Abstract
Introduction
Achilles tendinopathy is characterized by scar formation or ectopic ossification, both of which result in pain and worsened physical function in athletes and older people. Although cell therapy using adipose-derived stem cells (ASCs) has been shown to be effective for tendinopathy, the underlying mechanisms by which ASCs result in tendon healing in vivo have not yet been fully clarified.
Methods
ASCs were obtained from the fat pads of EGFP transgenic mice by collagenase digestion. C57BL/6 mice were used in a collagenase-induced injury model. ASCs were transplanted into injury sites at 1 week after injury. Tendons were harvested at 9 days, 2 weeks, and 4 weeks after transplantation, and analyzed by histological examination and μCT scanning.
Results
Histological analysis and μCT scanning revealed greater recovery of collagen fibers and suppression of ectopic ossification in the ASC-treated group than in the control group at 2 and 4 weeks after injury. Immunohistochemical analysis identified transplanted ASCs in the tendon core close to peritenon and connective tissue at 2 days and 1 week after transplantation, but not at 3 weeks. Furthermore, while the expression levels of IL-1β, GLUT1, and CA9 were significantly reduced in the ASC group compared to the control group at 9 days after injury, those of VEGF and the number of CD31 positive vessels were significantly increased.
Conclusion
The efficacy of ASCs for tendon repair and the prevention of ectopic ossification in Achilles tendinopathy were demonstrated. Our data suggest that ASCs can modulate inflammation and induce neovascularization in the early stage of tendon injury.
Keywords: Tendinopathy, Ectopic ossification, ASCs, IL-1β, Hypoxia, Neovascularization
Abbreviations: ASCs, adipose-derived stem cells; IL-1β, interleukin-1β; VEGF, vascular endothelial growth factor; Glut1, glucose transporter 1; CA9, carbonic acid 9
1. Introduction
Achilles tendinopathy is one of the most prevalent musculoskeletal disorders in athletes and older people. It impairs physical function and causes pain, which result in early retirement in athletes and reduced activity in older adults. Overuse, aging, and glucose metabolism disorder are associated with a high risk of tendinopathy [1,2]. Due to the hypocellularity and hypovascularity of tendons, their natural healing ability is extremely poor and inefficient [3,4].
Inflammation plays an essential role in tendon injuries and healing. Proinflammatory cytokines, including interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) promote expression of matrix-metalloproteinases (MMPs) and decrease collagen synthesis in tenocytes [5]. IL- 1β, which is upregulated at tendon injury sites, inhibits tenogenic differentiation of tenocytes derived from injured tendons [6].
Hypoxia is involved in the pathogenesis of tendinopathy. Tenocytes respond to hypoxia in vitro by activating classical hypoxia-induced factor-1α (HIF-1α)–driven pathways, and total hypoxia causes tenocyte apoptosis [7]. Injured tendons demonstrate acutely increased glycolysis and lactate synthesis after injury, and the inhibition of lactate synthesis improves collagen fiber alignment and inhibits mucoid accumulation and ectopic calcification [8].
Although HIF-1α also induces vascular endothelial growth factor (VEGF), which promotes vascularization [9], the role of angiogenesis in tendon repair is controversial. While angiogenesis is essential for the repair of tissues other than tendons, by facilitating the delivery of nutritive substances, chronic tendinopathy exhibits neovascularization that is associated with innervation and pain [10]. Understanding the mechanisms and temporal changes of angiogenesis will be beneficial for establishing novel therapeutic approaches.
The use of regenerative medicine as a new strategy for tendinopathy treatment has gathered significant attention among researchers and clinicians, and many studies using in vivo models have reported the efficacy of cell therapy in tendon healing [11,12]. Adipose-derived stem cells (ASCs) are a type of mesenchymal stem cell often used for tissue engineering or cell therapy. Compared to bone marrow-derived MSCs, ASCs are easily isolated, and a substantial amount of ASCs are obtained from the processing of adipose tissue. ASCs promote tissue regeneration by secreting cytokines and growth factors that stimulate the restoration of normal tissue function and reduce tissue damage [13,14]. Previous studies have reported that ASCs improve biomechanical properties and induce the organization of collagen fibers in tendon injury models [15,16]. ASCs have also been found to differentiate into tenocytes and modulate the inflammatory environment [17,18]. However, one studies have reported the effects of ASCs on ectopic ossification in tendinopathy [15], and the mechanisms whereby ASCs promote tendon healing and prevent ectopic ossification in vivo are poorly understood. We hypothesized that ASCs improve tendon repair by regulating inflammation and hypoxia, and by modulating angiogenesis in tendinopathy.
To test this hypothesis, we investigated whether the transplantation of ASCs improved the histological features of tendons and decreased ectopic ossification, and sought to elucidate ASCs' therapeutic mechanisms.
