Abstract
Emotion regulation impacts the expected emotional responses to the outcomes of risky decisions via activation of cognitive control strategies. However, whether the regulation of emotional responses to preceding, incidental stimuli also impacts risk-taking in subsequent decisions is still poorly understood. In this study, we investigated the interplay between the regulation of incidentally induced emotional responses and subsequent choice behavior using a risky decision-making task in two independent samples (behavioral and functional magnetic resonance imaging experiment). We found that overall, emotion regulation was followed by less risky decisions, which was further reflected in an increase in activation in brain regions in dorsolateral and ventrolateral prefrontal cortex and cingulate cortex. These findings suggest that altering incidental emotions using reappraisal strategies impacts on subsequent risk-taking in decision-making.
Keywords: fMRI, neuroimaging, emotion regulation, reappraisal, valuation, cognitive control, decision-making, risk-taking
Introduction
Trading off risk and reward, for example when working as a stockbroker or making financial decisions as a company manager, has long been thought to be driven by a purely cognitive and logical assessment of risk (Westbrook and Braver, 2015). However, contrary to financial economic accounts of traders as rational utility maximizers, evidence is building that internal factors such as emotions (Lerner et al., 2004; Phelps et al., 2014) and the ability to regulate emotions (Lerner et al., 2015; Heilman and Houser, 2016) have important effects on their financial decisions.
Thus, effective emotion regulation might be a relevant facet of trader expertise as it diminishes susceptibility to cognitive and psychological biases (Sokol-Hessner et al., 2009; Heilman et al., 2010; Fenton-O’Creevy et al., 2012). Most studies investigating the interplay of emotion regulation and decision-making focused on the regulation of emotions that participants attach to possible outcomes that they anticipate as a consequence of making decisions (Delgado et al., 2008; Grecucci et al., 2013; Gu et al., 2013; Hutcherson et al., 2012; Martin and Delgado, 2011; Sokol-Hessner et al., 2013; Sokol-Hessner et al., 2009; Staudinger et al., 2011; Staudinger et al., 2009; van’t Wout et al., 2010; van’t Wout et al., 2013; Yang et al., 2013). For instance, before making a risky decision, decision-makers can actively attempt to change the way they will perceive potential choice outcomes to minimize the emotional impact on decision-making. In this case, such emotions directly related to choice outcomes could either occur during decision-making (current emotions) or after decision-making (expected emotions) (Loewenstein et al., 2001; Knutson and Greer, 2008; Schlösser et al., 2013; Lerner et al., 2015).
Only few studies have investigated the effect of regulation of experimentally induced emotion on risky financial decisions. One prominent way to minimize the effects of expected emotions on decision-making is reappraisal, the reframing of the meaning of an emotional stimulus to alter its emotional impact (Gross and Thompson, 2007). Engaging in reappraisal of the decision-situation has been shown to effectively reduce loss aversion (Sokol-Hessner et al., 2009) and increase risk-taking (Braunstein et al., 2014). Other studies revealed that greater habitual use of cognitive reappraisal was associated with a performance advantage in financial decision-making (Fenton-O’Creevy et al., 2011) and with increased risk-taking, accompanied by decreased sensitivity to changes in risk-related probabilities and task configurations (Panno et al., 2013). In contrast, the implementation of imagery-based emotion regulation (i.e. imagining relaxing scenes) before financial decision-making was associated with reduced risk-taking (Martin and Delgado, 2011).
However, there is also a rich literature showing the influence of incidental emotional stimuli on decision-making in general (Lerner and Keltner, 2001; Loewenstein and Lerner, 2003; Lerner et al., 2004; Winkielman et al., 2005; Han et al., 2007; Pham, 2007; Seo and Barrett, 2007; Schulreich et al., 2014). Similarly, several studies highlighted the carry-over effects of emotionally loaded stimuli, such as erotic images, brand logos and other highly valenced stimuli, on subsequent financial decisions (e.g. Murawski et al., 2012; Wilson and Daly, 2004). Surprisingly, while these stimuli are designed to elicit strong emotional responses, the effect of emotion regulation for such incidental stimuli on subsequent decisions has not received much attention (Augustine and Larsen, 2011; Kahneman and Frederick, 2007; Miu and Crişan, 2011). A few studies, however, have suggested that reappraisal of incidental emotions could be associated with increased risk-taking in a subsequent decision-making task (Heilman et al., 2010; Szasz et al., 2016).
The cognitive control network typically underlying emotion regulation involves lateral prefrontal cortex (dorsolateral and ventrolateral prefrontal cortex, dlPFC and vlPFC), parietal and temporal regions as well as somatosensory cortex (Kohn et al., 2014; Morawetz et al., 2017). Increased responses within this prefrontal network are usually found to be related to decreased responses in the amygdala and striatum (Phillips et al., 2008; Ochsner et al., 2012). Neural networks underlying financial decision-making and preference formation are similarly spread across multiple brain regions, including dlPFC, vlPFC, medial PFC, orbitofrontal cortex, the ventral striatum, anterior cingulate cortex and posterior parietal regions (Bartra et al., 2013; Clithero and Rangel, 2014; Clithero et al., 2009; Kable and Glimcher, 2007; Kuhnen and Knutson, 2005; Levy and Glimcher, 2012; Voigt et al., 2018). The regulation of emotions during monetary incentive tasks has been linked to activity in prefrontal regions, such as the dlPFC and the vmPFC (Sokol-Hessner et al., 2013; Staudinger et al., 2011) and the striatum and the amygdala (Martin and Delgado, 2011; Sokol-Hessner et al., 2013; Staudinger et al., 2011). This suggests that the prefrontal regions might be involved in implementing the reappraisal-related changes in the decision-making circuitry by modulating activity in subcortical areas. Based on these findings, it has been suggested that when emotion regulation is used to change current and expected emotions related to a decision, this engages the same neural circuitry that is regularly found in typical emotion regulation studies where individuals down-regulate their emotions in response to aversive stimuli (Phelps et al., 2014). Some of these regions, however, are also part of the decision-making network, leaving it an open question as to how much of this network is shared between functions.
The primary goal of this study was to investigate the effect of regulation of emotional responses to preceding, incidental stimuli and subsequent choice behavior. This research question, which markedly differs from investigating the regulation of decision-related emotional responses to anticipated decision outcomes, allowed for exploring the impact of emotional spill-over effects on decision-making. In everyday life, we are very likely to constantly encounter emotional stimuli that are not directly related to the next decision. This means, understanding how dealing with such emotions can impact the next, apparently unrelated decision process is of utmost importance.
