Abstract
Aim
The current investigation focused on the therapeutic role of the administration of taurine on hypertensive rats to reduce or cure the hazard effects of hypertension problems.
Methodology
This research included 2 experiments; 1st was done to survey the variations that might occur in blood pressure (BP) of male rats because of the fed 8% NaCl diet for 4 weeks. 2nd experiment, it contains normal control rats', hypertensive rats were served as hypertension recovery group and hypertensive rats were took orally by the help of gastric tube 50 mg taurine/100 g b.wt/day for four weeks and served as taurine group.
Results
1st experimental, clarified a significant elevation in BP, body weight, serum cholesterol, triglycerides, LDL, activities of serum cardiac enzymes, endothelin-1, ADMA, MDA and TNF-α in hypertensive rats' group. On contrary, there is a significant reduction in serum level of TNO and antioxidant enzymes level in relation to the control group. A numerical variation but not statistically significant was happened in HDL in hypertensive rats' group as compared to their matching results in control rats' group. 2nd experimental taurine significantly reduced the BP as compared with hypertensive control. Furthermore, a significant improvement occurred in the mean value of most investigation parameters in hypertensive animal group which treated with taurine.
Conclusion
The previous data could be concluded that, there is an obvious amelioration effects of taurine on hypertensive rats by reducing the hazard effects of hypertension problems. The primary mechanisms were discussed according to existing published investigations.
Keywords: Biochemistry, Regenerative medicine, Public health, Pharmacology, Hypertensive rats, Taurine, Lipid profile, Cardiac enzymes, Antioxidant enzymes, TNO, Endothelin-1, MDA, TNF-α, ADMA
Biochemistry; Regenerative Medicine; Public Health; Pharmacology; hypertensive Rats; taurine; lipid profile; Cardiac enzymes; antioxidant enzymes; TNO; Endothelin-1; MDA; TNF-α; ADMA.
1. Introduction
In recent decades, patterns of illness are changing dramatically all over the world. Specifically, infectious diseases are becoming less common, and the occurrence of non-infectious chronic diseases, such as hypertension, are increasing [1].
Hypertension, generally mentioned as "high blood pressure", is a medical disorder usually linked with contraction of the blood vessel. This causes blood to be propelled with extreme strength against the blood vessel walls. Hypertension is entitled “Silent killer" since most individuals doesn't think they are hypertensive [2].
The role of hypertension undoubtedly is undervalued from illness and death statistics, which are mainly constructed on death certificates. After a patient dies because a stroke, angina, or renal failure all records in a straight line attributed these results to the stroke, the angina, or the renal failure, not the hypertension, usually is recorded as the reasons of death [3].
Hypertension is one of the greatest common illnesses worldwide, it considers a main reason of death after cardiovascular diseases. Because of hypertension linked with illness and death, so it is a community health problematic [4], so necessity to investigate for suitable protective, management plans must be the focus of health care centres. Amplified vascular oxidative tension might be the cause of harmful effects of hypertension [5, 6] and consider the main hazard for cardiovascular sickness & death in the industrialized and poor countries. The start of hypertension is produced by multifaceted connections between genetic tendency & ecological factors [7]. Enlarged sodium chloride (NaCl) consumption can worsen increasing hypertension which increase of significant end-organ injury [8].
The original theory that physical and functional irregularities in the circulatory system, containing endothelial malfunction, amplified oxidative pressure & reduced antioxidant actions, could predate BP and contribute to its harmful effects, has increased care in last years [9]. Experimental researches have largely reinforced theory that augmented hypertension is linked with amplified oxidative stress [10]. Raised lipid peroxidation by-products & reduced action of antioxidant systems already described in BP subjects [11]. Numerous researches have showed a rise in O2- levels in BP subjects [12] and associate NADPH oxidase as a cause of additional O2- [13], [14].
Angiotensin II has been revealed to be a strong activator of NADPH oxidase action in blood vessels smooth muscle, endothelial cells, and cardiac muscle cells [15]. A communal discovery in all kinds of BP as well as diabetes and metabolic condition is endothelial malfunction, characterized by an inequality in the appearance of and sensitivity to vessels dilator and vessels constrictor factors causing in amplified vessels muscle tone and thus rise in struggle to flow [16, 17].
Taurine is a sulfur-holding nonessential amino acid, endogenously produced & providing by food, specially eggs, meat, & seafood. It has been reported that taurine play an important role as antioxidant & anti-inflammatory mediator [18, 19] as well as its vessel's dilator proprieties [20]. In endothelial cells, taurine prevents apoptosis, inflammation and oxidative stress whereas increment nitric oxide productions [21].
Furthermore, taurine was described to possess valuable effects in numerous physiological and pathological circumstances by primarily lessening manufacture of reactive oxygen species (ROS) [22].
Hypertension and heart failure are pathological conditions with high illness & death, the full consideration and suitable treatment of these sicknesses remain a challenge. Treatment by taurine may have valuable effect on the cardiovascular problems made by hypertension. So, the current research emphases on the possible amelioration roles of taurine on hypertensive rats to reduce or cure the hazard effects of hypertension problems.
2. Material and methods
2.1. Animals
40 male rats Rattusrattus, body weight 120 ± 10 g, were obtained from the serum and antigen laboratories (Helwan). The rats were housed throughout the experiment in metallic cages and permitted to adapt to workroom atmosphere for ten days to circumvent any problems along the period of the experiment. Rats were kept under controlled conditions of temperature at 28±2 °C and 50% relative humidity, regular light-dark cycles and take food & water ad-libitum with new supplies offered every day. The experimental protocol was approved by the Ethical Committee of the Ain Shams University, Cairo, Egypt.
The current investigation was included 2 experimentations carried out as the following: 1st experiment, the animals were randomly separated into 2 main groups according to the type of diet. 1st group, 15 animals were fed on a standard diet only [starch (51.55%), soya bean (18.15%), sucrose (17.93%), corn oil (5.15%) and vitamin & mineral mixture which containing (g/kg): thiamin HCl 0.6, riboflavin 0.6, pyridoxine HCl 0.7, niacin 3, calcium pantothenate 1.6, folic acid 0.2, biotin 0.02, vitamin B12 (0.1% trituration in mannitol) 1, dry vitamin A palmitate (500,000 U/g) 0.8, dry vitamin E acetate (500 U/g) 10, vitamin D3 trituration (4,000,000 U/g), 0.25, manadione sodium bisulfite complex 0.15, calcium phosphate diabasic 500, sodium chloride 14, potassium citrate 220, potassium sulfate 52, magnesium oxide 24, mangnous carbonate 3.5, ferric citrate 6, zinc carbonate 1.6, cupric carbonate 0.3, potassium iodate 0.01, sodium selenite 0.01, chrominium potassium sulfate 0.55] and consider as control [23]. Whereas, 2nd group, 25 animals were fed on high-salt diet (8% NaCl) as labelled by [24]. Next four weeks, 5 rats from each previous group were taken to evaluate the alteration in the lipid profile, cardiac profile and hormonal profile.
2nd experiment, (start at 5th week), the remaining of standard diet rats' group (10 rats) was consider as normal control rats' group (group No. 1) while, the remaining 20 hypertensive rats were not further fed on high-salt diet and separated into 2 equal groups:
Group (No. 2): 10 hypertensive rats were served as hypertension recovery group.
