Abstract
Purpose:
To evaluate if jugular vein flow restoration in various venographic defects indicative of chronic cerebrospinal venous insufficiency (CCSVI) in multiple sclerosis (MS) patients can have positive effects on cerebral lesions identified using magnetic resonance imaging (MRI).
Materials and Methods:
The Brave Dreams trial (ClinicalTrials.gov identifier NCT01371760) was a multicenter, randomized, parallel group, double-blind, sham-controlled trial to assess the efficacy of jugular venoplasty in MS patients with CCSVI. Between August 2012 and March 2016, 130 patients (mean age 39.9±10.6 years; 81 women) with relapsing/remitting (n=115) or secondary/progressive (n=15) MS were randomized 2:1 to venography plus angioplasty (n=86) or venography (sham; n=44). Patients and study personnel (except the interventionist) were masked to treatment assignment. MRI data acquired at 6 and 12 months after randomization were compared to the preoperative scan for new and/or >30% enlargement of T2 lesions plus new gadolinium enhancement of pre-existing lesions. The relative risks (RR) with 95% confidence interval (CI) were estimated and compared. In a post hoc assessment, venograms of patients who underwent venous angioplasty were graded as “favorable” (n=38) or “unfavorable” (n=30) for dilation according to the Giaquinta grading system by 4 investigators blinded to outcomes. These subgroups were also compared.
Results:
Of the 130 patients enrolled, 125 (96%) completed the 12-month MRI follow-up. Analysis showed that the likelihood of being free of new cerebral lesions at 1 year was significantly higher after venoplasty compared to the sham group (RR 1.42, 95% CI 1.00 to 2.01, p=0.032). Patients with favorable venograms had a significantly higher probability of being free of new cerebral lesions than patients with unfavorable venograms (RR 1.82, 95% CI 1.17 to 2.83, p=0.005) or patients in the sham arm (RR 1.66, 95% CI 1.16 to 2.37, p=0.005).
Conclusion:
Expanded analysis of the Brave Dreams data that included secondary/progressive MS patients in addition to the relapsing/remitting patients analyzed previously showed that venoplasty decreases new cerebral lesions at 1 year. Post hoc analysis confirmed the efficacy of the Giaquinta grading system in selecting patients appropriate for venoplasty who were more likely to be free from accumulation of new cerebral lesions at MRI.
Keywords: angioplasty, cerebral drainage, cerebral lesion, chronic cerebrospinal venous insufficiency, echo Doppler, internal jugular vein, jugular flow, magnetic resonance imaging, multiple sclerosis, stenosis, vein defects, venography, venoplasty
Introduction
When hypoplasia, external compression, and/or intraluminal defects (eg, defective valve and/or septum) of the internal jugular and/or azygos vein are diagnosed, the condition is known as chronic cerebrospinal venous insufficiency (CCSVI).1-9 The reported prevalence of CCSVI in multiple sclerosis (MS) patients and in healthy controls is highly heterogeneous in the literature, as reported by 2 meta-analyses.10,11 This has generated considerable scientific controversy about the association between MS and CCSVI and therefore about the role of venous balloon angioplasty in improving symptoms and/or the natural history of MS.12 However, National Institute for Health and Care Excellence (NICE) guidance encouraged further research in the form of robust randomized controlled trials (RCT).13 On this basis, the Department of Public Health of the Emilia Romagna Region in Italy funded and organized a multicenter RCT to study the efficacy and safety of venoplasty in MS patients with CCSVI (Brave Dreams: Brain Venous Drainage Exploited Against Multiple Sclerosis).12 In both the test and control arms, the patients were under immunomodulatory treatment.
Two primary outcomes were assessed in the Brave Dreams trial: (1) a combined measurement of 5 functional indexes (walking, balance, manual dexterity, bladder control, visual acuity) and (2) the accumulation of new cerebral lesions detected by magnetic resonance imaging (MRI). The first analysis of the Brave Dreams trial presented data exclusively from the MS population with a relapsing/remitting clinical course, which is the most common type of MS. The trial failed to show that venous angioplasty had any effects on either measure in the relapsing-remitting MS patients at 12-month follow-up.14 However, between 6 and 12 months after angioplasty, patients showed a reduction in new cerebral lesions compared to patients in the sham arm.14 Moreover, a significant rate of lesion-free status was observed in treated patients who had restored cerebral outflow demonstrated on Doppler ultrasound.15 These observations led us to hypothesize that angioplasty can have a positive effect on new lesions in the central nervous system in a subgroup of MS patients.
In a recent paper measuring the preprocedural and postprocedural flow in almost 800 CCSVI patients who underwent angioplasty of the internal jugular veins, Giaquinta et al16 demonstrated that patients with transverse endoluminal defects or with segmental wall stenosis with or without endoluminal defects were more likely to respond well to treatment compared to those patients with hypoplasia, external compression, or long endoluminal defects. Commenting on this paper, Moneta17 suggested that additional post hoc analysis of the Brave Dreams outcomes from the perspective of “favorable” or “unfavorable” jugular lesions according to the Giaquinta venography grading system might help guide future investigations.
