Abstract
The corrosion inhibition of Luffa cylindrica Leaf Extract (LCLE) was investigated using gravimetric, depth of attack and surface analysis techniques. Effect of inhibitor concentrations (0.50–1.00 g/l), temperatures (30–60 °C) and immersion time (4–12 h) was studied on the Inhibition Efficiency (IE) of the extract on Mild Steel (MS) immersed in a 0.5 M HCl solution. The constituents of the proposed inhibitor were identified by using a GC-MS. The media solutions and adsorbed film on MS were characterized using FTIR Spectrophotometer. SEM microgram and surface tester were applied for studying surface morphology and depth of attack profile. The optimum IE of 87.89% was obtained. The LCLE adsorption on MS followed Langmuir isotherm and pseudo-second-order adsorption kinetics. Activation energy (28.71 kJ/mol), entropy (- 0.15 kJ/mol. K), average enthalpy (-28.00 kJ/mol) and Gibbs free energy (-11.43 kJ/mol) obtained at optimum condition indicate exothermic process and physical adsorption mechanism. The result obtained in this study compared well with many reported green inhibitors for MS corrosion.
Keywords: Chemical engineering, Chemical synthesis, Materials characterization, Electrochemical engineering, Adsorption, Luffa cylindrica, Corrosion inhibition, Efficiency, Corrosion rate, Optimization, Isotherms, Thermodynamics, Kinetics
Chemical engineering; Chemical synthesis; Materials characterization; Electrochemical engineering; Adsorption; Luffa cylindrica; Corrosion inhibition; Efficiency; Corrosion rate; Optimization; Isotherms; Thermodynamics; Kinetics
1. Introduction
In many industrial operations, the addition of inhibitors to process fluids to minimize the rate of metal corrosion is very common. Chemicals are usually applied on metal surfaces as part of the final finishing procedures prior to plating, painting, or storage (Bentiss et al., 1999). According to Patricia et al. (2017), the chemicals are capable of removing scales, soil and light rust from the metal surfaces. Apart from this, they often contained about 1 % organic corrosion inhibitors by volume of the acid such as hydrochloric acid. Synthetic inhibitors have been widely applied to protect metal surfaces against corrosion (Zhang et al., 2012; Markhali et al., 2013). However, these inhibitors are toxic, expensive with environmental and safety issues. Alternative sources including natural products, extracts from plants, and other environmental benign organic sources have been widely reported (Sharma et al., 2015).
Many organic compounds have been tested for corrosion inhibition. The functionality of these compounds has been attributed to their electronegative functional groups and presence of π electrons in triple or conjugated double bonds. They also contain nitrogen, sulphur or oxygen atoms in their structures. Their mode of operation is by physical or chemical interactions between their molecules and the metal surfaces (Patricia et al., 2017). Several plant extracts including an extract from Carica papaya, Rosmarinus Officinalis, Damsissa, Murrayakoenigii, cashew, mango, Uncaria gambir and Fiscusycomorus had been investigated (Ebenso and Ekpe, 1996; Kliskic et al., 2000; Abdel-Graber et al., 2006; Ashish and Quraishi, 2010; Da Rocha et al., 2010; Hussain and Kassim, 2011; Ogwo et al., 2017; Ogunleye et al., 2018). According to Helen et al. (2014), these plants possess adequate cyclic organic phytochemicals, nitrogen, sulphur and oxygen atoms that are responsible for their inhibition properties. The large scale synthesis of various natural plant extract is faced with many challenges. The chief of which is the isolation of specific components of the plant extract with inhibitory characteristics. Nevertheless, many natural plant extracts have proven efficient as corrosion inhibitors (Ji et al., 2011; Kamal and Sethuraman, 2012; Yaro et al., 2013). To gain insight on the working and applicability of natural extracts for commercial purposes, inhibition mechanism, kinetics and process thermodynamics was investigated on a pilot scale. The extract, after careful concentration under controlled temperature, may however, be added in the right mix to paints of organic solvent base for easy dissolution and dispersion.
Corrosion of metal takes place whenever there is an interaction of two different electrochemical reactions on the material surface. With detail knowledge of these electrochemical processes, potential theory is commonly applied for the prediction of corrosion rate. In many cases, these data are not available which limit the application of mixed potential theory with confidence. Laboratory measurements are therefore made and interpreted in terms of mixed potential theory such as polarization resistance, electrochemical impedance spectroscopy (EIS) and electrochemical noise (Markhali et al., 2013; Ostovari et al., 2009; Kliskic et al., 2000). These methods involve the use of advanced instruments with expert advice that are often not available to many researchers. Gravimetric-based method such as weight loss provides integrated mass loss information from corrosion that has occurred over some period of time. Because of simplicity and economic friendliness, gravimetric techniques are commonly used to measure general corrosion rate (Ogunleye et al., 2018). However, gravimetric-based techniques are unsuitable for continuous field monitoring of corrosion rates.
This present study deployed gravimetric and qualitative techniques to evaluate another eco-friendly material (LCLE), for use as an engineering inhibitor on MS submerged in a 0.5 M HCl solution. The optimum condition, the kinetics and thermodynamic parameters for maximum corrosion inhibition using LCLE were established.
2. Materials and equipment
The metal coupons utilized, chemical reagents, consumables and Luffa cylindrical plant were all sourced locally. Sensitive equipment such as soxhlet apparatus, evaporator, dryer, water bath, weighing balance and surface tester (PCE-RT 11) were employed for the test. Characterisation of materials and coupons was achieved by Gas Chromatography equipped with mass spectrophotometer (GC-MS; AGILENT 5789A), Fourier Transform Infra-Red (FTIR; BRUKER TENSOR 27) and Scanning Electron Microscopy (SEM; ZEISS equipment).
2.1. Extraction and analysis of Luffa cylindrica leaf extract
The Luffa cylindrical plant is a member of the cucurbitaceous family with smooth and rounded shaped fruits. It is called sponge gourd, vegetable sponge, bath sponge or dishcloth gourd (Velmurugan et al., 2011). Luffa plants grow by climbing on other physical solid materials. Typical leaves of a matured Luffa cylindrical plant is shown in Figure 1. The dried leaves of Luffa cylindrica plant was pulverized mechanically and screened to approximately 20 μm prior to extraction. Approximately 100 g of LCEC powder was soaked in 1000 ml of ethanol in a soxhlet extraction apparatus. The extract was then concentrated in a rotary evaporator and stored in an air-tight sterile container. The detail description of the extraction process is available elsewhere (Noyel et al., 2015). The constituent of the extract was identified using GC-MS. The dominant functional groups present in the extract before and after the corrosion study were identified using FT-IR.
Figure 1.
Luffa cylindrica plant.
2.2. Corrosion medium and coupon preparation
A 36 w/w % HCl (S.G = 1.18 gdm−3) was serially diluted with distilled water to obtain desired 0.5 M HCl solution. Different concentrations (0.2–1.0 g/L) of LCLE were prepared for corrosion inhibition study following experimental design technique. The experiment without inhibitor (zero inhibitor) served as the control for all experiments. The corrosion media was sonicated and extract-HCl solution was homogenized using ultrasonic homogenizer (150VT) before the experiments in accordance with Singh et al. (2016).