2. Materials and methods
2.1. Isolation and culture of adipose stem cells
All procedures were approved by the Research Ethics Committee of The University of Tokyo Hospital (ethical permission number 622). ASCs were isolated from 6- or 7-week-old EGFP transgenic mice (C57BL/6-Tg(CAG-EGFP)C14–Y01-FM131Osb; RIKEN BioResource Center, Japan). Adipose tissue was harvested from the inguinal fat pads, minced, and digested by shaking with 0.1% collagenase solution for 30 min at 37 °C. The solution was filtered through a cell strainer (70-μm pore size; BD Falcon, Japan) and centrifuged at 250G for 5 min, and the supernatant was removed to obtain the stromal vascular fraction. Cells were seeded at 1.0 × 106 cells/dish in 100-mm dishes, and cultured in medium consisting of Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12; Sigma–Aldrich,USA), 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin (Sigma–Aldrich) at 37 °C in 5% CO2. The medium was changed every 3–4 days and cells were passaged using trypsin–EDTA (Sigma–Aldrich), when they reached 80–90% confluence. After 2 passages, ASCs were used for transplantation.
2.2. Collagenase-induced mouse Achilles tendon injury model
Thirty-six 6-week-old male C57BL/6J mice (Nippon Bio-Supp. Center, Japan) were used in this study. The mice were anesthetized by inhalation of isoflurane. After all limbs were shaved and disinfected, 1% type I collagenase (Wako, Japan) dissolved in 20 μL of phosphate-buffered saline (PBS) was percutaneously injected into the Achilles tendon of the left limbs with a 30-gauge needle, and 20 μL PBS was injected into the right limbs.
2.3. Transplantation of ASCs
The mice were randomly divided into 2 groups: the ASC group (ASC-treated tendons, n = 18) and the control group (PBS-treated tendons, n = 18). One week after collagenase injection (i.e., after injury), the mice were anesthetized. ASCs were resuspended at a concentration of 1 × 107 cells/mL in PBS. Twenty microliters of ASC solution or PBS was injected into the collagenase-induced lesion with a 24-gauge needle. At 9 days, 2 weeks, and 4 weeks after collagenase injection (2 days, 1 week, and 3 weeks after transplantation), mice were sacrificed and Achilles tendons were harvested for evaluation (n = 6 tendons per time point and per group).
2.4. μCT analysis
Injured tendons were fixed with 4% paraformaldehyde for 24 h at 4 °C and subjected to μCT analysis using a scanner at 75 kV and 140 mA (InspeXio; Shimadzu Science East Corporation, Tokyo, Japan). Three-dimensional(3D) images were reconstructed with TRI/3D-BON (RATOC, Japan). Total volume of ectopically mineralized components in Achilles tendon was calculated.
2.5. Histology
Tendons were fixed in 4% paraformaldehyde for 24 h at 4 °C. They were then dehydrated, embedded in paraffin, and cut into 5-μm longitudinal sections. The slides were stained with hematoxylin and eosin (H&E), toluidine blue, and Alizarin Red to compare the tendon morphology of the ASC group with that of the control group. Photomicrographs of tissue were obtained using an optical microscope (DP 70; Olympus, Tokyo, Japan). Longitudinal sections of Achilles tendons were histopathologically analyzed using a modified, semi-quantitative histopathological score as previously described [19]. This scale consists of 4 features, each of which is graded from 0 to 3: (1) fiber structure, (2) rounding of the nuclei, (3) ground substance, and (4) vascularity (Table 1). The mean score of each specimen was used for analysis.
Table 1.
Scale degree of histology.
| 0 | 1 | 2 | 3 | ||
|---|---|---|---|---|---|
| (1) | Fiber structure | Continuous,long fiber | Slightly fragmented fiber | Moderately fragmented fiber | Severely fragmented fiber |
| (2) | Rounding of the nuclei | Long spindle-shape cells | Slightly rounded | Moderately rounded | Severely rounded |
| (3) | Ground substance (Toluidin Blue staining) | No stainable ground substance | Stainable mucin between fibers but bundle still discrete | Stainable mucin between fibers with loss clear demacration of bundles | Abundant mucin throughout with inconspicuous collagen staining |
| (4) | Vascularity | <10% | 10–20% | 20–30% | >30% |
2.6. Immunohistochemical staining
Immunohistochemical analysis was performed to track the locations of transplanted ASCs and to assess the inflammatory response, hypoxia, and neovascularization in tendon injury. Paraffin-embedded sections were dried at 60 °C for a few hours, deparaffinized in xylene, and dehydrated through a graded alcohol series. To activate antigens, the sections were incubated with protease solution for 15 min at 37 °C, then blocked in 3% H2O2 in methanol for 10 min and in 5% bovine serum for 20 min at room temperature. The sections were incubated at 4 °C overnight with primary antibody against anti–GFP IgG (dilution:1:1000; Life Technologies Corporation, Carlsbad, CA, USA), IL-1β (dilution:1:200; Abcam, Cambridge, USA), glucose transporter 1 (Glut1) (1:200; Abcam), carbonic acid 9 (CA9) (1:1000; Abcam), VEGF (1:100; Abcam), and CD31 (1:20; Abcam). After the application of secondary antibodies, sections were washed 3 times for 2 min and then developed in diaminobenzidine (DAB; Sigma–Aldrich), and then counterstained with hematoxylin. The sections were examined under light microscopy. DAB intensity was determined by image analysis software (Image J; NIH, Bethesda, MD, USA). Two randomly chosen 200x microscopic fields of each section were observed.
