Abstract
Background
Characterization of the mesenchymal stromal cell (MSC) safety profile is important as this novel therapy continues to be evaluated in clinical trials for various inflammatory conditions. Due to an increase in published randomized controlled trials (RCTs) from 2012–2019, we performed an updated systematic review to further characterize the MSC safety profile.
Methods
MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials and Web of Science (to May 2018) were searched. RCTs that compared intravascular delivery of MSCs to controls in adult populations were included. Pre-specified adverse events were grouped according to: (1) immediate, (2) infection, (3) thrombotic/embolic, and (4) longer-term events (mortality, malignancy). Adverse events were pooled and meta-analyzed by fitting inverse-variance binary random effects models. Primary and secondary clinical efficacy endpoints were summarized descriptively.
Findings
7473 citations were reviewed and 55 studies met inclusion criteria (n = 2696 patients). MSCs as compared to controls were associated with an increased risk of fever (Relative Risk (RR) = 2·48, 95% Confidence Interval (CI) = 1·27–4·86; I2 = 0%), but not non-fever acute infusional toxicity, infection, thrombotic/embolic events, death, or malignancy (RR = 1·16, 0·99, 1·14, 0·78, 0·93; 95% CI = 0·70–1·91, 0·81–1·21, 0·67–1·95, 0·65–0·94, 0·60–1·45; I2 = 0%, 0%, 0%, 0%, 0%). No included trials were ended prematurely due to safety concerns.
Interpretations
MSC therapy continues to exhibit a favourable safety profile. Future trials should continue to strengthen study rigor, reporting of MSC characterization, and adverse events.
Funding
Stem Cell Network, Ontario Institute for Regenerative Medicine and Ontario Research Fund
Keywords: Mesenchymal stem cells, Safety, Adverse events, Systematic review
Research in context.
Evidence before this study
Several small clinical trials have investigated the efficacy and safety of MSCs in diseases, including chronic heart failure, acute myocardial infarction, hematological malignancies, graft versus host disease and the acute respiratory distress syndrome, and found some benefit with MSC therapy compared to controls. A previous systematic review examined the safety of intravascular administration of MSC therapy in heterogeneous adult patient populations. The review included eight RCTs and identified fever as the only adverse event that was significantly associated with MSC therapy. Since that publication in 2012, several reviews of MSC efficacy and or safety have included safety as part of the review objective. However, only one review included a detailed and systematic examination of the efficacy and safety of intravascular MSC administration that was limited to acute myocardial infarction and ischemic heart failure conditions and found no association between MSC therapy and acute adverse events (less than 24 h after study treatment); however MSC therapy compared to controls was associated with delayed neurological events.
Added value of this study
In our updated systematic review that now includes over 40 additional RCTs and over 2000 additional patients, aside from fever, we continue to detect no significant reported safety signals associated with MSC treatment.
Implications of all the available evidence
Our findings suggest that with the accumulation RCT evidence, the administration of MSCs continues to appear safe. The findings from our review should provide additional assurance to researchers, clinicians, health regulators and patients and families that, with this updated evidence, the administration of MSCs continues to appear safe. Future trials should continue to strengthen study rigor, reporting of MSC characterization and functionality, and adverse events as clinical indications as well as manufacturing processes evolve and second generation MSC products make their way to clinical trials.
Alt-text: Unlabelled box
1. Introduction
Mesenchymal stromal cells (mesenchymal stem cells; MSCs) are multipotent stem cells that can be isolated from many adult tissues (e.g. bone marrow, adipose tissue). First described in 1974 [1], they have recently received attention in a number of different clinical fields for their potential therapeutic effects. While often described as ‘adult stem cells’, MSCs have limited cellular differentiation ability as compared to other types of stem cells. Pre-clinical evidence suggests that MSCs exert their beneficial effects primarily through immunomodulatory and paracrine mechanisms. MSCs target sites of inflammation and secrete bioactive molecules [2] and there is a growing body of literature demonstrating the efficacy of MSC therapy in a variety of pre-clinical models, including acute lung injury [3,4], sepsis [5] and acute myocardial infarction [6]. Indeed, evidence of the immune-modulatory ability of MSC therapy in pre-clinical models has led to interest in the possible therapeutic role for MSCs in a variety of acute and chronic inflammatory conditions.
To date, several small clinical trials have investigated the efficacy and safety of MSCs for a variety of conditions including chronic heart failure, acute myocardial infarction, hematological malignancies, graft versus host disease and acute respiratory distress syndrome. While the results of some trials suggest benefit, larger trials with clinically important endpoints are needed before more definitive conclusions can be drawn. Thus, as more and more patients are being asked to participate in the studies, the safety of MSC therapy is of increasing importance and any risk of adverse events could represent a significant barrier to their successful translation into clinical practice. These potential risks include neoplastic potential due to MSCs’ proliferative capacity, susceptibility to infection given their immunomodulatory effects, embolism of the cells, zoonoses associated with cell culture reagents, and acute or chronic immunogenicity of the cells themselves [7]. A previous systematic review published by our group in 2012 included eight randomized controlled trials (RCTs) (n = 369 patients) and identified fever as the only adverse event that was significantly associated with MSC therapy [8]. Given the increase in published RCTs and patients enrolled in MSC trials since that time, we decided to conduct and update our systematic review to further characterize the safety profile of MSC-based therapy and descriptively summarize primary and secondary efficacy outcomes in MSC RCTs.
2. Methods
The methods of this systematic review and meta-analysis are similar to our previously published review [8] with a few modifications; these are the addition of key words in our search strategy to capture placenta derived MSC trials, the inclusion of only randomized controlled trials, a focus on reporting adverse events that were pre-specified and that are potentially relevant to MSC administration, the addition of one additional pre-specified adverse event category (thrombotic and thromboembolic events) and one additional sub group analysis according placental MSCs, documentation of all reported serious adverse events and their relatedness to study treatment (in the MSC or control group), pooling of pre-specified adverse event estimates according to relative risks and 95% confidence intervals, and a descriptive summary of primary and secondary efficacy outcomes in the included RCTs. This report follows the PRISMA guidelines (complete checklist can be found in Appendix 2) [9] and because our review is an update of a previously published review, no protocol was registered.
2.1. Search strategy and selection criteria
We conducted electronic searches of Ovid MEDLINE (1950 to April 2019), EMBASE (1980 to April 2019) and Cochrane Central Register of Controlled Trials (April 2019). Given the non-standard terminology associated with MSCs, a number of terms were used (Appendix I, search strategy). ClinicalTrials.gov was searched for ongoing or recently completed trials. Abstracts and proceedings from clinical conferences were identified and searched using Web of Science (April 2019). Bibliographies of retrieved articles and relevant reviews were manually searched. All searches were performed without any language restrictions; if included, any non-English studies were subsequently translated for data extraction.
We included RCTs that examined the intravascular (venous and arterial) administration of MSCs compared to a control group that did not receive MSCs in adult populations. We excluded studies that exclusively used non-intravascular routes of administration (e.g. injection into a joint), ex vivo differentiated MSCs, or MSCs co-administered with other experimental cells or treatments.
Study screening and selection, data extraction and risk of bias assessments were all performed in duplicate by three independent reviewers (DW, MT, ED) using standardized forms.
2.2. Data analysis
Data were extracted under the following subheadings using a standardized spreadsheet: RCT characteristics and patient populations, MSC preparation and administration, assessment of risk of bias, and primary (safety) and secondary (efficacy) outcome measures. We recorded primary and secondary efficacy endpoints as reported in the RCTs. We contacted authors via email correspondence when data relevant to our systematic review was not reported in the included studies.
Safety was examined according to pre-specified incident adverse events according to the following categories: (1) immediate events (i.e., fever and non-fever acute infusional toxicity that occurred within 24 h of study drug administration) that captured the potential for MSCs to embolize or cause hypersensitivity reactions, (2) infection events that occurred at any time post-infusion because MSCs are known to immune-modulate in pre-clinical models, (3) thrombotic or thrombo-embolic events because MSCs can express or secrete tissue factor and other coagulation proteins [10], [11], [12], [13], [14] and therefore there is a theoretical risk of activation of coagulation and consequent adverse clinical events (i.e. deep venous thrombosis, pulmonary embolism, arterial thrombosis etc.), and (4) longer-term events including death and malignancy, the latter of which was captured due to the theoretical risk that MSCs could engraft.
Adverse event data were extracted based on the longest follow-up point. Adverse event data from RCTs with more than one MSC study arm (ex: dose escalation trial) were combined into one MSC study group. Meta-analyses for each pre-specified adverse event category was performed using OpenMetaAnalyst (for Windows 7). Data were analyzed using DerSimonian-Laird random effects models with a correction factor of 0.5 added to both arms for studies with 0 counts. Pooled events were described using Relative Risks (RR) and 95% confidence intervals (95% CI).
For all pre-specified adverse events we documented whether the events were reported as serious and if they were related to the study treatment (in either the MSC group or control group); we also captured other serious adverse events that were not pre-specified in our review and their reported relatedness to the study treatment. Finally, we captured the number of studies that were aborted pre-maturely due to safety concerns.
We used the CONSORT approach to harm reporting as a guide to capture the quality of adverse event reporting [15]. Specifically, we examined whether the reported approach to monitoring/recording adverse events (a priori plan to monitor events, types of events, frequency, and follow-up duration for events) were defined in the methods sections of the included studies.
Data related to MSC characterization as defined by the Dominici criteria were also recorded [16]. These included MSC cell source and origin, tri-lineage differentiation potential, cell surface markers, and cell morphology and adherence to plastic. We also described measures of MSC production (MSC viability, MSC potency, culture medium, and cryopreservation technique) because these measures could potentially impact both therapeutic efficacy and safety.
Heterogeneity between RCTs was evaluated using the I2 as well as the P-value from X2 test. Sub group analyses for each pre-specified adverse event category were planned according to the individual patient populations (cardiovascular, neurological, hematological/oncological, endocrine, renal, liver, respiratory, infectious, immune-deficient/inflammatory, other), MSC characteristics (type, origin, source), and MSC preparation (fresh versus cryopreserved, xenogeneic versus xeno-free culture media). No adjustments for multiple comparisons were made for these sub group analyses as they were considered exploratory. A post-hoc sensitivity analysis of the pre-specified adverse event pooled estimates that excluded studies published in abstract form only was also conducted to evaluate the robustness of the study findings. The secondary efficacy outcomes were not pooled but rather summarized descriptively in table format for the reader.