Following this suggestion, the Brave Dreams data were reanalyzed, this time including the secondary/progressive MS patients who had been excluded during the initial analysis. Furthermore, the analysis was expanded to determine whether patients with “favorable” venographic findings had better response to angioplasty than patients with “unfavorable” venographic findings or controls.
Materials and Methods
Study Design
Brave Dreams was a multicenter, randomized, parallel group, double-blind, sham-controlled trial to evaluate the clinical efficacy and safety of balloon angioplasty of the extracranial veins (internal jugular and azygos) in MS patients with CCSVI. The study was conducted in 6 Italian MS centers (see the Appendix) with echo-color Doppler (ECD) and angiographic units accredited by the National Health Service. All the investigators, including the endovascular surgeons and interventional radiologists who performed the procedures, participated in a training period followed by an accreditation procedure carried out by a commission appointed by the study Steering Committee. Study monitoring was entrusted to a contract research organization (CRO), while a Data Coordinating Center oversaw data quality.14
The study adhered to the Helsinki Declaration and the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice guidelines. The study protocol was approved by the ethics committees of the central coordinating center (University of Ferrara Hospital) and each of the participating centers. The trial was registered on the National Institutes of Health website (ClinicalTrials.gov; identifier NCT01371760).
Recruitment and Randomization
The study participants were recruited by the neurologist in charge at the participating MS centers according to the inclusion/exclusion criteria previously published12 and summarized in Table 1.
Table 1.
Inclusion/Exclusion Criteria for the Brave Dreams Trial.
| Inclusion criteria |
| Adults 18 to 65 years old |
| Subjects with multiple sclerosis (MS) affected by CCSVI screened with echo-Doppler according to a consensus protocol |
| Relapsing/remitting and\or secondary/progressive MS |
| Expanded Disability Status Scale 2–5.5 |
| Disease duration ≤15 years |
| Care provided by the recruiting center for at least 2 years |
| No relapse in the 30 days preceding the procedure |
| Clinical stability in the last 6 months with disease modification treatments |
| Subjects under the best available therapy |
| Exclusion criteria |
| Previous treatment for CCSVI or involvement in other clinical trials in the prior 3 months |
| Under treatment with fingolimod, cladribine, laquinimod, or botulinum toxin or had infusion pump or neurostimulator implantation |
| Pregnant or refusing to adopt contraception |
| Presence of significant comorbidities |
| Alcohol or drug abuse |
| Thrombophilia |
| Contraindication to magnetic resonance imaging |
A web-based computerized central randomization procedure stratified by participating centers and with variable length blocks was defined by the Data Coordinating Center and implemented by the CRO. The participants were assigned to angioplasty or a sham procedure with an allocation ratio of 2:1. The treatment assignment was automatically available to the operator in electronic case report form only on the scheduled intervention date. Both patients and all the investigators involved in the study (neurologists, outcome assessors, ECD operators, MRI evaluators, statisticians) as well as personnel in the operating and hospital rooms, except the interventionist, were masked to the assigned treatment. In order to ensure patient blinding, the interventionists in charge were trained to perform a special protocol for venography in order to simulate venous angioplasty, making the 2 procedures as similar as possible, as explained in detail in the protocol.14
Figure 1 summarizes the distribution of patients enrolled in this study. Of 204 patients assessed by ECD, 58 showed no evidence of CCSVI on ultrasound, so the prevalence of CCSVI in the screening cohort was 71.6%. Of the 146 patients assessed for eligibility, 16 were ineligible for venography/angioplasty according to the inclusion/exclusion criteria (Table 1). A total of 130 patients (mean age 39.9±10.6 years; 81 women) were randomized to angioplasty (n=86) or a sham procedure (n=44). The baseline characteristics of the recruited patients are given in Table 2.
Figure 1.
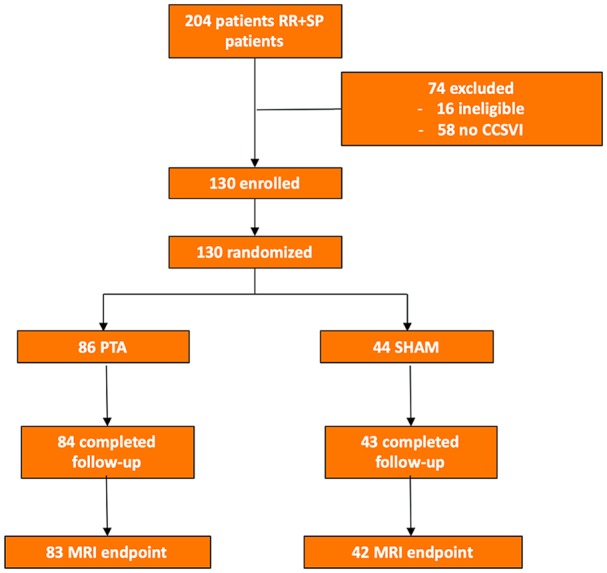
Flow diagram of the Brave Dreams trial including both relapsing/remitting (RR) and secondary/progressive (SP) multiple sclerosis patients. Chronic cerebrospinal venous insufficiency (CCSVI) was diagnosed using Doppler ultrasound. MRI, magnetic resonance imaging; PTA, percutaneous transluminal angioplasty.
