Abstract
Purpose
Sleep is essential for life, as well as having a major impact on quality of life. Not much attention has been given to this important factor in the care of lung cancer patients.
Patients and Methods
We retrospectively analyzed a cohort of 404 lung cancer patients treated in our institute between 2010 and 2018. Data about sleep quality, distress and pain were self-reported by questionnaires administered to patients at their first clinic visit to the Institute of Oncology. Sex, age, histology, stage, smoking and marital status were extracted from the patients’ charts. Uni- and multi-variate analyses were carried out to evaluate the correlation of these factors with survival.
Results
Most patients reported some level of distress and pain. Sleep abnormalities were reported by 58.7% of patients. Distress, pain and bad sleep were correlated with shorter survival in univariate analyses; however, only sleep remained associated with survival in multivariate analysis. Patients reporting bad sleep had a median survival of 16 months, compared to 27 months for patients reporting good sleep (hazard ratio 1.83, 95% C.I. 1.27–2.65). Frequent arousals at night were more tightly correlated with survival than difficulty falling asleep.
Conclusion
Sleep quality, as reported by lung cancer patients, is highly correlated with survival. Further studies are required to comprehend whether poor sleep quality is directly impacting survival or is a result of the cancer aggressiveness and patients’ conditions.
Keywords: lung cancer, sleep quality, survival, distress, pain, patient-reported outcomes
Introduction
Sleep remains one of the great mysteries of biology, comprising a period of vulnerable apparent inactivity, observed basically in all animals with a nervous system.1,2 The overarching need for sleep across species substantiates its essentially for life. In humans, sleep quality and length are associated with mortality in a complex manner.3 Both short4 and long sleep periods were associated with increased mortality and morbidity including obesity, diabetes and cardiovascular diseases.5 Sleep disturbances are correlated with increased blood levels of inflammatory mediators.6 In a mice model, sleep disruption caused accelerated atherosclerosis and impacted hematopoiesis.7 Sleep is essential for memory consolidation and other cognitive functions.8 Protein phosphorylation and various molecular correlates of the need-for-sleep, and of sleep-wake cycles have been described.9 Recent reports of sleep impact on chromatin modulations and repair of double-strand DNA breaks in neurons might offer an explanation to the essentially of sleep.10
Complex interactions exist between sleep abnormalities and cancer. Sleeping disturbances clearly violate the circadian rhythm.11 Association of the circadian rhythm with various aspects of cancer biology,12 gene expression,13 and cancer metabolism14 has been identified and studied. Disturbed circadian rhythm in mice increased the risk of liver damage and hepatic cancer.15 Insomnia and disturbances in the circadian rhythm can lead to reduced melatonin secretion.16 Melatonin in turn impacts the immune system favorably, potentially opposing cancer through various mechanisms17 such as antioxidative activity and modulation of the immune response.18 Sleep disturbances have been reported to correlate with increased cancer risk.19,20
Cancer patients commonly report abnormal sleep.21,22 Short sleep duration and snoring prior to cancer diagnosis were correlated with shorter survival of breast cancer patients.23 Poor sleep quality was correlated with reduced survival among advanced breast cancer patients,24 with most deaths being breast cancer-related rather than other co-morbidities.25 This association was independent of potential confounders as depression, cancer treatment, and cortisol levels. In that study, survival was not associated with sleep duration but rather with sleep efficiency (total sleep time/time spent in bed). Sleep disturbances were correlated with more aggressive cancer features among breast cancer patients.26 Deciphering cause and effect here is difficult, as cancer may negatively impact sleep. A model of breast cancer in immune-competent mice demonstrated cancer-induced abnormal orexin signaling causing fragmented sleep as well as altered glucose metabolism.27 Several mechanisms may thus explain an association of reduced sleep quality with cancer aggressiveness.22,28–30 Importantly, although disturbed sleep, depression, fatigue and stress seem closely correlated, each of these symptoms may endow a separate pathogenesis.29
Lung cancer is the most common cause of cancer-related death worldwide.31 Night shift work, known to be associated with poor sleep quality, has been correlated with increased incidence of several cancers including lung cancer.32 Melatonin was demonstrated to induce apoptosis, reduce cell proliferation of lung cancer cell lines and reduced metastatic spread in mouse models of lung cancer.33 In a mouse model of lung cancer, circadian rhythm disruption accelerated lung cancer progression, with evidence of a direct tumor-suppressive role for circadian clock genes.34 Sleep disturbances are common in lung cancer patients.35 The psychological, physiological and molecular correlates of poor sleep may impact lung cancer progression and response to therapy. In this study, we aimed to examine whether sleep quality correlated with survival of lung cancer patients. We evaluated sleep based on the reply to a single question, and demonstrate a significant correlation of sleep quality assessed this way with patients’ survival. To the best of our knowledge, this is the first report of the association of sleep quality with survival in lung cancer patients, as well as a unique demonstration of the utility of a simple self-reported sleep quality in this context.
