Abstract
Most of concrete structural failures are attributed to poor workmanship and poor engineering designs. Some microorganisms present in sewer systems can degrade the concrete and/or mortar. Concrete failures due to microbial attack has not attracted much attention especially in developing countries such as Kenya. This study investigated the effect of Thiobacillus intermedius bacteria on the performance of Ordinary Portland Cement (OPC). Preparation of test mortar prisms was done using the bacterial solution as either mix water, curing water or both. The control mortar prisms were prepared and cured in distilled water. Compressive strength test was done after 7th, 28th, 56th and 90th days of curing respectively. Results showed significant drop in compressive strength for the mortar prisms prepared and cured in bacterial solution as compared to the control mortar samples. Soundness and normal consistency increased significantly for the bacterial treated cement paste as compared to the control sample. Scanning Electron Microscopy (SEM) analysis showed severe damage on the bacterial treated cement mortar. This was characterized by formation of deleterious expansive products like ettringite and gypsum. Control mortar sample exhibited even formation of hydration products within the pore system.
Keywords: Bioengineering, Civil engineering, Concrete, Degradation, Microorganisms, Bacteria
Bioengineering; Civil engineering; Concrete; Degradation; Microorganisms; Bacteria
1. Introduction
Concrete/mortar has been classified as the second most used material on earth after water [1]. It is the most preferred construction material due to its unique properties such as high compressive strength and its flexibility to be applied in a wide range of environments. However, during its service life, concrete structures are subject to degradation/failures. While most of the concrete failures/degradation has been associated with ingress of aggressive agents such as carbon dioxide, chloride and sulphate ions [2, 3, 4], it is important to consider the contribution of microorganisms in degradation of concrete structures.
Concrete structures placed in chemically aggressive environments such as sewer systems are susceptible to microbial degradation. Microorganisms present in sewer systems secret corrosive metabolites that are chemically aggressive to the concrete structures. This form of concrete degradation is referred to as microbial induced concrete corrosion (MICC). The chemicals produced by the microorganisms present in such environments damage the microstructure of the concrete/mortar resulting to reduced service life of the structure [5].
The structural failures of the sewer systems associated with MICC forms part of the key problem of the subsurface infrastructure due to increased cost of repair [6, 7, 8]. Apart from interfering with the durability and the integrity of the concrete structures, microbial degradation has a negative impact on environmental health and safety due to production of harmful gases such as hydrogen sulphide [9]. According to Alexandra, 2014 [8], the main microorganisms involved in biodeterioration of concrete are bacteria and fungi. The metabolic processes of bacteria and fungi produce biogenic carboxylic acids in agro-industrial conditions that adversely impairs concrete floors and storage structures [5].
The range of acidic media capable of reacting with cement-based materials is comprehensive. It includes gaseous pollutants such as carbon dioxide, sulphur dioxide and nitrogen oxides, Organic acids such as lactic or acetic acid and mineral acids such as hydrochloric acid and/or sulphuric acid [10, 11, 12]. Since microorganisms have the ability to constantly discharge either organic or inorganic acids, their effect becomes more intense than a one-time application of acid on the surface of the concrete [13, 14].
Sulphur oxidizing bacteria such Acidithiobacillus species has been identified as corrosive agents in sewer collection systems. These microorganisms have the ability to produce biogenic sulphuric acid through their microbial activities [15]. The produced acid induces attack on the concrete surface by reacting with hydration products such as calcium hydroxide (CH) and calcium silicate hydrate (CSH) to form expansive products like gypsum and ettringite. As a result, internal cracking occurs due to increased volume and pressure. This leads to failure of such concrete structures [16].
Assessment of concrete/and or mortar durability is of great significance for the structural integrity. While a lot of studies have focused on the ingress of aggressive materials such as carbon dioxide, sulphate and chloride ions into concrete structures, little attention has been given to effects associated with microbial activities. Bettina Huber et al., 2016 [15] in their work showed that the concrete deterioration as a result of biogenic sulphuric acid was comparable to the chemical sulfuric acid. Cleary, it implies that some microorganisms have the ability to degrade concrete surfaces. This paper presents a laboratory simulated analysis conducted to investigate the effect of Thiobacillus intermedius on compressive strength, setting time, soundness and sorptivity of ordinary portland cement. While this work focused only on OPC mortar, the authors are looking into further research on the influence of the Thiobacillus intermedius on pozzolanic and slag based cements in sewage set up systems.