We used a typical emotion regulation task (e.g. Morawetz et al., 2016a and 2016b), which was followed by a financial risky decision-making task (Mohr et al., 2010; Majer et al., 2016). Participants’ neural responses were measured using functional magnetic resonance imaging (fMRI) as well as participants’ skin conductance to quantify physiological arousal responses to relate those to emotional reactivity and regulation.
Given that the engagement of highly similar neural circuitry is found in typical emotion regulation tasks and tasks involving reappraisal to change current/expected emotions and choices, we hypothesized that carry-over effects of incidental emotions and their regulation during decision-making would be represented in systematic activity changes in shared regions.
Materials and methods
Here we report two studies: a behavioral experiment to investigate the effect of emotion regulation on decision-making, followed by an fMRI experiment, which served to investigate the neural correlates of this process. In two independent samples, the two experiments used identical experimental paradigms, which are therefore described together in this section.
Participants
Behavioral experiment: We tested 22 right-handed, healthy participants with normal or corrected to normal vision (18 female, mean age = 22.77 years, SD = 5.28).
fMRI experiment: We tested 33 right-handed, healthy participants with normal or corrected to normal vision. Two participants had to be excluded due to technical problems with data acquisition and two other participants were not able to finish the experiment. The final sample consisted of 29 participants (13 females, mean age = 24.52 years, SD = 4.25).
Participants in both experiments gave written, informed consent to participate. The studies were approved by the local ethics committee of the Department of Education and Psychology at Freie Universität Berlin.
Stimuli
Stimuli for emotion regulation
Stimuli consisted of 96 aversive [normative International Affective Picture System (IAPS) ratings on a Likert scale from 1 (very negative/very calm) to 9 (very positive/very arousing): mean valence = 2.36, mean arousal = 6.30] and 48 neutral (normative IAPS ratings: mean valence = 5.26, mean arousal = 3.37) pictures from the IAPS (Bradley and Lang, 2007). During the experiment, images were presented in the center of the screen with an 800 × 600 pixel display subtending 32° × 24° visual angle on dual display goggles (VisuaStim, MR Research, USA) using the stimulation software Presentation (Version 14.1, Neurobehavioral Systems, USA). Pictures subtended a 24° × 18° visual angle, presented against a black background.
Experimental design and procedure
The first part of each experimental trial was a classical emotion regulation task, which has been adapted from previous studies on emotion regulation (Morawetz et al., 2016a, 2016b, 2017). This was followed by a risky decision-making task. During the instruction phase of the experiment, we explained the emotion regulation task and the risky decision-making task in great detail to each participant in written format as well as verbally. Participants performed a short training session before the actual experiment, and they could ask questions if they were uncertain about any aspects of the task.
Emotion regulation task
We used a well-established emotion regulation task, in which participants regulated their emotions in response to viewing one of the pictures in each trial. Three task conditions were implemented (Figure 1): in the Look-Negative condition, participants were presented with aversive pictures and were asked to view the stimuli attentively and allow themselves to experience/feel any emotional responses, which these might elicit without manipulating them. The Look-Neutral condition was identical; however, the stimuli presented did not elicit negative emotions. In contrast, in the Decrease condition, participants viewed negatively valenced images and were asked to actively reduce the intensity of negative emotions by distancing themselves from the image by becoming a detached observer, e.g. through thinking that the depicted situation is not real, by reducing the personal relevance of the image or by telling themselves that the depicted situation is ‘only a picture’ (Ochsner et al., 2004; Eippert et al., 2007; Urry et al., 2009). Importantly, participants were told not to substitute negative emotions with positive emotions.
Fig. 1.
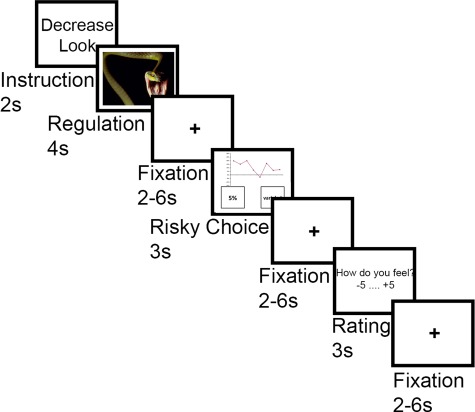
Task design. The first part of each trial was an emotion regulation task, which started with an instruction for 2 s, instructing the participants to either down-regulate (Decrease) their emotional responses or to simply experience them (Look). This was followed by a negative (Decrease and Look) or neutral (Look only) picture presented for 4 s during which the instructed strategy was applied. After a fixation period (2–6 s), the risky decision-making task followed. Participants were presented with a possible investment in a portfolio. The past performance of this portfolio was shown. Participants were asked to choose between the presented portfolio with varying risk (risky option) or a portfolio with a fixed 5% return (safe option, not shown) within a 3 s period. After the decision phase, a fixation period followed (2–6 s). Finally, participants rated their current emotional state (very negative to very positive) within 3 s. The trial concluded with a fixation period (2–6 s).
Risky decision-making task
The emotion regulation task was followed by an adjusted version of the Risk Perception in Investment Decisions task (Mohr et al., 2010; Majer et al., 2016). In this task, participants were presented with the course of returns of an investment, i.e. the past performance of a possible investment. The standard deviation of the return streams and the expected returns varied parametrically with four standard deviations (σ = 2%, 4%, 6% and 8%) and four expected returns (μ = 5%, 7%, 9% and 11%), resulting in 16 different combinations of standard deviations and expected returns (see Supplementary Material, Figure S1, for probability distributions). On each trial, participants made a choice between an investment with 5% fixed return (safe investment) and the investment represented by the return stream shown on the screen (risky investment). Participants received a flat payment of 15 euros for their participation in the experiment and a virtual endowment of 100 euros to invest. They were explicitly told that the returns they observe during the experiment were randomly drawn from Gaussian distributions. They were further instructed that after the experiment, one of their 144 choices would be randomly chosen to determine decision-dependent payments. For example, if the participant would choose the safe option in the respective trial, she would receive 5 euros (5% of 100 euros) in addition to the 15 euros flat payment. If a participant would choose the risky option in this trial, a random return was drawn from a Gaussian distribution with the same mean and standard deviation as the respective return stream. The resulting outcome (return times 100 euros) was added to or subtracted from the flat payment. Thus, participants could either win or lose when choosing the risky option. The optimal strategy was therefore to treat each decision as a real decision as each had the same probability to be paid out in the end. Participants could gain a maximum of 21 euros (in addition to the reimbursement for participation) or lose up to 4 euros.