RCTs that met inclusion criteria were assessed for risk of bias according to the Cochrane Collaboration methods [17].
2.3. Role of funding source
The funders had no role in the analysis, interpretation of the study results, or drafting of the manuscript. The authors independently designed the study, collected data, had access to the raw data, did the statistical analysis, and were responsible for the decision to submit for publication.
3. Results
Our search identified 7473 unique titles and 55 RCTs met inclusion criteria (see Fig. 1 for PRISMA flow diagram). All 55 RCTs were included for review (n = 2696 patients) [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66] (Table 1); six of the 55 RCTs were published in abstract form only [19,45,61,64,65,67]. Included RCTs were conducted in 12 different countries and 20 (36·4%) were multi-center [[20], [21], [22], [23],26,27,29,38,40,46,47,49,55,[62], [63], [64],66,[68], [69], [70]]. Sample sizes ranged from nine to 135 patients (49·9 ± 31·3, mean ± standard deviation). The follow-up period ranged from one day to 60 months (14·2 ± 13·5, mean ± standard deviation). Thirteen (23·6%) reported funding from a for-profit manufacturer of MSCs (i.e. Osiris Therapeutics, Inc., FCB-Pharmacell Company Limited, Celgene Cellular Therapeutics, etc.) [[20], [21], [22], [23],27,29,49,52,55,56,65,69,70].
Fig. 1.
Literature search and study inclusion. MSC= mesenchymal stem cell; *Figure is reflective of search data from 2012 to present and does not include search data from the prior publication, including the 8 RCTs previously included.
Table 1.
Characteristics of included RCTs.
| Source | Country | Patient Population (Sample Size) | Single-center vs multi-center (Number of centers) | Follow Up Duration (months) | Intervention | Control Comparison | Patients Evaluated (n (% male)) |
Age (years ± SD) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | ||||||||
| Cardiovascular | |||||||||||
| Chen et al., 2004 | PRC | Acute myocardial infarction (69) | Single-center | 6 | Autologous BM-MSCs | Saline, IC | 34 (94) | 35 (97) | 58 ± 7 | 57 ± 5 | |
| Chen et al., 2006 | PRC | Ischemic heart failure (45) | Single-center | 12 | Autologous BM-MSCs | Maximal medical therapy | 22 (88) | 23 (92) | 59 ± 7 | 57 ± 7 | |
| Chullikana et al., 2015 | India | Acute ST-elevation myocardial infarction (20) | Multi-center (4) | 24 | Unmatched allogeneic BM-MSCs | Multiple electrolytes (Plasma-lyte A), IV | 10 (100) | 10 (80) | 47·3 ± 12·1 | 47·8 ± 6·5 | |
| Gao et al., 2013 | PRC | Acute ST-elevation myocardial infarction (43) | Multi-center (4) | 24 | Autologous BM-MSCs | Routine therapy | 21 (100) | 22 (86) | 55·0 ± 1·6 | 58·6 ± 2·5 | |
| Gao et al., 2015 | PRC | Acute ST-elevation myocardial infarction (116) | Multi-center (11) | 18 | Unmatched allogeneic UC-MSCs | Saline with heparin, IC | 58 (95) | 58 (88) | 57·3 ± 1·3 | 56·7 ± 1·7 | |
| Hare et al., 2009 | USA | Acute myocardial infarction (53) | Multi-center (10) | 6 | Unmatched allogeneic BM-MSCs | Vehicle, IV | 34 (82) | 19 (79) | 59 ± 12 | 55 ± 10 | |
| Lee et al., 2014 | ROK | Acute myocardial infarction (58) | Multi-center (3) | 6 | Autologous BM-MSCs | Standard treatment | 30 (90) | 28 (89) | 53·9 ± 10·5 | 54·2 ± 7·7 | |
| Wang et al., 2006⁎⁎ | PRC | Idiopathic dilated cardiomyopathy (24) | Single-center | 6 | Autologous MSCs | Saline, IC | 12 (75) | 12 (67) | 54 ± 11 | 58 ± 11 | |
| Wang et al., 2014 | PRC | Acute myocardial infarction (58) | Single-center | 6 | Autologous BM-MSCs | Saline, IC | 28 (68) | 30 (53) | 58·0 ± 10·2 | 56·1 ± 9·8 | |
| Zhao et al., 2015 | PRC | Chronic systolic heart failure (59) | Single-center | 6 | Unmatched allogeneic UC-MSCs | No IC injection, only drug therapy alone | 30 (80) | 29 (66) | 52·9 ± 16·3 | 53·2 ± 11·5 | |
| Bartolucci et al., 2016/2017 | Chile | Stable heart failure (30) | Multi-center (2) | 12 | Unmatched allogeneic UC-MSCs | Placebo NR | 15 (80·0) | 15 (93·3) | 57·33 ± 10·05 | 57·20 ± 11·64 | |
| Xiao et al., 2017 | PRC | Dilated cardiomyopathy (37) | Single-center | 12 | Autologous BM-MSCs | Placebo (saline) | 17 (70·6) | 20 (70·0) | 51·6 ± 12·2 | 54·4 ± 11·6 | |
| Neurological | |||||||||||
| Ibrahim et al., 2016* | Malaysia | Acute middle cerebral artery stroke (17) | NR | 12 | Autologous BM-MSCs | Standard treatment | NR | NR | NR | NR | |
| Lee et al., 2008 | ROK | Multiple system atrophy (29) | Single-center | 12 | Autologous BM-MSCs | NR | 11 (73) | 18 (67) | 58 ± 7 | 57 ± 7 | |
| Lee et al., 2010 | ROK | Ischemic stroke (52) | Single-center | 60 | Autologous BM-MSCs | Rehabilitation alone | 16 (50) | 36 (72) | 64 ± 12 | 65 ± 15 | |
| Lee et al., 2012 | ROK | Multiple system atrophy (31) | Single-center | 12 | Autologous BM-MSCs | Saline, IV and IA | 14 (65) ⁎⁎⁎⁎ | 17 (63) ⁎⁎⁎⁎ | 56·1 ± 8·9 ⁎⁎⁎⁎ | 55·8 ± 6·1 ⁎⁎⁎⁎ | |
| Xie et al., 2007⁎⁎ | PRC | Spinal cord injury (24) | Single-center | 3 | Autologous BM-MSCs | Rehabilitation alone | 11 (81) | 13 (77) | (18–49) | (21–53) | |
| Xie et al., 2016 | PRC | Encephalopathy (22) | Single-center | 6 | Unmatched allogeneic UC-MSCs | Saline, IV | 12 (67) | 10 (60) | 58·0 ± 7·4 | 63·3 ± 6·11 | |
| Fernandez et al., 2018 | Spain | Secondary progressive multiple sclerosis (30) | Multi-center (2) | 12 | Autologous Adipose-MSCs | Placebo (Ringer's lactate) | 10 (40) ⁎⁎⁎⁎ | 11 (27) ⁎⁎⁎⁎ | 44·8 ± 8·0 ⁎⁎⁎⁎ | 46·3 ± 8·9 ⁎⁎⁎⁎ | |
| 9 (22) ⁎⁎⁎⁎ | 47·8 ± 9·7 ⁎⁎⁎⁎ | ||||||||||
| Tsang et al., 2017 | PRC | Chronic stroke/vegetative state (9) | Single-center | Day of or day after | Autologous BM-MSCs | Placebo (5% normal human albumin) | 5 (40) | 4 (75) | 52·8 (48–56) | 51·5 (41–59) | |
| Kim et al., 2018* | ROK | Cerebral infarction (12) | Single-center | 6 | Unmatched allogeneic UC-MSCS | Placebo | 8 (NR) | 4 (NR) | NR | NR | |
| Lublin et al., 2014 | USA/Canada | Multiple sclerosis (16) | Multi-center (8) | 12 | Placenta-derived mesenchymal-like cells | Placebo | 6 (33) | 4 (50) | 52·5 (41–58) | 47·5 (40–52) | |
| 6 (17) | 47·5 (36–56) | ||||||||||
| Oncological/Hematological | |||||||||||
| Gao et al., 2016 | PRC | Stem cell transplantation for hematologic malignancy (124) | Multi-center (5) | 51 (24–70) | Unmatched allogeneic UC-MSCs | Saline, IV | 62 (47) | 62 (48) | NR | NR | |
| Liu et al., 2011 | PRC | Stem cell transplantation for leukemia (55) | Single-center | 24 | Matched allogeneic BM-MSCs | Stem cell transplant alone | 27 (74) | 28 (68) | 30 (14–46) | 31.5 (12–48) | |
| Ning et al., 2008 | PRC | Stem cell transplantation for hematologic malignancy (25) | Single-center | 36 | Matched BM-MSCS | Stem cell transplant alone | 10 (90) | 15 (87) | 36 ± 11 | 39 ± 12 | |
| Kuzmina et al., 2012 | Russia | Recipients of allogeneic bone marrow transplants for hematological malignancies (37) | Single-center | 32 | Unmatched BM-MSCs | Standard aGVHD prophylaxis | 19 (42) | 18 (39) | 34 (20–63) | 29 (19–60) | |
| Shipounova et al., 2014 | Russia | Recipients of allogeneic bone marrow transplants for hematological malignancies (77) | Single-center | 60 | Matched BM-MSCS | Standard aGVHD prophylaxis | 39 (NR) | 38 (NR) | NR | NR | |
| Endocrine | |||||||||||
