Abstract
Introduction
Diabetic patients are often accompanied by complications of diabetic vascular disease, which could lead to heart failure or stroke. In this work, we explored the role of miR-503/Apelin-12 in diabetic angiopathy (DA) in vitro.
Methods
ELISA and qPCR were applied to assess the expression of miR-503 and Apelin-12 in high glucose (HG)-treated microvascular endothelial cells (HMEC-1). The effects of miR-503 on apoptosis, inflammation and oxidative stress were assessed by flow cytometry, western blotting, qPCR, and ELISA. The interaction between miR-503 and Apelin-12 was evaluated by dual-luciferase reporter assay, qPCR and ELISA, respectively. Western blotting was performed to examine the function of miR-503/Apelin-12 on JNK and p38MAPK activation.
Results
MiR-503 was markedly increased and Apelin-12 was decreased in HG-treated HMEC-1 cells. MiR-503 inhibitor significantly assuaged apoptosis, inflammation and oxidative stress in HMEC-1 cells. MiR-503 could specifically bind to the 3′UTR of Apelin and inversely downregulate Apelin-12 expression. Furthermore, Apelin-12 suppressed apoptosis, inflammation and oxidative stress. Inhibition of Apelin-12 could partially reverse the decrease of p-JNK and p-p38 expression levels induced by miR-503 suppression.
Conclusion
In HG-induced microvascular cells injury, miR-503/Apelin-12 enhances inflammation and oxidative stress by regulating JNK and p38MAPK pathway, suggesting a potential therapeutic target for DA.
Keywords: Diabetic angiopathy, miR-503, Apelin-12, JNK, p38MAPK
Abbreviations: Diabetic angiopathy, DA; High glucose, HG; MicroRNAs, miRNAs; malondialdehyde, MDA; superoxide dismutase, SOD; wild type, WT; mutant, Mut; Quantitative Real-time-PCR, qPCR; Enzyme linked immunosorbent assay, ELISA; reactive oxygen species, ROS
1. Introduction
Diabetic angiopathy (DA), a severe microvascular disease, is one of the most common complications of diabetes, with higher morbidity and mortality than other complications [1]. The common vascular diseases include cardiovascular disease, cerebrovascular disease, microvascular diseases of kidney, retina and skin. Several existing conventional treatment methods mainly prevent or limit the disease progression of DA by inducing pretreatment, post-treatment and pharmacological agents. However, there is no targeted treatment method with clear therapeutic mechanism and curative effect [2]. Therefore, it is of great significance to explore the mechanisms of diabetic vascular injury and to explore ideal treatment methods and strategies for DA.
MicroRNAs (miRNAs) have been identified as key factors regulating the expression of oxidative stress and inflammatory response-related proteins in cells, which closely related to DA [3,4]. MiRNAs are a group of low molecular weight, approximately 22 nucleotides in length, endogenous non-coding RNA. MiRNAs participate in a variety of physiological processes by binding to target mRNA at the post-transcriptional level, in other words, during protein translation, and regulating the protein expression of the target gene [5]. In recent years, more and more experimental studies have shown that miRNAs play an important role in diabetes and its complications, including DA [6]. Previous study have shown the overexpressed miR-145 could protects retinal microvasculature from HG-induced inflammation and oxidative stress through TLR4/NF-κB signaling [7]. Besides, miR-503 is also an important regulator of cardiac function and cardiovascular diseases [8].
Apelin and APJ receptors are essential for cardiovascular development and regeneration, and may also be involved in pathological processes related to cardiovascular diseases [9]. Apelin could enhances phosphorylation of AKT and eNOS to attenuates abnormal reaction of angiotensin II and acetylcholine in diabetic mice, indicating that Apelin/APJ system may act as a crucial regulator of diabetic vascular function [10]. It has been reported that Apelin/APJ showed a significant downward trend in the pathological process of severe and decompensated heart failure [11]. Moreover, Apelin/APJ could diminish HG-induced ROS formation and apoptosis in endothelial cell [12]. The precursor of Apelin consists of 77 amino acids, which are degraded to form active forms, such as Apelin-12, Apelin-13, Apelin-17 and Apelin-36. Apelin-12, one of the most potent short peptide substances of Apelin, has a protective effect on cardiovascular damage, corresponds to the oxidative stress [13]. Previous study demonstrated that the plasma Apelin-12 significantly reduced in people with type 2 diabetes mellitus [14]. Besides, Apelin-12 protects against ischemia-reperfusion injury in mice by inhibiting JNK and p38MAPK signaling pathways [15]. However, the function and mechanisms of miR-503/Apelin-12 on DA have received little attention.
In the current study, we investigated whether miR-503/Apelin-12 participate in the HG-induced microvascular endothelial cell injury through the JNK and P38MAPK signaling pathway to clarify its specific mechanism and provide a theoretical basis for targeted therapy in DA.
2. Materials and methods
2.1. Cell culture and treatments
The human microvascular endothelial cell line (HMEC-1) was obtained from China Center for Type Culture Collection. The cells were cultured with endothelial cell medium (ECM; ScienCell, USA) containing 10% fetal bovine serum (FBS; Gibco), 10 mM l-glutamine (Sigma), 1 μg/mL hydrocortisone (Sigma), 1% penicillin/streptomycin (Gibco) and 10 ng/mL epidermal growth factor (EGF; Sigma) in a cell culture flask at 37 °C under 5% CO2. HG (25 mM) was incubated with cells to establish a DA model in vitro [16]. And Apelin-12 (100 nM) was used in the future experiments.
