Abstract
Background
The preterm birth rate in Germany has remained unchanged at 8–9% since 2009. Preterm birth is the most common cause of neonatal morbidity and mortality. In the absence of a causal treatment, it is important to lower the risk of preterm birth by preventive measures in prenatal outpatient care.
Methods
This review is based on pertinent publications from the years 2000–2019 that were retrieved by a selective search in PubMed.
Results
The clinical risk factors for preterm birth—known mainly from retrospective cohort studies—include previous preterm birth (adjusted odds ratio [aOR]: 3.6), multiple pregnancy (relative risk [RR]: 7.7), nicotine consumption (aOR: 1.7), and a short uterine cervix, i.e., <25 mm in the second trimester (aOR: 6.9). In women with a short cervix, vaginally administered progesterone significantly lowers the preterm birth rate (22.5% vs. 14.1% for birth before 33 weeks of gestation, RR: 0.62; 95% confidence interval [0.47; 0.81]). Nicotine abstinence is associated with a lower preterm birth rate as well (aOR: 0.91; [0.88; 0,.94]), while working more than 40 hours per week (aOR: 1.25; [1,.01; 1,.54]) and heavy lifting during pregnancy (hazard ratio [HR]: 1.43; [1.13; 1.80]) are associated with a higher preterm birth rate. Avoidance of physical exertion, or bed rest, in the face of impending preterm birth does not lower the preterm birth rate, but it does increase the risk of complications, such as thromboembolism.
Conclusion
The meticulous assessment and elimination of treatable risk factors at the outset of ambulatory prenatal care can help lower the preterm birth rate. Further progress can be expected to include the development of causally directed treatments (e.g., changes of relevant environmental and epigenetic factors).
To this day preterm birth continues to constitute one of the biggest problems in obstetrics. It is defined as a birth taking place before completion of 37 weeks’ gestation. Rates of preterm births in Europe range from 5.3% (Latvia) to 14.7% (Cyprus) (1). In Germany in 2009, the rate was 9.38% (2) and in 2017, 8.60 (3)—which means it has remained high, with no major changes.
Preterm births account for 75% of perinatal mortality (≤ 7 days after the birth) and for 35% of neonatal mortality (≤ 28 days after the birth), as well as for 16% of deaths in children younger than 5 years (e1– e3). In Germany in 2017, perinatal mortality before completion of the 28th week of gestation was 33.4% (N=1498), in babies born between 28 and 31 weeks’ gestation it was 8.0% (N=568), and in those born between 32 and 36 weeks’ gestation, 1.6% (N=891) (3).
Extremely preterm neonates in particular present a massive psychosocial burden for affected families and a substantial financial burden for the healthcare system (4). Furthermore, preterm birth is considered one of the main risk factors for disability adjusted life years (DALYs, life years lost owing to sickness, disability, or early death) (5).
This article studies the risk factors and prevention of spontaneous preterm birth in singleton pregnancies. It has a particular focus on outpatient/ambulatory care.
Method
We conducted a selective literature search of the years 2000 through March 2019 in PubMed, using the search terms “preterm birth”, “preterm delivery”, “risk factors”, “prediction”, and “prevention”. We included publications of crucial importance that pre-date 2000.
Epidemiology/risk factors
The careful documentation of risk factors at the start of antenatal care is the essential prerequisite for individual risk assessment and the basis of prevention. Particular attention during pregnancy should be given to avoiding risk factors that can be influenced—such as smoking, an unhealthy diet or malnutrition, and severe professional stress. The studies included in our review used odds ratios (OR) or relative risks (RR) to describe risks. Where we adjusted these for confounding factors, we denoted this by adding the letter “a” as a prefix (aOR, aRR).
The length of the cervix can be determined by vaginal ultrasonography. Shortening of the cervix to <25 mm between 16 and 24 week’s of gestation is considered the strongest independent risk factor for spontaneous preterm birth <35 weeks’ gestation in women without premature labor with singleton pregnancies (RR: 6.9; 95% confidence interval [4.3; 11.1]) (table 1) (6). In such cases the risk of preterm birth is 25–30%; this rises to >35% in pregnant women who had a prior preterm birth (7), and if the cervical length is <15 mm on ultrasonography, it rises to 50% (6, 8).
Table 1. Risk factors for preterm birth.
| Risk factors | 95% CI | Source (reference) | ||
| Multiple pregnancy | RR | 7.7 | Birth register (11) | |
| Cervical length <25 mm between 16–24 weeks‘ gestation | RR | 6.9 | [4.3; 11.1] | Prospective cohort study (6) |
| Vaginal bleeding in late pregnancy | aOR | 5.6 | [4.9; 6.6] | Retrospective cohort study (e4) |
| Pre-eclampsia | aOR | 4.2 | [4.1; 4.3] | Retrospective cohort study (12) |
| Interval between pregnancies <12 months | aOR | 4.2 | [3.0; 6.0] | Retrospective cohort study (e35) |
| Prior spontaneous abortion | aOR | 3.6 | [3.2; 4.0] | Retrospective cohort study (e36) |
| Periodontal disease | aRR | 2.0 | [1.2; 3.2] | Prospective cohort study (e37) |
| Vaginal bleeding in early pregnancy | aOR | 1.9 | [1.6; 2.2] | Retrospective cohort study (e4) |
| Unfavorable socioeconomic circumstances/ conditions | RII | 1.75 | [1.65; 1.86] | Retrospective cohort study (e38) |
| Pregnant woman <18 years | aOR | 1.7 | [1.02; 3.08] | Retrospective cohort study (e39) |
| Smoking | aOR | 1.7 | [1.3; 2.2] | Case-control study (e40) |
| Prior cone biopsy | pRR | 1.7 | [1.24; 2.35] | Meta-analysis of retrospective cohort studies (e41) |
| Single mother | aOR | 1.61 | [1.26; 2.07] | Case-control study (e42) |
| Prior medically indicated preterm birth | aOR | 1.6 | [1.3; 2.1] | Retrospective cohort study (e36) |
| Anemia | aOR | 1.5 | [1.1; 2.2] | Retrospective cohort study (e5) |
| Bacterial vaginosis | aOR | 1.4 | [1.1; 1.8] | Prospective cohort study (e6) |
| Asymptomatic bacteriuria | aOR | 1.3 | [1.0; 1.6] | Retrospective cohort study (e4) |
95% CI, 95% confidence interval; aOR, adjusted odds ratio; aRR, adjusted relative risk; pRR, pooled relative risk;
RII, relative index of inequality; RR, relative risk;
the letter a as prefix a (aOR, aRR) denotes adjustment for confounding factors.