Group (No. 3): 10 hypertensive rats were received orally by the aid of gastric hose fifty milligram taurine (Sigma Chem. Co., St. Louis, Mo., USA)/100 g body weight/day for 4 weeks according to [25] and served as taurine group (T group).
At the end of each experimental period (2 weeks), 5 rats from each previous group were 12-hour fasted & slaughtered by decapitation. Blood samples were collected in a clean test tube to determine serum lipid profile [cholesterol (Chol.), triglycerides (TG), high density lipoprotein-cholesterol (HDL) and low density lipoprotein-cholesterol (LDL)], antioxidant enzymes [Catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD)], cardiac profile [aspartate aminotransferase (AST), creatine kinase (CK) and lactate dehydrogenase (LDH) activities] and the levels of total nitric oxide (TNO), endothelin-1 and asymmetric dimethyl arginine (ADMA). Moreover, the levels of Malondialdehyde (MDA) and tumor necrosis factor-α (TNF-α) were estimated.
2.2. Measurement of blood pressure
Blood pressure was monitored weekly by the tail-cuff technique using noninvasive Ugo Basile, series 58500 BP Recorder. 4 readings average were taken for each single rat. Mean arterial BP was generally calculated from brachial systolic and diastolic BP according to the next formula:
| Mean arterial pressure = DP + (SP - DP) / 3. |
where SP and DP are systolic and diastolic pressure, respectively [26].
2.3. Estimation of serum lipid profile
Serum total Chol., TG and HDL according to [27, 28, 29] respectively were estimated enzymatically using a commercial kit from Randox, Ltd., Co. (UK). LDL-cholesterol was estimated as per Freidewald's equation [29].
| LDL-Chol. = Total Chol. – [TG/5 – HDL- Chol.]. |
2.4. Determination of antioxidant enzymes
GPx, SOD and catalase activity were assessed according to methods of [30, 31] and [32] respectively by using commercial kits, purchased from Cayman Chemical Company, Ann Arbor, U.S.A.
2.5. Determination of serum cardiac profile
The actions of serum LDH, CK and AST were estimated kinetically according to the method of [33, 34] and [35] respectively. The kinetically commercial kits were purchased from Sclavo Bio-diagnostic Co. (Italy).
Serum rat-ADMA was assessed via commercial ELISA kit and purchased from American Laboratory Products Company (Alpco Diagnostics, USA) according to the method of [36].
Furthermore, the concentrations of serum rat endothelin-1 [37], TNO [38] and the level of rat TNF-α [39] were evaluated by ELISA (Sandwich Immunoassay Technique) via commercial kits (IBL-Hamburg, Co. Germany).
Colorimetric technique was used for the assessment of tissue malondialdehyde [40].
2.6. Statistical analysis
Student “t” test was used to test the difference in parameters tested herein between experimentally hypertensive rats and normal control rats in preliminary experiment according to [41]. Whereas, the comparison between the effects of taurine on biochemical parameters documented here were statistically analysed using analysis of variance (ANOVA) followed by Duncan's multiple range tests as described by [42, 43].
3. Results
From the inspection of the obtained data in Table 1 were reported significant (P < 0.001) increases in the blood pressure and body weight as a result of salt diet treatment. The mean values recorded 187.98 ± 1.73 mm Hg and 145.69 ± 2.81 (g) for blood pressure and body weight in hypertensive rats regarding to 108.12 ± 0.61 & 110.42 ± 2.59 in normotensive rats' group respectively.
Table 1.
Comparison between normal and hypertensive rats in BP, body weight and some blood variables related to hypertension risks.
| Parameters | Groups |
|
|---|---|---|
| Normal Rats Mean ± S.E. |
Hypertensive Rats Mean ± S.E. |
|
| Systolic BP (mm Hg) | 108.12 ± 0.61 | 187.98* ±1.73 |
| Body weight (g) | 110.42 ± 2.59 | 145.69* ±2.81 |
| Total cholesterol (mg/dL) | 62.129 ± 1.898 | 114.182* ± 1.483 |
| Triglycerides (mg/dl) | 63.925 ± 1.107 | 138.333* ± 1.979 |
| HDL- cholesterol (mg/dl) | 23.634 ± 0.952 | 24.103 ± 0.977 |
| LDL- cholesterol (mg/dl) | 25.600 ± 0.946 | 62.410* ± 0.445 |
| Catalase (nmol/min/mL) | 25.920 ± 1.360 | 14.210* ± 1.090 |
| GPx (nmol/min/mL) | 94.850 ± 1.220 | 27.190* ± 1.480 |
| SOD (U/mL) | 6.080 ± 1.150 | 3.170* ± 0.430 |
| LDH (U/L) | 230.205 ± 1.754 | 386.801* ± 1.556 |
| CK (U/L) | 93.053 ± 0.656 | 175.970* ± 1.121 |
| AST (U/L) | 122.059 ± 1.001 | 191.504* ± 1.683 |
| TNO (μmol/L) | 45.877 ± 1.673 | 21.806* ± 1.267 |
| MDA (nmol/mg tissue) | 0.371 ± 0.050 | 1.320* ± 0.190 |
| Endothelin-1 (pg/ml) | 0.376 ± 0.005 | 0.726* ± 0.044 |
| ADMA (μmol/L) | 1.115 ± 0.110 | 2.794* ± 0.321 |
| TNF-α (pg/ml) | 4.923 ± 0.011 | 8.895* ± 0.022 |
Values are expressed as means ± S.E.
* Significant at p < 0.001 between the groups in the same rows.
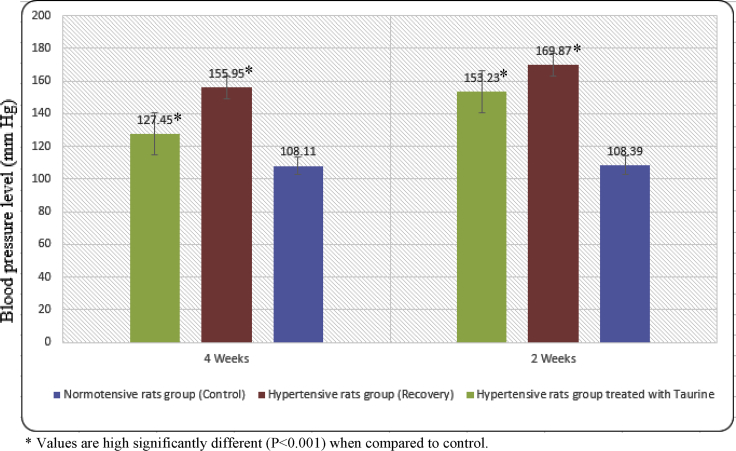
Figure 1 were summarized the main value of tail systolic blood pressure (TSBP) data at different time intervals. The mean value of TSBP level of normotensive rats showed no changes throughout the experimental period (4 weeks).
Figure 1.
The mean value of BP level (mm Hg) in normotensive (Control), hypertensive (Recovery) and hypertensive treated with taurine rats' groups at various time intervals. * Values are high significantly different (P < 0.001) when compared to control.
In hypertensive rats, the mean value of TSBP level was decreased and reached to 169.87 ± 1.61 mm Hg after 2 weeks recovery regarding to 108.39 ± 0.63 in normotensive rats' group. As rats were allowed to 4 weeks recovery, the mean value of TSBP level was more decreased and reached to 155.95 ± 1.41 mm Hg. The per cent of this decline was 44.25 at the last interval (4 weeks).