MiR-503 mimics/inhibitor or corresponding negative control (mimics NC/inhibitor NC), small interfering RNA of Apelin (si-Apelin) or negative control (si-NC) were purchased from GenePharma (Shanghai, China). When cells were reached to 80–90% confluence, transfection was performed using Lipofectamine 2000 (Invitrogen).
2.2. MTT assay
Cell viability under HG conditions was determined using the MTT assay. HMEC-1 (1 × 104 cells/well) was seeded on a 96-well plate, and each well was parallelized three times under the same conditions. After treatment under HG conditions, 20 μl of MTT (5 mg/mL, Sigma)/100 μL of medium was added to the cells. Incubation for 4 h at 37 °C, discard the medium in each well and add 150 μL of DMSO instead, then shake the plate on the shaker for 10 min at room temperature. The absorbance of each well was then measured using a microplate reader (Bio-Rad 680, Hercules, CA, USA) and the detection wavelength was set at 490 nm. Absorbance is directly proportional to cell viability or the number of viable cells cultured, and the final data is expressed as a percentage relative to control cells.
2.3. Annexin V/PI staining for apoptosis detection
The percentage of early and late apoptotic HMEC-1 cells induced by HG was determined by Annexin-V-FITC/PI staining. The cells were harvested 48 h after HG treatment, centrifuged at 200 g, and suspended in an appropriate buffer. Then, 5 μL of V-FITC-labeled Annexin and 5 μL of PI solution were incubated at 25 °C for 5 min, followed by analysis by flow cytometry.
2.4. Quantitative Real-Time PCR (qPCR)
In terms of the manufacturer's protocol, TRIzol (Invitrogen, Carlsbad, CA, USA) was added to the HMEC-1 cells for lysis and total RNA was extracted. Total RNA concentration and integrity were determined by UV spectrophotometry (NANODROP 2000C, Thermo, USA). The reverse transcriptase reaction was carried out using a Thermo Revert AidTM First Strand cDNA Synthesis Kit (K1622, Thermo, USA). qPCR reactions were performed using 2 × SYBR Select Master Mix (Invitrogen, USA) and a Real-time PCR system (Piko Real 96 PCR system, Thermo Scientific, USA). Each sample was measured in three wells. The data was normalized to the human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or U6. The relative expression of miR-503 and mRNA of genes were calculated and quantified using 2−ΔΔCt method.
2.5. Western blotting
HMEC-1 cells were prepared using RIPA lysate (Beyotime, Shenzhen, Guangdong). The supernatant after centrifugation was collected, and the protein lysate was assayed using a double myosin assay kit (Pierce). Equal amount of proteins were isolated using SDS-PAGE and electrophoretically transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, United States). Then, the membranes were blocked in 5% nonfat milk for 1 h followed by incubation with primary antibodies overnight at 4 °C. After incubation with secondary antibody for 1 h, proteins were visualized with enhanced chemiluminescence (ECL) substrates (PerkinElmer, Inc., MA, USA). The primary antibody is shown as follow: anti-Bcl-2, anti-Bax, anti-JNK1/2/3, anti-p-JNK1/2/3 (phospho T183 + T183 + T221), anti-p38, anti-p-p38 (phospho T180 + Y182) (Abcam, 1:1000 dilution) and anti-cleaved Caspase3 (c-Caspase3, Abcam, 1:500 dilution), anti-β-actin (Abcam, 1:5000 dilution). Each experiment was repeated at least three times.
2.6. Enzyme linked immunosorbent assay (ELISA)
Supernatants of cell culture medium were collected after the experiment. According to the protocol of the manufacturer, expression of Apelin-12 (phoenix pharmaceuticals, USA), tumor necrosis factor-α (TNF-α), interleukin-1beta (IL-1β) and interleukin-6 (IL-6) (Boster, Wuhan, China) were detected in the supernatant.
2.7. Measurement of ROS generation
We used dichloro-dihydro-fluorescein-diacetate (DCFH-DA), a membrane-permeable and Ross-sensitive dye to determine the amount of ROS accumulated. DCFH-DA is first converted to 2,7-dichlorodihydrofluorescein by intracellular esterase and then oxidized by ROS into highly fluorescent 2,7-dichlorofluorescein molecules. The assay was performed according to the manufacturer's protocol by first washing these cells twice with ice-cold phosphate buffered saline (PBS) and incubating with DMEM medium containing 10 μM DCFH-DA. The sample was then centrifuged at 800 g for 5 min, washed twice with ice-cold PBS, and each group was measured by flow cytometry.
2.8. Measurements of the activities of antioxidant enzymes
Malondialdehyde (MDA) and superoxide dismutase (SOD) are important biomarkers of oxidative stress. We processed the cell supernatants according to the manufacturer's protocol for detection and measured the activity of these enzymes in a microplate reader. The kit for measuring MDA and SOD was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.9. Luciferase reporter assays
The wild-type- (WT-) Apelin 3′UTR and the mutated- (MUT-) Apelin 3′UTR were synthesized by Sangon Biotech (Shanghai, China) and amplified by PCR. The WT and MUT exons of Apelin were inserted downstream of the firefly luciferase reporter gene in the psiCHECK-2 vector. The luciferase reporters constructed were psiCHECK-2-WT-Apelin-3′UTR and psiCHECK-2-MUT-Apelin-3′UTR. For luciferase assays, cells were seeded into 24-well plates and transfected with miR-503 mimics (stable miR-503-overexpression) and the control (mimics NC) using Lipofectamine 2000 (Invitrogen). After 48 h, luciferase activity was measured using a Clarity TM Luminescent Microplate Reader.
