfully used in clinical trials for the treatment of B cell malignancies.3, 4, 5, 6, 7 Tisagenlecleucel (Kymriah; Novartis Pharma, Basel, Switzerland) is the first CD19-specific autologous CAR-T cell product approved by the US Food and Drug Administration (FDA) and by the European Commission for the treatment of patients up to 25 years of age with B cell precursor acute lymphoblastic leukemia (B-ALL) that is refractory or in second or later relapse and for adult patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL), after ≥2 lines of systemic therapy.8,9 Another CD19 CAR-T cell product, axicabtagene ciloleucel (Yescarta; Kite Pharma, Santa Monica, CA, USA), is also approved by the FDA and the European Commission for the treatment of adult patients with relapsed or refractory DLBCL, after ≥2 lines of systemic therapy.10,11 Several other CAR-T cell products are in various stages of clinical development.12
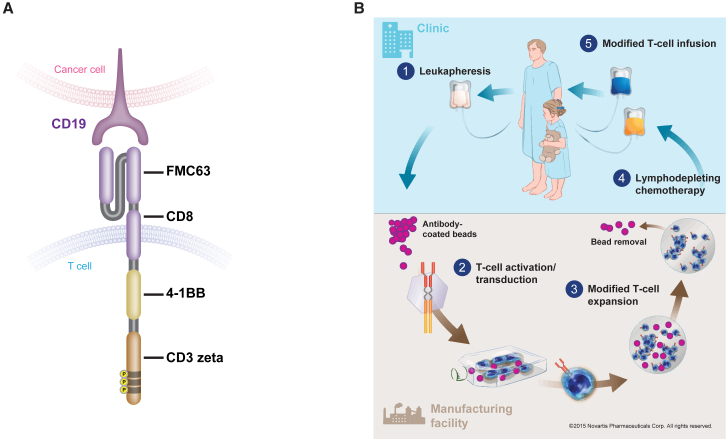
The tisagenlecleucel CAR is comprised of a murine single-chain antibody fragment specific for CD19 (FMC63), followed by a CD8-α hinge and transmembrane region, fused to the intracellular CD3-ζ signaling domain and 4-1BB costimulatory domain (Figure 1A). The mechanism of action of tisagenlecleucel has been described previously.3,13 The 4-1BB domain serves as the costimulatory signal for T cell activation and is important for the expansion, persistence, and antitumor activity of tisagenlecleucel.3,13,14 Tisagenlecleucel undergoes expansion following infusion into patients. When the CAR binds to CD19 on the target cells in vivo, signal transduction is initiated, leading to T cell activation, cytokine production, and initiation of target cell destruction. Robust expansion and long-term persistence of tisagenlecleucel, in addition to durable clinical responses following infusion, have been demonstrated in patients with B-ALL, chronic lymphocytic leukemia, and DLBCL in clinical trials.5,15, 16, 17
Figure 1.
Tisagenlecleucel Structure and Manufacturing Process
(A) Structure of tisagenlecleucel CAR. (B) Tisagenlecleucel manufacturing process. CAR, chimeric antigen receptor.
The initial tisagenlecleucel manufacturing process was developed at an academic center, the University of Pennsylvania (Philadelphia, PA, USA). This process was then transferred to Novartis and subsequently optimized and scaled out to supply global clinical trials. Optimization and process improvements were necessary to meet the increased demand, address donor variability, and improve product quality. These improvements focused on enhancing compliance, aiding scalability, and allowing for global distribution, and they resulted in the current manufacturing process. Tisagenlecleucel, manufactured at two centralized facilities, was successfully used in global clinical trials (ELIANA [ClinicalTrials.gov: NCT02435849]5 and JULIET [ClinicalTrials.gov: NCT02445248]6) involving >50 clinical centers in 12 countries. In this paper, we describe the tisagenlecleucel manufacturing process, initial challenges encountered during scale-out, and continuous improvements made during pivotal trials to address the challenges that finally led to the tisagenlecleucel commercial manufacturing process.
