Abstract
The proposed molecular mechanisms underlying neurodegenerative pathogenesis are varied, precluding the development of effective therapies for these increasingly prevalent disorders. One of the most consistent observations across neurodegenerative diseases is the phosphorylation of eukaryotic initiation factor 2α (eIF2α). eIF2α is a translation initiation factor, involved in cap-dependent protein translation, which when phosphorylated causes global translation attenuation. eIF2α phosphorylation is mediated by 4 kinases, which, together with their downstream signaling cascades, constitute the integrated stress response (ISR). While the ISR is activated by stresses commonly observed in neurodegeneration, such as oxidative stress, endoplasmic reticulum stress, and inflammation, it is a canonically adaptive signaling cascade. However, chronic activation of the ISR can contribute to neurodegenerative phenotypes such as neuronal death, memory impairments, and protein aggregation via apoptotic induction and other maladaptive outcomes downstream of phospho-eIF2α-mediated translation inhibition, including neuroinflammation and altered amyloidogenic processing, plausibly in a feed-forward manner. This review examines evidence that dysregulated eIF2a phosphorylation acts as a driver of neurodegeneration, including a survey of observations of ISR signaling in human disease, inspection of the overlap between ISR signaling and neurodegenerative phenomenon, and assessment of recent encouraging findings ameliorating neurodegeneration using developing pharmacological agents which target the ISR. In doing so, gaps in the field, including crosstalk of the ISR kinases and consideration of ISR signaling in nonneuronal central nervous system cell types, are highlighted.
Keywords: Cell fate, eIF2α, phosphorylation (p-eIF2α), Integrated stress response (ISR), Neurodegeneration, Neurodegenerative disease pathogenesis, Stress signaling
INTRODUCTION
In the current era, the prevalence of neurodegenerative diseases such as Alzheimer disease (AD) and Parkinson disease (PD) is becoming an increasingly heavy burden (1). This is highlighted by the fact that for many disorders the available therapies, if any, are only palliative and not curative or even preventative. This is largely because the proposed molecular mechanisms underlying disease pathogenesis are many, varied, untargetable, or unknown. Many of the known mechanisms converge on a pathway that results in global translation inhibition through phosphorylation of the eukaryotic initiation factor 2α (eIF2α), via competitive inhibition of the guanine exchange factor eukaryotic initiation factor 2B (eIF2B), as part of stress response signaling. In recent years, elevated and/or dysregulated phosphorylation of eIF2α (p-eIF2α) has been extensively associated with many neurodegenerative pathologies (2, 3). Phosphorylation of eIF2α lies downstream of 4 kinases which collectively contribute to the integrated stress response (ISR): protein kinase R (PKR), PKR-like endoplasmic reticulum (ER) kinase (PERK), general control nonderepressible 2 (GCN2), and heme-regulated inhibitor (HRI) (4). Activation of these kinases is aimed to protect against certain cellular stresses, including ER stress, oxidative stress, viral infection, inflammation, and amino acid deprivation (Fig. 1). Intriguingly, many of these stresses are common hallmarks of pathology in numerous neurodegenerative diseases. However, the ISR is traditionally considered an adaptive response to cellular stress, and while downstream signaling is known to include maladaptive and even apoptotic responses that could explain many neurodegenerative phenotypes, there remains debate on whether dysregulated eIF2α phosphorylation is a driver for or a sign of chronic neurodegeneration. Regardless, targeting the pathways associated with eIF2α phosphorylation has yielded encouraging results in attempts to ameliorate neurodegeneration.
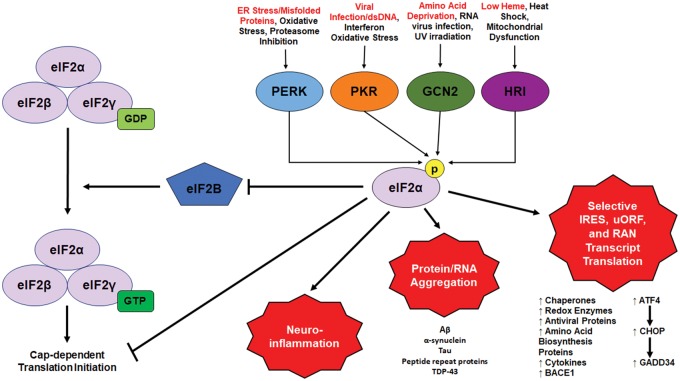
Figure 1.
Canonical integrated stress response signaling. The canonical integrated stress response (ISR) is made up of signaling by 4 stress-activated kinases: protein kinase R (PKR), PKR-like endoplasmic reticulum (ER) kinase (PERK), general control nonderepressible 2 (GCN2), and heme-regulated inhibitor (HRI). Each kinase has a distinct stress which is known to most directly activate it (in red); however, there is extensive overlap between the inductions of each kinase. Also, while each kinase has distinct targets, their main substrate when activated is the alpha subunit of eukaryotic initiation factor 2 (eIF2α). eIF2, when bound to GTP, is a component of the ternary complex required for the initiation of cap-dependent translation. However, p-eIF2α is a competitive inhibitor of eIF2B, the guanine exchange factor responsible for exchanging GDP for GTP on the γ subunit of eIF2 to allow repeat translation initiation. Thus, eIF2α phosphorylation attenuates global translation, while selectively upregulating translation of transcripts with internal ribosome entry sites (IRESs) or upstream open reading frames (uORFs), such as activating transcription factor 4 (ATF4). In turn this induces the upregulation of genes import for resolving stress and, over time with unresolvable stress, inducing apoptosis.
In this review, we summarize the canonical roles of each branch of the ISR and follow up with consideration and evidence of their implications in neurodegenerative pathologies. We also examine the maladaptive outcomes of ISR kinase activation and eIF2α phosphorylation, which might play mechanistic roles in neurodegeneration. Finally, we examine recent findings with emerging pharmacological agents to manipulate these pathways.
THE ISR-SIGNALING BY 4 DISTINCT STRESS-ACTIVATED KINASES
The ISR is a signaling network of 4 kinases that converges on phosphorylation of eIF2 at serine 51 of the α-subunit, which results in global repression of cap-dependent protein translation (5, 6) (Fig. 1). Composed of 3 subunits, α, β, and γ, eIF2 comprises a ternary complex that binds to the 40S ribosomal subunit to form part of the 43S preinitiation complex when bound to GTP and Met-tRNA (7, 8). After binding to the eIF4F cap recognition complex at the 5′ end of mRNAs, the pre-initiation complex scans the 5′ leader sequence for an AUG start codon. Once an AUG is recognized within a favorable sequence for translation initiation, GTP is hydrolyzed which releases eIF2 from the preinitiation complex and allows the 60S ribosomal subunit to bind the mRNA to initiate the elongation phase of translation (9). Phosphorylation of eIF2α inhibits this process by blocking the dissociation of eIF2 from eIF2B, a guanine nucleotide exchange factor (GEF) that is needed for the exchange of GDP for GTP in the γ subunit of eIF2 (10) and converts eIF2 from a substrate into a competitive inhibitor of eIF2B, thereby slowing the exchange rate of GDP for GTP and limiting the availability of competent ternary complexes to form the preinitiation complex (11).
Importantly, eIF2-mediated repression of protein translation is not universal; a subset of genes is translationally upregulated in response to eIF2α phosphorylation-mediated translational attenuation. These mRNAs escape translational repression through a variety of mechanisms including the presence of internal ribosome entry sites (IRESs) within the 5′ untranslated region (UTR). IRESs are cis-activating elements, composed of complex RNA structures, such as hairpins, in the 5′ UTR, which directly recruit the small ribosomal subunit in a cap-independent manner to internal codons with the help of trans-acting cellular proteins known as IRES trans-acting factors. Another mechanism of this selective upregulation involves small upstream open reading frames (uORFs) of approximately 30 codons in the 5′ UTR of gene transcripts (4, 12). When eIF2α phosphorylation is low and eIF2-GTP levels are high, scanning ribosomes translate the first uORF they encounter, assuming the AUG codon is in an optimal context for initiation. If a transcript contains uORFs upstream of the authentic start codon, such as that observed with activating transcription factor (ATF) 4, most of the ribosomes will initiate translation at one of the proximal uORFs, which decreases the likelihood that scanning ribosomes will reinitiate in time to begin translation at the authentic AUG start codon; this results in suppression of protein translation. However, when p-eIF2α levels are high and eIF2-GTP is limiting, scanning ribosomes, albeit still initiating translation at the first uORF, are more likely to scan through subsequent uORFs and reinitiate at the authentic start codon due to decreased availability of the preinitiation complex. Thus, decreased availability of the ternary complex/preinitiation complex reduces the translation rate of most cellular mRNAs that contain 1 start codon while simultaneously increasing the translation efficiency of mRNAs with multiple uORFs. This mechanism was first characterized in the yeast homolog of the transcription factor ATF4, GCN4, which contains 4 uORFs, and was subsequently linked to upregulation of a variety of genes involved in stress response, survival, and apoptosis (4, 5, 13–16). Specifically, ATF4 upregulates distinctive genes and transcription factors such as DDIT3, the gene for C/EBP homologous protein (CHOP) in an ongoing cascade. Approximately 35%–49% of human gene transcripts are predicted to contain uORFs, indicating that protein translation control via p-eIF2α is likely a widespread mechanism of regulation involved in many cellular functions and an area ripe for investigation of therapeutic interventional targets in human diseases (17).
The first eIF2α kinase identified was HRI, which serves the critical function of balancing the amount of globin production with the available heme levels by inhibiting protein translation in reticulocytes in response to low heme levels (4, 18–20). HRI binds heme at its N-terminus, which triggers the formation of stable HRI dimers (20). When heme levels are low, HRI dimers undergo autophosphorylation, activating the HRI kinase domain and leading to eIF2α phosphorylation and inhibition of global protein synthesis (18). HRI knockout mice display hypersensitivity to heme deficiency but no other physiological abnormalities (21–23).
The second ISR kinase, double-stranded RNA (dsRNA)-dependent PKR, is a cytosolic, constitutively expressed, mammalian kinase that was discovered after the initial observation that extracts from vaccinia virus-infected cells treated with interferon showed enhanced sensitivity to translational inhibition after addition to a cell-free system of exogenous mRNAs or synthetic dsRNA (24–26). Follow-up studies revealed that PKR activation occurred through binding of dsRNA at its N-terminal dsRNA-binding domains, leading to dimerization and autophosphorylation of its kinase domain (25). Downstream p-eIF2α limits translation of viral mRNAs while enhancing the expression of antiviral proteins (4, 27, 28). PKR can also be activated by a variety of dsRNA-independent stressors, including type-1 interferons, oxidative stress, and ER stress, via interaction with the PKR activator PACT (29). PACT is phosphorylated under various stress conditions and utilizes one of its 3 dsRNA-binding domains to interact with and activate PKR (4). Overexpression of PACT sensitizes cells to viral infection and other stresses, whereas knockdown of PACT mitigates stress-induced PKR activation and promotes clonal cell growth. Following activation by PACT, PKR phosphorylates eIF2α and phosphorylates or interacts with a variety of other targets, including signal transducers and activators of transcription, interferon regulatory factor 1, Jun-N terminal protein kinase, ATF3, IkB kinase, and p53, which may mediate some of the observed tumor suppressor activity of PKR (25, 30, 31). Overexpression of PKR leads to potent activation of apoptosis, likely as a protective response against the spread of viral infection. However, despite the ability of PKR to regulate cell survival and death, PKR knockout mice develop normally and display no phenotypic abnormalities (32).
The third ISR kinase, GCN2, was originally identified in yeast as a protein that senses and responds to amino acid deprivation, although it appears to be sensitive to other stressors including viral infection and UV irradiation in mammals (33, 34). GCN2 is activated when uncharged tRNAs bind to its histidyl-tRNA synthetase-related domain and induce dimerization and autophosphorylation to activate the GCN2 kinase domain (4). GCN2-mediated p-eIF2α results in translational upregulation of ATF4, which induces the transcription of numerous genes involved in the amino acid biosynthetic pathway, and protection against oxidative stress (12, 35). GCN2 can sense infection by RNA viruses, such as Sindbis virus and Semliki Forest virus, by binding viral RNA with its histidyl-tRNA synthetase-related domain and inhibits translation via eIF2α phosphorylation (4, 36). One recent study also demonstrated that GCN2 inhibited replication of human immunodeficiency virus (HIV) in vitro and in vivo and was cleaved directly by the HIV-1 protease (37).
The last ISR kinase, PERK, responds to misfolded protein stress in the ER as part of the unfolded protein response (UPR), a homeostatic signaling cascade that responds to ER stress via 3 master regulators: inositol-requiring enzyme (IRE) 1, ATF6, and PERK. These proteins act as sensors and initiate signaling that reestablishes protein homeostasis by increasing the cellular folding capacity (38–40). During UPR activation, following dissociation from binding protein (BiP, also known as GRP78), PERK dimers oligomerize to activate the kinase domain via trans-autophosphorylation; this activating phosphorylation results in the subsequent, PERK-mediated p-eIF2α. Repression of global protein synthesis ultimately reduces the production of new mRNAs, with concomitant selective upregulation of protein-coding transcripts containing IRESs or uORFS, such as ATF4; the PERK cascade likewise also leads to the upregulation of folding enzymes, molecular chaperones, and the ER-associated degradation pathway, all aimed to reduce the burden of misfolded proteins and prevent cellular toxicity (4, 41). Importantly, prolonged PERK activation and ATF4 activity are known to induce the proapoptotic transcription factor CHOP. CHOP induces the expression of growth arrest and DNA damage-inducible protein (GADD34), a nonenzymatic cofactor that targets protein phosphatase 1 (PP1) to dephosphorylate eIF2α to provide a negative feedback loop against chronic activation in cases of acute stress (42). PERK plays an important physiological role in secretory cell types, such as pancreatic β cells, which are frequently under a large biosynthetic load in response to varying demands for insulin production and thus have a larger and more developed ER. Missense mutations in EIF2AK3, the gene that encodes PERK, have been linked to the Wolcott-Rallison syndrome in humans, which is characterized by permanent neonatal diabetes, growth retardation, and pancreatic and skeletal system deficits, accompanied by mental retardation in some cases (43, 44). Similar phenotypic abnormalities were also observed in EIF2AK3 knockout mice. In addition to alleviating ER stress, PERK plays a critical role in limiting oxidative stress via activation of nuclear factor (erythroid-derived 2)-like 2 (NRF2), a transcription factor that regulates the protein expression of a battery of genes involved in the antioxidant response including detoxifying enzymes such as glutathione S-transferase A2 (GSTA2), heme oxygenase-1 (HO-1), and NADPH quinone oxidoreductase (NQO1) (45). Under normal conditions, NRF2 is kept inactive in the cytoplasm via interaction with and constitutive targeting for degradation by Kelch-like ECH-associated protein 1 (KEAP1) (46). Following phosphorylation by PERK, NRF2 dissociates from KEAP1, migrates to the nucleus, and activates transcription of genes that contain the antioxidant response element in their promoter (47). The significance of the link between ER stress and NRF2 activation is best highlighted by the hypersensitivity of cells lacking NRF2 to compounds that activate ER and oxidative stress (48).
