Abstract
The neurotransmitter metabolite 3,4-dihydroxy-phenylglycolaldehyde (dopegal) damages neurons and the myocardium by protein cross-linking, resulting in conglomerations and cell death. We investigated this process on a synthetic scale, leading to the discovery of an Amadori-type rearrangement of dopegal in the reaction with several amino acids and neuropeptides. This alkylation also occurs with neurotransmitters, suggesting an influence of dopegal on neurochemical processes. The rearrangement occurs readily under physiological conditions.
Monoamine oxidase (MAO) is known to catalyze the metabolism of the tyrosine-derived neurotransmitters dopamine 1, epinephrine 5, and norepinephrine 6 with the formation of 3,4-dihydroxyphenylacetaldehyde 2 (dopal) and 3,4-dihydroxyphenylglycolaldehyde 7 (dopegal), respectively (Scheme 1). Further metabolic transformation of these highly reactive aldehydes takes place via reducing (AR/ALR) or oxidizing (ALDH) enzymes arriving at the much less harmful alcohols or acids. When these enzymatic detoxification processes become less efficient, the residing catecholaldehydes give rise to unwanted reactions, eventually leading to protein alkylation and cross-linking.1,2 Neurodegenerative diseases such as Parkinson’s or Alzheimer’s diseases, as well as cardiovascular diseases, are associated with the presence of these reactive aldehydes.3 Meanwhile, knowledge about protein alkylation by the dopamine-derived aldehyde dopal (2) is growing, but the exact reaction mechanism of this process is not completely established.4 Oxidation of the catechol part of dopal 2 to a strongly electrophilic ortho-quinone with reactive oxygen species (ROS) likely plays an important role in irreversible protein alkylation.5
Scheme 1. Dopal and Dopegal: Formation and Protein Alkylation.
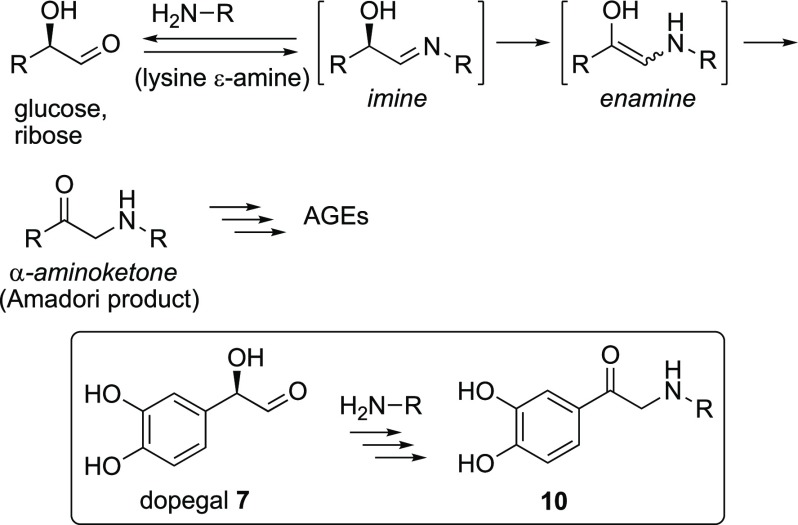
In the early stages of protein alkylation, Schiff’s bases 3 are readily formed in the reaction between the aldehyde of dopal and the lysine side chain amine residues (Scheme 1). The imines thus obtained equilibrate to conjugated enamines 4, a type of intermediate that is frequently observed during Pictet–Spengler reactions.6 In general, under aqueous conditions, imine/enamine adducts are not stable and swiftly hydrolyze to the starting compounds. Recently, a successful identification by mass spectroscopy of some of these imine/enamine intermediates derived from dopal (2) and some small amines was reported.4e The properties of dopegal (7) have not been studied in detail due to the limited stability and availability of this highly reactive aldehyde.7 Dopegal (7) contains an additional α-hydroxyl substituent compared to dopal (2), and this hydroxyl group plays an important role in the reactivity. While dopal requires an additional oxidation step of the catechol part to acquire irreversible reactivity with proteins after Schiff’s base formation, the α-hydroxyl substituent in dopegal actually presents a higher oxidation level, giving rise to direct irreversible alkylation of proteins as will be shown (Scheme 1).
The Amadori rearrangement is a well-known process that starts with imine formation in the reaction of primary amino substituents in proteins with reducing sugars such as ribose and glucose (Scheme 2).8 Initially, an imine is formed, which slowly tautomerizes to the enamine and, after reprotonation, relatively stable α-aminoketones are formed. The Amadori rearrangement is most known because this occurs during food preparations at elevated temperatures. Under physiological conditions, this reaction also takes place slowly and leads after further oxidation of these Amadori products to so-called advanced glycation end products (AGEs), which are harmful in many biological systems.9 Enhanced glucose levels as in diabetes mellitus-2 stimulate the process and result in irreversible damage to tissues and organs.
Scheme 2. Amadori Rearrangement.
The responsible sugars contain an α-hydroxyaldehyde part that resembles the glycolaldehyde part of dopegal. Nevertheless, the remarkably high reactivity of dopegal toward amines, and in particular, toward lysine residues in proteins, has never been recognized as an Amadori-type rearrangement. In a recent publication, a fast reaction between enzymatically (MAO) synthesized dopegal and the therapeutical dipeptide carnosine10 (βAla-His) was mentioned without giving the structure of the reaction product.11 Therefore, the sequestration of neural dopegal is essential to protect proteins and tissues from alkylation. As a possible solution, amines that are more nucleophilic toward dopegal than the target lysine residues in proteins could be of therapeutic interest. To gain further understanding of this neurochemistry, we started a synthetic project by reacting dopegal with biologically relevant primary amines and amino acids such as β-alanine and lysines, as well as dipeptides and a series of more complex biogenic amines.