Tisagenlecleucel Manufacturing Process
The tisagenlecleucel manufacturing process (Figure 1B) begins with collection of nonmobilized peripheral blood mononuclear cells from a patient by leukapheresis. This leukapheresed material is cryopreserved within 24 h after collection and stored below –120°C. The cryopreserved material is then shipped to the manufacturing facility and stored below –120°C until it is ready for further processing. Upon availability of a manufacturing slot, the patient leukapheresis material is thawed under controlled conditions, followed by cell wash to remove the cryomedium. The T cells are enriched, selected, and activated using anti-CD3/CD28 antibody-coated paramagnetic beads, followed by transduction with a self-inactivating lentiviral vector containing the anti-CD19 CAR transgene.2,13 Following transduction, the excess vector and other residuals are washed from the culture and the cells are expanded in static cultures and then in bioreactors. Cell expansion continues ex vivo until there are sufficient cells to meet the final product dose requirements. For cell harvest, the transduced T cells are isolated by separating them from the beads, washed, formulated in infusible media, transferred into infusion bags, and cryopreserved. Testing of the critical quality attributes (CQAs) of tisagenlecleucel (Table 1) is performed and, after final product release, cryopreserved tisagenlecleucel is shipped to the treatment site. Once the patient is ready to receive tisagenlecleucel, the cells are thawed and immediately infused into the same patient who provided the leukapheresed cells.
Table 1.
Tisagenlecleucel Product Critical Quality Attributes
| Parameter | Attributes |
|---|---|
| Appearance and description |
|
| Safety |
|
| Purity |
|
| Impurities |
|
| Identity |
|
| Quantity |
|
| Potency |
|
CAR, chimeric antigen receptor; IFN, interferon; qPCR, quantitative polymerase chain reaction; RCL, replication-competent lentivirus; VSV-G, vesicular stomatitis virus G.
Tisagenlecleucel Manufacturing Process Development History
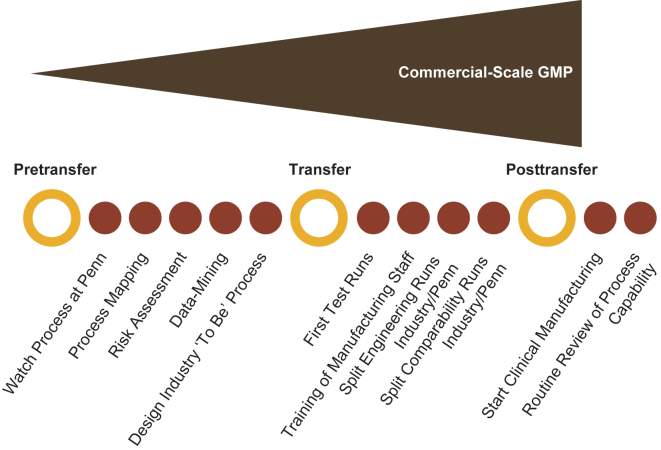
The early-phase tisagenlecleucel studies enrolled relatively few patients, and products used in these studies were manufactured at the University of Pennsylvania using the processes described previously.3,4,18, 19, 20 Transition from flexible processes at a single academic institution to global studies enrolling patients from numerous clinical sites, with manufacturing performed at two centralized manufacturing facilities that ultimately led to commercial manufacturing, required extensive coordination across many apheresis and treatment sites as well as the manufacturing facilities.2 Some of the challenges of scaling out production from the initial process included (1) standardization of the overall manufacturing process and characterization of the process and product; (2) meeting specific regulatory requirements from various countries and regions concomitantly; (3) identifying validated, automated manufacturing solutions for manual processes; and (4) managing complex logistics during the transition from phase 1/2 clinical trials to pivotal global trials. A stepwise approach to the technology transfer process was developed through collaboration of diverse technology transfer teams from academia, manufacturing, technical development, quality assurance, and regulatory departments (Figure 2). This collaboration led to the successful transfer of the tisagenlecleucel manufacturing process from academia to industry.
Figure 2.