OR: Ratio of affected persons with (a) and without risk factor (b) divided by the ratio of non-sick persons with (c) and without risk factor (d): a × d/ b × c;
RR: Ratio of the probabilities of a condition in persons with risk factor (a/a+c) versus a condition in persons without risk factor (b/b+d): a × (b+d)/ b × (a+c)
A further strong risk factor for spontaneous preterm birth is a prior spontaneous preterm birth (aOR: 3.6; [3.2; 4.0]) (table 1) (9). Depending on the number of prior spontaneous preterm births and the timing of their manifestation, the absolute risk of a repeat preterm birth is 30%, in two or more prior preterm births <32+0 weeks’ gestation, it is as high as 57% (10).
Further important risk factors are vaginal bleeding in late pregnancy (e.g. placenta previa) and pre-eclampsia (table 1); in both scenarios the pregnancy is often terminated for medical reasons. Such iatrogenic preterm births (early termination of pregnancy for medical reasons, mostly done by caesarean section, for example, because of pre-eclampsia) now account for 30–35% of all preterm births (e7).
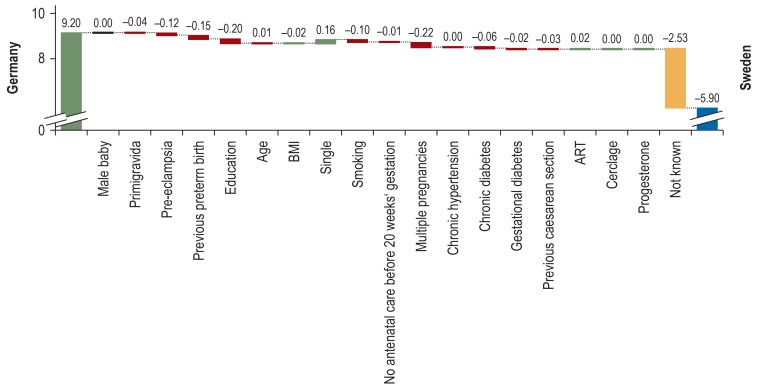
In the setting of pregnancy counseling for women considering subsequent pregnancies, the more than quadruple aOR for a preterm birth if the interval between two pregnancies is <12 months is important (9). Multiple pregnancies are associated with a 7.7 times increase in the RR for a preterm birth (absolute risk up to 60%). Their rate among the overall rate of preterm births is 10–20% (11). This should be considered especially for in vitro fertilization, which is associated with a raised incidence in multiple pregnancies (e8). Factors associated with a lower risk of preterm birth, such as nulliparity and male sex, affect to a lesser degree the individual risk than the overall preterm birth rate in the population, with rates of 13–28% and 6–8% (12). The difference in the rate of preterm births between Germany and Sweden is 3.3%; only 25% of this can be explained by differences in the known risk factors (figure). These include mainly the higher level of education and the lower rate of multiple pregnancies in Sweden (12).
Figure.
Contribution of individual risk factors and clinical measures to the different rates of preterm births in Germany (9.2 %) and Sweden (5.9 %) (modified from [12])
ART, assisted reproductive technology; BMI, body mass index
To lower the preterm birth rate to a significant degree, further in-depth research is needed into the causes of preterm birth, with the aim of developing effective, patient-centered therapeutic concepts.
Prevention
Progesterone
Progesterone has shown anti-inflammatory and immunomodulatory effects at the fetomaternal interface. Among others it inhibits uterine contraction and the production of prostaglandins, which induce labor and cervical maturation (e9).
To prevent preterm birth, natural progesterone is administered (vaginal or oral, dosage mostly 200 mg/day); in the US the substance of choice is 17-alpha-hydroxyprogesterone caproate (intramuscular, 250 mg/week; in Germany this is available only via the international pharmacy). Their use is considered off-label use.
A recent meta-analysis of individual patient data from randomized controlled trials (RCTs) showed in women with asymptomatic singleton pregnancies and cervical shortening (≤25 mm), confirmed on ultrasound before 24+0 weeks’ gestation, who were treated with vaginal progesterone (daily dose 90–200 mg) a significant reduction in the preterm birth rate before 33 weeks’ gestation (22.5% versus 14.1%, RR: 0.62; [0.47; 0.81]) and an improved neonatal treatment result (13). According to guideline 015/025 (prevention and therapy of preterm birth) of the Association of the Scientific Medical Societies in Germany (AWMF) such pregnant women should be given vaginal progesterone every day (as a 200 mg capsule, for example) up to 36+6 weeks‘ gestation (14).
In pregnant women who had had a previous spontanous preterm birth, administration of progesterone (starting from 16+0 weeks‘ gestation up to 36+0 weeks‘ gestation) should be decided on an individual basis as the data are not consistent.
Except for increased vaginal discharge, no adverse effects are known for vaginal progesterone. The neurological development of exposed children up to age 6 years is not negatively affected (e10).
Cervical pessary
“Invasive” cervical cerclage, which entails placing a non-resorbable sling around the cervix, usually requires inpatient admission and anesthesia and carries a risk of peri(post)operative complications (for example, infections) of up to 6% (e11). By contrast, placing a cervical pessary constitutes a non-invasive option to prevent preterm birth that can be done on an outpatient basis and is low in complications. The indication is sonographically confirmed cervical shortening ≤ 25 mm in the second trimester.