Before the corrosion study, the coupon samples were carefully washed with a soft brush in high purity water, rinsed with acetone and then oven dried. This was as well done before measurements were done to minimize experimental error.
2.3. Weight loss method
A 3.5 cm × 3.0 cm x 0.3 cm MS coupons of composition 0.13, 0.18, 0.39, 0.40, 0.04 and 0.025, for C, Si, Mn, P, S, and Cu, respectively were used. The iron constituent makes up the composition to 100%. The corrosion measurements were made by recording the weight losses of MS coupons submerged in 100 ml corrosion medium in the presence and absence of inhibitor. A full factorial design was employed with all test carried out at 27 (±0.5) °C. The inhibitor concentrations were varied between 0 and 1.2 g/L at an interval of 0.2 g/L for periods of 24, 48, 72, 96, 144 and 168 h. After the experiment, equilibrium parameters were established and used for comprehensive batch corrosion studies. For each experiment, the coupon was weighed before the experiment (m1) and after designated period, it was removed from test solution, washed, dried and then reweighed (m2). The measurements were made in triplicate using OHAUS AX124 analytical balance of sensitivity ±0.0001 g. The mean values were noted and utilized for weight loss calculation using following equations:
| (1) |
| (2) |
| (3) |
| (4) |
Where, CR is the corrosion rate of the coupon of surface area (A, cm2) over time t. W is the weight loss. θ is the degree of inhibitor surface coverage and IE the inhibition efficiency.
2.4. Surface depth profiles
The surface morphologies of MS in the absence and presence of LCLE were studied to evaluate the nature and extent of surface roughness using SEM. To further determine the capability of LCLE as inhibitor, surface depth profiles were measured using surface tester (PCE-RT11). Its mode of operation is provided in Cai et al (2010). The peak height of irregularities (Rz) over a 2.5 mm sampling length was recorded. Similar to weight loss, the corrosion rate of MS in terms of Rz was estimated using the following equations:
| (5) |
| (6) |
| (7) |
2.5. Batch corrosion inhibition experiment
A set of experiments were carried out to study the effects of solution volume to coupon are ratio. This was done by varying the SVAR between 10 – 50 ml/cm3 while temperature immersion time and inhibition concentration were varied between (30–60 °C) (4–12 h) and (0.2–1.0 g/L), respectively. Another set of experiments were performed using 3- level factorial design to determine the optimal process parameters that maximize the inhibition of MS by LCLE in 0.5 M HCl solution. Here, the factors selected include concentration of inhibition, temperature and time of immersion. The SVAR was fixed at 40 ml/cm3 for all experimental runs. Table 1 shows the summary statistics for the three selected factors.
Table 1.
Process variable and their levels.
| Factor | Name | Units | Low Actual | High Actual | Low Coded | High Coded |
|---|---|---|---|---|---|---|
| Concentration | g/l | 0.1 | 0.5 | -1 | 1 | |
| Temperature | K | 303 | 363 | -1 | 1 | |
| Time | h | 4 | 12 | -1 | 1 |
For each experimental run, corrosion rate (CR) of the coupon of surface area (A, cm2) over time t was calculated using both the weight loss (W) and depth profile techniques. The degree of inhibitor surface coverage (θ) and efficiency of inhibition (IE) were calculated.
2.6. Data analysis
Analysis of data and development of response surface models for CR and IE was achieved by Analysis of variance (ANOVA) and response surface methodology. The statistics considered are the F and p- values obtained at 95% confidence level. The goodness of models was ascertained using correlation coefficients (R2 and adjusted R2).
2.7. Adsorption isotherm
Adsorption isotherms explain the degree of interaction of molecules of various inhibitors with the metal surface (Ashish and Quraishi, 2010). Since corrosion inhibition using organic inhibitor occurs with the development of protective films caused by the adsorbed extract molecules on metal surfaces. Isotherm equations were used to confirm that the inhibition mechanism is truly adsorption. Also, to establish the closest equation that relates the concentration of inhibitors to the adsorbed concentration at saturation, the empirical equations such as exponential, hyperbolic, logarithmic and power are difficult to associate with the given mechanisms of the adsorption process. The Freundlich, Langmuir, and Temkin isotherms apart from their simplicity, are easy to apply to derive complete information from their parameters to characterize the corrosion inhibition system.
Customarily, adsorption isotherms are expressed in the form of Eq. (8) (Ameer et al., 2000).
| (8) |
The inhibition mechanism is invariably accompanied by the adsorption of molecules on the metal surface (Yaro et al., 2013; Fallavena et al., 2006). The fraction of the surface covered (θ)at any time was calculated using Eq. (4). For proper understanding of the mechanism involved for the case of LCLE and MS, the following isotherms were investigated:
2.7.1. Langmuir isotherm
The following equations describe the Langmuir adsorption isotherm. The 999 in Eq. (10) is the concentration of water (g/L) in solution. R is universal gas constant and T, temperature in (K).
| (9) |
| (10) |
2.7.2. Freundlich isotherm
Eq. (11) is a linearized form of Freundlich isotherm. A plot of ϴ against C of data obtained at room temperature (303 K) was made on a log-log graph and isotherm parameters n and KAds, were obtained as slope and intersect of the straight line. This was repeated for 318 and 333 K at (4, 8 and 12 h) using optimum concentration of LCLE.
| (11) |
2.7.3. Tempkin Isotherm
Eq. (12) related the degree of surface coverage (θ) and inhibition concentration (C). After linearization using logarithmic transformation, the constant K and parameter “a” were obtained for Tempkin Isotherm at various temperatures and time from Eq. (13). This was achieved by making a plot of θ against C.
| (12) |
| (13) |
2.8. Corrosion inhibition kinetics
To determine optimum concentration of LCLE, it is necessary to explore dynamics of its inhibition. Corrosion rate in the presence of inhibitor and nature of its kinetics parameters dictate the selection of strategies suitable for effective corrosion control and management. The two kinetics models considered in this study are pseudo-first and second-order kinetic models represented by Eqs. (14) and (15), respectively.
| (14) |
| (15) |
K1 and K2 (min−1) are the rate constants obtained graphically following integration within necessary boundary limits. θe and θt represent degree of surface coverage at equilibrium and at a given time t.
2.9. Thermodynamic study
To determine the energy required, the heat involved and mechanism of corrosion in the presence or absence of inhibitor, the following thermodynamics properties were determined experimentally: the activation energy (Ea), enthalpy (ΔHa°), and entropy (ΔSa°). By fitting experimental data to linearized form of Arrhenius and transition state equations, plots were particularly employed to determine these parameters using the Arrhenius Eq. (16) and the transition state Eq. (17):
| (16) |
| (17) |
| (18) |
T represents the absolute temperature. A is the pre- exponential factor. N is Avogadro's number (6.02×1023 mol−1), h is Plank's constant (6.63×10 −34 J s−1), K is equilibrium constant and the constant denoted by 55.5 denotes the molar heat of adsorption of water.