Step-Based Approach for Process Transfer
GMP, good manufacturing practice.
Transfer of Tisagenlecleucel Manufacturing
During the transition from academic to industrial manufacturing for use in global clinical trials, several steps were taken to improve process performance and robustness while maintaining the quality of the tisagenlecleucel product (Table 1). Some of the key process changes included enhancement of process control to ensure consistency, replacement of manual processes with automation to ensure reproducibility, validation of analytical methods to increase consistency, and use of closed systems to prevent contamination. Increased understanding of analytical method performance by method validation and implementation of more robust and/or faster methods were areas of focus during technology transfer and scale-out. A new quantitation method for CAR protein expression using an anti-idiotypic antibody improved the robustness and linear range of the assay used for dose determination. Optimization of the vector used for the CAR-T cell transduction step helped to reduce variability, maximize transduction efficiency, and allow for scaling out of the manufacturing process. The lentiviral vector manufacturing process was also improved to allow for large-scale vector production in good manufacturing practice (GMP) settings.2
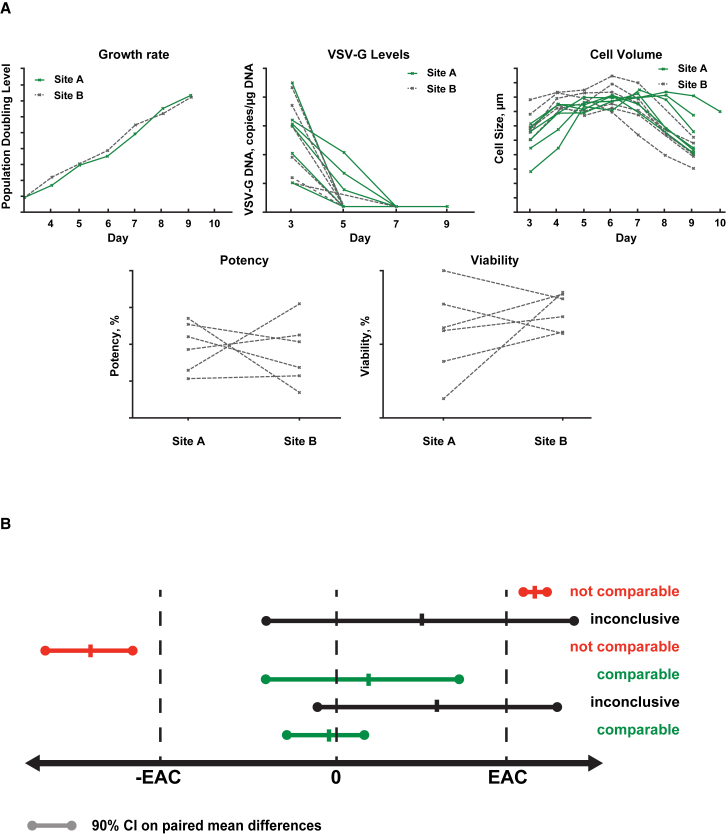
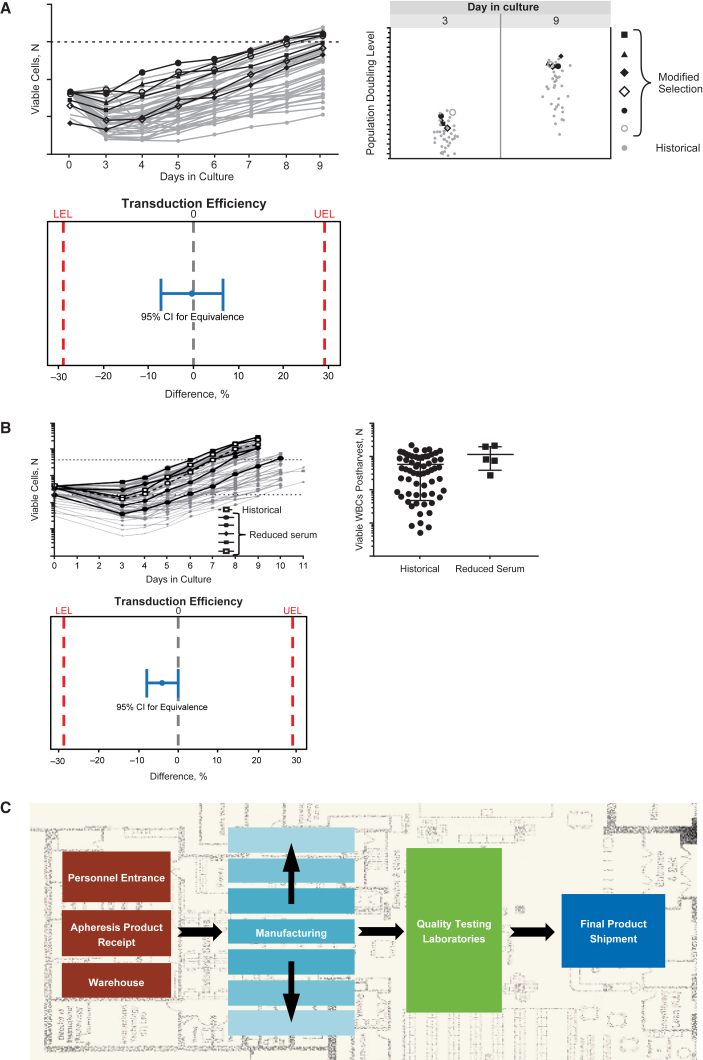
Comparability of process performance and product quality during the technical transfer was established through the assessment of selected parameters such as cellular growth rate, cell size, potency, viability, and removal of vector residuals (Figure 3A). Equivalency testing was carried out for each CQA to demonstrate analytical comparability (Figure 3B). The equivalence acceptance criteria setting are dependent on manufacturing process understanding, and can be based on the variability in the method and/or process.