A 2019 meta-analysis by Perez-Lopez (15) evaluated three RCTs (N=1612) with a defined outcome measure (preterm birth rate <34+0 weeks’ gestation). Women with singleton pregnancies were included, who had sonographically confirmed cervical shortening ≤ 25 mm between 18+0 weeks’ gestation and 22+6 weeks’ gestation, with pessary placement versus watch-and-wait management. All three RCTs found that pessary placement did not result in a significant reduction in the preterm birth rate before 34+0 weeks’ gestation (11.6% versus 18.4%) but it did result in a significant reduction in the preterm rate before 37+0 weeks’ gestation (20.8% versus 47.6%, RR: 0.46; [0.28; 0.77]). This was not confirmed in an additional 2019 meta-analysis (preterm birth rate <34 weeks’ gestation: OR: 0.68; [0.2; 2.29]; preterm birth rate <37 weeks’ gestation: OR 0.36; [0.09; 1.48]) (16).
In case of a cervical length ≤ 25 mm before 24 weeks’ gestation, a recently published RCT showed non-inferiority for placing a cervical pessary compared with vaginal administration of progesterone before 34 weeks’ gestation (14% versus 14%; risk difference: 0.11%; [–8.85; –8.62]) (17). Additional application of vaginal progesterone did not reduce the preterm birth rate before 34 and 37 weeks’ gestation compared with the pessary alone (18). The most common adverse effect of pessary placement was increased vaginal discharge in 40% of pregnant women.
Because of contradictory data, the AWMF guideline 015/025 2019 (14) considers placement of a cervical pessary in women with singleton pregnancies and a cervical length ≤ 25 mm confirmed by vaginal ultrasound before 34 weeks’ gestation as a decision to be made on a case by case basis. In our opinion, this has been confirmed by the meta-analyses published subsequently (15, 16).
Bacterial vaginosis
During pregnancy the prevalence of bacterial vaginosis (BV) is 7–22% (e12). In 50–75% of patients, BV does not cause complications. Pregnant women with symptomatic BV should be treated with antibiotics—for example, oral clindamycin (14).
BV is a further risk factor for preterm birth (table 1). A 2013 Cochrane analysis (19) showed, however, that treatment with antibiotics does not lower the rate of preterm births <37+0 weeks’ gestation (OR: 0.88; [0.71; 1.09]), even if treatment is initiated before 20 weeks’ gestation (OR: 0.85; [0.62; 1.17]) (20). In a randomized placebo-controlled double blinded study (PREMEVA) in pregnant women at low risk of preterm birth, systematic screening (N = 88 530) before 14 weeks’ gestation and subsequent treatment of BV (n=5630) with oral clindamycin (300 mg 2–3 time daily for four days) did not result in a significant reduction in the rate of late miscarriages between 16 and 21 weeks’ gestation and early preterm births between 22 and 32 weeks’ gestation (0.8% versus 1.0%; RR: 1.10; [0.53; 2.32], p=0.82) nor in spontaneous preterm births between 32+0 and 36+6 weeks’ gestation (4.6% versus 4.1%; RR: 1.17; [0.81; 1.69], p=0.40) (21).
Asymptomatic bacteriuria
Asymptomatic bacteriuria (ABS), defined as >100 000 pathogens/ml in midstream urine without clinical symptoms, is considered an independent risk factor for preterm birth (e13). Its prevalence in pregnancy is 2–7% and that of pyelonephritis 0.5% (e14).
According to a 2015 Cochrane review (22) and a systematic review (23), screening for asymptomatic bacteriuria is associated with a reduced rate of pyelonephritis (RR: 0.28; [0.15; 0.54]), but it does not lower the preterm birth rate <37 weeks’ gestation (RR: 8.70; [0.32; 240.07]).
The situation is similar for treatment. Administration of antibiotics—for example, ampicillin or nitrofurantoin—is associated with a reduced incidence of pyelonephritis compared with placebo/no treatment (RR: 0.24); [0.13; 0.41]), but not with a reduction in the preterm birth rate before 37 weeks’ gestation (RR: 0.57; [0.21; 1.56]). These results included four studies (n=533), of which three were published between 1960 and 1990 (e15– e17). The evidence regarding a reduction in the preterm birth rate is not sufficient to support routine screening for ABS in pregnancy by using urinary culture, nor for treating ABS with antibiotics (23) (e18). This result is consistent with the final report of the IQWIG (Institute for Quality and Efficiency in Health Care) of 2015 regarding screening for ABS (24).
Supplements
Supplementation with calcium; iron with or without folic acid, folic acid alone, vitamins A, D, E, and multivitamin preparations does not result in a significant reduction in the preterm birth rate before 37 weeks’ gestation, according to Cochrane analyses and systematic reviews of RCTs (25).
Fish oil and polyunsaturated omega-3 fatty acids (n-3 long chain polyunsaturated fatty acids [n-3 LC-PUFA], eicosapentaenoic acid [EPA], and docosahexaenoic acid [DHA]) have an anti-inflammatory effect owing to several mechanisms of action (e19– e20) and have been studied in terms of whether they are suitable for preventing preterm births. A pregnant woman’s daily requirement is 200 mg and is met by consuming more than one meal of fatty fish (ca 200 g/week) (caution: mercury exposure). Some (not all) multivitamin preparations contain 200 mg DHA. Since the studies of the effects of fish oils and n-3 LC-PUFA are heterogeneous—for example, regarding the risk profiles for preterm birth, dosages, start of administration, and duration of use—the results are also heterogeneous (table 2) (26) (e21– e25).