2. Materials and methods
2.1. Materials
Ordinary Portland cement of the grade 42.5 conforming to Kenya Standard KS EAS 18:1-2017 [17] and ISO standard sand conforming to EN196-1 [18] were used. The chemical composition of the cement used was as presented in Table 1. Chemicals of analytical grade (AR) were used to prepare the culture media and were purchased from Kobian Scientific Kenya Limited. The chemicals included Ammonium sulphate, (NH4)2SO4, Dipotassium phosphate, K2HPO4, Calcium Chloride, CaCl2, Agar, Manganese (II) sulphate monohydrate, MnSO4.H2O, Sodium thiosulfate pentahydrate, Na2S2O3.5H2O, Magnesium sulphate heptahydrate, MgSO4.7H2O, Iron (III) chloride hexahydrate, FeCl3.6H2O. Distilled water was used to prepare the medium solution. Thiobacillus intermedius bacteria (DSM18155) was obtained from Leibniz-Institut DSMZ Deutsche Sammlung Von Mikroorganismen und Zellkulture GmbH in Germany.
Table 1.
Composition of OPC test cement grade 42.5
| Test Parameter | Result in % |
|---|---|
| Al2O3 | 5.2 |
| SiO2 | 21 |
| Fe2O3 | 3.2 |
| CaO | 64.5 |
| MgO | 1.2 |
| SO3 | 2.2 |
| K2O | 0.25 |
| Na2O | 0.2 |
| Free CaO | 0.6 |
| IR | 1.35 |
| LOI | 0.3 |
2.2. Culturing of Thiobacillus intermedius
Pure spores of Thiobacillus intermedius was cultured at the microbiology laboratory of university of Embu, Kenya. Liquid culture media for the bacteria was prepared in accordance with the supplier's manual. Distilled water of pH 7 and conductivity of 5.5 μS/m was prepared using industrial laboratory distiller model A4000D- Stuart Aquatron as per the supplier's manual. Specific quantities of each of the analytical grade materials were accurately weighed and dissolved in a 1000 mL distilled water as shown in Table 2. The resultant pH of the mixture was adjusted to 6.6 by using a mixture of sodium carbonate (Na2CO3) and sodium bicarbonate (NaHCO3) to conform to the recommended optimal growth pH of the bacterium [19]. Autoclaving was done to sterilize the solution at a temperature of 125 °C. The solution was cooled to a room temperature and the spore of Thiobacillus intermedius introduced followed by 10.0 g of sodium thiosulphate and 12.0g of agar as the source of nutrients to the bacteria. Incubation of the bacteria was done in a shaker maintained at 30 °C for a period of 7 days. Concentration of the bacterial solution was maintained at 1.0 × 107 mL/cell. The obtained bacterial solution was used for mixing cement mortar as well as curing the prepared prisms (see Table 3).
Table 2.
Composition of Liquid Culture media for DSM 18155.
| Reagent | Amount in grams |
|---|---|
| (NH4)2SO4 | 0.1 |
| K2HPO4 | 4.0 |
| K2HPO4 | 4.0 |
| MgSO4.7H2O | 0.1 |
| CaCl2 | 0.1 |
| FeCl3.6H2O | 0.0 |
| MnSO4.H2O | 0.0 |
| Agar | 12.0 |
| Na2S2O3.5H2O | 10.0 |
Table 3.
Setting Time, Normal consistency and Soundness Test Results.
| Setting Time (Minutes) |
Normal Consistency (%) | Soundness (mm) | ||
|---|---|---|---|---|
| IST | FST | |||
| OPC H2O (H2O) | 100 | 180 | 28 | 1 |
| OPC TI (TI) | 130 | 230 | 28.4 | 3.2 |
2.3. Casting/mortar preparation
Mortar prisms were casted in moulds of size 40 mm × 40 mm x 160 mm as described in KS EAS 148:1-2000 [20]. Three sets of test were conducted. The first set was the control sample which was prepared and cured in distilled water. The sample was labelled as OPC H2O (H2O). The second set of test samples involved the use of bacterial solution as mix water and distilled water as the curing media. The sample was labelled as OPC TI (H2O). The third set of test samples involved the use of bacteria solution both as the mix water and the curing media. The sample was labelled as OPC TI (TI). In all the sets, water cement ratio (w/c) of 0.5 was adopted. After casting, the test prisms were placed in a humidity cabinet set at 20°C ± 2 °C with relative humidity above 95% for 24 h. Demoulding was done and the prisms placed in respective curing media maintained at 25°C ± 1 °C.
2.4. Determination of compressive strength
The compressive strength of the cured mortar prisms was determined in accordance with KS EAS 148:1-2000 [20] at 7th, 28th, 56th, and 90th day of curing respectively. The compressive strength machine model YAW-300 was used. The obtained strength was calculated and expressed in Mpa.