| Carlsson et al., 2015 | Sweden | Type 1 diabetes mellitus (18) | Single-center | 12 | Autologous BM-MSCs | Insulin-only treatment | 9 (89) | 9 (56) | 24 ± 2 | 27 ± 2 | |
| Hu et al., 2013 | PRC | Type 1 diabetes mellitus (29) | Single-center | 24 | Unmatched allogeneic UC-MSCs | Saline, IV | 15 (60) | 14 (57) | 17·6 ± 8·7 | 18·2 ± 7·9 | |
| Hu et al., 2016 | PRC | Type 2 diabetes mellitus (61) | Single-center | 36 | Unmatched allogeneic UC-MSCs | Saline, IV | 31 (55) | 30 (53) | 52·43 ± 4·88 | 53·21 ± 8·22 | |
| Skyler et al., 2015 | USA | Type 2 diabetes mellitus (61) | Multi-center (18) | 3 | Unmatched allogeneic BM-MPCs | Saline, IV | 15 (67) | 16 (75) | 57·7 ± 8·2 | 58·7 ± 7·3 | |
| 15 (60) | 55·3 ± 11·4 | ||||||||||
| 15 (60) | 57·2 ± 6·6 | ||||||||||
| Renal disease | |||||||||||
| Swaminathan et al., 2018 | USA | Patients undergoing cardiac surgery using cardiopulmonary bypass, who developed acute kidney insufficiency (135) | Multi-center (27) | 3 | Unmatched allogeneic BM-MSCs | Placebo | 67 (65·7) | 68 (82·4) | 65·6 ± 11·9 | 67·0 ± 9·9 | |
| Korotkov et al., 2018* | Belarus | Renal transplantation (NR) | Single-center | 7 days | Matched allogeneic MSCs | Standard treatment | NR | NR | NR | NR | |
| Sun et al., 2018 | PRC | Renal allograft (42) | Multi-center (3) | 12 | Unmatched allogeneic UC-MSCs | Standard treatment | 21 (67) | 21 (52) | 40·8 ± 9·2 | 47·1 ± 10·2 | |
| Liver disease | |||||||||||
| Suk et al., 2016 | ROK | Alcohol-related liver cirrhosis (68) | Multi-center (12) | 12 | Autologous BM-MSCs | Standard treatment | 21 (83) ⁎⁎⁎ | 24 (94) ⁎⁎⁎ | 53·1 ± 8·7 ⁎⁎⁎ | 53·7 ± 8·2 ⁎⁎⁎ | |
| 23 (89) ⁎⁎⁎ | 54·4 ± 7·9 ⁎⁎⁎ | ||||||||||
| Shi et al., 2012 | PRC | Acute-on-chronic liver failure (43) | Single-center | 18 | Unmatched allogeneic UC-MSCs | Placebo (saline) | 24 (83) | 19 (79) | 40 (24–59) | 45 (26–62) | |
| Salama et al., 2014 | Egypt | Post-HCV end-stage liver disease (40) | Multi-center (2) | 6 | Autologous BM-MSCs | Antiviral therapy (no hepatic artery infusion) | 20 (85) | 20 (80) | 50·27 ± 6·05 | 50·90 ± 7·23 | |
| Xu et al., 2014 | PRC | Hepatitis B virus-related liver cirrhosis (56) | Single-center | 6 | Autologous BM-MSCs | Standard care | 27 (65) ⁎⁎⁎ | 29 (58) ⁎⁎⁎ | 44 ± 12 ⁎⁎⁎ | 45 ± 10 ⁎⁎⁎ | |
| Lin et al., 2017 | PRC | Hepatitis B virus-related acute-on-chronic liver failure (110) | Single-center | 6 | Unmatched allogeneic BM-MSCS | Standard treatment | 56 (91·1) | 54 (98·2) | 40 ± 9.9 | 42.8 ± 8.4 | |
| Zhang et al., 2017 | PRC | Liver fibrosis induced by hepatolenticular degeneration (60) | Single-center | 3 | Autologous BM-MSCs | Standard treatment | 30 (53·3) | 30 (56·7) | 30·98 ± 11·25 | 32·1 ± 10·36 | |
| Shi et al., 2017 | PRC | First cadaveric liver transplantation (27) | Single-center | 6 | Unmatched allogeneic UC-MSCs | Standard treatment | 14 (92·9) | 13 (92·3) | 57 ± 12 | 55 ± 11 | |
| Respiratory | |||||||||||
| Weiss et al., 2013 | USA | Moderate to severe chronic obstructive pulmonary disease (62) | Multi-center (6) | 24 | Unmatched allogeneic BM-MSCs | Vehicle solution, IV | 30 (60) | 32 (56) | 68·1 ± 7·54 | 64·1 ± 8·76 | |
| Zheng et al., 2014 | PRC | Acute respiratory distress syndrome (12) | Single-center | 1 | Unmatched allogeneic adipose-MSCs | Saline, IV | 6 (100) | 6 (83) | 66·7 ± 20·4 | 69·8 ± 9·1 | |
| Matthay et al., 2018 | USA | Acute respiratory distress syndrome (60) | Multi-center (5) | 2 | Unmatched allogeneic BM-MSCs | Plasma-Lyte A, IV | 40 (58) | 20 (50) | 55 (17) | 55 (20) | |
| Infectious | |||||||||||
| Galstian et al., 2015/2016* | Russia | Patients with severe neutropenia and severe sepsis (30) | Single-center | 3 | Unmatched allogeneic BM-MSCs | Standard treatment | 15 (43) ⁎⁎⁎⁎⁎ | 15 (54) ⁎⁎⁎⁎⁎ | 48 (30–75) ⁎⁎⁎⁎⁎ | 55 (33–81) ⁎⁎⁎⁎⁎ | |
| Immune-deficient/auto-immune/inflammatory | |||||||||||
| Alvaro-Garcia et al., 2016 | Spain | Refractory rheumatoid arthritis (53) | Multi-center (18) | 6 | Unmatched allogeneic adipose-MSCs | Lactated Ringer's solution, IV | 20 (10) | 7 (14) | 54·15 ± 7·79 | 58·43 ± 14·25 | |
| 20 (10) | 57·40 ± 11·01 | ||||||||||
| 6 (0) | 50·33 ± 15·62 | ||||||||||
| Zhang et al., 2013 | PRC | HIV-1 infection (13) | Multi-center (NR) | 12 | Unmatched allogeneic UC-MSCs | Saline, IV | 7 (71) | 6 (83) | 30 (26–49) | 38 (19–55) | |
| Hu et al., 2016 | PRC | Ulcerative colitis (70) | Single-center | 24 | Unmatched allogeneic UC-MSCs | Saline, IV and IA | 34 (62) | 36 (61) | 42·9 ± 23·1 | 43·7 ± 28·7 | |
| Deng et al., 2017 | PRC | Systemic lupus erythematosus (18) | Single-center | 12 | Unmatched allogeneic UC-MSCs | Placebo | 12 (8) | 6 (0) | 29 ± 10 | 29 ± 7 | |
| Zhang et al., 2018 | PRC | Crohn's disease (82) | Single-center | 12 | Unmatched allogeneic UC-MSCS | Standard treatment | 41 (58.5) | 41 (63.4) | 34·3 (21–44) | 32·7 (20–41) | |
| Arturo et al., 2017* | Columbia | Crohn's disease (26) | Single-center | 6 | Autologous BM-MSCs | Standard treatment | NR | NR | NR | NR | |
| Panes et al., 2017* | Spain | Crohn's disease (131) | Multi-center (NR) | 12 | Unmatched allogeneic adipose-MSCs | Placebo | 70 (NR) | 61 (NR) | NR | NR | |
| Yang et al., 2018 | PRC | Rheumatoid arthritis (105) | Single-center | 11 | Unmatched allogeneic UC-MSCs | Placebo | 28 (25) | 53 (19) | 50·7 | 49·8 | |
| 24 (21) | 51·2 | ||||||||||
| Melmed et al., 2015 | USA | Crohn's disease (46) | Multi-center (NR) | 24 | Placenta-derived mesenchymal-like cells | Placebo | 8 (53) | 7 (44) | 35·3 ± 14·0 | 36·5 ± 7·3 | |
| 5 (33) | 36·2 ± 11·6 | ||||||||||
| Other | |||||||||||
| Tompkins et al., 2017 | USA | Frailty (30) | Single-center | 12 | Unmatched allogeneic BM-MSCs | Placebo | 10 (60) | 10 (60) | 75·0 ± 7·4 | 75·3 ± 6·8 | |
PRC= People's Republic of China; USA= United States of America; ROK= Republic of Korea; T= treatment; C= control; BM= bone marrow; UC= Umbilical cord; MSCs= mesenchymal stromal cells; MPCs= mesenchymal precursor cells; IA= intra-arterial; IC= intracoronary; IV= intravenous; NR= not reported.
Abstract form only.
Foreign language text only.
Age and gender demographic data reported only for patients that completed trial follow-up.
Age and gender demographic data reported for all patients randomized, not necessarily infused with study treatment.
Age and gender demographic data reported only in earlier abstract, not updated to current abstract.
Patient populations were diverse and included cardiovascular (12 trials, n = 612 patients) [21,[24], [25], [26], [27],29,37,40,42,44,49,58], neurological (10 trials, n = 242 patients) [[30], [31], [32],36,45,48,57,62,65,69], renal (three trials, n = 177 patients) [55,63,67], liver (seven trials, n = 404 patients) [35,43,47,53,54,59,66], respiratory (three trials, n = 134 patients) [18,23,68] and endocrine diseases (four trials, n = 169 patients) [22,28,39,50], hematological/oncological malignancies (five trials, n = 318 patients) [33,34,41,46,71], immune deficient or inflammatory conditions (nine trials, n = 544 patients) [20,38,51,52,60,61,64,70,72], general frailty (one trial, n = 30 patients) [56], and severe sepsis in severely neutropenic patients with hematologic malignancies (one trial, n = 30 patients) [19].