Table 2. Meta-analyses regarding fish oil and polyunsaturated omega-3 fatty acids versus placebo/no supplementation for preventing preterm birth.
| Preterm births <37 weeks‘ gestation | Preterm births <34 weeks‘ gestation | |||||
| Author (year) | RCT (N) | Pregnant women (N) | Supplement | Absolute risk (%) | RR [95% CI] | RR [95% CI] |
| Salvig and Lamont (2011) (e21) | 3 | 1187 | n-3-LC-PUFA | 8.9 vs. 16.3 | 0.62 [0.4; 0.93] | 0.32 [0.09; 0.95] |
| Newberry et al. (2016) (e22) | 7 | N/A | n-3-LC-PUFA | N/A | 0.87 [0.66; 1.15] | N/A |
| Kar et al. (2016) (e23) | 9 | 5980 | n-3-LC-PUFA | N/A | 0.83 [0.70; 0.98] | 0.42 [0.27; 0.66] |
| Chen et al. (2016) (e24) | 21 | 10 802 | Fish oil | N/A | 0.90 [0.81; 1.00] | 0.78 [0.64; 0.95] |
| Saccone et al. (2015) (e25) | 9 | 3854 | n-3-LC-PUFA | 7.7 vs. 9.1 | 0.90 [0.72; 1.11] | N/A |
| Middleton et al. (2018) (26) | 70 | 19 927 | n-3-LC-PUFA Fish oil | 11.9 vs. 13.4 | 0.89 [0.81; 0.97] | 0.58 [0.44; 0.77] |
N/A, not available; n-3-LC-PUFA, polyunsaturated omega-3 fatty acids; RCT, randomized controlled trial; RR (95% CI), relative risk (95% confidence interval).
According to a 2018 Cochrane review (26), intake of n-3 LC-PUFA compared with placebo did not bring about a significant reduction in the preterm birth rate before 37 weeks’ gestation and before 34 weeks’ gestation (table 2), but the incidence of post-term births beyond 42 weeks’ gestation was increased from 1.6% to 2.5% (six RCTs).
Most of the studies reported dosages of 600–900 mg/day. It was suggested to start taking these supplements at 12–20 weeks’ gestation and continue up to 36 weeks’ gestation or to term (e19). For definite recommendations, the results of two currently running RCTs from the US and Australia should be awaited (N >7000), which are prospectively investigating administration of n-3 LC-PUFA versus placebo or standard care (200 mg/day): ClinicalTrials.gov.NCT02626299 (e26); Australian/New Zealand Clinical Trial Registry Number 12613001142729 (e19).
Nicotine withdrawal
In the US and Europe, 5–25% of smoking women continue to smoke during pregnancy, mostly pregnant women between the 20th and 25th year of life (e27, e28). Smoking is an avoidable independent risk factor for intrauterine growth restriction and preterm birth, even extreme preterm birth <28 weeks’ gestation.
Compared with non-smokers, the risk of preterm birth is raised in smokers (OR: 1.7; [1.3; 2.2]) (9) and correlates over the entire course of pregnancy with the number of cigarettes consumed (e29). Compared with non-smoking pregnant women, abstinence from nicotine consumption in the first trimester is not associated with an increased rate in all preterm births <37 weeks’ gestation (27) (e30), but with a 20% increase <28 weeks’ gestation (0.61 versus 0.93, aOR: 1.20; [1/03; 1.40]) (table 3) (27). Abstaining from nicotine after the second trimester or nicotine consumption during the entire pregnancy are associated with an increased risk for preterm birth <37 week’s gestation (27) (e30), which also applies to pregnant women with a prior preterm birth (e31).
Table 3. Smoking and preterm birth risk.
| Non-smoker | Abstinence from nicotine before conception | Abstinence from nicotine after the first trimester | Abstinence from nicotine after the second trimester | No nicotine abstinence | |||||
| N (%) | n (%) | aOR (95% CI) | n (%) | aOR (95% CI) | n (%) | aOR (95% CI) | n (%) | aOR (95% CI) | |
| Preterm birth <37 weeks‘ gestation | 69 794 (10.01) | 5096 (9.55) | 0.91 [0.88; 0.94] | 2477 (11.36) | 1.02 [0.98; 1.07] | 1590 (18.07) | 1.70 [1.60; 1.80] | 18 053 (13.62) | 1.21 [1.19; 1.24] |
| Preterm birth 20–27 weeks‘ gestation | 4230 (0.61) | 312 (0.58) | 0.87 [0.77; 0.98] | 202 (0.93) | 1.20 [1.03; 1.40] | N/A | 870 (0.66) | 0.90 [0.83; 0.97] | |
| Preterm birth 28–36 weeks‘ gestation | 65 564 (9.40) | 4784 (8.97) | 0.91 [0.88; 0.94] | 2275 (10.43) | 1.01 [0.96; 1.05] | 1268 (14.41) | 1.46 [1.37; 1.55] | 17 183 (12.96) | 1.24 [1.21; 1.26] |
Modified from (27), reproduced with permission from Mosby-Elsevier Publishers. aOR (95% CI). adjusted odds ratio (95% confidence interval); N/A not available.
Passive smoking is associated with an increase of 36% in the risk of preterm birth (OR: 1.20; [1.07; 1.32]) (28). In psychosocial intervention programs, an increased proportion of pregnant women who refrained from nicotine consumption was observed, as was a reduced rate of babies with a low birth weight. The effect on the preterm birth rate is not known (e32). Legal measures such as smoking prohibition or tobacco taxes were associated with a reduction in the preterm birth rate in the US and some European countries (e33). A Canadian study found that using nicotine patches during pregnancy was associated with a reduced preterm birth rate before 37 weeks’ gestation (aOR: 0.21; [0.13; 0.34]) (29).
Work load, physical rest, bed rest
Pregnant women’s working hours and occupations are regulated by Germany’s Maternity Protection Act. In healthy women with singleton pregnancies, long hours (>40 hours/week) are associated with a slightly increased risk of preterm birth <37 weeks’ gestation (aOR: 1.25; [1.01; 1.54]) (30, 31).