2.7. Statistics
Data are expressed as mean ± SD and statistically analyzed using Student's t-test. A value of p < 0.05 was used to indicate statistical significance.
3. Results
3.1. Histopathological analysis
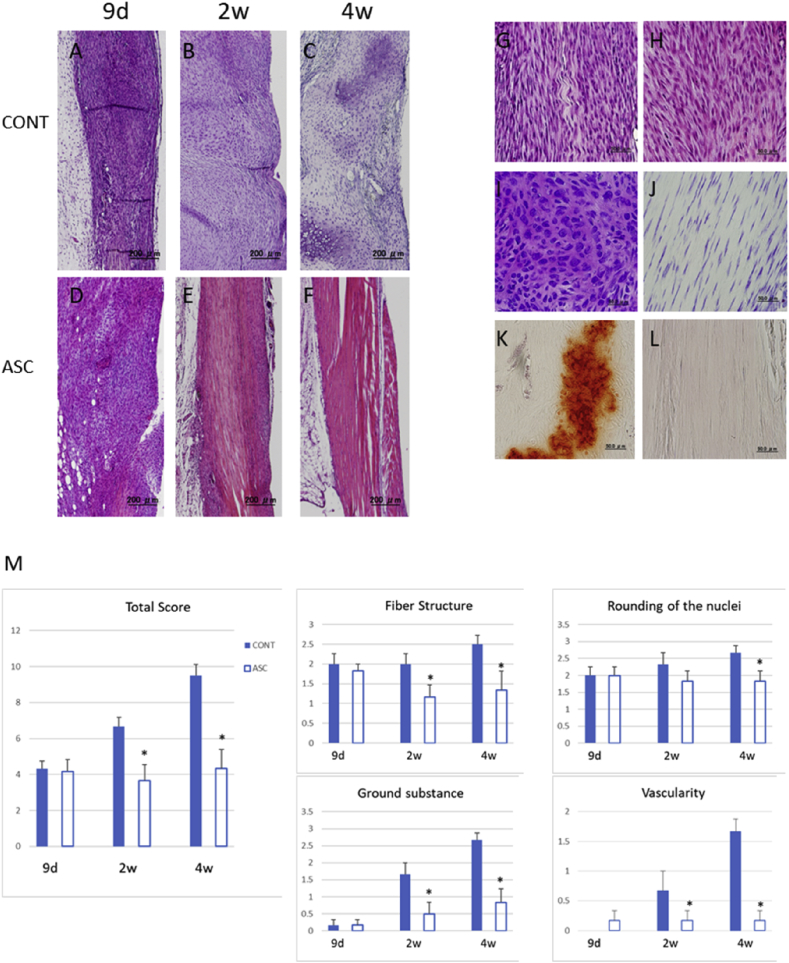
To examine the general tendon composition and organization during the healing stage, tendons and surrounding tissues were analyzed histologically by staining with H&E, toluidine blue, and Alizarin Red. In H&E staining, the ASC group showed less severe degenerative changes in tendon than the control group at 2 and 4 weeks after injury (Fig. 1A–F). While the ASC group demonstrated improved alignment of collagen fibers at 2 and 4 weeks compared with the control group, both groups showed high cellularity at 9 days after injury (Fig. 1G and H). In toluidine blue–stained samples, the control group exhibited more ground substance deposition between collagen fibers than the ASC group at 2 weeks after injury (Fig. 1I and J). In Alizarin Red–stained samples, the control group exhibited a larger area of calcification than the ASC group at 4 weeks after injury (Fig. 1K and L). H&E-stained sections were semi-quantitatively analyzed according to 4 histological parameters of tendon quality. The median scale scores in the ASC and control groups were 3.6 and 6.67, respectively, at 2 weeks after injury, and 4.3 and 9.5 at 4 weeks after injury. The ASC group exhibited a significantly lower degree of tendon degeneration than the control group at both time points (Fig. 1M).
Fig. 1.
Representative images of longitudinal tendon sections and Bonar Scale scoring. (A–F) HE staining of the ASC and control group with low magnification. (G,H) HE staining of at 9 days. (I,J) Toluidin blue staining at 2 weeks after injury. (K,L) Alizarin red staining at 4 weeks after injury. (M) Bonar scale score. (A-F)Scale bar = 500 μm, (G–L) Scale bar = 50 μm, (M) *p < 0.05.
