Abstract
Relational bullying and victimization are common social experiences during adolescence, but relatively little functional magnetic resonance imaging (fMRI) research has examined the neural correlates of bullying and victimization in adolescents. The aim of the present study was to address this gap by examining the association between amygdala activity to angry and fearful faces and peer relational bullying and victimization in a community-based sample of adolescents. Participants included 49 adolescents, 12–15 years old, who underwent fMRI scanning while completing an emotional face matching task. Results indicated that interactions between amygdala activity to angry and fearful faces predicted self-reported relational bullying and victimization. Specifically, a combination of higher amygdala activity to angry faces and lower amygdala activity to fearful faces predicted more bullying behavior, whereas a combination of lower amygdala activity to angry faces and lower amygdala activity to fearful faces predicted less relational victimization. Exploratory whole-brain analyses also suggested that increased rostral anterior cingulate cortex activity to fearful faces was associated with less bullying. These results suggest that relational bullying and victimization are related to different patterns of neural activity to angry and fearful faces, which may help in understanding how patterns of social information processing predict these experiences.
Keywords: fMRI, amygdala, bullying, victimization, adolescence
Introduction
Peer bullying and victimization are relatively common social experiences for adolescents. Current estimates suggest that the prevalence of being a bully or a victim of bullying in adolescence is between 25% and 50% (Wang et al., 2009; Schneider et al., 2012; Modecki et al., 2014). Additionally, many adolescents are both bullies and victims of bullying, often referred to as bully-victims (Wang et al., 2009). Being either a victim of bullying or a bully (or both) is associated with a number of negative consequences for mental health and well-being, including increased psychological distress, depression and anxiety, as well as decreased school engagement and academic achievement (Hawker and Boulton, 2000; Sweeting et al., 2006; Klomek et al., 2007; Schneider et al., 2012). This highlights an important need to understand the predictors of bullying and victimization in order to identify ways to reduce these experiences for adolescents. In particular, understanding the neural correlates of bullying and victimization during adolescence will increase our understanding of how these social processes develop and would yield insight into potential avenues for intervention.
Predictors of bullying in adolescence
Few functional magnetic resonance imaging (fMRI) studies have examined the neural correlates of bullying behavior specifically, but fMRI research has examined the neural correlates of broad antisocial behavior measures, many of which include items on bullying or aggressive behavior. In these studies, a common predictor of antisocial behavior in adolescents is amygdala activity to threatening (angry or fearful) faces (Marsh et al., 2008; Jones et al., 2009; Viding et al., 2012a; Hyde et al., 2013; Blair et al., 2014; Hyde et al., 2016; Dotterer et al., 2017). Research frameworks suggest that amygdala activity to angry faces and to fearful faces predicts different aspects or subtypes of antisocial behavior (Viding et al., 2012a; Hyde et al., 2013; Blair et al., 2014; Dotterer et al., 2017). Specifically, higher amygdala activity to angry faces with directed eye gaze has been associated with increased antisocial behavior in adolescents and is thought to relate to increased forms of reactive aggression in which antisocial behavior is in response to a perceived interpersonal threat (Dotterer et al., 2017). In contrast, decreased amygdala activity to fearful faces is associated with callous–unemotional traits (e.g. lack of guilt) in children and adolescents (Marsh et al., 2008; Jones et al., 2009; Viding et al., 2012b), which is thought to be due to lower empathy and ability to process others’ fear or distress (Viding et al., 2012a; Blair et al., 2014). Moreover, although most research suggests that this pattern of amygdala activity is unique to callous–unemotional traits, other research in young adults has shown that higher antisocial behavior (not specific to callous–unemotional traits) is correlated with lower amygdala activity to fearful faces (Hyde et al., 2016).
These patterns of brain activity are thought to characterize different pathways toward antisocial behavior (e.g. antisocial behavior with vs without callous–unemotional traits), but it is also possible that they may interact. For example, an adolescent with increased amygdala activity to angry faces and decreased amygdala activity to fearful faces may be at higher risk for aggressive behavior than an adolescent displaying only one of these patterns of activity, although to our knowledge this type of interaction has not been tested previously. Moreover, most of this previous research has examined antisocial behavior broadly (which includes aggression but also includes other behaviors such as rule-breaking and delinquency) and has focused on clinical samples selected for very high levels of antisocial behavior. Thus, there are gaps in our knowledge regarding how amygdala activity relates to a dimensional measure of bullying in a community-based sample of adolescents and whether the two patterns of amygdala activity previously associated with antisocial behavior (increased amygdala activity to angry faces and decreased amygdala activity to fearful faces) may interact to predict increased risk of bullying.
Although neuroimaging research on the neural correlates of bullying, specifically, has been relatively limited, in behavioral research, a wide range of factors at both the individual and contextual level have been associated with bullying. At the individual level, one of the most consistent predictors of bullying is problems with social and emotional processing. For instance, a meta-analysis of longitudinal predictors of bullying in adolescence found that ‘social problems’ (defined as social immaturity or having antisocial friends) was one of the stronger predictors of bullying compared to other predictors examined such as conduct problems and school problems (Kljakovic and Hunt, 2016). In another meta-analysis that included both children and adolescents and cross-sectional research, ‘other-related cognitions’ (defined as thoughts or feelings about others including empathy and perspective taking) was one of the two strongest predictors of bullying, in addition to externalizing behavior (Cook et al., 2010). As expected, other-related cognitions predicted less bullying, whereas externalizing behavior predicted more bullying. A third meta-analysis also found that bullies evidence decreased cognitive and affective empathy and increased callous–unemotional traits (Zych et al., 2019). Likewise, research on social information processing biases has shown that adolescent bullies exhibit a hostile attribution bias and expect more hostile behavior from peers in ambiguous social situations (Crick et al., 2002; Ziv et al., 2013; Wright, 2017). Moreover, emotion dysregulation (a latent construct that included poor emotional understanding, dysregulated expression of anger and sadness and rumination), higher impulsivity and anger have also been found to predict increased bullying (Herts et al., 2012; Wright, 2017; Espelage et al., 2018). In sum, behavioral research on the psychological predictors of bullying has strongly pointed to problems with social and emotional processing as risk factors for bullying, suggesting that neural activity to emotional faces may be an important correlate to examine as well.