Methods
Epidemiological, clinical and pathological data were retrospectively collected from medical charts of lung cancer patients treated at the Sheba Medical Center during 2010–2018. Patients were identified from the working database of the Institute’s lung cancer unit. Recognized prognostic factors were extracted from the medical charts and included age, sex, performance status and stage at diagnosis, histology, smoking status and marital status.36,37 Performance status (PS) was scored from 0 to 5 based on the Eastern Cooperative Oncology Group score.38,39 Last follow-up or date of death were extracted from the electronic medical record software.
Self-filled questionnaires which are part of the standard intake of patients on their first visit to the Oncology Institute were utilized to determine sleeping quality, distress and pain levels. Sleep quality was reported as any of: no problems; difficulty falling asleep; frequent arousals at night. The information about pain was self-reported by the same questionnaire by a visual analog scale (range 0–10), relating to three different situations: usual pain level, worst pain level and pain level on analgesics. In addition, the questionnaires queried level of distress, graded from 0 to 5, also by visual analog scale.
Inclusion criteria for the dataset of this study were a pathologic or cytologic diagnosis of thoracic malignancy of any type and stage; the availability of the sleep quality data; and availability of potential prognostic factors data.
Statistical Analysis
Overall survival (OS) was calculated from date of diagnosis till death or censured at last follow-up. All variables are described by median and a 95% confidence interval (CI) or median and range as appropriate. For the initial analysis, visual analog scale of pain was converted to a categorical parameter where a grade of zero or one out of 10 on analgesics was considered as “no pain” and all the rest as “in pain”. Distress was converted to a categorical parameter where distress level zero out of five was considered as “no distress” and all the rest as “distressed”. Further analyses of pain and distress regarded these as ordinal scales utilizing the precise grade as provided by patients. Sleep quality was converted to two categories: good sleep and bad sleep (including any of: difficulties falling asleep, frequent arousals at night). Correlation between sleep status and other parameters was assessed by Fisher’s exact test, two-sided; odds ratio was calculated where appropriate. Age was considered as a continuous variable, while stage, performance status, distress and pain were considered as ordinal variables, all other factors were nominal. As a sensitivity analysis, pain and distress were considered also as continuous variables. All smoking status types were considered as smoker besides never-smokers. Cox-regression was calculated, based on forward stepwise selection model (likelihood ratio), including all assessed potential risk factors. Survival was evaluated using the Kaplan Meier method and groups compared by log-rank test, hazard ratio was calculated by cox-regression analysis. Statistical analysis was performed using IBM SPSS statistics version 25.
Ethics
This study was approved by the Sheba ethics committee. Since this study did not include any interventional component, a waiver from informed consent was granted from the Sheba Ethics Committee. Patient data confidentiality was strictly kept according to accepted guidelines. All study procedures were conducted in compliance with the Declaration of Helsinki.
Results
Patients Characteristics
We analyzed questionnaires that were filled by 476 patients on their first visit to a Medical Oncologist from 2010 to 2018. Cutoff date of data extraction was 1st February 2019. Of the 476 questionnaires, 404 (84.9%) included data about sleep quality, distress and pain. These 404 patients were considered the study cohort and were analyzed further. Median follow up of these patients was 26.0 months (95% C.I. 18.7, 33.3). Median age was 66 years, mostly males, diagnosed at stage IV, married, smokers (Table 1). The most common histologic type was NSCLC and specifically Adenocarcinoma histology.
Table 1.