2.5. Scanning electron microscopy (SEM) analysis
Scanning electron microscopy analysis was determined for each set of the test samples after 28th day of curing. The SEM model used was Zeiss Ultra Plug FEG-SEM. Mortar samples obtained from OPC H2O (H2O), OPC TI (H2O) and OPC TI (TI) were prepared for SEM analysis as described in Scrivener et al., 2017 [21] guide for microstructural analysis of cementitious materials. Isopropanol alcohol was used to stop the hydration while resin of the type ERL-4206 was used to impregnate the hardened cement mortar.
2.6. Normal consistency, setting time and soundness
KS EAS 148:3-2000 [22] methods were adopted to carry out normal consistency, setting time and soundness tests. Cement pastes were separately prepared using bacterial solution and distilled water respectively. The cement paste prepared using bacterial solution was labelled as OPC TI (TI) while the control cement paste was labelled as OPC H2O (H2O). Normal consistency was determined and expressed in percentage whereas setting time was expressed in minutes and soundness was calculated and expressed in millimeters (mm).
2.7. Sorptivity testing
Vimal, 2009 [23] defined sorptivity as the function of the increase in mass of test sample as a result of water absorption relative to the time that one surface is exposed to water. ASTM C 1585-04 [24] method was used to carry out sorptivity test. The aim was to investigate the rate of water absorption by both microbial and control mortar prisms. The test mortar prisms cured for 28 days were dried in an oven set at 100 °C for 24 h. Each test prism was submerged in water at a level height of 5mm above the base of test mortar prisms with the 40 mm × 40 mm facing downwards at a regular intervals of 0.25 h, 0.5 h, 1 h, 1.5 h, 3 h, 5 h, 8 h, 24 h, 72 h, 96 h, 120 h, 144 h and 168 h. Each mortar prism was removed and new weight determined after drying the submerged surface with a clean towel. This was repeated up to 168th hour when the saturation of water infiltration was reached. The sorptivity coefficient (k) was determined using Eq. (1) below. The values of k were obtained graphically by plotting Q/A against the square root of time [23].
| (1) |
Q represents the amount of water absorbed in grams, A is the cross-section of the specimen in contact with water in cm2 and t is time of exposure in hours.
3. Results and discussion
3.1. Scanning Electron Microscopy (SEM)
SEM test results for OPC H2O (H2O), OPC TI (H2O) and OPC TI (TI) are as represented in Figures 1, 2, and 3 below for SEM images (a), (b) and (c) respectively. The test samples were exposed in bacteria solution for a period of 28 days. These SEM micrographs compared very well with other researcher's works already published [25, 26, 27, 28].
Figure 1.
SEM image (a) OPC H2O (H2O).
Figure 2.
SEM image (b) OPC TI (H2O).
Figure 3.
SEM (c) OPC TI (TI).
OPC H2O (H2O) as shown in Figure 1 was dominated by high levels of hexagonal calcium hydroxide (CH) plates and calcium silicate hydrates (C-S-H) as the main hydration products. These hydration phases are filled in pore spaces initially occupied by water and unhydrated cement grains. The formed ettringite (Aft) could be as a result of the sulphate content from gypsum added to control the setting of the cement. There was no evidence of any crack formation in the control sample since the hydration products were compact and well filled in the pore system of the hydrated cement mortar.
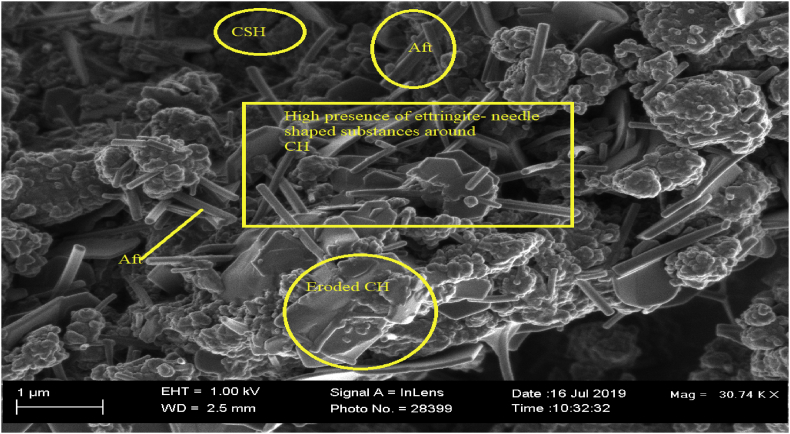
OPC TI (H2O), as shown in Figure 2, was dominated by high levels of ettringite (Aft) crystal formation. This was attributed to ingress of sulphates from the microbial activity. Sand and Bock, 1984 [29] and Diercks et al., 1991 [30] identified sulphur oxidizing bacteria present in sewage systems as accountable for production of biogenic sulphuric acid. Sulphuric acid when present in a cementitious material triggers dissolution and formation of expansive products like ettringite and gypsum. As described by Gutberlet et al., 2015 [31], the formed biogenic sulphuric acid from microbial activity reacts with the CH and C-S-H as the main hydration products of cement to form precipitates of gypsum and ettringite. The formation of ettringite reduces the quantity of calcium hydroxide and tricalcium aluminate content in the concrete/mortar matrix [32]. Presence of ettringite contributes to increased stress within the pores of the mortar/concrete resulting to spalling and cracking. This observation could be associated with internal sulphate attack as described by Ihab, 2017 [33].