Task procedures
In the experiment, both tasks were combined in each trial, i.e. the emotion regulation task was presented first, followed by the risky decision-making task. Each trial started with an instruction cue (2 s) indicating the experimental condition by displaying Decrease or Look (note that Look included both the negative and neutral image condition). Subsequently, an image was presented for 4 s during which the instructed strategy had to be applied. This was followed by a fixation cross for a jittered duration of 2–6 s. After this, participants were presented with the past returns of a new possible investment (risky choice option) on the top of the screen. Underneath, two boxes were displayed on the left or the right side of the fixation cross, indicating the two choice options (safe investment vs risky investment). Participants were asked to choose between the safe and risky option by pressing a button on a two-button fiber optic response pad (fORP, Cambridge Research Systems Ltd). The response window was 3 s, and the location of the choice options on the screen was pseudo-randomized between trials to avoid order effects. This task phase was again followed by a jittered fixation period for 2–6 s. Next, subjects were asked to rate their current emotional state on a scale from −5 to +5 (extremely negative to extremely positive) by pressing a button on the two-button fORP, providing a measure of trial-by-trial emotion regulation success. The extremes of the emotional state ratings were not labeled; only the scale from −5 to +5 was presented. The response window for the rating was again 3 s. Finally, a fixation cross was presented in the middle of the screen for a jittered duration of 2–6 s concluded the trial.
Participants performed three runs of the experiment. Each run consisted of 48 trials, and each of the 16 resulting return streams was used in each emotion regulation condition in each run. One trial lasted 24 s on average, one run lasted about 19 min and thus one scanning session consisted of 144 trials, which resulted in ~1 h of scanning.
fMRI data acquisition
Whole brain functional and anatomical images were acquired using a 3.0 T Magnetom TrioTim MRI scanner (Siemens, Erlangen, Germany) and a 12-channel head coil. A high-resolution 3D T1-weighted dataset was acquired for each subject (176 sagittal sections, 1 × 1 × 1 mm3; 256 × 256 data acquisition matrix). Functional images were acquired using a T2*-weighted, gradient-echo echo planar imaging (EPI) pulse sequence recording 37 sections oriented parallel to the anterior and posterior commissure at an in-plane resolution of 3 × 3 × 3 mm3 (interslice gap = 0; TE = 30 ms; TR = 2 s; FA = 90°; FoV = 192 × 192 mm2; 64 × 64 data acquisition matrix). For each experimental run 573 whole brain volumes were recorded.
Data analyses
Behavioral data
Behavioral task performance
We calculated reappraisal success scores based on the affect ratings acquired after each trial. Overall reappraisal success was defined as the mean decrease in reported emotion when applying cognitive reappraisal (Decrease) relative to the mean affect ratings of the control condition (Look-Negative), the latter representing the ‘natural’ emotional response to the stimuli (Morawetz et al., 2016a, 2016b; Wager et al., 2008). Reappraisal success scores for Decrease (Decrease minus Look-Negative) for each participant were calculated and used to analyze how these related to decision-making, i.e. whether emotion regulation per se affected choice behavior or whether the effect of emotion regulation on choice behavior depended on regulation success.
As a sanity check, we also analyzed electrodermal activity in each condition. Details can be found in the Supplementary Materials (Figure S2).
fMRI data
Preprocessing
Functional imaging data analysis was performed using SPM12 (Wellcome Institute for Cognitive Neurology, London, UK). As interleaved slice acquisition was used, slice time correction was included during the preprocessing of the fMRI data (Sladky et al., 2011). In addition, standard preprocessing involved realignment to the mean image, spatial normalization to the standard EPI template (MNI template, as implemented in SPM8) and spatial smoothing with an 8 mm full-width at half-maximum isotopic Gaussian kernel.
General linear models
We used several general linear models (GLMS) to analyze the data
GLM1. A first GLM was estimated to investigate risky decision-making and identify the emotion regulation network. This model included the following regressors: instruction cue (duration 2 s), emotion regulation conditions (Decrease, Look-Negative, Look-Neutral) (duration 4 s), type of choice (Risky Choices, Safe Choices) (duration 3 s), rating phase (duration 3 s). This model included motion parameters as nuisance covariates. The regressors were convolved with a canonical form of the hemodynamic response. Contrast images of brain activations associated with decision-making (Risky Choices > Safe Choices and Safe Choices > Risky Choices) were calculated for each participant and used in a second-level analysis. Contrast images of brain activations associated with emotion regulation (Decrease > Look-Negative; Decrease > Look-Neutral; Look-Negative > Decrease; Look-Neutral > Decrease) and emotion reactivity (Look-Negative > Look-Neutral; Look-Neutral > Look-Negative) were produced for each participant. T-statistics for each voxel were thresholded at P < 0.05 corrected for multiple comparisons across whole brain with family wise error (FWE) rate.
GLM2. This model was designed to identify regions whose activity increased during risky decision-making as a function of the emotion regulation condition. For this, the regressors for the choice phase were split into more specific regressors compared to the previous GLMs, specifying the choice outcomes in relation to the preceding emotion regulation condition. It included the following regressors: instruction cue (duration 2 s), emotion regulation conditions (Decrease, Look-Negative, Look-Neutral) (duration 4 s), type of choice (Risky ChoicesDecrease, Risky ChoicesLook-Negative, Risky ChoicesLook-Neutral, Safe ChoicesDecrease, Safe ChoicesLook-Negative, Risky ChoicesLook-Neutral) (3 s) and rating (duration 3 s). This model again included motion parameters as nuisance covariates. The regressors were convolved with a canonical form of the hemodynamic response. Contrast images of brain activations associated with risky decision-making following experiencing negative emotions (Risky ChoicesLook-Negative > Risky ChoicesLook-Neutral; Risky ChoicesLook-Neutral > Risky ChoicesLook-Negative) and emotion regulation (Risky ChoicesDecrease > Risky ChoicesLook-Negative; Risky ChoicesDecrease > Risky ChoicesLook-Neutral; Risky ChoicesLook-Negative> Risky ChoicesDecrease; Risky ChoicesLook-Neutral > Risky ChoicesDecrease) were calculated and used in a second-level analysis. T-statistics for each voxel were thresholded at P < 0.05 corrected for multiple comparisons across whole brain with FWE rate.
Results
Behavioral study
Emotion regulation task
A significant main effect of task was found (F(1,21) = 157.87, P < 0.001). Post-hoc t-tests revealed significantly more negative emotional state ratings for Look-Negative compared to Look-Neutral (t(21) = −14.37, P < 0.001, Cohen’s d = 4.61) and for Look-Negative compared to Decrease (t(21) = −10.61, P < 0.001, Cohen’s d = 1.24). Decrease also resulted in more negative emotional state ratings than Look-Neutral (t(21) = −10.71, P < 0.001, Cohen’s d = 3.22).