2.10. Statistical analysis
Differences between the two groups were compared using Student's t test. Differences between multi-groups were assessed using one-way analysis of variance (ANOVA, Prism version 5.0). All results are expressed as mean ± standard deviation (SD). A p value less than 0.05 was considered statistically significant.
3. Result
3.1. The expression of miR-503 and Apelin-12 in DA model in vitro
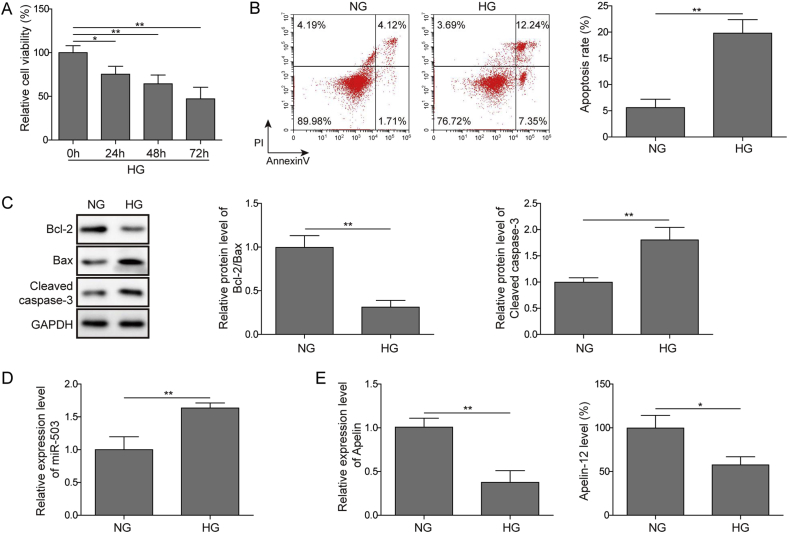
First, we used MTT assays and flow cytometry to assess cell damage in HG-induced in vitro model. The results showed that HG significantly reduced cell viability in a time dependence (Fig. 1A). In addition, HG induced cell apoptosis obviously (Fig. 1B), consistently, increased c-Caspase3 expression, and decreased Bcl-2/Bax (Fig. 1C). Next, the expression of miR-503 and Apelin-12 was examined by qPCR and ELISA. In our results, miR-503 expression was markedly increased in HG treated HMEC-1 cells compared to the normal glucose (NG) group (Fig. 1D). However, the expression of Apelin-12 level was certainly reduced (Fig. 1E). Collectively, miR-503 may be highly expressed while Apelin-12 lowly expressed in DA.
Fig. 1.
The expression of miR-503 and Apelin-12 in HG-induced HMEC-1 cells. (A) Cell viability detected by MTT assay. (B) Cell apoptosis assessed by flow cytometry. (C) Detection of Bcl-2, Bax and cleaved Caspase3 expression by Western blotting. (D) The expression level of miR-503 determined by qPCR. (E) The level of Apelin-12 determined by qPCR and ELISA. All data were represented by 3 independent experiments. *P < 0.05; **P < 0.01.
3.2. Suppression of miR-503 attenuates HG-induced inflammation and oxidative stress in HMEC-1 cells
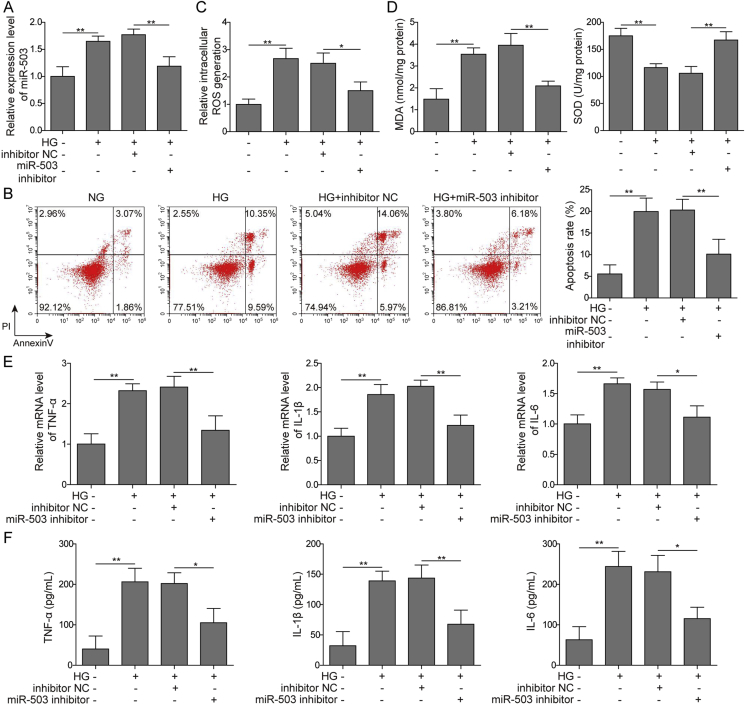
We transfected miR-503 inhibitor and inhibitor-NC into HMEC-1 cells and performed loss-of-function experiments to further investigate the role of miR-503 in HG-induced microvascular endothelial injury. According to our results, the addition of miR-503 inhibitor significantly down-regulated the expression of miR-503 in HG-treated cells and reduced apoptosis (Fig. 2A–B). Furthermore, miR-503 inhibition decreased the HG-induced ROS level (Fig. 2C). Additionally, MDA level was restrained, whereas the activity of SOD was increased in miR-503 supressing cells under HG condition (Fig. 2D). Treatment of miR-503 inhibitor produced a remarkable reduction in the expression of TNF-α, IL-1β and IL-6 at both mRNA and protein levels (Fig. 2E–F). This suggested that inhibition of miR-503 expression can alleviate oxidative stress and inflammation in HG-induced HMEC-1 cells.