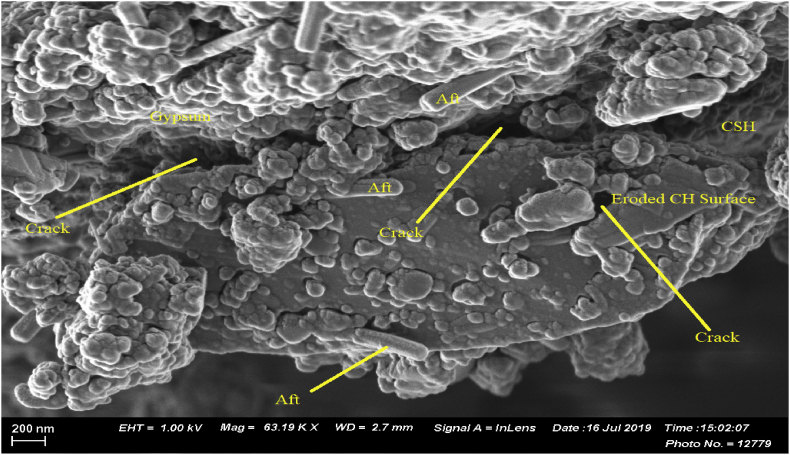
Adverse effect of Thiobacillus intermedius was observed in OPC TI (TI) as shown in Figure 3. There was decrease in CH with an evidence of erosion on the hydration products and formation of cracks. The crack formation was attributed to expansive products like gypsum and ettringite (Aft) while reduced CH amounts was due to dissolution of CH by sulphuric acid as a result of bacterial activity [14]. Sumit et al., 2019 [4] in their work associated spalling and cracking on concrete surfaces to accumulation of gypsum and ettringite crystals in the pores of the cementitious matrix. Several other authors have associated crack formation and expansion of mortar/concrete to gypsum and ettringite formation [34, 35, 36]. The levels of calcium hydroxide plates decreased significantly in bacterial samples. This could be attributed to gypsum and ettringite formation as shown in Eqs. (2) and (3) below.
| Ca (OH)2 + H2SO4 → CaSO4.2H2O (Gypsum) | (2) |
| (3) |
3.2. Compressive strength performance
The compressive strength test for control and microbial mortar prisms was as shown in Figure 4 below.
Figure 4.
Compressive strength Performance of control mortar vis a vis microbial mortar at different curing ages.
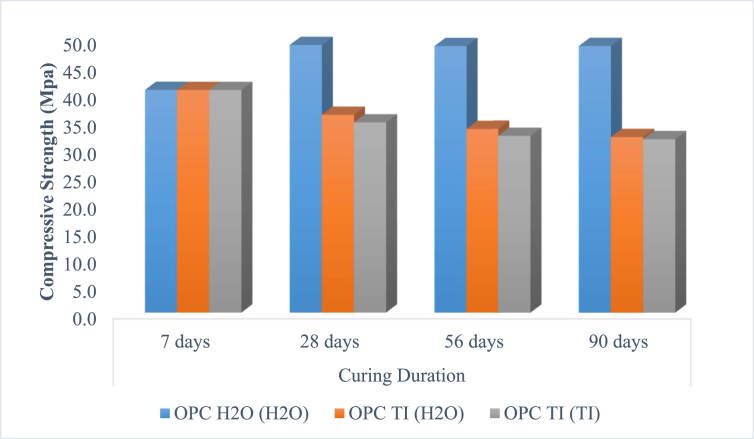
The test results shows that the strength of control mortar, OPC H2O (H2O) increased with increase in curing period. This was as expected because the rate of hydration increases with increase in curing period. In reference to SEM images for OPC H2O (H2O), the continual increase in compressive strength would as well be attributed to the formation of calcium silicate hydrate (CSH) which acts as the binding centers between the remaining unhydrated parts of the cement grains. The SEM image showed good formation of CH and C-S-H as hydration products, an indication that hydration had taken place well. Further, it was observed that the compressive strength of OPC H2O (H2O) did not increase significantly after 28 days. This is attributed to slow hydration process after 28 days for OPC based cements. Similar observations were made by Muthengia, 2009 [37], Munyao, 2015 [38] and Othmane et al., 2012 [39].