The production of dopegal (7) by chemical or enzymatic methods is troublesome, and the limited commercial availability hampers extensive synthetic research.1,7 Recently, a one-step preparation of the acetal precursor 13 from catechol (11) and glyoxal dimethyl acetal (12) was described, as an intermediate in a short synthesis of hydroxytyrosol (Scheme 3).12 The authors described a partial hydrolysis of acetal 13 with aqueous acetic acid and demonstrated a clean conversion to hydrated dopegal in D2O solution (50% conversion after 3 days at 60 °C). We upscaled the alkylation of catechol with glyoxal dimethyl acetal to multigram quantities and obtained dopegal precursor 13 readily in pure form by direct crystallization. Complete hydrolysis of the dimethyl acetal in 13 was effected with 1 equiv of TFA in D2O, at a concentration of 0.1 M (16 h at 40°). Under these conditions, dopegal (7) is almost entirely present in its hydrated form and contains only 5% of the free aldehyde, together with small amounts of its dimer as a complex mixture of several diastereomers. This dimer is formed reversibly, and the ratio of monomer/dimer is concentration-dependent. To obtain dopegal free from water and acid, the aqueous hydrolysis mixture was freeze-dried. However, in the solid residue, we could not detect the free aldehyde either by NMR, both in DMSO and D2O, or by IR (no carbonyl absorption in the solid state), indicating that dopegal is completely converted to its cyclic dimer upon concentration. In the literature, a comparable dimerization of the phenyl analogue of dopegal, i.e., mandelaldehyde, is described, in support of our observations.13 These properties are different from previously synthesized dopegal, which was reported to display aldehyde signals in both NMR and IR spectra.1,7 In solution, the dimer is in equilibrium with its (hydrated) monomer but still shows the aldehyde-derived reactivity in further chemistry. After storage of the freeze-dried dopegal for several months, analysis by NMR spectroscopy did not show any decomposition.
Scheme 3. Synthesis of Dopegal.
With dopegal in hand, in multigram amounts in a stable form, the condensation reaction with several amines was conducted (Table 1). These condensation reactions proceeded readily in water under ambient temperatures (rt to 40 °C) and at reasonable rates with reaction times ranging from 4 to 24 h, depending on the basicity and nucleophilicity of the amines. A pH range from 6 to 8 proved to be optimal. Above pH 8, the darkening of the solution became stronger due to side reactions that always occur when working with these types of catechols. Slightly acidic conditions (pH 4–6) were also effective, so the acidic aqueous solutions obtained from the TFA-catalyzed hydrolysis can be used directly for condensation reactions, adding one equivalent of a base resulted in a pH range of 7–8. Amino acids preferably react with freeze-dried, acid-free dopegal, which results in a pH of around 7 (entries 1–7). The condensation products were isolated by direct crystallization from the reaction mixtures or were obtained by trituration or precipitation after evaporation of the reaction solvents.
Table 1. Reaction of Dopegal with Amines.
Ar = catechol.
Isolated yield.
Conditions: H2O (0.1 M), 1:1 ratio, 20–40 °C, 18 h.
The product was contaminated due to follow- up side reactions. By LCMS analysis, the Pictet–Spengler products of 22 with dopega were detected.
The product could not be isolated in pure form.
The α-aminoketones 14–17 derived from βAla, Gly-Gly, Ac-Lys, and Gln, respectively, were obtained in good yields (entries 1–4). It should be noted that proteins that are targeted by dopegal contain lysine-rich areas that can be considerably more nucleophilic than isolated Ac-Lys (entry 3). Cysteine condenses with dopegal in a different way and produces a diastereomeric mixture of catechol-substituted thiazolidines 18, which is a well-known reaction with aldehydes (entry 5).14 Although the formation of this type of thiazolidine is reversible, the thiazolidine adducts with dopegal were stable. Carnosine (entry 6) is a naturally occurring dipeptide that is abundant in the brain and displays a variety of positive effects as an antioxidant, and more importantly, as an antiglycant, preventing the formation of AGEs from sugars and protecting against neurodegenerative diseases such as Alzheimer’s disease.10,11,15 Carnosine and the common neurotransmitters GABA and phenethylamine reacted smoothly, providing α-aminoketones 19, 20, and 21, respectively, as solids in pure form (entries 6, 7, and 8). The Amadori products derived from the very important neurotransmitters dopamine (entry 9) and serotonin (not shown) were prone to follow-up reactions and could not be obtained in pure form. As can be concluded from the 1H and 13C NMR spectra, dopamine-derived aminoketone 22 was obtained in >80% purity. LCMS analysis showed that, most probably, a follow-up Pictet–Spengler reaction of 22 with dopegal occurs, followed by further oxidation reactions. Both dopamine and serotine are discriminated from the other neurotransmitters by their highly π-nucleophilic catechol or indole moieties. Also, histamine behaved differently, and although the reaction took place, we could not obtain the Amadori product 23 in pure form (entry 10). Finally, also of pyridoxamine, which is under investigation for the depletion of glucose in diabetes mellitus, the Amadori product 24 was isolated in pure form, albeit in a low yield (entry 11).15
Because the aromatic ring of the catechol part within the condensation products is conjugated with the carbonyl group, the stability of the catechol part is strongly increased toward oxidation as compared to precursors such as dopamine, dopal, and dopegal. Some reactivity still remains in the α-aminoketones as condensation in methanol with phenyl acetaldehydes readily yields pyrroles.16,17 A representative example of the reactivity of the catecholaldehydes is the condensation of dopamine-derived aminoketone 22 with dopal (2) at a neutral pH to give pyrrole 25 (43% yield) without the use of oxidants and or catalysts (Scheme 4). The isolation and characterization of pure pyrrole 25 is a further structure proof of the starting aminoketone 22 (vide supra). Pyrrole 25 could also be obtained by a one-pot, two-step condensation of dopegal and dopal18 with dopamine in a 1:1:1 ratio, at a neutral pH, albeit in a lower yield. This type of bis-aryl pyrroles and their further oxidized metabolites are well-known bioactive natural products, e.g., the lamellarines and ningaline.19 Bax et al. described an interesting, related pyrrole-forming reaction, whereby 2 equiv of dopal react with Ac-Lys under oxidative conditions, suggesting a mechanism for cross-linking processes in AD-related synuclein aggregation.4c,4d,5
Scheme 4. Pyrrole Formation.