Fig. 2.
Effects of miR-503 on oxidative stress and inflammation induced by HG in HMEC-1 cells. (A) qPCR detection of miR-503 expression level. (B) Cell apoptosis assessed by flow cytometry. (C) Measuring of ROS generation by DCFH-DA assay. (D) The ELISA kit detects MDA and SOD levels. (E) TNF-α, IL-1β, IL-6 mRNA expression levels detected by qPCR. (F) ELISA measurement of protein expression levels of TNF-α, IL-1β and IL-6. All data were represented by 3 independent experiments. *P < 0.05; **P < 0.01.
3.3. Apelin-12 is a target of miR-503
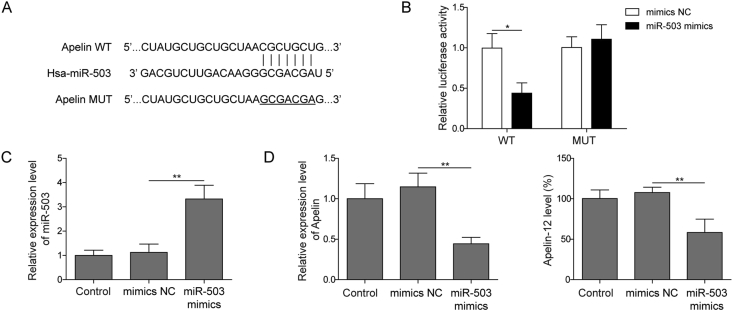
We next investigated the role of up-regulation of miR-503 induced by HG in the regulation of Apelin-12 expression in HMEC-1. First, screening using web-based predictive software starBase (http://starbase.sysu.edu.cn/index.php) revealed the presence of a binding site between miR-503 and Apelin 3′UTR (Fig. 3A). To verify this prediction, Apelin-WT/MUT were added to the dual luciferase reporter vector, respectively. The results indicated that miR-503 mimics obviously restrain the luciferase activity of Apelin-WT transfected cells (Fig. 3B). In addition, the expression of miR-503 was increased significantly after transfection of miR-503 mimics into HMEC-1 cells (Fig. 3C), subsequently inhibited Apelin-12 level (Fig. 3D). These data indicated that miR-503 directly targeting Apelin-12, prompting miR-503 may affect the HG-induced HMEC-1 cells injury through Apelin-12.
Fig. 3.
The connection between miR-503 and Apelin-12. (A) starBase predicts miR-503 and Apelin binding sites. (B) HMEC-1 were transfected with luciferase plasmids of Apelin-WT or Apelin-MUT for 48 h. After that, a dual-luciferase reporter assay was performed. (C) qPCR detection of miR-503 expression level. (D) Apelin-12 level measured by qPCR and ELISA assay. All data were represented by 3 independent experiments. *P < 0.05; **P < 0.01.
3.4. Effects of Apelin-12 on inflammation and oxidative stress in HG-induced HMEC-1 cells
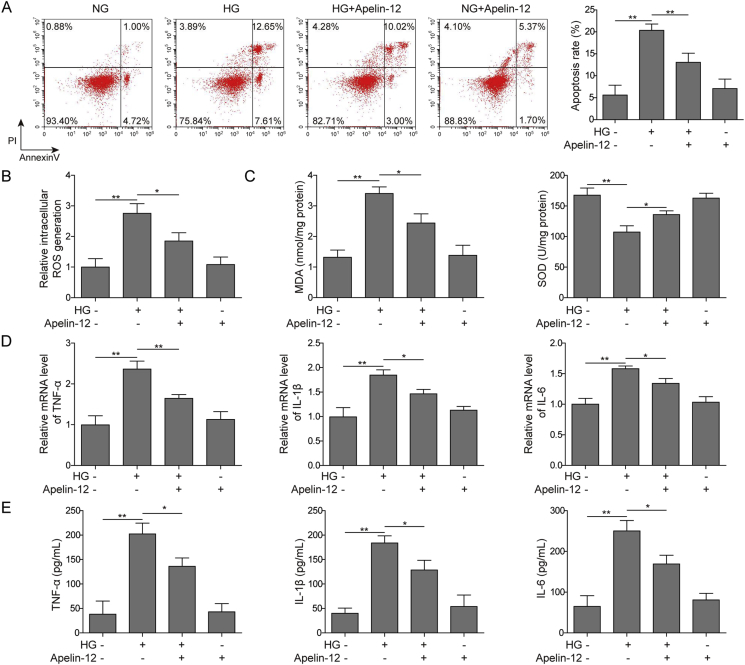
As shown, Apelin-12 decreased cell apoptosis remarkably in HG-stimulated cells (Fig. 4A). We examined ROS production, MDA levels and SOD activity in HMEC-1 cells to reflect oxidative stress in cells. In Fig. 4B, the accumulation of ROS is significantly increased when HMEC-1 cells are under HG conditions, which was inhibited by Apelin-12 incubation. After HG treatment, our study demonstrated that SOD activity was significantly decreased in HMEC-1 cells compared with the control group, while the content of MDA was significantly increased. Interestingly, Apelin-12 partially reversed these results (Fig. 4C). These results indicate that Apelin-12 can attenuate oxidative stress in HG-induced HMEC-1 cells. In addition, compared with the HG group, the expression of TNF-α, IL-1β, IL-6 were decreased significantly in Apelin-12 group, which indicates that Apelin-12 could inhibit inflammation in HMEC-1 cells induced by HG. From the above statement, miR-503/Apelin-12 significantly attenuated inflammation and oxidative stress in HG-induced HMEC-1 cells.