Characteristics of Patients Included in the Study
| Parameters | All Patients | Good Sleep | Bad Sleep | Odds Ratio (95% CI) |
P-value |
|---|---|---|---|---|---|
| N | 404 | 167 | 237 | ||
| Age – median (range), yearsa | 66 (23–93) | 66 (23–89) | 67 (28–93) | 1.20 (0.80–1.78) | 0.419 |
| Sex – Men N (%) | 239 (59.2) | 97 (58.1) | 142 (59.9) | 0.93 (0.62–1.39) | 0.758 |
| Marital Status N (%) | |||||
| Married | 283 (70.0) | 116 (69.5) | 167 (70.5) | 0.95 (0.62–1.47) | 0.829 |
| Divorced | 53 (13.1) | 17 (10.2) | 36 (15.2) | 0.63 (0.34–1.17) | 0.178 |
| Single | 22 (5.4) | 12 (7.2) | 10 (4.2) | 1.76 (0.74–4.17) | 0.265 |
| Widowed | 46 (11.4) | 22 (13.2) | 24 (10.1) | 1.35 (0.73–2.49) | 0.345 |
| Stage of Diseaseb N (%) | |||||
| I | 45 (11.1) | 25 (15.0) | 20 (8.4) | 1.91 (1.02–3.57) | 0.053 |
| II | 43 (10.6) | 20 (12.0) | 23 (9.7) | 1.26 (0.67–2.39) | 0.514 |
| III | 86 (21.3) | 37 (22.2) | 49 (20.7) | 1.09 (0.67–1.77) | 0.805 |
| IV | 230 (56.9) | 85 (50.9) | 145 (61.2) | 0.66 (0.44–0.98) | 0.042 |
| Pathologic Subtype N (%) | |||||
| NSCLC –Adenocarcinoma | 241 (59.7) | 102 (61.1) | 139 (58.6) | 1.10 (0.74–1.66) | 0.681 |
| NSCLC – Squamous cell | 66 (16.3) | 25 (15.0) | 41 (17.3) | 0.84 (0.49–1.45) | 0.586 |
| NSCLC – NOS | 32 (7.9) | 10 (6.0) | 22 (9.3) | 0.62 (0.29–1.35) | 0.265 |
| NSCLC – Large cell neuroendocrine | 10 (2.5) | 5 (3.0) | 5 (2.1) | 1.29 (0.70–2.37) | 0.434 |
| SCLC | 37 (9.2) | 17 (10.2) | 20 (8.4) | ||
| Thymoma | 10 (2.5) | 3 (1.8) | 7 (3.0) | 1.14 (0.44–2.96) | 0.810 |
| Mesothelioma | 5 (1.2) | 2 (1.2) | 3 (1.3) | ||
| Carcinoid | 3 (0.7) | 3 (1.8) | - | ||
| ECOG PS N (%) | |||||
| 0 | 219 (54.2) | 104 (62.3) | 115 (48.5) | 1.75 (1.17–2.62) | 0.008 |
| 1 | 103 (25.5) | 36 (21.6) | 67 (28.3) | 0.70 (0.44–1.11) | 0.134 |
| 2 | 45 (11.1) | 14 (8.4) | 31 (13.1) | 0.61 (0.31–1.18) | 0.151 |
| 3 | 28 (6.9) | 10 (6.0) | 18 (7.6) | 0.75 (0.37–1.52) | 0.486 |
| 4 | 9 (2.2) | 3 (1.8) | 6 (2.5) | ||
| Smoking – yes | 288 (71.3) | 110 (65.9) | 178 (75.1) | 0.66 (0.42–1.02) | 0.073 |
| Pain – yesa | 183 (45.3) | 50 (29.9) | 133 (56.1) | 2.99 (1.97–4.55) | <0.001 |
| Distress – yesa | 344 (85.1) | 117 (70.1) | 227 (95.8) | 9.70 (4.75–19.82) | <0.001 |
Notes: aCategorial as described in methods; bAJCC version 8th. Data regarding all of the study cohort together, followed by separate columns for patients reporting good sleep or bad sleep (difficulty falling asleep and/or frequent awakening). The odds ratio and the p-value are demonstrated for each factor.