The compressive strength of mortar prisms prepared with bacterial solution, OPC TI (H2O), OPC TI (TI) compared with the strength of control, OPC H2O (H2O) at 7 days. Perhaps, it was early to observe the deleterious effect. However, the strength of the bacterial prepared mortar decreased significantly after 28 days of curing. This could be associated with the deleterious effect associated with Thiobacillus intermedius. As observed from SEM results, these test samples had significant amounts of ettringite and gypsum and reduced Ca(OH)2 content. Cracks were as well observed. These are clear indication of deleterious attack as a result of bacterial activity [14].
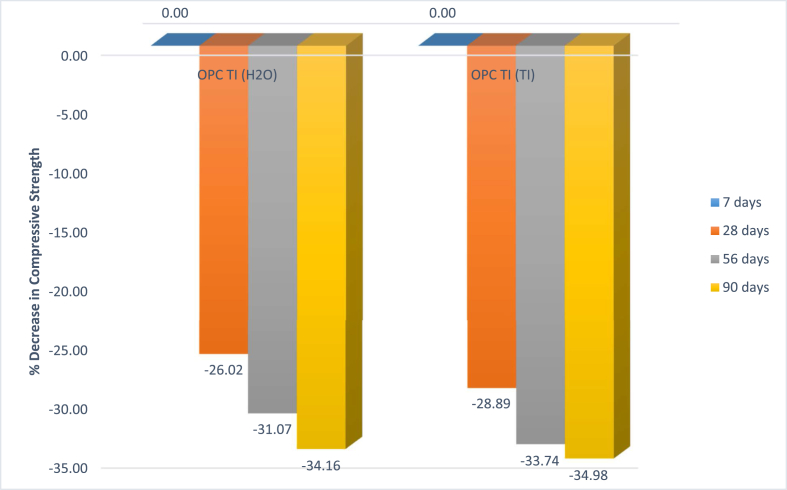
The compressive strength decrease in microbial mortar was as presented in Figure 5. In both scenarios, OPC TI (H2O) and OPC TI (TI) there was a significant decrease in compressive strength with increase in curing period. Drastic decrease in strength of 34.98 % was observed after 90th day of curing. The decrease could be attributed to the formation of biogenic sulphuric acid from the microbial activities. The formed sulphuric acid reacts with calcium hydroxide to form gypsum and ettringite. These products are expansive and may lead to crack formation and hence failure of the concrete/cement structure. Further, the formed acid lowers the pH of the resultant mortar. Low pH is unfavorable for cement reaction which leads to low strength development. According to Bettina et al., 2016 [15], the damage of concrete surface associated with acid attack was dependent on pH. The authors further reported that microbially derived sulphuric acid had similar corrosion damage on the surface of hardened cement paste as chemical sulphuric acid.
Figure 5.
Percent Compressive strength decrease of microbial mortar prisms.
3.3. Setting time, normal consistency and soundness
The tests showed conformity in both OPC H2O (H2O) and OPC TI (TI) for setting time, normal consistency and soundness as described in KS EAS 148-3 [22]. However, it was noted that there was appreciable increase in setting time and soundness for OPC TI (TI).This could be attributed to the effect of some reagents used to prepare the media culture for the bacterial growth. Some reagents used to prepare the bacteria culture were sulphate salts as indicated in Table 2. Sulphates are known to retard the setting time of cement mortar through formation of a diffusion barrier around the tricalcium aluminate phase. Presence of Mg (OH)2 from MgSO4.7H2O could have also contributed to the increase in soundness for the microbial paste. Mg (OH)2 causes cement soundness.