Risky decision-making task
A significant main effect of task was observed (F(1,21) = 4.21, P < 0.05). Post-hoc t-tests showed that participants chose the safe option more often after emotion regulation (Decrease) as compared to Look-Negative (t(21) = −2.11, P < 0.05, Cohen’s d = 0.24) and as compared to Look-Neutral (t(21) = −3.45, P < 0.01, Cohen’s d = 0.56). Reaction times during the risky decision-making task did not differ after emotion induction and regulation (all P > 0.1, data not shown).
fMRI study
Emotion induction
After the fMRI experiment, participants rated all the images on valence and arousal using a nine-point Likert scale from 1 (very negative/very calm) to 9 (very positive/very arousing). The images a priori selected to be perceived as ‘negative’ were indeed rated as more negative (t(25) = 15.10, P < 0.001, Cohen’s d = 4.48) and more arousing (t(25) = 11.36, P < 0.001, Cohen’s d = 3.05) than the ‘neutral’ images, confirming the normative ratings (Bradley and Lang, 2007). The available skin conductance data provided support for the success of the emotion induction (Figure S2).
Emotion regulation task
A significant main effect of task was found (F(1,28) = 103.53, P < 0.001) (Figure 2A). Post-hoc t-tests revealed significantly more negative emotional state ratings for Look-Negative as compared to Look-Neutral (t(28) = −11.00, P < 0.001, Cohen’s d = 3.48) and for Look-Negative as compared to Decrease (t(28) = −7.13, P < 0.001, Cohen’s d = 1.12). Decrease also resulted in more negative emotional state ratings than Look-Neutral (t(28) = −9.75, P < 0.001, Cohen’s d = 2.76). These results again confirm that emotion regulation was successful.
Fig. 2.

Physiological and behavioral results. (A) Emotional state ratings after each trial (fMRI study). The subjective ratings of emotional states indicated fewer negative emotions after the down-regulation of emotions compared to Look-Negative. During the Look-Neutral condition, participants rated their emotional state to be significantly less negative compared to the Decrease and Look-Negative condition. (B) Percent risky choices as a function of preceding emotion regulation (fMRI study). After emotion regulation, participants showed less risk-taking behavior, i.e. they chose the safe option more often compared to the Look-Negative condition. * indicates P < 0.05; ** indicates P < 0.01; *** indicates P < 0.001.
Risky decision-making task
First, we confirmed that participants again preferred the safe over the risky option after Decrease compared to Look-Negative (t(28) = −2.34, P < 0.05, Cohen’s d = 0.16). The differences between Decrease and Look-Neutral (t(28) = −1.48, P = 0.15, Cohen’s d = 0.01) as well as Look-Negative and Look-Neutral (t(28) = 0.16, P = 0.88, Cohen’s d = 0.14) were non-significant (Figure 2B). Reaction times during the risky decision-making task did not differ after Look-Negative and Decrease (all P > 0.1; data not shown).
Second, we performed two control analyses to investigate (i) the effect of emotion regulation success on subsequent choices and (ii) the effect of arousal on choices during the decision phase. The results can be found in the Supplementary Materials.
fMRI results
In a first step (based on GLM1), we performed two control analyses that mainly served as a general sanity check. First, we tested for neuronal correlates of emotion regulation. For this, we performed a conjunction analysis [(Decrease > Look-Negative) & (Decrease > Look-Neutral)]. The results revealed increased activity in the left IFG and the supplementary motor area (SMA) (Table 1). Contrasting the Decrease condition with Look-Neutral revealed increased activity in a widespread network of regions including frontal (IFG), temporal and parietal regions (Table 1). The observed network aligns with our previous findings on the general emotion regulation network (Morawetz et al., 2017). Second, we investigated which regions were implicated in risk-related decision-making in general by contrasting risky vs safe decisions (Risky Choices > Safe Choices), independent of the emotion regulation conditions, and found an increase in activity in the vmPFC, the right dlPFC as well as the thalamus (Table 2). These findings are in line with a previous meta-analysis on value-based decision-making (Bartra et al., 2013).
Table 1.
Emotion regulation task
| Contrast | Region | L/R | Size | t-value | P (FWE-corr.) | Coordinates | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Conjunction: | SMA | L | 58 | 2.74 | 0.03 | −3 | 8 | 62 |
| (Decrease > look-Negative) & (Decrease > Look-Neutral) | Inferior frontal gyrus | L | 73 | 2.63 | 0.01 | −54 | 26 | 5 |
| Decrease > Look-Negative | Caudate | R | 112 | 4.89 | 0.02 | 9 | 23 | 2 |
| Cerebellum | R | 97 | 4.88 | 0.04 | 15 | −88 | −19 | |
| Decrease > Look-Neutral | Inferior occipital gyrus | L | 713 | 7.79 | <0.001 | −42 | −67 | −4 |
| Inferior temporal gyrus | R | 321 | 7.32 | <0.001 | 45 | −64 | −7 | |
| Supramarginal gyrus | L | 182 | 7.08 | <0.001 | −63 | −31 | 35 | |
| Precentral gyrus | L | 884 | 5.80 | <0.001 | −42 | −1 | 35 | |
| Superior parietal lobe | R | 317 | 5.58 | <0.001 | 30 | −52 | 62 | |
| Superior parietal lobe | L | 342 | 5.40 | <0.001 | −27 | −55 | 59 | |
| SMA | L | 180 | 4.99 | <0.001 | −3 | 11 | 56 | |