Predictors of victimization in adolescence
Of the fMRI studies that have examined the correlates of peer victimization, the majority have examined the associations between chronic peer victimization and neural activity in response to social exclusion, risk-taking or reward processing. This research has shown that in adolescents, peer victimization is associated with enhanced sensitivity of regions that process social pain during social exclusion tasks (Rudolph et al., 2016; Will et al., 2016), heightened activity in emotion and motivation regions during a risk-taking task (Telzer et al., 2018) and decreased activity in the medial prefrontal cortex during a reward anticipation task (Casement et al., 2014). However, no research to our knowledge has examined how neural activity to emotional face expressions relates to peer victimization.
In regards to psychological predictors of peer victimization, the meta-analysis of longitudinal research in adolescents reviewed above also suggested that ‘social problems’ (defined as social isolation, peer rejection and conflict with friends), was a significant predictor of peer victimization, in addition to conduct problems and internalizing problems (Kljakovic and Hunt, 2016). In the meta-analysis that included cross-sectional research on children and adolescents also discussed above, ‘peer status’ (defined as the quality of peer relationships, including peer rejection) and social competence were the strongest predictors of victimization, with better quality peer relationships and higher social competence predicting decreased peer victimization (Cook et al., 2010). Research on social information processing biases has also shown that adolescent victims tend to be more avoidant in ambiguous social situations, exhibit hostile attribution bias, exhibit self-blame and negative self-evaluations and exhibit difficulties with emotion recognition (Woods et al., 2009; Ziv et al., 2013; van Reemst et al., 2016; Guy et al., 2017). Moreover, a meta-analysis demonstrated that higher internalizing problems were both predictors and consequences of peer victimization (Reijntjes et al., 2010). Thus, behavioral research suggests that social and emotional problems are one of the strongest predictors of victimization in adolescents, pointing to the importance of examining neural activity during emotional face processing as a correlate of peer victimization.
The present study
The goal of the present study is to address prior gaps in the literature by examining associations between amygdala activity during an emotional face matching task and self-reported peer relational bullying and victimization. We focused specifically on relational bullying and victimization (e.g. social exclusion, spreading rumors) because it is a relatively more common form of bullying. For example, one study indicated that the 2 month prevalence of relational bullying/victimization was 51.4%, compared to 20.8% for physical bullying/victimization (Wang et al., 2009).
Although no research to our knowledge has specifically examined bullying behavior in relation to amygdala activity in adolescents, research on the broader construct of antisocial behavior leads us to predict that either increased amygdala activity to angry faces, decreased amygdala activity to fearful faces or a combination of these two patterns of neural activity will be associated with increased relational bullying in adolescents. Based on the theoretical frameworks proposed for antisocial behavior, higher amygdala activity to angry faces could contribute to the emotion dysregulation, anger problems and hostile attribution biases associated with bullying, whereas lower amygdala activity to fearful faces could contribute to the decreased empathy associated with bullying.
Given that victims also show social information processing difficulties (e.g. decreased social competence, expectations of hostility from peers, peer rejection) we expected the neural correlates of peer victimization might be similar to those for bullying. In particular, we expected that increased amygdala activity to angry faces would be associated with victimization, given that victims expect more hostility from peers (Ziv et al., 2013; Guy et al., 2017). We did not have strong directional hypotheses for amygdala activity to fearful faces. On the one hand, given that victims often have difficulty with processing ambiguous social situations and exhibit decreased emotion recognition (Woods et al., 2009), it is possible victimization would be correlated with decreased amygdala activity to fearful faces. But on the other hand, given that internalizing problems are also a risk factor for victimization (Reijntjes et al., 2010; Kljakovic and Hunt, 2016), victimization may be associated with increased amygdala activity to fearful faces, which has often been associated with internalizing problems in adolescents (Yang et al., 2010; Hall et al., 2014; Swartz et al., 2017). Finally, although our region of interest (ROI) analyses were focused on amygdala activity, we also planned to conduct exploratory whole-brain regressions to identify any regions outside of the amygdala in which the processing of fearful or angry faces is associated with bullying or victimization in adolescents.
Methods
Participants
Participants were recruited from the community for the ongoing Adolescent Health and Brain Study, which aims to examine associations between biological and environmental influences on mental health. Participants were recruited through a variety of methods including fliers, tables at community events, internet advertisements, mail advertisements and word of mouth. Inclusion criteria were that participants were between 12 and 15 years old, spoke English and were capable of understanding all study procedures, providing informed assent and lying still in the MRI scanner. Exclusion criteria included a diagnosis of autism spectrum disorder, attention-deficit/hyperactivity disorder, schizophrenia or bipolar disorder (based on parent report of whether the child had ever received one of these diagnoses from a mental health professional); chronic disease or condition that could affect cerebral blood flow such as hypertension or diabetes; use of psychotropic medications such as selective serotonin reuptake inhibitors; and any contraindications to MRI scanning (e.g. braces, metal in the body, etc.). All procedures were approved by the University of California, Davis IRB; parents provided informed consent and adolescents provided informed assent before beginning study procedures.
