Abstract
Primary biliary cholangitis (PBC) is a chronic, cholestatic condition associated with symptoms that directly impact the quality of life in those afflicted with the disease. In addition to pruritus and fatigue, patients with PBC may develop metabolic bone disease from reduced bone density, such as osteopenia and osteoporosis. Osteoporosis increases the risk of fractures, as well as morbidity and mortality. The prevalence of osteoporosis in PBC is expected to increase in conjunction with the rising prevalence of PBC as a whole. Timely diagnosis, prevention and management of osteoporosis are crucial in order to optimize the quality of life. There is a paucity of data evaluating the management of osteoporosis in PBC. The optimal timing for diagnosis and monitoring is not yet established and is guided by expert opinion. National guidelines recommend screening for osteoporosis at the time of diagnosis of PBC. Monitoring strategies are based on results of initial screening and individual risk factors for bone disease. Identifying reduced bone density is imperative to institute timely preventive and treatment strategies. However, treatment remains challenging as efficacious therapies are currently lacking. The data on treatment of osteoporosis in PBC are mostly extrapolated from postmenopausal osteoporosis literature. However, this data has not directly translated to useful treatment strategies for PBC-related osteoporosis, partly because of the different pathophysiological mechanisms of the two diseases. The lack of useful preventive measures and efficacious treatment strategies remains the largest pitfall that challenges the management of patients with PBC. In this review, we comprehensively outline the epidemiology, clinical implications and challenges, as well as management strategies of PBC-related osteoporosis.
Keywords: osteoporosis, metabolic bone disease, primary biliary cholangitis, fractures, cholestatic liver disease
Introduction
Primary biliary cholangitis (PBC) is a chronic immune-mediated, cholestatic liver condition.1 It is associated with debilitating symptoms that reduce the quality of life for those afflicted with the disease. Common symptoms that affect patients with PBC include but are not limited to fatigue, pruritus and bone disease.2–5 Prevention and modulation of these symptoms remain the cornerstone of management in PBC.
Metabolic bone disease is a fraught complication of chronic liver disease, particularly in PBC.6 The spectrum of metabolic bone disease that affects those with PBC includes osteopenia and osteoporosis. Osteoporosis is a disease of decreased bone density that results in an increased risk of fractures. It is four times more common in patients with PBC compared to age and gender-matched controls.7,8 Osteoporosis leads to falls resulting in fractures, which increases morbidity and mortality in patients with PBC and other chronic liver conditions.8–10
The World Health Organization (WHO) defines osteoporosis (at the spine or proximal femur) as having a bone mineral density (BMD) expressed as a T-score of less than 2.5 standard deviations (SD) below the mean on dual X-ray absorptiometry (DEXA).11 Osteopenia refers to a T-score between −1.0 and −2.5 SDs below the mean. Prevention and timely diagnosis of osteopenia and osteoporosis are imperative to reduce its associated complications.
The burden of bone loss in patients with PBC remains significant. Prevention by reducing bone loss and improving bone formation is essential. Unfortunately, therapeutic options are limited in efficacy.4 In this comprehensive review, we aim to outline the epidemiology, clinical impact and challenging management implications that are associated with osteoporosis in PBC.
Epidemiology of Disease
It is important to highlight the growing prevalence of PBC as a disease prior to considering the epidemiology of PBC-related osteoporosis. Overall, PBC occurs primarily in women with an annual incidence and prevalence of 0.3–5.8 and 1.9–40.2 per 100,000, respectively.12,13 Multiple studies, including data from a 2012 systematic review and a multicenter PBC consortium within the United States, demonstrate the rising prevalence of the disease.14–16 Hence, ensuring timely diagnosis and appropriate treatment strategies are imperative as the prevalence of PBC increases.
Osteoporosis is a known complication in multiple etiologies of liver disease, but its epidemiology has been studied most extensively in the context of cholestatic liver disease. The prevalence of osteoporosis in PBC ranges from 20% to 45%7,17–20 with the highest prevalence in those with cirrhosis on the liver transplant list.21 The prevalence of osteoporosis is higher than in age-matched postmenopausal women without PBC or cirrhosis.7,8,22 In addition, the incidence of fractures in PBC ranges from 0% to 14% over a 2-year period,19 whereas the prevalence ranges from 9% to 22%.7,8,18,21,23 The incidence and prevalence of PBC-related osteoporosis is expected to rise as the prevalence of the disease grows overall.