Furthermore, a significant improvement occurred in the mean value of TSBP level in hypertensive animal group which treated with taurine. The improvement was reached to 127.45 ± 1.37 at the last interval (4 weeks).
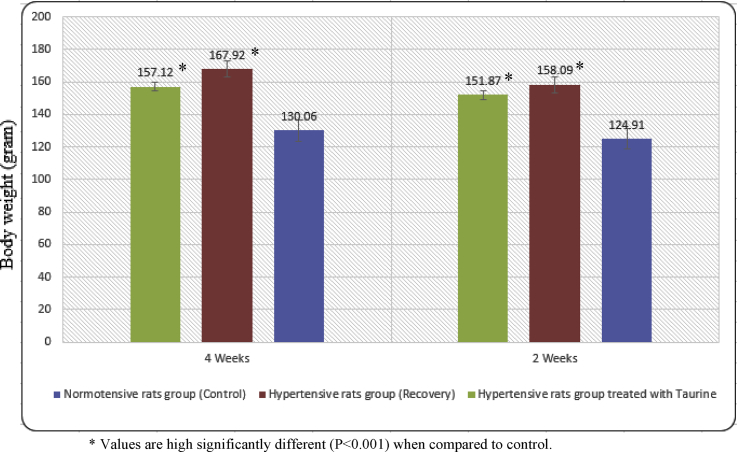
The mean body weight of normotensive rats' group was increased gradually throughout the experimental period. These data are given in Figure 2. Moreover, the mean body weight of hypertensive rats group showed a significant increase dependant on time intervals reaching 167.92 ± 3.38 regarding to 130.06 ± 2.29 in normotensive rats' group at the end of the experimental period. A significant reduction in the mean body weight of hypertensive rats group occurred after treatment with taurine.
Figure 2.
The mean value of body weight (gram) in normotensive (Control), hypertensive (Recovery) and hypertensive treated with taurine rats' groups at various time intervals. * Values are high significantly different (P < 0.001) when compared to control.
In contrast, the obtained data in Table 1 of the first experimental, elucidated a significant (P < 0.001) increasing in serum Chol., TG and LDL levels in hypertensive rats. The mean values recorded were 114.182 ± 1.483, 138.333 ± 1.979 and 62.410 ± 0.445 mg/dL regarding to 62.129 ± 1.898, 63.925 ± 1.107 and 25.600 ± 0.946 mg/dL in normal control rats respectively. A numerical change but not statistically significant was occurred in HDL in hypertensive rats' group as compared to their corresponding results in control rats' group.
On detecting antioxidant enzymes level Cat, GPx and SOD via the information presented in Table 1. It is documented that rats fed on high-salt diet showed a significant (P < 0.001) reduction in antioxidant enzymes level as compared to the corresponding normal control rats.
The activities of all estimated serum cardiac enzymes AST, CK and LDH were significantly (P < 0.001) raised in hypertensive rats' group. Likewise, the levels of endothelin-1, ADMA, MDA and TNF-α were significantly (P < 0.001) increased in hypertensive rats' group. In relation to the control rats, a significant (P < 0.001) reduction in the serum level of TNO was described in hypertensive rats' group Table 1.
In the second experimental, a significant (P < 0.05) reduction was occurred in the levels of serum cholesterol, triglycerides and LDL levels in hypertensive rats after treatment with taurine. The maximum improvement result was reported in the hypertensive rats which treated with taurine dependent on the time of treatment (4 weeks) Table 2.
Table 2.
Effects of giving taurine on serum lipid profile level of hypertensive rats.
| Parameters | Groups |
|||
|---|---|---|---|---|
| Control | Hypertensive Recovery group | Hypertensive with T group | ||
| Total cholesterol (mg/dL) | 2 weeks | |||
| 4 weeks | ||||
| Triglycerides (mg/dL) | 2 weeks | |||
| 4 weeks | ||||
| HDL - Cholesterol (mg/dL) | 2 weeks | |||
| 4 weeks | ||||
| LDL - Cholesterol (mg/dL) | 2 weeks | |||
| 4 weeks | ||||
Values are expressed as means ± S.E.
A, B, C = means bearing different superscripts within the same row are differ significantly (P < 0.05).
a, b = means bearing different subscripts within the same column are differ significantly (P < 0.05).
A significant (P < 0.05) recovery occurred in the serum antioxidant enzymes Cat, GPx and SOD activities of hypertensive rats which were treated with taurine dependent on the time of treatment (2 & 4 weeks). This improvement was reported in Table 3. The best improvement in these parameters was dependent on the time of treatment.
Table 3.
Effects of giving taurine on serum antioxidant enzymes activity of hypertensive rats.
| Parameters | Groups |
|||
|---|---|---|---|---|
| Control | Hypertensive Recovery group | Hypertensive with T group | ||
| Catalase (nmol/min/mL) | 2 weeks | |||
| 4 weeks | ||||
| GPx (nmol/min/mL) | 2 weeks | |||
| 4 weeks | ||||
| SOD (U/mL) | 2 weeks | |||
| 4 weeks | ||||
Values are expressed as means ± S.E.
A, B, C = means bearing different superscripts within the same row are differ significantly (P < 0.05).
a, b = means bearing different subscripts within the same column are differ significantly (P < 0.05).
A significant (P < 0.05) amelioration occurred in the serum AST, CK and LDH activities of hypertensive rats which were treated with taurine dependent on the time of treatment (2 & 4 weeks). This improvement was reported in Table 4. The best improvement in these parameters was distinguished in the hypertensive rat's group which treated with taurine and dependent on the duration of treatment.
Table 4.
Effects of giving taurine on serum Cardiac enzymes profile activity of hypertensive rats.
| Parameters | Groups |
|||
|---|---|---|---|---|
| Control | Hypertensive Recovery group | Hypertensive with T group | ||
| LDH (U/L) | 2 weeks | |||
| 4 weeks | ||||
| C.K (U/L) | 2 weeks | |||
| 4 weeks | ||||
| 2 weeks | ||||
| 4 weeks | ||||
Values are expressed as means ± S.E.
A, B, C = means bearing different superscripts within the same row are differ significantly (P < 0.05).
a, b = means bearing different subscripts within the same column are differ significantly (P < 0.05).
Next the hypertensive rats treated with taurine for 2 & 4 weeks, the data recorded a significant (P < 0.05) rise in the serum level of TNO dependent on duration of treatment Table 5. The maximum elevation was occurred in the serum level of TNO in hypertensive rats' group which take taurine. This improvement still not reached to their corresponding values in the normal control rats' group.
Table 5.
Effects of giving taurine on serum TNO, MDA, Endothelin-1, ADMA and TNF-α levels of hypertensive rats.
| Parameters | Groups |
|||
|---|---|---|---|---|
| Control | Hypertensive Recovery group | Hypertensive with T group | ||
| TNO (μmol/L) | 2 weeks | |||
| 4 weeks | ||||
| MDA (nmol/mg tissue) | 2 weeks | |||
| 4 weeks | ||||
| Endothelin-1 (pg/ml) | 2 weeks | |||
| 4 weeks | ||||
| ADMA (μmol/L) | 2 weeks | |||
| 4 weeks | ||||
| TNF-α (pg/ml) | 2 weeks | |||
| 4 weeks | ||||
Values are expressed as means ± S.E.