Fig. 4.
Inhibition of Apelin-12 on oxidative stress and inflammation induced by HG in HMEC-1 cells. (A) Cell apoptosis assessed by flow cytometry. (B) Measuring of ROS generation by DCFH-DA assay. (C) The ELISA kit detects MDA and SOD levels. (D) qPCR detection of TNF-α, IL-1β, IL-6 mRNA expression levels. (E) ELISA measurement of protein expression levels of TNF-α, IL-1β and IL-6. All data were represented by 3 independent experiments. *P < 0.05; **P < 0.01.
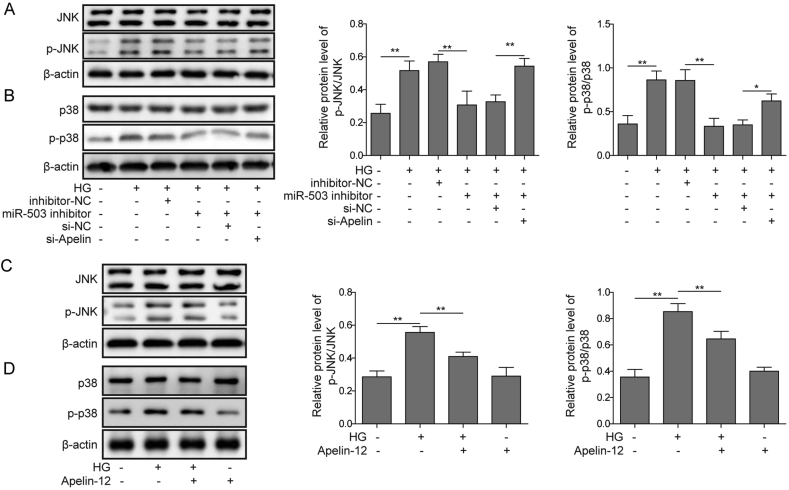
3.5. MiR-503/Apelin-12 mediates JNK and p38MAPK signaling activation
We next investigated whether MAPK is involved in HG-induced HMEC-1 cells damage. As depicted in Fig. 5A, B, HG apparently induced JNK and p38 phosphorylation in HMEC-1 cells, indicating the activation of JNK and p38. To further demonstrate whether miR-503 and Apelin-12 can trigger phosphorylation of JNK and p38 in HG-treated HMEC-1 cells, miR-503 inhibitor and si-Apelin were transfected into cells. MiR-503 inhibitor significantly abolished phosphorylation of JNK and P38 induced by HG, while si-Apelin partially reversed these changes (Fig. 5A, B). Furthermore, Apelin-12 significantly inhibited HG-induced JNK and p38 phosphorylation (Fig. 5C, D). The above results indicate that miR-503/Apelin-12 mediates HG-induced HMEC-1 cells injury via JNK and p38MAPK signaling pathway.
Fig. 5.
miR-503/Apelin-12 mediates activation of JNK and p38MAPK signaling pathways. (A&C) JNK phosphorylation level evaluated by western blotting. (B&D) p38 phosphorylation level evaluated by western blotting. All data were represented by 3 independent experiments. *P < 0.05; **P < 0.01.
4. Discussion
In diabetic patients, complications associated with DA usually occur, which can lead to heart failure or stroke [17]. Especially in patients with vascular disease and type 2 diabetes, it is more prone to peripheral microvascular diseases and peripheral nervous system diseases in the kidney, retina and other parts [18]. In addition, cardiovascular complications are a common complication of diabetes. Clinical statistics and experimental studies have demonstrated that microvascular endothelial injury is widespread in human patients or diabetic animal models [19]. In this study, HG-induced HMEC-1 injury were assessed by cell viability, morphological observation and apoptosis. Then, the levels of oxidative stress and inflammation related indicators and related pathway proteins were detected after treated by miR-503 inhibitor and Apelin-12. These results indicate that miR-503 inhibitor or Apelin-12 can alleviate HMEC-1 oxidation stress and inflammation by inhibiting JNK and p38MAPK pathway.