When stress is prolonged and cannot be resolved by the above-mentioned signaling cascades, the ISR enters a second phase of maladaptive signaling and apoptotic induction. ATF4 upregulates the transcription and subsequent expression of the proapoptotic BCL-2 protein NOXA, a plasma membrane proton ATPase, as well as CHOP (3). CHOP induces the expression of the proapoptotic BH3-only BCL-2 family members as well as death receptor 5 (3, 49). Additionally, sustained CHOP expression results in the upregulation of ER oxidoreductase 1α (ERO1α), which further sensitizes cells to death (49–51). ERO1α causes calcium release from the ER by activating the inositol-1,2,5-triphosphate receptor; such calcium dysregulation can induce apoptosis and necrosis (49). Furthermore, translational attenuation downregulates cyclin D1 and BCL-2, thereby sensitizing cells to apoptosis (49). These and other ISR-mediated death pathways, both downstream of and tangential to CHOP, have been reviewed extensively (3, 49, 50, 52, 53).
Altogether, these findings demonstrate that eIF2α phosphorylation by HRI, PKR, GCN2, and PERK is a critical cellular and physiological mechanism of protein translational control that protects cells against a wide variety of stressors but can, however, leads to adverse outcomes when activated in a chronic, uncontrolled fashion. While the precise determinants of the switch from homeostatic to cytotoxic signaling are still being investigated, eIF2α phosphorylation appears to act as a convergence point of many stress inputs, the resultant level of which may dictate cell fate.
THE ROLE OF ISR KINASES IN NEURODEGENERATION
Elevated and/or dysregulated eIF2α phosphorylation in association with neurodegenerative conditions has been a major focus of research for many years with good reason: Classic neurodegenerative conditions, such as AD, PD, amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), Huntington disease (HD), and prion disorders, are characterized both clinically and in postmortem patient pathology by chronic neuroinflammation, disturbances in proteostasis, and oxidative stress (41, 54–59), which have been shown to be ISR activators as well. While ISR activation converges on eIF2α phosphorylation, which leads to global translational attenuation with concomitant upregulation of select protein coding transcripts, such as ATF4, as a homeostatic response to certain stimuli, chronic or extreme stress can augment the signaling pathways downstream of eIF2α phosphorylation and result in maladaptive responses and even cell death.
Canonical ATF4 signaling includes induction of cytotoxic signals, such as CHOP upregulation, under conditions of unresolved stress and prolonged eIF2α phosphorylation, leading to cell death (3). Furthermore, sustained translational attenuation by itself is disruptive to neuronal function on several levels (41, 42, 59, 60). Formation of stress granules and liquid droplet aggregates, such as that seen in certain neurodegenerative conditions, has been shown to be dependent on elevated eIF2α phosphorylation as it leads to the accumulation of mRNAs (61, 62). Neurons also suffer from translation inhibition due to the fact that synapses turn over large amounts of proteins, and synaptic cell-to-cell transport/communication is critical to the function of neuronal networks. A large body of research highlights the necessity of translation, and the related bidirectional role of eIF2α phosphorylation, in long-term memory potentiation (LTP) and synaptic plasticity (63–66). Specifically, mice heterozygous for an alanine mutation at serine 51 of eIF2α exhibited enhanced LTP and memory consolidation, whereas pharmacological induction of eIF2α phosphorylation had the reverse effect (67). Additionally, pharmacological downregulation of ATF4 in mice resulted in enhanced synaptic plasticity and long-term memory (68). Treatment with a nonspecific, general translation inhibitor, anisomycin, however, did not lead to differences in LTP (69). Mice knocked out for EIF2AK4 or EIF2AK2, the genes encoding GCN2 or PKR also exhibited enhanced memory consolidation (70, 71), whereas forebrain-specific ablation of EIF2AK3 in adult mice resulted in impairment of cognitive and information processing (72). These signaling pathways and outcomes of prolonged eIF2α phosphorylation might partially explain the many neurodegenerative phenotypes with which it is associated, such as aggregate-associated pathology, synaptic dysfunction and loss, neuronal death, and memory deficits (2, 40, 49).
Several studies have also demonstrated that sustained eIF2α phosphorylation is correlated with cognitive deficits in neurodegenerative disease models. For example, translation, synaptic deficits, and neuronal loss were rescued by overexpression of GADD34 in a mouse model of prion disease (60). Intriguingly, GADD34 was the only factor downstream of CHOP that was not upregulated during disease progression in this disease model (60). Interestingly, DDIT3 knockout ameliorated cell death in a mouse model of PD (73). Additionally, an eIF2α siRNA protected against cytotoxicity induced by rotenone, a mitochondrial inhibitor used to model PD by inducing oxidative stress resulting in phosphorylation of PERK, PKR, and eIF2α as well as cell death (74). While these are common effects of all ISR kinases, each kinase also induces its own specific downstream signaling pathway and has distinct pursuant implications in neurodegeneration.
PKR-Like ER Kinase
PERK is 1 branch of the UPR, activation of which is one of the most pervasive observations across neurodegenerative diseases. Specifically, eIF2α phosphorylation attributed to PERK has been observed in the postmortem brains, and sometimes the cerebrospinal fluid (CSF), of clinical patients with AD, PD, ALS, HD, progressive supranuclear palsy (PSP), and HIV-associated neurocognitive disorders (HAND), a neurodegenerative condition which persists in up to 50% of patients on antiretroviral therapy (ART) with suppressed virus (38, 41, 59, 75–78). Thus, misfolded proteins such as amyloid beta (Aβ), α-synuclein, and PrP, which are major hallmarks of many neurodegenerative diseases, may act in a feed-forward mechanism, inducing chronic PERK signaling via the UPR. Akin to the ISR, a large body of evidence suggests that the UPR can switch from an adaptive/proteostatic profile to maladaptive/cytotoxic signaling in response to severe or prolonged stress such as that seen in neurodegenerative pathologies (38, 41, 49, 51, 53). While this switch is believed to be dependent on the context and strength of the stress, the precise mechanism underlying this protective/toxic switch remains an ongoing question in the field.
The PERK arm of the UPR has the most well-established connections to maladaptive processes and has even been suggested to act as an adaptive signaling pathway only in coordination with the other arms of the UPR (4, 40, 49, 50, 59, 79, 80). In fact, IRE1 activity results in increased p58 levels, which inhibit PERK, suggesting the presence of tight regulation aimed to prevent overactivation (81). Additionally, PERK is the only branch of the UPR that remains active under chronic stress conditions when the UPR is presumably in the maladaptive phase (82). Many studies support the direct role of PERK dysregulation in elevated eIF2α phosphorylation in neurodegeneration (41, 83). PERK signaling is upregulated early in neurodegenerative disorders such as AD and PD, as shown in animal models and human specimens (84–94). Moreover, genetic ablation of EIF2AK3 in the central nervous system (CNS) was shown to be protective in prion-inoculated mice and in a mouse model of AD (91, 94). Increased eIF2α phosphorylation was also observed in postmortem cortex of patients with HAND compared to neurocognitively normal individuals (95). Additionally, a recent transcriptome analysis by Venkatachari et al (96) identified EIF2AK3 among the genes associated with neurodegeneration in HIV-infected males.
Several recent findings on PERK haplotypes provide further evidence for dysregulated PERK activity and elevated eIF2α phosphorylation levels as contributors to neurodegeneration. Of the 5 known PERK haplotypes, the most prominent are haplotypes A and B, which are present in 67.6% and 31.1% of the general population, respectively (97). Four single-nucleotide polymorphisms differentiate these 2 major haplotypes; 3 of which result in changes in amino acids, 2 of which are within the luminal domain and 1 of which is within the kinase domain of PERK (98). Intriguingly, experiments in cultured lymphocytes from homozygous donors for either haplotype showed that the B haplotype of PERK led to higher eIF2α phosphorylation levels in response to the ER stress inducer thapsigargin, compared with the A haplotype (97). This enhanced response is likely the result of the amino acid changes conferring differences in PERK activation, activity, and/or downregulation between the 2 haplotypes. As noted, eIF2α phosphorylation level is a potential rheostat for downstream signaling pathways. Thus, a cell with more active PERK, such as that might be the case with PERK haplotype B, is expected to be biased toward maladaptive signaling. Conversely, 1 study in a tauopathy model for PSP reported that PERK haplotype B was a hypomorph at low stress levels, which also led to maladaptive outcomes due to ER stress sensitivity (99). Nonetheless, PERK haplotype B has been identified as a risk factor for AD and PSP, a neurodegenerative tauopathy (78, 100–105). This risk factor status makes it a compelling target for pharmacogenetic studies, particularly for the development of personalized medicine in these at-risk populations.
Despite all these disease implications, it remains that PERK signaling acts as an adaptive response during acute ER stress and plays numerous physiological and prosurvival roles, as outlined above (79, 106, 107). In following, many studies have convincingly showed that ablation of PERK signaling was toxic and that augmentation of eIF2α phosphorylation conferred protection from cell death (38, 59). For example, genetic ablation of EIF2AK3, resulted in increased sensitivity to cell death following stress induction, which was expected due to the prevention of the initial adaptive phase and ablation of PERK before stress onset instead of during chronic stress (106). In another study, allicin, a garlic extract, was used to upregulate the antioxidant pathway via PERK phosphorylation of NRF2, which ameliorated ER stress-mediated cognitive deficits in rats (108, 109). As previously noted, functionally null mutations in EIF2AK3, lead to Wolcott-Rallison syndrome which characterized by early neurodegeneration (110), which is expected based on the previously described role of PERK in neuronal wiring. The extrinsic morphogenesis factor semaphorin-3A induces acute local translation of proteins involved in proteostasis and the actin skeleton, which leads to PERK activation concurrent with ERK1/2-PP1-mediated dephosphorylation of eIF2B that increases its GEF activity and overcomes the translational block, illustrating the role of this signaling pathway in axon guidance and branching (111). Furthermore, beyond its contribution to protein synthesis-dependent memory consolidation, PERK signaling is implicated in cellular calcium dynamic-dependent working memory, which was found to be impaired in mice with pharmacologically inhibited PERK or in brain-specific EIF2AK3, knockout mice (71).
PERK signaling is also important to a concept known as hormesis, whereby a constant state of nonlethal ER stress preconditions and provides protection of cells against further stresses (41). This is very important for cells that synthesize and secrete large amounts of proteins, such as pancreatic β cells, lymphocytes, and oligodendrocytes. Hormesis is achieved by upregulation of the PP1 phosphatase cofactor GADD34, which targets PP1 to phosphorylated eIF2α (41). A study, in which eIF2α phosphorylation levels were increased artificially by PERK activation independent of stress, demonstrated that this effect was enhanced by upregulation of GADD34, which persisted after treatment and mediated future protection (112). These beneficial roles of PERK highlight that studies elucidating the role of PERK in neurodegeneration must be cautious of its dichotomous roles.
Additional studies and reviews have proposed PERK as decisionmaker in cellular fate (2), with outcomes depending on stress levels and the interaction of PERK with various modulators (79, 80). One study, using selenite-induced PERK activation, showed that phosphorylated PERK facilitated p38 dissociation from the inhibitory Hsp90-PERK complex such that it could be activated by autophosphorylation. Additionally, phosphorylated p38 induced p53 phosphorylation and activation, which in turn induced eIF2α phosphorylation and eIF4E dephosphorylation. Thus, ATF4 association with the DDIT3, promotor was enhanced and association with the MAP1LC3B, the gene encoding LC3, promotor suppressed, thereby promoting apoptosis by attenuating autophagy (80, 113). Furthermore, autophagy and pursuant lysosomal degradation are frequently reported to be impaired in neurodegenerative diseases, which might partially explain the predominance of maladaptive outcomes during PERK activation in these conditions (57, 93, 114, 115). Conversely, a study on cancer highlighted that PERK mutants reported in the Human Cancer Genome Atlas were consistently hypomorphic and demonstrated that the EIF2AK3 levels dictated the tumor suppressive versus the oncogenic properties of PERK via pharmacological and genetical modulation (106). Thus, the opposing effects of its activity lend further support for PERK as an ideal candidate for regulating the aforementioned switch between homeostatic and proapoptotic roles.
One final aspect that is commonly overlooked in evaluating PERK and other kinases of the ISR during neurodegenerative processes is that neurons do not exist in isolation in vivo. Any insult triggering ISR in the CNS will also result in ISR activation in glial cells, which are resistant to ER stress-mediated cell death. Instead, astrocytes produce cytokines as part of an innate immune response (116); these cytokines create a neuroinflammatory environment which, over time, can lead to neuronal dysfunction both directly and by inducing ER stress in the neurons themselves. PERK signaling, specifically, has been linked to the induction of interleukin (IL)-6, C-C motif chemokine ligand (CCL)-2, and CCL-20 expression in astrocytes (116). Sustained eIF2α phosphorylation was necessary for the cytokine induction via PERK signaling, whereas ablation of the PERK signaling by genetic haploinsufficiency was shown to attenuate the expression of cytokines (117).