Abbreviations: NS, non-significant; NOS, non-other specified; ECOG-PS, Eastern Cooperative Oncology Group performance status.
Pain, Distress and Sleep
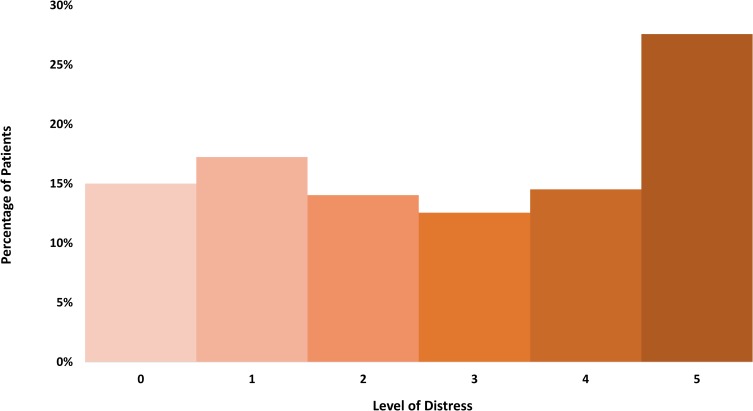
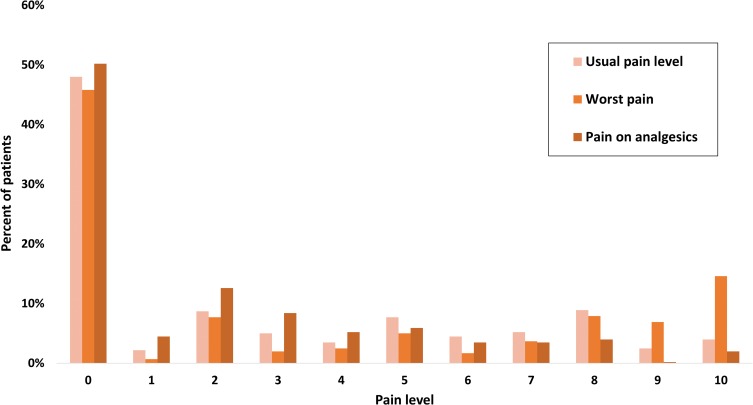
Most of the patients reported some level of distress and pain. As seen in Table 2, more than half of the cohort (237 of 404; 58.7%) had sleep abnormalities. As seen in Figure 1 and in Table S1, 54.2% (219 of 404) reported a distress level of 3 or higher. As seen in Figure 2, pain score of 2 or more (with analgesics) was reported by 45.3% of patients (183 of 404, Table S2), a score of 4 or more was reported by 24.3% (98 of 404). As can be expected, pain and distress were tightly correlated (p-value <0.001 Fisher’s exact test). In addition, pain and distress were highly correlated with sleep abnormalities (p-value <0.001 Fisher’s exact test for each of these factors correlation with sleep). Potential associations between PS, stage of disease, histology, marital status and sleep were examined. The only significant correlations found were between stage IV disease and sleep abnormalities (Fisher’s exact test p-value 0.042, Odd Ratio 1.52, 95% CI 1.02–2.27) and between PS 0 and good sleep (Fisher’s exact test p-value 0.004, Odd Ratio 1.75, 95% CI 1.17–2.62).
Table 2.
Univariate Cox-Regression Analysis of Self-Reported Sleep Quality Correlation with Overall Survival for the Entire Study Cohort (n=404)
| Sleep Status | n (%) | Hazard Ratio (95% CI) | P-value |
|---|---|---|---|
| No sleep problems | 167 (41.3) | Reference | |
| Difficulty falling asleepa | 55 (13.6) | 1.34 (0.76–2.34) | 0.303 |
| Frequent arousals at nighta | 135 (33.4) | 2.04 (1.37–3.05) | <0.001 |
| Both | 47 (11.6) | 1.80 (1.02–3.20) | 0.042 |
Notes: “Both” refers to report of both difficulty falling asleep and frequent arousals at night. aNot including cases of “both”. P value for the correlation is 0.005, with a higher risk of death for patients with sleep problems. Shown are also p values of correlations of each sub-category with overall survival.
Figure 1.
Distribution of distress levels. The percentage of patients within each category of stress as self-defined in the admission questionnaire. The most common level of distress was five out of five.