Table 2.
Demographics and Baseline Clinical and Disease Characteristics of Patients Enrolled in the Brave Dreams Trial.a
| Variable | Angioplasty (n=86) | Sham (n=44) |
|---|---|---|
| Age, y | 41.0±10.6 | 37.8±10.3 |
| Women | 50 (58.1) | 31 (70.5) |
| EDSS score | ||
| 2 or 2.5 | 51 (59.3) | 24 (54.5) |
| 3 or 3.5 | 22 (25.6) | 12 (27.3) |
| 4 or 4.5 | 10 (11.6) | 3 (6.8) |
| 5 or 5.5 | 3 (3.5) | 5 (11.4) |
| Duration of MS in relapsing/remitting patients, y | 4.3 (2.8, 8.4) | 6.1 (3.7, 9) |
| Duration of MS in secondary/progressive patients, y | 7.9 (4.7, 11.8) | 8 (7.6, 9) |
| Months from progression | 37.5 (22, 53) | 38 (26, 44) |
| Relapses in previous 2 years | ||
| 0 | 10 (11.6) | 5 (11.4) |
| 1 | 44 (51.2) | 26 (59.1) |
| 2 | 24 (27.9) | 4 (9.1) |
| ≥3 | 8 (9.3) | 9 (20.4) |
| Intraluminal obstacle at ECD in at least 1 IJV | 80 (93.0) | 42 (95.4) |
| Bidirectional and/or absent flow at ECD in at least 1 IJV in 2 positions | 82 (95.3) | 43 (97.7) |
| Patients with gadolinium-enhancing T1 lesions | 18 (20.9) | 7 (15.9) |
| Immunomodulatory therapy | 35 (41.0) | 24 (54.6) |
Abbreviations: ECD, echo-Doppler; EDSS, Expanded Disability Status Scale; IJV, internal jugular vein; MS, multiple sclerosis.
Continuous data are presented as the mean ± standard deviation or median (interquartile range Q1, Q3); categorical data are given as the number (percentage).
Procedures
The participants underwent catheter venography of the ascending lumbar, the left renal, the azygos, and the internal jugular veins via a percutaneous left femoral venous approach to evaluate the presence and location of CCSVI, as previously reported.4,5,8,16 Participants randomized to the angioplasty arm received the intervention during the diagnostic venography session, while those allocated to the sham group underwent venography and simulated angioplasty. All the patients were given a prophylactic dose of low-molecular-weight heparin for the following 3 weeks.
MRI Protocol and Outcomes
In each center the MRI scans were acquired at baseline and at 6 and 12 months using at least 1.5-T equipment and the same protocol for the duration of the study. Before starting the study, each participating specialist completed a “dummy scan” consisting of 2 separate acquisitions of the brain of the same individual. Quality parameters of the images, as well as repositioning accuracy and the signal-noise ratio, were evaluated by the independent MRI center at the University of Florence, which performed blinded review of all MRI scans.14
The primary MRI outcome measure was the same as in the first report, ie, the proportion of MS patients free from new brain lesions for 12 months after venoplasty. A new lesion was any of the following: (1) new lesions not seen previously on T2-weighted images, (2) enlargement of pre-existing lesions by >30%, and (3) new gadolinium enhancement of pre-existing lesions on T1-weighted images. As secondary outcome measures, the results of the scans were evaluated separately for any new lesion or change in a pre-existing lesion between months 0 and 6, 6 and 12, and 0 and 12.
Venogram Classification
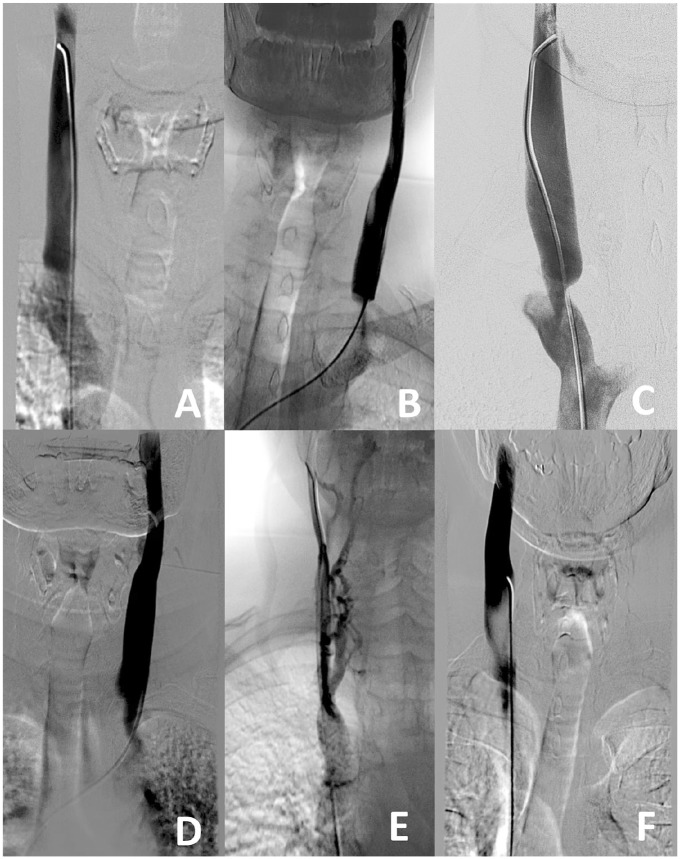
In the post hoc study, specific training was carried out to obtain uniformity in classifying the cases according to the Giaquinta classification16 of favorable or unfavorable for angioplasty. Illustrations demonstrating the salient features that distinguish the various appearances described by Giaquinta are presented in Figure 2. CCSVI jugular lesions are characterized by significant narrowing of the contrast column consistent with stenosis. Features of favorable lesions were horizontal endoluminal defects without wall stenosis (Figure 2A), segmental stenosis with horizontal endoluminal defects (Figure 2B), and segmental stenosis with short endoluminal lesions (Figure 2C). Unfavorable lesions were identified by long defects (Figure 2D), hypoplasia (Figure 2E), or external compression (Figure 2F).