A total of 61 participants underwent fMRI scanning; 2 participants ended the scan early, 7 were excluded due to not meeting quality control criteria (see below in pre-processing section) and three participants were missing data on the self-report measures, leaving a total of 49 participants (24 female, 24 male, 1 non-binary) with valid data on both fMRI and self-report measures. The sample was diverse based on self-reported race/ethnicity: 4 participants were African American, 2 were Asian, 4 were Hispanic/Latino, 1 was Pacific Islander, 15 were White and 23 participants (47% of the sample) identified as two or more races/ethnicities. Participant characteristics are reported in Table 1. There were five sets of siblings in the sample. All analyses conducted in Mplus controlled for the nested nature of the data for siblings by using the cluster command to nest participants within families.
Table 1.
Participant characteristics
| Males | Females | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Group difference | |
| Age | 13.36 | 1.04 | 13.46 | 1.02 | t(47) = −0.34, P = 0.739 |
| Relational bullying | 1.01 | 0.83 | 1.16 | 0.80 | t(47) = −0.63, P = 0.533 |
| Relational victimization | 1.42 | 1.02 | 1.52 | 0.86 | t(47) = −0.40, P = 0.690 |
| Mean accuracy (%) | 96.4 | 3.1 | 98.1 | 3.0 | t(47) = −1.96, P = 0.056 |
| Mean RT (ms) | 1376.5 | 314.2 | 1169.8 | 246.9 | t(47) = 2.55, P = 0.014 |
Note: Relational bullying and relational victimization were square-root transformed due to skew in the distributions. Mean accuracy and RT are reported for the face matching task (mean behavioral data include face and shape matching trials). For the purposes of testing sex differences and dummy-coding, the participant who identified as non-binary was coded as male.
Self-report measures: relational bullying and victimization
Participants completed all self-report measures on tablets using Qualtrics survey software. One participant was missing data due to technical difficulties with the tablets and two participants were excluded from analyses because they skipped four out of the five questions on the relational peer victimization scale. Participants completed the relational aggression subscale of the Peer Experiences Scale (Prinstein et al., 2001), which measures experiences of relational peer aggression (both as the bully and the victim). The relational bullying and relational victimization subscales each included five items assessing the frequency of different forms of relational bullying or victimization (e.g. being left out of a conversation or activity) over the past 12 months on a scale ranging from ‘Never’ (0) to ‘A few times a week’ (4). Scores on these subscales were totaled and used as the measures of relational bullying and relational victimization for analyses. The relational bullying subscale, Cronbach’s α = 0.69 and the relational victimization subscale, Cronbach’s α = .80, each demonstrated adequate internal consistency. These variables were square-root transformed because they were both positively skewed. Relational bullying and relational victimization were significantly correlated (Table 2).
Table 2.
Bivariate correlations
| Age | Relational bullying | Relational victimization | Amygdala activity, angry | Amygdala activity, fearful | Mean accuracy | Mean RT | |
|---|---|---|---|---|---|---|---|
| Age | 1 | ||||||
| Relational bullying | 0.08 | 1 | |||||
| Relational victimization | −0.11 | 0.43** | 1 | ||||
| Amygdala activity, angry | −0.13 | −0.04 | 0.17 | 1 | |||
| Amygdala activity, fearful | −0.10 | −0.28* | 0.15 | 0.20 | 1 | ||
| Mean accuracy | 0.17 | 0.10 | −0.11 | −0.05 | −0.33* | 1 | |
| Mean RT | −0.03 | −0.13 | 0.20 | −0.12 | 0.22 | −0.50*** | 1 |
Note: Accuracy and RT are reported for the face matching task and include all face matching and shape matching trials. *P < 0.05, **P < 0.01, ***P < 0.001.
Emotional face matching task
During fMRI scanning, participants completed an emotional face matching task that has been used in prior research in adolescents (Swartz et al., 2015; Dotterer et al., 2017; Swartz et al., 2017). In a face matching trial, participants viewed a trio of faces, with a target face on the top row and two faces on the bottom row. The participant’s task was to select which of the two faces on the bottom row matched the target face on the top row by pressing their index finger for the left face or middle finger for the right face on an MRI-compatible button box. In the version used in the current study, face matching trials were presented in one run with blocks of faces that were fearful, angry or happy, and the order of presentation was counterbalanced across participants. Face stimuli were taken from the NimStim Set of Facial Expressions (Tottenham et al., 2009) and included an equal number of male and female faces, as well as faces of different races (Caucasian, African American and Asian). Participants viewed two blocks each of the fearful, angry and happy faces with six trials in each block presented for 4 s each and a variable interstimulus interval (ISI) between 2 s and 6 s. For the control condition, shape matching blocks were interleaved between the face matching blocks. The task was similar, although for these blocks, participants selected which shape out of two on the bottom row matched a shape on the top row. Shape stimuli were presented in blocks of six trials for 4 s each with an ISI of 2 s. Accuracy and response times for all trials were recorded. Mean accuracy and reaction time (RT) for the emotional face matching task are reported in Table 1. Participants completed a short practice version of this task during a mock scanner session before scanning in order to ensure their comprehension of the task.
fMRI data acquisition
Scanning took place at the UC Davis Imaging Research Center on a research-dedicated 3T Siemens TIM Trio MRI system. Data acquisition included collection of a magnetization-prepared rapid gradient-echo image, blood-oxygen-level dependent (BOLD) images during the face matching task and a field map. Further details regarding acquisition parameters are provided in the Supplementary Methods.