In a Japanese study of pregnant doctors in their first trimester, who were working >51–70 hours/week, the risk of preterm birth was 2.5 times (aOR: 2.5; [1.2; 5.2]) higher, and for working hours in excess of 71 hours/week, it was 4.2 times higher (aOR: 4.2; [1.0; 9.1]) (32). No increased risk of preterm birth was found for shift work or standing for long periods of time (>6 hours/day) (30, 31). Lifting or carrying heavy loads in pregnancy was—dependent on the load (≤ 200 kg/day)—associated with a 1.4-fold increase in the preterm birth rate (HR: 1.43; [1.13; 1.80]) (33).
In a systematic review from 2014 (34) and subsequent study, physical rest could not be shown to reduce the preterm birth rate. Two studies of pregnant women at risk of preterm birth even found a 2.1–2.4-fold increase in the preterm birth rate <34 weeks’ gestation or <37 weeks’ gestation (aOR: 2.37; [1.60; 3.53]) (35, 36).
In Germany, the individual prohibition of employment can be issued by the caring physician, and the general prohibition of employment by the employer. We are not aware of any studies of the effect of these measures on the preterm birth rate.
No evidence exists to support the prescription of bed rest in women under threat of preterm birth (37); according to two RCTs, bed rest at home did not lower the preterm birth rate before 37 weeks’ gestation but was associated with increased risks, for example thromboembolism, loss in muscle mass, weight loss, and psychological sequalae, such as anxiety and depression (34). In the individual case—for example, in prolapse of the amniotic sac or in bleeding placenta previa—bed rest can make sense.
Prevention programs
A 2011 systematic review did not provide any evidence in support of the nationwide introduction of prevention programs (38). Two meta-analyses showed no reduction—or only a slightly significant reduction—in the preterm birth rate <37 weeks’ gestation in the context of prevention programs—for example, screening examinations by specialized obstetricians—compared with standard care (39, e34).
In Australia, the introduction of a multimodal prevention program that entailed, among others, information/education for counseling pregnant women and doctors or the admission of women with high-risk pregnancies into a specialized perinatal center, led to a reduction in the preterm birth rate from 7.5% to 6.9% (40).
Conclusions
In Germany and many European countries, preterm birth rates have not fallen for almost 10 years. Because of lacking causal therapeutic modalities, clinical-scientific research thus far has focused on the identification of risk factors and their prevention. Many risks are avoidable by counseling pregnant women at the start of antenatal care and by changing lifestyle habits and job-related stressed in pregnancy, but others are not.
Sonographic measuring of the cervical length in the second trimester enables an effective approach to prevention if vaginal progesterone is applied once cervical shortening has been confirmed.
The proportion of iatrogenic preterm births among the overall preterm birth rate is increasing. It is therefore appropriate to evaluate the indications for early termination of pregnancy more critically than has been done to date. Crucial progress can be expected in future from in-depth research into the causes of preterm birth and the effective therapeutic strategies that can be derived from this.
Key Messages.
The preterm birth rate in Germany has remained almost unchanged, at between 8% and 9%, in more than 10 years.
Careful assessment of risk factors at the outset of antenatal care is the prerequisite for individual risk assessment and the basis of prevention.
Appropriate measures for reducing the preterm birth risk in the outpatient/ambulatory care sector are the vaginal application of progesterone if the cervix is found to be shortened in the second trimester, abstinence from nicotine in smokers, and avoidance of long hours and heavy physical work during pregnancy.
In healthy pregnant women at risk of preterm birth, physical rest and bed rest are not evidence-based measures for preventing preterm birth.
The development of causal therapies should be the objective of future research, so as to lower the preterm birth rate effectively.
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Footnotes
Conflict of interest statement
Prof. Berger was reimbursed for conference delegate fees and travel expenses and received lecture honoraria from Eickeler.
PD Dr Kuon was reimbursed for conference delegate fees and travel expenses and received lecture honoraria from Dr Kade/Besins GmbH.
PD Dr Maul received consultancy fees from Horologic. He received travel expenses and lecture honoraria from Kade.
The remaining authors declare that no conflict of interest exists.
References
- 1.Zeitlin J, Szamotulska K, Drewniak N, et al. Preterm birth time trends in Europe: a study of 19 countries. BJOG. 2013;120:1356–1365. doi: 10.1111/1471-0528.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SQG-Bundesauswertung zum Verfahrensjahr 2009 16/1 - Geburtshilfe [Google Scholar]
- 3.IQTIG. Bundesauswertung zum Erfassungsjahr 2017 - Geburtshilfe Qualitätsindikatoren. https://iqtig.org/downloads/auswertung/2017/16n1gebh/QSKH_16n1-GEBH_2017_BUAW_V02_2018-08-01.pdf (last accessed on 28 August 2019) [Google Scholar]