It is known that vascular-related complications are closely associated with a higher risk in patients with type 2 diabetes [20]. MiRNAs are involved in many key metabolic processes in the body, such as transcription and protein level regulation of inflammation and anti-angiogenic pathways, which may lead to common disease complications, including retinal microangiopathy caused by type 2 diabetes, neuropathy, nephropathy and ischemic diseases [21]. For instance, miR-146a is down-regulated in both HG-induced endothelial cell model and streptozotocin-induced rat diabetes model [22]. MiR-181b not only plays a significant role in the regulation of microvascular inflammation, but also inhibits inflammation of vascular epithelial cells in the macrovasculature [[23], [24], [25]]. MiR-503 is a member of the miRNA-16 family and is expressed in a variety types of diabetes, especially in its associated complications. Further studies have demonstrated that phase II enzyme-inducing factor (CPDT) regulates Nrf2/ARE signaling pathway by affecting miR-503 activity and protects against diabetic cardiomyopathy [26]. Therefore, miR-503 may be a new potential therapeutic target for the treatment of diabetic complications. Likewise, in our study, we found that miR-503, which increased in DA, closely related to the increased levels of oxidative stress index and inflammatory cytokines. Exhilaratingly, we also found that Apelin-12 was lowly expressed in DA and miR-503 overexpression inhibited its expression, indicating Apelin-12 is a target of miR-503.
Apelin was first purified by Tatemoto and co-workers from bovine stomach and identified as a novel endogenous peptide ligand for APJ [27]. Apelin and APJ are expressed in the endothelial cells of blood vessels [28]. Studies have shown that the expression of Apelin in plasma of diabetes mellitus patients is dramatically increased [29]. Our results of qPCR and ELISA verified that Apelin is involved in DA development. Increased Apelin level also significantly reduces macrophage load in the arterial wall, thereby exerting a direct anti-inflammatory effect [30]. Apelin-13 infusion effectively inhibits vascular lesion formation and suppresses the expression of TNF-α and IL-1β, such anti-inflammatory and vaso-relaxation effects reduce the atherosclerosis formation and lower the risk of heart ischemia in diabetics [31,32]. Equally, according to our data, Apelin-12 weakened the expression of TNF-α, IL-1β, IL-6, which can act both locally and systemically; therefore, their production and secretion need to be tightly regulated [33], exerted anti-inflammatory effect in HG-induced HMEC-1 cells injury. Increasing antioxidant activity is important for mitigating cell damage caused by ROS, which can change cell membrane permeability, lead to cell structure destruction and lipid peroxidation, and finally induce cell damage attribute to loss of key enzymes [34,35]. SOD can decompose H2O2 and O2 and prevent them from becoming more reactive hydroxyl radicals, is the first defense line against oxidative damage [36]. In our results, Apelin-12 also increased SOD level and decrease MDA and ROS levels in HMEC-1 cells, indicated potential ability on anti-oxidation. Moreover, Bcl-2/Bax in cells, resulting in increased mitochondrial membrane permeability, cytochrome c release, Caspase3 activation, and apoptosis [37], were significantly down-regulated by Apelin-12 intervention.
It's clearly proved that the generation of ROS significantly aggravated the activation of glucose-stimulated oxidative stress and MAPKs signaling, including p38, ERK1/2 and JNK [38]. In the p38 pathway, p38 is activated by MKK3 or MKK6, and these MAP2Ks are activated by the same MAP3Ks that function in the JNK pathway [39]. The JNK and p38 signaling pathways are activated by various types of cellular stress such as oxidative and by proinflammatory cytokines such as TNF-α and IL-1β [39]. Recently, it has been reported that the activity of p38MAPK in Type 2 Diabetes was increased, subsequently contributed to the increase of inflammatory cytokine gene expression [40]. In addition, studies have shown that phosphorylation level of p38MAPK are significantly increased in vascular endothelial cells exposed to HG [41]. Taken together, it can be inferred from these results that excessive ROS accumulated under HG conditions and stimulated the production of inflammation and oxidative stress, which in turn causes DA through p38MAPK pathway. In the current study, our results indicated that miR-503 significantly upregulated the phosphorylation of JNK and p38MAPK in HG-treated HMEC-1 cells, but Apelin-12 showed the opposite effects. Therefore, the regulation of inflammation and oxidative stress by Apelin-12 and miR-503 might be related to its effects on the phosphorylation of JNK and p38MAPK. However, the effect of miR-503/Apelin-12 on diabetic vascular disease has not been validated at the animal level. The study of the effects of miR-503/Apelin-12 in animal models of DA would be our further research program.
5. Conclusions
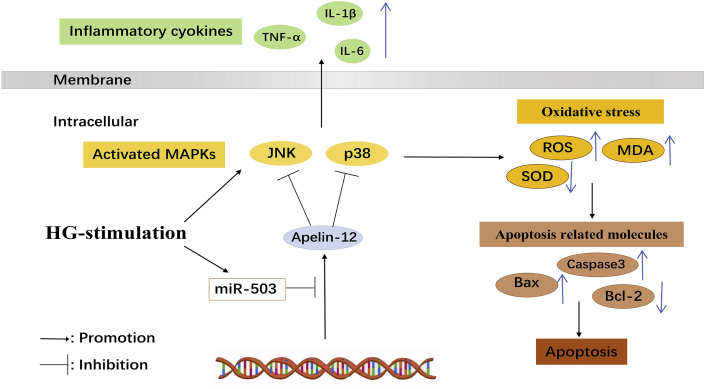
MiR-503 inhibited Apelin-12 level by targeting the promoter region of the Apelin gene by binding to its 3′UTR and moderate the HG-induced HMEC-1 injury through JNK and p38MAPK signaling pathway (Fig. 6). These findings enrich the pathology of DA and provide a theoretical basis for innovative therapeutic strategies of DA based on regulating miR-503/Apelin-12 level.