Pre-processing and quality control criteria
Pre-processing of fMRI data was conducted in SPM12 (update revision number 6906) (Friston et al., 2007). BOLD images were realigned to the first volume in the time series to correct for head motion and the field map was used to unwarp the images. The mean BOLD image created during realignment was co-registered to the high-resolution anatomical image. The high-resolution anatomical image was then segmented and spatially normalized into a standard stereotactic space [Montreal Neurological Institute (MNI) template] using a 12-parameter affine model (final resolution of functional images = 2 mm isotropic voxels). The warps for the co-registered high-resolution anatomical image were saved and applied to the BOLD functional images to normalize these into MNI space. Finally, images were smoothed with a Gaussian filter, set at 6 mm full-width at half-maximum.
Artifact detection software (http://www.nitrc.org/projects/artifact_detect) was used to create nuisance regressors for volumes exhibiting significant mean-volume signal intensity variation 4 standard deviations above or below the mean signal of all volumes in the time series and individual volumes where scan-to-scan movement exceeded 2 mm translation or 2° rotation in any direction. Quality control criteria for inclusion of a participant’s imaging data were <10% volumes exceed Artifact detection criteria for motion or signal intensity outliers, ≥90% coverage of signal within the anatomically-defined bilateral amygdala ROI and accuracy ≥75% on the matching task performed during scanning. A total of 61 participants underwent fMRI scanning; of these, 2 participants ended the scan early, 2 were excluded for exceeding the Artifact motion criteria, 4 were excluded for <90% coverage of signal within the amygdala and 1 was excluded for accuracy. Thus, a total of 52 participants met all quality control criteria for fMRI data; as mentioned above, 3 participants were excluded due to missing data on the self-report responses, leaving a total of 49 participants available for analysis.
Individual level analyses
All analyses were conducted with SPM12 software. For the individual-level model, boxcar regressors were used to model the effect of condition (Angry Face blocks, Fearful Face blocks, Happy Face blocks and Shape blocks) for each individual. Regressors from the Artifact toolbox (described above) were included in the model as nuisance covariates. Next, individual contrast images for effects of each expression (e.g. Angry Faces > Shapes) were generated at the first level for each participant. These contrast images were then entered into second-level random effects models.
Statistical analysis: ROI analyses
To test our hypotheses that amygdala activity to angry and/or fearful faces would be associated with relational bullying and victimization, we used an ROI approach to examine associations between amygdala activity and these measures. The ROI was defined as the bilateral amygdala from the Automated Anatomical Labeling atlas. We conducted group analyses to examine the main effect of task for each emotional face expression vs shape matching control. Using the Wake Forest University Pickatlas v2.4 (Maldjian et al., 2003), we identified functional clusters within the left and right amygdala that were activated to each condition at P < 0.05 family-wise error (FWE) small-volume corrected for the amygdala ROI, with a minimum cluster threshold of 10 voxels. We then extracted mean contrast values from these functional clusters and submitted them to further statistical analyses in Mplus v7.4 software (Muthén and Muthén, 1998–2012). Because left and right amygdala activity to each face expression was highly correlated (angry faces: r = 0.81, P < 0.001; fearful faces: r = 0.83, P < 0.001), we calculated the mean of activity between the left and right amygdala for each condition to reduce the number of comparisons performed.
To test for interactions between amygdala activity to angry and fearful faces, we centered each predictor (mean amygdala activity to angry faces and mean amygdala activity to fearful faces) and then calculated the interaction term with the centered predictors. We conducted two separate regressions in Mplus: one in which amygdala activity (main effects and interaction) predicted relational bullying and one in which amygdala activity (main effects and interaction) predicted relational victimization. Regressions were estimated with maximum likelihood estimation with robust standard errors (MLR), which provides standard errors robust to non-normality. We included covariates for age and sex. Significant interactions were plotted and probed using simple slopes calculators available online (http://www.jeremydawson.co.uk/slopes.htm). Simple slopes analyses were conducted to aid in the interpretation of continuous interaction effects and were not further corrected for multiple comparisons. Because there were six potential effects of interest (the two main effects of amygdala activity to fearful and angry faces and their interaction for the outcomes of bullying and victimization) we used a Benjamini–Hochberg false discovery rate (FDR) correction (Benjamini and Hochberg, 1995) to correct for six statistical tests.
Statistical analysis: whole-brain regressions
We also conducted exploratory whole-brain analyses to identify any other regions throughout the brain related to relational bullying or victimization. Whole-brain regressions were conducted in SPM12 by entering either bullying or victimization as a predictor of brain activity for the contrasts of Angry Faces > Shapes and Fearful Faces > Shapes. Age and sex were entered as covariates. We used AFNI version 19.2.04 (Cox, 1996) 3dClustSim function to identify a minimum cluster size to achieve a whole-brain corrected P < 0.05 threshold using an uncorrected voxelwise P < 0.005 threshold (see details in Supplementary Methods). The minimum cluster size for the Angry Faces > Shapes bullying regression was 390, for the Angry Faces > Shapes victimization regression was 392, for the Fearful Faces > Shapes bullying regression was 386 and for the Fearful Faces > Shapes victimization regression was 371. Since these analyses were intended to be exploratory, we did not further correct for multiple comparisons for these regressions.