3.2. μCT analysis
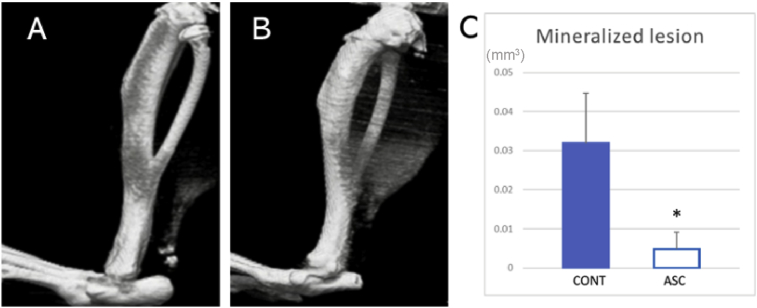
Next, we sought to evaluate the inhibition of ectopic ossification by ASC treatment. The tendons harvested at 4 weeks post-injury were used in μCT analysis. Ectopic mineralization was detected in the all tendons of the control group (Fig. 2A). In the ASC group, ectopic mineralization was undetectable in some tendons (Fig. 2B). Quantification analysis of the μCT images revealed that the volume of mineralized materials was significantly smaller in the ASC group than in the control group (Fig. 2C).
Fig. 2.
RμCT analysis of tendons 4 weeks post-injury. (A,B) Representative images of control group (A) and ASC group (B). (C)The volume of mineralized materials. (*p < 0.05).
3.3. Tracking of transplanted ASCs in the injury site
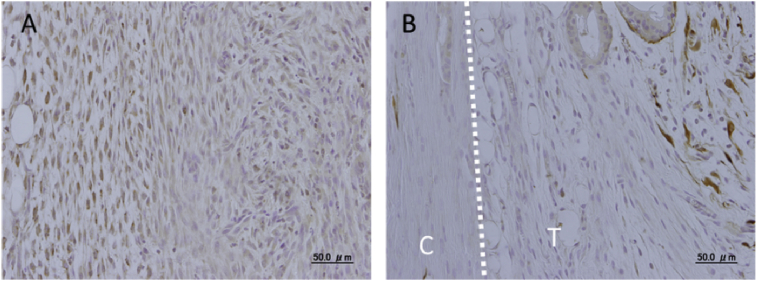
The transplanted ASCs could be detected by immunohistochemical staining of the GFP. We found GFP-positive cells in the tendon core close to the peritendon at 2 days after transplantation, and in the connective tissue around the tendon at 1 week (Fig. 3A and B). We could not detect GFP-positive cells at 3 weeks after transplantation (data not shown). These findings indicate that the GFP-ASCs migrate around peritendon in the early stage of injury and may play an important paracrine role in tendon repair before they disappear.
Fig. 3.
Tracking of transplanted ASCs in the injury site with immunohistochemical staining of GFP. (A) Two days after transplantation (9 days after transplantation). (B) One week after transplantation (2 weeks after transplantation). T: tendon, C: connective tissue, Scale bar = 50 μm.
3.4. Early inflammatory responses in tendon healing
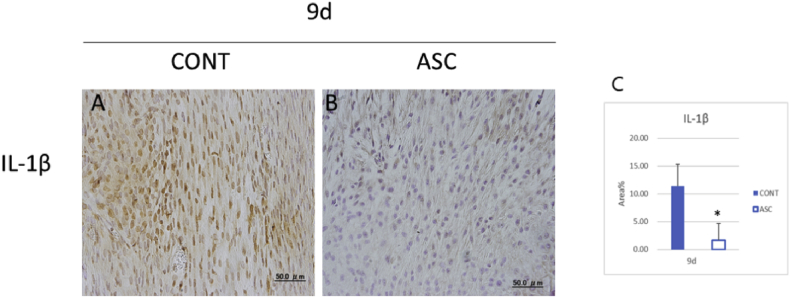
Since high cellularity was shown at 9 days after injury in both groups, we hypothesized that the inflammatory response would occur at the injury site. We immunostained IL-1β as a pro-inflammatory cytokine. The ASC group showed significantly decreased expression of IL-1β compared to the control group (Fig. 4A, B, C). This finding suggest that the transplantation of ASC reduced the expression of IL-1β in acute injury.
Fig. 4.
Early inflammatory response by immunohistochemical staining in tendon healing 9 days after injury. (A,B) IL-1β expression. (C) Total area of IL-1β expression. Scale bar = 50 μm, *p < 0.05.