Protein Kinase R
Given its ability to be activated by inflammatory cytokines, ER stress, and oxidative stress in addition to the canonical viral infection, it is unsurprising that PKR activation is observed during various stages of many clinical neurodegenerative conditions. PKR’s ability to dictate cell fate and its function as a proapoptotic kinase and an inducer of downstream inflammatory cascades support a role for PKR activation in disease pathogenesis. Furthermore, besides eIF2α, PKR has several substrates implicated in neurodegeneration (25, 118, 119). For example, NF-kB activation downstream of IKK interaction results in the production of several cytokines widely believed to be pathogenic in neurodegenerative as well as apoptotic pathways.
Several studies in in vitro and animal models and patient samples suggest that PKR may play a key role in AD pathogenesis: In these various AD-related contexts, phospho-PKR (p-PKR) and p-eIF2α are elevated and correlate with cognitive decline (84, 114, 120–123). In particular, 1 study showed that extracellular Aβ led to PKR-dependent induction of neuronal apoptosis that involved eIF2α phosphorylation without UPR activation (118, 124). Additionally, PKR phosphorylation was reduced in postmortem brain tissue of patients who received Aβ immunotherapy for AD compared to untreated AD patients (125). In that study, p-PKR levels correlated with Aβ load and markers of neurodegeneration. The authors also observed a decrease in microglial activation, while what microglial activity remained correlated positively with PKR phosphorylation, suggesting a role for PKR in the induction of microglia-associated inflammatory response (125). Another study using Aβ treatment of SH-SY5Y neuroblastoma cells in vitro and in APPSLPS1 knock-in mice demonstrated increased levels of p-PKR and the proapoptotic protein FADD in parallel with caspase-3 and caspase-8 activation, all of which were blocked with a PKR inhibitor (118, 126). Relatedly, PKR activation was reported in both brain tissue and peripheral blood mononuclear cells isolated from patients with AD (119, 126); PKR inhibition in these cells decreased the production of several proinflammatory cytokines and inhibited caspase-3 activation, encouraging further investigation of the role of peripheral cells in neuropathogenesis. In multiple models, PKR was also shown to directly bind and phosphorylate α-synuclein, whereas pharmacological inhibition or silencing of PKR was shown to alleviate cellular degeneration in correlation with reduced α-synuclein phosphorylation, implicating a role of PKR activation in PD and similar synucleinopathies (127). Increased p-PKR levels were observed in hippocampal tissue specimens of patients with PD and HD, suggesting an association of PKR activation with extrastriata degeneration (128). Finally, intracytoplasmic PrP is proposed to activate PKR: p-PKR levels were reported to correlate with neuronal apoptosis, microglia activation, and markers of astrocytosis as well as disease severity in samples from patients with Creutzfeldt-Jakob disease (129).
Interestingly, EIF2AK2, was identified as one of the factors associated with neurodegeneration in the transcriptome study of HIV-infected men by Venkatachari et al (96). In another study, treatment of neuroglial cultures with the HIV-1 surface protein gp120, which can induce neuronal damage and death, resulted in increased PKR protein levels in both primary rat neurons and astrocytes, with PKR activation observed only in neurons similar to that seen in patients with HIV-associated dementia, the most severe form of HAND (130). The authors also showed that pharmacological PKR inhibition and genetic EIF2AK2 knockout were neuroprotective in the same experimental paradigm. Another study examined the role of PKR in viral infections in the context of a prion model of neurodegeneration; to that end, the authors mimicked the inflammatory response to systemic viral infections using toll-like receptor 3 to induce type 1 interferons and observed an accelerated disease progression accompanied with increased apoptosis, which correlated with EIF2AK2 and FAS transcription (131); the authors inferred that neurodegeneration sensitized the CNS to exaggerated inflammatory responses by microglia, supporting the role of inflammation in exacerbating neurodegeneration.
General Control Nonderepressible 2
GCN2 is prominently expressed in the brain and is a known regulator of synaptic plasticity, learning, and memory in the hippocampus, as demonstrated in a study where knockout of EIF2AK4 resulted in altered LTP profiles in response to electrical stimuli. These results were correlated with memory-related behavioral deficits, and, as expected, reduced ATF4 and increased CREB expression (70). In a separate study linking ribosomal stalling, which can be triggered by oxidative stress, to neurodegeneration, GCN2 activation, eIF2α phosphorylation, and induction of ATF4-regulated genes were seen as intermediate steps (132). However, in the model used in that study, which utilized mutations in translational factors to promote ribosome stalling, the GCN2-ATF4 signaling appeared to be neuroprotective. Still, given that the type of stress that induces the ISR can dictate the outcome, GCN2 is likely to play a role in chronic neurodegenerative pathologies, although studies investigating GCN2 in this context are limited.
In 1 study, amino acid deprivation-induced GCN2 activity was shown to result in the ATF4-dependent upregulation of presenilin-1, the catalytic component of γ-secretase (133). Presenilin-1 is involved in amyloid precursor protein (APP) processing, including the generation of both soluble APP and Aβ peptide from APP, implicating GCN2 activity in AD and other conditions with Aβ pathology. As GCN2 can also be activated in response to oxidative stress, GCN2-presinilin signaling may also constitute a feed-forward pathway playing a role in the pathogenesis of progressive neurodegenerative conditions. For example, a study using okadaic acid treatment to model AD in neurons demonstrated GCN2 activation (84). Additionally, 1 study utilizing mouse models of familial AD observed that the deletion of GCN2 rescued synaptic plasticity and spatial memory (91). GCN2 is also activated in response to viral infections and was previously described to interact directly with HIV, raising the possibility of its involvement in virus-related neurodegenerative conditions such as HAND.
Heme-Regulated Inhibitor
HRI is not thought to play a significant role in neuronal p-eIF2α levels given that it is primarily expressed in erythroid cells and has no known physiological roles in other cell types. Albeit severely understudied, it is clear that the roles EIF2AK1 plays in cell types that interact with neurons have potential implications in neurodegeneration. For example, knockdown of HRI in macrophages, which express HRI, was shown to result in defects in macrophage maturation and inflammatory responses to LPS (23). The same study also highlighted the role of macrophages in recycling iron from senescent red blood cells, a process inhibited by the upregulation of hepcidin under conditions of inflammation and oxidative stress. In the EIF2AK1 knockout mice, hepcidin levels were reduced in both control and LPS-treated animals, potentially due to decreased cytokine production, leading to increased serum iron levels and impairing erythropoiesis. (23). Intriguingly, dysregulated iron homeostasis has been extensively implicated in neurodegeneration (134, 135). Particularly, the disruption of iron homeostasis in microglia and its contribution to chronic inflammation mediated by microglia were reported previously (136). Whether HRI signaling plays a role in microglia akin to that reported in macrophages remains unclear.
In Schwann cells, disruption of mitochondrial activity, a common hallmark of neurodegenerative diseases, was shown to induce a maladaptive ISR (137). Specifically, HRI is exclusively activated by this noncanonical stress, which leads to the remodeling of lipid metabolism to downregulate fatty acid synthesis and upregulate β-oxidation. This results in decreases in myelin components, which likely contributes to pathological demyelination, as well as accumulation and release of acylcarnitines, which can cause axonal degeneration via disruption of the calcium homeostasis. A study on oligodendrocytes also reported that mitochondrial deficits led to changes in metabolism in the form of increased reliance on glycolysis; however, the shift was sufficient to meet the energy needs of the cells, and no demyelination or axonal degeneration was observed (138). The relevance of these studies and the need for further investigations are highlighted by the observations that energy depletion/metabolism remodeling, fatty acid oxidation, and altered calcium homeostasis are commonly observed, and are believed to play a role, in neurodegenerative conditions.
ISR Kinase Crosstalk
Importantly, studies in neurodegenerative disease often attribute the outcomes of manipulating eIF2α phosphorylation levels to the signaling events related to the specific ISR kinase that is most directly impacted within the disease model in question. However, there is a broad and continually expanding overlap among the ISR kinases beyond their canonical activation schemes, as previously highlighted. Many of these studies do not consider and/or control for contributions of the other kinases in the family. Furthermore, even for those studies that account for potential crosstalk, concerns remain regarding the specificity of many of the commonly employed tools to manipulate these kinases. For example, in a study where the inflammatory molecule 15-deoxy-Δ12,14-prostaglandin J2 was shown to activate multiple eIF2α kinases, potentially partially and not exclusively through proteasomal inhibition, the investigators attempted to identify the specific responsive kinases (139). However, they found that thapsigargin, considered to be a PERK inducer, also activated PKR and that PKR inhibitors inhibited PERK as well. In the same study, a PERK inhibitor reduced stress granules in response to sodium selenite, which was traditionally considered to only activate HRI. Additionally, it is likely that in addition to the presence of a crosstalk among the 4 ISR signaling branches, inhibition of one of the kinases can induce the ISR, which in turn stimulates another ISR kinase in a feed-forward cascade. Going forward, it will be pertinent for the field to examine whether activation of the ISR during neurodegeneration initiates in response to a specific stress, such as ER stress, oxidative stress, or inflammation, which in turn tends to drive the others, or if different diseases initiate different stresses, and/or if the various branches of the ISR are triggered concomitantly in pathology. Similarly, it will be illuminating to examine whether there are compensatory mechanisms among the ISR kinases. Furthermore, whether the order of induction of ISR kinases is dependent on disease and/or whether they are indeed activated independently in disease will shed a significant light in elucidating the complex network of ISR kinase-associated signaling.
MALADAPTIVE OUTCOMES OF eIF2α PHOSPHORYLATION
The consequences of increased ISR kinase activity have largely been focused on apoptosis as a mechanism of neurodegeneration and memory impairments due to inhibition of general protein translation. However, there are multiple novel pathways that are being explored as mechanisms downstream of ISR kinase activation, mainly PERK and PKR, that potentially contribute to axonal damage, neurodegeneration, and the consequent cognitive impairment. Amyloid and tau pathology, as well as repeat-associated non-AUG (RAN) translation and neuroinflammation, have been linked to activation and/or dysregulation of ISR kinases and altering the activity of ISR kinases appears to modulate these distinct pathological pathways. In this section, we review the evidence linking ISR kinases with downstream detrimental mechanisms observed in different neurodegenerative diseases.
Repeat-Associated Non-AUG Translation
Neurodegenerative diseases such as HD, ALS, and FTD are included in a class of disorders known as microsatellite expansion disorders, due to their association with such mutations (140–142). Specifically, hexanucleotide repeat expansions in C9ORF72 and trinucleotide repeat expansions in HTT have been extensively linked to the pathology of these diseases, and have recently been demonstrated to undergo RAN translation (143–146). RAN translation refers to translation which occurs without the involvement of the canonical AUG start codon and, intriguingly, has been demonstrated to be intertwined with ISR signaling.
RAN translation was first observed in the context of spinocerebellar ataxia type 8 (SCA8), in a study where transfection of cells with ATXN8 containing a CAG repeat expansion, which is a microsatellite mutation associated with the SCA8, resulted in the production of polyglutamine proteins, even when the ATXN8 start codon was mutated (147). The investigators also found that this noncanonical translation could initiate in all 3 reading frames, producing polyalanine and polyserine proteins in addition to the clinically observed polyglutamine (147). The existence of these alternative RAN translation products in clinical disease was validated by positive staining in the brains of SCA8 patients for polyalanine (147). Furthermore, the investigators also validated, by staining, the existence of another alternative RAN translation product, polyglutamine, in the brains of myotonic dystrophy 1 and 2 patients, and found polyglutamine staining associated with caspase 8 staining, suggesting the ability of these proteins to induce apoptosis (147). Indeed, staining of multiple RAN products in HD-affected brains colocalized with neuronal loss, microglial activation, and apoptosis, and many more studies have pointed toward to the toxicity of a continually growing list of RAN products (140, 141, 143, 148).
Since its identification, RAN translation has been determined to require hairpin formation, a common feature of nucleotide repeat expansions, and to have some degree of length dependence, while follow-up studies have further shown it to be capable of bidirectional translation, utilizing both the sense and antisense transcripts (147, 149–151). While the precise mechanisms of RAN translation remain largely ambiguous, it has been proposed to operate similarly to IRES translation, given the dependence on RNA hairpins, which may create a blockade for scanning ribosomes and facilitate noncanonical initiation in the context p-eIF2α-induced limited preinitiation complex availability (147, 152–155). Controversially, different studies have suggested both cap-dependent and cap-independent mechanisms regarding RAN translation of different expansions in different diseases. Specifically, 1 group showed RAN translation of the C9ORF72 hexanucleotide expansion associated with ALS and FTD was cap-dependent, and selectively enhanced by canonical ISR activation, utilizing a near cognate CUG start codon during p-eIF2α-dependent alteration in start codon fidelity (156). Separately, ISR activation by pharmacological induction, glutamate mediated toxicity, and optogenetically altered neuronal excitation were all shown to drive RAN translation in primary cortical neurons and ALS-patient-derived spinal motor neurons (157). Thus, RAN is a novel, specifically upregulated translation mechanism downstream of eIF2α phosphorylation, with potentially maladaptive outcomes.
However, it should be noted that while Green et al (156) made these observations across multiple cell types, using multiple methods and transfections to support the same conclusion, it also utilized linear mRNA repeat constructs, which may be processed differently than when they are in their physiological intronic context. The study further demonstrated that the produced dipeptide repeat proteins themselves also triggered the ISR and induced stress granules, which could constitute a feed-forward mechanism of toxicity in pathology (156). Similarly, another study linked RAN translation of a C9ORF72 expansion to the use of a CUG start codon and IRES-like mechanisms and described the ISR-mediated enhancement of RAN translation as well as dipeptide repeat protein-mediated induction of the ISR (158). However, that study also identified dependence on the noncanonical initiation factor eIF2A for RAN translation and noted that RAN translation persisted during the ISR-mediated decline in cap-dependent translation (158). Green et al (156) made reference to RAN-translated proteins in which no near cognate start codons have been identified, suggesting that different repeats may have distinct RAN initiation mechanisms.