In summary, we have unraveled an efficient amine alkylation process with the endogenous neurotoxin dopegal in the absence of an external oxidant. This Amadori-type rearrangement, which is actually an intramolecular redox reaction, is much faster than the natural Amadori reaction of amino residues in peptides with sugars. The resulting catechol-aminoketones are not known in the literature, and further (oxidative) conversion into harmful AGE-like derivatives cannot be ruled out. The potential value for these compounds can be found in the application as biomarkers for the accumulation of dopegal in biological systems. Finally, our scalable synthesis of dopegal enables its further use in neurobiochemical studies, which will be carried out in due course.
Experimental Section
General Information
All reactions concerning catechol derivatives were performed with degassed solvents and under argon to minimize darkening. Reagents and precursors were purchased with the highest purity (usually >98%) from Sigma-Aldrich and Fluorochem and used as received. Reactions were monitored by thin-layer chromatography (TLC) carried out on 0.25 mm E. Merck silica gel plates (60F-254). SilaFlash P60 (particle size 40–63 μm) was used for silica column chromatography. NMR spectra were recorded on Bruker DRX-500, -400, and -300 MHz instruments and calibrated on residual undeuterated solvent signals as an internal standard. High-resolution mass spectra (HRMS) were recorded with a TOF mass spectrometer of the type AccuTOF GC v 4g, JMST100GCV (JEOL, Japan). LCMS analysis has been carried out on a Finnigan LXQ (ESI-MS and Surveyor plus UV detectors) using a Shim-Pack GIST C18-AQ (Shimadzu) column. As the solvent system, A (H2O (Milli-Q quality) + 0.1% TFA) and B (MeOH + 0.1% TFA) were used. With a continuous flow of 1 mL/min, in 10 min, a gradient of 10% B was added and held for 5 min, followed by back to 100% A in 5 min. This was continued for 10 min. An FD/FI probe was equipped with an FD emitter of 10 μm. A current rate of 51.2 mA/min over 1.2 min using field desorption (FD) was used as an ionization method. IR spectra were recorded on a Bruker Alpha FTIR machine.
Synthetic Procedures
2-(3,4-Dihydroxyphenyl)acetaldehyde (2, Dopal)
KOtBu (1.35 g, 12.0 mmol) was added in one portion to a stirred suspension of (methoxymethyl)triphenylphosphonium chloride (4.12 g, 12.0 mmol) in THF (30 mL) at 0 °C. After 20 min, a solution of 3,4-bis((tetrahydro-2H-pyran-2-yl)oxy)benzaldehyde20 (3.67 g, 10.0 mmol) was added, and the reaction was stirred for 1 h at 0 °C and the next 4 h at room temperature. Silica was added, and the solvent was evaporated. Flash chromatography (silica, EtOAc/PE mixtures, 1/4 and 1/3) provided the enol ether (syrup, 2.3 g, 6.89 mmol, 69%) as a ca. 1:1 mixture of E/Z isomers. 1H NMR (400 MHz, CDCl3, selected signals) δ 6.95 (d, J = 12.9 Hz, 1H), 6.07 (d, J = 7.0 Hz, 1H), 5.76 (d, J = 12.9 Hz, 1H), 5.17 (d, J = 7.0 Hz, 1H), 3.77 (s, 3H), 3.67 (s, 9H); 13C{1H} NMR (101 MHz, CDCl3) δ 148.1, 148.1, 147.8, 147.6, 147.1, 146.9, 146.9, 146.8, 145.7, 145.6, 145.5, 145.4, 131.3, 131.2, 130.9, 130.8, 122.8, 122.7, 119.4, 119.3, 119.2, 118.8, 118.8, 118.5, 118.4, 118.1, 115.5, 115.2, 105.4, 105.4, 104.7, 104.7, 97.9, 97.8, 97.7, 97.7, 97.7, 97.6, 97.5, 97.3, 97.3, 77.6, 77.2, 76.9, 61.9, 61.9, 61.8, 61.8, 61.7, 61.7, 60.5, 56.4, 30.5, 30.4, 30.4, 25.4, 25.4, 18.8, 18.7, 18.7, 18.7, 18.6, 18.6; HRMS (FD+) m/z calcd for C19H26O5 [M•+] 334.1780, found 334.1772.
Then, 37% aqueous HCl (1 mL) was added dropwise to a stirred solution of this enol ether (1.02 g, 3.0 mmol) in acetone (20 mL) at 0 °C. After 20 min, the reaction was quenched with aqueous NaHCO3, extracted with EtOAc, and dried over Na2SO4. Chromatography (3% and 4% MeOH in DCM) gave dopal 2 (0.202 g, 1.33 mmol, 44%) as a thick syrup: 1H NMR (400 MHz, DMSO-d6) δ 9.59 (t, J = 2.3 Hz, 1H), 8.91 (s, 1H), 8.84 (s, 1H), 6.71 (d, J = 8.0 Hz, 1H), 6.62 (d, J = 2.1 Hz, 1H), 6.48 (dd, J = 8.0, 2.1 Hz, 1H), 3.52 (d, J = 2.3 Hz, 2H); 13C{1H} NMR (101 MHz, DMSO) δ 201.1, 145.9, 144.8, 123.5, 120.9, 117.4, 116.3, 49.5.