3.4. Water sorptivity
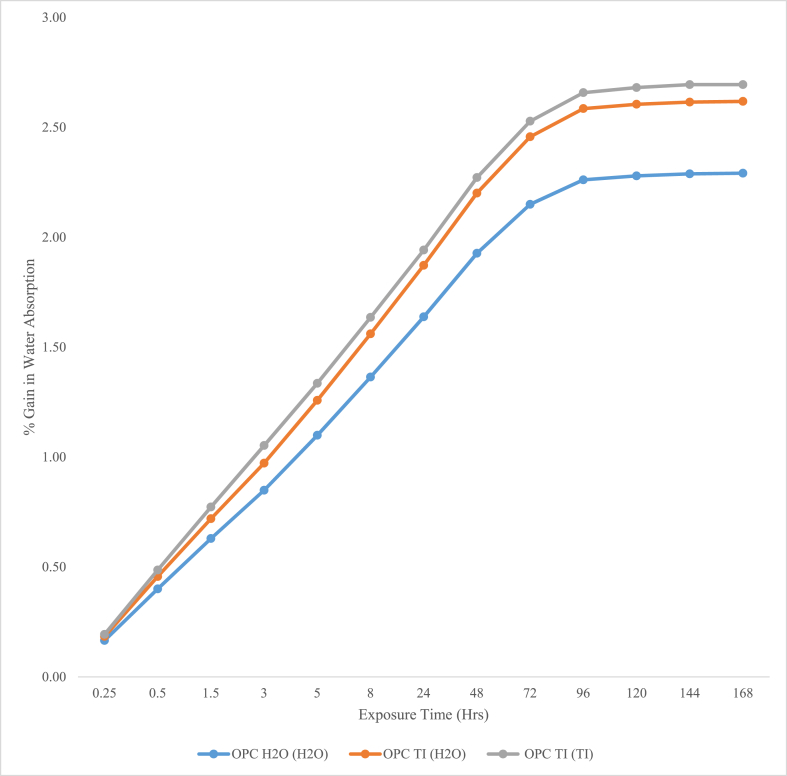
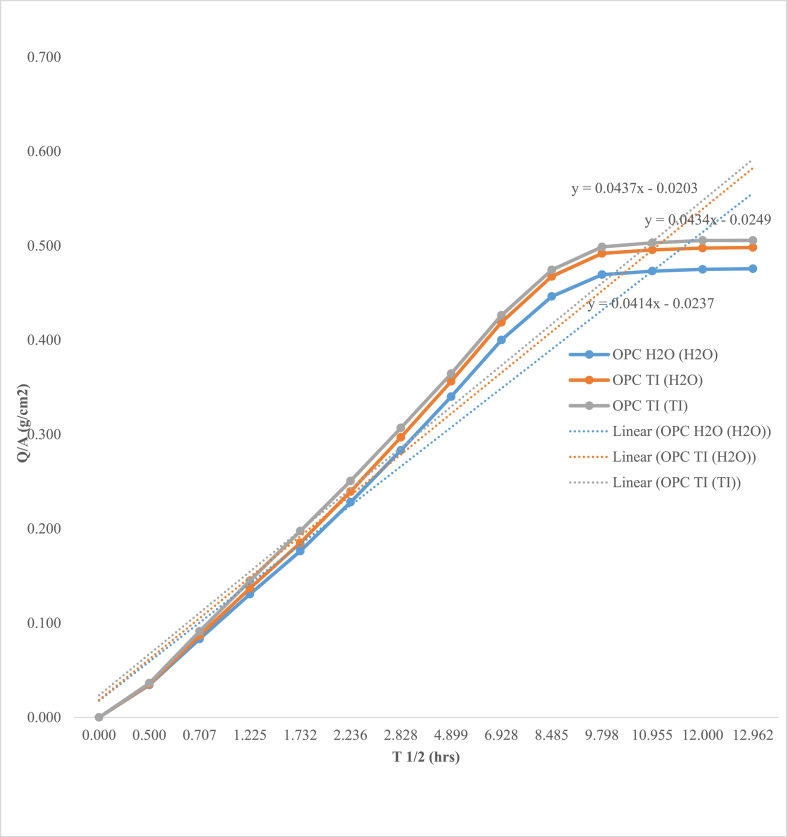
Mortar prisms were subjected to sorptivity test for a period of up to 168 h. The results are as represented in Figures 6 and 7.
Figure 6.
Percentage gain in water absorption.
Figure 7.
Sorptivity Coefficients for Control and Microbial test mortars.
From test results presented in Figures 6 and 7, OPC TI (H2O) and OPC TI (TI) exhibited both high water absorption rates and sorptivity coefficients as compared to OPC H2O (H2O). This could be as a result of increased deterioration from the microbial attack. From SEM results, erosion of calcium hydroxide plates was observed with severe formation of multiple cracks. The formed cracks could have resulted from the formation of expansive products like gypsum and ettringite. Presence of cracks made the mortar to be more porous and hence increased water absorption rates. The observed phenomenon agreed with other observations made on sulfate attack [4].
The low absorption rates and sorptivity coefficient observed in OPC H2O (H2O) could have been attributed to continued hydration. The formed hydration products was assumed to have sealed the pores within the hydrated mortar making it denser and hence reduced water absorption. As confirmed from the SEM results, OPC H2O (H2O) exhibited uniform distribution of hydration products with no evidence of any cracks. There was no appreciable increase in water absorption after 96th hour of exposure on both control and microbial mortars. This was attributed to decreased capillary suction due to saturation of the test mortars.
4. Conclusion
From the results of this work, the following conclusions were drawn:
-
1.
Thiobacillus intermedius bacteria affects the microstructure of the cement mortar. This could affect the durability of cement based structures
-
2.
Thiobacillus intermedius bacteria reduces the compressive strength of cement mortar.
-
3.
Setting time, normal consistency and soundness of OPC are greatly affected by the attack of Thiobacillus intermedius bacteria
-
4.