3. Results and discussion
3.1. Chemical constituents of LCLE
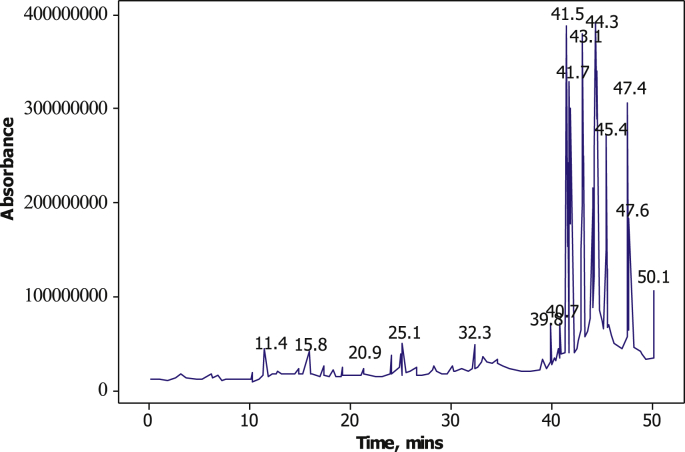
The GC-MS chromatograph showing the composition of LCLE is presented in Figure 2. The result shows that the principal active ingredient of LCLE is n-Hexadecanoic acid Dodecanoic acid with the highest peak value (22.18%) and retention time (39.894 min). The presence of carboxylic acid, hydroxyl and phenolic compounds suggested that the extract has a good corrosion inhibitory potential (Verma and Fahmida, 2016).
Figure 2.
GC-MS chromatograph for LCLE.
3.2. FTIR analysis of LCLE
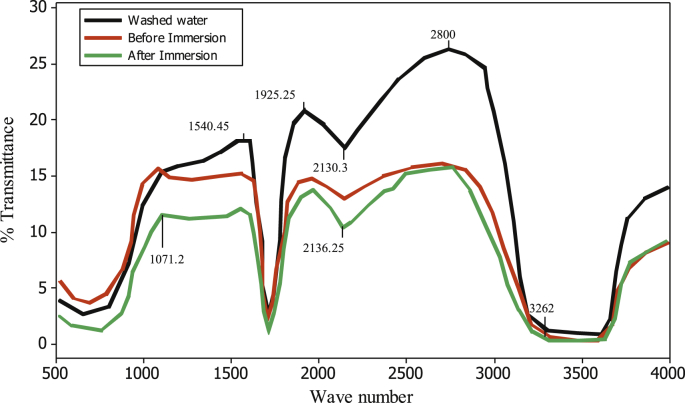
The IR spectra of corrosion medium were captured before and after the corrosion. In addition, IR of the films adsorbed on the MS surface was taken by washing the coupon with distilled water. These results are presented as shown in Figure 3. The IR Peak observed in the test solution before immersion of mild steel ranged from 538.30 – 3594.05 cm−1 while the IR peak of test solution after immersion ranged from 602.64 – 3620.05 cm−1. The coupon washed solution has IR Peak ranged from 588.13 – 3601.06 cm−1. The dominant functional groups present in the three solutions are the wide broad band O–H group (3594.85–3262.40 cm−1), C ≡ C stretch (2090.91cm−1), C = O Carboxyl group (1638.14 cm−1) and C–H bend alkyl group (605.80 cm−1). Similar observations were also reported in the previous study (Okewale et al., 2016). According to Owate et al. (2014), the prevalence of carbonyl and double bond of carbon group in extracts of plants is an indication of corrosion inhibition characteristics.
Figure 3.
FTIR spectra for LCLE test solution (a) before immersion with IR peak ranged from 538.30 – 3594.05 cm-1 (b) after immersion with IR peak ranged from 602.64 – 3620.05 cm-1 and (c) washed coupon film with IR peak ranged from 588.13 – 3601.06 cm-1. These ranges suggest the presence and synergetic effect of O–H, C ≡ C stretch, C = O, Carboxyl and C–H groups in corrosion inhibition process.
The FTRIR analysis of the LCLE indicated the prevalence of oxygen- and nitrogen-containing compounds (hydroxy aromatic compound) such as flavonoids, tannins, pectins, and other organic compounds. The high value of corrosion inhibition efficiency recorded using LCLE in the 0.5 M HCl solution containing MS is attributed to the presence of hydroxy aromatic compounds. Tannins, for example, would form a passivating layer of tannates on the MS surface thereby slow down the corrosion rate. In addition, several OH groups in the extract could form strong links with other molecules which result in complexes formation with metals that causes blockage of micro anodes generated on the metal surfaces when in contact with electrolytes, and, hence, retard subsequent dissolution of the metal.
3.3. Surface morphology of test coupons
The surface characteristic of polished MS, the MS immersed in 0.5 M HCl without inhibitor (control) and MS immersed in 0.5 M HCl for 12 h in the presence of 1.0 g/L of LCLE at 60 °C are shown in Figure 4. The SEM image of MS coupon before immersion in the 0.5 M HCl solution containing no inhibitor (Figure 4b), revealed the presence of porous layers full of micro cracks. Consequently, the Chemicals can easily permeate the steel and can corrode the plate. However, in the presence of inhibitor (Figure 4c), the MS surface is notably improved considering its smoothness. It is also observed to be less porous with very minimal micro-cracks which are an indication of a reduction in corrosion rate. This improvement in surface morphology is due to the formation of a protective layer by LCLE on the surface of the metal. Therefore, using surface analysis it is shown that LCLE has a great tendency to adsorb to the MS surface and can be regarded as a good inhibitor for MS used in acidic medium.
Figure 4.
SEM images showing (a) unexposed polished MS (b) corroded surfaces of the exposed MS to 0.5 M HCl with no inhibitor (blank) and (c) the protected surfaces of the immersed MS in 0.5 m HCl +1 g/L of extract in 0.5 M HCl solution at test temperature of 60 °C after 12 h.
4. Sensitivity of CR to SVR, time and temperature
Corrosion rate was observed to vary with coupons SVR. This was investigated experimentally for varied range of SVR (10–50 ml/m2), concentration (0.2–1.0 g/L) over corrosion period of 168 h at 27 °C. The optimum SVR was obtained at 40 ml/m2, beyond which the CR remains relatively constant for irrespective of inhibition concentration used as shown in Figure 5a. The effect of time on corrosion rates for different inhibitor concentrations (0.2–1.2 g/L) at 27 °C for fixed SVR 40 ml/m2 is shown in Figure 5b. The CR was observed to fall as immersion time and concentration of LCLE increases. However, a rapid reduction from 54.2 – 21.7 g/m2.h of CR after 120 h of immersion was observed for control experiment with zero inhibitions. Upon addition of a mere 0.2 g/L LCLE, a tremendous reduction of CR from 24.16 – 4.38 g/m2.h was observed over equal time interval between 0 and 120 h.
Figure 5.