In accordance, another study, utilizing HeLa cells stably expressing various translation reporters, published on cap-independent RAN translation of the C9ORF72 hexanucleotide repeat expansion from the endogenously spliced first intron, but only after its translocation to the cytoplasm (159). The study showed that this cap-independent RAN translation was also upregulated in response to ISR stressors, and was dependent on eIF2α phosphorylation, which was necessary and sufficient for the promotion of RAN translation (159). In addition, the investigators found that upon expression the prion-like domain of TDP-43 mislocalized to the cytoplasm, formed inclusions, and induced stress granule formation, eIF2α phosphorylation, and RAN translation of C9ORF72 expansions (159).
TDP-43 as well as several other RNA-binding proteins (RBPs) are frequently mutated, and commonly mislocalized and/or aggregated in ALS and FTD, including in cases involving C9ORF72 expansions; thus, this may constitute another feed-forward mechanism of disease pathogenesis (142). However, a separate study found that the expression of wild-type human disease-associated RBPs, including TDP-43, FUS, and hnRNPA2B1, resulted in their binding to and alteration of the structure of a SCA type 31-associated pentanucleotide repeat expansion (160). This interaction was shown to be direct and suppressed toxicity, specifically eye degeneration in Drosophila, by acting as an RNA chaperone, dispersing downstream, clinically observed RNA foci and diminishing RAN-mediated pentapeptide production (160). Furthermore, RNAi knockdown of endogenous TDP-43 exacerbated eye degeneration (160). On the other hand, short repeat peptides were found to ameliorate toxic TDP-43 expression, indicating that there is intricate crosstalk required for ribonucleoprotein homeostasis which can be unbalanced from either side in pathology, and suggesting a possible physiological function for such repeats (160). In summary, several aspects of the RBPs implicated in neurodegeneration, including their disordered, misfolding prone domains, their common sequestration in stress granules, and the emerging evidence of the intricate, seemingly bidirectional crosstalk between them, ISR signaling, and RAN translation, suggest that therapeutically modulating them will, in effect, target a stress signaling intersection that may beneficially combat multiple drivers of pathology simultaneously. For example, targeting RNA folding homeostasis has preliminarily demonstrated therapeutic benefit. Indeed, RNA helicase DDX3X was identified as a repressor of RAN translation in a CRISPR screen (161). Additionally, targeting repeat expansions with antisense oligonucleotides has been a promising area of therapeutic development (140, 141, 161). Furthermore, the discovery of the bidirectional capacity of RAN translation suggests that further development of such efforts to target both strands may enhance therapeutic benefit.
The pathogenesis of microsatellite expansion disorders was classically presumed to be driven by a mixture of RNA gain- and loss-of-function mechanisms, in accordance with the observation of RNA foci and aggregates in postmortem diseased tissue, as well as the toxicity of the corresponding homopolymeric expansion proteins, which are also known to be aggregated in diseased brains (140, 142). The discovery of RAN translation overturned the traditional thinking and expanded the possible players of pathogenesis to include not only proteins encoded by alternate reading frames but also those which could be produced from intronic and noncoding repeat expansions. The field of RAN translation is a fairly new and still rapidly evolving area of research with many questions still unresolved, as evidenced by the continually emerging plethora of reviews and perspectives (140–142, 148). A recent high throughput screen identified 5 diverse, bioactive small molecules which were able to dose-dependently inhibit RAN translation of repeats associated with ALS, FTD, and fragile X-associated tremor ataxia syndrome (162). Importantly, while the molecules were shown to interact with different aspects of RAN translation, the mechanisms of some were elusive, highlighting the gaps in our understanding of RAN translation (162). Furthermore, while none of the molecules drastically affected canonical translation, proving selective targeting of RAN translation to be feasible, limited investigation still suggested that these molecules were toxic to cells (162). Thus, while emerging interventions such as antibodies targeting RAN proteins are theoretically promising, significantly better understanding of the mechanisms of RAN induction and identification of toxic contributors in the context of various repeat expansions are desperately needed if RAN is to be successfully targeted for therapeutic purposes. Further, understanding the potential interplay between ISR translation attenuation and RAN translation in microsatellite expansion disorders will be critical for effective treatment.
BACE1 Upregulation and Amyloidogenesis
Amyloidogenesis is another possible downstream consequence of chronic ISR kinase dysregulation that might mechanistically contribute to neurodegeneration. The amyloidogenic pathway of AD, which results in the generation and accumulation of toxic Aβ species, is initiated by the cleavage of APP by the protease β-site APP cleaving enzyme-1 (BACE1). BACE1 protein levels are increased in clinical AD and HAND in vivo as well as in response to excitotoxic injury and ARTs in vitro (163–168). Additionally, BACE1 mediates Aβ deposition and neuronal loss in mouse models of AD, underlining its importance in neurodegeneration (169). Studies exploring the link between ISR and BACE1 in AD demonstrated that p-eIF2α levels correlated with BACE1 protein levels in the temporal and frontal cortices and with Aβ in the frontal cortex (170). Interestingly, the transcript of BACE1 was identified in a subset of mRNAs that were induced in response to pharmacological induction of eIF2α phosphorylation in both BACE1-overexpressing HEK293 cells and, more importantly, in primary neurons, underlying the possibility that inducing eIF2α phosphorylation might be sufficient for the upregulation of BACE1 protein levels in neurons (171). Furthermore, a study using ethanol treatment of the human-derived neuroblastoma cell line SK-N-MC concluded that increased p-eIF2α led to upregulation of BACE1 protein levels and Aβ secretion through the stimulation of cyclooxygenase-2 and prostaglandin 2 production (172). These studies suggest that activating the ISR might have detrimental effects via the amyloidogenic pathway. The observed increase in BACE1 levels was shown to consequently lead to a significant increase in Aβ production in primary neurons and a trend toward increased amyloidogenesis in the Tg2576 mouse model of AD. Therefore, in the presence of stress conditions, increased levels of p-eIF2α can potentially lead to Aβ production and aggregation through BACE1 upregulation. Additional studies identified PERK as the kinase mediating the BACE1 upregulation in mouse models of AD. Similarly, 5XFAD mice, which carry mutations in the APP and PSEN-1 genes and have advanced amyloid pathology, were reported to have increased levels of p-eIF2α as well as phosphorylated PERK (88, 173). Furthermore, crossing PERK haploinsufficient mice with 5XFAD mice resulted in reversal of increased BACE1 levels and amelioration of Aβ plaque burden. These findings were recapitulated in other contexts as well. For example, upregulation of BACE1 by certain antiretroviral drugs was shown to be mediated by PERK, suggesting its critical role in mediating an increase in BACE1 levels and potentially amyloidogenesis of HIV-infected individuals (167).
Other ISR kinases have also been shown to be involved in regulating BACE1 expression. Particularly, p-PKR levels were increased in AD patients, and CSF p-PKR levels correlated with cognitive decline in AD patients (120, 174–178). In 1 study, activation of PKR and consequent eIF2α phosphorylation led to derepression of BACE1 expression in herpes simplex virus type 1-infected neuron-like SH-SY5Y cells (174). Similarly, inhibition of PKR attenuated BACE1 protein levels that were increased in response to oxidative stress in SH-SY5Y cells and in LPS-tread mice (170, 179). One recent study elucidated the link between PKR and BACE1: In a mouse model utilizing thiamine deficiency to recapitulate oxidative stress in AD, activation of the PKR-p-eIF2α axis and increased levels of BACE1 and Aβ were observed in the thalamus, effects which were blocked by knocking out EIF2AK2 or treatment with a PKR inhibitor (180). In a similar manner, a study found that crossing EIF2AK2 knockout mice with the 5XFAD mouse model led to improved memory consolidation (181); Aβ accumulation and BACE1 activity were significantly decreased in the double-mutant mice, indicating that PKR modulated amyloidogenesis in vivo. HRI has also been mechanistically linked with BACE1 induction. Although few studies reported the presence of HRI in the brain, 1 study found HRI mRNA and protein in both human and mouse hippocampus; interestingly, HRI colocalized with synaptic spines (182). Further examination revealed that inhibiting HRI blocked nitric oxide-induced BACE1 upregulation in SH-SY5Y cells. These mechanisms could potentially be linked to AD pathology and other neurodegenerative diseases that are characterized by Aβ aggregates.
Although these studies suggest that ISR kinase activation and consequent p-eIF2α lead to increases in BACE1 protein levels, another study found that GCN2 deletion in 5XFAD mice further exacerbated BACE1 levels, which was due to the compensatory overactivation of the PERK pathway with a consequent increase in p-eIF2α levels (183). Additionally, as addressed previously, Aβ aggregates might directly activate 1 or more of the ISR kinases to exacerbate neurodegeneration via a positive feedback loop (184).
While the above findings highlight the ISR kinase-p-eIF2α-BACE1 pathway as an important therapeutic target, BACE1 inhibitors have unfortunately been largely disappointing in AD clinical trials (185). For example, a phase III clinical trial (EPOCH) of verubecestat, the first BACE1 inhibitor to reach late-stage clinical trials in AD patients, was recently terminated early due to observed adverse effects (186, 187). This is a common theme as inhibition of BACE1 has proven challenging and questionable in terms of safety, presumably due to mechanism-based side effects (188, 189). It is speculated that BACE1 inhibitors might be failing for a combination of reasons: inhibition of the beneficial roles of BACE1, such as important functions at the synapse (188, 190); administration too late in neuropathological progression; stabilization of BACE1 (191). Additionally, BACE2 is largely nonneuronal, but highly homologous to BACE1, and thus some of the detrimental effects of BACE1 inhibition may be accounted for by the off-target effects on BACE2, as all thus far tested molecules have not been selective between the 2 isoforms (185, 188, 192). Despite these failures, ongoing research has been aimed at resolving these issues related to BACE1 inhibition. In fact, AM-6494, a cyclopropylthiazine molecule recently discovered by structure-based design, demonstrated specificity for BACE1 over BACE2 in an in vitro assay, passed a 14-day toxicity screen in rats and dogs, and proved to be able to reduce CSF Aβ in rats and rhesus monkeys (193). Additionally, phage-display and biopanning were recently used to identify a 12-mer peptide which tightly bound BACE1, with selectivity over BACE2, prevented the cleavage of APP in in vitro assays and produced no detrimental morphological changes when administered to SHY5Y cells (194). While it remains to be seen if such competitive peptide inhibitors can be fine-tuned to target the BACE1-APP interaction over other BACE1 substrates, these recent developments highlight that there is still therapeutic potential for BACE1 inhibition.
Still, while off-target effects may explain the toxicity of previously evaluated BACE1 inhibitors, and thus allow for improvement and refinement, the failure of these drugs to provide clinical and cognitive benefit in disease contexts, even when efficaciously reducing CSF Aβ levels, highlights the need to critically examine the amyloid hypothesis and assess whether targeting amyloidogenesis is a viable therapeutic option, particularly in isolation (185, 189). For the development of BACE1-targeting drugs to be successful in clinical trials, 2 important issues should be resolved. First, a better understanding of the connection between Aβ accumulation and cognitive decline is necessary to prioritize the administration of these drugs to amenable cases. Similarly, the physiological functions of BACE1 should be elucidated further to improve the selectivity of the drugs. It is possible that inhibiting chronic ISR activation upstream of amyloidogenic processing may encounter similar obstacles as those observed with BACE1 inhibitors. Thus, inhibition of ISR to treat neurological disease should be optimized accordingly to avoid the negative consequences observed with BACE1 inhibitors.
Tau
Integrated stress response kinases and activation of the eIF2α pathway are extensively associated with a range of clinical diseases termed tauopathies, which are characterized by the presence of neurofibrillary tangles. Phosphorylation of tau, a microtubule associated protein, at 1 or more epitopes leads to its aggregation into insoluble tangles, which correlate with cognitive decline in AD (195). Evidence suggests that ISR kinases might be mediating tau pathology in tauopathies, including AD and PSP. First, both PERK and PKR are activated in clinical PSP and AD (85, 90, 92, 114, 196). Similarly, increased p-PERK expression was observed in neurons and glia in the tissue specimens of patients with frontotemporal lobar degeneration and early tau pathology in the hippocampus, while markers of UPR activation correlated with phospho-tau in AD patient tissue (92). Activation of PERK and PKR was reported to closely correlate with levels of phosphorylated tau, observed primarily in early pretangle neurons as well as in astrocytes and oligodendrocytes in AD tissue (197). In the hippocampus of AD, one of the most severely affected areas in disease, p-PERK colocalized with AT8, a marker of hyperphosphorylated tau, in neurons (198). Furthermore, a genome-wide association study reported that EIF2AK3 was a genetic risk factor for PSP (103). These findings all together suggest ISR dysregulation as an underlying mechanism of pathology and/or a consequence of aberrant tau protein aggregation in tauopathies. However, mechanistic studies provide evidence for ISR kinases as upstream mediators of tau pathology. For example, using the ER stress inducers tunicamycin and thapsigargin, which activate the UPR and PERK as a consequence, albeit by different mechanisms, several studies observed that chemically induced ER stress was sufficient to increase the phosphorylated tau levels (199–201). Pharmacological inhibition of PERK reduced the levels of phosphorylated tau and alleviated cell death in the rTg4510 mouse model of tauopathy, wherein mice harboring the P301L tau mutation exhibit pathological findings mimicking AD and FTD (202). A possible mechanism underlying this finding is alteration in the levels and/or activation of tau kinases such as cyclin-dependent kinase 5 and glycogen-synthase kinase-3 beta (GSK-3β). Several studies found that ER stress as well as specific PERK activation induced GSK-3β activity (115, 198, 203, 204). Activation of tau kinases leads to tau hyperphosphorylation, which affects microtubule-binding properties and facilitates aggregation; tau aggregation might also be mediated by p-eIF2α, which was found to be elevated and colocalized with phosphorylated tau in the brains of patients with sporadic AD (114). Several studies implicate PKR in tau pathology as well. Specifically, inhibiting PKR either pharmacologically or via siRNA inhibited tunicamycin-induced tau phosphorylation in SH-SY5Y cells, suggesting that PKR might also be upstream of tau pathology in the context of disease (198).