Burden of Disease
There is an estimated four-fold increase in osteoporosis in PBC compared to those without PBC.7,8 Osteoporosis leads to falls and fractures, which significantly impacts morbidity and mortality.4 In one study, fractures were shown to have a perioperative morbidity and mortality near 80% and 60%, respectively.9 PBC patients with a T-score on DEXA less than 1.5 have an increased risk of vertebral fractures.8
Liver transplantation in PBC is also associated with heightened fracture risk. Patients after liver transplantation are prone to osteopenia and osteoporosis, as they have an expected bone loss of 8% to 18% in the first 3–6 months after liver transplantation24–26 and a 20% to 40% incidence of fractures in the first year post-transplant.24,27–29 Reducing the burden of disease from osteoporosis before and after transplantation is imperative in the overall management of PBC.
Diagnosis and Monitoring
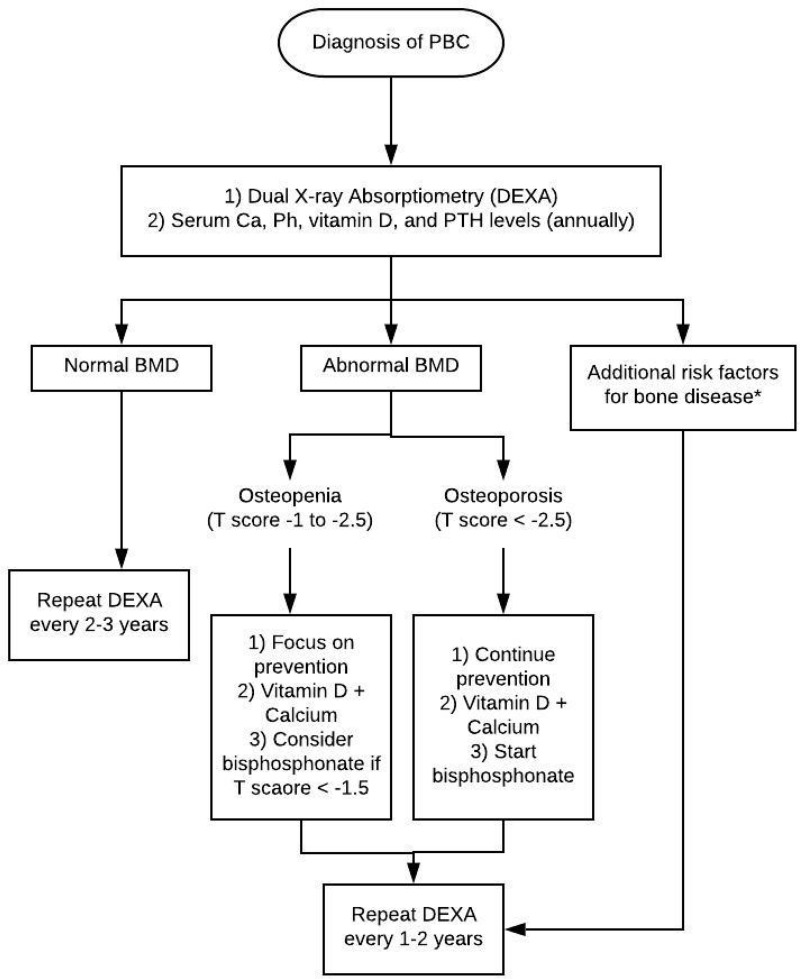
The optimal timing for diagnosis and monitoring of osteoporosis in PBC is not well established. However, expert consensus recommends screening with DEXA in all patients with PBC at the time of diagnosis (Figure 1).30 Commonly, patients with PBC have vitamin D deficiency, which may propagate their bone disease.31 Hence, patients with PBC should have serum calcium, phosphorus, vitamin D and parathyroid hormone levels checked at diagnosis and annually thereafter.32–34 Patients with normal BMD on initial DEXA can be monitored every 2 to 3 years. However, patients with abnormal BMD or additional risk factors of osteoporosis should be monitored more closely. These additional risk factors include but are not limited to severe cholestasis, corticosteroid use, low BMI, postmenopausal women, early menopause, smoking or alcohol abuse.17 In patients who are on treatment, repeat DEXA should be undergone every 1 to 2 years.35
Figure 1.
Management algorithm for bone disease in primary biliary cholangitis. *Additional risk factors include but are not limited to severe cholestasis, corticosteroid use, low BMI, postmenopausal women, early menopause, smoking or alcohol abuse.
Abbreviations: PBC, primary biliary cholangitis, Ca, calcium, Ph, phosphorus, PTH, parathyroid hormone, BMD, bone mineral density.
Management Challenges
Management of osteoporosis in PBC remains challenging. Therapeutic options are limited in efficacy. There is an incomplete understanding of the pathophysiology resulting in the paucity of studies evaluating treatments within the context of PBC.4 Useful treatment options exist for postmenopausal patients without PBC. However, these therapies have not been found efficacious in the context of PBC. Preventing osteoporosis in this patient population is therefore essential.