A, B, C = means bearing different superscripts within the same row are differ significantly (P < 0.05).
a, b = means bearing different subscripts within the same column are differ significantly (P < 0.05).
A notable improvement was reported in the serum levels of MDA, endothelin-1, ADMA and TNF-α after the hypertensive rats treated with taurine for two & four weeks. These significant (P < 0.05) improvements were noticeable in the hypertensive rat's group which treated with taurine. This improvement in the serum levels of MDA, endothelin-1 and ADMA were reached to the control rats' group whereas, the improvements in the serum level of TNF-α and TNO were still not reached to the corresponding in the control rats' group Table 5.
4. Discussion
Hypertension remains a common and serious problem, contributing in a major way to the most common causes of morbidity and mortality all over the world [44]. In the current study, a food with 8 % sodium chloride was used to induce hypertension in Male albino rats Rattus rattus for 4 weeks after that treated with taurine for additional 4 weeks.
It is evident from this investigation that high salt diet led to a marked elevation in the TSBP Table 1 [45]. The mechanism by which high-salt foods initiate hypertension may be due to rise in the level of circulating sodium (Na) which push cells to release water because of osmotic pressure which raises the pressure on blood vessel walls [46]. Also, increasing Na concentration in circulation can turn activates sympathetic nervous system and renin-angiotensin-aldesterol-system. Furthermore, to increased signaling through the mineralocorticoid receptors [47]. These may cause increasing production of ROS which result to oxidative stress. Further possible mechanisms could be in part via a rising in the plasma's capacity to prevent adenosine triphosphatase (ATP) which increases the BP by preventing the Na–Ca exchange pump in blood vessels smooth muscle [45], or that Na diet is linked with amplified intrarenal angiotensin II [46] which may cause in renal vessels constriction and amplified renal production due to activation of NADPH oxidase. Overproduction of superoxide anions and other free radicals due to activation of NADPH oxidase may overcome the antioxidant ability and cause imbalances between oxidant and antioxidant status which may cause in oxidative stress. Moreover, NO may cause a critical role in the regulation of arterial pressure by influencing vascular tone throughout the cardiovascular system and by serving as a mediator of the changes induced by the arterial pressure in tubular sodium re-absorption [46]. The authors attributed the rise of TSBP in hypertensive rats to the constriction effect of angiotensin II on both afferent and efferent arterioles.
Treating the rats with high salt food induced a significant elevation in the body weight of hypertensive rats compared to the normotensive ones. This elevation may be attributed to a rapid drop in glomerular filtration rates (GFR). However, the majority of administered NaCl enters specifically to the proximal tubular epithelial cells, causing abnormalities in the function and metabolism of various intracellular membranes and organelles. Moreover, these disturbances may be due to rapid breakdown of protein and elevation in the level of phospholipidosis in the lysosomes. Hence, these enzymes inhibit Na+ K+ -ATPase which cause a rise of the free radical agents which accelerate cell injury. These results are in harmony with [48, 49].
Also, this study was indicated that in addition to increase in BP and body weight, salt-loaded rats' serum lipid profile levels were significant higher in serum cholesterol, triglycerides and LDL levels of hypertensive rats' group. However, HDL level wasn't significant different between hypertensive rats' group and control normotensive rats' group. The increased cholesterol, triglycerides and LDL levels rises the hazard of coronary heart disease and rise serum lipid profiles were significant risks for hypertension. Researchers have reported association of serum/plasma lipids and lipoproteins with hypertension [50, 51].
Moreover, it is obvious in this investigation that after rats fed on high salt food it was raised the activity of cardiac enzymes (AST, LDH and CK) levels in serum of hypertensive rats than control normotensive rats (Table 1). Such clinical condition promotes the initiation of cardiac diseases such as infarction and atherosclerosis since the elevation in the activity of CK is liable to occur if there is damage in skeletal or cardiac muscles. LDH leaks into the serum from damaged organs. An elevation of its activity in serum suggests subclinical infarction or at least cellular damage. The height and duration of the increased activity depends on the size of lesion. Therefore, LDH is used as an index of membrane damage of cardiac muscle or vascular endothelial cells [52]. In 2001, Researchers discovered that when vascular endothelial cells were exposed to oxidized LDL, there was a significant increase of LDH release indicating cell membrane injury and disturbance in the lipid peroxidation of cardiac tissues [53].
In the current study, antioxidant enzymes (GPx, CAT and SOD) was significantly (P < 0.001) decreased in hypertensive rats as compared with their relevant level in control normotensive rats Table 1. Such clinical condition promotes the initiation of hypertension since the depletion of antioxidant enzymes catalyzes the H2O2 to H2O and O− at the expense of reduced glutathione. The lowering antioxidant enzymes activity may be attributed to decreased synthesis of these enzymes which can cause a rise in reactive oxygen species that inactivates NO and promotes endothelial dysfunction [54, 55, 56].
The current results in Table 1, there was a significant reduction in the TNO concentration was detected with a significant raise in the levels of MDA, endothelin-1, ADMA and TNF-α in hypertensive rats than control normotensive rats. The current data could be because of excessive myocardial infraction as a result of free radical production, epigenetic gene alteration, inhibition in the activity of total nitric synthase (TNOs), raise the level of serum endothelin-1 accompanied with elevation in serum ADMA level and appearance of hypertension. These results are in parallel with those gotten by [57].
Total nitric oxide level was significantly (P < 0.001) reduced in hypertensive rats as mentioned above and these results are in parallel with [58, 59, 60]. They reported that the raise of oxidative stress in hypertensive rats is associated with the release of nitric oxide from hypertensive rat kidneys. They attributed these results to the disturbance in the endothelin type B receptor endothelin nitric oxide synthesis (eNOs) depletion in the glutathione system and elevation in the levels of high reactive oxidant peroxynitrite (ONOO–).
Furthermore, peroxynitrite has numerous harmful effects such as starting lipid peroxidation, causing DNA injury and nitration of protein deposits, including magnesium superoxide dismutase. Moreover, increasing vascular ROS activity can cause decrease bioavailable nitric oxide and diminished endothelium-dependent relaxation [61].
In contrast, the pro-oxidant MDA level in serum was significantly (P < 0.001) increased in hypertensive rats and these results may be due to NaCl has the ability to transfer electron to oxygen thus generating oxygen radical species such as superoxide (O2.) radical and eventually hydroxyl (OH.) radical which might be responsible for the cytotoxic effect [62, 63, 64].
ADMA level was significantly (P < 0.001) amplified in hypertensive rats as compared with relevant level in control normotensive rats. Moreover, it is obvious that induced hypertension raised ADMA level in serum which causes inhibitions in all three isoforms of nitric oxide synthesis (NOS) due to inhibition of eNOS. Also, the elevation of ADMA concentration is linked to impaired endothelium-dependent vasodilation, a feature that is indicative of increased cardiovascular risk. These results are in harmony with evidence gathered from several experimental researches occurred by [65, 66, 67, 68, 69]. They indicated that ADMA is a powerful endogenous NOS inhibitor. Therefore, chronically raised serum ADMA levels may be of certain importance in human vascular pathology. Also, the authors reported that there is a strong correlation between increased ADMA levels and cardiovascular morbidity and mortality in different populations. Some researchers attributed these results to that higher ADMA doses cause a larger rise in BP. In addition, of significant renal vasoconstriction with ADMA infusion, causing acute plasma levels in the supra-physiological range is important of the action of angiotensin II, which exerts renal vasoconstrictor effects at blood levels that are above those found in patients with hypertension and/or cardiovascular disease. However, angiotensin II has several effects encouraging atherogenesis, maybe due to high tissue concentrations. The last one might be correct for ADMA too, and chronically raised ADMA blood and/or tissue levels may be a reason for vascular damage at the endothelial cell level [70].