As mentioned previously, PERK haplotype B is associated with AD and PSP, 2 neurodegenerative diseases characterized by neurofibrillary tangles composed of tau proteins. PERK haplotype B was found to be associated with increased PERK activation in the brains of PSP cases, suggesting that PERK coding variants associated with haplotype B lead to the chronic activation of the PERK pathway and subsequent detrimental outcomes (196). This mechanism is in agreement with studies showing that PERK haplotype B has increased kinase activity and responds more robustly to ER stress compared to PERK haplotype A (97). It remains unclear exactly how PERK haplotype B and higher PERK activity may lead to increased risk of developing tauopathies. Possibly, patients homozygous for PERK haplotype B have increased kinase activity, enhanced chronic ISR response, and consequently increased tau kinase activation. In turn, this may lead to increased tau phosphorylation and aggregation. Contradicting earlier studies, Yuan et al (99) found that PERK haplotype B was a hypomorph in neurons and that neurons derived from induced pluripotent stem cells from PERK haplotype B PSP patients exhibited increased vulnerability to ER stress. The study also showed that induced pluripotent stem cell-derived neurons from PERK haplotype B PSP patients had higher amounts of AT8 and PHF-1 phosphoepitopes in response to tunicamycin, suggesting that decreased PERK activity due to the PERK haplotype B variants led to increased tau pathology. However, the study did not normalize the levels of hyperphosphorylated tau to levels of total tau, which was also increased after tunicamycin treatment in PERK haplotype B PSP neurons when compared to control neurons. Therefore, it is still necessary to accurately dissect the effect of PERK coding variants on PERK activity as well as their downstream consequences in neurons as well as glia.
In contrast to reports suggesting that PERK dysregulation exacerbates tau aggregation and that alleviating PERK activation might be beneficial in tau pathology, 1 study demonstrated that pharmacological PERK activation or PERK overexpression led to a reduction in hyperphosphorylated tau levels in cultured human neurons expressing wild-type 4R tau in vitro and rescued behavioral deficits and neurodegeneration in the P301S transgenic mouse model of tauopathy in vivo (205). One explanation for this discrepancy is differential activation of downstream targets of PERK, wherein the beneficial effects of PERK activation in mitigating tau pathology might be occurring through Nrf2 rather than eIF2α phosphorylation. There is evidence supporting a beneficial role for Nrf2 in tau pathology, which involves the induction of autophagy for clearance of insoluble tau aggregates in mouse models of tauopathy (206). Nrf2 activation by PERK is independent of eIF2α phosphorylation (45) indicating that the link between PERK and tau pathology might be dependent on the pathway that is activated. Although PERK and PKR were studied to elucidate tau pathology, the relationship of tau pathology with the other 2 ISR kinases, GCN2 and HRI, has yet to be studied. While whether augmented eIF2α phosphorylation plays a direct and key role in tau pathology remains unclear, evidence suggests that the dysregulation in eIF2α phosphorylation may be an underlying pathological mechanism.
Other Protein Aggregates
Similar to tau and Aβ aggregation, ISR kinases have also been assigned important roles in mediating α-synuclein pathology in PD (207). Tunicamycin treatment of the human neuroblastoma cell line BE2-M17D overexpressing wild-type human α-synuclein was shown to increase α-synuclein oligomers compared to untreated cells. Whether this effect occurs via the activation of PERK and induction of p-eIF2α remains to be elucidated. However, these results as well as the accumulating literature illustrating the role of ISR kinases in mediating multiple pathways of protein aggregation suggest that p-eIF2α and/or kinases that lead to its generation might be contributing to α-synuclein oligomerization and aggregation as well.
Neuroinflammation
Induction of inflammation in the CNS is increasingly recognized as an important component of neurodegenerative diseases, particularly during the chronic stage, as inflammatory markers have been extensively observed in the brains of diseased patients (56, 58). Several stress response pathways have been linked with neuroinflammatory processes, raising the possibility that ISR kinases might play contributory roles as mediators of inflammation. For example, in LPS-administered mice, genetic EIF2AK2 downregulation blocked neuroinflammation as observed by normalization in the levels of increased Iba-1, a marker of microgliosis, as well as a normalization tumor necrosis factor (TNF)-α and IL-6 levels (179). Other proinflammatory cytokines were also found to be modulated by PKR. Intraperitoneal injection of C16, a pharmacological PKR inhibitor, prevented quinolinic acid-induced increase in IL-1β levels in rats (208). In another study, EIF2AK2 knockout in 5XFAD mice attenuated Iba1 immunoreactivity in the hippocampus, whereas the levels of IL‐1β, TNF-α, interferon-γ, and IL‐6 returned to the levels measured in wild-type mice (181). The authors further investigated whether the effect of PKR was via neurons or microglia using mixed neuronal and microglial cocultures from wild‐type and EIF2AK2 knockout mice. Upon LPS treatment, cytokine release was attenuated in cocultures where PKR was knocked out only in the microglia when compared to wild-type. However, the effect was completely abolished only when PKR was knocked out in both cell types, suggesting PKR-mediated cytokine release involves both neurons and microglia.
Although PKR is the main ISR kinase known to mediate inflammatory responses, several studies also examined the role of PERK in cytokine expression. Particularly, genetic as well as pharmacologic inhibition of PERK decreased the expression of CCL-20 and the secretion of IL-6 and CCL-2 from astrocytes under ER stress (117).
Paradoxically, other studies demonstrated that ISR downregulation augmented neuroinflammation and that ISR activation ameliorated neuroinflammation. Specifically, knocking out EIF2AK3, in experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis (MS), led to exacerbation of inflammation as determined by an increase in the number CD3-positive T cells and CD11b-positive macrophages/microglia in the lumbar spinal cord (209). Additionally, neuron-specific EIF2AK3 knockout augmented axon degeneration and impaired clinical recovery in this model. The authors suggested that the potential mechanism underlying the exacerbated inflammation associated with EAE in EIF2AK3 knockout mice was not mediated by the peripheral immune cells but rather arose via the facilitation of tissue damage in the CNS, mainly axonal degeneration. In a model of intracerebral hemorrhage which included increased p-eIF2α levels, treatment with recombinant GCN2 was associated with decreases in the levels of IL-1β and TNF-α, inhibition of neutrophil infiltration, and improvement in neurological function (210). These beneficial effects of GCN2 were abolished by blocking eIF2α, indicating that this process was dependent on eIF2α. Furthermore, in a mouse model, GCN2-deficient regulatory T cells displayed impaired migration in the presence of a CCL2 gradient, suggesting that the role of GCN2 in mediating neuroinflammation might occur via peripheral immune cells (211). No studies to date have addressed the role of HRI in neuroinflammation. Nonetheless, studies increasingly ascribe neuroprotective roles for the other ISR kinases in neurodegenerative diseases with an inflammatory component such as MS. Importantly, these proposed roles also implicate that, in addition to their functional roles in neurons, ISR kinases may also play key roles in nonneuronal cells via the modulation of inflammatory processes that are persistent in neurological disease.
PHARMACOLOGICAL INTERVENTIONS FOR MODULATING eIF2α PHOSPHORYLATION
Given the overwhelming evidence for dysregulation of eIF2α phosphorylation as a common mechanism in several diseases, substantive efforts have been spent to identify and develop pharmacological compounds that target different components of this pathway. In this section, we review the inhibitors and activators of ISR kinases as well as other compounds that impact downstream targets (Fig. 2). The evidence thus far strongly implicates ISR signaling as an important mediator of neurodegeneration. However, whether activating or inhibiting interventions are more beneficial remains a slightly ambiguous question of stress context.
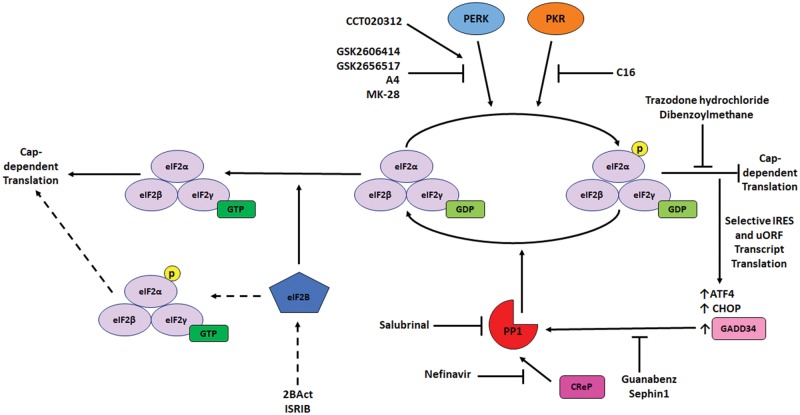
Figure 2.
Pharmacological interventions targeting integrated stress response (ISR) signaling. The ISR can be pharmacologically targeted and modulated at many levels. Specific inhibitors of 2 of the branch kinases, protein kinase R (PKR), PKR-like endoplasmic reticulum (ER) kinase (PERK) have been developed to reduce eIF2α phosphorylation and prevent downstream ISR signaling. On the other hand, inhibitors of protein phosphatase 1 (PP1) and the cofactors which direct it to p-eIF2α under different conditions have been developed to raise p-eIF2α levels and prolong ISR signaling. On a more encompassing level, activators of eIF2B have been developed to allow it to interact with eIF2 even when phosphorylated, thus removing the translational block from ISR signaling. Additional repurposed drugs have also been shown to alleviate the translational block cause by eIF2 phosphorylation without dephosphorylation; however, the mechanism of this reversal is not known, whether through eIF2B or otherwise.
PERK Inhibitors
All ISR kinases converge on eIF2α phosphorylation, which, as elucidated above, is considered a central hub that determines cell-fate outcomes in conditions of neurodegeneration. However, as the most extensively studied ISR kinase in efforts to mitigate neurodegenerative diseases, PERK has been the main target for the development of pharmacological inhibitors aimed at suppressing the ISR (212). GSK2606414, a specific PERK inhibitor, is a highly selective and potent compound that can effectively cross the blood-brain barrier and thus was used as the main pharmacological agent to assess the role of PERK in neurodegeneration (213). A year later, the same group reported the optimized PERK inhibitor GSK2656157 (214). The first study investigated the utility of GSK2606414 in prion-infected mice, which exhibit sustained eIF2α phosphorylation and neurodegeneration in response to misfolded prion proteins (60). In this prion model, pharmacological PERK inhibition using GSK2606414 at early disease stage rescued prion-induced neurodegeneration and reversed the behavioral abnormalities determined by the novel object recognition test and burrowing activity (94). Similarly, GSK2606414-mediated PERK inhibition prevented substantia nigra degeneration in a toxin-induced PD model in mice (215). Moreover, the PERK/eIF2α inhibition rescued the synthesis of critical synaptic proteins which allowed neuronal recovery. Furthermore, GSK2606414 led to decreased levels of hyperphosphorylated tau and neurotoxicity in the rTg4510 mouse model of tauopathy, underlining the significance of PERK in mediating downstream protein aggregation (202). GSK2606414 has also been shown to reverse RAN translation in sodium arsenite- and MG132-treated cells, as well as in cells exposed to an excitotoxic glutamate insult (157, 159). In another study, intracerebroventricular injection of GSK2646157, the optimized inhibitor, reduced inflammatory and apoptotic factors associated with early brain injury after acute subarachnoid hemorrhage model in rats, as well as improved prognosis (216). This study did not show improvements in mortality or neurological deficits, however, perhaps because of the acute nature of the stress. Therefore, PERK inhibition can rescue neurodegenerative phenotypes in various disease models and does not appear specific to 1 type of neuropathological hallmark or neurodegenerative disease, suggesting that controlling PERK activity might be beneficial for a wide range of diseases.
However, in all the aforementioned studies, mice treated with GSK2606414 exhibited mild hyperglycemia and reduced body weight. Given the major role of PERK as a regulator of glucose homeostasis in the pancreas, effective systemic PERK inhibition might be associated with significant blood glucose dysregulation. Although GSK2606414 was reported to be specific for PERK and was shown to be effective in ameliorating neurodegeneration in several disease models, the associated alterations in glucose metabolism precludes its evaluation in humans and highlights the complexities of identifying CNS-PERK inhibitors without peripheral adverse effects. Intriguingly, MK-28, a derivative of an older PERK inhibitor molecule, A4, which was developed based on structural determinants was recently shown to have low pancreatic cell toxicity in mouse pancreatic cells and whole animals, to have no effect on blood glucose in wild-type mice, and to decrease elevated blood glucose found in the R2/6 transgenic mouse model of HD (217, 218). The study also demonstrated that MK-28 reduced cytotoxicity in a cellular HD model, and improved cognitive and motor behaviors as well as survival in a mouse model (218).
PKR Inhibitors
In a screen aimed to identify specific inhibitors of PKR, from 26 ATP-binding site-directed inhibitors an oxindole/imidazole compound termed C16 was confirmed to inhibit PKR autophosphorylation, which is necessary for its activation; furthermore, C16 treatment rescued the PKR-induced translational block in rabbit reticulocytes (219). C16 has been shown to be effective in inhibiting PKR-induced toxicity in the SH-SY5Y neuronal cell line, both when mediated by treatment with tunicamycin or β-amyloid peptide (126, 220). The beneficial effects in response to a traditional ER stressor, tunicamycin, as compared to how PERK inhibitors sensitize cells to ER-stress-mediated death, highlight PKR’s more traditional apoptotic roles. Also, in contrast to PERK inhibitors, acute systematic administration of C16 in rats encouragingly inhibited apoptotic PKR signaling without simulating proliferative mTOR signaling (221). As mentioned in one of the previously discussed studies, Couturier et al (119) showed that C16 reduced levels of caspase 3 and inflammatory cytokines such as TNF-α, IL-1α, IL-1β, and IL-6 in peripheral blood mononuclear cells with activated PKR, isolated from AD patients. Also mentioned previously, the study by Tronel et al (208) showed that PKR inhibition using C16 attenuated neuroinflammation in an excitotoxic neuroinflammatory model where rats were treated with quinolinic acid. In that study, the authors demonstrated that C16, administered intraperitoneally 24 and 2 hours before as well as 24 hours after quinolinic acid injection, crossed the blood-brain barrier and decreased quinolinic acid-mediated neuronal loss. There were no reported deleterious side effects of this C16 treatment, although this was not a chronic treatment paradigm, and it remains possible that longer treatment periods with PKR inhibitors might recapitulate the adverse effects observed PERK inhibitors. Therefore, it remains uncertain whether long-term systemic treatment with ISR kinase inhibitors such as C16 might have systemic adverse effects.