Many of the treatment strategies for osteoporosis in PBC are derived from therapeutic approaches in postmenopausal osteoporosis. The American Association for the Study of Liver Disease (AASLD) recommends vitamin D, calcium supplementation and alendronate based on postmenopausal osteoporosis literature.30 However, extrapolating from the postmenopausal osteoporosis literature may be unreliable since the underlying pathophysiological processes are likely varied. PBC-related osteoporosis is driven primarily by decreased bone formation compared to postmenopausal osteoporosis, which is secondary mostly to increased bone resorption.17 Investigating therapies for PBC-specific osteoporosis is needed. Table 1 illustrates the therapies that have been evaluated for PBC.
Table 1.
Summary of Randomized-Controlled Trials of Active Agents versus Placebo or No Treatment (Adapted from Danford et al17)
| Agent | Treatment | No. of Patients (n) | BMD Changes at 1 Year (%) | BMD changes at 2 Years (%) | Fractures (n) |
|---|---|---|---|---|---|
|
Etidronate Wolfhagen 199754 |
400 mg/d (3 month cycles) | 6 (etidronate) 6 (no treatment)a |
+1.0 (L), +0.2 (F) -1.7 (L), +0.4 (F) |
N/A | 0 0 |
| Lindor 200053 | 400 mg/d (3 month cycles) | 29 (etidronate) 31 (placebo) |
+0.7 (L), +1.3 (F) -0.6 (L), +0.9 (F) |
+1.0 (L), +0.5 (F) +2.6 (L), +0.8 (F) |
4 (V) 4 (V) |
|
Alendronate Zein 200552 |
70 mg/wk | 15 (alendronate) 13 (placebo) |
±10.4 (L), +1.4 (F) -0.1 (L), −2.1 (F) |
N/A | 1 (V), 0 (P) 0 (V), 1 (P) |
|
HRT Ormarsdottir 200450 |
50 mcg twice weekly TD estradiol + 2.5 mg/d progestin | 8 (HRT) 9 (no treatment)a |
±3.1 (L), ±1.7 (F) +1.0 (L), −0.6 (F) |
N/A | 0 0 |
| Boone 200651 | 0.05 mg/d TD estradiol + 0.25 mg/d TD progestin | 8 (HRT) 14 (placebo) |
N/A | −0.6 (L), +0.2 (F) -0.8 (L), −3.7 (F) |
0 (V) 2 (V) |
|
Sodium fluoride Guañabens 199258 |
50 mg/d sodium fluoride | 8 (fluoride) 8 (placebo) |
N/A |
+2.9 (L) -6.6 (L) |
0 0 |
|
Calcitriol Shiomi 199944 |
0.5 mcg/d BID calcitriol | 17 (calcitriol) 17 (no treatment)a |
+0.1 (L) -3.1 (L) |
N/A | N/A |
|
Vitamin K Nishiguchi 200160 |
45 mg/d vitamin K2 | 15 (vitamin K) 15 (no treatment)a |
+0.3 (L) -3.5 (L) |
−0.8 (L) -6.9 (L) |
N/A |
Notes: Bold denotes statistical significance (p < 0.05) between groups; Underline denotes statistical difference (p < 0.05) from baseline; aPatients in no treatment groups received vitamin D and calcium supplementation.
Abbreviations: L, lumbar; F, femoral; V, vertebral; P, peripheral.
Prevention
Data on the prevention of bone disease in PBC are also extrapolated mostly from postmenopausal osteoporosis literature. Preventive strategies revolve around lifestyle measures and dietary supplementation. In fact, exercise is associated with significant improvement in BMD in a 16-year prospective study evaluating postmenopausal women with osteopenia compared to controls.36 Other data suggest lifestyle factors such as alcohol consumption, tobacco use, low levels of exercise and reduced dietary calcium intake can lead to reduced bone density and portend an increased fracture risk.11
In patients with liver disease, we recommend alcohol and smoking cessation, in addition to practicing routine weight-bearing exercises and consuming a balanced diet. Patients who are at-risk for metabolic bone disease should consume 1200 mg of dietary calcium and 800 international units (IU) of vitamin D daily.11 It may be particularly challenging for patients with cirrhosis to meet their daily dietary requirements, and hence, we recommend routine nutritional counseling in order to prevent malnourishment, which may further propagate osteoporosis and fracture risk.37
PBC-Specific Therapies
The current therapies for PBC are associated with significant improvement in PBC-related outcomes overall. Ursodeoxycholic acid (UDCA) remains the gold standard therapy for PBC. UDCA, obeticholic acid, and fibrates all significantly reduce alkaline phosphatase levels, a surrogate marker of disease activity in PBC.38–42 UDCA also reduces the progression to cirrhosis and need for liver transplantation.1 Additional therapies are currently undergoing evaluation for the treatment of PBC.1 Despite concrete evidence on improvement in important clinical outcomes in PBC with these drugs, their efficacy in osteopenia or osteoporosis has not been established.