Figure 3.
Assessment of Comparability during Technical Transfer
(A) Comparison of process performance. (B) Demonstration of statistical comparability. EAC, equivalence acceptance criteria; VSV-G, vesicular stomatitis virus G. Population doubling level is a metric that indicates the cumulative number of cell doublings over the course of the culture.
Cryopreserved leukapheresis was selected as the preferred starting material over fresh material to meet the demands of centralized manufacturing for a global trial while allowing for maximal flexibility in patient management. Cryopreserved leukapheresis allows for extended storage of starting material in the event of manufacturing delays, allows for reliable and consistent manufacturing, and, most importantly, provides flexibility for patients to undergo leukapheresis at a time when it is most convenient and appropriate for the patient, as opposed to aligning with manufacturing slot availability. The cryopreservation process (including cryopreservation media, cryobags, storage, cryoshippers, thawing conditions, and stability of postthaw leukapheresis material) were evaluated extensively, and the manufacturing process using cryopreserved starting material was optimized and validated for use in pediatric and young adult patients with B-ALL and adult patients with DLBCL.
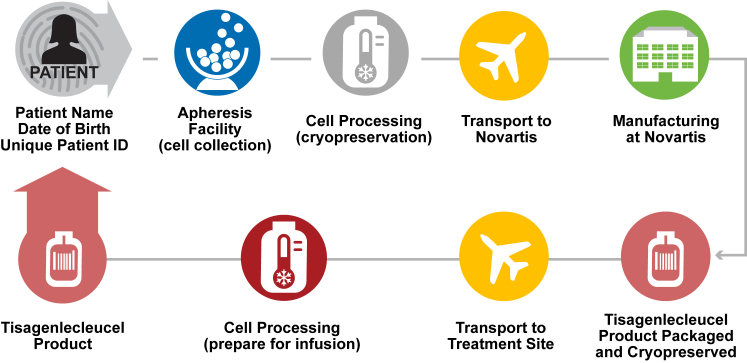
Logistical controls were planned and implemented to meet projected patient demand. Vendors and service providers were qualified according to GMP standards, and partnerships were established to ensure long-term supply of critical raw materials. A system to control chain of custody was developed using customized software to track patient identity during transport of leukapheresis material from the clinic to the return of manufactured tisagenlecleucel, ensuring patients are infused with their own cells (Figure 4).