4-(1-Hydroxy-2,2-dimethoxyethyl)benzene-1,2-diol (13)
Catechol 11 (4.4 g, 40 mmol) and dimethoxy acetaldehyde 12 (5.96 mL of a 60% solution in water, 40 mmol) were added to a solution of sodium hydroxide (1.6 g, 40 mmol) in water (60 mL), and the resulting solution was stirred under nitrogen at 80 °C for 6 h. After cooling, acetic acid (2.29 mL, 40 mmol) was added with stirring, followed by solid NaCl (ca. 3 g). The resulting solution was extracted with a mixture of ethyl acetate and methanol (95/5, 5 × 40 mL) The combined organic layers were dried (Na2SO4), evaporated to dryness, and coevaporated with ethyl acetate (50 mL) at 50 °C and 10 mbar. The resulting light brown syrup was stirred with dichloromethane (30 mL), which induced crystallization of the product, leaving starting material, and the ortho isomer in solution. (If the product refuses to crystallize, a small amount should be purified by chromatography). The mixture was kept at 4 °C overnight, after filtration, washing with dichloromethane (3 × 5 mL), and air-drying. Product 13 (3.02 g, 14.1 mmol, 35%) was obtained as a free-flowing solid: mp 124–127 °C; IR 3390, 3266, 1624, 1605 cm–1; 1H NMR (400 MHz, D2O) δ 6.82 (m, 2H), 6.75 (d, J = 8.2 Hz, 1H), 4.46 (d, J = 6.7 Hz, 1H), 4.42 (d, J = 6.7 Hz, 1H), 3.40 (s, 3H), 3.19 (s, 3H); 13C{1H} NMR (126 MHz, D2O) δ 144.0, 143.9, 131.8, 120.0, 116.1, 115.1, 107.0, 73.1, 55.7, 55.3; HRMS (FD+) m/z calcd for C10H14O5 [M•+] 214.0836, found 214.0835.
2-(3,4-Dihydroxyphenyl)-2-hydroxyacetaldehyde (7, Dopegal)
A solution of acetal 13 (0.856 g, 4 mmol) in water (40 mL) was degassed with a stream of nitrogen gas at 45 °C during 1 h. Trifluoroacetic acid (0.308 mL, 4 mmol) was added; the reaction flask was stoppered and stirred during 24 h at 45 °C. The required reaction time and temperature were obtained from earlier D2O experiments, in an NMR tube, also at c = 0.1 M. At this concentration, dopegal is 95% present in the hydrated form. Small amounts of dimeric forms are probably also present, but these give no characteristic signals (see below). This 0.1 M solution of dopegal in 0.1 M aqueous TFA is suitable for further chemistry. Freeze-drying, followed by dissolving in water (20 mL) and repeated freeze-drying, gave the dimer as a mixture of isomers and conformers (beige solid, 0.62 g, 92%), which has the same reactivity as the 1% TFA solution.
Dopegal Hydrate:12
hydrate/free aldehydes 95/5; 1H NMR (400 MHz, 0.1 M TFA in D2O) δ 6.80 (s, 1H), 6.77 (d, J = 7.7 Hz, 1H), 6.73–6.66 (m, 1H), 4.93 (d, J = 6.2 Hz, 1H), 4.24 (d, J = 6.2 Hz, 1H), 3.21 (s, 6H); 13C{1H} NMR (101 MHz, D2O) δ 143.6, 132.0, 119.8, 115.9, 114.9, 91.9, 75.8, 48.7. Dopegal free aldehyde: 1H NMR (400 MHz, 0.1 M TFA in D2O) δ 9.38 (m, 1H), 5.91 (m, 1H). Dopegal dimeric mixture: mp 70–95 °C; IR 3200–3400, 1607, 1520 cm–1; 1H NMR (400 MHz, D2O) δ 7.0–6.5 (broad, 3H–Ar), 5.6–4.2 (m, 2H); HRMS (FD+) m/z calcd for C8H8O4 [M•+] 168.0418, found 168.0419 (monomer).
3-((2-(3,4-Dihydroxyphenyl)-2-oxoethyl)amino)propanoic Acid (14) from βAla
Dopegal 7 (70.6 mg, 0.42 mmol) was added to a solution of β-alanine (35.6 mg, 0.40 mmol) in water (1.5 mL), and the resulting solution was stirred at 40 °C for 18 h. Cooling to room temperature, filtration and washing with water (3×), and with a mixture of isopropanol/diethyl ether, (1/1) gave 14 (52.3 mg, 0.218 mmol, 54%) as a beige solid: mp:239–241 °C; IR 3358, 3300–2400, 1688, 1602 cm–1; 1H NMR (400 MHz, DMSO-d6) δ 7.38 (m, 2H), 6.84 (d, J = 8.0 Hz, 1H), 4.14 (s, 2H), 2.86 (t, J = 6.6 Hz, 2H), 2.38 (t, J = 6.6 Hz, 2H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 195.5, 174.1, 151.9, 145.9, 127.3, 121.5, 115.8, 115.3, 53.9, 44.9, 33.9; HRMS (FD+) m/z calcd for C11H14NO5 [M + H]+ 240.0867, found 240.0871.
(2-(3,4-Dihydroxyphenyl)-2-oxoethyl)glycylglycine (15) from Gly-Gly
Dopegal 7 (75.6 mg, 0.45 mmol) was added to a solution of glycinylglycine (52.8 mg, 0.4 mmol) in water (1 mL), and the resulting solution was stirred at 40 °C for 18 h. After cooling, the precipitated product was filtered and washed successively with water (0.5 mL), isopropanol, and ether to give 15 (73.7 mg, 0.261 mmol, 65%): mp 232–234 °C; IR 3353, 1669, 1589 cm–1; 1H NMR (400 MHz, DMSO-d6) δ 8.18 (t, J = 5.8 Hz, 1H), 7.33–7.35 (m, 2H), 6.83 (d, J = 8.1 Hz, 1H), 4.07 (s, 2H), 3.79 (d, J = 5.8 Hz, 2H), 3.28 (s, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 195.1, 171.8, 170.4, 151.7, 145.8, 127.1, 121.3, 115.7, 115.0, 54.2, 51.2, 41.4; HRMS (FD+) m/z calcd for C12H15N2O6 [M + H]+ 283.0925, found 283.0930.