Thiobacillus intermedius bacteria degrades cement and hence increases the porosity of the cement material making it vulnerable to ingress of any potentially harmful substance
Declarations
Author contribution statement
Onesmus Mulwa Munyao: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Joseph Karanja Thiong'o & Jackson Wachira Muthengia: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Daniel Karanja Mutitu & Romano Mwirichia: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Genson Muriithi & Joseph Mwiti Marangu: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the African Development Bank grant in collaboration with the Ministry of Education, Science & Technology- Kenya.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank the African Development Bank (AFDB) in collaboration with the Ministry of Education, Science &Technology- Kenya for the award of PhD scholarship. Sincere gratitude to my supervisors for their guidance and support throughout this work. Access of scholarly repository and library materials from Kenyatta University and University of Embu, Kenya is highly acknowledged. Many thanks to the management of Savannah Cement Limited- Kenya for facilitating laboratory equipment's and allowing me to use their cement laboratory in carrying out some tests. Special thanks to Anton Weavind of Starex (Pyt) limited through their collaboration with the University of Pretoria, South Africa allowed me to carry out part of this research work in their laboratories.
References
- 1.Krishnapriya S., Babu D.L.V., Arulraj G.P. Isolation and Identification of bacteria to improve the strength of concrete. Microbiol. Res. 2015;174:48–55. doi: 10.1016/j.micres.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Bastidas-Arteaga E., Sánchez-Silva M., Chateauneuf A., Ribas-Silva M. Coupled reliability model of biodeterioration, chloride ingress and cracking for reinforced concrete structures. Struct. Saf. 2008;30:110–129. [Google Scholar]
- 3.Niloofar P., Davood M., Davood P. Use of bacteria to improve electrical resistivity and chloride penetration of air-entrained concrete. Constr. Build. Mater. 2019;210:588–595. [Google Scholar]
- 4.Sumit J., Shweta G., Abhijit M., Sudhakara M.R. Protection of concrete structures under sulphate environments by using calcifying bacteria. Constr. Build. Mater. 2019;209:156–166. [Google Scholar]
- 5.De Windt L., Devillers P. Modelling the degradation of Portland cement pastes by biogenic organic acids. Cement Concr. Res. 2010;40:1165–1174. [Google Scholar]
- 6.Jensen H.S. Aalborg University; 2009. Hydrogen Sulfide Induced concrete Corrosion of Sewer Networks. PhD Thesis. [Google Scholar]
- 7.O’Conwell M., McNally C., Richardson M.G. Biochemical attack on concrete in waste water applications: a state of the art review. Cement Concr. Compos. 2010;32:479–485. [Google Scholar]
- 8.Bertron A. Understanding interactions between cementitious materials and microorganisms: a key to sustainable and safe concrete structures in various contexts. Mater. Struct. 2014;47:1787–1806. [Google Scholar]
- 9.Grengg C., Mittermary F., Koraimann G., Konrad F., Szabό M., Demeny A., Dietzel M. The decisive role of acidophilic bacteria in concrete sewer networks: a new model for fast progressing microbial concrete corrosion. Cement Concr. Res. 2017;101:93–101. [Google Scholar]
- 10.Beddoe R., Hilbig H. Modelling the evolution of damage to concrete by acid attack, simulation of time dependent degradation of porous materials: final report on priority program. 2009;1122:275–293. [Google Scholar]
- 11.Zivica V.R., Bajza A. Acidic Attack of cement based-materials- a review: Part 1. Principle of acidic attack. Constr. Build. Mater. 2001;15:331–340. [Google Scholar]
- 12.Alexander M., Bertron A., De Belie N. Springer; 2013. Performance of Cement-Based Materials in Aggressive Aqueous Environments. State of the Art Report, Rilem TC 211-PAE. [Google Scholar]
- 13.Rogers R.D., Hamilton M.A., McConnell J.W. U.S. Nuclear Regulatory Commission; Washington DC, USA: 1996. Microbial Degradation of Low Level Radioactive Waste-Final Report, NUREG/CR-6341. [Google Scholar]
- 14.Guadalupe Ma., Gutie’rrez-Padilla D., Bielefeldt A., Ovtchinnikov S., Hernandez M., Silverstein J. Biogenic sulphuric acid attack on different types of commercially produced concrete sewer pipes. Cement Concr. Res. 2010;40:293–301. [Google Scholar]