Figure 2.
Favorable jugular lesions for angioplasty include (A) focal intraluminal and transverse defects without wall stenosis, (B) focal intraluminal transverse defects producing narrowing >80%, or (C) segmental stenosis with a short vertical shoulder. Unfavorable jugular lesions are (D) intraluminal defects >1 cm long, (E) internal jugular vein hypoplasia with significant collateral circulation, or (F) extrinsic compression by the omohyoid muscle.
Readers met twice in 4-hour sessions to read and classify 50 CCSVI venograms not belonging to the Brave Dreams series. Three teleconferences among numerous experts were held in which CCSVI venograms were screened, discussed, and finally classified. After this training, 2 investigators (R.G. and A.G.), who performed the vast majority of the Brave Dreams venograms, characterized venograms according to the Giaquinta CCSVI subtypes.16 Subsequently, all images and video clips were sent at random to one of the 2 central experts (C.S. and S.S.) who performed a second blinded reading. If the CCSVI subtype diagnosis (favorable vs unfavorable) matched, the final report was issued for that patient. If there was no agreement between the local and central examiners, the case was excluded from further analysis.
Statistical Analysis
The analyses were performed on an intention to treat basis by an independent statistician (E.M.). The proportion of patients who were free of new brain lesions on MRI were compared using the chi-square test, followed by estimation of the relative risk (RR) with 95% confidence interval (CI). Post hoc analysis also compared the distributions of lesion-free patients in the favorable vs unfavorable venogram groups vs controls using the Fisher exact test with Bonferroni correction for multiple comparisons. Interobserver variability in venogram classification among the 4 raters was calculated using the Fleiss ĸ test. The level of significance was set at 5%, and the analyses were done using Stata statistical software (version 13; StataCorp, College Station, TX, USA).
Results
Venography confirmed the CCSVI diagnosis by ECD in 96% (125/130) patients, with 5 false positives (3 in the treatment group and 2 in the sham group). There were 2 adverse events (vagal reaction and an episode of transient neck pain) but no serious adverse events reported.
All 125 patients with confirmed CCSVI had MRI follow-up. Regarding the primary outcome, the present analysis showed significantly fewer patients with new combined MRI lesions at 12 months in the group that underwent angioplasty compared to the patients who underwent a sham procedure (p=0.032; Figure 3). In the angioplasty arm (n=83), 56 (67%) patients were lesion free vs 20 (48%) in the sham arm (n=42). The 1.42 relative risk (95% CI 1.00 to 2.01) comparing angioplasty and sham groups was significantly different (p=0.032). In other words, patients undergoing venous angioplasty were 42% more likely to be free of a new lesion at 12 months after treatment compared to those who underwent a sham procedure.
Figure 3.

The proportions of patients free from new cerebral lesions on magnetic resonance imaging vs those with new lesions at 0 to 12 months from randomization in the angioplasty (PTA) vs sham arms (primary outcome), in the favorable vs unfavorable for PTA subgroups, and in the PTA favorable subgroup vs the sham arm.
For the post hoc analysis to identify CCSVI lesions favorable to angioplasty, the local and central venography reviewers did not agree on the classification in 15 of the 83 venograms (inter-rater variability ĸ=0.89); these cases were excluded from this part of the analysis. Of the 68 patients with categorized CCSVI lesions, 38 (56%) patients had venograms that were favorable for angioplasty vs 30 (44%) that were unfavorable. The favorable subtypes of CCSVI lesions (Figure 3) were 12 (18%) horizontal endoluminal defects without wall stenosis and 26 (38%) segmental stenoses with short endoluminal lesions. In the unfavorable subgroup, there were 13 (19%) long defects, 4 (6%) hypoplastic lesions, and 13 (19%) with external compression.