Fig. 6.
Schematic illustration of the effects of miR-503/Apelin-12 on HG-induced HMEC-1 cells damage.
Declaration of Competing Interest
Authors have declared no conflict of interests.
Acknowledgements
This work was supported by Effect in nCPAP on glycometabolism and Apelin-12 level of diabetic patients with OSAHS (Hunan Science and Technology Department, 2013SK3222) and Changes of MnSOD and MDA in OSA patients with T2DM and the influence of nCPAP (Hunan Science and Technology Department, 2014TZ2014).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Lin X., Wang J., Yun L., Jiang S., Li L., Chen X. Association between LEKR1-CCNL1 and IGSF21-KLHDC7A gene polymorphisms and diabetic retinopathy of type 2 diabetes mellitus in the Chinese Han population. J Gene Med. 2016;18(10):282–287. doi: 10.1002/jgm.2926. [DOI] [PubMed] [Google Scholar]
- 2.Semeraro F., Morescalchi F., Cancarini A., Russo A., Rezzola S., Costagliola C. Diabetic retinopathy, a vascular and inflammatory disease: therapeutic implications. Diabetes Metab. 2019;45(6):517–527. doi: 10.1016/j.diabet.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W., Wang Y., Kong Y. Exosomes derived from mesenchymal stem cells modulate miR-126 to Ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Investig Ophthalmol Vis Sci. 2019;60(1):294–303. doi: 10.1167/iovs.18-25617. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z., Liu Y., Han N., Chen X., Yu W., Zhang W. Profiles of oxidative stress-related microRNA and mRNA expression in auditory cells. Brain Res. 2010;1346:14–25. doi: 10.1016/j.brainres.2010.05.059. [DOI] [PubMed] [Google Scholar]
- 5.Xiao J., Chen Y.H. MicroRNAs: novel regulators of the heart. J Thorac Dis. 2010;2(1):43–47. [PMC free article] [PubMed] [Google Scholar]
- 6.Shantikumar S., Caporali A., Emanueli C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc Res. 2012;93(4):583–593. doi: 10.1093/cvr/cvr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hui Y., Yin Y. MicroRNA-145 attenuates high glucose-induced oxidative stress and inflammation in retinal endothelial cells through regulating TLR4/NF-kappaB signaling. Life Sci. 2018;207:212–218. doi: 10.1016/j.lfs.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y., Deng L., Zhao D., Chen L., Yao Z., Guo X. MicroRNA-503 promotes angiotensin II-induced cardiac fibrosis by targeting Apelin-13. J Cell Mol Med. 2016;20(3):495–505. doi: 10.1111/jcmm.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu X.H., Tang Z.B., Liu L.J., Qian H., Tang S.L., Zhang D.W. Apelin and its receptor APJ in cardiovascular diseases. Clin Chim Acta. 2014;428:1–8. doi: 10.1016/j.cca.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Hou X., Zeng H., He X., Chen J.X. Sirt3 is essential for apelin-induced angiogenesis in post-myocardial infarction of diabetes. J Cell Mol Med. 2015;19(1):53–61. doi: 10.1111/jcmm.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalzell J.R., Rocchiccioli J.P., Weir R.A., Jackson C.E., Padmanabhan N., Gardner R.S. The emerging potential of the apelin-APJ system in heart failure. J Card Fail. 2015;21(6):489–498. doi: 10.1016/j.cardfail.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Zeng H., He X., Hou X., Li L., Chen J.X. Apelin gene therapy increases myocardial vascular density and ameliorates diabetic cardiomyopathy via upregulation of sirtuin 3. Am J Physiol Heart Circ Physiol. 2014;306(4):H585–H597. doi: 10.1152/ajpheart.00821.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pisarenko O.I., Lankin V.Z., Konovalova G.G., Serebryakova L.I., Shulzhenko V.S., Timoshin A.A. Apelin-12 and its structural analog enhance antioxidant defense in experimental myocardial ischemia and reperfusion. Mol Cell Biochem. 2014;391(1–2):241–250. doi: 10.1007/s11010-014-2008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdem G., Dogru T., Tasci I., Sonmez A., Tapan S. Low plasma apelin levels in newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116(5):289–292. doi: 10.1055/s-2007-1004564. [DOI] [PubMed] [Google Scholar]
- 15.Liu D.R., Hu W., Chen G.Z. Apelin-12 exerts neuroprotective effect against ischemia-reperfusion injury by inhibiting JNK and P38MAPK signaling pathway in mouse. Eur Rev Med Pharmacol Sci. 2018;22(12):3888–3895. doi: 10.26355/eurrev_201806_15273. [DOI] [PubMed] [Google Scholar]
- 16.Ye E.A., Steinle J.J. miR-146a attenuates inflammatory pathways mediated by TLR4/NF-kappaB and TNFalpha to protect primary human retinal microvascular endothelial cells grown in high glucose. Mediat Inflamm. 2016;2016:3958453. doi: 10.1155/2016/3958453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett E.J., Liu Z., Khamaisi M., King G.L., Klein R., Klein B.E.K. Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab. 2017;102(12):4343–4410. doi: 10.1210/jc.2017-01922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lastra G., Syed S., Kurukulasuriya L.R., Manrique C., Sowers J.R. Type 2 diabetes mellitus and hypertension: an update. Endocrinol Metab Clin North Am. 2014;43(1):103–122. doi: 10.1016/j.ecl.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lotfy M., Adeghate J., Kalasz H., Singh J., Adeghate E. Chronic complications of diabetes mellitus: a mini review. Curr Diabetes Rev. 2017;13(1):3–10. doi: 10.2174/1573399812666151016101622. [DOI] [PubMed] [Google Scholar]