Furthermore, it has been reported that tumor necrosis factor-α displays a cardio depressive characteristic. Moreover, tumor necrosis factor-α has been exposed to be independent predictors of death in heart failure [71]. Hence, the current research suggested that the association of raised tumor necrosis factor-α as proinflammatory cytokines with hypertension may place the rats at complex hazard for the evolution of suggestive cardiomyopathy.
There is a very fine equilibrium between cellular systems that generate various free radicals and those responsible for the maintenance of antioxidant defence mechanisms [72]. It appears that hypertension tips this equilibrium by helping of oxidants, both by raising the creation of free radicals and by decreasing endogenous antioxidant [73]. Endogenous antioxidants, like SOD, CAT and GPx are no longer able to cope with this rise in free radical creation especially in the tissues with less developed antioxidant defence mechanisms, such as the cardiac muscle, hence it is a highly vulnerable to damage that is induced by free radical creation. Since lack of nitric oxide bioactivity is a vital feature of endothelial malfunction in hypertension, providing additional substrate to support nitric oxide creation has been suggested as a rational treatment method. Administration of taurine enhanced endothelium-dependent vasodilation because it is the substrate for the synthesis of endothelium-derived NO, an effective vasodilator that controls blood pressure and renal hemodynamic in the basal state. Taurine acts as nitrogen donor for synthesis of nitric oxide, which is deficient during hypertension and atherosclerosis [74].
From Tables 2, 3, 4, and 5 and Figures 1 and 2, showed that the administration of taurine to hypertensive rats caused significant ameliorative effects on the blood pressure, body weight and all biochemical parameters. These results may be due to the several mechanisms for taurine improvement of endothelial functions which have been suggested to include: (1) a reduction in the inhibitory effects of L-glutamine, (2) a decline in the inhibitory effects of ADMA, (3) an antioxidant action, (4) free radicals scavenger, (5) stimulation of insulin release, (6) improved production of vasoconstrictor intermediaries like endothelin-1 and (7) nonenzymatic & nonstereo-specific creation of nitric oxide [75, 76].
From the data presented in the current study, it is obvious that; the depression in the TSBP in hypertensive rats treated with 50 mg/kg b.wt taurine reached its maximum degree of amelioration in the last interval period. Nevertheless, values still failed to reach the value of TSBP of normotensive rats Figure 1. These results may be due to the capability of taurine to act as a hypolipidemic agent, membrane stabilizer and free radical scavenger. Also, taurine plays an important role in declining the activities of cardiac enzymes and the kinetic potential of β-oxidation of fatty acids in the mitochondrial matrix by the aid of Krebs cycle. These data are in harmony with [77, 78].
Moreover, the administration of taurine to the hypertensive rats led to decrease in the body weight. This improvement in the body weight may be due to the bio-chemical powerful of taurine which works as a good antioxidant by decreasing the creation of oxygen free radicals, inhibition of the formation of thromboxane A2, vasodilator of coronary vessels, due to inhibition of certain intramuscular enzymes and improvement in the cyclic AMP of cardiac tissue [79, 80].
Hypertensive rats treated with taurine 50 mg taurine/100 g b.wt/day for 4 weeks showed a significant (P < 0.05) reduction in the levels of serum Chol., TG and LDL levels when compared with control rats. It was reported that the capability of taurine to lower cholesterol could be due to its effect on the change of cholesterol to bile acids [81]. A 31% reduction in aortic lesions in hyperlipidemic rabbits given 0.3 % taurine in drinking water for twenty-four weeks has also been described [82]. Clinically, taurine treatment 3 g per day has been demonstrated to recover lipid metabolism and decrease body weight in weighty subjects, in addition to decrease triglycerides levels and the atherogenic index [83]. In other clinical research, taurine supplementation six gram per day in normal young men consuming a huge amount of fat significantly decrease serum total cholesterol and low-density lipoprotein levels [84]. It must be distinguished that taurine has an antioxidant effect, so it may also decrease the oxidation of low-density lipoprotein and thereby reduce the process of atherosclerosis [85, 86].
The possible health benefits of taurine in hypertension are quickly emergent. Though additional research requires to be achieved, several experimental and numerous clinical researches established that taurine aids the cardiovascular system via a variety of mechanisms including an improved lipid profile, modulation of Ca2+ ion, antioxidant effects and antagonism of angiotensin II action. Since oxidative stress is recognized to cause intracellular Ca2+ overload, it is probable that the variation of Ca2+ ion by taurine may be intermediated via its antioxidant effects. Moreover, angiotensin II generates ROS, so it can be argued that the antagonism of angiotensin II actions by taurine might also because of its antioxidant effects [87, 88]. In 2007 it is reported that taurine can prevent endothelial cell malfunction induced by high glucose and oxidized low-density lipoprotein. Therefore, this action of taurine may be a vital mechanism for providing [89].
The obtained data in this study showed that, the activities of cardiac enzymes (CK, LDH and AST) in taurine animal group were significantly lower than in recovery group. With the progress of time the reduction was more marked at last interval. These results were confirmed by [90, 91].
Moreover, the administration of taurine to the hypertensive rats led to increase in the GPx. This improvement as mentioned above may be due to the bio-chemical powerful of taurine which works as a free radical scavenger, good antioxidant by decreasing the manufacture of oxygen free radicals, inhibition of the formation of thromboxane A2, decrease platelet aggregation, vasodilator of coronary vessels, due to inhibition of certain intramuscular enzymes, and improvement in the cyclic AMP of cardiac tissue. This is logic because of the molecular function of taurine, is to bring back endothelial nitric oxide creation to normal, in that way normalizing vascular function [90].
On the other hand, the treatment of hypertensive rats with taurine led to an obvious reduction in the level of MDA. These results might be attributed to the ability of the antioxidant taurine which play as a free radical scavenger to decrease the polarization of cell membrane, lipid peroxidation and increase the immune system defence of body and the β oxidation in mitochondrial matrix. The administration of taurine enhanced endothelin nitric oxide synthesis function, causing an enhancement of nitric oxide activity and a diminishing of O2· and ONOO· generation, in the same line with raising of taurine content in endothelium of hypertensive rats [91].
The increased levels of MDA, Endothelin-1, ADMA, and TNF-α and decrease in the Catalase, SOD TNO observed in hypertensive rats compared to normal group corroborated with several studies [50, 51]. The amelioration effects of taurine on hypertensive rats might be because of amplified synthesis or reduced depletion of high-density lipoprotein that may decline the oxidized lipid species in Low density lipoprotein particles, so protective them from atherogenesis. This may indeed reflect the beginning for the protection against atherosclerosis. The data also presented reduced in the levels of MDA and rise NO of the supplemented groups as compared with hypertensive control. The enhanced endothelial function detected in the taurine groups confirms the role of taurine antioxidant properties in the treatment of hypertension. Therefore, the molecular mechanisms underlying antioxidant effects of taurine on endothelial function might be mediated through oxidative stress suppression which caused a recovering antioxidant status and endothelial function as showed by the reduced level of lipid peroxidation index, MDA, and increased NO.