- 15.Huber B., Hilbirg H., Mariana M.M., Jörg E.D., Műller E. Comparative analysis of biogenic and chemical sulfuric acid attack on hardened cement paste using laser ablation- ICP-MS. Cement Concr. Res. 2016;87:14–21. [Google Scholar]
- 16.Roberts D.J., Nicaa D., Zuoa G., Davisa J.L. Quantifying microbially induced deterioration of concrete initial studies. Int. Biodeterior. Biodegrad. 2002;49:227–234. [Google Scholar]
- 17.KS EAS 18-1 . Kenya Bureau of Standards; Nairobi, Kenya: 2017. Kenya Standard Test Method for Oxides Specification of Hydraulic Cement; pp. 59–61. [Google Scholar]
- 18.EN 196-1 Cement Part I: Determination of Strength. European Union of Standards; Beckum, Germany: 2016. [Google Scholar]
- 19.London J.P. Thiobacillus Intermedius nov. sp. A novel type of facultative Autotroph. Arch. Mikrobiol. 1963;46:329–337. [Google Scholar]
- 20.KS EAS 148:1 . Kenya Bureau of Standards; Nairobi, Kenya: 2000. Kenya Standard Test Method for Determination of Cement Strength. [Google Scholar]
- 21.Scrivener K., Snellings R., Lothenbach B. first ed. CRC Press; July, 26, 2017. A Practical Guide to Microstructural Analysis of Cementitious Materials. [Google Scholar]
- 22.KS EAS 148-3 . Kenya Bureau of Standards; Nairobi, Kenya: 2000. Kenya Standard Test Method for Determination of Setting Time and Soundness. [Google Scholar]
- 23.Vimal N.P. McGill University, Department of Civil Engineering and Applied Mathematics, Montreal Canada; August 2009. Sorptivity Testing to assess durability of concrete against freeze thaw cycling; pp. 66–68. Thesis. [Google Scholar]
- 24.ASTM C 1585-04 . ASTM International; West Conshohocken, PA: 2004. Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic Cement Concretes.www.astm.org [Google Scholar]
- 25.Mutitu D.K., Wachira J.M., Mwirichia R., Thiong’o J.K., Munyao O.M., Muriithi G. Influence of Lysinibacillus sphaericus on compressive strength and water sorptivity in Microbial cement mortar. Heliyon. 2019;5:e02881. doi: 10.1016/j.heliyon.2019.e02881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee B.Y., Kurtis K.E. Effect of pore structure on salt crystallization damage of cement-based materials: consideration of w/b and nanoparticle use. Cement Concr. Res. 2017;98:61–70. [Google Scholar]
- 27.Achal V., Mukherjee A. A review of microbial precipitation for sustainable construction. Constr. Build. Mater. 2015;93:1224–1235. [Google Scholar]
- 28.Feng P., Garboczi E.J., Miao C., W Bullard J. Microstructural origins of cement paste degradation by external sulphate attack. Constr. Build. Mater. 2015;96:391–403. doi: 10.1016/j.conbuildmat.2015.07.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sand W., Bock E. Concrete corrosion in the Hamburg sewer system. Environ. Technol. Lett. 1984;5:517–528. [Google Scholar]
- 30.Diercks M., Sand W., Bock E. Microbial corrosion of concrete. Experientia. 1991;47:514–516. [Google Scholar]
- 31.Gutberlet T., Hilbig H., Beddoe R.E. Acid attack on hydrated cement- effect of mineral acids on the degradation process. Cement Concr. Res. 2015;74:35–43. [Google Scholar]
- 32.Aye T., Oguchi C.T. Resistance of Plain and Blended cement mortars exposed to severe sulfate Attacks. Constr. Build. Mater. 2011;25:2988–2996. [Google Scholar]
- 33.Saleh I.S. Effect of external and internal sulphate on compressive strength of concrete. Int. J. App. Eng. Res. 2017;12:10324–10333. [Google Scholar]
- 34.Maes M., De Belie N. Resistance of concrete and mortar against combined attack of chloride and sodium sulphate. Cement Concr. Compos. 2014;53:59–72. [Google Scholar]
- 35.Tian B., Cohen M.D. Does gypsum formation during sulphate attack on concrete lead to expansion? Cement Concr. Res. 2000;30:117–123. [Google Scholar]
- 36.Roziere E., Loukili A., El Hachem R., Grondin F. Durability of concrete exposed to leaching and external sulphate attacks. Cem. Concr. Res. 2009;39:1188–1198. [Google Scholar]
- 37.Muthengia J.W. Department of Chemistry, Kenyatta University; Kenya: 2009. Effects of Selected Media on Novel Portland Pozzolana Cement. PhD Thesis. [Google Scholar]
- 38.Munyao O.M. Department of Chemistry, Kenyatta University; Kenya: 2015. Effects of Surface and Subsurface Mixing Water of Nairobi, Machakos and Kajiado Counties on Cement Mortar Performance. Msc. Thesis. [Google Scholar]
- 39.Othmane B., El-Hadj K., Said K. Effects of granulated blast furnace slag on superplasticizer type on the fresh properties and compressive strength of self-compacting concrete. Cement Concr. Res. 2012;34:583–590. [Google Scholar]