Figure 4.
Implementation of Logistical Controls and Maintaining Chain of Identity
Formal process validation occurred during the clinical trials. The CQAs of tisagenlecleucel were identified based on standard tests for patient safety along with product-specific markers for clinical efficacy. Process performance was characterized and the critical control parameters were established using a risk-based approach. A robust product release process was established to verify safety, purity, identity, and potency of each patient-specific tisagenlecleucel product (Table 1) according to commercial standards.
Early Challenges during Tisagenlecleucel Clinical Trials and Subsequent Process Improvements
Early on, logistical challenges related to meeting manufacturing demands for multicenter trials and adapting to the observed patient-to-patient variability in leukapheresis material led to higher than expected dropout rates between enrollment and infusion. To address these challenges, enhancements were made to improve manufacturing capacity, process robustness, process success rate, and product quality, as well as to reduce throughput time, as described below.
Manufacturing experience gained during clinical trials combined with process characterization helped identify specific areas for improvement. For example, the initial process for T cell enrichment used multiple pathways to accommodate for variability in cell population composition of incoming patient leukapheresis material. Experimentation and evaluation of different options led to optimization of the T cell enrichment step into a single improved pathway that is used for a range of incoming cell populations. These modifications to the T cell enrichment process led to streamlined manufacturing with improved cellular growth kinetics and equivalent product quality. As shown in Figure 5A, the cell growth curves and population doubling were comparable using this modified selection method versus historical methods; comparability of these methods was demonstrated based on equivalence tests that showed transduction efficiency of cells generated using the modified process was comparable to or higher than those generated using historical processes.
Figure 5.
Examples of Process Improvements
(A) Improved T cell enrichment: cell growth curves and population doubling were comparable, and transduction efficiency was comparable or higher using the modified T cell enrichment process versus historical methods. (B) Improved culture media: cell growth, white blood cell (WBC) counts, and transduction efficiency were comparable in batches that used reduced serum versus historical batches. (C) Facility and operational improvements; modular facility design. LEL, lower equivalence limit; UEL, upper equivalence limit. Population doubling level is a metric that indicates the cumulative number of cell doublings over the course of the culture.
The amount of human AB serum used in the media was identified as a potential rate-limiting step and needed to be reduced to overcome global supply limitations. Reducing serum concentrations in the culture media resulted in a more sustainable process with equivalent growth kinetics and product quality. As shown in Figure 5B, the cell growth profiles and number of viable white blood cells in reduced serum batches were within the ranges of historical manufacturing runs. Equivalence tests demonstrated comparability of the transduction efficiency of cells generated using the different processes.
Another rate-limiting step in the manufacturing process was the time needed to perform sterility testing by the standard compendial methods. The validation and use of a more rapid quantitative polymerase chain reaction-based mycoplasma sterility assay reduced the time required for sterility testing before product release. Rigorous sterility testing in the tisagenlecleucel manufacturing process is part of an overall sterility assurance strategy to ensure that tisagenlecleucel is free of potential contaminants. This validated method ensures quality standards meet regional and global regulatory requirements.
Facility and operational improvements were necessary to meet worldwide clinical demand. These improvements included measures such as increasing manufacturing capacity, reducing throughput time, and decreasing patient dropout rates. Staffing was optimized for receipt of an increased incoming volume of samples with a corresponding increase in clean-room capacity. A modular facility design allowed for flexibility for scale-out with evolving manufacturing platforms and the ability to manufacture products for multiple indications (Figure 5C). As patient demand increased, processing times were reduced through optimization of training programs, increasing personnel, shift rotations, optimizing process flows, and logistics. An automated electronic ordering system was introduced to ensure the chain of identity and chain of custody were maintained from leukapheresis collection, through manufacturing, and finally to transportation of manufactured product to the clinical site. Shipping logistics, which are key to ensuring that patients receive the drug in a timely manner, were evaluated. This evaluation also included the design of cryoshippers, temperature control mechanisms, and couriers providing the shipment service. Identifying areas that needed improvement and addressing them with process improvements supported by suitable studies helped to optimize the manufacturing process during pivotal trials.