With respect to MSC preparation and administration, of the 55 included RCTs, 31 (56·4%) examined bone marrow [19,[21], [22], [23], [24], [25], [26], [27],[29], [30], [31], [32], [33], [34],36,39,[41], [42], [43],45,47,53,[55], [56], [57], [58], [59],61,66,68,71], 16 (29·1%) umbilical cord [28,35,38,40,44,46,[48], [49], [50], [51], [52],54,60,63,65,72], four (7·3%) adipose-derived MSCs [18,20,62,64], two (3·6%) placenta-derived cells [69,70]; and in two RCT (3·6%) the source of MSCs was unclear [37,67]. See Supplementary Table 1 for expanded detail. Twenty (36·4%) RCTs used autologous MSCs [[24], [25], [26],[29], [30], [31], [32],36,37,39,42,43,45,47,[57], [58], [59],61,62,66], 29 (52·7%) used third party unmatched allogeneic MSCs [[18], [19], [20], [21], [22], [23],27,28,35, 38,40,44,46,[48], [49], [50], [51], [52], [53], [54], [55], [56], 60,[63], [64], [65]], and four (7·3%) used allogeneic MSCs from matched donors [33,34,41,67]. Two (3·6%) RCTs used placenta-derived mesenchymal-like cells [69,70] and one (1·8%) RCT used mesenchymal precursor cells (MPC) rather than MSCs [22]. Twelve (21·8%) RCTs cultured the MSCs in a xeno-free medium [21,27,28,39,41,49,50, 52,55,58,59,71], whereas the remainder either used a xenogenic product (40·0%, n = 22) [18,22,23,25,26,[29], [30], [31], [32], [33], [34], [35],42,46,47,53,56,57,60,62, 66,68] or did not report the medium used (38·1%, n = 21) [19,20,24,[36], [37], [38],40,[43], [44], [45],48,51,54,61,[63], [64], [65],67,69,70,72]. Fifteen (27·3%) RCTs cryopreserved MSCs prior to administration [[19], [20], [21], [22], [23],27,30,39,52,53,55,56,60,69,70], 32 (58·2%) used fresh MSCs [18,[24], [25], [26],28,29,[31], [32], [33], [34], [35],38,40,[42], [43], [44],[46], [47], [48], [49], [50], [51],54,[57], [58], [59],[62], [63], [64],66,68,72], two (3·6%) used both a fresh and cryopreserved product [41,71] and in six (10·9%) it was unclear [36,37,45,61,65,67]. One trial that used both a fresh and cryopreserved product (1·8%) [71] and five of the 32 RCTs that used fresh MSCs (9·1%) used a cryopreserved cell product that was thawed and cultured prior to injection for a fresh cell product [18,47,49,68,72]. Of the 22 RCTs that reported cryopreserving their product, 14 (25·6%) used dimethyl sulfoxide as the cryoprotectant solution at a concentration of 10% or less [18,19,[21], [22], [23],27, 41,47,52,55,56,60,68,71]; the type cryoprotectant was unclear for the eight other RCTs (14·5%). Seven (12·7%) of the included RCTs reported all three Dominici criteria for MSC characterization [20,21,40, 41,68,71,72]. Twenty-nine (52·7%) RCTs reported on cell viability [[20], [21], [22], [23],25,26,[29], [30], [31], [32], [33],37,39,40,43,[47], [48], [49],52,[54], [55], [56], [57], [58],62,68,71,72] and eight (14·5%) reported on a measure of MSC potency [20,22,23,25, 29,47,62,68].
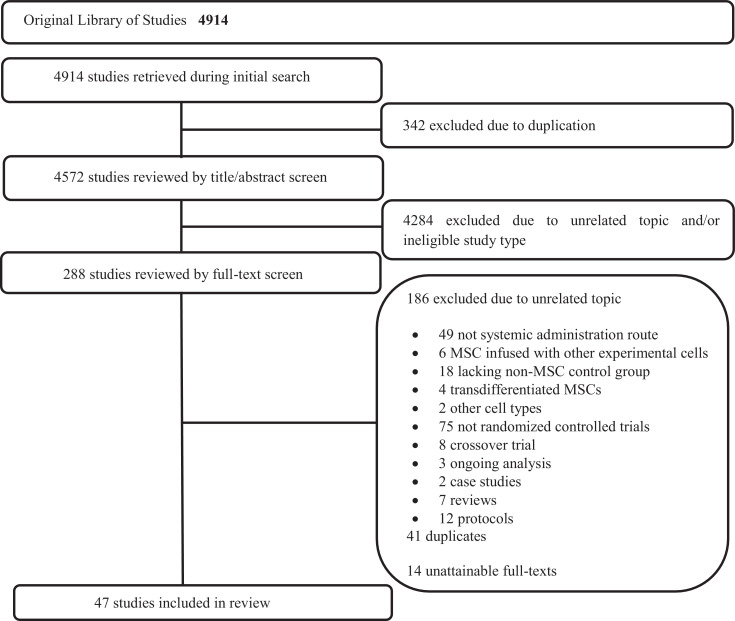
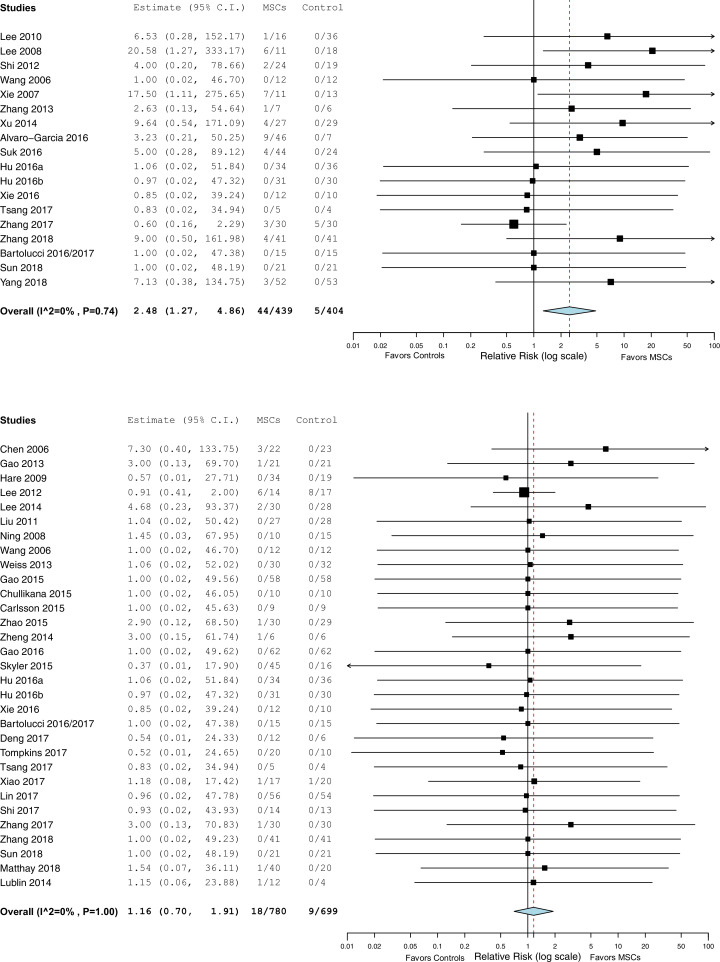
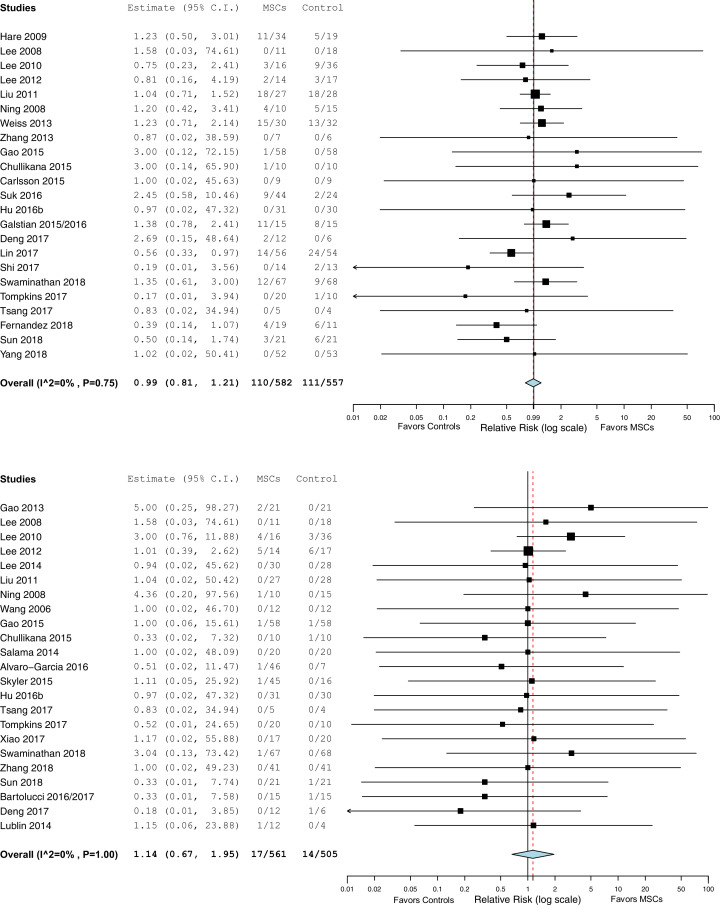
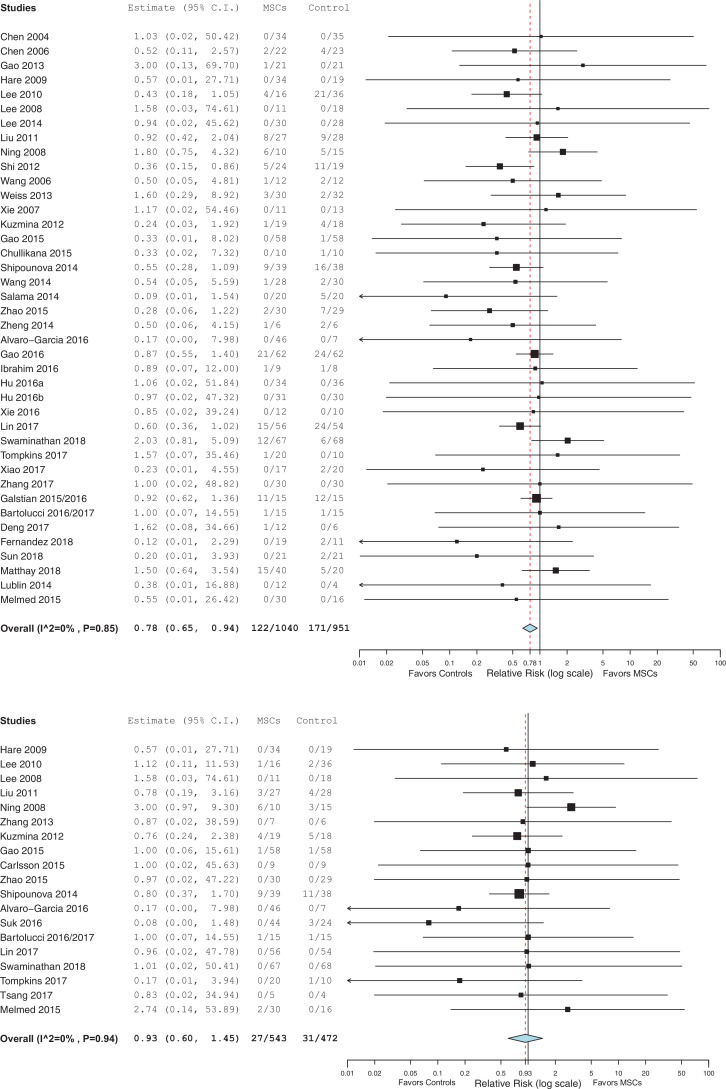
A description and frequency of the pre-specified incident adverse events defined in our systematic review (infusional toxicity: fever and non-fever, infection, thrombotic or thromboembolic events, death and malignancy) for each included RCT is provided in Supplementary Table 2 and a summary of pooled data presented as forrest plots for each pre-specified adverse event category are summarized in Figs. 2A–F.