Certain studies have evaluated BMD with these therapies. In a randomized controlled trial with UDCA, patients had annual BMD evaluation with DEXA while on therapy over a period of 3 years, and there was no significant difference in lumbar density compared to placebo.43 In a Phase 3 pilot study of obeticholic acid, patients had BMD evaluation at baseline and at 12 months. Although there was less of a decline in patients on treatment, there was no statistically significant difference.40 More conclusive evidence of these treatments for the bone-specific complications of PBC is warranted.
Vitamin D and Calcium
Data on vitamin D and calcium in PBC are sparse. However, supplementation is still routinely recommended in these patients given the low side-effect profile of these medications.33,34 Interestingly, a single trial on calcitriol supplementation did not show a benefit in BMD at 1 year compared to baseline, but the untreated group did have a significantly worse BMD.44 AASLD and the European Association for the Study of Liver Disease (EASL) suggest consideration of calcium and vitamin D supplementation.45,46 AASLD advises 1,500 mg/day of calcium and 1000 IU/day of vitamin D in patients without a history of renal stones. We also believe that calcium and vitamin D supplementation should be considered, particularly in those with low serum vitamin D levels or other high-risk factors for osteoporosis (i.e. corticosteroid use).
Hormone Replacement Therapy
Hormonal replacement therapy (HRT) was previously used in postmenopausal women to reduce bone resorption and decrease bone loss.47 Initial trials of HRT in liver disease suggested possible worsening of liver disease, but subsequent studies proved this not to be true.48–51 However, randomized trials have failed to prove benefit in terms of reducing fracture risk, but a single trial did show improvement in BMD.50,51 Although HRT’s side effect profile is tolerable in the context of liver disease, the non-liver related side effects severely limit the routine use of these agents.
In our recent systematic review, HRT did not show benefit in fracture risk reduction, but differentially met the secondary outcome of change in BMD.4 There was a significant improvement in lumbar BMD with HRT at 24 months compared to placebo or no intervention (standard mean difference [SMD] 0.69, 95% confidence interval [CI] 0.11 to 1.26, p=0.02), but no improvement in femoral BMD at 24 months (SMD 1.79, 95% CI −0.96 to 4.55, p=0.2). However, HRT was associated with significant adverse events leading to withdrawal of patients from studies. For these reasons, HRT is not a recommended therapy in patients with PBC.
Bisphosphonates
Bisphosphonates reduce bone resorption and have been tested in PBC-related osteoporosis. A double-blinded, randomized, placebo-controlled trial of 34 patients evaluating alendronate, a third-generation bisphosphonate, demonstrates a significant improvement in spinal (0.09 ± 0.03 g/cm2 SD from baseline vs −0.003 ± 0.02 g/cm2 SD from baseline, p=0.005) and femoral BMD (0.012 ± 0.04 g/cm2 vs −0.019 ± 0.04 g/cm2 SD from baseline, p=0.046) compared to placebo at 1 year.52 In contrast, the two studies evaluating etidronate, a first-generation bisphosphonate, do not show significant changes in BMD.53,54 In our review, a combined pooled analysis of bisphosphonates did not demonstrate any improvement on BMD compared to placebo or no intervention (lumbar SMD 0.41, 95% CI −0.95 to 1.45, p=0.68; femoral SMD 0.21, 95% CI −0.39 to 0.82, p=0.49).4 Overall, the quality of current evidence is low. Hence, the use of bisphosphonates in PBC still requires further investigation.
Additional Treatments
Less common therapies, which have proven useful in postmenopausal osteoporosis, have been considered in the context of PBC. For example, a single pilot study of 9 patients with PBC evaluated the use of raloxifene, a selective estrogen receptor modulators (SERM) that reduce bone resorption.55 This study notes a small, but significant improvement in lumbar BMD compared to the age-matched controls (0.72 g/cm2, 95% CI 0.62–0.87 vs 0.74 g/cm2, 95% CI 0.63–0.97; p-value=0.02), but there was no difference in femoral BMD. Calcitonin has been evaluated against calcium supplementation and control without any noted significant improvement in BMD.56,57 Sodium fluoride, which increases bone formation, was initially thought to increase BMD compared to placebo in patients with PBC,58 but subsequent investigation actually observed a decrease in femoral BMD associated with its use after 2 years of therapy.59 In addition, a small randomized trial evaluated the effect of vitamin K, which is involved in bone formation, and found that it was also not effective in improving BMD.60 Human parathyroid hormone is approved for postmenopausal osteoporosis, but has not been evaluated in humans with PBC. A recombinant form of parathyroid hormone called teriparatide, which stimulates bone formation, has only been evaluated in animal studies.61 Overall, our recent systematic review and meta-analysis of individual studies evaluating sodium fluoride, calcitriol, vitamin K, and cyclosporine A did not show any meaningful improvement in BMD from baseline. Routine use of these therapies is therefore not recommended for PBC-related osteoporosis.