Results
Main effects of the emotional face matching task
As expected, the emotional face matching task elicited significant activation in the bilateral amygdala for both emotional face expressions vs the shape matching control (Figure 1). Specifically, the contrast of Angry Faces > Shapes elicited significant activity in the left amygdala, t(48) = 6.90, P-corrected<0.001, peak MNI coordinates: (−20, −6, −18) and in the right amygdala, t(48) = 7.17, P-corrected<0.001, (22, −4, −18). The contrast of Fearful Faces > Shapes also elicited significant activity in the left amygdala, t(48) = 5.56, P-corrected<0.001, (−20, −6, −18) and in the right amygdala, t(48) = 6.59, P-corrected<0.001, (24, −6, −14).
Figure 1.
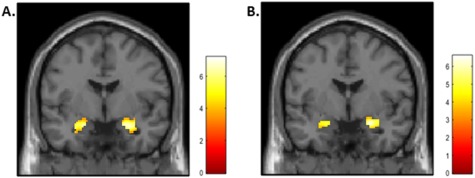
Main effects of task for Angry Faces > Shapes (A) and Fearful Faces > Shapes (B). Effects were evaluated with a P < 0.05 FWE small-volume correction for the bilateral amygdala ROI.
ROI analysis: associations between amygdala activity to fearful and angry faces and relational bullying and victimization
Contrast values were extracted from the functional clusters identified in the amygdala ROI, averaged between the left and right amygdala and submitted to further statistical analyses in MPlus. There was a significant interaction between amygdala activity to angry faces and amygdala activity to fearful faces in predicting relational bullying, B = −6.02, SE = 2.26, standardized beta = −.30, P = 0.008, change r2 = 0.08 (Table 3), which survived FDR correction for multiple comparisons. As shown in Figure 2, increased amygdala activity to angry faces predicted higher levels of bullying when amygdala activity to fearful faces was 1 SD below the mean (P = 0.034).
Table 3.
Results of multiple regressions
| B | SE | Standardized beta | P-value | |
|---|---|---|---|---|
| Dependent variable: Relational Bullying | ||||
| Sex | 0.06 | 0.21 | 0.04 | 0.773 |
| Age | 0.04 | 0.11 | 0.05 | 0.728 |
| Amygdala activity to angry faces | 0.05 | 0.46 | 0.01 | 0.915 |
| Amygdala activity to fearful faces | −0.85 | 0.62 | −0.19 | 0.168 |
| Amygdala to angry x amygdala to fearful | −6.02 | 2.26 | −0.30 | 0.008 |
| Dependent variable: Relational Victimization | ||||
| Sex | 0.03 | 0.26 | 0.02 | 0.904 |
| Age | −0.08 | 0.12 | −0.09 | 0.518 |
| Amygdala activity to angry faces | 0.50 | 0.45 | 0.11 | 0.260 |
| Amygdala activity to fearful faces | 1.05 | 0.71 | 0.21 | 0.139 |
| Amygdala to angry x amygdala to fearful | −7.43 | 2.27 | −0.32 | 0.001 |
Note: SE = standard error.
Figure 2.
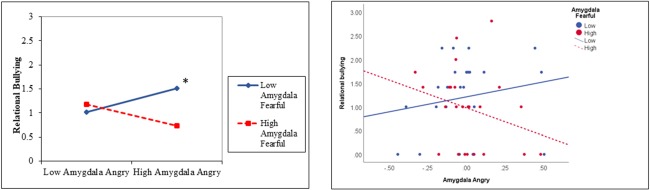
Interaction between amygdala activity to angry and fearful faces predicts relational bullying. Results are displayed for a simple slopes analysis for low levels of amygdala activity to fearful faces (1 SD below the mean) and high levels of amygdala activity to fearful faces (1 SD above the mean) (A) and plotting data points with a median split for amygdala activity to fearful faces (B). The relational bullying variable was square root-transformed for all analyses. *P < 0.05 in simple slopes analysis.
There was also a significant interaction between amygdala activity to angry faces and amygdala activity to fearful faces as a predictor of relational peer victimization, B = −7.43, SE = 2.27, standardized beta = −.32, P = 0.001, change r2 = 0.10 (Table 3), which survived FDR correction for multiple comparisons. As shown in Figure 3, lower levels of amygdala activity to angry faces predicted lower victimization when amygdala activity to fearful faces was 1 SD below the mean (P < 0.001).
Figure 4.
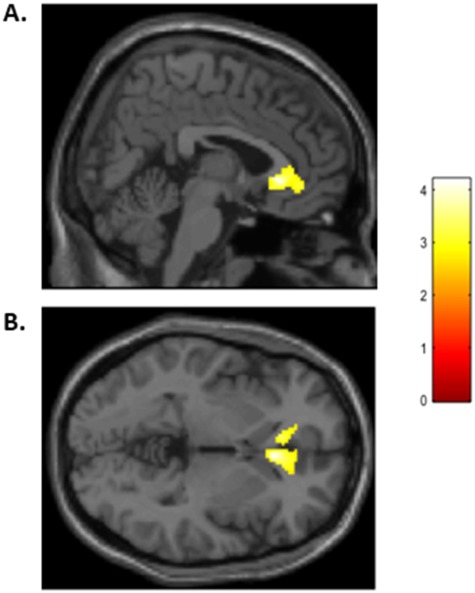
rACC activity to fearful faces is negatively associated with relational bullying. Results are displayed at P < 0.005 uncorrected voxelwise with a minimum cluster extent threshold of 386 voxels. (A) demonstrates activity in sagittal view and (B) demonstrates activity in axial view.
Figure 3.