N2-Acetyl-N6-(2-(3,4-dihydroxyphenyl)-2-oxoethyl)-l-lysine (16) from Ac-Lys
Dopegal 7 (75.6 mg, 0.45 mmol) was added to a solution of N2-acetyl-lysine (75.2 mg, 0.40 mmol) in water (1.5 mL), and the resulting solution was stirred at 40 °C for 18 h. Evaporation of the solvent and trituration of the residue with methanol (3 mL) at 50 °C gave 16 as a beige solid (75.0 mg, 0,222 mmol, 55%): mp 194–197 °C; IR 3500–2500, 1662, 1585 cm–1; 1H NMR (400 MHz, DMSO-d6) δ 7.80 (d, J = 7.7 Hz, 1H), 7.37 (m, 2H), 6.85 (d, J = 8.1 Hz, 1H), 4.32 (s, 2H), 4.07 (m, 1H), 2.73 (t, J = 7.5 Hz, 2H), 1.84 (s, 3H), 1.77–1.43 (m, 4H), 1.43–1.19 (m, 2H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 194.5, 174.7, 169.3, 152.5, 146.2, 127.1, 121.6, 116.0, 115.5, 53.9, 53.2, 48.6, 32.0, 28.1, 25.9, 23.4, 23.0; HRMS (FD+) m/z calcd for C16H23N2O6 [M + H]+ 339.1551, found 339.1554.
(2-(3,4-Dihydroxyphenyl)-2-oxoethyl)-l-glutamine (17) from Gln
Dopegal 7 (75.6 mg, 0.45 mmol) was added to a solution of glutamine (58.4 mg, 0.40 mmol) in water (1 mL), and the resulting solution was stirred at 40 °C for 18 h. After cooling, the precipitated product was filtered and washed with water (0.5 mL), isopropanol, and ether. The filtrate was concentrated to obtain a second batch of off-white product 17 (85.4 mg, 0.289 mmol, 72%): mp 184–185 °C; IR 3409, 3323, 3197, 2900–2300, 1663, 1600, cm–1; 1H NMR (400 MHz, DMSO-d6) δ 9.45 (bs, 2H), 7.43–7.39 (m, 2H), 7.37 (s, 1H), 6.87 (m, 1H), 6.84 (s, 1H), 4.27 (d, J = 18.1 Hz, 1H), 4.12 (d, J = 18.1 Hz, 1H), 3.27 (m, 1H), 2.25 (m, 2H), 1.94 (m, 1H), 1.85 (m, 1H); 13C{1H} NMR (101 MHz, DMSO) δ 193.0, 174.9, 172.6, 152.3, 146.0, 126.5, 121.6, 115.8, 115.2, 61.0, 52.1, 31.9, 26.6; HRMS (FD+) m/z calcd for C13H17N2O6 (M+H)+ 297.1081, found 297.1077.
(4R)-2-((3,4-Dihydroxyphenyl)(hydroxy)methyl)thiazolidine-4-carboxylic acid (18) from Cys
A solution of dopegal 7 (38.6 mg, 0.23 mmol) and l-cysteine (24.2 mg, 0.2 mmol) in water (1 mL) was kept at rt overnight. The solvent was evaporated, and the residue was triturated with a mixture of methanol and isopropanol to give thiazolidine 14 as a mixture of isomers (24.9 mg, 0.108 mmol, 54%): mp 154–164 °C; IR 3400–2200, 1608, 1576 cm –1; 1H NMR (400 MHz, DMSO-d6, main isomer, selected signals) δ 8.83 (broad, 2H), 6.80 (m, 1H), 6.62 (m, 2H), 5.12 (broad, 1H), 4.72 (d, J = 7.2 Hz, 1H), 4.29 (d, J = 7.2 Hz, 1H), 3.89 (t, J = 6.6 Hz, 1H), 3.01 (dd, J = 10.1, 6.5 Hz, 1H); HRMS (FD+) m/z calcd for C11H14NO5S [M + H]+ 272.0587, found 272.0588.
(3-((2-(3,4-Dihydroxyphenyl)-2-oxoethyl)amino)propanoyl)-l-histidine (19) from βAla-His (Carnosine)
Carnosine (45.0 mg, 0.20 mmol) was added to a solution of 0.1 M dopegal in 0.1 M TFA (2.5 mL, 0.25 mmol), and the solution was stirred for 16 h at rt. The solvent was evaporated, the residue was dissolved in methanol, and the addition of isopropanol gave precipitation of product 19 as a solid (TFA-salt, 76.0 mg, 0.136 mmol, 68% after drying at 60 °C and 0.1 mbar): mp 156–159 °C; IR 3300–2300, 1668, 1585 cm–1; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (m, 1H), 7.49–7.28 (m, 2H), 6.99 (s, 1H), 6.90 (d, J = 8.2 Hz, 1H), 4.57 (s, 2H), 4.38 (dt, J = 8.3, 3.8 Hz, 1H), 3.19 (t, J = 7.0 Hz, 2H), 3.05 (dd, J = 15.0, 4.7 Hz, 1H), 2.88 (dd, J = 14.9, 8.6 Hz, 1H), 2.69–2.57 (m, 2H); 13C{1H} NMR (126 MHz, DMSO) δ 190.7, 172.8, 169.8, 152.8, 146.1, 134.7, 131.3, 125.8, 122.2, 118.7, 117.3, 116.4, 116.0, 115.8, 115.2, 62.5, 62.5, 52.3, 52.3, 43.7, 31.3, 27.6; HRMS (FD+) m/z calcd for C17H21N4O6 [M + H]+ 377.1456, found 377.1457.