Future Therapies in the Pipeline
The future for osteoporosis therapy in PBC remains uncertain. Recently, a parathyroid hormone-related peptide analog, abaloparatide, was approved by the Food and Drug Administration (FDA) for postmenopausal osteoporosis. It differs from teriparatide in that it actually promotes bone formation, is generally better tolerated, and could potentially have a role in PBC once investigations are undergone in this population. Unfortunately, large gaps in knowledge on the pathophysiology of PBC-related osteoporosis limit the advent of novel, efficacious and useful therapies. In comparison to treatments used for postmenopausal osteoporosis, which is due to increased bone resorption, future therapies for PBC should be targeted to improve bone formation.
Putting It All Together
Osteoporosis is a common manifestation in PBC and leads to an impaired quality of life. Osteoporosis heightens the risk of fractures and negatively impacts morbidity and mortality in patients with PBC, including in those who undergo liver transplantation. The prevalence of osteoporosis in PBC is expected to increase as the overall prevalence of the disease continues to rise.
Instituting timely diagnosis and intervention is imperative in preventing the progression of osteoporosis and optimizing the quality of life for patients with PBC. However, management of this potentially debilitating complication is not without challenges. Methods in prevention, monitoring and treatment of osteoporosis in PBC are sparse and limited in efficacy.
The optimal strategy for the diagnosis, prevention and treatment of osteoporosis in PBC is not well established. Majority of the data is extrapolated from the literature on the management of postmenopausal osteoporosis, and may not generalize well to patients with PBC as the pathophysiological mechanisms of the two diseases vary. While postmenopausal osteoporosis is largely driven by an increased bone resorption, PBC-related osteoporosis is mostly from reduced bone formation.
Preventing the reduction of bone density is important to decrease the risk of fractures and improve morbidity and mortality. Unfortunately, the data in treatments of PBC-related osteoporosis are inadequate as the overall quality of evidence is low. In our recent systematic review and meta-analysis of 11 randomized studies and 584 patients, we evaluated multiple treatments for osteoporosis including bisphosphonates (etidronate, alendronate), hormonal replacement therapy (HRT), UDCA, obeticholic acid, cyclosporine A, vitamin K, calcitriol, and sodium fluoride.4 None of the 11 studies met the primary outcome of fracture reduction, including in the pooled-analysis of bisphosphonates, but HRT did differentially meet the secondary outcome of change in BMD. Figure 1 provides a proposed algorithm for the diagnosis and management of osteoporosis in PBC. Although we have learnt a lot about PBC-related therapies for osteoporosis from the postmenopausal osteoporosis literature, future studies investigating PBC-specific therapies with a focus on improving bone formation are warranted.
Disclosure
HDT receives educational funding from NIH T32 (5T32DK007760-19). CJD, DG and AB have no financial disclosures. There are no conflicts of interest in the creation of this manuscript.
References
- 1.Trivedi HD, Lizaola B, Tapper EB, et al. Primary biliary cholangitis: new treatments for an old disease. Frontline Gastroenterol. 2017;8(1):29–36. doi: 10.1136/flgastro-2016-100741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JY, Danford CJ, Trivedi HD, et al. Treatment of fatigue in primary biliary cholangitis: a systematic review and meta-analysis. Dig Dis Sci. 2019. doi: 10.1007/s10620-019-5457-5 [DOI] [PubMed] [Google Scholar]
- 3.Trivedi HD, Lizaola B, Tapper EB, et al. Management of pruritus in primary biliary cholangitis: a narrative review. Am J Med. 2017;130:744.e1–744.e7. doi: 10.1016/j.amjmed.2017.01.037 [DOI] [PubMed] [Google Scholar]