Salubrinal
Salubrinal is a small molecule identified during a screen for compounds that protected cells from ER stress-mediated apoptosis (222). Salubrinal inhibits eIF2α dephosphorylation by PP1, regardless of whether it is mediated by either of its specificity cofactors: the constitutively expressed CReP or by ER stress-induced GADD34. This inhibition is sufficient to induce elevated eIF2α phosphorylation and upregulation of downstream genes even in the absence of stress (222).
Several studies to date demonstrated the efficacy of salubrinal in ER stress and neurodegeneration: For example, in a study using SH-S5Y5 neuronal cells treated with rotenone, which induces ER stress and reduces the levels of parkin protein, to recapitulate PD, salubrinal treatment led to an increase in ATF4 expression and a reduction in cell death (223). Salubrinal also attenuated disease in a mutant α-synuclein transgenic mouse model of PD; however, in that model, the disease onset was correlated with the induction of an atypical UPR that resulted in chaperone upregulation in the absence of eIF2α phosphorylation (75). In models of ALS, specifically Caenorhabditis elegans and zebrafish expressing mutant TARDBP, encoding mutant TDP-43, salubrinal treatment caused reduced oxidative stress, neurodegeneration, and paralysis (224). Additionally, in cultured rat neurons and mouse microglia treated with Aβ peptide to model AD, salubrinal attenuated both neuronal death and microglial activation (225). Of note, the benefits of salubrinal were attributed not to the alleviation of ER stress but inhibition of both IKK and subsequent NF-kB activation. In a study investigating mild traumatic brain injury (TBI) in a mouse model, salubrinal rescued memory deficits as well as neuronal integrity after injury; however, the mice also exhibited reduced eIF2α phosphorylation after injury (226). Another study using blast-induced injury in rats demonstrated that salubrinal was neuroprotective in TBI by reducing markers of stress and ameliorating behavioral deficits (227).
Of note, most of these studies are showing salubrinal treatment to be beneficial in models which activate an atypical ISR, or in cells, using acute treatments to model disease. In these instances, eIF2α phosphorylation levels can be low, and perhaps even suboptimal for an adaptive stress response. Traditionally, unresolved ER stress signaling has been proposed as a mechanistic link between acute neurotrauma and progressive neurodegenerative disease. Thus, these data strongly suggest that salubrinal might be beneficial in acute but not chronic neurodegenerative conditions in which increased eIF2α phosphorylation is observed, as may be expected from the initial adaptive versus prolonged maladaptive phases of the ISR. Indeed, salubrinal treatment of prion-infected mice 8 weeks postinfection led to exacerbated disease progression, including increased neurotoxicity and decreased survival (94). Intriguingly, the authors noted the absence of GADD34 upregulation during disease progression despite chronic ER stress, which might at least partially explain the neurodegenerative phenotype that was enhanced by salubrinal.
A related molecule, sephin1, which inhibits the PP1 cofactor GADD34, was shown to prolong disease onset in mice with EAE, modeling MS, and correlated with increased eIF2α phosphorylation and myelination (228). However, eIF2α was only increased at earlier timepoints that were assessed; at late-stage disease, sephin1 led to a significant increase in inflammation. Another GADD34 inhibitor, guanabenz, was demonstrated to have both beneficial and detrimental roles in different models of ALS (224, 229, 230) and was shown to rescue neuronal toxicity in models of SCA and PD (231, 232). Additionally, nelfinavir, an antiretroviral drug that inhibits the HIV protease, inhibited constitutive eIF2α dephosphorylation via downregulation of the PP1 cofactor CReP (233). This aligns with the theory that persistence of HAND in patients on ART may be partially due to neurotoxicity of the ART drugs themselves over time and with aging (234, 235).
In primary neurons, basal eIF2α phosphorylation was found to increase the antioxidant capacity downstream of ATF4 induction in neurons and, in following, salubrinal treatment led to resistance to oxidative glutamate toxicity (236). However, persistently high levels of p-eIF2α are evidently toxic. Studies utilizing salubrinal capture the complexity of this dichotomy and illustrate that while the outcome might be dependent on the type and duration of stress, there remains ambiguity concerning the determinants of the switch between adaptive and maladaptive outcomes.
PERK Activator
CCT020312, also known as PERK activator (PA), was discovered in a cancer study screen to identify small molecules that activate the G1/S cell cycle checkpoint (237). In that study, PA was determined to act through PERK-mediated p-eIF2α, with the pursuant translational block triggering cell cycle arrest in G1. Intriguingly the observed PERK activation happened independent of ER stress and without activation of the other UPR branches, demonstrating its PERK selectivity (237). In that context, induction of apoptosis by PERK activation was beneficial, facilitating proliferation control and sensitizing cells to chemotherapy-associated stress. This initial study falls in line with the overall hypothesis that extensive eIF2α phosphorylation and PERK activation, particularly in the absence of activation of the other arms of the UPR, are cytotoxic. However, in another study, mentioned above, PA was used to mitigate tau pathology both in vitro and in vitro (205). Of note, this study also reported downregulation of both total and phosphorylated eIF2α protein levels in the cortex of PSP patients, in comparison to healthy controls, while both PERK and NRF2 were upregulated in both their total and phosphorylated forms (205). As discussed above, the benefit of PERK activation in this case seems to stem from upregulation of adaptive ISR signals distinct from eIF2α phosphorylation.
Even with these caveats, successful studies utilizing salubrinal and PA to ameliorate neurodegenerative phenotypes demonstrate that activation of the ISR can be beneficial in the right context. Going forward, targeting/upregulating the adaptive ISR is of therapeutic interest. PA appears to achieve this goal, but the mechanism is unknown, not to mention unvalidated in the context of elevated eIF2α phosphorylation. The small molecule discussed next, integrated stress response inhibitor (ISRIB), is another potential example. Thus far lacking nonsmall molecule modalities, such as CRISPR-mediated adaptive upregulation, may lead to more fruitful therapeutic interventions enhancing adaptive signaling rather than inhibiting maladaptive.
Integrated Stress Response Inhibitor
Pathological implications of the substantial overlaps among the ISR kinase pathways in neurodegenerative conditions are the direct effects of eIF2α phosphorylation. A small molecule, ISRIB, which was discovered in a cell-based screen for PERK inhibitors, was found to increase cellular resistance to eIF2α phosphorylation without a decrease in PERK phosphorylation (238). Sidrauski et al also determined the trans-ISRIB isoform to be about more than a 100-fold more effective than cis-ISRIB, and as such the trans isoform has been used since. ISRIB interacts with eIF2B, allowing GEF activity even in the presence of p-eIF2α (239). Specifically, cryoelectron microscopy showed that ISRIB bound the β and δ subunits of the core regulatory complex, stabilizing a rate-limiting intermediate in the assembly of the decamer, which is the active form (240, 241). The binding of ISRIB to eIF2B not only prevents but also reverses the formation of stress granules, which are a common feature of neurodegenerative conditions, following ER stress (242). Additionally, ISRIB was shown to reverse RAN translation in sodium arsenite- and MG132-treated cells (159). Cells treated with ISRIB were sensitized to PERK-driven ER stress; however, ISRIB-treated mice exhibited enhanced spatial and fear-associated learning, in line with the role of translation in memory consolidation (238). Thus, ISRIB highlights both the beneficial and malignant aspects of eIF2α signaling and demonstrates the ability to target the latter.
Indeed, follow-up studies using ISRIB demonstrated its beneficial effects in various models of neurodegeneration. Specifically, in prion-infected mice ISRIB treatment partially restored translation rates and reduced ATF4 levels, resulting in neuroprotection and increased survival without toxicity to ER stress-prone pancreatic cells (243). The same study also demonstrated that ISRIB was able to cross the blood-brain barrier, illustrating its potential as a therapeutic agent. Another study, investigating TBI modeled in mice, showed that ISRIB reversed cognitive deficits, even when administrated weeks after injury, and that these effects persisted even after the termination of treatment (244). Among studies investigating the maladaptive outcomes of eIF2α phosphorylation, Briggs et al (245) found that ISRIB treatment of the PS19 mice expressing the mutant P301S tau led to the augmentation of the tau pathology, as evidenced by increased AT8 levels and phosphorylated tau-positive inclusions, albeit a modest restoration of spatial acquisition deficits reported in other studies. Additionally, in cells overexpressing APP as a model of AD, ISRIB was able to ameliorate cell death in a manner independent of Aβ production (246). Overall, these data suggest that ISRIB might be beneficial by rescuing the translational block induced by p-eIF2α rather than by a direct effect on amyloidogenesis or tau pathology. Of note, ISRIB was also shown to alleviate the neuronal toxicity of elvitegravir, an antiretroviral drug proposed to play a role in the pathogenesis of HAND, providing additional neurodegenerative conditions where it might have utility as an adjunctive therapy (247).
Intriguingly, a recent study examining the effects of ISRIB during viral infection found that ISRIB was able to restore translation only during early infection when eIF2α phosphorylation levels were low, and that its effect was overcome above a critical threshold, independent of the trigger (248). Critically, the authors suggested that infection represented an acute stress condition where the beneficial outcomes of ISR activation are predominant, in comparison to neurodegeneration, in which chronic low-level stress facilitates predominance of the maladaptive ISR. Thus, the suggested eIF2α phosphorylation threshold may act to limit ISRIB activity to a window of chronic stress that could account for its lack of overt toxicity in vivo. In following, 2BAct, a recently developed derivative of ISRIB with increased solubility and pharmacokinetics, was also shown to restore translation under chronic ER stress conditions and sustained eIF2α phosphorylation (249). In a mouse model of vanishing white matter disease, which arises from mutations in EIF2B1, encoding mutant that diminish its activity, treatment with 2BAct or ISRIB prevented myelin loss, reactive gliosis, and motor deficits (250). These accumulating data not only emphasize the central role of eIF2α phosphorylation in a myriad of neurodegenerative conditions affecting distinct cells in the CNS but also raise hope for treatment strategies that can be utilized to alleviate or resolve damage in these diseases with complex pathological mechanisms.
Repurposed Compounds Targeting eIF2α Phosphorylation
Due to the pancreatic toxicity of common PERK inhibitors, Halliday et al examined the efficacy of other compounds for their inhibitory potential in the eIF2α pathway to identify potential therapeutic compounds. In a screen of 1040 FDA-approved drugs, the authors assessed the candidates based on their ability to overcome the tunicamycin-induced development block in C. elegans as well as their potency in inhibiting CHOP expression. They also examined the efficacy in attenuating neurodegeneration and neurological deficits in prion-infected mice and the rTg4510 mouse model of tauopathy, in the absence of pancreatic toxicity (83). In that study, the authors identified 2 drugs, trazodone, an antidepressant, and dibenzoylmethane, a curcumin analog with anticancer properties, that were effective in inhibiting the PERK/eIF2α signaling. Both compounds were able to rescue neurodegeneration and increase survival in the mouse models that were tested. They were also shown to mitigate MG132, sodium arsenite, and glutamate-induced RAN translation (157). Thus, these 2 molecules have been identified as new potential therapeutic agents in mediating dysregulation of p-eIF2α in disease without the adverse side effects of previously developed compounds targeting eIF2α phosphorylation.
Conclusions and Perspectives
While the ISR is a canonically adaptive signaling cascade, chronic ISR activation and dysregulated p-eIF2α can contribute to neurodegenerative phenotypes via canonical apoptotic induction as well as translation-dependent deficits that result in neuronal dysfunction. Given this sensitive dichotomy, and the prevalence of genetic implications of the ISR in neurodegeneration, targeting the ISR kinases and their downstream signaling components could make for fruitful pharmacogenetic studies. Furthermore, extensive compelling evidence suggests the downstream induction of, or feed-forward contribution to, amyloidogenesis, tau pathology, and neuroinflammation as major mechanistic drivers of neurodegeneration. Still, while the myriad pathways involved in eIF2α phosphorylation make compelling targets for pharmacological intervention, supported by promising studies, the homeostatic activities of these targets and signaling pathways must be considered and adjusted for when they are being manipulated, in order to better infer their implications for therapeutic development.
As the field moves forward, efforts should be made to elucidate the roles and contributions of the lesser-studied ISR kinases to neurodegeneration. In this vein, it is imperative to consider the crosstalk and/or compensatory upregulation of the ISR kinases that might exist under canonical activation or inhibition of the others. Finally, the intersection of these signals across different cell types is highly likely to play a role in disease pathogenesis and warrants extensive further attention.
Sarah Bond and Claudia Lopez-Lloreda contributed equally to this work.
This study was supported by NIH NIMH R01 MH109382 (role of PERK haplotypes in HIV-associated neurocognitive disorder); NIH NIMH R01 MH098742 (oligodendrocyte damage and dysfunction in HIV-associated neurocognitive disorder); NIH NIGMS T32GM008275 (structural biology & molecular biophysics training program); and NIH NIGMS T32 GM008076 (Predoctoral Training Grant in Pharmacology).