4-((2-(3,4-Dihydroxyphenyl)-2-oxoethyl)amino)butanoic Acid (20) from GABA
Dopegal 7 (75.6 mg, 0.45 mmol) was added to γ-aminobutyric acid (41.2 mg, 0.40 mmol) in water (1 mL), and the resulting solution was stirred at 40 °C for 18 h. The sticky product was converted into crystalline material by concentration and trituration. The product was filtered and washed successively with water (0.5 mL), isopropanol, and ether to give 20 (53.9 mg, brownish solid, 0.213 mmol, 53%): mp 181–184 °C; IR 3300–2300, 1676, 1600 cm–1; 1H NMR (400 MHz, DMSO-d6) δ 9.0–6.0 (broad, 2H), 7.45–7.33 (m, 2H), 6.82 (d, J = 7.9 Hz, 1H), 4.10 (s, 2H), 2.68 (t, J = 6.8 Hz, 2H, 2.29 (t, J = 6.8 Hz, 2H), 1.70 (m, 2H); 13C{1H} NMR (101 MHz, DMSO) δ 193.6, 176.0, 153.0, 146.2, 126.2, 121.7, 115.7, 115.0, 53.2, 48.3, 34.0, 23.6; HRMS (FD+) m/z calcd for C12H16NO5 [M + H]+ 254.1023, found 254.1024.
1-(3,4-Dihydroxyphenyl)-2-(phenethylamino)ethan-1-one (21) from Phenethylamine
A solution of phenethylamine (48.4 mg, 0.4 mmol) in methanol (2 mL) was mixed with dopegal 7 (4 mL of a 0.1 M solution in 0.1 M TFA, 0.40 mmol) and stirred at 45 °C overnight. Evaporation of the solvents and trituration with isopropanol gave 23 (TFA-salt, 97.9 mg, 0.266 mmol, 66%): mp 179–181 °C; IR 3374, 1676, 1669 1605 cm–1; 1H NMR (400 MHz, DMSO-d6) δ 10.24 (broad, 1H), 9.58 (broad, 1H), 9.02 (broad, 2H), 7.49–7.34 (m, 4H), 7.29 (m, 3H), 6.91 (d, J = 8.2 Hz, 1H), 4.70 (s, 2H), 3.22 (t, J = 8.3 Hz, 2H), 3.09–2.93 (m, 2H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 190.8, 159.0, 158.7, 152.8, 146.1, 137.6, 129.1, 129.1, 127.3, 125.9, 122.2, 115.8, 115.2, 52.2, 48.3, 31.9; HRMS (FD+) m/z calcd for C16H18NO3 [M + H]+ 272.1281, found 272.1278.
2-((3,4-Dihydroxyphenethyl)amino)-1-(3,4-dihydroxyphenyl)ethan-1-one (22) from Dopamine
A solution of dopamine·HCl (151.6 mg, 0.8 mmol), ascorbic acid (35.2 mg, 0.20 mmol), and dopegal 7 (123.9 mg, 0.81 mmol) in a mixture of degassed water (3 mL) and methanol (1 mL) was purged with argon for 30 min. Sodium hydrogen carbonate (84.0 mg, 1.0 mmol) was added; degassing was continued for 10 min. The reaction flask was wrapped in aluminum foil, and the reaction mixture was stirred at rt for 72 h. The precipitate was collected, washed with water (3 times ) and a 1/1 mixture of isopropanol and diethyl ether. The brown product 21 (157.6 mg, 0.521 mmol, 65%) was stored at 4 °C: mp 183–192 °C IR 3500–2500, 1645, 1589 cm –1; 1H NMR (400 MHz, DMSO-d6) δ 7.39–7.30 (m, 2H), 6.78 (d, J = 8.2 Hz, 1H), 6.68–6.58 (m, 2H), 6.46 (dd, J = 8.0, 2.0 Hz, 1H), 3.98 (s, 2H), 2.73 (t, J = 7.3 Hz, 2H), 2.58 (t, J = 7.3 Hz, 2H); 13C{1H} NMR (101 MHz, DMSO) δ 196.0, 153.1, 146.2, 145.4, 143.8, 131.1, 126.5, 121.5, 119.6, 116.4, 115.8, 115.4, 114.3, 54.6, 51.1, 35.3; HRMS (FD+) m/z calcd for C16H18NO5 [M + H]+ 304.1180, found 304.1182.
2-((2-(1H-Imidazol-4-yl)ethyl)amino)-1-(3,4-dihydroxyphenyl)ethan-1-one (23) from Histamine
NaHCO3 (40 mg, 0.44 mmol) was added to a solution of histamine dihydrochloride (73.6 mg, 0.4 mmol) and dopegal 7 (74.0 mg, 0.42 mmol) in water (2 mL). After stirring at 40 °C overnight, the dark reaction mixture was acidified with 2 M HCl (1 mL) and evaporated to dryness. The residue was coeavaporated 3 times with methanol, redissolved in methanol (ca. 2 mL), and filtered to remove inorganic salts. Evaporation of the filtrate and triturating the residue with mixtures of ethanol, isopropanol, and diethyl ether gave 24·2HCl (67.0 mg, 0.20 mmol, 50%) as a dark, waxy solid: IR 3500–2500, 1674, 1596 cm–1; 1H NMR (300 MHz DMSO-d6) δ 14.57 (s, 3H), 10.38 (s, 1H), 9.64 (s, 1H), 9.37 (s, 2H), 9.09 (s, 2H), 8.24 (s, 1H), 7.56 (s, 1H), 7.41 (m, 2H), 6.93 (d, J = 8.6 Hz, 1H), 4.99 (s, 1H), 4.69 (s, 2H), 3.3–3.4 (m, 2H), 3.25–3.11 (m, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 190.3, 189.7, 152.9, 152.7, 146.1, 134.2, 129.3, 129.2, 125.8, 125.5, 122.3, 117.2, 115.9, 115.3, 62.5, 52.0, 45.6, 37.7, 25.9, 22.7, 21.3; HRMS (FD+) m/z calcd for C13H16N3O3 [M + H]+ 262.1186, found 262.1189.