Figure 2.
Distribution of pain scale. Distribution of the visual analog scale grades given to each of the three different pain categories in the admission questionnaire. The most prevalent level of pain was zero, although a substantial proportion of patients reported pain at higher levels.
Factors Predictive of Survival
Univariate analysis of sleep quality, pain and distress demonstrated a significant correlation of each of these factors with survival (Table 2, S1, S2). As a sensitivity analysis, pain and distress were examined also as continuous variables, both found to be significantly correlated with survival (univariate cox regression: pain, HR 1.12, 95% CI 1.06–1.19, p <0.0001; distress, HR 1.15, 95% CI 1.051.27, p=0.004). Of the sub-categories of sleep abnormalities, frequent arousals at night demonstrated a clear correlation with a higher risk of death, while difficulties falling asleep on its own did not (Table 2). Multivariate cox-regression analysis demonstrates significant association of survival with PS, stage, smoking and sleep abnormalities (Table 3). The correlation of distress and pain with survival was not significant in this analysis, whether examined a ordinal or as continuous variables.
Table 3.
Cox Multivariate Regression Analysis, Correlation of Potential Prognostic Factors with Survival
| Risk Factors | Hazard Ratio (95% CI) | P-value (Cox Regression) |
|---|---|---|
| Clinical Stage | <0.001 | |
| I | Reference | |
| II | 1.15 (0.07–18.49) | 0.922 |
| III | 9.28 (1.22–70.56) | 0.031 |
| IV | 30.86 (4.27–223.10) | 0.001 |
| ECOG-PS | <0.001 | |
| 0 | Reference | |
| 1 | 1.08 (0.69–1.67) | 0.744 |
| 2 | 0.98 (0.56–1.72) | 0.935 |
| 3 | 3.28 (1.97–5.47) | <0.001 |
| 4 | 3.35 (1.57–7.11) | 0.002 |
| Smokinga | 2.18 (1.39–3.41) | 0.001 |
| Sleep abnormalitiesb | 1.78 (1.22–2.59) | 0.003 |
| Pain | NS | |
| Distress | NS | |
| Age | NS | |
| Sex | NS | |
| Marital status | NS | |
| Histology | NS |
Notes: aRelative to no smoking. bRelative to no sleep abnormalities.
Abbreviations: ECOG-PS, Eastern Cooperative Oncology Group performance status; NS, non-significant.
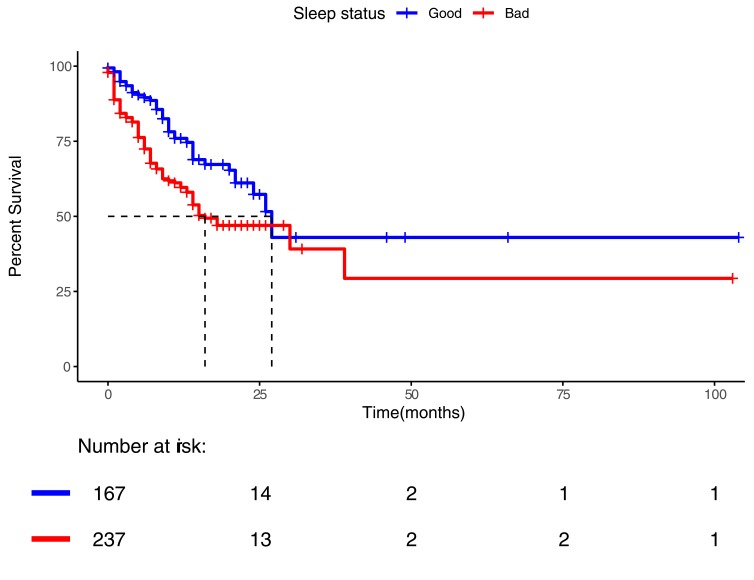
Overall survival was worse for the 237 patients reporting sleep abnormalities compared to 167 patients reporting no sleep abnormalities (Figure 3; median survival 16.0 months, 95% CI 7.85–24.14 versus 27.0 months, 95% CI 22.81–31.19; univariate cox-regression hazard ratio 1.83 (1.27–2.65), log-rank p-value 0.001).
Figure 3.