Fig. 2.
a) Infusional toxicity: Fever. b) Infusional toxicity: Non-fever. c) Infection. d) Thrombotic/thrombo-embolic events. e) Mortality. f) Malignancy or ectopic tissue formation.
RR, relative risk; CI, confidence interval; MSC, mesenchymal stem cell.
With respect to the occurrence immediate adverse events, a total of 19 RCTs (n = 880 patients) reported on fever infusional toxicity [20,31,32,[35], [36], [37], [38],43,[47], [48], [49], [50], [51],57,59,60,63,71,72]. In the pooled analysis, the risk of fever was significantly greater in the MSC group as compared to the control group (Relative Risk (RR) =2·48, 95% Confidence Interval (CI) = 1·27–4·86, I2 = 0%; see Fig. 2a). Pooled analysis of reported non-fever infusional toxicity events in a total of 32 RCTs (n = 1525 patients) however did not reveal any significant increase in risk for the MSC as compared to the control group (RR = 1·16, 95% CI = 0·70–1·91, I2 = 0%; see Fig. 2b) [18,[21], [22], [23],[25], [26], [27],29,30, 33,34,37,39,40,44,46,[48], [49], [50], [51], [52], [53], [54],[56], [57], [58], [59], [60],63,[68], [69], [70]].
A total of 27 RCTs (n = 1315 patients) reported on infection [[19], [20], [21], [22], [23],27,[30], [31], [32], [33], [34],[38], [39], [40],47,50,[52], [53], [54], [55], [56], [57],62,63,69,70,72]. In the pooled analysis, there was no significant increase in the risk of infection for the MSC as compared to the control group (RR =0·99, 95% CI = 0·81–1·21, I2 = 0%; see Fig. 2c).
The occurrence of thrombotic or thrombo-embolic events were reported in a total of 24 RCTs (n = 1112 patients) [20,21,26,29,32,33, 37,40,50,56,58,60,63,66,69,70]. In the pooled analysis there was no significant increase in the risk of thrombotic/thrombo-embolic events for MSCs as compared to the control group (RR = 1·14, 95% CI = 0·67–1·95, I2 = 0%; see Fig. 2d).
A total of 40 (n = 1991 patients) and 19 (n = 1015 patients) RCTs reported on death [[18], [19], [20], [21],[23], [24], [25], [26], [27],29,[31], [32], [33], [34], [35], [36], [37],[40], [41], [42],[44], [45], [46],[48], [49], [50], [51], [52], [53],55,56, 58,59,62,63,66,[68], [69], [70], [71]] and malignancy and or ectopic tissue formation respectively [20,27,[31], [32], [33], [34],[38], [39], [40], [41],44,47,49,53, [55], [56], [57],70,71]. In the pooled analysis, the risk of death was significantly lower for the MSC group as compared to the control group (RR = 0·78, 95% CI = 0·65–0·94, I2 = 0%; see Fig. 2e). There was no significant increase found in the risk of malignancy or ectopic tissue formation for the MSC as compared to the control group (RR = 0·93, 95% CI = 0·60–1·45, I2 = 0%; see Fig. 2f).
The results of the risk of bias assessment found that only six (10·9%) RCTs fulfilled all six criteria for low risk of bias (Table 2) [23,27,30,40,49,62]. Nine (16·4%) RCTs met five of six primary criteria [21,28,32,46,50,52,56,68,69]. The allocation lists were concealed in 24 (43·6%) [[21], [22], [23],27,28,[30], [31], [32],39,40,46,47,49,50,52,53,55,56,59,60,62, 63,68,69]; 21 (38·1%) were double blinded [18,21,23,27,28,30,40,46, [49], [50], [51], [52],[55], [56], [57],62,64,65,[68], [69], [70]] and three (5·5%) had an open label intervention but blinded outcome measures [29,32,54]. In terms of other potential sources of bias, 35 (63·6%) of the RCTs were registered with either clinicaltrials.gov or their own regional registration program [[18], [19], [20], [21], [22], [23],26,29,30,35,[38], [39], [40], [41],43,[46], [47], [48], [49],[51], [52], [53], [54], [55], [56], [57],60,[62], [63], [64], [65], [66],68,70,72]. Thirty-eight (69·0%) RCTs did not report an a priori sample size calculation or provide a rationale for the sample size [18,19,21, [24], [25], [26], [27], [28],[31], [32], [33], [34], [35], [36], [37], [38], [39],41,42,44,45,[48], [49], [50], [51], [52],54,[57], [58], [59],61,[64], [65], [66], [67],69,71,72].
Table 2.
Risk of bias assessments (expanded detail provided in Supplementary Table 2).
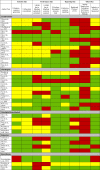 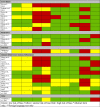
|
Sub-groups were meta-analyzed for the six pre-specified adverse event outcome categories and are summarized in Supplementary Table 3. Briefly, the risk of fever related acute infusional toxicity in the MSC group was increased in the neurological and immune/inflammatory populations, when unmatched allogeneic and autologous, bone marrow, umbilical, or fresh MSCs were administered, and when the MSC culture medium was xenogenic or unclear. The risk of death was significantly reduced in the MSC group in three clinical populations (cardiovascular, neurological, and liver disease), and with autologous, umbilical and freshly cultured MSCs. The sensitivity analysis which excluded RCTs published in abstract form only did not affect the strength or direction of the pre-specified pooled adverse event estimates (see Table 4).
Table 4.
Post-hoc sensitivity analysis of Safety Outcomes Reporting Findings in all studies versus only full-text publications.
| Safety Outcomes | All studies |
Only full-test publications |
||
|---|---|---|---|---|
| # of RCTs* | Findings (RR, 95% CI) | # of RCTs* | Findings (RR, 95% CI) | |
| Infection | 23/55 | 0·99 (0·81–1·21) | 22/55 | 0·94 (0·76–1·17) |
| Mortality | 40/55 | 0·78 (0·65–0·94) | 38/40 | 0·74 (0·60–0·92) |
That reported the adverse event.
A description of all reported serious adverse events (pre-specified and not pre-specified in our review) and their relatedness to study treatment in the MSC or control group is also provided in Supplementary Table 4. Of all reported serious adverse events (SAEs), a total of 1 SAE in the control group (ventricular tachycardia post-infusion in a trial that administered study drug intravenously) [21] and 3 SAEs in the MSC group (treatment related fever [47], in-stent thrombosis with death and acute coronary artery occlusion [26], the latter two of which were also associated with intra-coronary injection of study drug) were considered related to study treatment. Four other SAEs in the MSC group (grade 1 anaphylactoid reaction [69], gastric ulcer perforation [70], hypersensitivity reaction [70], and anal cancer [70]) were also judged to be possibly related to study treatment [70]. None of the included RCTs were ended prematurely due to safety concerns.
A total of 43 (78·2%) of the 55 RCTs reported an a priori plan to monitor safety [18,[20], [21], [22], [23],[26], [27], [28], [29], [30], [31], [32], [33], [34], [35],[38], [39], [40],[42], [43], [44],46,47,[49], [50], [51],53, [55], [56], [57], [58], [59], [60],62,63,65,[67], [68], [69], [70], [71], [72]]; 20 (36·4%) of the RCTs also reported an a priori plan to monitor for expected adverse events to be monitored [26,[29], [30], [31], [32],35,[38], [39], [40],42,46,49,50,53,[55], [56], [57], [58],63,68] (see Supplementary Table 4). Forty-five (81·8%) RCTs provided an a priori description of follow-up frequency for adverse events [18,[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35],[38], [39], [40], [42], [43], [44],[47], [48], [49], [50], [51], [52], [53], [55], [56], [57], [58], [59], [60],62,63,[65], [66], [67], [68], [69], [70],72].
When comparing the pre-specified adverse event profile from 2012 compared to our updated systematic review, the risk of fever is the safety outcome that remains significantly associated with MSC administration (see Table 3). There now appears to be a reduction in the risk of death in association with MSC therapy. In comparison to 2012, our updated review found that more of the included RCTs reported an a priori plan to monitor for the occurrence of adverse events (37·5% versus 78·2%, respectively).
Table 3.
Comparison of 2012 versus 2019 Safety Outcomes and Quality of Safety Reporting Findings.
| Safety Outcomes | 2012 SafeCell SR |
2018 SafeCell SR Update |
||
|---|---|---|---|---|
| # of RCTs* | Findings (RR, 95% CI) | # of RCTS* | Findings (RR, 95% CI) | |
| Infusional toxicity- non-fever | 4/8 | 2·01 (0·34–11·77) | 32/55 | 1·16 (0·70–1·91) |
| Infusional toxicity- fever | 4/8 | 9·28 (2·02–42·71) | 19/55 | 2·48 (1·27–4·86) |
| Infection | 4/8 | 1·09 (0·61–1·94) | 27/55 | 0·99 (0·81–1·21) |
| Malignancy or ectopic tissue formation | 4/8 | 2·21 (0·85–5·74) | 19/55 | 0·93 (0·60–1·45) |
| Mortality | 8/8 | 1·22 (0·71–2·10) | 40/55 | 0·78 (0·65–0·94) |
| Thrombotic or embolic events | 4/8⁎⁎ | 2·71 (0·86–8·48) | 24/55 | 1·14 (0·67–1·95) |
| Quality of Safety Reporting | # of RCTs* | Findings (%) | # of RCTs* | Findings (%) |
| A priori plan to monitor adverse events | 3/8 | 37·5% | 43/55 | 78·2% |
That reported the adverse event.