(a) Showing SVAR effects on CR. There is no more CR changes beyond SVAR of 40 ml/cm3 for different inhibitor dosages (b) Showing CR versus immersion time for different inhibitor dosage. As immersion time increased, more protective film layer are formed on MS thereby slowed down the CR (c) Showing CR increases with temperature. More CR reduction observed with inhibited system compared with the control.
However, much more reduction of CR was observed after 120 h when inhibitor concentrations were increased above 0.2 g/L. In all the experiments, between 120 and 168 h, there was a gentle CR reduction from 21.7 to 19.5 g/m2.h for control experiment and 4.36 to 2.4 g/m2.h for the experiment with 0.2 g/L of LCLE. These observations agreed to Yaro et al. (2013) and Nathiya and Raj (2017).
Figure 5c show that CR of MS increases with temperature. The rate of corrosion, however, goes up more rapidly in the blank solution compared with inhibited systems. This can be explained by the increasing tendency of metals to dissociate quickly thereby causing partial desorption of the inhibitor from the metal surface as temperature increases. After 4 h of immersion, doubling the concentration of the extract from 0.5 to 1 g/l at 30, 45 and 60 °C, the corrosion rate decreases by 12.7, 23.7 and 18.0%, respectively. After 8 h at 0.5, 0.75 and 1.0 g/l of LCLE at 30 °C, the corrosion rate decreases by 9.7, 11.8 and 9.1% respectively as against 68.0, 67.4 and 66.0% when the temperature increases from 30 – 60 °C for 0.5 0.75 and 1.0 g/l of the LCLE respectively.
5. Corrosion depth measurements
Table 2 shows the measured CR and IE as a function of surface maximum depth of attack (Rz). It was observed at all temperature and immersion time that the pitting of the MS was reduced drastically as the concentration of LCLE increases compared to the control solution. This indicates that the corrosion of MS was effectively inhibited in the presence of the molecules of LCLE, and the etching of the MS surface is sensitive to the concentration of LCLE.
Table 2.
Surface maximum depth parameter at different temperature, concentration of Luffa cylindrica extract and time of immersion of MS in 0.5 MHCl.
| Temp K |
Inh. Conc. (g/L) |
Time (hr) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 |
8 |
12 |
||||||||
| RZ |
CR |
IE |
RZ |
CR |
IE |
RZ |
CR |
IE |
||
| (μm) | (μm/hr) | (%) | (μm) | (μm/hr) | (%) | (μm) | (μm/hr) | (%) | ||
| 303 | 0.00 | 1.01 | 0.2525 | 1.84 | 0.2306 | 2.83 | 0.2360 | - | ||
| 0.50 | 0.30 | 0.0752 | 70.24 | 0.55 | 0.0684 | 70.34 | 0.81 | 0.0676 | 71.34 | |
| 0.75 | 0.29 | 0.0714 | 71.73 | 0.51 | 0.0639 | 72.27 | 0.76 | 0.0630 | 73.32 | |
| 1.00 | 0.26 | 0.0661 | 73.83 | 0.47 | 0.0587 | 74.53 | 0.67 | 0.0562 | 76.20 | |
| 0.00 | 3.02 | 0.7558 | 5.35 | 0.6681 | 7.40 | 0.6163 | - | |||
| 318 | 0.50 | 0.68 | 0.1694 | 77.58 | 1.19 | 0.1487 | 77.75 | 1.56 | 0.1301 | 78.89 |
| 0.75 | 0.61 | 0.1532 | 79.73 | 1.09 | 0.1361 | 79.64 | 1.43 | 0.1188 | 80.72 | |
| 1.00 | 0.53 | 0.1331 | 82.39 | 0.91 | 0.1138 | 82.97 | 1.21 | 0.1011 | 83.59 | |
| 0.00 | 5.93 | 1.4822 | 10.75 | 1.3443 | 15.97 | 1.3311 | - | |||
| 333 | 0.50 | 0.93 | 0.2314 | 84.38 | 1.57 | 0.1960 | 85.42 | 2.28 | 0.1899 | 85.73 |
| 0.75 | 0.88 | 0.2193 | 85.21 | 1.48 | 0.1848 | 86.25 | 2.11 | 0.1760 | 86.78 | |
| 1.00 | 0.78 | 0.1955 | 86.81 | 1.36 | 0.1697 | 87.38 | 1.92 | 0.1600 | 87.98 | |
Rz = maximum depth r = rate of attack IE = inhibition efficiency.
More so, it was evident that the IE of the LCLE increases as concentration increases. Inhibition efficiency recorded ranges from 70.24 to 88% at test conditions. Maximum IE (87.98%) was obtained with LCLE concentration 1.0 g/L for temperature up to 60 °C over 12 h of corrosion test.
6. Adsorption study
The result obtained from the various isotherms and thermodynamics was sufficient to explain the adsorption mechanism and inhibition potential of the LCLE molecules at the interface between the corrosion medium and MS. Table 3 shows the estimated parameters from the following isotherm graphs: Freundlich, Langmuir, and Temkin at different temperature and immersion time. The result revealed that the mechanism of LCLE adsorption on the surfaces of MS obeyed Langmuir isotherm at all studied temperature and time due to high values of R2 and with the slope close to unity. This implies monolayer coverage and homogenous distribution of the LCLE molecules on the MS surface as assumed by Langmuir equation (Meroufel et al., 2013).
Table 3.
Adsorption parameters for inhibition of MS in 0.5M HCl in the presence of LCLE at different temperature and time.