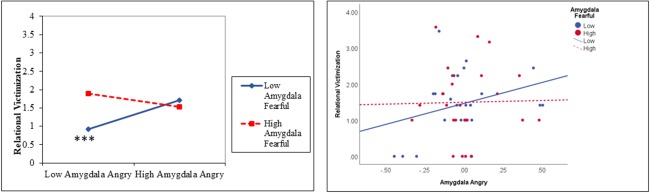
Interaction between amygdala activity to angry and fearful faces predicts relational victimization. Results are displayed for a simple slopes analysis for low levels of amygdala activity to fearful faces (1 SD below the mean) and high levels of amygdala activity to fearful faces (1 SD above the mean) (A) and plotting data points with a median split for amygdala activity to fearful faces (B). The relational victimization variable was square root-transformed for all analyses. ***P < 0.001 in simple slopes analysis.
Whole-brain analysis
For the contrast of Fearful Faces > Shapes, there were no regions positively associated with relational bullying. However, activity in the bilateral rostral anterior cingulate cortex (rACC) was negatively associated with relational bullying, t(45) = 4.28, P-uncorrected<0.001, cluster size = 586 voxels, peak MNI coordinates: (6, 26, −2), as displayed in Figure 4. No effects were significant in the other whole-brain regressions.
Discussion
The main findings of the present study were that interactions between amygdala activity to angry and fearful faces were associated with relational bullying and relational victimization in a community-based sample of adolescents. Results indicated that a combination of high amygdala activity to angry faces and low amygdala activity to fearful faces was associated with higher self-reported bullying. For relational victimization, a combination of low amygdala activity to angry faces and low amygdala activity to fearful faces predicted lower levels of peer victimization. Finally, results of the exploratory whole-brain regressions indicated that higher rACC activity to fearful faces was associated with lower relational bullying.
The results for relational bullying are consistent with prior research finding that both increased amygdala activity to angry faces and decreased amygdala activity to fearful faces is associated with antisocial behavior. This suggests that results from clinical samples may extend dimensionally into milder forms of aggressive behavior such as relational bullying. This also extends prior research by showing that both of these patterns of amygdala activity can interact to predict bullying behavior. Although we did not collect measures on potential mediators that could explain this association, we speculate here about potential pathways that could be tested in future research. Consistent with interpretations from antisocial behavior research, increased amygdala activity to angry faces may relate to bullying because it may contribute to a hostile attribution bias in which adolescents with this pattern of amygdala activity interpret peers in ambiguous social situations as intentionally hostile. Another related possibility is that increased amygdala activity to angry faces may overwhelm the ability to regulate emotions in emotionally charged situations, leading to more relational conflict. Likewise, decreased amygdala activity to fearful faces may relate to decreased ability to perceive or process others’ distress and could lead to reduced empathy or perspective-taking, which may be an important protective factor against relational bullying. Relatedly, decreased amygdala activity to fearful faces is associated with callous–unemotional traits (Marsh et al., 2008; Jones et al., 2009; Viding et al., 2012b), which are predictors of bullying behavior (Viding et al., 2009; Fanti and Kimonis, 2012).
For relational victimization, a combination of low amygdala activity to angry faces and low amygdala activity to fearful faces predicted lower levels of relational victimization. In other words, either higher amygdala activity to angry faces and/or higher amygdala activity to fearful faces was associated with higher levels of peer victimization. This could potentially indicate multiple pathways to victimization. Higher amygdala activity to angry and fearful faces has been associated with higher internalizing symptoms in adolescents (Yang et al., 2010; Hall et al., 2014; Swartz et al., 2017), suggesting that adolescents with heightened activity to both facial expressions or either threatening facial expression may exhibit an internalizing pathway in which heightened amygdala activity to threatening faces is related to increased social avoidance, leading to increased peer rejection and victimization. Another possibility is that adolescents with higher amygdala activity to angry faces (but lower amygdala activity to fearful faces) could experience victimization through a bully-victim pathway since bullying often co-occurs with victimization. In sum, adolescents who displayed lower levels of amygdala activity to angry faces and lower levels of amygdala activity to fearful faces reported the lowest levels of peer victimization, whereas higher amygdala activity to fearful and/or angry faces predicted higher peer victimization. Proposed mediators on both the bullying and victimization pathways will need to be tested in future research that directly measures these.
Results of the exploratory whole-brain regressions also revealed an additional region, the rACC, in which increased activity to fearful faces related to decreased bullying. The rACC is functionally connected with the amygdala and is involved in processing emotional stimuli, and some frameworks suggest it plays a specific role in regulating responses to emotional stimuli and resolving emotional conflict (Etkin et al., 2011). It is possible that rACC activity during this task reflects an implicit form of emotion regulation associated with performing the goal-directed matching task with emotional stimuli. Alternatively, since the ACC is broadly involved in integration of social and emotional information (Lavin et al., 2013), this activity could reflect further processing of signals of others’ distress, which may relate to decreased bullying through similar mediators as amygdala activity to fearful faces, such as empathy. Since this is an exploratory whole-brain analysis and this task was not designed to measure emotion regulation or empathy explicitly, these potential explanations are speculative and will need to be tested in future research.