Kaplan Meier analysis of survival of lung cancer patients reporting sleep abnormalities (red, “bad”) or reporting no sleep problems (blue, “good”). Log-rank test p value of the difference is <0.001.
Discussion
We report here a detailed analysis of sleep, distress and pain in patients with thoracic malignancies. As expected, these issues are highly prevalent in this population. In addition, we investigated the correlation between these factors and overall survival of lung cancer patients. Interestingly, while each of these factors was correlated with survival on a univariate analysis, only sleep remained significant on multivariate analysis. Although the number of patients of the sub-categories of sleep abnormalities are small, patients reporting frequent arousals at night stood out as having a clear higher risk of death. Importantly, although sleep assessment in our study was performed by a very simple questionnaire, the results are strongly correlated with survival, stressing the robustness of the sleep-survival correlation. This report is the first to our knowledge to highlight sleep as a major prognostic factor for lung cancer patients. Further studies are needed to gain insight into the mechanisms at play, and to try to understand if the presence of an aggressive cancer impacts sleep quality, or whether poor sleep reduces patients resilience or otherwise impacts directly their survival. Our results should encourage a focused approach on sleep quality as part of the multi-disciplinary treatment of lung cancer patients. Patients and medical teams should be aware of the high prevalence of sleep disturbances and its potential importance.40 Risk factors of sleep disruptions in cancer patients are multifactorial.22 Accordingly, various interventions can be utilized for sleep improvement, including hypnotics, cognitive behavior therapy41 and cannaboids42 among other options. Continuously assessing individually patients’ sleep status and identifying potential disruptive factors such as pain or psychological stress is likely to improve sleep quality for many patients. As we report, pain and distress are both highly correlated to abnormal sleep. All these factors should be regarded and treated as part of the general palliative care of cancer patients. Further research is required to demonstrate the efficacy of various interventions on sleep quality in lung cancer patients. Most importantly, such studies may identify specific sleep restorative interventions as improving patients’ outcome.
Our study is a retrospective, single-center study, with inherent limitations. No formal objective assessment of sleep status was performed in this study. Reliance on patients’ report is likely to be not accurate, as has been demonstrated,43 but the general assessment of good versus bad sleep is plausibly reliable. Several detailed questionnaires were reported and validated44–48 and their use would have allowed better understanding of the nature of the sleep problems our patients endure. No biological correlates such as melatonin levels or immune system function were assessed, limiting our ability to infer causality and to understand the mechanisms at play. In addition, we had no data about sleep quality changes with treatment as well as with progression of disease, since the questionnaires are currently being administered only at the first clinic visit. Patient reported outcomes (PRO) are now gaining more attention,49 commonly being evaluated as part of the outcomes of clinical trials interventions.50 Routine PRO evaluations are becoming an expected standard, utilizing electronic devices and social media platforms, in some cases linked to medical teams real-time response options. Our results and the available reported evidence indicate that sleep quality should be persistently integrated among PROs collected and assessed. Shining a spotlight on sleep as critically important aspect of life may allow further improvements in the outcome of lung cancer patients.
Conclusions
Sleep quality, as self-reported by a simple questionnaire, was found to be a robust prognostic factor among lung cancer patients. Although sleep, distress and pain were correlated with each other, only bad sleep remained significantly associated with survival in multivariate analysis. The factors impacting sleep quality require further studies. Sleep should be potentially regarded as a therapeutic target among lung cancer patients.
Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. In the interest of full transparency: D.U. reports honoraria from Boehringer Ingelheim, Roche, BMS, MSD, Teva, AstraZeneca and Takeda. Y.R.L. reports research support from Checkmate Pharmaceuticals and BMS. A.O. reports research support from Boehringer Ingelheim, Roche, Sanofi-Aventis, Xcovery, AstraZeneca, BMS and MSD, advisory fees from Boehringer Ingelheim, MSD, Takeda and AstraZeneca, and honoraria from Takeda, Roche and Boehringer Ingelheim. J.B. reports honoraria from AstraZeneca, MSD, Boehringer Ingelheim, Roche, BMS, Takeda, Abbvie, Pfizer and VBL, and research support from MSD, AstraZeneca and Pfizer. All disclosures reported above are unrelated to the submitted work. All other authors declare no potential conflicts of interest in this work.