- 4.Danford CJ, Ezaz G, Trivedi HD, et al. The pharmacologic management of osteoporosis in primary biliary cholangitis: a systematic review and meta-analysis. J Clin Densitom. 2019. doi: 10.1016/j.jocd.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 5.Reshetnyak VI. Concept on the pathogenesis and treatment of primary biliary cirrhosis. World J Gastroenterol. 2006;12(45):7250–7262. doi: 10.3748/wjg.v12.i45.7250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay JE. Bone disease in cholestatic liver disease. Gastroenterology. 1995;108(1):276–283. doi: 10.1016/0016-5085(95)90033-0 [DOI] [PubMed] [Google Scholar]
- 7.Guanabens N, Parés A, Ros I, et al. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J Hepatol. 2005;42(4):573–577. doi: 10.1016/j.jhep.2004.11.035 [DOI] [PubMed] [Google Scholar]
- 8.Guanabens N, Cerdá D, Monegal A, et al. Low bone mass and severity of cholestasis affect fracture risk in patients with primary biliary cirrhosis. Gastroenterology. 2010;138(7):2348–2356. doi: 10.1053/j.gastro.2010.02.016 [DOI] [PubMed] [Google Scholar]
- 9.Cohen SM, Te HS, Levitsky J. Operative risk of total hip and knee arthroplasty in cirrhotic patients. J Arthroplasty. 2005;20(4):460–466. doi: 10.1016/j.arth.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Guanabens N, Parés A, Mariñoso L, et al. Factors influencing the development of metabolic bone disease in primary biliary cirrhosis. Am J Gastroenterol. 1990;85(10):1356–1362. [PubMed] [Google Scholar]
- 11.Office of the Surgeon, G. Reports of the Surgeon General, in Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville (MD): Office of the Surgeon General (US); 2004. [PubMed] [Google Scholar]
- 12.Kim WR, Lindor KD, Locke GR, et al. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology. 2000;119(6):1631–1636. doi: 10.1053/gast.2000.20197 [DOI] [PubMed] [Google Scholar]
- 13.Hurlburt KJ, McMahon BJ, Deubner H, et al. Prevalence of autoimmune liver disease in Alaska natives. Am J Gastroenterol. 2002;97(9):2402–2407. doi: 10.1111/j.1572-0241.2002.06019.x [DOI] [PubMed] [Google Scholar]
- 14.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181–1188. doi: 10.1016/j.jhep.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 15.Lu M, Zhou Y, Haller IV, et al. Increasing prevalence of primary biliary cholangitis and reduced mortality with treatment. Clin Gastroenterol Hepatol. 2018;16(8):1342–1350.e1. doi: 10.1016/j.cgh.2017.12.033 [DOI] [PubMed] [Google Scholar]
- 16.Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danford CJ, Trivedi HD, Papamichael K, et al. Osteoporosis in primary biliary cholangitis. World J Gastroenterol. 2018;24(31):3513–3520. doi: 10.3748/wjg.v24.i31.3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menon KV, Angulo P, Weston S, et al. Bone disease in primary biliary cirrhosis: independent indicators and rate of progression. J Hepatol. 2001;35(3):316–323. doi: 10.1016/S0168-8278(01)00144-1 [DOI] [PubMed] [Google Scholar]
- 19.Guanabens N, Parés A, Navasa M, et al. Cyclosporin A increases the biochemical markers of bone remodeling in primary biliary cirrhosis. J Hepatol. 1994;21(1):24–28. doi: 10.1016/S0168-8278(94)80132-0 [DOI] [PubMed] [Google Scholar]
- 20.Newton J, Francis R, Prince M, et al. Osteoporosis in primary biliary cirrhosis revisited. Gut. 2001;49(2):282–287. doi: 10.1136/gut.49.2.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guichelaar MM, Kendall R, Malinchoc M, et al. Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver Transpl. 2006;12(9):1390–1402. doi: 10.1002/(ISSN)1527-6473 [DOI] [PubMed] [Google Scholar]
- 22.Seki A, Ikeda F, Miyatake H, et al. Risk of secondary osteoporosis due to lobular cholestasis in non-cirrhotic primary biliary cholangitis. J Gastroenterol Hepatol. 2017;32(9):1611–1616. doi: 10.1111/jgh.2017.32.issue-9 [DOI] [PubMed] [Google Scholar]
- 23.Pares A, Guañabens N, Alvarez L, et al. Collagen type Ialpha1 and vitamin D receptor gene polymorphisms and bone mass in primary biliary cirrhosis. Hepatology. 2001;33(3):554–560. doi: 10.1053/jhep.2001.22758 [DOI] [PubMed] [Google Scholar]
- 24.Guichelaar MM, Malinchoc M, Sibonga JD, et al. Bone histomorphometric changes after liver transplantation for chronic cholestatic liver disease. J Bone Miner Res. 2003;18(12):2190–2199. doi: 10.1359/jbmr.2003.18.12.2190 [DOI] [PubMed] [Google Scholar]