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. Jin K, Simpkins JW, Ji X, et al. The critical need to promote research of aging and aging-related diseases to improve health and longevity of the elderly population. Aging Dis 2015;6:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hetz C, Saxena S.. ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 2017;13:477–91 [DOI] [PubMed] [Google Scholar]
- 3. Pakos-Zebrucka K, Koryga I, Mnich K, et al. The integrated stress response. EMBO Rep 2016;17:1374–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Donnelly N, Gorman AM, Gupta S, et al. The eIF2alpha kinases: Their structures and functions. Cell Mol Life Sci 2013;70:3493–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wek RC, Cavener DR.. Translational control and the unfolded protein response. Antioxid Redox Signal 2007;9:2357–71 [DOI] [PubMed] [Google Scholar]
- 6. Harding HP, Zhang Y, Ron D.. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999;397:271–4 [DOI] [PubMed] [Google Scholar]
- 7. Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev Biol 2005;16:3–12 [DOI] [PubMed] [Google Scholar]
- 8. Balvay L, Lopez Lastra M, Sargueil B, et al. Translational control of retroviruses. Nat Rev Microbiol 2007;5:128–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pavitt GD. eIF2B, a mediator of general and gene-specific translational control. Biochem Soc Trans 2005;33:1487–92 [DOI] [PubMed] [Google Scholar]
- 10. Jennings MD, Zhou Y, Mohammad-Qureshi SS, et al. eIF2B promotes eIF5 dissociation from eIF2*GDP to facilitate guanine nucleotide exchange for translation initiation. Genes Dev 2013;27:2696–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gebauer F, Hentze MW.. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol 2004;5:827–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ameri K, Harris AL.. Activating transcription factor 4. Int J Biochem Cell Biol 2008;40:14–21 [DOI] [PubMed] [Google Scholar]
- 13. Holcik M, Sonenberg N.. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 2005;6:318–27 [DOI] [PubMed] [Google Scholar]
- 14. Clemens MJ. Initiation factor eIF2 alpha phosphorylation in stress responses and apoptosis. Prog Mol Subcell Biol 2001;27:57–89 [DOI] [PubMed] [Google Scholar]
- 15. Hinnebusch AG. Evidence for translational regulation of the activator of general amino acid control in yeast. Proc Natl Acad Sci U S A 1984;81:6442–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wek RC, Jiang HY, Anthony TG.. Coping with stress: EIF2 kinases and translational control. Biochem Soc Trans 2006;34:7–11 [DOI] [PubMed] [Google Scholar]
- 17. Wethmar K, Smink JJ, Leutz A.. Upstream open reading frames: Molecular switches in (patho)physiology. Bioessays 2010;32:885–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bauer BN, Rafie-Kolpin M, Lu L, et al. Multiple autophosphorylation is essential for the formation of the active and stable homodimer of heme-regulated eIF2alpha kinase. Biochemistry 2001;40:11543–51 [DOI] [PubMed] [Google Scholar]
- 19. Levin D, Ranu RS, Ernst V, et al. Regulation of protein synthesis in reticulocyte lysates: Phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A 1976;73:3112–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen JJ. Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: Relevance to anemias. Blood 2007;109:2693–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han AP, Yu C, Lu L, et al. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J 2001;20:6909–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han AP, Fleming MD, Chen JJ.. Heme-regulated eIF2alpha kinase modifies the phenotypic severity of murine models of erythropoietic protoporphyria and beta-thalassemia. J Clin Invest 2005;115:1562–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu S, Suragani RN, Wang F, et al. The function of heme-regulated eIF2alpha kinase in murine iron homeostasis and macrophage maturation. J Clin Invest 2007;117:3296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Metz DH, Esteban M.. Interferon inhibits viral protein synthesis in L cells infected with vaccinia virus. Nature 1972;238:385–8 [DOI] [PubMed] [Google Scholar]
- 25. Garcia MA, Gil J, Ventoso I, et al. Impact of protein kinase PKR in cell biology: From antiviral to antiproliferative action. Microbiol Mol Biol Rev 2006;70:1032–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friedman RM, Metz DH, Esteban RM, et al. Mechanism of interferon action: Inhibition of viral messenger ribonucleic acid translation in L-cell extracts. J Virol 1972;10:1184–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakayama Y, Plisch EH, Sullivan J, et al. Role of PKR and Type I IFNs in viral control during primary and secondary infection. PLoS Pathog 2010;6:e1000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saunders LR, Barber GN.. The dsRNA binding protein family: Critical roles, diverse cellular functions. FASEB J 2003;17:961–83 [DOI] [PubMed] [Google Scholar]
- 29. Patel CV, Handy I, Goldsmith T, et al. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J Biol Chem 2000;275:37993–8 [DOI] [PubMed] [Google Scholar]
- 30. Yoon CH, Lee ES, Lim DS, et al. PKR, a p53 target gene, plays a crucial role in the tumor-suppressor function of p53. Proc Natl Acad Sci U S A 2009;106:7852–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bennett RL, Pan Y, Christian J, et al. The RAX/PACT-PKR stress response pathway promotes p53 sumoylation and activation, leading to G(1) arrest. Cell Cycle 2012;11:407–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang YL, Reis LF, Pavlovic J, et al. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J 1995;14:6095–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robert F, Williams C, Yan Y, et al. Blocking UV-induced eIF2alpha phosphorylation with small molecule inhibitors of GCN2. Chem Biol Drug Des 2009;74:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deng J, Harding HP, Raught B, et al. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr Biol 2002;12:1279–86 [DOI] [PubMed] [Google Scholar]
- 35. Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 2003;11:619–33 [DOI] [PubMed] [Google Scholar]
- 36. Berlanga JJ, Ventoso I, Harding HP, et al. Antiviral effect of the mammalian translation initiation factor 2alpha kinase GCN2 against RNA viruses. EMBO J 2006;25:1730–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. del Pino J, Jimenez JL, Ventoso I, et al. GCN2 has inhibitory effect on human immunodeficiency virus-1 protein synthesis and is cleaved upon viral infection. PLoS One 2012;7:e47272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noack J, Brambilla Pisoni G, Molinari M.. Proteostasis: Bad news and good news from the endoplasmic reticulum. Swiss Med Wkly 2014;144:w14001. [DOI] [PubMed] [Google Scholar]
- 39. Ron D, Walter P.. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007;8:519–29 [DOI] [PubMed] [Google Scholar]
- 40. Walter P, Ron D.. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011;334:1081–6 [DOI] [PubMed] [Google Scholar]
- 41. Hetz C, Mollereau B.. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 2014;15:233–49 [DOI] [PubMed] [Google Scholar]
- 42. Marciniak SJ, Ron D.. Endoplasmic reticulum stress signaling in disease. Physiol Rev 2006;86:1133–49 [DOI] [PubMed] [Google Scholar]
- 43. Delepine M, Nicolino M, Barrett T, et al. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet 2000;25:406–9 [DOI] [PubMed] [Google Scholar]
- 44. Senee V, Vattem KM, Delepine M, et al. Wolcott-Rallison Syndrome: Clinical, genetic, and functional study of EIF2AK3 mutations and suggestion of genetic heterogeneity. Diabetes 2004;53:1876–83 [DOI] [PubMed] [Google Scholar]
- 45. Cullinan SB, Zhang D, Hannink M, et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 2003;23:7198–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nguyen T, Nioi P, Pickett CB.. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 2009;284:13291–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cullinan SB, Diehl JA.. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem 2004;279:20108–17 [DOI] [PubMed] [Google Scholar]
- 48. Cullinan SB, Diehl JA.. Coordination of ER and oxidative stress signaling: The PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol 2006;38:317–32 [DOI] [PubMed] [Google Scholar]
- 49. Urra H, Dufey E, Lisbona F, et al. When ER stress reaches a dead end. Biochim Biophys Acta 2013;1833:3507–17 [DOI] [PubMed] [Google Scholar]
- 50. Tabas I, Ron D.. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 2011;13:184–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Herbert TP. PERK in the life and death of the pancreatic beta-cell. Biochem Soc Trans 2007;35:1205–7 [DOI] [PubMed] [Google Scholar]
- 52. Shore GC, Papa FR, Oakes SA.. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol 2011;23:143–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Y, Guo Y, Tang J, et al. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 2014;46:629–40 [DOI] [PubMed] [Google Scholar]
- 54. Bankston AN, Mandler MD, Feng Y.. Oligodendroglia and neurotrophic factors in neurodegeneration. Neurosci Bull 2013;29:216–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bonda DJ, Bajic VP, Spremo-Potparevic B, et al. Review: Cell cycle aberrations and neurodegeneration. Neuropathol Appl Neurobiol 2010;36:157–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen WW, Zhang X, Huang WJ.. Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Rep 2016;13:3391–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dasuri K, Zhang L, Keller JN.. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic Biol Med 2013;62:170–85 [DOI] [PubMed] [Google Scholar]
- 58. Kempuraj D, Thangavel R, Natteru PA, et al. Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine 2016;1:1003. [PMC free article] [PubMed] [Google Scholar]
- 59. Scheper W, Hoozemans JJ.. The unfolded protein response in neurodegenerative diseases: A neuropathological perspective. Acta Neuropathol 2015;130:315–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moreno JA, Radford H, Peretti D, et al. Sustained translational repression by eIF2alpha-P mediates prion neurodegeneration. Nature 2012;485:507–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kedersha N, Chen S, Gilks N, et al. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell 2002;13:195–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kedersha NL, Gupta M, Li W, et al. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol 1999;147:1431–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Costa-Mattioli M, Sossin WS, Klann E, et al. Translational control of long-lasting synaptic plasticity and memory. Neuron 2009;61:10–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Klann E, Antion MD, Banko JL, et al. Synaptic plasticity and translation initiation. Learn Mem 2004;11:365–72 [DOI] [PubMed] [Google Scholar]
- 65. Klann E, Dever TE.. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci 2004;5:931–42 [DOI] [PubMed] [Google Scholar]
- 66. Davis HP, Squire LR.. Protein synthesis and memory: A review. Psychol Bull 1984;96:518–59 [PubMed] [Google Scholar]
- 67. Costa-Mattioli M, Gobert D, Stern E, et al. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 2007;129:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen A, Muzzio IA, Malleret G, et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron 2003;39:655–69 [DOI] [PubMed] [Google Scholar]
- 69. Jiang Z, Belforte JE, Lu Y, et al. eIF2alpha Phosphorylation-dependent translation in CA1 pyramidal cells impairs hippocampal memory consolidation without affecting general translation. J Neurosci 2010;30:2582–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Costa-Mattioli M, Gobert D, Harding H, et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature 2005;436:1166–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhu S, Henninger K, McGrath BC, et al. PERK regulates working memory and protein synthesis-dependent memory flexibility. PLoS One 2016;11:e0162766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Trinh MA, Kaphzan H, Wek RC, et al. Brain-specific disruption of the eIF2alpha kinase PERK decreases ATF4 expression and impairs behavioral flexibility. Cell Rep 2012;1:676–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Silva RM, Ries V, Oo TF, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem 2005;95:974–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen YY, Chen G, Fan Z, et al. GSK3beta and endoplasmic reticulum stress mediate rotenone-induced death of SK-N-MC neuroblastoma cells. Biochem Pharmacol 2008;76:128–38 [DOI] [PubMed] [Google Scholar]
- 75. Colla E, Jensen PH, Pletnikova O, et al. Accumulation of toxic alpha-synuclein oligomer within endoplasmic reticulum occurs in alpha-synucleinopathy in vivo. J Neurosci 2012;32:3301–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Atkin JD, Farg MA, Walker AK, et al. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol Dis 2008;30:400–7 [DOI] [PubMed] [Google Scholar]
- 77. Hoozemans JJ, van Haastert ES, Eikelenboom P, et al. Activation of the unfolded protein response in Parkinson’s disease. Biochem Biophys Res Commun 2007;354:707–11 [DOI] [PubMed] [Google Scholar]
- 78. Saylor D, Dickens AM, Sacktor N, et al. HIV-associated neurocognitive disorder–pathogenesis and prospects for treatment. Nat Rev Neurol 2016;12:234–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hetz C, Papa FR.. The unfolded protein response and cell fate control. Mol Cell 2018;69:169–81 [DOI] [PubMed] [Google Scholar]
- 80. Liu Z, Lv Y, Zhao N, et al. Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate. Cell Death Dis 2015;6:e1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yan W, Frank CL, Korth MJ, et al. Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci U S A 2002;99:15920–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lin JH, Li H, Zhang Y, et al. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One 2009;4:e4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Halliday M, Radford H, Zents KAM, et al. Repurposed drugs targeting eIF2ɑ-P-mediated translational repression prevent neurodegeneration in mice. Brain 2017;140:1768–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kim SM, Yoon SY, Choi JE, et al. Activation of eukaryotic initiation factor-2 alpha-kinases in okadaic acid-treated neurons. Neuroscience 2010;169:1831–9 [DOI] [PubMed] [Google Scholar]
- 85. Hoozemans JJ, van Haastert ES, Nijholt DA, et al. The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus. Am J Pathol 2009;174:1241–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gavilan MP, Pintado C, Gavilan E, et al. Dysfunction of the unfolded protein response increases neurodegeneration in aged rat hippocampus following proteasome inhibition. Aging Cell 2009;8:654–65 [DOI] [PubMed] [Google Scholar]
- 87. Salminen A, Kauppinen A, Suuronen T, et al. ER stress in Alzheimer's disease: A novel neuronal trigger for inflammation and Alzheimer's pathology. J Neuroinflammation 2009;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Devi L, Ohno M.. PERK mediates eIF2alpha phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer's disease. Neurobiol Aging 2014;35:2272–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hoozemans JJ, van Haastert ES, Nijholt DA, et al. Activation of the unfolded protein response is an early event in Alzheimer's and Parkinson's disease. Neurodegenerative Dis 2012;10:212–5 [DOI] [PubMed] [Google Scholar]