3.5. Hypoxia and angiogenesis in tendon healing
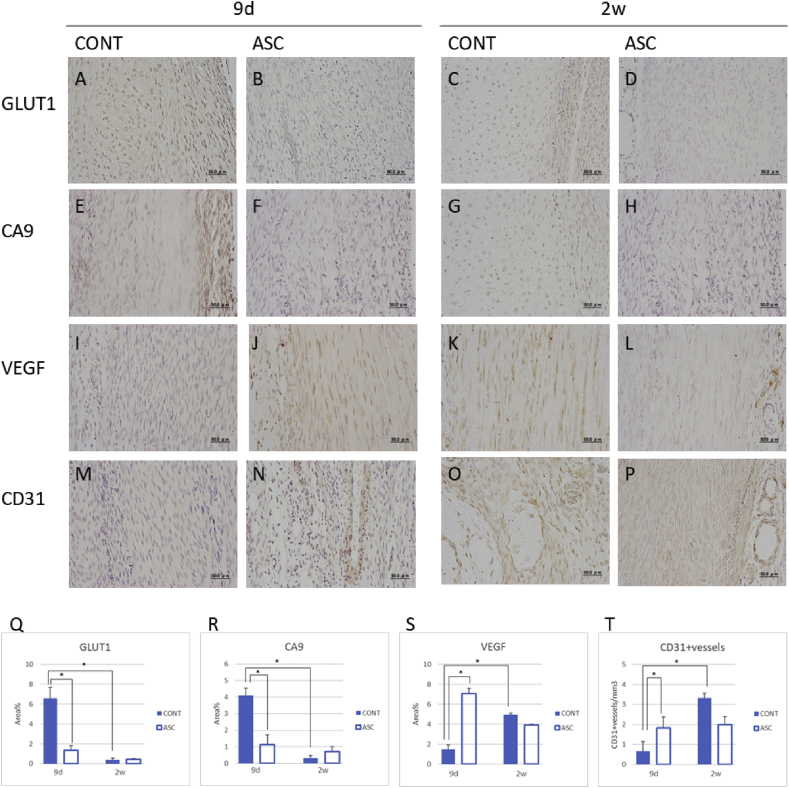
To test whether ASCs affect hypoxia and neovascularization during early healing in tendon injury, we immunostained GLUT1 and CA9 as hypoxia markers, and VEGF and endothelial CD31 to examine angiogenesis at 9 days and 2 weeks after injury. The ASC group exhibited significantly decreased expression of Glut1 and CA9 at 9 days after injury compared to the control group (Fig. 5A–H, Q, R). On the other hand, the ASC group showed significantly increased expression of VEGF and endothelial CD31 at 9 days after injury compared to the control group. The control group, but not the ASC group, demonstrated significantly increased expressions of VEGF and endothelial CD31 at 2 weeks after injury compared to 9 days (Fig. 5 I–P, S, T). These findings suggest that ASC may modulate hypoxia and induce angiogenesis in the early stage of tendon healing.
Fig. 5.
Hypoxia and angiogenesis by immunohistochemical staining and their changes in tendon repair. (A-D)The expression of GLUT1 at 9 days and 2 weeks after injury. (E–H) The expression of CA9 at 9 days and 2 weeks after injury. (I–L) The expression of VEGF at 9 days and 2 weeks after injury. (M–P) The expression of VEGF at 9 days and 2 weeks after injury. (Q–T) Total area of GLUT1, CA9, VEGF positive areas and CD31 + vessels at 9 days and 2 weeks after injury. Scale bar = 50 μm, *p < 0.05.
4. Discussion
We demonstrated that transplanted ASCs promoted tendon repair and decreased ectopic ossification in a collagenase-induced Achilles tendinopathy mouse model, by modulating IL-1β expression and hypoxic conditions and increasing neovascularization early in the tendon healing process. The novelty of this research is 1) we clearly showed ASCs are effective for inhibition of ectopic ossification in tendon injury and 2) we suggested that the neovasucularization by ASCs in the early stage of injury may improve tendon healing. Neovascularization in chronic tendinopathy has been characterized as one of the degenerative changes. On the other hand, we showed neovascularization at the acute stage in the ASCs group, which was followed by the lower degree of degeneration than in the control group at the late stage. We originally propose the benefit of ASCs inducing neovasculatization in the early stage of injury for the tendon healing.
Histological and μCT analyses demonstrated that the ASC group showed superior tendon tissue regeneration compared to the control group, and that tendons from control mice demonstrated degenerative changes such as chondrogenic ground substance and calcific depositions. At 9 days after injury, however, both ASC and control groups showed high cellularity, a phenomenon that is associated with inflammatory cell infiltration and an increased number of TSPCs in the early stage of the healing process [20,21]. While stem/progenitor cells are necessary for tissue repair, TSPCs might undergo aberrant differentiation to chondrocyte-like cells in the inflammatory environment of acute tendon injury. It has been shown that tendon progenitor cells in injured tendons have strong chondrogenic potential and may contribute to ectopic mineralization that can be caused by endochondral ossification [22]. Our data suggest that the transplantation of ASCs influences inflammatory cells and TPSCs in the early stage of tendon repair.
Tracking of transplanted ASCs revealed that these cells appeared in the tendon core close to the peritendon and in the connective tissue around the tendon at 2 days and 1 week after transplantation. Additionally, we identified no labeled ASCs at 3 weeks after transplantation, a finding that coincides with the results of previous studies [20,23]. Numerous studies have shown that in response to inflammatory stimuli, ASCs secrete cytokines and growth factors that promote the local regenerative process [24]. Our results support the concept that ASCs are capable of tendon repair in a paracrine manner.