More recently, variability has been observed in the percentage of viable cells in commercial products manufactured for adult patients with DLBCL, due to increased variability of incoming patient material. Despite this variability, most of these products are still able to be administered to the patients.
Manufacturing Success and Low Throughput Time with Optimized Tisagenlecleucel Commercial Manufacturing
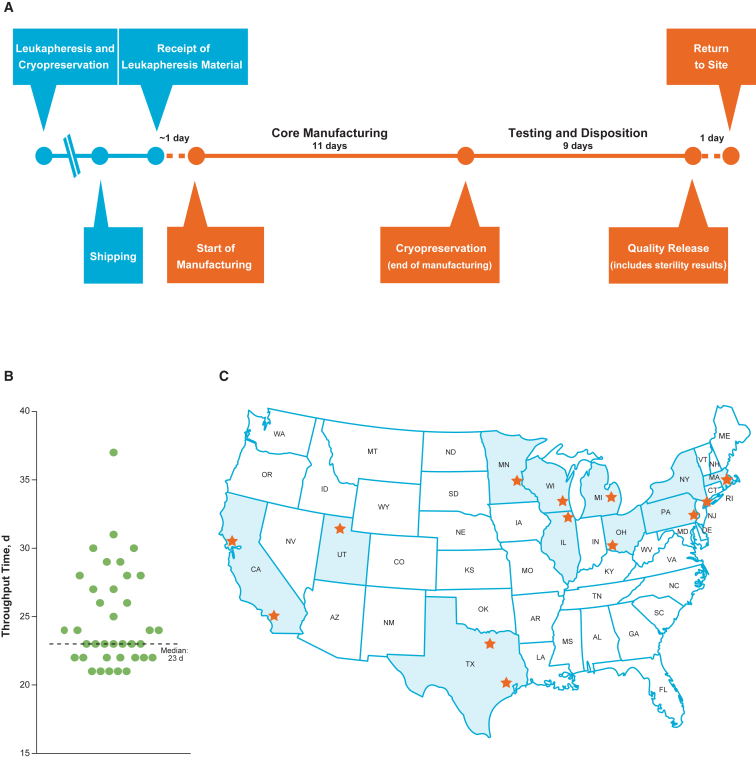
Continuous process improvements in manufacturing during ongoing clinical trials resulted in a consistent and optimized process for tisagenlecleucel that was able to adapt to the variations observed in incoming leukapheresis material during the trial (Figure 6A). This enabled the establishment of a 22-day target for the manufacturing cycle and resulted in a reasonably high manufacturing success rate in the commercial setting. These changes were first evaluated in development studies and then introduced during clinical trials after demonstrating comparability of the post-change product, which was necessary to ensure that patients would receive product of consistent quality. Based on the first 37 commercially manufactured tisagenlecleucel products for B-ALL (cutoff date, January 30, 2018), the current process showed a median throughput time of 23 days (range, 21–37 days) from receipt of the leukapheresed material at the manufacturing facility to return of tisagenlecleucel to the clinical site (including shipping time; Figure 6B). During this time period, the commercial patient orders were from 13 treatment sites across a large geographical area in the United States (Figure 6C). Ongoing process improvements are also expected to further reduce manufacturing throughput times.
Figure 6.
Commercial Manufacturing Process for Tisagenlecleucel
(A) Commercial manufacturing target timelines (median throughput time, 23 days; range, 21–37 days). (B) Throughput time in the first 37 commercial patients. (C) US sites that received commercially manufactured tisagenlecleucel. Stars in (C) indicate locations of the 13 unique sites where 37 patients received commercially manufactured tisagenlecleucel.
Patients aged <3 years were excluded from the ELIANA study;5 however, the youngest patient for whom tisagenlecleucel was manufactured in the commercial setting was 2 years old. Throughput time for this patient was 24 days. Manufacturing in patients <3 years of age will continue to be monitored and evaluated to ensure availability of T cells to start manufacturing and also to ensure the ability to deliver an appropriate dose in such young patients.