1-(3,4-Dihydroxyphenyl)-2-(((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methyl)amino)ethan-1-one (24) from Pyridoxamine
NaHCO3 (37 mg, 0.44 mmol) was added to a solution of pyridoxamine dihydrochloride (96.4 mg, 0.4 mmol) and dopegal 7 (73.9 mg, 0.44 mmol) in water (2 mL). After stirring at rt overnight, the dark reaction mixture was evaporated, acidified with 2 M HCl (1 mL), and evaporated to dryness. The residue was coeavaporated with water (1 mL), redissolved in water (0.5 mL), and kept in the fridge overnight. The crystalline products was isolated by filtration and washed successively with a few drops of ice-cold water with isopropanol and diethyl ether to give 25 (42.1 mg, 0.108 mmol, 27% in 2 crops): mp 142–148 °C; IR 3500–2500, 1674, 1589 cm–1; 1H NMR (400 MHz, DMSO-d6) δ 10.37 (br, 1H), 9.64 (br, 2H), 8.27 (s, 1H), 7.41 (m, 2H), 6.94 (d, J = 8.8 Hz, 1H), 4.82 (s, 2H), 4.74 (s, 2H), 4.48 (s, 2H), 2.70 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 190.2, 154.1, 152.7, 146.0, 143.0, 140.2, 134.3, 130.5, 125.6, 122.2, 115.8, 115.2, 58.6, 52.5, 41.7, 16.0; HRMS (FD+) m/z calcd for C16H19N2O5 [M + H]+ 319.1289, found 319.1306.
4,4′-(1-(3,4-Dihydroxyphenethyl)-1H-pyrrole-3,4-diyl)bis(benzene-1,2-diol) (25)
A mixture of 22 (60.6 mg, 0.2 mmol) and dopal 2 (33.5 mg, 0.21) in 1.5 mL of MeOH (degassed with argon) was stirred in the dark for 42 h under O2-free conditions. Evaporation and flash chromatography (DCM/MeOH 90/10 and 87/13) were performed quickly. Product 25 (36.4 mg, 0.0869 mmol, 43%) was obtained after drying (0.1 mbar, 40 °C) as a colorless glass, turning red/brown upon standing. A one-pot reaction of the three components in a 1/1 mixture of methanol and water, with 48 h intervals, gave 25 in a slightly lower yield: 1H NMR (500 MHz, DMSO-d6) δ 8.63 (bs, 6H), 6.72 (s, 2H), 6.69–6.64 (m, 2H), 6.63–6.56 (m, 4H), 6.51 (dd, J = 8.1, 2.1 Hz, 1H), 6.44 (dd, J = 8.1, 2.1 Hz, 2H), 3.99 (dd, J = 9.0, 6.6 Hz, 2H), 2.96–2.78 (m, 2H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 145.5, 145.2, 144.1, 143.5, 129.9, 128.2, 122.3, 119.8, 119.8, 119.3, 116.6, 116.0, 116.0, 115.8, 50.8, 37.1; HRMS (FD+) m/z calcd for C24H21NO6 [M•+] 419.1363, found 419.1355.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.9b01948.
1H and 13C NMR spectra of all new products and intermediates; LCMS traces of the aminoketones 14–24 (PDF)
The authors declare no competing financial interest.
Dedication
We dedicate this contribution to the memory of Prof. Gerrit-Jan Koomen, who sadly passed away on January 16, 2019 as a consequence of Alzheimer’s disease.
Supplementary Material
References
- Burke W. J.; Li S. W.; Chung H. D.; Ruggiero D. A.; Kristal B. S.; Johnson E. M.; Lampe P.; Kumar V. B.; Franko M.; Williams E. A.; Zahm D. S. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. NeuroToxicology 2004, 25, 101–115. 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Marchitti S. A.; Deitrich R. A.; Vasiliou V. Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: The role of aldehyde dehydrogenase. Pharmacol. Rev. 2007, 59, 125–150. 10.1124/pr.59.2.1. [DOI] [PubMed] [Google Scholar]
- Goldstein D. S.; Kopin I. J.; Sharabi Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol. Ther. 2014, 144, 268–282. 10.1016/j.pharmthera.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Burke W. J.; Kumar V. B.; Pandey N.; Panneton W. M.; Gan Q.; Franko M. W.; O’Dell M.; Li S. W.; Pan Y.; Chung H. D.; Galvin H. D. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008, 115, 193–203. 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]; b Rees J. N.; Florang V. R.; Eckert L. L.; Doorn J. A. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem. Res. Toxicol. 2009, 22, 1256–1263. 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Werner-Allen J. W.; DuMond J. F.; Levine R. L.; Bax A. Toxic Dopamine Metabolite DOPAL Forms an Unexpected Dicatechol Pyrrole Adduct with Lysines of alpha-Synuclein. Angew. Chem., Int. Ed. 2016, 55, 7374–7378. 10.1002/anie.201600277. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Werner-Allen J. W.; Monti S.; DuMond J. F.; Levine R. L.; Bax A. Isoindole Linkages Provide a Pathway for DOPAL-Mediated Cross-Linking of alpha-Synuclein. Biochemistry 2018, 57, 1462–1474. 10.1021/acs.biochem.7b01164. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Kumar V. B.; Hsu F.-F.; Lakshmi V. M.; Gillespie K. N.; Burke W. J. Aldehyde adducts inhibit 3,4-dihydroxyphenylacetaldehyde-induced alpha-synuclein aggregation and toxicity: Implication for Parkinson neuroprotective therapy. Eur. J. Pharmacol. 2019, 845, 65–73. 10.1016/j.ejphar.2018.12.027. [DOI] [PubMed] [Google Scholar]