In this study, investigation of the inflammatory response to acute tendon injury revealed that the expression of IL-1β was reduced in the ASC group compared to the control group. Although IL-1β is released by tendon cells in response to mechanical stretching, the addition of IL-1β to these cells results in the production of MMPs and decreased type I collagen production [25]. It was reported that the use of an IL-1 receptor antagonist limited the pathologic changes in an animal tendon injury model [26]. M1 macrophages, along with leukocytes and other cell types, secrete proinflammatory factors such as IL-1β and TNF-α, which result in poor healing. Tsuzaki et al. showed that exogenous IL-1β exposure increased endogenous IL-1β gene expression in tendon cells through a positive feedback loop [27]. ASCs can modulate the inflammatory response by regulating macrophage polarization toward the generative M2 phenotype and by suppressing the pro-inflammatory M1 phenotype [28,29]. To determine whether ASCs reduce the expression of IL-1β by modulating pro-inflammatory macrophage or tendon cells, further studies are needed.
Evaluation of the presence of hypoxia revealed that the expression levels of Glut1 and CA9 were reduced in the ASC group at 9 days after injury compared to the control group. Glut1 is usually expressed in erythrocytes and endothelial cells in the blood–brain barrier. Hypoxia upregulates the expression of GLUT1, and overexpression of Glut1 is associated with tumor progression [30]. Surface transmembrane CA9 is expressed at low levels in the healthy kidney and most other normal tissues, and CA9 are also induced by HIF-1α and overexpressed in many tumors [31]. Hypoxia is associated with chondrogenesis in mesenchymal stem cell culture, and studies have demonstrated that ectopic calcification in Achilles tendon rupture models is caused by ectopic chondrogenesis-mediated endochondral ossification [32,33]. It was reported that the expression of HIF-1, Sox-9, Runx2, transforming growth factor(TGF)-β, and bone morphogenetic proteins(BMP) protein were seen in ectopic ossification in Achilles tenotomy models [34]. The administration of ASCs may reverse or prevent hypoxia in the early phase of tendon healing, which would be attributable to suppression of the inflammation and increased neovascularization by ASCs in the early phase.
The initial vascular response is essential since it has been shown that a decreased blood supply impairs healing [35]. On the other hand, there is a continuing debate on whether neovascularization in tendon injury is degenerative or regenerative [36]. In this study, neovascularization was increased in the control group compared to the ASC group at 4 weeks after injury. However, the expression levels of VEGF and the number of CD31 positive vessels were significantly increased in the ASC group compared to the control group at 9 days after injury. We also found that although VEGF expression was similar in both groups at 2 weeks after injury, it increased from 9 days to 2 weeks after injury in the control group but significantly decreased in the ASC group. This is the first report of ASC administration leading to early neovascularization during tendon healing. Whereas the expression of VEGF, a hypoxic marker, is induced by HIF-1α, some studies have reported that VEGF protein is expressed following the cellular expression of GlutT1 and CA9 [37,38]. In addition to hypoxia, Fibroblast growth factor (FGF) is known to be a potent stimulator of VEGF [39]. Previous studies have demonstrated that under inflammatory conditions, ASCs secrete VEGF, HGF, TGF-beta, and FGF2, which explains their potent angiogenic properties [25,40]. Our data suggest that the early angiogenesis promoted by ASCs induces VEGF expression by TPSCs, and is useful for tendon healing. Takebe et al. reported that transient vascularization in the early stage of chondrogenesis plays an essential role in the regeneration of adult cartilage, which is an avascular tissue like tendon [41]. We speculate that ASCs induce angiogenesis that improves tendon repair, and the timing of angiogenesis may affect degenerative changes after tendon injury.
The main limitation of this study is that tendon healing was evaluated at relatively few time points, although these did demonstrate chronological repair. Since the local environment changes during disease progression or during the healing process after acute injury, the time point of cell delivery (i.e., in the acute, subacute, or chronic stage) will impact therapeutic efficacy [42,43]. Further investigations to evaluate the expression of cytokines and growth factors and to track ASCs in vivo using time-lapse or live imaging will shed light on the pathogenesis of tendon injury and the mechanisms of tendon healing, which will contribute to the development of cell therapy for tendon repair. Another limitation of this study is our research design using this animal model. Although collagenase-induced tendinopathy model mimics the natural injury to some extent, it is still different from natural course of the disease. In addition, tendinopathy in mice should be different from the human tendon injury. Cares should be taken to interpret the results obtained in this study to human diseases.
5. Conclusions
In conclusion, ASCs improve tendon repair and prevent ectopic ossification by inhibiting inflammation in acute tendon injury and inducing neovascularization in the early phase of tendon healing. These findings may be useful for choosing therapeutic time points and establishing effective cell therapies for tendinopathy.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Hikita A holds an endowed chair supported by FUJISOFT INCORPORATED.