5. Conclusion
The data of the current research proposed that high NaCl diet loading encourages high blood pressure through oxidative stress, because it causes lipid peroxidation and impact the actions of antioxidant enzymes in the experimental animals. This is showed by the rising of total antioxidant status and actions of SOD, CAT, and GPx resulting from supplementation of taurine which was overcome the oxidative stress happened by sodium chloride. Also, the current data supported the role of oxidative stress in high blood pressure, and supplementations of taurine have the capability potential to avoid or heal the cardiovascular complications of hypertension.
Declarations
Author contribution statement
Marwan A. Ibrahim, Mostafa M. Eraqi, Faiz A. Alfaiz: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Oparil S., Weber M. second ed. Vol. 21. J Card Surg; 2006. p. 116. (Hypertension). [Google Scholar]
- 2.Guyton A.C. Blood pressure control: special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan N.M. eighth ed. Lippincott Williams & Wilkins Publishers; 2002. Kaplan's Clinical Hypertension. By. [Google Scholar]
- 4.Murray C.J.L., Lopez A.D. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349(9061):1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigo R., Passalacqua W., Araya J., Orellana M., Rivera G. Implications of oxidative stress and homocysteine in the pathophysiology of essential hypertension. J. Cardiovasc. Pharmacol. 2003;42(4):453–461. doi: 10.1097/00005344-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Miyajima K., Minatoguchi S., Ito Y. Reduction of QTc dispersion by the angiotensin II receptor blocker valsartan may be related to its anti-oxidative stress effect in patients with essential hypertension. Hypertens. Res. 2007;30(4):307–313. doi: 10.1291/hypres.30.307. [DOI] [PubMed] [Google Scholar]
- 7.Winkelmann B.R., Hager J., Kraus W.E. Genetics of coronary heart disease: current knowledge and research principles. Am. Heart J. 2000;140(4):S11–S26. doi: 10.1067/mhj.2000.109636. [DOI] [PubMed] [Google Scholar]
- 8.Rassler B. The renin-angiotensin system in the development of salt-sensitive hypertension in animal models and humans. Pharmaceuticals. 2010;3(4):940–960. doi: 10.3390/ph3040940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oparil S., Zaman M.A., Calhoun D.A. Pathogenesis of hypertension. Ann. Intern. Med. 2003;139(9):761–776. doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]
- 10.Grossman E. Does increased oxidative stress cause hypertension? Diabetes Care. 2008;31:S185–S189. doi: 10.2337/dc08-s246. [DOI] [PubMed] [Google Scholar]
- 11.Yasunari K., Maeda K., Nakamura M., Yoshikawa J. Oxidative stress in leukocytes is a possible link between blood pressure, blood glucose, and C-reacting protein. Hypertension. 2002;39(3):777–780. doi: 10.1161/hy0302.104670. [DOI] [PubMed] [Google Scholar]
- 12.Lacy F., O'Connor D.T., Schmid-Schönbein G.W. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J. Hypertens. 1998;16(3):291–303. doi: 10.1097/00004872-199816030-00006. [DOI] [PubMed] [Google Scholar]
- 13.Berry C., Hamilton C.A., Brosnan M.J. Investigation into the sources of superoxide in human blood vessels: angiotensin II increases superoxide production in human internal mammary arteries. Circulation. 2000;101(18):2206–2212. doi: 10.1161/01.cir.101.18.2206. [DOI] [PubMed] [Google Scholar]
- 14.Touyz R.M., Schiffrin E.L. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J. Hypertens. 2001;19(7):1245–1254. doi: 10.1097/00004872-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Taniyama Y., Griendling K.K. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42(6):1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 16.Versari D., Daghini E., Virdis A., Ghiadoni L., Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br. J. Pharmacol. 2009;157(4):527–536. doi: 10.1111/j.1476-5381.2009.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Félétou M., Köhler R., Vanhoutte P.M. Endothelium-derived vasoactive factors and hypertension: possible roles in pathogenesis and as treatment targets. Curr. Hypertens. Rep. 2010;12(4):267–275. doi: 10.1007/s11906-010-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueki I., Roman H.B., Valli A., Fieselmann K., Lam J., Peters R., Hirschberger L.L., Stipanuk M.H. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am. J. Physiol. Endocrinol. Metab. 2011;301:E668–E684. doi: 10.1152/ajpendo.00151.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Yang R., Liu X., Zhou Y., Qu C., Kikuiri T., Wang S., Zandi E., Du J., Ambudkar I.S., Shi S. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca (2+) channel sulfhydration. Cell Stem Cell. 2014;15:66–78. doi: 10.1016/j.stem.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abebe W. Effects of taurine on the reactivity of aortas from diabetic rats. Life Sci. 2008;82:279–289. doi: 10.1016/j.lfs.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Tan B., Jiang D.J., Huang H., Jia S.J., Jiang J.L., Hu C.P., Li Y.J. Taurine protects against low-density lipoprotein-induced endothelial dysfunction by the DDAH/ADMA pathway. Vasc. Pharmacol. 2007;46:338–345. doi: 10.1016/j.vph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Chiba Y., Ando K., Fujita T. The protective effects of taurine against renal damage by salt loading in Dahl salt-sensitive rats. J. Hypertens. 2002;20:2269–2274. doi: 10.1097/00004872-200211000-00027. [DOI] [PubMed] [Google Scholar]
- 23.NRC, National Research Council . Nation. Acad. Sci.; Washington, DC., U.S.A: 1977. Nutrient Requirements of Domestic Animals, Nutrient Requirements of Rat. [Google Scholar]
- 24.Tian N., Thrasher K.D., Gundy P.D., Hughson M.D., Jr Manning R.D. Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitivity hypertension. Hypertension. 2005;45(5):934–939. doi: 10.1161/01.HYP.0000160404.08866.5a. [DOI] [PubMed] [Google Scholar]
- 25.Byung I.C., Ah Y.K., Joon K.H. Hepatobiliary and pancreatic: unusual pancreatic lesions. J. Gastroenterol. Hepatol. 2001;14:827. doi: 10.1046/j.1440-1746.1999.1942a.x. [DOI] [PubMed] [Google Scholar]
- 26.Smeltzer S.C., Bare B.G., Hinkle J.L., Cheever K.H. twelfth ed. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelpha: 2010. Brunner & Suddarth's Textbook of Medical-Surgical Nursing. [Google Scholar]
- 27.Watson D. Calorimetric test for cholesterol lipid clearing factor. Clin. Chem. Acta. 1960;5:637. [Google Scholar]
- 28.Fossati P., Burghen G.A., Li H., Hudson M.M., Kun L.E. Serum triglycerides determined calorimetrically with an enzyme that produce hydrogen peroxide. Clin. Chem. 1982;8:2078–2082. [PubMed] [Google Scholar]