| Supramarginal gyrus | R | 76 | 4.92 | 0.04 | 63 | −25 | 44 | |
| Precentral gyrus | R | 96 | 4.84 | 0.01 | 48 | 8 | 38 | |
| Inferior frontal gyrus | R | 232 | 4.82 | <0.001 | 48 | 26 | 5 | |
| Thalamus | L | 76 | 4.50 | 0.04 | −6 | −10 | −1 | |
| Look-Negative > Decrease | Middle temporal gyrus | L | 857 | 5.42 | <0.001 | −57 | −37 | 5 |
| Insula | R | 520 | 4.73 | <0.001 | 36 | −25 | 17 | |
| Cerebellum | L | 115 | 4.21 | 0.02 | −18 | −46 | −28 | |
| Inferior frontal gyrus | L | 95 | 4.10 | 0.04 | −45 | 38 | −7 | |
| Look-Neutral > Decrease | Superior temporal gyrus | R | 782 | 7.32 | <0.001 | 63 | −13 | 5 |
| Medial orbitofrontal gyrus | R | 1144 | 6.53 | <0.001 | 3 | 50 | −7 | |
| Superior temporal gyrus | L | 870 | 6.47 | <0.001 | −57 | −10 | 2 | |
| Precentral gyrus | L | 133 | 5.34 | 0.005 | −24 | −25 | 77 | |
| Superior frontal gyrus | R | 86 | 5.07 | 0.02 | 30 | 29 | 53 | |
| Cuneus | R | 101 | 5.03 | 0.01 | 15 | −58 | 20 | |
| Postcentral gyrus | R | 271 | 4.85 | <0.001 | 15 | −34 | 80 | |
| Cuneus | L | 76 | 4.20 | 0.04 | −15 | −58 | 20 | |
| Look-Negative > Look-Neutral | Supramarginal gyrus | L | 144 | 9.08 | <0.001 | −63 | −31 | 38 |
| Middle temporal gyrus | L | 462 | 8.78 | <0.001 | −48 | −67 | 2 | |
| Inferior temporal gyrus | R | 210 | 7.63 | <0.001 | 48 | −64 | −7 | |
| Vermis | 153 | 7.31 | <0.001 | −3 | −31 | −4 | ||
| Superior occipital gyrus | R | 48 | 6.78 | <0.001 | 27 | −70 | 32 | |
| Superior parietal lobe | R | 102 | 6.67 | <0.001 | 27 | −61 | 56 | |
| Inferior frontal gyrus | R | 32 | 5.92 | <0.001 | 36 | 29 | 2 | |
| Superior occipital gyrus | L | 28 | 5.88 | <0.001 | −24 | −73 | 32 | |
| Precentral gyrus | L | 46 | 5.83 | <0.001 | 48 | 8 | 38 | |
| Precentral gyrus | R | 41 | 5.77 | <0.001 | 48 | 8 | 38 | |
| Medial superior frontal gyrus | R | 15 | 5.74 | 0.001 | 6 | 47 | 44 | |
| Superior parietal lobe | L | 63 | 5.74 | <0.001 | −24 | −61 | 56 | |
| Supramarginal gyrus | R | 15 | 5.73 | 0.001 | 63 | −22 | 38 | |
| Inferior frontal gyrus | R | 29 | 5.44 | <0.001 | 45 | 23 | 20 | |
| Insula | R | 11 | 5.30 | 0.002 | 42 | 8 | −16 | |
| Look-Neutral > Look-Negative | SMA | L | 777 | 6.21 | <0.001 | −9 | −16 | 56 |
| Superior temporal gyrus | L | 138 | 5.83 | 0.004 | −54 | −10 | −1 | |
| Superior temporal gyrus | R | 289 | 5.33 | <0.001 | 60 | −13 | 2 | |
| Insula | L | 122 | 4.63 | 0.007 | −36 | −22 | 23 | |
L = left. R = right. Coordinates refer to MNI coordinate system. P < 0.05 FWE corrected (k = 10).
Table 2.
Risky decision-making task
| Contrast | Region | L/R | size | t-value | P (FWE-corr.) | Coordinates | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Risky Choices > Safe Choices | Anterior cingulum/vmPFC | L | 196 | 5.08 | 0.001 | −3 | 41 | 8 |
| Thalamus | R | 105 | 4.50 | 0.02 | 6 | −7 | 5 | |
| Superior frontal gyrus/dlPFC | R | 80 | 4.25 | 0.05 | 18 | 41 | 44 | |
| Safe Choices > Risky Choices | No significant clusters | |||||||
| Risky ChoicesDecrease > Risky ChoicesLook-Neutral | Superior frontal gyrus/dlPFC | L | 176 | 4.55 | 0.003 | −24 | 41 | 32 |
| Cingulum | L | 109 | 4.50 | 0.02 | −21 | −46 | 29 | |
| Inferior occipital gyrus | R | 94 | 4.20 | 0.03 | 39 | −88 | −4 | |
| Inferior frontal gyrus/vlPFC | L | 117 | 3.87 | 0.01 | −60 | 17 | 17 | |
| Risky ChoicesLook-Neutral > Risky ChoicesDecrease | No significant clusters | |||||||
| Risky ChoicesDecrease > Risky ChoicesLook-Negative | No significant clusters | |||||||
| Risky ChoicesLook-Negative > Risky ChoicesDecrease | No significant clusters | |||||||
| Risky ChoicesLook-Negative > Risky ChoicesLook-Neutral | Superior frontal gyrus/dlPFC | R | 116 | 4.37 | 0.01 | 21 | 59 | 20 |
| Superior frontal gyrus/dlPFC | L | 82 | 4.38 | 0.05 | −24 | 50 | 29 | |
| Risky ChoicesLook-Neutral > Risky ChoicesLook-Negative | No significant clusters | |||||||
| Safe ChoicesDecrease > Safe ChoicesLook-Neutral | No significant clusters | |||||||
| Safe ChoicesLook-Neutral > Safe ChoicesDecrease | No significant clusters | |||||||
| Safe ChoicesDecrease > Safe ChoicesLook-Negative | No significant clusters | |||||||
| Safe ChoicesLook-Negative > Safe ChoicesDecrease | No significant clusters | |||||||
| Safe ChoicesLook-Negative > Safe ChoicesLook-Neutral | No significant clusters | |||||||
| Safe ChoicesLook-Neutral > Safe ChoicesLook-Negative | No significant clusters | |||||||
L = left. R = right. Coordinates refer to MNI coordinate system. P < 0.05 FWE corrected (k = 10).
In a second step (based on GLM2), we identified the brain regions linked to risky decision-making when regulating emotions. More specifically, we tested whether the experience of emotional responses affected neural activity during risky decision-making (Risky ChoicesLook-Negative > Risky ChoicesLook-Neutral). The results revealed increased activity in the bilateral dlPFC (Figure 3A, Table 2). Next, we investigated the neural activity related to the preference for risky options over safe options after emotion regulation. For this, we contrasted (Risky ChoicesDecrease > Risky ChoicesLook-Neutral) and observed enhanced activity in left dlPFC, left vlPFC and cingulate cortex (Figure 3B, Table 2). The reverse contrasts for neural activity during safe decision-making did not reveal any significant differences in a whole-brain analysis (Table 2).
Fig. 3.