References
- 1.Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-X [DOI] [PubMed] [Google Scholar]
- 2.Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–1253. doi: 10.1093/sleep/30.10.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabanayagam C, Shankar A. Sleep duration and cardiovascular disease: results from the NATIONAL HEALTH INTERVIEW SURVEy. Sleep. 2010;33:1037–1042. doi: 10.1093/sleep/33.8.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2018;39:25–36. doi: 10.1016/j.smrv.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 6.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAlpine CS, Kiss MG, Rattik S, et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019. doi: 10.1038/s41586-019-0948-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawangjit A, Oyanedel CN, Niethard N, Salazar C, Born J, Inostroza M. The hippocampus is crucial for forming non-hippocampal long-term memory during sleep. Nature. 2018;564:109–113. doi: 10.1038/s41586-018-0716-8 [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Ma J, Miyoshi C, et al. Quantitative phosphoproteomic analysis of the molecular substrates of sleep need. Nature. 2018;558:435–439. doi: 10.1038/s41586-018-0218-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zada D, Bronshtein I, Lerer-Goldshtein T, Garini Y, Appelbaum L. Sleep increases chromosome dynamics to enable reduction of accumulating DNA damage in single neurons. Nat Commun. 2019;10. doi: 10.1038/s41467-019-08806-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007;30(11):1484–1501. doi: 10.1093/sleep/30.11.1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savvidis C, Koutsilieris M. Circadian rhythm disruption in cancer biology. Mol Med. 2012;18(9):1. doi: 10.2119/molmed.2012.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2016;18:164. doi: 10.1038/nrg.2016.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9:886. doi: 10.1038/nrc2747 [DOI] [PubMed] [Google Scholar]
- 15.Kettner NM, Voicu H, Finegold MJ, et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell. 2016;30:909–924. doi: 10.1016/j.ccell.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci. 2007;64(10):1174. doi: 10.1007/s00018-007-6529-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50(4):1129–1146. [PubMed] [Google Scholar]
- 18.Pohanka M. Impact of melatonin on immunity: a review. Cent Eur J Med. 2013;8(4):369–376. doi: 10.2478/s11536-013-0177-2 [DOI] [Google Scholar]
- 19.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13(4):257–264. doi: 10.1016/j.smrv.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 20.Reed VA, Work S. Light at night, and the risk of breast cancer. AAOHN J. 2010;59(1):37–45. doi: 10.3928/08910162-20101216-01 [DOI] [PubMed] [Google Scholar]
- 21.Fiorentino L, Ancoli-Israel S. Sleep dysfunction in patients with cancer. Curr Treat Options Neurol. 2007;9(5):337–346. doi: 10.1007/s11940-007-0019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ancoli-Israel S. Sleep disturbances in cancer: a review. Sleep Med Res. 2015;6(2):45–49. doi: 10.17241/smr.2015.6.2.45 [DOI] [Google Scholar]
- 23.Phipps AI, Bhatti P, Neuhouser ML, et al. Pre-diagnostic sleep duration and sleep quality in relation to subsequent cancer survival. J Clin Sleep Med. 2016;12(4):495–503. doi: 10.5664/jcsm.5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trudel-Fitzgerald C, Zhou ES, Poole EM, et al. Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses’ Health Study. Br J Cancer. 2017;116(9):1239–1246. doi: 10.1038/bjc.2017.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palesh O, Aldridge-Gerry A, Zeitzer JM, et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37:837–842. doi: 10.5665/sleep.3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soucise A, Vaughn C, Thompson CL, et al. Sleep quality, duration and breast cancer aggressiveness. Breast Cancer Res Treat. 2017;164(1):169–178. doi: 10.1007/s10549-017-4245-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borniger JC, Walker WH, Surbhi S, et al. A role for hypocretin/orexin in metabolic and sleep abnormalities in a mouse model of non-metastatic breast cancer. Cell Metab. 2018;28:118–129.e5. doi: 10.1016/j.cmet.2018.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimsdale J, Fiorentino L, Parker BA, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2005;14(3):201–209. doi: 10.1007/s00520-005-0861-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bower JE. Behavioral symptoms in breast cancer patients and survivors: fatigue, insomnia, depression, and cognitive disturbance. J Clin Oncol. 2008;26(5):768–777. doi: 10.1200/JCO.2007.14.3248.Behavioral [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895–908. doi: 10.1200/JCO.2001.19.3.895 [DOI] [PubMed] [Google Scholar]