This study should be interpreted with respect to several limitations. First, the sample size was relatively small, and so results should be considered preliminary until replicated in a larger sample. Second, as described above, although we have suggested potential mediators that may explain the observed effects, many of these (e.g. hostile attribution bias, empathy) were not measured in the current data set. Thus, these proposed mediating pathways will need to be tested in future research in which these variables are measured. Third, the NimStim Set of Facial Expressions used in the face task includes actors who are 21–30 years old (Tottenham et al., 2009) and thus older than adolescents’ peers. It is unclear whether similar patterns of neural activity would be observed when using same-aged peer faces as stimuli for the face task. This will be an important direction for future research. Fourth, due to the small sample size, we were not able to test for additional moderators of these effects, such as sex (although sex was controlled for as a covariate in analyses). Fifth, this was a cross-sectional study and so the direction of effects is unclear. It is possible that these patterns of amygdala activity led to bullying or victimization, or resulted from bullying or victimization, or that they have bi-directional effects on each other over time. This will be an important question to address in future longitudinal research. Sixth, although the blocked fMRI paradigm had the advantage of maximizing signal-to-noise ratio and our statistical power to detect amygdala activity, blocked paradigms can be more predictable for participants than an event-related design, and we were unable to control for potential covariates at the individual trial level such as accuracy and RT. And finally, because this was a community-based sample of adolescents and was not recruited specifically based on bullying or peer victimization and excluded for certain forms of psychopathology that might be related to bullying and victimization such as attention-deficit/hyperactivity disorder, rates of each of these experiences were relatively low in the sample. It is unclear if these results would generalize to more extreme forms of bullying, other forms of bullying (e.g. physical, verbal or cyberbullying) or other forms of antisocial behavior.
In sum, these results indicate that bullying and victimization in adolescents are related to different combinations of amygdala activity to angry and fearful faces. Further research is needed to test the psychological mediators of these effects. If these effects are replicated and extended to longitudinal research in the future, they will help to elucidate how biased patterns of social and emotional processing may increase risk for bullying and victimization in adolescents and could lead to more tailored intervention approaches.
Funding
This work was supported by UC Davis, Prop. 63, the Mental Health Services Act and the Behavioral Health Center of Excellence at UC Davis (J.R.S.) and the USDA National Institute of Food and Agriculture, Hatch project 1013485 (J.R.S.). Part of this research was conducted at the UC Davis Clinical and Translational Science Center Clinical Research Center, which is supported by the National Center for Advancing Translational Sciences and the National Institutes of Health through grant number UL1 TR001860.
Supplementary Material
Acknowledgements
The authors would like to thank the participants of the study for their time. De-identified data from this manuscript and Eprime files for the fMRI task are available by request to the first author.
References
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, 57, 289–300. [Google Scholar]
- Blair R.J.R., Leibenluft E., Pine D.S. (2014). Conduct disorder and callous–unemotional traits in youth. The New England Journal of Medicine, 371, 2207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casement M.D., Guyer A.E., Hipwell A., et al. (2014). Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Developmental Cognitive Neuroscience, 8, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C.R., Williams K.R., Guerra N.G., Kim T.E., Sadek S. (2010). Predictors of bullying and victimization in childhood and adolescence: a meta-analytic investigation. School Psychology Quarterly, 25(2), 65–83. [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Crick N.R., Grotpeter J.K., Bigbee M.A. (2002). Relationally and physically aggressive children’s intent attributions and feelings of distress for relational and instrumental peer provocations. Child Development, 73(4), 1134–42. [DOI] [PubMed] [Google Scholar]
- Dotterer H.L., Hyde L.W., Swartz J.R., Hariri A.R., Williamson D.E. (2017). Amygdala reactivity predicts adolescent antisocial behavior but not callous–unemotional traits. Devevelopmental Cognitive Neuroscience, 24, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelage D.L., Merrin G.J., Hong J.S., Resko S.M. (2018). Applying social cognitive theory to explore relational aggression across early adolescence: a within- and between-person analysis. Journal of Youth and Adolescence, 47, 2401–13. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti K.A., Kimonis E.R. (2012). Bullying and victimization: the role of conduct problems and psychopathic traits. Journal of Research on Adolescence, 22(4), 617–31. [Google Scholar]
- Friston K.J., Ashburner J.T., Kiebel S.J., Nichols T.E., Penny W.D. (2007). Statistical Parametric Mapping: The Analysis of Functional Brain Images, London: Academic Press. [Google Scholar]
- Guy A., Lee K., Wolke D. (2017). Differences in the early stages of social information processing for adolescents involved in bullying. Aggressive Behavior, 43, 578–87. [DOI] [PubMed] [Google Scholar]
- Hall L.M.J., Klimes-Dougan B., Hunt R.H., et al. (2014). An fMRI study of emotional face processing in adolescent major depression. Journal of Affective Disorders, 168, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker D.S.J., Boulton M.J. (2000). Twenty years’ research on peer victimization and psychosocial maladjustment: a meta-analytic review of cross-sectional studies. Journal of Child Psychology and Psychiatry, 41(4), 441–55. [PubMed] [Google Scholar]