- 4.Voss W, Hobbiebrunken E, Ungermann U, Wagner M, Damm G. The development of extremely premature infants. Dtsch Arztebl Int. 2016;113:871–878. doi: 10.3238/arztebl.2016.0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newton JN, Briggs AD, Murray CJ, et al. Changes in health in England, with analysis by English regions and areas of deprivation, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;386:2257–2274. doi: 10.1016/S0140-6736(15)00195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glover AV, Manuck TA. Screening for spontaneous preterm birth and resultant therapies to reduce neonatal morbidity and mortality: areview. Semin Fetal Neonatal Med. 2018;23:126–132. doi: 10.1016/j.siny.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iams JD, Goldenberg RL, Mercer BM, et al. The preterm prediction study: recurrence risk of spontaneous preterm birth National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1998;178:1035–1040. doi: 10.1016/s0002-9378(98)70544-7. [DOI] [PubMed] [Google Scholar]
- 8.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. N Engl J Med. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 9.Murphy DJ. Epidemiology and environmental factors in preterm labour. Best Pract Res Clin Obstet Gynaecol. 2007;21:773–789. doi: 10.1016/j.bpobgyn.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Phillips C, Velji Z, Hanly C, Metcalfe A. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ open. 2017;7 doi: 10.1136/bmjopen-2016-015402. e015402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin JA, Hamilton BE, Osterman MJK, et al. Births: final data for 2015. National Vital Statistics Reports; 2017;66 [PubMed] [Google Scholar]
- 12.Ferrero DM, Larson J, Jacobsson B, et al. Cross-country individual participant analysis of 41 million singleton births in 5 countries with very high human development index confirms known associations but provides no biologic explanation for 2/3 of all preterm births. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162506. e0162506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161–180. doi: 10.1016/j.ajog.2017.11.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AWMF. Prävention und Therapie der Frühgeburt 015-025. www.awmf.org/leitlinien/detail/ll/015-025.html (last accessed on 8 May 2019) [Google Scholar]
- 15.Perez-Lopez FR, Chedraui P, Perez-Roncero GR, Martinez-Dominguez SJ. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215–1231. doi: 10.1007/s00404-019-05096-x. [DOI] [PubMed] [Google Scholar]
- 16.Jarde A, Lutsiv O, Beyene J, McDonald SD. Vaginal progesterone, oral progesterone, 17-OHPC, cerclage, and pessary for preventing preterm birth in at-risk singleton pregnancies: an updated systematic review and network meta-analysis. BJOG. 2019;126:556–567. doi: 10.1111/1471-0528.15566. [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Melguizo S, San-Frutos L, Martinez-Payo C, et al. Cervical pessary compared with vaginal progesterone for preventing early preterm birth: a randomized controlled trial. Obstet Gynecol. 2018;132:907–915. doi: 10.1097/AOG.0000000000002884. [DOI] [PubMed] [Google Scholar]
- 18.Stricker N, Timmesfeld N, Kyvernitakis I, Goerges J, Arabin B. Vaginal progesterone combined with cervical pessary: achance for pregnancies at risk for preterm birth? Am J Obstet Gynecol. 2016;214:739.e1–739e10. doi: 10.1016/j.ajog.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2013;2013 doi: 10.1002/14651858.CD000262.pub4. CD000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P, Ota E. Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD002250.pub2. Cd002250. [DOI] [PubMed] [Google Scholar]
- 21.Subtil D, Brabant G, Tilloy E, et al. Early clindamycin for bacterial vaginosis in pregnancy (PREMEVA): a multicentre, double-blind, randomised controlled trial. Lancet. 2018;392:2171–2179. doi: 10.1016/S0140-6736(18)31617-9. [DOI] [PubMed] [Google Scholar]
- 22.Smaill FM, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD000490.pub3. Cd000490. [DOI] [PubMed] [Google Scholar]
- 23.Wingert A, Pillay J, Sebastianski M, et al. Asymptomatic bacteriuria in pregnancy: systematic reviews of screening and treatment effectiveness and patient preferences. BMJ. 2019;9 doi: 10.1136/bmjopen-2017-021347. e021347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.IQWIG. Screening auf asymptomatische Bakteriurie im Rahmen der Mutterschafts-Richtlinien unter besonderer Berücksichtigung der Testmethoden Abschlussbericht S13-02. IQWIG. 2015 [Google Scholar]
- 25.Matei A, Saccone G, Vogel JP, Armson AB. Primary and secondary prevention of preterm birth: a review of systematic reviews and ongoing randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2019;236:224–239. doi: 10.1016/j.ejogrb.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst Rev. 2018;11 doi: 10.1002/14651858.CD003402.pub3. Cd003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore E, Blatt K, Chen A, Van Hook J, DeFranco EA. Relationship of trimester-specific smoking patterns and risk of preterm birth. Am J Obstet Gynecol. 2016;215:109.e1–109e6. doi: 10.1016/j.ajog.2016.01.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui H, Gong TT, Liu CX, Wu QJ. Associations between passive maternal smoking during pregnancy and preterm birth: evidence from a meta-analysis of observational studies. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147848. e0147848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berard A, Zhao JP, Sheehy O. Success of smoking cessation interventions during pregnancy. Am J Obstet Gynecol. 2016;215:611.e1–611e8. doi: 10.1016/j.ajog.2016.06.059. [DOI] [PubMed] [Google Scholar]
- 30.van Melick MJ, van Beukering MD, Mol BW, Frings-Dresen MH, Hulshof CT. Shift work, long working hours and preterm birth: a systematic review and meta-analysis. Int Arch Occup Environ Health. 2014;87:835–849. doi: 10.1007/s00420-014-0934-9. [DOI] [PubMed] [Google Scholar]