- 29.Freidewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 30.Rotruk J.T., Pope A.L., Gauther H.E. Selenium: biochemical roles as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 31.Malstrom B., Andreasson L., Reinhammer B. In: The Enzymes. Boyer P., editor. XIIB, Academic Press; New York: 1975. p. 533. [Google Scholar]
- 32.Johansson L.H., Borg L.A.H. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988;174:331–336. doi: 10.1016/0003-2697(88)90554-4. [DOI] [PubMed] [Google Scholar]
- 33.Reitman S., Frankel S. A colorimetric methods for the glutamic pyruvate transaminase. Am. J. Clin. Pathol. 1957;18:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 34.Szasz G., Gerhardt W., Gruber W. Creatine kinase in serum: further study of adenylate kinase inhibitors. Clin. Chem. 1977;23:1888–1892. [PubMed] [Google Scholar]
- 35.Weisshaar H.D., Prasad M.C., Parker R.S. Estimation of lactate dehydrogenase in serum/plasma. Med. Welt. 1975;26:387–391. [Google Scholar]
- 36.Böger R.H., Bode-Böger S.M., Szuba A., Tangphao O., Tsao P., Chan J.R., Blaschke T.F., Cooke J.P. Asymmetric dimethylarginine: a novel risk factor for endothelial dysfunction. Its role in hypercholesterolemia. Circulation. 1998;98:1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 37.Wakisaka S., Terui N., Suzuki H. Endothelin-1 kinetics in plasma urine, and blister fluid in burn patients. Ann. Plast. Surg. 1996;37(3):305–309. doi: 10.1097/00000637-199609000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 39.Beutler B., Greenwald D., Hulmes J.D. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- 40.Niehaus W.G., Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968;6(1):126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 41.Milton J.S., Corbert J.J., Teer P.M. D.C. Health and Company; Cannada: 1986. Introduction to Statistics 3rded. [Google Scholar]
- 42.Duncan D.B. Multiple ranges and multiple F- test. Biometrics. 1955;11:1–42. [Google Scholar]
- 43.Snedecor G.W., Cochran W.G. seventh ed. Two state University press; Ames Iowa, U.S.A: 1982. Statistical Methods. [Google Scholar]
- 44.Adedaraa Isaac A., Alakea Sanmi E., Adeyemoa Mercy O., Olajidea Laide O., Ajibadeb Temitayo O., Farombi Ebenezer O. Taurine enhances spermatogenic function and antioxidant defense mechanisms in testes and epididymis of L-NAME-induced hypertensive rats. Biomed. Pharmacother. 2018;97(2018):181–189. doi: 10.1016/j.biopha.2017.10.095. [DOI] [PubMed] [Google Scholar]
- 45.Meneton P., Jeunemaitre X., De Wardener H.E., Macgregor G.A. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol. Rev. 2005;85(2):679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 46.Kobori H., Nishiyama A., Abe Y., Navar L.G. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41(3 I):592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kagota S., Tamashiro A., Yamaguchi Y. Downregulation of vascular soluble guanylate cyclase induced by high salt intake in spontaneously hypertensive rats. Br. J. Pharmacol. 2001;134(4):737–744. doi: 10.1038/sj.bjp.0704300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cruz Antonio, Rodriguez-Gomez Isabel, Wangensteen Rosemary, Quesada Andres, Perez-Abud Rocıo, Osuna Antonio, Vargas Miguel Angel, Moreno Juan Manuel. Vol. 2011. Hindawi Publishing Corporation; 2011. Effects of clofibrate on salt loading-induced hypertension in rats; pp. 1–8. (J. Biomed. Biotechnol.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dandare A., Isah S.A., Ladan M.J., Mainasara A.S., Saidu Y. Serum glucose and lipid profile in salt induced metabolic syndrome rats treated with camel milk. Int. J. Sci. Eng. Res. 2015;6(9):734–740. [Google Scholar]
- 50.Lakshmana N.K., Deepthi J., Rao Y.N., Deedi K.M. Study of lipid profile, serum magnesium and blood glucose in hypertension. Biol. Med. 2010;2(1):6–16. [Google Scholar]
- 51.Biswas U.K., Kumar A. A study on lipid profile, oxidation stress and carbonic anhydrase activity in patients with essential hypertension. J. Clin. Diagn. Res. 2010;4(6):3414–3420. [Google Scholar]
- 52.Ide N., Lau B.H. Garlic compounds protect vascular endothelial cells from oxidized low-density lipoprotein- induced injury. J. Pharm. Pharmacol. 1997;49:905–911. doi: 10.1111/j.2042-7158.1997.tb06134.x. [DOI] [PubMed] [Google Scholar]
- 53.Lau B.H. Suppression of LDL oxigation by garlic. J. Nutr. 2001;131:9585–9590. doi: 10.1093/jn/131.3.985S. [DOI] [PubMed] [Google Scholar]
- 54.Clairbone A. Catalase activity. In: Green-Wald R.A., editor. Handbook of Methods for Oxygen Radical Research. CRC Press Inc; Boca Raton, FL: 1986. pp. 283–284. [Google Scholar]
- 55.Packer L. Cell regulation by thiol antioxidants: from glutathione to lipoate to anethole dithiolethione. In: Packer L., Traber M.G., Xin W., editors. Proceedings of the International Symposium on Natural Antioxidants Molecular Mechanisms and Health Effects. AOCS Press; Champaign, Ill: 1995. pp. 223–235. [Google Scholar]
- 56.Griffiths N.R., Moller L., Bartosz G., Bast A., Bertoni-Freddari C., Collins A., Cooke M., Haenen G., Hoberg A.M., Loft S., Lunec J., Olinski R., Parry J., Pompella A., Poulsen H., Verhagen H., Astley S.B. Biomarkers. Mol. Asp. Med. 2002;23:101–208. doi: 10.1016/s0098-2997(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 57.Mach F. Inflammation is a crucial feature of atherosclerosis and a potential target to reduce cardiovascular events. Handb. Exp. Pharmacol. 2005:697–722. doi: 10.1007/3-540-27661-0_26. [DOI] [PubMed] [Google Scholar]
- 58.Johnson R.A., Freeman R.H. Sustained Hypertension in the rat induced by chronic blockade of nitric oxide production. Am. J. Hypertens. 1992;5:919–922. doi: 10.1093/ajh/5.12.919. [DOI] [PubMed] [Google Scholar]
- 59.Sigmon D.H., Beierwaltes Nitric oxide influences blood flow distribution in renovascular hypertension. Hypertension. 1994;23(I):134–139. doi: 10.1161/01.hyp.23.1_suppl.i34. [DOI] [PubMed] [Google Scholar]
- 60.Vaziri N.D., Wang X.O., Oveisi F., Rad B. Induction of oxidative stress by glutathione depletion cases severs hypertensive in normal rats. Hypertension. 2000;36:142–146. doi: 10.1161/01.hyp.36.1.142. [DOI] [PubMed] [Google Scholar]
- 61.Berry C., Brosnan L., Fennell L., Hamilton A. Oxidative stress and vascular damage in hypertension. Nephrol. Hypertens. 2001;10:247–255. doi: 10.1097/00041552-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 62.Cross A.R., Jones O.T. Enzymatic mechanisms of superoxide production. Biochim. Biophys. Acta. 1991;1057:281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- 63.Rajagopalan S., Kurz S., Munzel T., Tarpey M., Freeman B. Angiotensin-II mediated hypertension in the rat increase vascular superoxide production via membrane NADH/NADPH oxidase activation. J. Clin. Investig. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia Y., Tsai A.L., Berka V., Zweier J.L. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J. Biol. Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 65.Cooke J.P. Does ADMA cause endothelial dysfunction? Arterioscler. Thromb. Vasc. Biol. 2000;20:2032–2037. doi: 10.1161/01.atv.20.9.2032. [DOI] [PubMed] [Google Scholar]