- 31.World Health Organisation. Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. Int Agency Res Cancer. 2018(September):13–15. [Google Scholar]
- 32.Parent MÉ, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012. doi: 10.1093/aje/kws318 [DOI] [PubMed] [Google Scholar]
- 33.Ma Z, Yang Y, Fan C, et al. Melatonin as a potential anticarcinogen for non-small-cell lung cancer. Oncotarget. 2016. doi: 10.18632/oncotarget.8776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papagiannakopoulos T, Bauer MR, Davidson SM, et al. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab. 2016;24(2):324–331. doi: 10.1016/j.cmet.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levin RD, Daehler MA, Grutsch JF, et al. Circadian function in patients with advanced non-small-cell lung cancer. Br J Cancer. 2005;93:1202–1208. doi: 10.1038/sj.bjc.6602859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rami-Porta R, editor. Staging Handbook in Thoracic Oncology 2nd ed. 2016. Available from: https://www.iaslc.org/Portals/0/staging_handbook_2016_hi-res_restricted_use_only_per_iaslc_permission.pdf?ver=2019-05-08-202520-383.
- 37.Wu Y, Ai Z, Xu G. Marital status and survival in patients with non-small cell lung cancer: an analysis of 70006 patients in the SEER database. Oncotarget. 2017;8(61):103518–103534. doi: 10.18632/oncotarget.21568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eastern Cooperative Oncology Group. ECOG performance status. 1982. Available from: https://ecog-acrin.org/resources/ecog-performance-status. Accessed December31, 2019.
- 39.Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer Part A. 1996;32(7):1135–1141. doi: 10.1016/0959-8049(95)00664-8 [DOI] [PubMed] [Google Scholar]
- 40.Perry GS, Patil SP, Presley-Cantrell LR. Raising awareness of sleep as a healthy behavior. Prev Chronic Dis. 2013;10(4):10–13. doi: 10.5888/pcd10.130081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma MP, Andrade C. Behavioral interventions for insomnia: theory and practice. Indian J Psychiatry. 2012;54(4):359–366. doi: 10.4103/0019-5545.104825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19(4). doi: 10.1007/s11920-017-0775-9 [DOI] [PubMed] [Google Scholar]
- 43.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Sleep duration: how well do self-reports reflect objective measures? The CARDIA Sleep Study. Epidemiology. 2008. doi: 10.1097/EDE.0b013e318187a7b0 [DOI] [Google Scholar]
- 44.Ibáñez V, Silva J, Cauli O. A survey on sleep assessment methods. PeerJ. 2018;6:e4849. doi: 10.7717/peerj.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 46.Violani C, Devoto A, Lucidi F, Lombardo C, Russo PM. Validity of a Short Insomnia Questionnaire: the SDQ. Brain Res Bull. 2004;63(5):415–421. doi: 10.1016/j.brainresbull.2003.06.002 [DOI] [PubMed] [Google Scholar]
- 47.Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh sleep quality index in cancer patients. J Pain Symptom Manage. 2004;27(2):140–148. doi: 10.1016/j.jpainsymman.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 48.Sweere Y, Kerkhof GA, De Weerd AW, Kamphuisen HA, Kemp B, Schimsheimer RJ. The validity of the Dutch Sleep Disorders Questionnaire (SDQ). J Psychosom Res. 1998;45: 549–55. doi: 10.1016/S0022-3999(98)00030-0 [DOI] [PubMed] [Google Scholar]
- 49.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197. doi: 10.1001/jama.2017.7156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willke RJ, Burke LB, Erickson P. Measuring treatment impact: a review of patient-reported outcomes and other efficacy endpoints in approved product labels. Control Clin Trials. 2004;25:535–552. doi: 10.1016/j.cct.2004.09.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Eastern Cooperative Oncology Group. ECOG performance status. 1982. Available from: https://ecog-acrin.org/resources/ecog-performance-status. Accessed December31, 2019.