- 31.Palmer KT, Bonzini M, Bonde JP, et al. Pregnancy: occupational aspects of management: concise guidance. Clin Med. 2013;13:75–79. doi: 10.7861/clinmedicine.13-1-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi M, Rahman M, Ishiguro A, Nomura K. Long working hours and pregnancy complications: women physicians survey in Japan. BMC Pregnancy Childbirth. 2014;14 doi: 10.1186/1471-2393-14-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mocevic E, Svendsen SW, Jorgensen KT, Frost P, Bonde JP. Occupational lifting, fetal death and preterm birth: findings from the Danish National Birth Cohort using a job exposure matrix. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090550. e90550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarty-Singleton S, Sciscione AC. Maternal activity restriction in pregnancy and the prevention of preterm birth: an evidence-based review. Clin Obstet Gynecol. 2014;57:616–627. doi: 10.1097/GRF.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 35.Grobman WA, Gilbert SA, Iams JD, et al. Activity restriction among women with a short cervix. Obstet Gynecol. 2013;121:1181–1186. doi: 10.1097/AOG.0b013e3182917529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levin HI, Sciscione A, Ananth CV, Drassinower D, Obican SG, Wapner RJ. Activity restriction and risk of preterm delivery. J Matern Fetal Neonatal Med. 2018;31:2136–2140. doi: 10.1080/14767058.2017.1337738. [DOI] [PubMed] [Google Scholar]
- 37.Sosa CG, Althabe F, Belizan JM, Bergel E. Bed rest in singleton pregnancies for preventing preterm birth. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD003581.pub3. Cd003581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollowell J, Oakley L, Kurinczuk JJ, Brocklehurst P, Gray R. The effectiveness of antenatal care programmes to reduce infant mortality and preterm birth in socially disadvantaged and vulnerable women in high-income countries: a systematic review. BMC Pregnancy Childbirth. 2011;11 doi: 10.1186/1471-2393-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catling CJ, Medley N, Foureur M, et al. Group versus conventional antenatal care for women. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD007622.pub3. Cd007622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newnham JP, White SW, Meharry S, et al. Reducing preterm birth by a statewide multifaceted program: an implementation study. Am J Obstet Gynecol. 2017;216:434–442. doi: 10.1016/j.ajog.2016.11.1037. [DOI] [PubMed] [Google Scholar]
- E1.Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3–12. doi: 10.1016/j.bpobgyn.2018.04.003. [DOI] [PubMed] [Google Scholar]
- E2.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- E3.UNICEF, WHO, World Bank Group and United Nations. United Nation Children`s Fund. New York: 2017. Levels and trends in child mortality: report 2017. Estimates developed by the UN inter-agency group for child mortality estimates. [Google Scholar]
- E4.Meis PJ, Michielutte R, Peters TJ, et al. Factors associated with preterm birth in Cardiff, Wales I. Univariable and multivariable analysis. Am J Obstet Gynecol. 1995;173:590–596. doi: 10.1016/0002-9378(95)90287-2. [DOI] [PubMed] [Google Scholar]
- E5.Yi SW, Han YJ, Ohrr H. Anemia before pregnancy and risk of preterm birth, low birth weight and small-for-gestational-age birth in Korean women. Eur J Clin Nutr. 2013;67:337–342. doi: 10.1038/ejcn.2013.12. [DOI] [PubMed] [Google Scholar]
- E6.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- E7.Gyamfi-Bannerman C, Ananth CV. Trends in spontaneous and indicated preterm delivery among singleton gestations in the United States, 2005-2012. Obstet Gynecol. 2014;124:1069–1074. doi: 10.1097/AOG.0000000000000546. [DOI] [PubMed] [Google Scholar]
- E8.Delnord M, Zeitlin J. Epidemiology of late preterm and early term births - An international perspective. Semin Fetal Neonatal Med. 2019;24:3–10. doi: 10.1016/j.siny.2018.09.001. [DOI] [PubMed] [Google Scholar]
- E9.Rath W, Kuon RJ. Progesterone-effective for tocolysis and long-term treatment following acute tocolysis? Critical analysis of evidence. Geburtshilfe Frauenheilkd. 2019 doi: 10.1055/a-0829-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Vedel C, Larsen H, Holmskov A, et al. Long-term effects of prenatal progesterone exposure: neurophysiological development and hospital admissions in twins up to 8 years of age. Ultrasound Obstet Gynecol. 2016;48:382–389. doi: 10.1002/uog.15948. [DOI] [PubMed] [Google Scholar]
- E11.Woensdregt K, Norwitz ER, Cackovic M, Paidas MJ, Illuzzi JL. Effect of 2 stitches vs 1 stitch on the prevention of preterm birth in women with singleton pregnancies who undergo cervical cerclage. Am J Obstet Gynecol. 2008;198:396.e1–396e7. doi: 10.1016/j.ajog.2007.10.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Desseauve D, Chantrel J, Fruchart A, et al. Prevalence and risk factors of bacterial vaginosis during the first trimester of pregnancy in a large French population-based study. Eur J Obstet Gynecol Reprod Biol. 2012;163:30–34. doi: 10.1016/j.ejogrb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- E13.Sheiner E, Mazor-Drey E, Levy A. Asymptomatic bacteriuria during pregnancy. J Matern Fetal Neonatal Med. 2009;22:423–427. doi: 10.1080/14767050802360783. [DOI] [PubMed] [Google Scholar]
- E14.Wing DA, Fassett MJ, Getahun D. Acute pyelonephritis in pregnancy: an 18-year retrospective analysis. Am J Obstet Gynecol. 2014;210:219.e1–219.e6. doi: 10.1016/j.ajog.2013.10.006. [DOI] [PubMed] [Google Scholar]
- E15.Furness ET, McDonald PJ, Beasley NV. Urinary antiseptics in asymptomatic bacteriuria of pregnancy. N Z Med J. 1975;81:417–419. [PubMed] [Google Scholar]
- E16.Thomsen AC, Morup L, Hansen KB. Antibiotic elimination of group-B streptococci in urine in prevention of preterm labour. Lancet. 1987;1:591–593. doi: 10.1016/s0140-6736(87)90234-0. [DOI] [PubMed] [Google Scholar]
- E17.Wren BG. Subclinical renal infection and prematurity. Med J Aust. 1969;2:596–600. doi: 10.5694/j.1326-5377.1969.tb107290.x. [DOI] [PubMed] [Google Scholar]