| 4hr |
8hr |
12hr |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 303K | 318K | 333K | 303K | 318K | 333K | 303K | 318K | 333K | |
| Freundlish isotherm | |||||||||
| slope | 0.0398 | 0.0984 | 0.0766 | 0.0321 | 0.100 | 0.072 | 0.038 | 0.0923 | 0.0819 |
| intercept | 0.0626 | 0.0849 | 0.1336 | 0.0592 | 0.083 | 0.128 | 0.055 | 0.0776 | 0.1202 |
| kf | 0.7352 | 0.1416 | 0.8658 | 0.7451 | 0.827 | 0.873 | 0.881 | 0.8364 | 0.7582 |
| n | 13.158 | 10.204 | 25.641 | 13.889 | 10.00 | 31.25 | 12.35 | 10.870 | 27.027 |
| R2 | 0.992 | 0.994 | 0.924 | 0.981 | 0.955 | 0.968 | 0.989 | 0.9450 | 0.9780 |
| Langmuir isotherm | |||||||||
| slope | 0.9234 | 0.9016 | 0.9602 | 0.928 | 0.900 | 0.968 | 0.918 | 0.9077 | 0.9622 |
| intercept | 0.1336 | 0.0849 | 0.0626 | 0.1278 | 0.083 | 0.059 | 0.120 | 0.0776 | 0.0550 |
| K (l/g) | 1.3602 | 1.2159 | 1.155 | 1.3421 | 1.209 | 1.146 | 1.319 | 1.1956 | 1.135 |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 1 | 0.999 | 0.999 | 1 |
| Temkin isotherm | |||||||||
| slope | 0.0549 | 0.0783 | 0.034 | 0.0522 | 0.080 | 0.028 | 0.060 | 0.0755 | 0.0329 |
| intercept | 0.7352 | 0.8224 | 0.8658 | 0.745 | 0.827 | 0.873 | 0.758 | 0.8363 | 0.8809 |
| B | 0.0549 | 0.0783 | 0.034 | 0.0522 | 0.080 | 0.028 | 0.060 | 0.075 | 0.0329 |
| KT (mol/J) | 45888 | 33767 | 81432 | 48261 | 33008 | 99952 | 41848 | 35253 | 84154 |
| AT | 6.5E+05 | 3.6E+04 | 1.1E+11 | 1.6E+06 | 3.0E+04 | 4.8E+13 | 2.9E+05 | 7.0E+04 | 4.2E+11 |
| R2 | 0.999 | 0.993 | 0.922 | 0.983 | 0.951 | 0.967 | 0.99 | 0.941 | 0.978 |
Also, the slope of the curve and its magnitude indicates existence and extent of electrostatic interaction of LCLE molecules with MS. Each of the molecules occupies one active site which agrees to Langmuir isotherm prediction of physisorption (Meroufel et al., 2013; Ogwo et al., 2017). Freundlich isotherm suggested a physical process as evident by the n values which ranged between 10 and 27.027. The value of n greater than unity at all temperature indicates adsorption of the extract on MS was based on heterogeneous interaction. However, the correlation coefficient R2 values obtained at different temperatures are quite low when compared with that from Langmuir isotherm. Similar observation was made for the Temkin isotherm. The Langmuir constant shows a higher constant value of 1.3602 L/g at 303K which indicates that the extract is strongly adhered on the MS surface at 303 K and 12 h (Aprael et al., 2013).
7. Thermodynamics studies
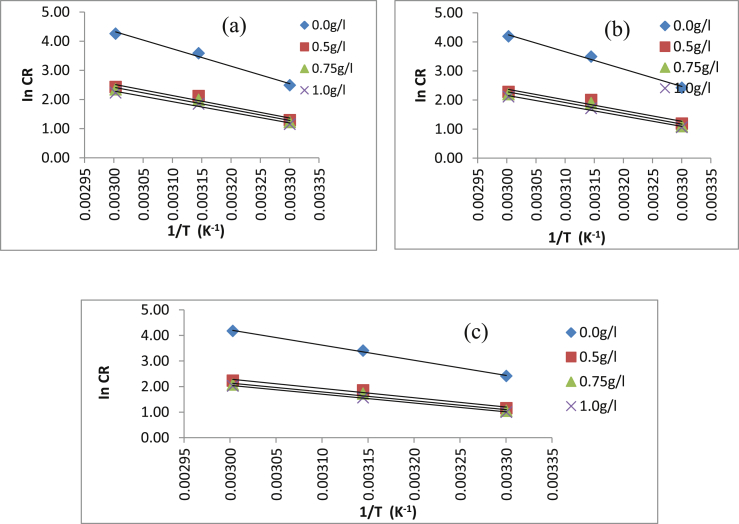
Figure 6 shows the Arrhenius plot used for estimating the Ea after 4, 8 and 12 h of immersion of MS in 0.5 M HCl in the presence and absence of different LCLE concentrations. The values of Ea estimated ranges from 28.7 - 32.07 kJ/mol for all concentrations of LCLE. According to Eddy and Ebenso (2008), Rani and selvaraj, (2010), the values of Ea< 80 kJ/mol indicate physical adsorption while Ea > 80 kJ/mol indicates chemical adsorption. Thus, the interaction of LCLE molecules that led to adsorption on MS surface followed physical mechanism.
Figure 6.
Arrhenius plot of ln CR vs 1/T for MS in the presence and absence of different concentrations of Luffa cylindrica extract after (a) 4 h (b) 8 h and (c) 12 h of immersion.
Figure 7 shows the transition state plot for control and inhibited system after 4, 8 and 12 h of corrosion process while Table 4 shows parameters estimated at different temperature and time. The enthalpy changes (ΔHAds) at different concentrations of LCLE and immersion time were negative which indicated exothermic (Iloamaeke et al., 2015). The change in enthalpy obtained ranged between -26.066 and -29.429 kJ/mol across test temperature (303–333 K) and indicated a physisorption mechanism (Meroufel et al., 2013). The negative values of entropy ΔSa revealed a decrease in disorderliness. The adsorption free energy (ΔGa) ranges between -11.56 and -11.45 kJ/mol at 333 K as shown in Table 5. Negative ΔGa explains the feasibility and spontaneous nature of the adsorption. The values obtained at 333 K shows good interaction between its molecules and the MS surface which confirms that the LCLE is strongly adsorbed on MS (Dakeshwar and Fahmida, 2016). According to Dakeshwar and Fahmida, (2016), ΔGa values of -20 kJ/mol or less negative are associated with physisorption while those of with more negative than -40 kJ/mol are associated with chemical adsorption. Thus, the adsorption of LCLE molecules on the surface of MS is said to follow physical adsorption mechanism.
Figure 7.
Transition state plot of In (CR/T) vs 1/T for MS in the presence and absence of different concentration of Luffa cylindrica extract after (a) 4 h (b) 8 h and (c) 12 h of immersion.
Table 4.
Thermodynamics parameters for inhibition of mild steel in 0.5M HCl in the presence of Luffa cylindrica extract at different temperature and time.
| Time |
||||
|---|---|---|---|---|
| 4hr |
||||
| IC g/l | ΔH KJ/mol | ΔS J/mol K | Ea KJ/mol | A |
| 0.00 | -47.001 | -68.792 | 49.640 | 4.59E+09 |
| 0.50 | -29.429 | -136.577 | 32.068 | 1.32E+06 |
| 0.75 | -28.897 | -139.025 | 31.536 | 9.85E+05 |
| 1.00 | -27.609 | -143.983 | 30.248 | 5.42E+05 |
| 8hr | ||||
| 0.00 | -46.996 | -69.449 | 49.635 | 4.24E+09 |
| 0.50 | -28.048 | -141.951 | 30.687 | 6.93E+05 |
| 0.75 | -28.258 | -142.068 | 30.897 | 6.83E+05 |
| 1.00 | -26.919 | -147.129 | 29.558 | 3.71E+05 |
| 12hr | ||||
| 0.00 | -46.612 | -70.970 | 49.251 | 3.53E+09 |
| 0.50 | -27.572 | -144.081 | 30.211 | 5.36E+05 |
| 0.75 | -26.085 | -149.832 | 28.724 | 2.68E+05 |
| 1.00 | -26.066 | -150.632 | 28.705 | 2.44E+05 |
Table 5.