Original review did not report on this outcome, but event rate is inserted for comparison purposes.
Thirty-five (63·6%) of the 55 RCTs included at least one efficacy outcome as a primary endpoint [21,[24], [25], [26],[28], [29], [30], [31],35,36,38,39, [41], [42], [43], [44], [45], [46], [47],49,[52], [53], [54], [55],[57], [58], [59], [60], [61],63,64,66,67,70,71], where the remaining RCTs focused on safety alone. Of the 36 RCTs that reported efficacy outcomes, 23 (41·8%) found that MSCs were efficacious in at least one of the primary efficacy outcomes [24,[28], [29], [30], [31],35,38,43,44, 46,47,49,[52], [53], [54],[58], [59], [60],64,66,67,70,71]. A more detailed description of each RCT's primary and secondary endpoints and their respective findings is provided in Supplementary Table 5.
4. Discussion
In our updated systematic review that now includes over 40 additional RCTs and over 2000 additional patients, we continue to detect no associations between MSC treatment and the development of non-fever acute infusional toxicity, infection, or malignancy, nor did we detect associations between MSC treatment and the development of thrombotic or thrombo-embolic events. There does continue to be a significant association between MSC administration and reported fever. However of the 19 RCTs (n = 880 patients) that reported on fever, only six were reported as serious, albeit all in MSC treated patients. In contrast, with an increase in the number of RCTs and patients in our updated review, the risk of death is now significantly reduced in the MSC as compared to the control group. In our updated review we also found that the approach to safety reporting was improved as many more authors reported an a priori plan to monitor for safety (78·2% versus 35·5%) and none of the trials were ended pre-maturely due to safety concerns. The findings of our updated review should provide additional assurance to researchers, clinicians, regulators, and patients and families that the administration of MSCs continues to appear safe.
Our systematic review will require future updates as scientists continue to unravel the multitude of mechanisms of actions associated with the cells, as the sources and origins of MSCs expand, and the manufacturing process and the development of second generation MSC products evolve. To illustrate, recent in vitro, pre-clinical, and clinical data has found that MSCs can express or increase secretion of proteins associated with coagulation (ex: tissue factor, thrombin anti-thrombin complexes) and with reports of thromboses [10], [11], [12], [13], [14]. Depending on the clinical population this potential pro-coagulant effect could result in a beneficial or harmful clinical effect. In our updated review, we began to address this concern with the inclusion of thrombotic/thromboembolic events as a pre-specified adverse event category. Our findings suggest that these incident events reported in the included RCTs are rare (31 events in 24 RCTs and 1112 patients studied), were reported in both study groups (n = 17 and 14 in the MSC and control groups respectively) and were not significantly associated with administration of MSCs. Although a significant association was not detected, it is likely that these events will be rare and as such we encourage investigators to a priori plan to monitor and report on these events to enable the detection of future thrombotic safety signals.
In contrast to our review from 2012, we found that safety reporting was improved in that more investigators reported an a priori plan to monitor for adverse events (78·2% versus 35·5% respectively). Serious adverse events that were reported as related to or as possibly related to study treatment (either in the MSC or control group) (n = 8 out of 2634 patients studied) were very rare. This could be because these events are indeed rare or because it can be challenging if not impossible to attribute an event to study treatment, especially when the event does not occur during or shortly after completion of the infusion. To address this challenge in adverse event reporting, we sought to capture and synthesize pre-specified adverse events and any other SAE, irregardless of relatedness to study treatment in each of the RCTs. Even using this approach, safety signals other than fever generation were not detected.
A significant impediment to understanding whether MSCs are efficacious and safe relates to the quality of trial design and transparent reporting. Of the 55 included RCTs, only six trials met all six criteria for low risk of bias whereas none of the RCTs from the 2012 review met all six criteria. Although an improvement from 2012, it is important for investigators to address these risk of bias elements at the design phase of these clinical trials to maximize the internal validity of their research findings. With regard to MSC characterization, only seven trials reported on all three Dominici criteria [16] which aim to provide minimal and standardized criteria to define a MSC. Furthermore, only 29 of the included trials (52·7%) reported some measure of MSC viability during the manufacturing process and even fewer (n = 8, 14·5%) reported on a measure of MSC potency or functionality. We strongly assert that it is critical for investigators to transparently report on MSC characteristics, potency and viability in order to help readers, researchers, health regulators, and the community to better understand why a given trial may have succeeded or failed to meet study endpoints and with the ultimate aim to help move the field forward.
Our systematic review has several strengths. We included a transparent search strategy, pre-defined a set of adverse events that were clinically relevant to MSC administration, and reported on all SAEs that were and were not identified as part of our a priori event categories irregardless of relatedness to study treatment to provide the most comprehensive and up to date evaluation of the safety profile of MSC therapy. Our review also has limitations. Six of the RCTs were published in abstract form only and as such contained limited information to populate in our review. However, we included these trials so that the readership is aware of them and can further evaluate the efficacy and safety of study results when the full trials are published. Furthermore, the strength or direction of our pooled apriori adverse outcome estimates were not influenced by removal of studies that were published in abstract form only. As in our 2012 review, we pooled incident adverse events from RCTs from diverse adult clinical populations, MSC characteristics and MSC manufacturing in an effort to obtain signals for harm. However, to begin to address this diversity and due to the increased number of included RCTs in this review, we conducted several a prior derived sub group analyses to examine for heterogeneity in our a priori derived adverse event estimates and acknowledged that these analyses should be considered hypothesis generating. Only a few of the included trials (10·9%) met all six low risk of bias criteria which threatens the internal validity of the study findings from the perspectives of both safety and efficacy and we strongly encourage investigators to address these biases at the design stage and during the conduct of these RCTs. Finally, pooling efficacy outcomes for all of the included RCTs was not feasible within the scope of this safety review. However, in an attempt to provide some measure of efficacy information for the readership, we summarized the primary and secondary efficacy endpoints and associated results descriptively in Supplementary Table 5.
In conclusion, our review provides a systematic examination for incident adverse events related to the use of MSCs. Aside from fever, we did not identify any significant reported safety signals. Results from our systematic review provide further assurance to readers, investigators, health regulators, and our patients and communities that, with this updated evidence, MSC therapy continues to appear safe.
Declaration of Competing Interests
LM reports grants from CIHR, OIRM and SCN during the conduct of this study. DJS and SHJM reports affiliations with Northern Therapeutics Inc., outside the submitted work. BH reports prior honoraria from Cornerstone Research Group for the provision of methodologic advice related to systematic review and meta-analysis, outside the submitted work. KRW reported grants for CIHR, outside the submitted work. No other authors have any affiliations to report in relation to this study.
Acknowledgments
Our systematic review was funded by the Ontario Research Fund, the Ontario Institute for Regenerative Medicine and the Stem Cell Network; none of whom were involved in study design, in the collection and interpretation of data, in the writing of the report, nor in the decision to submit the paper for publication. Our research team would like to thank Ms. Risa Shorr (Medical Information Specialist) for her assistance with building and conducting the electronic search strategy. We would also like to thank Ms. Elham Sabri for her statistical expertise and Ms. Marnie Gordon for the administrative assistance that she provided.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2019.100249.
Appendix 1. Search strategy
Medline
Database: Ovid MEDLINE(R) ALL 〈1946 to September 23, 2019〉
Search Strategy:
-
1.
exp Mesenchymal Stem Cells/ (33,741)
-
2.
exp Mesenchymal Stem Cell Transplantation/ (10,675)
-
3.
exp Multipotent Stem Cells/ (36,140)
-
4.
exp Mesenchymal Stromal Cells/ (33,741)
-
5.
(mesenchymal adj3 (stem or stroma$1 or progenitor*) and cell$1).tw. (47,264)
-
6.
(mesenchymal adj2 (stem or stromal or progenitor or multipotent or bone marrow or adipose or placenta*)).tw. (47,000)
-
7.
(MSC or MSCs or ADMSC or ADMSCs or BM-MSC or BM-MSCs or BMD-MSC or BMD-MSCs or BMDMSC or BMDMSCs).tw. (29,267)
-
8.
((multipotent or multi-potent) adj3 (stroma$1 cell$1 or stem cell$1)).tw. (4521)
-
9.
marrow stroma$1 cell$1.tw. (6975)
-
10.
(colony-forming unit fibroblast* or CFU-F$1).tw. (844)
-
11.
Mesoderm/cy (5710)
-
12.
or/1-11 (71,482)
-
13.
(ae or to or po or co).fs. (3,812,354)
-
14.
(safe or safety).ti,ab. (723,468)
-
15.
side effect$.ti,ab. (236,090)
-
16.
((adverse or undesirable or harm* or serious or toxic) adj3 (effect* or reaction* or event* or outcome*)).ti,ab. (494,272)
-
17.
exp product surveillance, postmarketing/ (14,736)
-
18.
exp adverse drug reaction reporting systems/ (7274)
-
19.
exp clinical trials, phase iv/ (289)
-
20.
exp poisoning/ (154,008)
-
21.
exp substance-related disorders/ (269,073)
-
22.
exp drug toxicity/ (111,624)
-
23.
exp abnormalities, drug induced/ (14,457)
-
24.
exp drug monitoring/ (19,962)
-
25.
exp drug hypersensitivity/ (44,888)
-
26.
(toxicity or complication* or noxious or tolerability or hypersensitivity or abnormal*).ti,ab. (1,919,833)
-
27.
exp Postoperative Complications/ (525,174)
-
28.
exp Intraoperative Complications/ (51,079)
-
29.
or/13-28 (6,186,659)
-
30.
12 and 29 (10,413)
-
31.
randomized controlled trial.pt. (489,730)
-
32.
controlled clinical trial.pt. (93,263)
-
33.
randomized.ab. (454,901)
-
34.
placebo.ab. (200,750)
-
35.
drug therapy.fs. (2,140,942)
-
36.
randomly.ab. (318,366)
-
37.
trial.ab. (476,933)
-
38.
groups.ab. (1,955,339)
-
39.
(clinical trial* or multicenter study).pt. (747,085)
-
40.
or/31-39 (4,746,525)
-
41.
exp animals/ not humans/ (4,617,450)
-
42.
40 not 41 (4,135,721)
-
43.