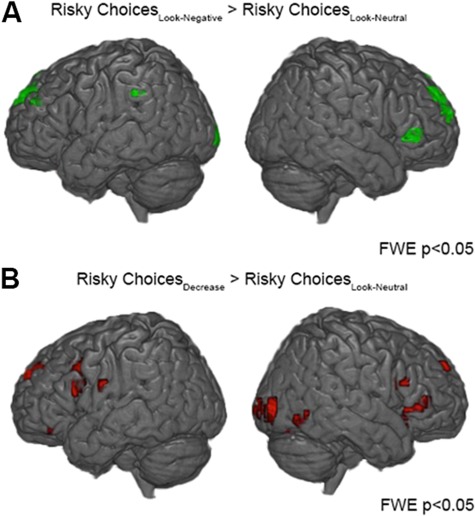
(A) Risky choices after Look-Negative. The contrast (Risky ChoicesLook-Negative > Risky ChoicesLook-Neutral) yielded increased activity in bilateral dlPFC. (B) Risky choices after emotion regulation. The effect of emotion regulation on risky choices (Risky ChoicesDecrease > Risky ChoicesLook-Neutral) yielded increased activity in the left dlPFC, left vlPFC and cingulate cortex.
In a third step, we determined in an explorative manner the overlap of regions implicated in the emotion regulation and risky decision-making in general, by combining the contrasts of both task phases. An overlap in activity between the contrasts (Risky Choices Decrease > Risky ChoicesLook-Neutral) and [(Decrease > Look-Negative) & (Decrease > Look-Neutral)] was found in the left vlPFC region. This suggests that the observed effect of emotion regulation on risky decision-making could be a direct carry-over effect from the emotion regulation task.
Discussion
Our findings showed that emotion regulation of incidental emotions effectively reduced the experience of negative emotions and subsequently resulted in decreased risk-taking in two independent samples. This carry-over effect of emotion regulation on decision-making was reflected in increased activity in ventrolateral and dorsolateral prefrontal regions and cingulate cortex. Furthermore, we found evidence for a shared neural substrate for emotion regulation and risky decision-making in left vlPFC. Together, these findings suggest that emotion reappraisal can alter risk-related decision-making even if the emotional stimulus is incidental.
Effect of reappraisal of incidental emotions on risky decision-making
Behaviorally, our results both confirm and contradict previous studies. On the one hand, previous studies support the idea that emotion regulation leads to increased risk-taking behavior (Heilman et al., 2010; Braunstein et al., 2014; Panno et al., 2013), as individuals who successfully regulate their negative emotions tend to make choices that maximize performance (Seo and Barrett, 2007) and place less weight on the outcome of a single decision, which in turn reduces loss aversion (Sokol-Hessner et al., 2013; Sokol-Hessner et al., 2009). On the other hand, Martin and Delgado (2011) reported that reappraisal leads to more goal-directed behavior and promotes less risk-taking behavior. Our findings indicate that reappraisal of current emotions was linked to less risky choices. One pathway for this effect might be that emotion regulation neutralizes the negative emotional state as the decision-maker becomes aware of, and actively manages, their negative affective experience (Forgas, 2000; Forgas and Ciarrochi, 2002). This could then lead to a carry-over effect of enhanced cognitive control into the decision stage and, as a by-product, decrease risk-taking. Note, however, that this interpretation depends on the definition of goal-directedness in this scenario and only holds if resisting the temptation of making a risky choice (as in gambling) is regarded as requiring more cognitive control than the other way around. Alternatively, one might argue that emotion regulation could simply neutralize the impact of the negative emotional experience that would otherwise carry-over into the decision-phase and increase risk-taking. However, our finding that there was no difference in risk-taking between the passive viewing conditions involving negative and neural images clearly contradicts this interpretation. This means, in our study, it was the regulation of a preceding negative experience rather than the negative experience per se that impacted subsequent incidental decision-making.
There are three major differences between our study and earlier studies investigating emotion regulation and decision-making that might explain some of the discrepancies in findings. Firstly, we induced incidental negative emotions before each decision, meaning that participants regulated emotional responses that were not related to the decision process per se. In contrast, in some previous studies participants were asked to emphasize or deemphasize the importance of an upcoming decision (e.g. Braunstein et al., 2014; Sokol-Hessner et al., 2009; Sokol-Hessner et al., 2013). This means that unlike in our study, in those previous studies no explicit emotional stimulus was present at the time of regulation, rather, the emotions were expected to arise from the decision-making process. Regulating emotions related to potential decision outcomes could arguably lead to a very different profile of choices. Secondly, we instructed participants to use reappraisal to regulate their emotions by implementing tactics such as reality change or distancing (McRae et al., 2012). However, other studies used different strategies to reduce emotions such as taking the perspective of a trader (Sokol-Hessner et al., 2009) or imagine a calming scene such as a sunny day in a park (Martin and Delgado, 2011). These differences in regulation strategy could potentially also lead to differences in choice profiles. Thirdly, we did not provide immediate feedback on participants’ choices that could have triggered emotional processes as in other studies (Sokol-Hessner et al., 2013; Sokol-Hessner et al., 2009; Staudinger et al., 2011; Staudinger et al., 2009). This again fully decoupled the emotional response from the decision-making process and allowed us to isolate incidental regulation-related processes. There is one other study that we are aware of that induced incidental emotions using negative film clips before risky decision-making (Heilman et al., 2010). These authors used the Balloon Analogue Risk Task in which participants can earn financial rewards by ‘pumping up’ balloons presented on the screen. However, as the balloons have variable, unknown explosion points, and participants lose when the breaking point is reached, emotion regulation might also extend to feedback/outcome-related emotions, or expectation of those. In contrast, in our task emotion regulation was more directly attributable to the emotional stimulus alone as no feedback was provided for risky choices.
It is important to note, however, that our study, like most others in the field, did not explicitly investigate the effect of a variety of specific negative (or positive) emotions, or the regulation of such, nor did it systematically vary the type of decision task and risk or include specific groups of decision-makers. It is also conceivable that in other decision scenarios, choosing the risky option might always be the optimal choice, and it is not clear whether the impact of emotion regulation on such choices would be different. It therefore remains to be seen how well our results generalize to other emotion and decision scenarios.
Neural networks underlying the effect of emotion regulation on risky decision-making
We found that the modulatory effect of cognitive reappraisal of incidental emotions on decision-making was primarily associated with increased activity in dlPFC and vlPFC. Our findings extend previous studies that only reported an increase in activity in the dlPFC when individuals engaged in reappraisal to change emotional responses and choices (Grecucci et al., 2013; Hutcherson et al., 2012; Sokol-Hessner et al., 2013; Staudinger et al., 2011). Moreover, we found that the left vlPFC was implicated in the emotion regulation process as well as in the decision-making process. In the context of emotion regulation, dlPFC has been associated with top-down cognitive control processes involved in attention and working memory (Corbetta and Shulman, 2002; Rottschy et al., 2012; Nee et al., 2013; Vossel et al., 2014; Cieslik et al., 2015), while vlPFC has been implicated in top-down outcome-based language-related appraisal processes (Kohn et al., 2014; Liakakis et al., 2011; Messina et al., 2015; Morawetz et al., 2016a). Both regions seem to be part of a self-regulating feedback loop during reappraisal (Morawetz et al., 2016b).