Free energy for inhibition of mild steel in 0.5M HCl in the presence of Luffa cylindrica extract at different temperature and time.
| Temp (K) | Langmuir ΔG (KJ/mol) |
Freundiich |
Temkin |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 4hr | 8hr | 12hr | 4hr | 8hr | 12hr | 4hr | 8hr | 12hr | |
| 303 | -10.88 | -10.85 | -10.81 | -9.36 | -9.39 | -9.43 | -37.90 | -38.16 | -38.03 |
| 318 | -11.25 | -11.20 | -11.17 | -10.00 | -10.04 | -10.08 | -40.55 | -40.40 | -40.25 |
| 333 | -11.56 | -11.49 | -11.45 | -10.69 | -10.75 | -10.80 | -40.73 | -33.99 | -40.30 |
8. Kinetics and optimization studies
Table 6 shows the result obtained after fitting the experimental data; concentration (0.5–1.0 g/l), temperatures (303–333 K) and time (0–12 h) to the two kinetic models selected for this study. With correlation coefficient (R-square values) criteria, it was observed that the corrosion of MS in the presence of LCLE obeys the pseudo-second-order kinetics with R-square value ranged between 0.9999 – 1.
Table 6.
Kinetic parameters for inhibition of MS in 0.5M HCl in the presence of LCLE at different temperature.
| 0.5 g/l |
0.75 g/l |
1.0 g/l |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 303K | 318K | 333K | 303K | 318K | 333K | 303K | 318K | 333K | |
| Pseudo-first order | |||||||||
| K1/hr | 0.3385 | 0.3431 | 0.3155 | 0.4583 | 0.2994 | 0.3869 | 0.4076 | 0.3892 | 0.3731 |
| θe | 0.0893 | 0.1102 | 0.0525 | 0.2038 | 0.0555 | 0.1427 | 0.1645 | 0.1185 | 0.0733 |
| θe exp. | 0.7167 | 0.7886 | 0.8585 | 0.7440 | 0.8082 | 0.8744 | 0.7576 | 0.8414 | 0.8804 |
| R2 | 0.933 | 0.829 | 0.999 | 0.921 | 0.724 | 0.895 | 0.839 | 0.870 | 0.896 |
| Pseudo-second order order | |||||||||
| K2/hr | 6.2191 | 7.6716 | 5.5551 | 11.4931 | 5.3523 | 4.6493 | 6.0896 | 6.9618 | 4.5099 |
| θe value | 0.7485 | 0.7994 | 0.8554 | 0.7468 | 0.8183 | 0.8749 | 0.7770 | 0.8251 | 0.9012 |
| θe exp. | 0.7167 | 0.7886 | 0.8585 | 0.7440 | 0.8082 | 0.8744 | 0.7576 | 0.8414 | 0.8804 |
| R2 | 1 | 1 | 0.999 | 1 | 1 | 1 | 0.999 | 1 | 0.999 |
The 32 experiments performed for optimization of IE and CR are presented in Table 7 which was analysed using analysis of variance. The ANOVA confirmed the suitability of quadratic models for the CR and IE with F-value of 373.88 and 236.909, respectively. The p – values recorded were less than 0.0001 for both CR and IE and attest to the reliability of the analysis with 95 percent confidence level. The final equations in terms of actual factors are presented in Eqs. (19) and (20). The values of correlation, adjusted and predicted R-squares (Table 8) show that the model equations are predictive and can reliably useful for sampling data within the experimental range of parameters. Figure 8 shows factor interaction as it affects the IE and CR of MS in 0.5M HCl.
| (19) |
| (20) |
Table 7.
Responses of experimental design for inhibition process of MS in the presence of Luffa cylindrica extract in 0.5M HCl.
|
Std |
Run | Factor 1 |
Factor 2 |
Factor 3 |
Response 1 |
Response 2 |
|---|---|---|---|---|---|---|
| X1:inhi.Conc |
X2:Temp |
X3:time |
Corrosion rate |
Inhibition efficiency |
||
| g/l | K | hr | g/m2 hr | % | ||
| 4 | 1 | 0.50 | 318 | 4 | 8.3238 | 76.92 |
| 18 | 2 | 1.00 | 333 | 8 | 8.2768 | 87.38 |
| 13 | 3 | 0.50 | 318 | 8 | 7.4133 | 77.42 |
| 15 | 4 | 1.00 | 318 | 8 | 5.5493 | 83.09 |
| 2 | 5 | 0.75 | 303 | 4 | 3.4062 | 71.73 |
| 28 | 6 | 0.75 | 318 | 8 | 6.6351 | 79.64 |
| 3 | 7 | 1.00 | 303 | 4 | 3.1755 | 73.64 |
| 10 | 8 | 0.50 | 303 | 8 | 3.2869 | 70.77 |
| 21 | 9 | 1.00 | 303 | 12 | 2.7185 | 75.68 |
| 22 | 10 | 0.50 | 318 | 12 | 6.3908 | 78.74 |
| 11 | 11 | 0.75 | 303 | 8 | 3.0055 | 73.27 |
| 14 | 12 | 0.75 | 318 | 8 | 6.6351 | 79.64 |
| 7 | 13 | 0.50 | 333 | 4 | 11.3705 | 84.38 |
| 19 | 14 | 0.50 | 303 | 12 | 3.1807 | 71.55 |
| 27 | 15 | 1.00 | 333 | 12 | 7.5813 | 87.98 |
| 16 | 16 | 0.50 | 333 | 8 | 9.7736 | 85.42 |
| 30 | 17 | 0.75 | 318 | 8 | 6.6351 | 79.64 |
| 9 | 18 | 1.00 | 333 | 4 | 9.3289 | 86.81 |
| 1 | 19 | 0.50 | 303 | 4 | 3.6382 | 69.80 |
| 31 | 20 | 0.75 | 318 | 8 | 6.6351 | 79.64 |
| 32 | 21 | 0.75 | 318 | 8 | 6.6351 | 79.64 |
| 6 | 22 | 1.00 | 318 | 4 | 6.3514 | 82.39 |
| 29 | 23 | 0.75 | 318 | 8 | 6.6351 | 79.64 |
| 23 | 24 | 0.75 | 318 | 12 | 5.6288 | 80.72 |
| 17 | 25 | 0.75 | 333 | 8 | 9.0117 | 86.81 |
| 5 | 26 | 0.75 | 318 | 4 | 7.3101 | 79.73 |
| 25 | 27 | 0.50 | 333 | 12 | 9.3299 | 85.73 |
| 8 | 28 | 0.75 | 333 | 4 | 10.4628 | 85.21 |
| 24 | 29 | 1.00 | 318 | 12 | 4.7898 | 84.06 |
| 26 | 30 | 0.75 | 333 | 12 | 7.9840 | 87.34 |
| 12 | 31 | 1.00 | 303 | 8 | 2.8853 | 74.34 |
| 20 | 32 | 0.75 | 303 | 12 | 2.8708 | 74.32 |
Table 8.
Analysis of variance for corrosion rate and inhibition of MS in 0.5 M HCl.
| MODEL | Sum of | df | Mean | F-value | p-value | |
|---|---|---|---|---|---|---|
| IE (%) | Quadratic | 910.984 | 9 | 101.220 | 236.909 | <0.0001 |
| R2 = 0.989 | Adj. R2 = 0.986 | Pred. R2 = 0.9768 | ||||
| CR (g/m2 hr) | Quadratic | 189.376 | 9 | 21.042 | 373.884 | <0.0001 |
| R2 = 0.993 | Adj. R2 = 0.991 | Pred. R2 = 0.9836 |
Figure 8.