30 and 42 (1815)
-
44.
limit 43 to yr="2012 -Current" (1346)
Cochrane
Search Name: McIntyre-Lauralynn-MSCS-Safety_2019-09-25
Date Run: 25/09/2019 19:04:03
Comment:
ID Search Hits
-
1.
MeSH descriptor: [Mesenchymal Stem Cells] explode all trees 97
-
2.
MeSH descriptor: [Mesenchymal Stem Cell Transplantation] explode all trees 183
-
3.
MeSH descriptor: [Multipotent Stem Cells] explode all trees 99
-
4.
(mesenchymal NEAR/3 (stem or stroma$1 or progenitor*)):ti,ab,kw 1250
-
5.
(mesenchymal NEAR/2 (stem or stromal or progenitor or multipotent or bone marrow or adipose or placenta*)):ti,ab,kw 1392
-
6.
(MSC or MSCs or ADMSC or ADMSCs or BM-MSC or BM-MSCs or BMD-MSC or BMD-MSCs or BMDMSC or BMDMSCs):ti,ab,kw 989
-
7.
(((multipotent or multi-potent) NEAR/3 (stroma$1 cell$1 or stem cell$1))):ti,ab,kw 29
-
8.
(marrow stroma* cell*):ti,ab,kw 281
-
9.
(colony-forming unit fibroblast*):ti,ab,kw 11
-
10.
(cfu f):ti,ab,kw 155
-
11.
(cfu fs):ti,ab,kw 2
-
12.
MeSH descriptor: [Mesoderm] this term only and with qualifier(s): [cytology - CY] 3
-
13.
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 1951
-
14.
MeSH descriptor: [] explode all trees and with qualifier(s): [adverse effects - AE, toxicity - TO, poisoning - PO, complications - CO] 167,040
-
15.
(safe or safety):ti,ab,kw 237,637
-
16.
(side effect*):ti,ab,kw 144,447
-
17.
(((adverse or undesirable or harm* or serious or toxic) NEAR/3 (effect* or reaction* or event* or outcome*))):ti,ab,kw 272,736
-
18.
MeSH descriptor: [Product Surveillance, Postmarketing] explode all trees 194
-
19.
MeSH descriptor: [Adverse Drug Reaction Reporting Systems] explode all trees 87
-
20.
MeSH descriptor: [Clinical Trial, Phase IV] explode all trees 0
-
21.
MeSH descriptor: [Poisons] explode all trees 31
-
22.
MeSH descriptor: [Substance-Related Disorders] explode all trees 13,818
-
23.
MeSH descriptor: [Drug-Related Side Effects and Adverse Reactions] explode all trees 3356MeSH descriptor: [Abnormalities, Drug-Induced] explode all trees 47
-
24.
MeSH descriptor: [Drug Monitoring] explode all trees 1685
-
25.
MeSH descriptor: [Drug Hypersensitivity] explode all trees 955
-
26.
(toxicity or complication* or noxious or tolerability or hypersensitivity or abnormal*):ti,ab,kw 291,059
-
27.
MeSH descriptor: [Postoperative Complications] explode all trees 37,166
-
28.
MeSH descriptor: [Intraoperative Complications] explode all trees 4123
-
29.
#14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR # 27 OR #28 OR #29 583,508
-
30.
#13 AND #30 1141
-
31.
Limit from 2012 – September 25, 2019 1044
Web of Science – January 31, 2018
5
169
#4 AND #3
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI Timespan=2017-2018
Edit
# 4
1207
((TS= ((Mesenchymal) OR (Multipotent Stem Cell*) OR (Stromal Cell*)))) AND DOCUMENT TYPES: (Meeting Abstract OR Proceedings Paper)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI Timespan=2017-2018
Edit
# 3
19,021
#2 OR #1
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI Timespan=2017-2018
Edit
# 2
13,741
((CF =((American Heart Association) OR (American College of Cardiology) OR (Transcatheter Cardiovascular Therapeutics) OR (European Society of Cardiology) OR (International Society for Heart Research) OR (World Congress of Cardiology) OR (Heart Failure Society of America) OR (American Society of Hematology) OR (American Society for Blood and Marrow) OR (European Bone Marrow Transplant) OR (International Society of Cellular Therapy) OR (International Society for Experimental Hematology) OR (European Respiratory Society) OR (American Thoracic Society) OR (American College Chest Physicians) OR (British Thoracic Society) OR (Canadian Respiratory Conference) OR (Asia-Pacific Society of Respirology) OR (International Union against Tuberculosis and Lung Disease) OR (Aspen Lung Conference) OR (American Academy of Neurology) OR (American Association of Neurologists) OR (European Federation of Neurological Societies) OR (World Federation of Neurology) OR (European Committee for Treatment and Research in Multiple Sclerosis) OR (Society of Critical Care Medicine) OR (International Symposium on Intensive Care and Emergency Medicine)))) AND DOCUMENT TYPES: (Meeting Abstract OR Proceedings Paper)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI Timespan=2017-2018
Edit
# 1
16,768
((Source = (Blood) OR Source = (Biology of Blood and Marrow Transplantation) OR Source = (Bone Marrow Transplantation) OR Source = (Cytotherapy) OR Source = (Experimental Hematology) OR Source = (Circulation) OR Source = (Journal of the American College of Cardiology) OR Source = (European Heart Journal) OR Source = (Journal of Molecular and Cellular Cardiology) OR Source = (Circulation) OR Source = (Journal of Cardiac Failure) OR Source = (European Respiratory Journal) OR Source = (American Journal of Respiratory and Critical Care Medicine) OR Source = (Chest) OR Source = (Thorax) OR Source = (Canadian Respiratory Journal) OR Source = (Respirology) OR Source = (International Journal of Tuberculosis and Lung Disease) OR Source = (Neurology) OR Source = (Annals of Neurology) OR Source = (European Journal of Neurology) OR Source = (European Journal of Neurology) OR Source = (Multiple Sclerosis) OR Source = (Critical Care Medicine) OR Source = (Critical Care))) AND DOCUMENT TYPES: (Meeting Abstract OR Proceedings Paper)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI Timespan=2017-2018
Appendix B. Supplementary materials
References
- 1.Friedenstein A.J., Petrakova K.V., Kurolesova A.I., Frolova G.P. Heterotopic transplants of bone marrow. Transplantation. 1968;6:17. [PubMed] [Google Scholar]
- 2.Caplan A.I. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthay M.A., Goolaerts A., Howard J.P., Lee J.W. Mesenchymal stem cells for acute lung injury: preclinical evidence. Crit Care Med. 2010;38:S569–S573. doi: 10.1097/CCM.0b013e3181f1ff1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mei S.H., McCarter S.D., Deng Y., Parker C.H., Liles W.C., Stewart D.J. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei S.H., Haitsma J.J., Dos Santos C.C. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 6.Boyle A.J., McNiece I.K., Hare J.M. Mesenchymal stem cell therapy for cardiac repair. Methods Mol Biol. 2010;660:65–84. doi: 10.1007/978-1-60761-705-1_5. [DOI] [PubMed] [Google Scholar]
- 7.Prockop D.J., Brenner M., Fibbe W.E. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12:576–578. doi: 10.3109/14653249.2010.507330. [DOI] [PubMed] [Google Scholar]
- 8.Lalu M.M., McIntyre L., Pugliese C. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS ONE. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 10.Hoogduijn M.J., de Witte S.F., Luk F. Effects of freeze-thawing and intravenous infusion on mesenchymal stromal cell gene expression. Stem Cells Dev. 2016;25:586–597. doi: 10.1089/scd.2015.0329. [DOI] [PubMed] [Google Scholar]
- 11.Moll G., Rasmusson-Duprez I., von Bahr L. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells. 2012;30:1565–1574. doi: 10.1002/stem.1111. [DOI] [PubMed] [Google Scholar]
- 12.Tan L., Huang Y., Pan X. Administration of bone marrow stromal cells in sepsis attenuates sepsis-related coagulopathy. Ann Med. 2016;48:235–245. doi: 10.3109/07853890.2016.1157725. [DOI] [PubMed] [Google Scholar]
- 13.Moll G., Ankrum J.A., Kamhieh-Milz J. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25:149–163. doi: 10.1016/j.molmed.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Perlee D., van Vught L.A., Scicluna B.P. Intravenous infusion of human adipose mesenchymal stem cells modifies the host response to lipopolysaccharide in humans: a randomized, single-blind, parallel group, placebo controlled trial. Stem Cells. 2018;36:1778–1788. doi: 10.1002/stem.2891. [DOI] [PubMed] [Google Scholar]
- 15.Ioannidis J.P., Evans S.J., Gotzsche P.C. Better reporting of harms in randomized trials: an extension of the consort statement. Ann Intern Med. 2004;141:781–788. doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- 16.Dominici M., Le Blanc K., Mueller I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 17.Higgins L., Green S. England: John Wiley & Sons; West Sussex: 2011. Cochrane handbook for systematic reviews of interventions. 5.1.0 ed. [Google Scholar]
- 18.Zheng G., Huang L., Tong H. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galstyan G., Makarova P., Parovichnikova E.N. Administration of multipotent mesenchymal stromal cells (MSC) improves short term but not long term survival in oncohematological neutropenic patients (PTS) with septic shock (SS) Intensive Care Med Exp. 2016;4 [Google Scholar]
- 20.Alvaro-Gracia J.M., Jover J.A., Garcia-Vicuna R. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 2017;76:196–202. doi: 10.1136/annrheumdis-2015-208918. [DOI] [PubMed] [Google Scholar]
- 21.Chullikana A., Majumdar A.S., Gottipamula S. Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy. 2015;17:250–261. doi: 10.1016/j.jcyt.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Skyler J.S., Fonseca V.A., Segal K.R., Rosenstock J., Investigators M.-.D. Allogeneic mesenchymal precursor cells in type 2 diabetes: a randomized, placebo-controlled, dose-escalation safety and tolerability pilot study. Diabetes Care. 2015;38:1742–1749. doi: 10.2337/dc14-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss D.J., Casaburi R., Flannery R., LeRoux-Williams M., Tashkin D.P. A placebo-controlled, randomized trial of mesenchymal stem cells in copd. Chest. 2013;143:1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S.L., Fang W.W., Qian J. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl) 2004;117:1443–1448. [PubMed] [Google Scholar]
- 25.Chen S., Liu Z., Tian N. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol. 2006;18:4. [PubMed] [Google Scholar]
- 26.Gao L.R., Pei X.T., Ding Q.A. A critical challenge: dosage-related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int J Cardiol. 2013;168:3191–3199. doi: 10.1016/j.ijcard.2013.04.112. [DOI] [PubMed] [Google Scholar]