The MRI primary outcome of patients treated for favorable lesions compared to patients with unfavorable lesions or sham procedures (Figure 3) demonstrated a significantly higher probability of a lesion-free status among patients with favorable venograms than patients with unfavorable venograms (RR 1.82, 95% CI 1.17 to 2.83, p=0.005) or controls (RR 1.66, 95% CI 1.16 to 2.37, p=0.005). Freedom from any kind of new cerebral lesion on MRI was significantly more likely in the favorable subgroup (Table 3) at 0 to 12 months, whereas freedom from gadolinium-enhancing T1 lesions was most likely between months 0 and 6 and freedom from new/enlarged T2 lesions between months 6 and 12.
Table 3.
Distribution of New MRI Lesions After Randomization in the Favorable and Unfavorable Subgroups of the Treatment Arm, as Well as the Sham Arm.
| 0–12 Months |
0–6 Months |
6–12 Months |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Sham (n=42) |
Unfavorable (n=30) |
Favorable (n=38) |
Sham (n=42) |
Unfavorable (n=30) |
Favorable (n=38) |
Sham (n=42) |
Unfavorable (n=30) |
Favorable (n=38) |
|
| Freedom from new or enlarged T2 lesionsa | 23 (54.8); RR 1.49 (1.09 to 2.04), p=0.016 |
16 (53.3); RR 1.53 (1.06 to 2.21), p=0.018 |
31 (81.6) | 31 (73.8); RR 1.14 (0.91 to 1.43), p=0.287 |
19 (63.3); RR 1.33 (0.98 to 1.80), p=0.089 |
32 (84.2) | 28 (66.7); RR 1.46 (1.17 to 1.82), p<0.001 |
20 (66.7); RR 1.46 (1.13 to 1.89), p<0.001 |
37 (97.4) |
| Freedom from new Gd-enhancing T1 lesionsa | 25 (59.5); RR 1.55 (1.19 to 2.02), p<0.001 |
18 (60.0); RR 1.54 (1.13 to 2.09), p=0.003 |
35 (92.1) | 33 (78.6); RR 1.24 (1.05 to 1.46), p=0.016 |
22 (73.3); RR 1.33 (1.06 to 1.66), p=0.008 |
37 (97.4) | 30 (71.4); RR 1.29 (1.04 to 1.60), p=0.023 |
23 (76.7); RR 1.20 (0.97 to 1.49), p=0.094 |
35 (92.1) |
| Freedom from new combined lesionsa | 20 (47.6); RR 1.66 (1.16 to 2.37), p=0.005 |
13 (43.3); RR 1.82 (1.17 to 2.83), p=0.005 |
30 (79.0) | 29 (69.1); RR 1.22 (0.95 to 1.56), p=0.125 |
17 (56.7); RR 1.49 (1.06 to 2.09), p=0.016 |
32 (84.2) | 25 (59.5); RR 1.55 (1.19 to 2.02), p<0.001 |
19 (63.3); RR 1.45 (1.09 to 1.94), p=0.006 |
35 (92.1) |
Abbreviations: Gd, gadolinium; MRI, magnetic resonance imaging; PTA, percutaneous transluminal angioplasty.
Data are presented as the number (percentage); relative risk (RR) with the 95% confidence interval in parentheses, and the p value comparing the sham and the unfavorable groups to the favorable group.
Discussion
This new analysis of the Brave Dreams trial data demonstrated that angioplasty had a meaningful effect on disease activity, as measured by freedom from new cerebral lesion development on MRI and/or decreased activity of existing lesions at 12 months in a population that included secondary/progressive MS patients in addition to the relapsing/remitting MS cohort previously described.14 Because the initial Brave Dreams report did not show effectiveness of angioplasty in restoring venous flow from the brain in a large proportion of patients, a post hoc analysis was made to evaluate MRI outcomes according to the CCSVI appearance at venography. These venograms were re-interpreted using a recently reported set of distinctive venographic appearances that had been linked to favorable angioplasty treatment in CCSVI.16
Initially, the study demonstrated that interpretation of the venograms was reproducible by interventionists who had treated patients in Brave Dreams as well as by independent experts. Consensus was good, although some venograms could not be categorized because of inadequate image quality or incomplete recording of the procedures.
Secondly, patients who had venograms with favorable characteristics were indeed more likely to have improved MRI outcomes after angioplasty. The rate of MS patients protected from the development of new cerebral lesions or increased activity of pre-existing lesions 12 months after angioplasty was 36% greater in favorable patients than in subjects with features unlikely to respond to angioplasty and was 31% greater than in the sham arm. Since the proportions of patients under immunomodulatory treatment were similar in the 2 arms, it appears evident that angioplasty may provide a further modulation of fundamental MRI parameters widely used in clinical practice to monitor MS disease activity.14,15
Some might argue that catheter venography is too invasive a procedure to use as a determinant of treatment, although Brave Dreams and other safety trials have confirmed the safety of the procedure. Recent advances in ultrasound technology, which were published subsequent to the Brave Dreams protocol, may provide reliable information to screen patients who might benefit from angioplasty. Indeed, the findings described by Giaquinta et al16 are readily seen on ultrasound.18-22
From a pathophysiological perspective one can speculate that the modulating effect of restoring venous drainage on MRI lesions can be explained by recent discoveries. Balloon angioplasty causes significant reduction in pressure in the jugular vein and venous sinuses.23-25 Lowering of this fundamental circulatory parameter facilitates glymphatic drainage.26-28 This might explain the reduced accumulation of gadolinium-enhanced T1 lesions found in the present study, particularly evident immediately after the procedure in the subgroup with favorable venography. Furthermore, restoration of jugular flow has been shown to be beneficial to cerebral perfusion.18 There is less T2 lesion accumulation when cerebral perfusion is enhanced because improved blood flow facilitates myelin repair.28-30 Speculatively, this reparative process takes time, as suggested by more improvement in MRI between months 6 and 12 as compared to scans in T2 lesion accumulation at 0 to 6 months (Table 3).