- 20.Wei M., Gaskill S.P., Haffner S.M., Stern M.P. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care. 1998;21(7):1167–1172. doi: 10.2337/diacare.21.7.1167. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Sun X., Icli B., Feinberg M.W. Emerging roles for MicroRNAs in diabetic microvascular disease: novel targets for therapy. Endocr Rev. 2017;38(2):145–168. doi: 10.1210/er.2016-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng B., Chen S., McArthur K., Wu Y., Sen S., Ding Q. miR-146a-Mediated extracellular matrix protein production in chronic diabetes complications. Diabetes. 2011;60(11):2975–2984. doi: 10.2337/db11-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X., Icli B., Wara A.K., Belkin N., He S., Kobzik L. MicroRNA-181b regulates NF-kappaB-mediated vascular inflammation. J Clin Investig. 2012;122(6):1973–1990. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X., Sit A., Feinberg M.W. Role of miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc Med. 2014;24(3):105–112. doi: 10.1016/j.tcm.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin J., He S., Sun X., Franck G., Deng Y., Yang D. MicroRNA-181b inhibits thrombin-mediated endothelial activation and arterial thrombosis by targeting caspase recruitment domain family member 10. FASEB J. 2016;30(9):3216–3226. doi: 10.1096/fj.201500163R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miao Y., Wan Q., Liu X., Wang Y., Luo Y., Liu D. miR-503 is involved in the protective effect of phase II enzyme inducer (CPDT) in diabetic cardiomyopathy via Nrf2/ARE signaling pathway. BioMed Res Int. 2017;2017:9167450. doi: 10.1155/2017/9167450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatemoto K., Hosoya M., Habata Y., Fujii R., Kakegawa T., Zou M.X. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 28.Kleinz M.J., Skepper J.N., Davenport A.P. Immunocytochemical localisation of the Apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept. 2005;126(3):233–240. doi: 10.1016/j.regpep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Li Y., Bai Y.J., Jiang Y.R., Yu W.Z., Shi X., Chen L. Apelin-13 is an early promoter of cytoskeleton and tight junction in diabetic macular edema via PI-3K/Akt and MAPK/Erk signaling pathways. BioMed Res Int. 2018;2018:3242574. doi: 10.1155/2018/3242574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leeper N.J., Tedesco M.M., Kojima Y., Schultz G.M., Kundu R.K., Ashley E.A. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol. 2009;296(5):H1329–H1335. doi: 10.1152/ajpheart.01341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H., Liu C., Cheng C., Zheng L., Huang K. Effects of Apelin peptides on diabetic complications. Curr Protein Pept Sci. 2018;19(2):179–189. doi: 10.2174/1389203718666170918154728. [DOI] [PubMed] [Google Scholar]
- 32.Lee T., Park C.K., Ha S.Y. Prognostic role of Apelin receptor expression in hepatocellular carcinoma treated with curative surgical resection. Anticancer Res. 2019;39(6):3025–3031. doi: 10.21873/anticanres.13435. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X., Wang Y., Shen W., Ma S., Chen W., Qi R. Rosa rugosa flavonoids alleviate myocardial ischemia reperfusion injury in mice by suppressing JNK and p38 MAPK. Microcirculation. 2017;24(7) doi: 10.1111/micc.12385. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Li X., Wang X., Lau W., Wang Y., Xing Y. Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury via Akt/GSK-3beta signaling and inhibition of the mitochondria-dependent apoptotic pathway. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao M., Liu R. Molecular mechanism of CAT and SOD activity change under MPA-CdTe quantum dots induced oxidative stress in the mouse primary hepatocytes. Spectrochim Acta A Mol Biomol Spectrosc. 2019;220:117104. doi: 10.1016/j.saa.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Asweto C.O., Wu J., Alzain M.A., Hu H., Andrea S., Feng L. Cellular pathways involved in silica nanoparticles induced apoptosis: a systematic review of in vitro studies. Environ Toxicol Pharmacol. 2017;56:191–197. doi: 10.1016/j.etap.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Huang F., Sheng X.X., Zhang H.J. DUSP26 regulates podocyte oxidative stress and fibrosis in a mouse model with diabetic nephropathy through the mediation of ROS. Biochem Biophys Res Commun. 2019;515(3):410–416. doi: 10.1016/j.bbrc.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 39.Kim E.K., Choi E.J. Compromised MAPK signaling in human diseases: an update. Arch Toxicol. 2015;89(6):867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 40.Ciaraldi T.P., Ryan A.J., Mudaliar S.R., Henry R.R. Altered myokine secretion is an intrinsic property of skeletal muscle in type 2 diabetes. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0158209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazrouei S., Sharifpanah F., Caldwell R.W., Franz M., Shatanawi A., Muessig J. Regulation of MAP kinase-mediated endothelial dysfunction in hyperglycemia via arginase I and eNOS dysregulation. Biochim Biophys Acta Mol Cell Res. 2019;1866(9):1398–1411. doi: 10.1016/j.bbamcr.2019.05.004. [DOI] [PubMed] [Google Scholar]