Acknowledgments
We thank Tomoaki Sakamoto for his technical support. This work was partly supported by JSPS KAKENHI Grants-in-Aid for Scientific Research (C)(#16K11677) and (#19K22700).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Ganestam A., Kallemose T., Troelsen A., Barfod K.W. Increasing incidence of acute Achilles tendon rupture and a noticeable decline in surgical treatment from 1994 to 2013. A nationwide registry study of 33,160 patients. Knee Surg Sport Traumatol Arthrosc. 2016;24:3730–3737. doi: 10.1007/s00167-015-3544-5. [DOI] [PubMed] [Google Scholar]
- 2.Maffulli N., Via A.G., Oliva F. Springer; Cham: 2016. Achilles tendon rupture. Arthroscopy and sport injuries. [Google Scholar]
- 3.Nourissat G., Berenbaum F., Duprez D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol. 2015;11:223–233. doi: 10.1038/nrrheum.2015.26. [DOI] [PubMed] [Google Scholar]
- 4.Lui P.P.Y., Chan L.S., Fu S.C., Chan K.M. Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am J Sports Med. 2010;38:757–764. doi: 10.1177/0363546509355402. [DOI] [PubMed] [Google Scholar]
- 5.Millar N.L., Murrell G.A.C., Mcinnes I.B. Cytokines in tendon disease: a systematic review. Bone Jt Res. 2017;6:656–664. doi: 10.1302/2046-3758.612.BJR-2017-0112.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang K., Asai S., Yu B. IL-1beta irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem Biophys Res Commun. 2015 Aug 7;463(4):667–672. doi: 10.1016/j.bbrc.2015.05.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang M., Cornell H.R., Zargar Baboldashti N., Thompson M.S., Carr A.J., Hulley P.A. Regulation of hypoxia-induced cell death in human tenocytes. Adv Orthop. 2012;2012:1–12. doi: 10.1155/2012/984950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang K., Hast M.W., Izumi S., Usami Y., Shetye S., Akabudike N. Modulating glucose metabolism and lactate synthesis in injured mouse tendons: treatment with dichloroacetate, a lactate synthesis inhibitor, improves tendon healing. Am J Sports Med. 2018;46:2222–2231. doi: 10.1177/0363546518778789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahin H., Tholema N., Petersen W., Raschke M.J., Stange R. Impaired biomechanical properties correlate with neoangiogenesis as well as VEGF and MMP-3 expression during rat patellar tendon healing. J Orthop Res. 2012;30:1952–1957. doi: 10.1002/jor.22147. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P., Maffulli N. Tendinopathy and tendon injury: the future. Disabil Rehabil. 2008;30:1733–1745. doi: 10.1080/09638280701788274. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto N., Kushida T., Oe K., Umeda M., Ikehara S., Iida H. Treating Achilles tendon rupture in rats with bone-marrow-cell transplantation therapy. J Bone Joint Surg Am. 2010;92:2776–2784. doi: 10.2106/JBJS.I.01325. [DOI] [PubMed] [Google Scholar]
- 12.Uysal C., Tobita M., Hyakusoku H. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg. 2012;65(12):1712–1719. doi: 10.1016/j.bjps.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Mizuno H., Tobita M., Uysal A.C. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- 14.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 15.Oshita T., Tobita M., Tajima S., Mizuno H. Adipose-derived stem cells improve collagenase-induced tendinopathy in a rat model. Am J Sports Med. 2016;44:1983–1989. doi: 10.1177/0363546516640750. [DOI] [PubMed] [Google Scholar]
- 16.Lee S.Y., Kwon B., Lee K., Son Y.H., Chung S.G. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 2017;45:1429–1439. doi: 10.1177/0363546517689874. [DOI] [PubMed] [Google Scholar]
- 17.Uysal A.C., Mizuno H. Differentiation of adipose-derived stem cells for tendon repair. In: Gimble J., Bunnell B., editors. vol. 702. Humana Press; Totowa, NJ: 2011. (Adipose-derived stem cells. Methods in molecular biology (methods and protocols)). 15 November 2010. [DOI] [PubMed] [Google Scholar]
- 18.Shen H., Kormpakis I., Havlioglu N., Linderman S.W., Sakiyama-Elbert S.E., Erickson I.E. The effect of mesenchymal stromal cell sheets on the inflammatory stage of flexor tendon healing. Stem Cell Res Ther. 2016;7:1–13. doi: 10.1186/s13287-016-0406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook J.L., Feller J.A., Bonar S.F., Khan K.M. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes' patellar tendons. J Orthop Res. 2004;22:334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Tan Q., Lui P.P.Y., Lee Y.W. In vivo identity of tendon stem cells and the roles of stem cells in tendon healing. Stem Cells Dev. 2013;22:3128–3140. doi: 10.1089/scd.2013.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lui P.P.Y., Kong S.K., Lau P.M., Wong Y.M., Lee Y.W., Tan C. Allogeneic tendon-derived stem cells promote tendon healing and suppress immunoreactions in hosts: in vivo model. Tissue Eng A. 2014;20:2998–3009. doi: 10.1089/ten.TEA.2013.0713. [DOI] [PubMed] [Google Scholar]