Factor interaction as affects the IE of LCLE for the corrosion of MS in 0.5M HCl (a) The effect of temperature and inhibitor concentration. As inhibition concentration increased from minimum to maximum concentration, the IE increases with temperature (b) Effect of time and inhibition concentration. For any fixed amount of LCLE in the system, the IE increases with time but high for higher concentration of inhibitor (c) Effect of temperature and time. Temperature exhibited greater effects with maximum IE at higher temperature.
Eqs. (20) and (21) represent objective functions. CR was minimized while IE was maximized subjected to the constraints shown in Table 1. The maximum IE of 88.35% was obtained at 0.99 g/L of extract concentration, temperature of 332.81K and immersion time of 10.15 h using numerical optimization technique. The minimum CR of 7.63 g/m2h was obtained at optimum condition. The validity of these numerical values was established experimentally and the value of 87.89 ± 0.325 percent and 7.91 ± 0.0282 g/m2h for IE and CR respectively attested to the consistency and representativeness of the model for prediction purposes. Table 9 shows the result of comparative study of IE recorded from this study with other known green inhibitors. It is evident that the result obtained in this study compared well with some green inhibitors that have been applied to MS.
Table 9.
Comparison of inhibition efficiency of LCLE with other natural inhibitors on MS.
| Natural Products | Percentage Inhibition | References |
|---|---|---|
| Pectin from citrus peels | 94.20% | Fiori-Bimbi et al. (2015) |
| Punica granatum peel extract | 92.40% | Behpour et al. (2012) |
| Longan seed and peel extracts | 92.93% | Liao et al. (2017) |
| Griffonia simplicifolia extract | 91.73% | Ituen et al. (2017) |
| Water Melon rind extract | 83.35% | Odewunmi et al. (2015) |
| Justicia gendarussa extract | 93.00% | Satapathy et al. (2009) |
| Murraya Koenigii extract | 84.60% | Sharmila et al. (2010) |
| MIPE | 95.75% | Ogunleye et al. (2018) |
| LCLE | 87.89% | This study |
9. Conclusion
The capacity of LCLE as a green corrosion inhibitor has been assessed in this research by using the weight loss, depth of attack and surface analyses. The extract showed good inhibition characteristic for mild steel in 0.5 M HCl solution due to the presence of tannins, phenol, flavonoids, and alkanol groups according to the GC - MS and FTIR analyses. The inhibition efficiency improved with increasing inhibitor concentrations and decreased with increasing temperature. The result obtained using weight loss and depth of attack methods are consistent. At a concentration of 1.0 g/L, the extract proved to be an excellent inhibitor with an efficiency of 87.89 ±0.325% at 332.81K after 10 h. FTIR spectra revealed the presence of functional groups containing hetero-atoms. The SEM analysis showed that the inhibitor adsorption forms a protective film on the MS surface. The adsorption of extract film was found to follow Langmuir isotherm and pseudo-second-order kinetics. The values of the activation energy, enthalpy, Gibbs free energy and parameters estimated from the isotherm models suggested that the adsorption mechanism of LCLE on the MS is physisorption and exothermic.
Declarations
Author contribution statement
Ogunleye, O.O.: Conceived and designed the experiments; Wrote the paper.
Arinkoola, A.O. & Agbede, O.O.: Analyzed and interpreted the data; Wrote the paper.
Eletta O.A. & Hamed J.O.: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Osho, Y.A. & Morakinyo A.F.: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by the Tertiary Education Trust Fund (TETFUND), Ladoke Akintola University of Technology, Ogbomoso, Nigeria.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors acknowledge Chemical Engineering laboratories of both LAUTECH and UNILORIN for technical support.
References
- Abdel-Graber A.M., Abd-El-Nabey B.A., Sidahmed I.M., El-Zayady A.M., Saadawy M. Effect of temperature on inhibitive action of Damsissa extract on the corrosion of steel in acidic media. Corros. Sci. 2006;62(4):239–299. [Google Scholar]
- Ameer M.A., Khamis E., Al-Senani G. Adsorption studies of the effect of thiosemicarbazides on the corrosion of steel in phosphoric acid. Adsorpt. Sci. Technol. 2000;18(3):177–194. [Google Scholar]
- Aprael S.Y., Anees A.K., Rafal K.W. Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alexandria Eng. J. 2013;52:129–135. [Google Scholar]
- Ashish K.S., Quraishi M.A. Effect of cefazolin on the corrosion of mild steel in HCl solution. Corros. Sci. 2010;52:152–160. [Google Scholar]
- Behpour M., Ghoreishi S.M., Khayatkashani M., Soltani N. Green approach to corrosion inhibition of mild steel in two acidic solutions by the extract of Punica granatum peel and main constituents. Mater. Chem. Phys. 2012;131(3):621–633. [Google Scholar]
- Bentiss F., Lagrenee M., Traisnel M., Hornez J.C. The corrosion inhibition of mild steel in acidic media by a new triazole derivative. Corros. Sci. 1999;41:789–803. [Google Scholar]
- Cai B., Lui Y., Tian X., Wang F., Li H., Ji R. An experimental study of crevice behaviour of 316L stainless steel in artificial seawater. Corros. Sci. 2010;52:3235–3242. [Google Scholar]
- Dakeshwar K.V., Fahmida K. Green approach to corrosion inhibition of mild steel in hydrochloric acid medium using extract of spirogyra algae. Green Chem. Lett. Rev. 2016;9(1):52–60. [Google Scholar]
- Da Rocha J.C., Da Cunha P., Gomes J.A., Elia E.D. Corrosion inhibition of carbon steel in hydrochloric acid solution by fruit peel aqueous extract. Corros. Sci. 2010;52(7):2341–2348. [Google Scholar]
- Ebenso E.E., Ekpe U.J. Kinetic study of corrosion and corrosion inhibition of mild steel in sulphuric acid using Carica papaya leave extract. West Afr. J. Biol. Appl. Chem. 1996;41:21–27. [Google Scholar]
- Eddy N.O., Ebenso E.E. Adsorption and inhibitive properties of ethanol extracts of Musa sapientum peels as a green corrosion inhibitor for mild steel in H2SO4. Afr. J. Pure Appl. Chem. 2008;2(6):046–054. [Google Scholar]