- E18.Angelescu K, Nussbaumer-Streit B, Sieben W, Scheibler F, Gartlehner G. Benefits and harms of screening for and treatment of asymptomatic bacteriuria in pregnancy: a systematic review. BMC Pregnancy Childbirth. 2016;16 doi: 10.1186/s12884-016-1128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E19.Zhou SJ, Best K, Gibson R, et al. Study protocol for a randomised controlled trial evaluating the effect of prenatal omega-3 LCPUFA supplementation to reduce the incidence of preterm birth: the ORIP trial. BMJ. 2017;7 doi: 10.1136/bmjopen-2017-018360. e018360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E20.Chen CY, Chen CY, Liu CC, Chen CP. Omega-3 polyunsaturated fatty acids reduce preterm labor by inhibiting trophoblast cathepsin S and inflammasome activation. Clin Sci (Lond) 2018;132:2221–2239. doi: 10.1042/CS20180796. [DOI] [PubMed] [Google Scholar]
- E21.Salvig JD, Lamont RF. Evidence regarding an effect of marine n-3 fatty acids on preterm birth: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2011;90:825–838. doi: 10.1111/j.1600-0412.2011.01171.x. [DOI] [PubMed] [Google Scholar]
- E22.Newberry SJ, Chung M, Booth M, et al. Omega-3 fatty acids and maternal and child health: an updated systematic review. Evidence report/technology assessment. 2016:1–826. doi: 10.23970/AHRQEPCERTA224. [DOI] [PubMed] [Google Scholar]
- E23.Kar S, Wong M, Rogozinska E, Thangaratinam S. Effects of omega-3 fatty acids in prevention of early preterm delivery: a systematic review and meta-analysis of randomized studies. Eur J Obstet Gynecol Reprod Biol. 2016;198:40–46. doi: 10.1016/j.ejogrb.2015.11.033. [DOI] [PubMed] [Google Scholar]
- E24.Chen B, Ji X, Zhang L, Hou Z, Li C, Tong Y. Fish oil supplementation improves pregnancy outcomes and size of the newborn: a meta-analysis of 21 randomized controlled trials. J Matern Fetal Neonatal Med. 2016;29:2017–2027. doi: 10.3109/14767058.2015.1072163. [DOI] [PubMed] [Google Scholar]
- E25.Saccone G, Saccone I, Berghella V. Omega-3 long-chain polyunsaturated fatty acids and fish oil supplementation during pregnancy: which evidence? J Matern Fetal Neonatal Med. 2016;29:2389–2397. doi: 10.3109/14767058.2015.1086742. [DOI] [PubMed] [Google Scholar]
- E26.Carlson SE, Gajewski BJ, Valentine CJ, et al. Assessment of DHA on reducing early preterm birth: the ADORE randomized controlled trial protocol. BMC Pregnancy Childbirth. 2017;17 doi: 10.1186/s12884-017-1244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Dahlin S, Gunnerbeck A, Wikstrom AK, Cnattingius S, Edstedt Bonamy AK. Maternal tobacco use and extremely premature birth - a population-based cohort study. BJOG. 2016;123:1938–1946. doi: 10.1111/1471-0528.14213. [DOI] [PubMed] [Google Scholar]
- E28.Drake P, Driscoll AK, Mathews TJ. Cigarette smoking during pregnancy: United States, 2016. NCHS data brief. 2018:1–8. [PubMed] [Google Scholar]
- E29.Kondracki AJ, Hofferth SL. A gestational vulnerability window for smoking exposure and the increased risk of preterm birth: how timing and intensity of maternal smoking matter. Reprod Health. 2019;16 doi: 10.1186/s12978-019-0705-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E30.Soneji S, Beltran-Sanchez H. Association of maternal cigarette smoking and smoking cessation with preterm birth. JAMA network open. 2019;2 doi: 10.1001/jamanetworkopen.2019.2514. e192514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E31.Wallace JL, Aland KL, Blatt K, Moore E, DeFranco EA. Modifying the risk of recurrent preterm birth: influence of trimester-specific changes in smoking behaviors. Am J Obstet Gynecol. 2017;216:310.e1–310.e8. doi: 10.1016/j.ajog.2016.11.1034. [DOI] [PubMed] [Google Scholar]
- E32.Chamberlain C, O‘Mara-Eves A, Porter J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev. 2017;2 doi: 10.1002/14651858.CD001055.pub5. Cd001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E33.Faber T, Kumar A, Mackenbach JP, et al. Effect of tobacco control policies on perinatal and child health: a systematic review and meta-analysis. The Lancet Public health. 2017;2:e420–e437. doi: 10.1016/S2468-2667(17)30144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E34.Whitworth M, Quenby S, Cockerill RO, Dowswell T. Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD006760.pub2. Cd006760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E35.Hsieh TT, Chen SF, Shau WY, Hsieh CC, Hsu JJ, Hung TH. The impact of interpregnancy interval and previous preterm birth on the subsequent risk of preterm birth. J Soc Gynecol Investig. 2005;12:202–207. doi: 10.1016/j.jsgi.2004.12.004. [DOI] [PubMed] [Google Scholar]
- E36.Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol. 2006;195:643–650. doi: 10.1016/j.ajog.2006.05.022. [DOI] [PubMed] [Google Scholar]
- E37.Offenbacher S, Boggess KA, Murtha AP, et al. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 2006;107:29–36. doi: 10.1097/01.AOG.0000190212.87012.96. [DOI] [PubMed] [Google Scholar]
- E38.Fairley L, Leyland AH. Social class inequalities in perinatal outcomes: Scotland 1980-2000. J Epidemiol Community Health. 2006;60:31–36. doi: 10.1136/jech.2005.038380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E39.da Silva AA, Simoes VM, Barbieri MA, et al. Young maternal age and preterm birth. Paediatr Perinat Epidemiol. 2003;17:332–339. doi: 10.1046/j.1365-3016.2003.00515.x. [DOI] [PubMed] [Google Scholar]
- E40.Burguet A, Kaminski M, Abraham-Lerat L, et al. The complex relationship between smoking in pregnancy and very preterm delivery. Results of the Epipage study. BJOG. 2004;111:258–265. doi: 10.1046/j.1471-0528.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- E41.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–498. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- E42..Zeitlin JA, Saurel-Cubizolles MJ, Ancel PY. Marital status, cohabitation, and risk of preterm birth in Europe: where births outside marriage are common and uncommon. Paediatr Perinat Epidemiol. 2002;16:124–130. doi: 10.1046/j.1365-3016.2002.00396.x. [DOI] [PubMed] [Google Scholar]