- 25.Eastell R, Dickson ER, Hodgson SF, et al. Rates of vertebral bone loss before and after liver transplantation in women with primary biliary cirrhosis. Hepatology. 1991;14(2):296–300. doi: 10.1002/(ISSN)1527-3350 [DOI] [PubMed] [Google Scholar]
- 26.Bjoro K, Brandsæter B, Wiencke K, et al. Secondary osteoporosis in liver transplant recipients: a longitudinal study in patients with and without cholestatic liver disease. Scand J Gastroenterol. 2003;38(3):320–327. doi: 10.1080/00365520310000681a [DOI] [PubMed] [Google Scholar]
- 27.Haagsma EB, Thijn CJP, Post JG, et al. Bone disease after orthotopic liver transplantation. J Hepatol. 1988;6(1):94–100. doi: 10.1016/S0168-8278(88)80467-7 [DOI] [PubMed] [Google Scholar]
- 28.Meys E, Fontanges E, Fourcade N, et al. Bone loss after orthotopic liver transplantation. Am J Med. 1994;97(5):445–450. doi: 10.1016/0002-9343(94)90324-7 [DOI] [PubMed] [Google Scholar]
- 29.Butin S, Griffoul I, Espitalier F, et al. High incidence of vertebral osteoporotic fracture within the first year after liver transplantation. Clin Exp Rheumatol. 2017;35(6):913–918. [PubMed] [Google Scholar]
- 30.Lindor KD, Gershwin ME, Poupon R, et al. Primary biliary cirrhosis. Hepatology. 2009;50(1):291–308. doi: 10.1002/hep.22906 [DOI] [PubMed] [Google Scholar]
- 31.Reshetnyak VI. Primary biliary cirrhosis: clinical and laboratory criteria for its diagnosis. World J Gastroenterol. 2015;21(25):7683–7708. doi: 10.3748/wjg.v21.i25.7683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glass LM, Su GL. Metabolic bone disease in primary biliary cirrhosis. Gastroenterol Clin North Am. 2016;45(2):333–343. doi: 10.1016/j.gtc.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 33.Pares A, Guanabens N. Osteoporosis in primary biliary cirrhosis: pathogenesis and treatment. Clin Liver Dis. 2008;12(2):407–24; x. doi: 10.1016/j.cld.2008.02.005 [DOI] [PubMed] [Google Scholar]
- 34.Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124(3):795–841. doi: 10.1053/gast.2003.50106 [DOI] [PubMed] [Google Scholar]
- 35.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kemmler W, Engelke K, von Stengel S. Long-term exercise and bone mineral density changes in postmenopausal women–are there periods of reduced effectiveness? J Bone Miner Res. 2016;31(1):215–222. doi: 10.1002/jbmr.2608 [DOI] [PubMed] [Google Scholar]
- 37.Trivedi HD, Tapper EB. Interventions to improve physical function and prevent adverse events in cirrhosis. Gastroenterol Rep (Oxf). 2018;6(1):13–20. doi: 10.1093/gastro/gox042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poupon RE, Bonnand A-M, Chrétien Y, et al. Ten-year survival in ursodeoxycholic acid-treated patients with primary biliary cirrhosis. The UDCA-PBC Study Group. Hepatology. 1999;29(6):1668–1671. doi: 10.1002/hep.510290603 [DOI] [PubMed] [Google Scholar]
- 39.Pares A, Caballería L, Rodés J, et al. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. UDCA-Cooperative Group from the Spanish Association for the Study of the Liver. J Hepatol. 2000;32(4):561–566. doi: 10.1016/S0168-8278(00)80216-0 [DOI] [PubMed] [Google Scholar]
- 40.Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375(7):631–643. doi: 10.1056/NEJMoa1509840 [DOI] [PubMed] [Google Scholar]
- 41.Levy C, Peter JA, Nelson DR, et al. Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment Pharmacol Ther. 2011;33(2):235–242. doi: 10.1111/j.1365-2036.2010.04512.x [DOI] [PubMed] [Google Scholar]
- 42.Reig A, Sese P, Pares A. Bezafibrate: a novel and effective alternative for relieving pruritus in patients with primary biliary cirrhosis. Hepatology. 2015;62:508A. [Google Scholar]
- 43.Lindor KD, Janes CH, Crippin JS, et al. Bone disease in primary biliary cirrhosis: does ursodeoxycholic acid make a difference? Hepatology. 1995;21(2):389–392. [PubMed] [Google Scholar]
- 44.Shiomi S, Masaki K, Habu D, et al. Calcitriol for bone loss in patients with primary biliary cirrhosis. J Gastroenterol. 1999;34(2):241–245. doi: 10.1007/s005350050250 [DOI] [PubMed] [Google Scholar]