Few published data are available for comparison with our findings. One small (10 patients sham/9 patients treated) RCT was reported after follow-up at 6 months.31 The authors showed that clinical and MR endpoints were no better or worse in patients who received venous angioplasty compared to controls. Traboulsee et al32 performed a double-blind RCT showing no differences in the angioplasty arm compared to sham as far as quality of life was concerned. However, the demographics of the recruited patients were different from those reported in our study, especially regarding disease duration. Traboulsee’s patients had longer mean disease duration (18 years), with significant differences also in the mean disability scale (EDSS 4; Table 2). These differences in patient selection make the data from these 2 studies difficult to compare. A recent Cochrane review based on the RCTs described above identified moderate-quality evidence that venous balloon angioplasty did not provide benefit in patients with MS regarding disability, physical or cognitive functions, relapse, and quality of life.33 Finally, a recent RCT found significant improvement in several quality of life–related items in patients who underwent angioplasty.34
Limitations
Despite being the largest trial,14 this study had a small sample size. A further limitation is that MRI lesions have not consistently been shown to correlate with individual clinical outcomes in trials. In MS, MRI imaging outcomes are more sensitive to change than clinical measures, thus requiring smaller sample sizes and shorter study durations to detect treatment differences.35,36 Therefore, MRI is especially valuable for early proof of concept trials, such as the present trial, where the sample size was small and the follow-up was short.
It is unfortunate that the Giaquinta classification16 of jugular narrowing at venography was published after the beginning of the Brave Dreams trial.17 It would have been preferable to have used the classification to choose patients for the trial. However, post hoc analysis based on a blinded double review of venographic images suggests that the classification may improve patient selection when considering treatment of MS patients who have CCSVI. Indeed, some unfavorable venographic criteria can be detected by ultrasound, which might be useful in determining suitability for angioplasty. From this point of view, new larger phase 3 trials could be planned on patients with early onset and mild disability selected with ultrasound Doppler examination.
Conclusion
Our findings have confirmed that venography and angioplasty are safe endovascular procedures, and, in selected patients, the latter seems to improve protection against new lesions detected by MRI. Finally, the contribution of impaired cerebral venous drainage to the development of MS cerebral plaques warrants further investigation.28
Acknowledgments
The Authors thank the following charities for contributing to the trial: Fondazione Cassa di Risparmio di Macerata, Associazione CCSVI nella SM, Associazione Sportiva Dilettanti, Associazione CCSVI Campania, Associazioni Sportive Italiane FE, Centro Amministrativo Farmacie, Circolo Ricreativo Mun. Rovereto, Circolo Le Café Lenzi Davide, Circolo Officina Ferrarese del Motorismo Storico, Gruppo calcistico Portomaggiore, Lions Club Ravenna Host, Lions Club Ferrara Host, Musei Arte Antica, Panathlon International Club di Taranto, Parrocchia Santi Pietro e Paolo, Proloco di Poggiorenatico, Rotaract Club Ferrara, SPAL Ferrara, Società Adp-GsIi Italia, Società Byte Software House, Unione Agricoltori Ferrara in memoria Iole Giglioli Gulinelli, and UTEF Ferrara.
Appendix
Brave Dreams Investigators and Participating Centers
Anna University Hospital, Ferrara, Italy (Central Coordinating Center) and IRCCS Neurosciences, Bellaria Hospital, Bologna, Italy: Elena Barbarossa, Ilaria Bartolomei, Stefano Ceruti, Paolo Conforti, Anna Maria Malagoni, Erica Menegatti, Mirko Tessari, Lisa Pellegrino, Francesca Pancaldi, and Maria Elena Vanini. “S. Maria delle Croci” Hospital, Ravenna, Italy: Maria Grazia Piscaglia, Patrizia Cenni, Fabrizio Rasi, Mara Babini, Antonella Drea, Eugenia Guerrini, Enrico Maria Lotti, Agnese Morelli, Milena Peroni, Valentina Zalambani, and Sauro Zecchini. University Hospital “Policlinico Vittorio Emanuele,” Catania, Italy: Clara Chisari, Alessia Giaquinta, Ignazio Chiaramonte, Vincenzo Cimino, Luigi Di Pino, Gianni Failla,† and Pierfrancesco Veroux. “Maggiore della Carità” Hospital, Novara, Italy: Roberto Cantello, Maurizio Leone, Lorenzo Coppo, Giuseppe Guzzardi, Olga Raymkulova, Simona Ruggerone, Alessandro Stecco, and Domizia Vecchio. Neurological Institute “C. Besta” IRCCS Foundation, Milan, Italy: Paolo Agostino Confalonieri and Elisa Ciceri. University Hospital “Ospedale Riuniti,” Ancona, Italy, and Hospital of Civitanova Marche, Italy: Maura Danni, Salvatore Alborino, Carla Belleggia, Giuseppe Luccioni, Luigi Oncini, and Cristina Quatrini. †, deceased.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported mainly by Regione Emilia-Romagna Directorate-General for Health and Welfare and charitable donations.