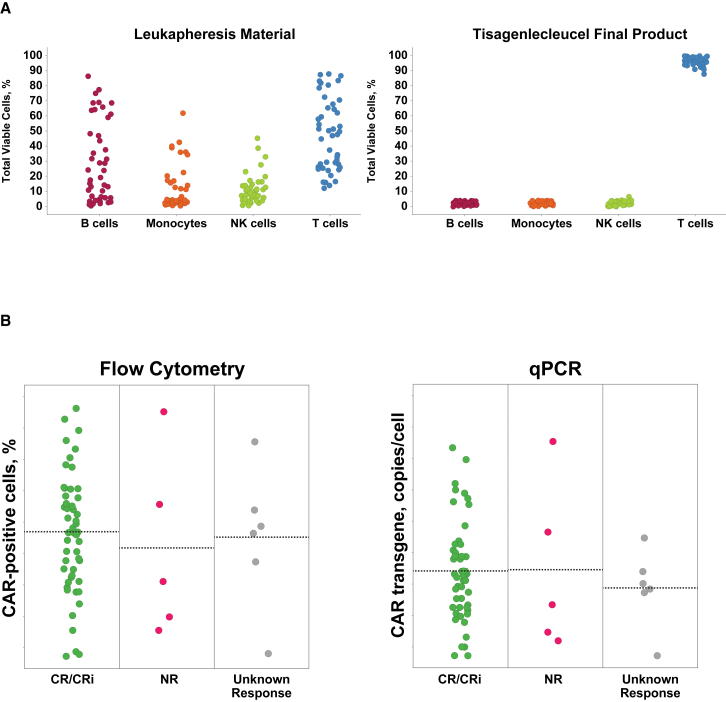
Tisagenlecleucel Product Characteristics
Consistent product quality during manufacturing has been demonstrated by extensive product testing. Assessment of T cell populations in the ELIANA trial (Figure 7A) showed consistent T cell product developed from variable patient leukapheresis starting materials. Analysis of CAR expression and transgene copies per cell showed stable vector integration with positive clinical outcomes observed across the allowable range (Figure 7B). Positive clinical outcomes were also observed across the complete range of acceptable potency assay results, and clinical response, safety, and in vivo expansion during the clinical studies were not affected by selected product characteristics.16,21
Figure 7.
Manufacturing Experience from the ELIANA Clinical Trial
(A) Consistent T cell product from variable patient leukapheresis material. (B) Positive patient outcomes across a range of transgene positive cells and copy numbers. CAR, chimeric antigen receptor; CR, complete remission; CRi, complete remission with incomplete blood count recovery; NK, natural killer; NR, nonresponder; qPCR, quantitative polymerase chain reaction.
Conclusions and Future Directions
The current manufacturing process for tisagenlecleucel is a reflection of how a cellular therapy with a complex manufacturing process was successfully scaled out, streamlined, and optimized to ensure supply of high-quality product to a global patient population. By focusing on key areas of enhancement, the manufacturing process was optimized and production efficiency was achieved for tisagenlecleucel manufacturing without compromising product integrity or potency. Considerable experience has been accrued in centralized manufacturing of tisagenlecleucel in the global multicenter trials. Through continuous evaluation and improvements, a consistent and robust commercial manufacturing process for tisagenlecleucel was developed. The current manufacturing process, based on the first 37 commercial patients (for B-ALL), resulted in a median 23-day period from receipt of leukapheresed material at the manufacturing facility to return of manufactured tisagenlecleucel to the clinical site, including shipping time. It is anticipated that ongoing, continuous process improvements will result in further incremental enhancements in the manufacturing of tisagenlecleucel and further decrease the throughput time from receipt of leukapheresis material to return of the manufactured product.
Author Contributions
All authors contributed to the acquisition, analysis, or interpretation of data and to the conception, design, drafting, and revising of the manuscript; approved the final version to be published; and agree to be accountable for all aspects of the work.