- 45.Lindor KD, Bowlus CL, Boyer J, et al. Primary biliary cholangitis: 2018 practice guidance from the American Association for the study of liver diseases. Hepatology. 2019;69(1):394–419. doi: 10.1002/hep.30145 [DOI] [PubMed] [Google Scholar]
- 46.EASL. EASL Clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67(1):145–172. doi: 10.1016/j.jhep.2017.03.022 [DOI] [PubMed] [Google Scholar]
- 47.Hillard TC, Whitcroft SJ, Marsh MS, et al. Long-term effects of transdermal and oral hormone replacement therapy on postmenopausal bone loss. Osteoporos Int. 1994;4(6):341–348. doi: 10.1007/BF01622195 [DOI] [PubMed] [Google Scholar]
- 48.Olsson R,Mattsson LÅ, Obrant K, Mellström D. Estrogen-progestogen therapy for low bone mineral density in primary biliary cirrhosis. Liver. 1999;19(3):188–192. doi: 10.1111/j.1478-3231.1999.tb00034.x [DOI] [PubMed] [Google Scholar]
- 49.Menon KV, Angulo P, Boe GM, et al. Safety and efficacy of estrogen therapy in preventing bone loss in primary biliary cirrhosis. Am J Gastroenterol. 2003;98(4):889–892. doi: 10.1111/j.1572-0241.2003.07341.x [DOI] [PubMed] [Google Scholar]
- 50.Ormarsdottir S, Mallmin H, Naessén T, et al. An open, randomized, controlled study of transdermal hormone replacement therapy on the rate of bone loss in primary biliary cirrhosis. J Intern Med. 2004;256(1):63–69. doi: 10.1111/j.1365-2796.2004.01342.x [DOI] [PubMed] [Google Scholar]
- 51.Boone RH, Cheung AM, Girlan LM, et al. Osteoporosis in primary biliary cirrhosis: a randomized trial of the efficacy and feasibility of estrogen/progestin. Dig Dis Sci. 2006;51(6):1103–1112. doi: 10.1007/s10620-006-8015-x [DOI] [PubMed] [Google Scholar]
- 52.Zein CO, Jorgensen RA, Clarke B, et al. Alendronate improves bone mineral density in primary biliary cirrhosis: a randomized placebo-controlled trial. Hepatology. 2005;42(4):762–771. doi: 10.1002/(ISSN)1527-3350 [DOI] [PubMed] [Google Scholar]
- 53.Lindor KD, Jorgensen RA, Tiegs RD, et al. Etidronate for osteoporosis in primary biliary cirrhosis: a randomized trial. J Hepatol. 2000;33(6):878–882. doi: 10.1016/S0168-8278(00)80118-X [DOI] [PubMed] [Google Scholar]
- 54.Wolfhagen FH, van Buuren HR, den Ouden JW, et al. Cyclical etidronate in the prevention of bone loss in corticosteroid-treated primary biliary cirrhosis. A prospective, controlled pilot study. J Hepatol. 1997;26(2):325–330. doi: 10.1016/S0168-8278(97)80048-7 [DOI] [PubMed] [Google Scholar]
- 55.Levy C, Harnois DM, Angulo P, et al. Raloxifene improves bone mass in osteopenic women with primary biliary cirrhosis: results of a pilot study. Liver Int. 2005;25(1):117–121. doi: 10.1111/liv.2005.25.issue-1 [DOI] [PubMed] [Google Scholar]
- 56.Camisasca M, Battezzati PM, Albisetti W, et al. Parenteral calcitonin for metabolic bone disease associated with primary biliary cirrhosis. Hepatology. 1994;20(3):633–637. doi: 10.1016/0270-9139(94)90098-1 [DOI] [PubMed] [Google Scholar]
- 57.Floreani A, Zappala F, Fries W, et al. A 3-year pilot study with 1,25-dihydroxyvitamin D, calcium, and calcitonin for severe osteodystrophy in primary biliary cirrhosis. J Clin Gastroenterol. 1997;24(4):239–244. doi: 10.1097/00004836-199706000-00012 [DOI] [PubMed] [Google Scholar]
- 58.Guanabens N, Parés A, Del Rio L, et al. Sodium fluoride prevents bone loss in primary biliary cirrhosis. J Hepatol. 1992;15(3):345–349. doi: 10.1016/0168-8278(92)90066-X [DOI] [PubMed] [Google Scholar]
- 59.Guanabens N, Pares A, Monegal A, et al. Etidronate versus fluoride for treatment of osteopenia in primary biliary cirrhosis: preliminary results after 2 years. Gastroenterology. 1997;113(1):219–224. doi: 10.1016/S0016-5085(97)70098-2 [DOI] [PubMed] [Google Scholar]
- 60.Nishiguchi S, Shimoi S, Kurooka H, et al. Randomized pilot trial of Vitamin K2 for bone loss in patients with primary biliary cirrhosis. J Hepatol. 2001;35(4): 543–545. [DOI] [PubMed] [Google Scholar]
- 61.Dresner-Pollak R, Gabet Y, Steimatzky A, et al. Human parathyroid hormone 1-34 prevents bone loss in experimental biliary cirrhosis in rats. Gastroenterology. 2008;134(1):259–267. doi: 10.1053/j.gastro.2007.10.025 [DOI] [PubMed] [Google Scholar]