ORCID iD: Paolo Zamboni  https://orcid.org/0000-0002-7107-888X
https://orcid.org/0000-0002-7107-888X
Contributor Information
for the Brave Dreams Investigators:
Elena Barbarossa, Ilaria Bartolomei, Stefano Ceruti, Paolo Conforti, Anna Maria Malagoni, Erica Menegatti, Mirko Tessari, Lisa Pellegrino, Francesca Pancaldi, Maria Elena Vanini, Maria Grazia Piscaglia, Patrizia Cenni, Fabrizio Rasi, Mara Babini, Antonella Drea, Eugenia Guerrini, Enrico Maria Lotti, Agnese Morelli, Milena Peroni, Valentina Zalambani, Sauro Zecchini, Clara Chisari, Ignazio Chiaramonte, Vincenzo Cimino, Luigi Di Pino, Gianni Failla, Roberto Cantello, Maurizio Leone, Lorenzo Coppo, Olga Raymkulova, Simona Ruggerone, Alessandro Stecco, Domizia Vecchio, Paolo Agostino Confalonieri, Elisa Ciceri, Maura Danni, Carla Belleggia, Giuseppe Luccioni, Luigi Oncini, and Cristina Quatrini
References
- 1. Lee BB, Baumgartner I, Berlien P, et al. ; International Union of Phlebology. Diagnosis and treatment of venous malformations. Consensus document of the International Union of Phlebology (IUP): updated 2013. Int Angiol. 2015;34:97–149. [PubMed] [Google Scholar]
- 2. Pascolo L, Gianoncelli A, Rizzardi C, et al. Calcium micro-depositions in jugular truncular venous malformations revealed by Synchrotron-based XRF imaging. Sci Rep. 2014;4:6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pedriali M, Zamboni P. The pathology of the internal jugular vein wall in multiple sclerosis. J Mult Scler (Foster City). 2015;2:160. doi: 10.4172/2376-0389.1000160 [DOI] [Google Scholar]
- 4. Zivadinov R, Bastianello S, Dake MD, et al. ; International Society for Neurovascular Disease. Recommendations for multimodal noninvasive and invasive screening for detection of extracranial venous abnormalities indicative of chronic cerebrospinal venous insufficiency: a position statement of the International Society for Neurovascular Disease. J Vasc Interv Radiol. 2014;25:1785–1794.e17. [DOI] [PubMed] [Google Scholar]
- 5. Zamboni P, Galeotti R, Menegatti E, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Utriainen D, Trifan G, Sethi S, et al. Magnetic resonance imaging signatures of vascular pathology in multiple sclerosis. Neurol Res. 2012;34:780–792. [DOI] [PubMed] [Google Scholar]
- 7. Veroux P, Giaquinta A, Perricone D, et al. Internal jugular veins out flow in patients with multiple sclerosis: a catheter venography study. J Vasc Interv Radiol. 2013;24:1790–1797. [DOI] [PubMed] [Google Scholar]
- 8. Zivadinov R, Marr K, Cutter G, et al. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology. 2011;77:138–144. [DOI] [PubMed] [Google Scholar]
- 9. Zhou D, Ding J, Asmaro K, et al. Clinical characteristics and neuroimaging findings in internal jugular venous outflow disturbance. Thromb Haemost. 2019;119(2):308–318. [DOI] [PubMed] [Google Scholar]
- 10. Laupacis A, Lillie E, Dueck A, et al. Association between chronic cerebrospinal venous insufficiency and multiple sclerosis: a meta-analysis. CMAJ. 2011;183:E1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zwischenberger BA, Beasley MM, Davenport DL, et al. Meta-analysis of the correlation between chronic cerebrospinal venous insufficiency and multiple sclerosis. Vasc Endovasc Surg. 2013;47:620–624. [DOI] [PubMed] [Google Scholar]
- 12. Zamboni P, Bertolotto A, Boldrini P, et al. Efficacy and safety of venous angioplasty of the extracranial veins for multiple sclerosis. Brave Dreams study (brain venous drainage exploited against multiple sclerosis): study protocol for a randomized controlled trial. Trials. 2012;13:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Institute for Health and Care Excellence. Research recommendation IPG420/1. Published April 2016. Available at https://www.nice.org.uk/about/what-we-do/research-and-development/research-recommendations/ipg420/1. Last accessed October 28, 2018.