Disclosures
All authors were employees of Novartis Pharmaceuticals at the time of manuscript development. T.S. is currently an employee of Ziopharm Oncology.
Acknowledgments
The authors acknowledge the contributions of Dr. Elizabeth Pratico for discussion of data and critical review of the manuscript. We also thank Yoko Momonoi, Elizabeth Pratico, Margit Jeschke, Florence Salmon, Cindy Riggins, and members of Novartis Cell and Gene Analytical and Process Sciences for providing data, materials, and technical insight. Medical writing assistance was provided by Beena John, PhD, of C4 MedSolutions (Yardley, PA, USA), a CHC Group company. This study and writing assistance were funded by Novartis Pharmaceuticals (East Hanover, NJ, USA).
References
- 1.Maus M.V., Levine B.L. Chimeric antigen receptor T-cell therapy for the community oncologist. Oncologist. 2016;21:608–617. doi: 10.1634/theoncologist.2015-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B.L., Miskin J., Wonnacott K., Keir C. Global manufacturing of CAR T cell therapy. Mol. Ther. Methods Clin. Dev. 2016;4:92–101. doi: 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A., June C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster S.J., Bishop M.R., Tam C., Waller E.K., Borchmann P., McGuirk J., Jäger U., Jaglowski S., Andreadis C., Westin J. Global pivotal phase 2 trial of the CD19-targeted therapy CTL019 in adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL)—an interim analysis. Hematol. Oncol. 2017;35(Suppl 2):27. [Google Scholar]
- 7.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novartis Pharmaceuticals Corporation . 2018. Kymriah. Tisagenlecleucel. https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert---KYMRIAH.pdf. [Google Scholar]
- 9.Novartis Europharm Limited . 2018. Kymriah. Tisagenlecleucel. https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert---YESCARTA.pdf. [Google Scholar]
- 10.Kite Pharma, Inc. 2017. Yescarta. Axicabtagene ciloleucel. https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert---YESCARTA.pdf. [Google Scholar]
- 11.Kite Pharma EU B.V. 2019. Yescarta. Axicabtagene ciloleucel. https://www.ema.europa.eu/en/documents/product-information/yescarta-epar-product-information_en.pdf. [Google Scholar]
- 12.Buechner J., Kersten M.J., Fuchs M., Salmon F., Jäger U. Chimeric antigen receptor-T cell therapy: practical considerations for implementation in Europe. Hemasphere. 2018;2:e18. doi: 10.1097/HS9.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milone M.C., Fish J.D., Carpenito C., Carroll R.G., Binder G.K., Teachey D., Samanta M., Lakhal M., Gloss B., Danet-Desnoyers G. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H., Snyder K.M., Suhoski M.M., Maus M.V., Kapoor V., June C.H., Mackall C.L. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J. Immunol. 2007;179:4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller K.T., Maude S.L., Porter D.L., Frey N., Wood P., Han X., Waldron E., Chakraborty A., Awasthi R., Levine B.L. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130:2317–2325. doi: 10.1182/blood-2017-06-786129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller K.T., Waldron E., Grupp S.A., Levine J.E., Laetsch T.W., Pulsipher M.A., Boyer M.W., August K.J., Hamilton J., Awasthi R. Clinical pharmacology of tisagenlecleucel in B-cell acute lymphoblastic leukemia. Clin. Cancer Res. 2018;24:6175–6184. doi: 10.1158/1078-0432.CCR-18-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuster S.J., Bishop M.R., Tam C.S., Waller E.K., Borchmann P., McGuirk J.P., Jäger U., Jaglowski S., Andreadis C., Westin J.R., JULIET Investigators Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 18.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter D.L., Hwang W.T., Frey N.V., Lacey S.F., Shaw P.A., Loren A.W., Bagg A., Marcucci K.T., Shen A., Gonzalez V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(2017). Tisagenlecleucel (CTL019) FDA Advisory Committee Briefing Document. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM566168.pdf.