- Fallavena T., Antonow M., Goncalves R.S. Caffeine as nontoxic corrosion inhibitor for copper in aqueous solutions of potassium nitrate. Appl. Surf. Sci. 2006;253:566–571. [Google Scholar]
- Fiori-Bimbi M.V., Alvarez P.E., Vaca H., Gervasi C.A. Corrosion inhibition of mild steel in HCl solution by pectin. Corros. Sci. 2015;92:192–199. [Google Scholar]
- Helen L.Y., Rahim A.A., Saad B., Saleh M.I., Bothi R.P. Aquilaria crassna leaves extracts – a green corrosion inhibitor for mild steel in 1.0 M HCl medium. Int. J. Electrochem. Sci. 2014;9:830–846. [Google Scholar]
- Hussain H.M., Kassim J.M. The corrosion inhibition and adsorption behaviour of Uncaria gambir extract on mild steel in 1M HCl. Int. J. Mater. Chem. Phys. 2011;12(5) 46-468. [Google Scholar]
- Iloamaeke I.M., Egwuatu C.I., Umeobika U.C., Edike H. Corrosion inhibition and adsorption studies of ethanol extract of Senna alata for mild steel in 2.0M H2SO4 solution. Int. J. Mater. Chem. Phys. 2015;1(3):295–299. [Google Scholar]
- Ituen E., Akaranta O., James A., Sun S. Green and sustainable local biomaterials for oilfield chemicals: Griffonia simplicifolia extract as steel corrosion inhibitor in hydrochloric acid. Sustain. Mater. Technol. 2017;11:12–18. [Google Scholar]
- Ji G., Shukla S.K., Dwivedi P., Sundaram S., Prakash R. Inhibitive effect of argimone mexicana plant extract on acid corrosion of mild steel. Ind. Eng. Chem. Res. 2011;50:11954–11959. [Google Scholar]
- Kamal C., Sethuraman M.G. Caulerpin-A bis-indole alkaloid as a green inhibitor for the corrosion of mild steel in 1 M HCl solution from the marine alga caulerpa racemose. Ind. Eng. Chem. Res. 2012;51:10399–10407. [Google Scholar]
- Kliskic M., Radosevic J., Gudic S., Katalinic V. Aqueous extract of osmarinus officianliis L. As inhibitor of Al-Mg alloy corrosion in chloride solution. J. Appl. Electrochem. 2000;30(7):823–830. [Google Scholar]
- Liao L.L., Mo S., Luo H.Q., Li N.B. Longan seed and peel as environmentally friendly corrosion inhibitor for mild steel in acid solution: experimental and theoretical studies. J. Colloid Interface Sci. 2017;499:110–119. doi: 10.1016/j.jcis.2017.03.091. [DOI] [PubMed] [Google Scholar]
- Markhali B.P., Naderi R., Mahdavian M., Sayebani M., Arman S.Y. Electrochemical impedance spectroscopy and electrochemical noise measurements as tools to evaluate corrosion inhibition of azole compounds on stainless steel in acidic media. Corros. Sci. 2013;75:269–279. [Google Scholar]
- Meroufel B., Benali O., Benyahia M., Benmoussa Y., Zenasni M.A. Adsorptive removal of anionic dye from aqueous solutions by algerian kaolin: characteristics, isotherm, kinetic and thermodynamic studies. J. Mater. Environ. Sci. 2013;3(4):482–491. [Google Scholar]
- Nathiya R.S., Raj V. Evaluation of Dryopteris cochleata leaf extracts as green inhibitor for corrosion of aluminium in 1 M H2SO4. Egypt J. Pet. 2017;26(2):313–323. [Google Scholar]
- Noyel V.S., Rohith P., Manivannan R. Psidium guajara Leaf extract as green corrosion inhibitor for mild steel in phosphoric acid. Int. J. Electrochem. Sci. 2015;10:2220–2238. [Google Scholar]
- Odewunmi N.A., Umoren S.A., Gasem Z.M. Utilization of watermelon rind extract as a green corrosion inhibitor for mild steel in acidic media. J. Ind. Eng. Chem. 2015;25:239–247. [Google Scholar]
- Ogunleye O.O., Eletta O.A., Arinkoola A.O., Agbede O.O. Gravimetric and quantitative surface morphological studies of Mangifera indica peel extract as a corrosion inhibitor for mild steel in 1 M HCl solution. Asia Pac. J. Chem. Eng. 2018 [Google Scholar]
- Ogwo K.D., Osuwa J.C., Udoinyang I.E., Nnanna L.A. Corrosion inhibition of mild steel and aluminium in 1 M hydrochloric acid by leaves extracts of Ficus sycomorus. Phys. Sci. Int. J. 2017;14(3):1–10. [Google Scholar]
- Okewale A.O., Omoruwou F., Ojaigho R. Alternative energy production for evironmental sustainability. Br. J. Renew. Energy. 2016;1(2):18–22. [Google Scholar]
- Ostovari A., Hoseinieh S.M., Peikari M., Shadizadeh S.R., Hashemi S.J. Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (lawsone, gallic acid, a-D-glucose and tannic acid) Corros. Sci. 2009;51:1935–1949. [Google Scholar]
- Owate I.O., Nwadiuko O.C., Dike I.I., Isu J.O., Nnanna L.A. Inhibition of mild steel corrosion by Aspilia africana in acidic solution. Am. J. Mater. Sci. 2014;4(3):144–149. [Google Scholar]
- Patricia E.A., Fiori-Bimbi M.V., Adriana N., Silvia A.B., Claudio A.G. Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution. J. Ind. Eng. Chem. 2017 [Google Scholar]
- Rani P.D., selvaraj S. Emblica officinalis (AMILA) leaves extract as corrosion inhibitor for copper and its alloy (Cu- 272N) in natural sea water. Archived Appl. Sci. Res. 2010;2(6):140–150. [Google Scholar]
- Satapathy A.K., Gunasekaran G., Sahoo S.C., Amit K., Rodrigues P.V. Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros. Sci. 2009;51(12):2848–2856. [Google Scholar]
- Sharmila A., Prema A.A., Sahayaraj P.A. Influence of Murraya koenigii (curry leaves) extract on the corrosion inhibition of carbon steel in HCl solution. Rasayan J. Chem. 2010;3(1):74–81. [Google Scholar]
- Sharma S.K., Anjali P., Obot I.B. Potential of Azadirachta indica as a green corrosion inhibitor against mild steel, aluminum, and tin: a review. J. Anal. Sci. Technol. 2015;6:26–42. [Google Scholar]
- Singh P., Srivastava V., Quraishi M.A. Novel quinoline derivatives as green corrosion inhibitors for mild steel in acidic medium: electrochemical, SEM, AFM, and XPS studies. J. Mol. Liq. 2016;216:164–173. [Google Scholar]
- Velmurugan V., Shiny G., Surya Surekha P. Phytochemical and biological screening of Luffa cylindrica linn. Fruit. Int. J. PharmTech Res. 2011;3(3):1582–1585. [Google Scholar]
- Verma D.K., Fahmida K. Green approach to corrosion inhibition of mild steel in hydrochloric acid medium using extract of spirogyra algae. Green Chem. Lett. Rev. 2016;9(1):52–60. [Google Scholar]
- Yaro A.S., Khadom A.A., Wael R.K. Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alexandria Eng. J. 2013;52:129–135. [Google Scholar]
- Zhang G.A., Zeng Y., Guo X.P., Jiang F., Shi D.Y., Chen Z.Y. Electrochemical corrosion behavior of carbon steel under dynamic high pressure H2S/CO2 environment. Corros. Sci. 2012;65:37–47. [Google Scholar]