- 14. Zamboni P, Tesio L, Galimberti S, et al. Efficacy and safety of extracranial vein angioplasty in multiple sclerosis: a randomized clinical trial. JAMA Neurol. 2018;75:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zamboni P, Zivadinov R. Extracranial veins in multiple sclerosis: is there a role for vascular surgery? Eur J Vasc Endovasc Surg. 2018;56:618–621. [DOI] [PubMed] [Google Scholar]
- 16. Giaquinta A, Beggs CB, Veroux M, et al. Factors influencing the hemodynamic response to balloon angioplasty in the treatment of outflow anomalies of internal jugular veins. J Vasc Surg Venous Lymphat Disord. 2017;5(6):777–788. [DOI] [PubMed] [Google Scholar]
- 17. Moneta GL. Optimism, enthusiasm, responsibility. J Vasc Surg Venous Lymphat Disord. 2017;5:775–776. [DOI] [PubMed] [Google Scholar]
- 18. Zamboni P, Menegatti E, Cittanti C, et al. Fixing the jugular flow reduces ventricle volume and improves brain perfusion. J Vasc Surg Venous Lymphat Disord. 2016;4:434–445. [DOI] [PubMed] [Google Scholar]
- 19. De Bonis P, Menegatti E, Cavallo MA, et al. JEDI (jugular entrapment, dilated ventricles, intracranial hypertension) syndrome: a new clinical entity? A case report. Acta Neurochir (Wien). 2019;161(7):1367–1370. [DOI] [PubMed] [Google Scholar]
- 20. Menegatti E, Tessari M, Vannini ME, et al. High resolution M-mode evaluation of jugular vein valves in patients with neurological and neurosensory disorders. Curr Neurovasc Res. 2017;14(4):316–322. [DOI] [PubMed] [Google Scholar]
- 21. Czyzewska D, Ustymowicz A, Kosel J. Internal jugular veins must be measured before catheterization. J Clin Anesth. 2015;27(2):129–131. [DOI] [PubMed] [Google Scholar]
- 22. Menegatti E, Tessari M, Gianesini S, et al. Human internal jugular valve M-mode ultrasound characterization. Curr Neurovasc Res. 2014;11(2):149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zamboni P, Galeotti R, Menegatti E, et al. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J Vasc Surg. 2009;50:1348–1358, e1341–1343. [DOI] [PubMed] [Google Scholar]
- 24. Gadda G, Majka M, Zieliński P, et al. A multiscale model for the simulation of cerebral and extracerebral blood flows and pressures in humans. Eur J Appl Physiol. 2018;118(11):2443–2454. [DOI] [PubMed] [Google Scholar]
- 25. Toro EF, Muller LO, Cristini M, et al. Impact of jugular vein valve function on cerebral venous haemodynamics. Curr Neurovasc Res. 2015;12(4):384–397. [DOI] [PubMed] [Google Scholar]
- 26. Gaitán MI, Shea CD, Evangelou IE, et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann Neurol. 2011;70:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zamboni P. The contribution of extracranial venous drainage to neuro-inflammation in multiple sclerosis. In: Minagar A, ed. Neuroinflammation. London: Elsevier; 2018:579–599. [Google Scholar]
- 29. Wuerfel J, Bellmann-Strobl J, Brunecker P, et al. Changes in cerebral perfusion precede plaque formation in multiple sclerosis: a longitudinal perfusion MRI study. Brain. 2004;127:111–119. [DOI] [PubMed] [Google Scholar]
- 30. Holland CM, Charil A, Csapo I, et al. The relationship between normal cerebral perfusion patterns and white matter lesion distribution in 1,249 patients with multiple sclerosis. J Neuroimaging. 2012;22:129–136. [DOI] [PubMed] [Google Scholar]
- 31. Siddiqui AH, Zivadinov R, Benedict RH, et al. Prospective randomized trial of venous angioplasty in MS (PREMiSe). Neurology. 2014;83:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Traboulsee AL, Machan L, Girard JM, et al. Safety and efficacy of venoplasty in MS: A randomized, double-blind, sham-controlled phase II trial. Neurology. 2018,30:e1660–e1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jagannath VA, Pucci E, Asokan GV, et al. Percutaneous transluminal angioplasty for treatment of chronic cerebrospinal venous insufficiency (CCSVI) in people with multiple sclerosis. Cochrane Database Syst Rev. 2019. May 31;5:CD009903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Napoli V, Berchiolli R, Carboncini MC, et al. Percutaneous venous angioplasty in patients with multiple sclerosis and chronic cerebrospinal venous insufficiency: a randomized wait list control study [published online ahead of print August 22, 2019]. Ann Vasc Surg. doi: 10.1016/j.avsg.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 35. Comi G, Rovaris M, Leocani L, et al. Clinical and MRI assessment of brain damage in MS. Neurol Sci. 2001;22 Suppl 2:S123–127. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y, Salter A, Cutter G, et al. Clinical trials in multiple sclerosis: milestones. Ther Adv Neurol Disord. 2018;11:1756286418785499. doi: 10.1177/1756286418785499. [DOI] [PMC free article] [PubMed] [Google Scholar]