- Herts K.L., McLaughlin K.A., Hatzenbuehler M.L. (2012). Emotion dysregulation as a mechanism linking stress exposure to adolescent aggressive behavior. Journal of Abnormal Child Psychology, 40(7), 1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Shaw D.S., Hariri A.R. (2013). Understanding youth antisocial behavior using neuroscience through a developmental psychopathology lens: review, integration, and directions for research. Developmental Review, 33, 168–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Shaw D.S., Murray L., Gard A., Hariri A.R., Forbes E.E. (2016). Dissecting the role of amygdala reactivity in antisocial behavior in a sample of young, low-income, urban men. Clinical Psychological Science, 4(3), 527–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.P., Laurens K.R., Herba C.M., Barker G.J., Viding E. (2009). Amygdala hypoactivity to fearful faces in boys with conduct problems and callous–unemotional traits. American Journal of Psychiatry, 166, 95–102. [DOI] [PubMed] [Google Scholar]
- Kljakovic M., Hunt C. (2016). A meta-analysis of predictors of bullying and victimisation in adolescence. Journal of Adolescence, 49, 134–45. [DOI] [PubMed] [Google Scholar]
- Klomek A.B., Marrocco F., Kleinman M., Schonfeld I.S., Gould M.S. (2007). Bullying, depression, and suicidality in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 46(1), 40–9. [DOI] [PubMed] [Google Scholar]
- Lavin C., Melis C., Mikulan E., Gelormini C., Huepe D., Ibanez A. (2013). The anterior cingulate cortex: an integrative hub for human socially-driven interactions. Frontiers in Neuroscience, 7, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- Marsh A.A., Finger E.C., Mitchell D.G.V., et al. (2008). Reduced amygdala response to fearful expressions in children and adolescents with callous–unemotional traits and disruptive behavior disorders. American Journal of Psychiatry, 165, 712–20. [DOI] [PubMed] [Google Scholar]
- Modecki K.L., Minchin J., Harbaugh A.G., Guerra N.G., Runions K.C. (2014). Bullying prevalence across contexts: a meta-analysis measuring cyber and traditional bullying. Journal of Adolescent Health, 55, 602–11. [DOI] [PubMed] [Google Scholar]
- Muthén L.K., Muthén B.O. (1998–2012). Mplus User’s Guide, Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Prinstein M.J., Boergers J., Vernberg E.M. (2001). Overt and relational aggression in adolescents: social-psychological adjustment of aggressors and victims. Journal of Clinical Child Psychology, 30(4), 479–91. [DOI] [PubMed] [Google Scholar]
- van Reemst L., Fischer T.F.C., Zwirs B.W.C. (2016). Social information processing mechanisms and victimization: a literature review. Trauma Violence, & Abuse, 17(1), 3–25. [DOI] [PubMed] [Google Scholar]
- Reijntjes A., Kamphuis J.H., Prinzie P., Telch M.J. (2010). Peer victimization and internalizing problems in children: a meta-analysis of longitudinal studies. Child Abuse & Neglect, 34, 244–52. [DOI] [PubMed] [Google Scholar]
- Rudolph K.D., Miernicki M.E., Troop-Gordon W., Davis M.M., Telzer E.H. (2016). Adding insult to injury: neural sensitivity to social exclusion is associated with internalizing symptoms in chronically peer-victimized girls. Social Cognitive and Affective Neuroscience, 11(5), 829–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S.K., O’donnell L., Stueve A., Coulter R.W.S. (2012). Cyberbullying, school bullying, and psychological distress: a regional census of high school students. American Journal of Public Health, 102(1), 171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz J.R., Williamson D.E., Hariri A.R. (2015). Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. The American Journal of Psychiatry, 172(3), 276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz J.R., Hariri A.R., Williamson D.E. (2017). An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents. Molecular Psychiatry, 22(2), 209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeting H., Young R., West P., Der G. (2006). Peer victimization and depression in early-mid adolescence: a longitudinal study. British Journal of Educational Psychology, 76, 577–94. [DOI] [PubMed] [Google Scholar]
- Telzer E., Miernicki M.E., Rudolph K.D. (2018). Chronic peer victimization heightens neural sensitivity to risk taking. Development and Psychopathology, 30(1), 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., et al. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168(3), 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E., Simmonds E., Petrides K.V., Frederickson N. (2009). The contribution of callous–unemotional traits and condcut problems to bullying in early adolescence. Journal of Child Psychology and Psychiatry, 50(4), 471–81. [DOI] [PubMed] [Google Scholar]
- Viding E., Fontaine N.M.G., McCrory E.J. (2012a). Antisocial behaviour in children with and without callous–unemotional traits. Journal of the Royal Society of Medicine, 105, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E., Sebastian C.L., Dadds M.R., et al. (2012b). Amygdala response to preattentive masked fear in children with conduct problems: the role of callous–unemotional traits. American Journal of Psychiatry, 169, 1109–16. [DOI] [PubMed] [Google Scholar]
- Wang J., Iannotti R.J., Nansel T.R. (2009). School bullying among US adolescents: physical, verbal, relational and cyber. The Journal of Adolescent Health, 45(4), 368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will G., van Lier P.A.C., Crone E.A., Guroglu B. (2016). Chronic childhood peer rejection is associated with heightened neural responses to social exclusion during adolescence. Journal of Abnormal Child Psychology, 44, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S., Wolke D., Nowicki S., Hall L. (2009). Emotion recognition abilities and empathy of victims of bullying. Child Abuse & Neglect, 33(5), 307–11. [DOI] [PubMed] [Google Scholar]
- Wright M.F. (2017). Adolescents’ emotional distress and attributions for face-to-face and cyber victimization: longitudinal linkages to later aggression. Journal of Applied Developmental Psychology, 48, 1–13. [Google Scholar]
- Yang T.T., Simmons A.N., Matthews S.C., et al. (2010). Adolescents with major depression demonstrate increased amygdala activation. Journal of the American Academy of Child and Adolescent Psychiatry, 49(1), 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y., Leibovich I., Schechtman Z. (2013). Bullying and victimization in early adolescence: relations to social information processing patterns. Aggressive Behavior, 39, 482–92. [DOI] [PubMed] [Google Scholar]
- Zych I., Ttofi M.M., Farrington D.P. (2019). Empathy and callous–unemotional traits in different bullying roles: a systematic review and meta-analysis. Trauma, Violence, & Abuse, 20(1), 3–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