Our findings suggest that the impact of emotion regulation on the decision process is modulated by this shared neural network. Of course, our results do not provide direct evidence that both processes are neurally integrated in these regions, but the shared cognitive functions, in particular in cognitive control processes, might suggest such an explanation. A pre-activation of cognitive control regions during emotion regulation could lead to spill-over effects to cognitive control in risk-related decision-making, again lending support to the view that it was the regulation process and not the absence of negative emotions that was related to the altered choice patterns. Thus, our findings suggest that emotion regulatory processes in general play a critical role in value computation, and that changes in emotional states can be associated with changes in choice outcomes, even when the emotions are not directly related to any aspects of the decisions per se.
Conclusions
Our results extend earlier work on emotion regulation and decision-making in two ways: first, we showed that emotion regulation of incidental emotions affected risk-taking in decision-making, potentially because cognitive control processes carried over to the decision stage in the absence of immediate outcome feedback. Second, our study provides insights into the possible neural networks underlying emotion regulation of incidental emotions and subsequent decision-making, supporting the view of multiple modulatory neural circuits (Phelps et al., 2014). Our study provides a first step toward a more nuanced understanding of the relationship between emotion regulation and decision-making.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft Grant MO 2041/2-1 to C.M. and an Australian Research Council Discovery Project grant DP160103353 to S.B.
Conflict of interest
None declared.
Supplementary Material
References
- Augustine A.a., Larsen R.J. (2011). Affect regulation and temporal discounting: interactions between primed, state, and trait affect. Emotion, 11, 403–12. [DOI] [PubMed] [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. (2007). The International Affective Picture System (IAPS) in the study of motion and attention In: Coan J.A., Allen J.J., editors. Handbook of Emotion Elicitation and Assessment. Series in Affective Science, New York: Oxford University Press, pp. 29–46. [Google Scholar]
- Braunstein L., Herrera S.J., Delgado M.R. (2014). Reappraisal and expected value modulate risk taking. Cognition & Emotion, 28, 172–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik E.C., Mueller V.I., Eickhoff C.R., et al. (2015). Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neuroscience and Biobehavioral Reviews, 48, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero J.a., Rangel A. (2014). Informatic parcellation of the network involved in the computation of subjective value. Social Cognitive and Affective Neuroscience, 9, 1289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero J.A., Carter R.M., Huettel S.A. (2009). Local pattern classification differentiates processes of economic valuation. NeuroImage, 45, 1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience, 3, 201–15. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Gillis M.M., Phelps E.A. (2008). Regulating the expectation of reward via cognitive strategies. Nature Neuroscience, 11, 880–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F., Veit R., Weiskopf N., et al. (2007). Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping, 28, 409–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton-O’Creevy M., Soane E., Nicholson N., et al. (2011). Thinking, feeling and deciding: the influence of emotions on the decision making and performance of traders. Journal of Organizational Behavior, 32, 1044–61. [Google Scholar]
- Fenton-O’Creevy M., Lins J.T., Vohra S., et al. (2012). Emotion regulation and trader expertise: heart rate variability on the trading floor. Journal of Neuroscience, Psychology, and Economics, 5, 227–37. [Google Scholar]
- Forgas J.P. (2000). Managing moods: toward a dual-process theory of spontaneous mood regulation. Psychological Inquiry, 11, 172–7. [Google Scholar]
- Forgas J.P., Ciarrochi J.V. (2002). On managing moods: evidence for the role of homeostatic cognitive strategies in affect regulation. Personality and Social Psychology Bulletin, 28, 336–45. [Google Scholar]
- Grecucci A., Giorgetta C., Van’t Wout M., et al. (2013). Reappraising the ultimatum: an fMRI study of emotion regulation and decision making. Cerebral Cortex, 23, 399–410. [DOI] [PubMed] [Google Scholar]
- Gross J.J., Thompson R.A. (2007). Emotion regulation: conceptual foundations In: Handbook of Emotion Regulation, New York, NY, US: The Guilford Press, pp. 3–24. [Google Scholar]
- Gu X., Kirk U., Lohrenz T.M., et al. (2013). Cognitive strategies regulate fictive, but not reward prediction error signals in a sequential investment task. Human Brain Mapping, 00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Lerner J.S., Keltner D. (2007). Feelings and consumer decision making: the appraisal-tendency framework. Journal of Consumer Psychology, 17, 158–68. [Google Scholar]
- Heilman R., Houser D. (2016). Emotion regulation and economic decision-making In: Reuter M., Montag C., editors. Neuroeconomics, Studies in Neuroscience, Psychology and Behavioral Economics, Berlin, Heidelberg: Springer. [Google Scholar]
- Heilman R.M., Crişan L.G., Houser D., et al. (2010). Emotion regulation and decision making under risk and uncertainty. Emotion, 10, 257–65. [DOI] [PubMed] [Google Scholar]
- Hutcherson C.a., Plassmann H., Gross J.J., et al. (2012). Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. Journal of Neuroscience, 32, 13543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. (2007). The neural correlates of subjective value during intertemporal choice. Nature Neuroscience, 10, 1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D., Frederick S. (2007). Frames and brains: elicitation and control of response tendencies. Trends in Cognitive Sciences, 11, 45–6. [DOI] [PubMed] [Google Scholar]
- Knutson B., Greer S.M. (2008). Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363, 3771–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., et al. (2014). Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. NeuroImage, 87, 345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen C.M., Knutson B. (2005). The neural basis of financial risk taking. Neuron, 47, 763–70. [DOI] [PubMed] [Google Scholar]
- Lerner J.S., Keltner D. (2001). Fear, anger, and risk. Journal of Personality and Social Psychology, 81, 146–59. [DOI] [PubMed] [Google Scholar]
- Lerner J.S., Small D.A., Loewenstein G. (2004). Heart strings and purse strings: carryover effects of emotions on economic decisions. Psychological Science, 15, 337–41. [DOI] [PubMed] [Google Scholar]
- Lerner J.S., Li Y., Valdesolo P., et al. (2015). Emotion and decision making. Annual Review of Psychology, 66, 799–823. [DOI] [PubMed] [Google Scholar]
- Levy D.J., Glimcher P.W. (2012). The root of all value: a neural common currency for choice. Current Opinion in Neurobiology, 22, 1027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakakis G., Nickel J., Seitz R.J. (2011). Diversity of the inferior frontal gyrus—a meta-analysis of neuroimaging studies. Behavioural Brain Research, 225, 341–7. [DOI] [PubMed] [Google Scholar]