- 27.Hare J.M., Traverse J.H., Henry T.D. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J., Yu X., Wang Z. Long term effects of the implantation of Wharton's jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J. 2013;60:347–357. doi: 10.1507/endocrj.ej12-0343. [DOI] [PubMed] [Google Scholar]
- 29.Lee J.W., Lee S.H., Youn Y.J. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014;29:23–31. doi: 10.3346/jkms.2014.29.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee P.H., Lee J.E., Kim H.S. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72:32–40. doi: 10.1002/ana.23612. [DOI] [PubMed] [Google Scholar]
- 31.Lee P.H., Kim J.W., Bang O.Y., Ahn Y.H., Joo I.S., Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008;83:723–730. doi: 10.1038/sj.clpt.6100386. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.S., Hong J.M., Moon G.J. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 33.Liu K., Chen Y., Zeng Y. Coinfusion of mesenchymal stromal cells facilitates platelet recovery without increasing leukemia recurrence in haploidentical hematopoietic stem cell transplantation: a randomized, controlled clinical study. Stem Cells Dev. 2011;20:1679–1685. doi: 10.1089/scd.2010.0447. [DOI] [PubMed] [Google Scholar]
- 34.Ning H., Yang F., Jiang M. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22:593–599. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- 35.Shi M., Zhang Z., Xu R. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725–731. doi: 10.5966/sctm.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie Z.W., Cui G.X., Li Y.Z. Curative effect of autologous mesenchymal stem cell transplantation on spinal cord injury. J Clin Rehabil Tissue Eng Res. 2007;11:2. [Google Scholar]
- 37.Wang J.A., Xie X.J., He H. [A prospective, randomized, controlled trial of autologous mesenchymal stem cells transplantation for dilated cardiomyopathy] Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34:107–110. [PubMed] [Google Scholar]
- 38.Zhang Z., Fu J., Xu X. Safety and immunological responses to human mesenchymal stem cell therapy in difficult-to-treat HIV-1-infected patients. AIDS. 2013;27:1283–1293. doi: 10.1097/QAD.0b013e32835fab77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlsson P.O., Schwarcz E., Korsgren O., Le Blanc K. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64:587–592. doi: 10.2337/db14-0656. [DOI] [PubMed] [Google Scholar]
- 40.Gao L.R., Chen Y., Zhang N.K. Intracoronary infusion of Wharton's jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015;13:162. doi: 10.1186/s12916-015-0399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shipounova I.N., Petinati N.A., Bigildeev A.E. Analysis of results of acute graft-versus-host disease prophylaxis with donor multipotent mesenchymal stromal cells in patients with hemoblastoses after allogeneic bone marrow transplantation. Biochemistry (Mosc) 2014;79:1363–1370. doi: 10.1134/S0006297914120104. [DOI] [PubMed] [Google Scholar]
- 42.Wang X., Xi W.C., Wang F. The beneficial effects of intracoronary autologous bone marrow stem cell transfer as an adjunct to percutaneous coronary intervention in patients with acute myocardial infarction. Biotechnol Lett. 2014;36:2163–2168. doi: 10.1007/s10529-014-1589-z. [DOI] [PubMed] [Google Scholar]
- 43.Xu L., Gong Y., Wang B. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol. 2014;29:1620–1628. doi: 10.1111/jgh.12653. [DOI] [PubMed] [Google Scholar]
- 44.Zhao X.F., Xu Y., Zhu Z.Y., Gao C.Y., Shi Y.N. Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet Mol Res. 2015;14:3010–3017. doi: 10.4238/2015.April.10.11. [DOI] [PubMed] [Google Scholar]
- 45.Ibrahim N.M., Tan H., Chin S., Law Z. BM-MSC accelerates acute stroke recovery in a randomized placebo-controlled clinical phase II/III study. Cytotherapy. 2016;18 [Google Scholar]
- 46.Gao L., Zhang Y., Hu B. Phase ii multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-Haploidentical stem-cell transplantation. J Clin Oncol. 2016;34:2843–2850. doi: 10.1200/JCO.2015.65.3642. [DOI] [PubMed] [Google Scholar]
- 47.Suk K.T., Yoon J.H., Kim M.Y. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: phase 2 trial. Hepatology. 2016;64:2185–2197. doi: 10.1002/hep.28693. [DOI] [PubMed] [Google Scholar]
- 48.Xie B., Gu P., Wang W. Therapeutic effects of human umbilical cord mesenchymal stem cells transplantation on hypoxic ischemic encephalopathy. Am J Transl Res. 2016;8:3241–3250. [PMC free article] [PubMed] [Google Scholar]
- 49.Bartolucci J., Verdugo F.J., Gonzalez P.L. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [Randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]) Circ Res. 2017;121:1192–1204. doi: 10.1161/CIRCRESAHA.117.310712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu J., Wang Y., Gong H. Long term effect and safety of wharton's jelly-derived mesenchymal stem cells on type 2 diabetes. Exp Ther Med. 2016;12:1857–1866. doi: 10.3892/etm.2016.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu J., Zhao G., Zhang L. Safety and therapeutic effect of mesenchymal stem cell infusion on moderate to severe ulcerative colitis. Exp Ther Med. 2016;12:2983–2989. doi: 10.3892/etm.2016.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng D., Zhang P., Guo Y., Lim T.O. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann Rheum Dis. 2017;76:1436–1439. doi: 10.1136/annrheumdis-2017-211073. [DOI] [PubMed] [Google Scholar]
- 53.Lin B.L., Chen J.F., Qiu W.H. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology. 2017;66:209–219. doi: 10.1002/hep.29189. [DOI] [PubMed] [Google Scholar]
- 54.Shi M., Liu Z., Wang Y. A pilot study of mesenchymal stem cell therapy for acute liver allograft rejection. Stem Cells Transl Med. 2017;6:2053–2061. doi: 10.1002/sctm.17-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swaminathan M., Stafford-Smith M., Chertow G.M. Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J Am Soc Nephrol. 2018;29:260–267. doi: 10.1681/ASN.2016101150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tompkins B.A., DiFede D.L., Khan A. Allogeneic mesenchymal stem cells ameliorate aging frailty: a phase II randomized, double-blind, placebo-controlled clinical trial. J Gerontol A Biol Sci Med Sci. 2017;72:1513–1522. doi: 10.1093/gerona/glx137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsang K.S., Ng C.P.S., Zhu X.L. Phase I/Ii randomized controlled trial of autologous bone marrow-derived mesenchymal stem cell therapy for chronic stroke. World J Stem Cells. 2017;9:133–143. doi: 10.4252/wjsc.v9.i8.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao W., Guo S., Gao C. A randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. Int Heart J. 2017;58:238–244. doi: 10.1536/ihj.16-328. [DOI] [PubMed] [Google Scholar]
- 59.Zhang D. A clinical study of bone mesenchymal stem cells for the treatment of hepatic fibrosis induced by hepatolenticular degeneration. Genet Mol Res. 2017;16 doi: 10.4238/gmr16019352. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J., Lv S., Liu X., Song B., Shi L. Umbilical cord mesenchymal stem cell treatment for crohn's disease: a randomized controlled clinical trial. Gut Liver. 2018;12:73–78. doi: 10.5009/gnl17035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arturo J., Lucena C., Perez C. Efficacy of autologous expanded bone marrow derived mesenchymal stem cells (axBM-MSC) in the treatment of patients with chron's disease who have failed to standard therapy: an open-label, randomized controlled clinical trial phase II. Cytotherapy. 2017;19 [Google Scholar]
- 62.Fernandez O., Izquierdo G., Fernandez V. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: a triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0195891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun Q., Huang Z., Han F. Allogeneic mesenchymal stem cells as induction therapy are safe and feasible in renal allografts: pilot results of a multicenter randomized controlled trial. J Transl Med. 2018;16:52. doi: 10.1186/s12967-018-1422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panes J., Garcia-Olmo D., Van Assche G. OP009 long-term efficacy and safety of Cx601, allogeneic expanded adipose-derived mesenchymal stem cells, for complex perianal fistulas in Crohn's disease: 52-week results of a phase III randomised controlled trial. J Crohn's Colitis. 2017;11 [Google Scholar]
- 65.Kim O.J. Abstract TP320: A Randomized, Double-blind, placebo-controlled, phase I/IIa clinical trial for evaluation of the safety and potential therapeutic effects after intravenous transplantation of human umbilical cord-derived mesenchymal stem cells in patients with cerebral infarction. Stroke. 2018;49 [Google Scholar]
- 66.Salama H., Zekri A.R., Medhat E. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther. 2014;5:70. doi: 10.1186/scrt459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korotkov S., Nosik A., Koritko A. The results of pilot study of the mesenchymal stem cells efficiency for induction of immunosuppressive therapy in the early postoperative period after kidney transplantation. Transpl. 2018;102:S207. [Google Scholar]
- 68.Matthay M.A., Calfee C.S., Zhuo H. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lublin F.D., Bowen J.D., Huddlestone J. Human placenta-derived cells (PDA-001) for the treatment of adults with multiple sclerosis: a randomized, placebo-controlled, multiple-dose study. Mult Scler Relat Disord. 2014;3:696–704. doi: 10.1016/j.msard.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Melmed G.Y., Pandak W.M., Casey K. Human placenta-derived cells (PDA-001) for the treatment of moderate-to-severe crohn's disease: a phase 1b/2a study. Inflamm Bowel Dis. 2015;21:1809–1816. doi: 10.1097/MIB.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 71.Kuzmina L.A., Petinati N.A., Parovichnikova E.N. Multipotent mesenchymal stromal cells for the prophylaxis of acute graft-versus-host disease-a phase II study. Stem Cells Int. 2012;2012 doi: 10.1155/2012/968213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Y., He X., Zhao R. Serum IFN-gamma levels predict the therapeutic effect of mesenchymal stem cell transplantation in active rheumatoid arthritis. J Transl Med. 2018;16:165. doi: 10.1186/s12967-018-1541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.