- 22.Asai S., Otsuru S., Candela M.E. Tendon progenitor cells in injured tendons have strong chondrogenic potential: the CD105-negative subpopulation induces chondrogenic degeneration. Stem Cells. 2014;32:3266–3277. doi: 10.1002/stem.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinnaird T., Stabile E., Burnett M.S., Shou M., Lee C.W., Barr S. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 24.Salgado A.J., Reis R.L., Sousa N.J., Gimble J.M. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 25.Millar N.L., Murrell G.A.C., Mcinnes I.B. Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol. 2017;13:110–122. doi: 10.1038/nrrheum.2016.213. [DOI] [PubMed] [Google Scholar]
- 26.Berkoff D.J., Kallianos S.A., Eskildsen S.M., Weinhold P.S. Use of an IL1-receptor antagonist to prevent the progression of tendinopathy in a rat model. J Orthop Res. 2016;34:616–622. doi: 10.1002/jor.23057. [DOI] [PubMed] [Google Scholar]
- 27.Tsuzaki M., Guyton G., Garrett W., Archambault J.M., Herzog W., Almekinders L. IL-1β induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1β and IL-6 in human tendon cells. J Orthop Res. 2003;21:256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 28.Manning C.N., Martel C., Sakiyama-Elbert S.E., Silva M.J., Shah S., Gelberman R.H. Adipose-derived mesenchymal stromal cells modulate tendon fibroblast responses to macrophage-induced inflammation in vitro. Stem Cell Res Ther. 2015;6 doi: 10.1186/s13287-015-0059-4. 1DUMMY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelberman R.H., Linderman S.W., Jayaram R., Dikina A.D., Sakiyama-Elbert S., Alsberg E. Combined administration of ASCs and BMP-12 promotes an M2 macrophage phenotype and enhances tendon healing. Clin Orthop Relat Res. 2017;475:2318–2331. doi: 10.1007/s11999-017-5369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szablewski L. Expression of glucose transporters in cancers. Biochim Biophys Acta Rev Canc. 2013;1835:164–169. doi: 10.1016/j.bbcan.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Ivanov S., Liao S.Y., Ivanova A., Danilkovitch-Miagkova A., Tarasova N., Weirich G. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leijten J., Georgi N., Teixeira L.M., Van Blitterswijk C.A., Post J.N., Karperien M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc Natl Acad Sci U S A. 2014;111:13954–13959. doi: 10.1073/pnas.1410977111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schipani E. Posttranslational modifications of collagens as targets of hypoxia and Hif-1α in endochondral bone development. Ann N Y Acad Sci. 2010;1192:317–321. doi: 10.1111/j.1749-6632.2009.05236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin L., Shen Q., Xue T., Yu C. Heterotopic ossification induced by Achilles tenotomy via endochondral bone formation: expression of bone and cartilage related genes. Bone. 2010;46:425–431. doi: 10.1016/j.bone.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 35.Fenwick S.A., Hazleman B.L., Riley G.P. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002;4:252–260. doi: 10.1186/ar416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H.-Y., Achilles Tendinopathy Hua Y.-H. Current concepts about the basic science and clinical treatments. BioMed Res Int. 2016;2016 doi: 10.1155/2016/6492597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Kathleen H., Andrew M., Shenglin C., Minghui X., David D. Nephron-deficient Fvb mice develop rapidly progressive renal failure and heavy albuminuria involving excess glomerular GLUT1 and VEGF. Lab Investig. 2010 January;90(1):83–97. doi: 10.1038/labinvest.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bencini L., Lastraioli E., Bianchini E., Romoli M.R., Crociani O., Giommoni E. hERG1 channels and glut-1 as independent prognostic indicators of worse outcome in stage I and II colorectal cancer : a pilot study. Transl Oncol. 2012;5:105–112. doi: 10.1593/tlo.11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masaki I., Yonemitsu Y., Yamashita A., Sata S., Tanii M., Komori K. Angiogenic gene therapy for experimental critical acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Cir Res. 2002:966–973. doi: 10.1161/01.res.0000019540.41697.60. [DOI] [PubMed] [Google Scholar]
- 40.Bajek A., Gurtowska N., Olkowska J., Kazmierski L., Maj M., Drewa T. Adipose-derived stem cells as a tool in cell-based therapies. Arch Immunol Ther Exp (Warsz) 2016;64:443–454. doi: 10.1007/s00005-016-0394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takebe T., Kobayashi S., Suzuki H., Mizuno M., Chang Y.M., Yoshizawa E. Transient vascularization of transplanted human adult–derived progenitors promotes self-organizing cartilage. J Clin Investig. 2014 Oct;124(10):4325–4334. doi: 10.1172/JCI76443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kavanagh D.P.J., Robinson J., Kalia N. Mesenchymal stem cell priming: fine-tuning adhesion and function. Stem Cell Rev Rep. 2014 Aug;10(4):587–599. doi: 10.1007/s12015-014-9510-7. [DOI] [PubMed] [Google Scholar]
- 43.Najar M., Krayem M., Merimi M., Burny A., Meuleman N., Bron D. Insights into inflammatory priming of mesenchymal stromal cells: functional biological impacts. Inflamm Res. 2018;67:467. doi: 10.1007/s00011-018-1131-1. [DOI] [PubMed] [Google Scholar]