- Loewenstein G., Lerner J.S. (2003). The role of affect in decision making In: Davidson R.J., Scherer K.R., Goldsmith H.H., editors. Series in affective science. Handbook of Affective Sciences, Oxford University Press, pp. 619–42. [Google Scholar]
- Loewenstein G.F., Weber E.U., Hsee C.K., et al. (2001). Risk as feelings. Psychological Bulletin, 127, 267–86. [DOI] [PubMed] [Google Scholar]
- Majer P., Mohr P.N.C., Heekeren H.R., et al. (2016). Portfolio decisions and brain reactions via the CEAD method. Psychometrika, 81, 881–903. [DOI] [PubMed] [Google Scholar]
- Martin L.N., Delgado M.R. (2011). The influence of emotion regulation on decision-making under risk. Journal of Cognitive Neuroscience, 23, 2569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K., Ciesielski B., Gross J.J. (2012). Unpacking cognitive reappraisal: goals, tactics, and outcomes. Emotion, 12, 250–5. [DOI] [PubMed] [Google Scholar]
- Messina I., Bianco S., Sambin M., et al. (2015). Executive and semantic processes in reappraisal of negative stimuli: insights from a meta-analysis of neuroimaging studies. Frontiers in Psychology, 6, 974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miu A.C., Crişan L.G. (2011). Cognitive reappraisal reduces the susceptibility to the framing effect in economic decision making. Personality and Individual Differences, 51, 478–82. [Google Scholar]
- Mohr P.N.C., Biele G., Krugel L.K., et al. (2010). Neural foundations of risk-return trade-off in investment decisions. NeuroImage, 49, 2556–63. [DOI] [PubMed] [Google Scholar]
- Morawetz C., Bode S., Baudewig J., Kirilina E., et al. (2016a). Changes in effective connectivity between dorsal and ventral prefrontal regions moderate emotion regulation. Cerebral Cortex, 26. [DOI] [PubMed] [Google Scholar]
- Morawetz C., Bode S., Baudewig J., et al. (2016b). Neural representation of emotion regulation goals. Human Brain Mapping, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C., Bode S., Derntl B., et al. (2017). The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: a meta-analysis of fMRI studies. Neuroscience & Biobehavioral Reviews, 72, 111–28. [DOI] [PubMed] [Google Scholar]
- Murawski C., Harris P.G., Bode S., et al. (2012). Led into temptation? Rewarding brand logos bias the neural encoding of incidental economic decisions. PLoS One, 7, e34155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee D.E., Brown J.W., Askren M.K., et al. (2013). A meta-analysis of executive components of working memory. Cerebral Cortex, 23, 264–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23, 483–99. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panno A., Lauriola M., Figner B. (2013). Emotion regulation and risk taking: predicting risky choice in deliberative decision making. Cognition & Emotion, 27, 326–34. [DOI] [PubMed] [Google Scholar]
- Pham M.T. (2007). Emotion and rationality: a critical review and interpretation of empirical evidence. Review of General Psychology, 11, 155–78. [Google Scholar]
- Phelps E.a., Lempert K.M., Sokol-Hessner P. (2014). Emotion and decision making: multiple modulatory neural circuits. Annual Review of Neuroscience, 263–90. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C., Drevets W.C. (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13, 829–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C., Langner R., Dogan I., et al. (2012). Modelling neural correlates of working memory: a coordinate-based meta-analysis. NeuroImage, 60, 830–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlösser T., Dunning D., Fetchenhauer D. (2013). What a feeling: the role of immediate and anticipated emotions in risky decisions. Journal of Behavioral Decision Making, 26, 13–30. [Google Scholar]
- Schulreich S., Heussen Y.G., Gerhardt H., et al. (2014). Music-evoked incidental happiness modulates probability weighting during risky lottery choices. Frontiers in Psychology, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M.-G., Barrett L.F. (2007). Being emotional during decision making—good or bad? An empirical investigation. Academy of Management Journal, 50, 923–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R., Friston K.J., Tröstl J., et al. (2011). Slice-timing effects and their correction in functional MRI. NeuroImage, 58, 588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol-Hessner P., Hsu M., Curley N.G., et al. (2009). Thinking like a trader selectively reduces individuals’ loss aversion. Proceedings of the National Academy of Sciences of the United States of America, 106, 5035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol-Hessner P., Camerer C.F., Phelps E.a. (2013). Emotion regulation reduces loss aversion and decreases amygdala responses to losses. Social Cognitive and Affective Neuroscience, 8, 341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger M.R., Erk S., Abler B., et al. (2009). Cognitive reappraisal modulates expected value and prediction error encoding in the ventral striatum. NeuroImage, 47, 713–21. [DOI] [PubMed] [Google Scholar]
- Staudinger M.R., Erk S., Walter H. (2011). Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cerebral Cortex, 21, 2578–88. [DOI] [PubMed] [Google Scholar]
- Szasz P.L., Hofmann S.G., Heilman R.M., et al. (2016). Effect of regulating anger and sadness on decision-making. Cognitive Behaviour Therapy, 45, 479–95. [DOI] [PubMed] [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., et al. (2009). Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage, 47, 852–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt K., Murawski C., Speer S., et al. (2018). Hard decisions shape the neural coding of preferences. The Journal of Neuroscience, 39, 718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Wout M., Chang L.J., Sanfey A.G. (2010). The influence of emotion regulation on social interactive decision-making. Emotion, 10, 815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Wout M., Faught S., Menino D. (2013). Does interoceptive awareness affect the ability to regulate unfair treatment by others? Frontiers in Psychology, 4, 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S., Geng J.J., Fink G.R. (2014). Dorsal and ventral attention systems. The Neuroscientist, 20, 150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., et al. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook A., Braver T.S. (2015). Cognitive effort: a neuroeconomic approach. Cognitive, Affective, & Behavioral Neuroscience, 15, 395–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M., Daly M. (2004). Do pretty women inspire men to discount the future? Proceedings. Biological Sciences, 271(Suppl 4), S177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkielman P., Berridge K.C., Wilbarger J.L. (2005). Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Personality and Social Psychology Bulletin, 31, 121–35. [DOI] [PubMed] [Google Scholar]
- Yang Q., Gu R., Tang P., et al. (2013). How does cognitive reappraisal affect the response to gains and losses? Psychophysiology, 50, 1094–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