- Werner-Allen J. W.; Levine R. L.; Bax A. Superoxide is the critical driver of DOPAL autoxidation, lysyl adduct formation, and crosslinking of alpha-synuclein. Biochem. Biophys. Res. Commun. 2017, 487, 281–286. 10.1016/j.bbrc.2017.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ruiz-Olalla A.; Würdemann M. A.; Wanner M. J.; Ingemann S.; van Maarseveen J. H.; Hiemstra H. Organocatalytic Enantioselective Pictet-Spengler Approach to Biologically Relevant 1-Benzyl-1,2,3,4-Tetrahydroisoquinoline Alkaloids. J. Org. Chem. 2015, 80, 5125–5132. 10.1021/acs.joc.5b00509. [DOI] [PubMed] [Google Scholar]; b Horst B.; Wanner M. J.; Ingemann Jørgensen S.; Hiemstra H.; van Maarseveen J. H. Total Synthesis of the Ortho-Hydroxylated Protoberberines (S)-Govaniadine, (S)-Caseamine, and (S)-Clarkeanidine via a Solvent Directed Pictet–Spengler Reaction. J. Org. Chem. 2018, 83, 15110–15117. 10.1021/acs.joc.8b02378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Li S. W.; Elliott W. H.; Burke W. J. Synthesis of a biochemically important aldehyde, 3,4-dihydroxyphenylglycolaldehyde. Bioorg. Chem. 1994, 22, 337–342. 10.1006/bioo.1994.1027. [DOI] [Google Scholar]; b Narayanan J.; Hayakawa Y.; Fan J.; Kirk K. L. Convenient syntheses of biogenic aldehydes, 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde. Bioorg. Chem. 2003, 31, 191–197. 10.1016/S0045-2068(03)00019-1. [DOI] [PubMed] [Google Scholar]
- a Hodge J. E. The Amadori rearrangement. Adv. in Carboh. Chem. 1955, 10, 169–205. 10.1016/S0096-5332(08)60392-6. [DOI] [PubMed] [Google Scholar]; b Amadori M. The condensation product of glucose and p-anisidine. Atti R Accad Naz Lincei 1929, 9, 226–230. [Google Scholar]
- Salahuddin P.; Rabbani G.; Khan R. H. The role of advanced glycation end products in various types of neurodegenerative disease: a therapeutic approach. Cell. Mol. Biol. Lett. 2014, 19, 407–437. 10.2478/s11658-014-0205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z.; Baba S. P.; Sweeney B. R.; Barski O. A. Detoxification of aldehydes by histidine-containing dipeptides: From chemistry to clinical implications. Chem.-Biol. Interact. 2013, 202, 288–297. 10.1016/j.cbi.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. A. M.; Builta Z. J.; Monroe T. B.; Doorn J. A.; Anderson E. J. Biochemical characterization of the catecholaldehyde reactivity of l-carnosine and its therapeutic potential in human myocardium. Amino Acids 2019, 51, 97–102. 10.1007/s00726-018-2647-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziosi P.; Paolucci C.; Santarelli F.; Tabanelli T.; Passeri S.; Cavani F.; Righi P. A Two-Step Process for the Synthesis of Hydroxytyrosol. ChemSusChem 2018, 11, 2202–2210. 10.1002/cssc.201800684. [DOI] [PubMed] [Google Scholar]
- Griffiths D. W.; Gutsche C. D. Synthesis of Mandelaldehyde Dimers. J. Org. Chem. 1971, 36, 2184–2186. 10.1021/jo00814a034. [DOI] [Google Scholar]
- Schmolka I. R.; Spoerri P. E. Thiazolidine Chemistry. II. The Preparation of 2-Substituted Thiazolidine-4-carboxylic Acids. J. Org. Chem. 1957, 22, 943–946. 10.1021/jo01359a023. [DOI] [Google Scholar]
- Ghodsi R.; Kheirouri S. Carnosine and advanced glycation end products: a systematic review. Amino Acids 2018, 50, 1177–1186. 10.1007/s00726-018-2592-9. [DOI] [PubMed] [Google Scholar]
- Voziyan P. A.; Hudson B. G. Pyridoxamine as a multifunctional pharmaceutical: targeting pathogenic glycation and oxidative damage. Cell. Mol. Life Sci. 2005, 62, 1671–1681. 10.1007/s00018-005-5082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov M.; Delpech B.; Aubert G.; Cresteil T.; García-Alvarez M. C.; Collin P.; Marazano C. A consice formation of N-substituted 3,4-diarylpyrroles - synthesis and cytotoxic activity. Org. Biomol. Chem. 2014, 12, 1518–1524. 10.1039/C3OB42309C. [DOI] [PubMed] [Google Scholar]
- Dopal 2 was prepared by a Wittig reaction/acid hydrolysis route, see Experimental Section.
- For a representative synthetic study on lamellarine and ningaline alkaloids, see:; Li Q.; Jiang J.; Fan A.; Cui Y.; Jia Y. Total Synthesis of Lamellarins D, H, and R and Ningalin B.. Org. Lett. 2011, 13, 312–315. 10.1021/ol1027877. [DOI] [PubMed] [Google Scholar]
- De Simone A.; Bartolini M.; Baschieri A.; Apperley K. Y. P.; Chen H. H.; Guardigni M.; Montanari S.; Kobrlova T.; Soukup O.; Valgimigli L.; Andrisano V.; Keillor J. W.; Basso M.; Milelli A. Hydroxy-substituted trans-cinnamoyl derivatives as multifunctional tools in the context of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 139, 378–389. 10.1016/j.ejmech.2017.07.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.