- 66.Valkonen V.P., Paiva H., Salonen J.T. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet. 2001;358:2127–2128. doi: 10.1016/S0140-6736(01)07184-7. [DOI] [PubMed] [Google Scholar]
- 67.Zoccali C., Bode-Böger S.M., Mallamaci F., Benedetto F.A., Tripepi G., Malatino L. Asymmetric dimethylarginine (ADMA): an endogenous inhibitor of nitric oxide synthase predicts mortality in end-stage renal disease (ESRD) Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 68.Vallance P., Leiper J. Cardiovascular biology of the asymmetric dimethylarginine: dimethylarginine dimethyl-aminohydrolase pathway. Arterioscler. Thromb. Vasc. Biol. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 69.Kielstein J.T., Donnerstag F., Gasper S., Menne J., Fliser D., Kielstein A., Martens-Lobenhoffer J., Scalera F., Bode-Böger S.M., Cooke J. Asymmetrical dimethylarginine inhibits shear stress-induced nitric oxide release and dilation and elicits superoxide-mediated increase in arteriolar tone. Stroke. 2006;37(12) 2871 – 2871. [Google Scholar]
- 70.Unger T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am. J. Cardiol. 2002;89:3A–10A. doi: 10.1016/s0002-9149(01)02321-9. [DOI] [PubMed] [Google Scholar]
- 71.Deswal A., Petersen N.J., Feldman A.M., Young J.B., White B.G., Mann D.L. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 72.Singal P.K., Kirshenbaum L.A. A relative deficit in antioxidant reserve may contribute in cardiac failure. Can. J. Cardiol. 1990;6:47–49. [PubMed] [Google Scholar]
- 73.Doroshow J.H., Locker G.Y., Myers C.E. Enzymatic defenses of the mouse heart against reactive oxygen metabolites. J. Clin. Investig. 1980;65:128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abebe W., Mozaffari M.S. Effects of chronic taurine treatment on reactivity of the rat aorta Amino Acids, 19: 615-623. Bidri M, Choay P (2003) Taurine: a particular amino acid with multiple functions. Ann. Pharm. Fr. 2000;61:385–391. doi: 10.1007/s007260070011. [DOI] [PubMed] [Google Scholar]
- 75.Chahine R., Hanna J., Bassil C., Rihana N., Mounayar A., Greige H. Beneficial effect of taurine in spontaneous hypertensive rats: implication of its antioxidant activity. Afr. J. Pharm. Pharmacol. 2011;4(12):874–877. [Google Scholar]
- 76.Ahmadian M., Roshan D., Ashourpore E. Taurine supplementation improves functional capacity, myocardial oxygen consumption and electrical activity in heart failure. J. Diet. Suppl. 2017;14:422–432. doi: 10.1080/19390211.2016.1267059. [DOI] [PubMed] [Google Scholar]
- 77.Bouckenooghe T., Remacle C., Reusens B. Is taurine a functional nutrient? Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:728–733. doi: 10.1097/01.mco.0000247469.26414.55. [DOI] [PubMed] [Google Scholar]
- 78.Rosa F.T., Freitas E.C., Deminice R., Jordao A.A., Marchini J.S. Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind placebo-controlled study. Eur. J. Nutr. 2014;53:823–830. doi: 10.1007/s00394-013-0586-7. [DOI] [PubMed] [Google Scholar]
- 79.Yamori Y., Taguchi T., Hamada A., Kunimasa K., Mori H., Mori M. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J. Biomed. Sci. 2010;17:S6. doi: 10.1186/1423-0127-17-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yanagita T., Han S.Y., Hu Y., Nagao K., Kitajima H., Murakami S. Taurine reduces the secretion of apolipoprotein B100 and lipids in HepG2 cells. Lipids Health Dis. 2008;7:38. doi: 10.1186/1476-511X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murakami S., Kondo Y., Sakurai T., Kitajima H., Nagate T. Taurine suppresses development of atherosclerosis in Watanabe heritable hyperlipidemic (WHHL) rabbits. Atherosclerosis. 2002;163:79–87. doi: 10.1016/s0021-9150(01)00764-x. [DOI] [PubMed] [Google Scholar]
- 82.Zhang M., Bi L.F., Fang J.H. Beneficial effects of taurine on serum lipids in overweight or obese non-diabetic subjects. Amino Acids. 2004;26:267–271. doi: 10.1007/s00726-003-0059-z. [DOI] [PubMed] [Google Scholar]
- 83.Mizushima S., Nara Y., Sawamura M., Yamori Y. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv. Exp. Med. Biol. 1996;403:615–622. doi: 10.1007/978-1-4899-0182-8_68. [DOI] [PubMed] [Google Scholar]
- 84.Murakami S., Sakurai T., Tomoike H., Sakono M., Nasu T., Fukuda N. Prevention of hypercholesterolemia and atherosclerosis in the hyperlipidemia- and atherosclerosis-prone Japanese (LAP) quail by taurine supplementation. Amino Acids. 2010;38:271–278. doi: 10.1007/s00726-009-0247-6. [DOI] [PubMed] [Google Scholar]
- 85.Gokce G., Ozsarlak-Sozer G., Oran I., Oktay G., Ozkal S., Kerry Z. Taurine suppresses oxidative stress-potentiated expression of lectin-like oxidized low-density lipoprotein receptor and restensosis in balloon-injured rabbit iliac artery. Clin. Exp. Pharmacol. Physiol. 2011;38:811–818. doi: 10.1111/j.1440-1681.2011.05612.x. [DOI] [PubMed] [Google Scholar]
- 86.Dhalla N.S., Temsah R.M., Netticadan T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 87.Guo X., Saini H.K., Wang J., Gupta S.K., Goyal R.K., Dhalla N.S. Prevention of remodeling in congestive heart failure due to myocardial infarction by blockade of the renin-angiotensin system. Expert Rev. Cardiovasc Ther. 2005;3:717–732. doi: 10.1586/14779072.3.4.717. [DOI] [PubMed] [Google Scholar]
- 88.Ulrich-Merzenich G., Zeitler H., Vetter H., Bhonde R.R. Protective effects of taurine on endothelial cells impaired by high glucose and oxidized low density lipoproteins. Eur. J. Nutr. 2007;46:431–438. doi: 10.1007/s00394-007-0682-7. [DOI] [PubMed] [Google Scholar]
- 89.Kaneyuki U., Ueda S., Yamagishi S. Pitavastatin inhibits lysophosphatidic acid-induced proliferation and monocyte chemoattractant protein-1 expression in aortic smooth muscle cells by suppressing Rac-1-mediated reactive oxygen species generation. Vasc. Pharmacol. 2007;46:286–292. doi: 10.1016/j.vph.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 90.Ripps H., Shen W. Review: taurine: a “very essential” amino acid. Mol. Vis. 2012;18:2673–2686. [PMC free article] [PubMed] [Google Scholar]
- 91.Wang G.G., Li W., Lu X.H., Zhao X., Xu L. Taurine attenuates oxidative stress and alleviates cardiac failure in type I diabetic rats. Croat. Med. J. 2013;54:171–179. doi: 10.3325/cmj.2013.54.171. [DOI] [PMC free article] [PubMed] [Google Scholar]