- 90. Hoozemans JJ, Veerhuis R, Van Haastert ES, et al. The unfolded protein response is activated in Alzheimer's disease. Acta Neuropathol 2005;110:165–72 [DOI] [PubMed] [Google Scholar]
- 91. Ma T, Trinh MA, Wexler AJ, et al. Suppression of eIF2alpha kinases alleviates Alzheimer's disease-related plasticity and memory deficits. Nat Neurosci 2013;16:1299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nijholt DA, van Haastert ES, Rozemuller AJ, et al. The unfolded protein response is associated with early tau pathology in the hippocampus of tauopathies. J Pathol 2012;226:693–702 [DOI] [PubMed] [Google Scholar]
- 93. Scheper W, Nijholt DA, Hoozemans JJ.. The unfolded protein response and proteostasis in Alzheimer disease: Preferential activation of autophagy by endoplasmic reticulum stress. Autophagy 2011;7:910–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Moreno JA, Halliday M, Molloy C, et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med 2013;5:206ra138. [DOI] [PubMed] [Google Scholar]
- 95. Akay C, Lindl KA, Shyam N, et al. Activation status of integrated stress response pathways in neurones and astrocytes of HIV-associated neurocognitive disorders (HAND) cortex. Neuropathol Appl Neurobiol 2012;38:175–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Venkatachari NJ, Jain S, Walker L, et al. Transcriptome analyses identify key cellular factors associated with HIV-1-associated neuropathogenesis in infected men. AIDS 2017;31:623–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu J, Hoppman N, O'Connell JR, et al. A functional haplotype in EIF2AK3, an ER stress sensor, is associated with lower bone mineral density. J Bone Miner Res 2012;27:331–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Carrara M, Prischi F, Nowak PR, et al. Crystal structures reveal transient PERK luminal domain tetramerization in endoplasmic reticulum stress signaling. EMBO J 2015;34:1589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yuan SH, Hiramatsu N, Liu Q, et al. Tauopathy-associated PERK alleles are functional hypomorphs that increase neuronal vulnerability to ER stress. Hum Mol Genet 2018;27:3951–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stutzbach LD, PERK Genetic Variation and Function in Progressive Supranuclear Palsy. Neuroscience, Philadelphia, PA: University of Pennsylvania; 2015 [Google Scholar]
- 101.Letendre S, Espinoza C, Bharti A, et al. Genetic variation in EIF2AK3 is associated with neurocognitive impairment in HIV+ adults [CROI Abstract 74] In Special Issue: Abstracts from the 2017 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med. 2017:25(supp1):28–9
- 102. Clifford DB, Ances BM.. HIV-associated neurocognitive disorder. Lancet Infect Dis 2013;13:976–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hoglinger GU, Melhem NM, Dickson DW, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet 2011;43:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu QY, Yu JT, Miao D, et al. An exploratory study on STX6, MOBP, MAPT, and EIF2AK3 and late-onset Alzheimer's disease. Neurobiol Aging 2013;34:1519.e13–7 [DOI] [PubMed] [Google Scholar]
- 105. Ferrari R, Ryten M, Simone R, et al. Assessment of common variability and expression quantitative trait loci for genome-wide associations for progressive supranuclear palsy. Neurobiol Aging 2014;35:1514.e1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pytel D, Gao Y, Mackiewicz K, et al. PERK is a haploinsufficient tumor suppressor: Gene dose determines tumor-suppressive versus tumor promoting properties of PERK in melanoma. PLoS Genet 2016;12:e1006518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cubillos-Ruiz JR, Mohamed E, Rodriguez PC.. Unfolding anti-tumor immunity: ER stress responses sculpt tolerogenic myeloid cells in cancer. J Immunother Cancer 2017;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Niture SK, Kaspar JW, Shen J, et al. Nrf2 signaling and cell survival. Toxicol Appl Pharmacol 2010;244:37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhu YF, Li XH, Yuan ZP, et al. Allicin improves endoplasmic reticulum stress-related cognitive deficits via PERK/Nrf2 antioxidative signaling pathway. Eur J Pharmacol 2015;762:239–46 [DOI] [PubMed] [Google Scholar]
- 110. Bruch J, Kurz C, Vasiljevic A, et al. Early neurodegeneration in the brain of a child without functional PKR-like endoplasmic reticulum kinase. J Neuropathol Exp Neurol 2015;74:850–7 [DOI] [PubMed] [Google Scholar]
- 111. Cagnetta R, Wong HH, Frese CK, et al. Noncanonical modulation of the eIF2 pathway controls an increase in local translation during neural wiring. Mol Cell 2019;73:474–89.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lu PD, Jousse C, Marciniak SJ, et al. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J 2004;23:169–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jiang Q, Li F, Shi K, et al. Involvement of p38 in signal switching from autophagy to apoptosis via the PERK/eIF2alpha/ATF4 axis in selenite-treated NB4 cells. Cell Death Dis 2014;5:e1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chang RC, Wong AK, Ng HK, et al. Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer's disease. Neuroreport 2002;13:2429–32 [DOI] [PubMed] [Google Scholar]
- 115. Nijholt DA, Nolle A, van Haastert ES, et al. Unfolded protein response activates glycogen synthase kinase-3 via selective lysosomal degradation. Neurobiol Aging 2013;34:1759–71 [DOI] [PubMed] [Google Scholar]
- 116. Meares GP, Liu Y, Rajbhandari R, et al. PERK-dependent activation of JAK1 and STAT3 contributes to endoplasmic reticulum stress-induced inflammation. Mol Cell Biol 2014;34:3911–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Guthrie LN, Abiraman K, Plyler ES, et al. Attenuation of PKR-like ER kinase (PERK) signaling selectively controls endoplasmic reticulum stress-induced inflammation without compromising immunological responses. J Biol Chem 2016;291:15830–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Couturier J, Morel M, Pontcharraud R, et al. Interaction of double-stranded RNA-dependent protein kinase (PKR) with the death receptor signaling pathway in amyloid beta (Abeta)-treated cells and in APPSLPS1 knock-in mice. J Biol Chem 2010;285:1272–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Couturier J, Page G, Morel M, et al. Inhibition of double-stranded RNA-dependent protein kinase strongly decreases cytokine production and release in peripheral blood mononuclear cells from patients with Alzheimer's disease. J Alzheimers Dis 2010;21:1217–31 [DOI] [PubMed] [Google Scholar]
- 120. Peel AL, Bredesen DE.. Activation of the cell stress kinase PKR in Alzheimer's disease and human amyloid precursor protein transgenic mice. Neurobiol Dis 2003;14:52–62 [DOI] [PubMed] [Google Scholar]
- 121. Paquet C, Dumurgier J, Hugon J.. Pro-apoptotic kinase levels in cerebrospinal fluid as potential future biomarkers in Alzheimer's disease. Front Neurol 2015;6:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Paccalin M, Pain-Barc S, Pluchon C, et al. Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer's disease. Dement Geriatr Cogn Disord 2006;22:320–6 [DOI] [PubMed] [Google Scholar]
- 123. Hugon J, Mouton-Liger F, Dumurgier J, et al. PKR involvement in Alzheimer's disease. Alzheimers Res Ther 2017;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yu MS, Suen KC, Kwok NS, et al. Beta-amyloid peptides induces neuronal apoptosis via a mechanism independent of unfolded protein responses. Apoptosis 2006;11:687–700 [DOI] [PubMed] [Google Scholar]
- 125. Paquet C, Amin J, Mouton-Liger F, et al. Effect of active Abeta immunotherapy on neurons in human Alzheimer's disease. J Pathol 2015;235:721–30 [DOI] [PubMed] [Google Scholar]
- 126. Page G, Rioux Bilan A, Ingrand S, et al. Activated double-stranded RNA-dependent protein kinase and neuronal death in models of Alzheimer's disease. Neuroscience 2006;139:1343–54 [DOI] [PubMed] [Google Scholar]
- 127. Reimer L, Vesterager LB, Betzer C, et al. Inflammation kinase PKR phosphorylates alpha-synuclein and causes alpha-synuclein-dependent cell death. Neurobiol Dis 2018;115:17–28 [DOI] [PubMed] [Google Scholar]
- 128. Bando Y, Onuki R, Katayama T, et al. Double-strand RNA dependent protein kinase (PKR) is involved in the extrastriatal degeneration in Parkinson's disease and Huntington's disease. Neurochem Int 2005;46:11–8 [DOI] [PubMed] [Google Scholar]
- 129. Paquet C, Bose A, Polivka M, et al. Neuronal phosphorylated RNA-dependent protein kinase in Creutzfeldt-Jakob disease. J Neuropathol Exp Neurol 2009;68:190–8 [DOI] [PubMed] [Google Scholar]
- 130. Alirezaei M, Watry DD, Flynn CF, et al. Human immunodeficiency virus-1/surface glycoprotein 120 induces apoptosis through RNA-activated protein kinase signaling in neurons. J Neurosci 2007;27:11047–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Field R, Campion S, Warren C, et al. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav Immun 2010;24:996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ishimura R, Nagy G, Dotu I, et al. Activation of GCN2 kinase by ribosome stalling links translation elongation with translation initiation. Elife 2016;5:e14295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Mitsuda T, Hayakawa Y, Itoh M, et al. ATF4 regulates gamma-secretase activity during amino acid imbalance. Biochem Biophys Res Commun 2007;352:722–7 [DOI] [PubMed] [Google Scholar]
- 134. Biasiotto G, Di Lorenzo D, Archetti S, et al. Iron and neurodegeneration: Is ferritinophagy the link? Mol Neurobiol 2016;53:5542–74 [DOI] [PubMed] [Google Scholar]
- 135. Apostolakis S, Kypraiou AM.. Iron in neurodegenerative disorders: Being in the wrong place at the wrong time? Rev Neurosci 2017;28:893–911 [DOI] [PubMed] [Google Scholar]
- 136. Nnah IC, Wessling-Resnick M.. Brain iron homeostasis: A focus on microglial iron. Pharmaceuticals (Basel) 2018;11:129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Viader A, Sasaki Y, Kim S, et al. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron 2013;77:886–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Funfschilling U, Supplie LM, Mahad D, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012;485:517–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tauber D, Parker R.. 15-Deoxy-Delta(12, 14)-prostaglandin J2 promotes phosphorylation of eukaryotic initiation factor 2alpha and activates the integrated stress response. J Biol Chem 2019;294:6344–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Cleary JD, Pattamatta A, Ranum L.. Repeat-associated non-ATG (RAN) translation. J Biol Chem 2018;293:16127–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Banez-Coronel M, Ranum L.. Repeat-associated non-AUG (RAN) translation: Insights from pathology. Lab Invest 2019;99:929–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Goodman LD, Bonini NM.. Repeat-associated non-AUG (RAN) translation mechanisms are running into focus for GGGGCC-repeat associated ALS/FTD. Prog Neurobiol 2019;183:101697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Banez-Coronel M, Ayhan F, Tarabochia AD, et al. RAN translation in Huntington disease. Neuron 2015;88:667–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mori K, Weng SM, Arzberger T, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 2013;339:1335–8 [DOI] [PubMed] [Google Scholar]
- 145. Ash PE, Bieniek KF, Gendron TF, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 2013;77:639–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zu T, Liu Y, Banez-Coronel M, et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci U S A 2013;110:E4968–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zu T, Gibbens B, Doty NS, et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci U S A 2011;108:260–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Green KM, Linsalata AE, Todd PK.. RAN translation-What makes it run? Brain Res 2016;1647:30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mori K, Arzberger T, Grasser FA, et al. Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol 2013;126:881–93 [DOI] [PubMed] [Google Scholar]
- 150. Reddy K, Zamiri B, Stanley SY, et al. The disease-associated r(GGGGCC)n repeat from the C9orf72 gene forms tract length-dependent uni- and multimolecular RNA G-quadruplex structures. J Biol Chem 2013;288:9860–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Fratta P, Mizielinska S, Nicoll AJ, et al. C9orf72 hexanucleotide repeat associated with amyotrophic lateral sclerosis and frontotemporal dementia forms RNA G-quadruplexes. Sci Rep 2012;2:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Palam LR, Baird TD, Wek RC.. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem 2011;286:10939–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Starck SR, Tsai JC, Chen K, et al. Translation from the 5′ untranslated region shapes the integrated stress response. Science 2016;351:aad3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Schwab SR, Shugart JA, Horng T, et al. Unanticipated antigens: Translation initiation at CUG with leucine. PLoS Biol 2004;2:e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zu T, Pattamatta A, Ranum L.. Repeat-associated non-ATG translation in neurological diseases. Cold Spring Harb Perspect Biol 2018;10:a033019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Green KM, Glineburg MR, Kearse MG, et al. RAN translation at C9orf72-associated repeat expansions is selectively enhanced by the integrated stress response. Nat Commun 2017;8: 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Westergard T, McAvoy K, Russell K, et al. Repeat-associated non-AUG translation in C9orf72-ALS/FTD is driven by neuronal excitation and stress. EMBO Mol Med 2019;11:e9423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sonobe Y, Ghadge G, Masaki K, et al. Translation of dipeptide repeat proteins from the C9ORF72 expanded repeat is associated with cellular stress. Neurobiol Dis 2018;116:155–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Cheng W, Wang S, Mestre AA, et al. C9ORF72 GGGGCC repeat-associated non-AUG translation is upregulated by stress through eIF2alpha phosphorylation. Nat Commun 2018;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ishiguro T, Sato N, Ueyama M, et al. Regulatory role of RNA chaperone TDP-43 for RNA misfolding and repeat-associated translation in SCA31. Neuron 2017;94:108–24.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Cheng W, Wang S, Zhang Z, et al. CRISPR-Cas9 screens identify the RNA helicase DDX3X as a repressor of C9ORF72 (GGGGCC)n repeat-associated non-AUG translation. Neuron 2019;104:885–98.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Green KM, Sheth U, Flores BN, et al. High-throughput screening yields several small-molecule inhibitors of repeat-associated non-AUG translation. J Biol Chem 2019;294:18624–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Holsinger RM, McLean CA, Beyreuther K, et al. Increased expression of the amyloid precursor beta-secretase in Alzheimer's disease. Ann Neurol 2002;51:783–6 [DOI] [PubMed] [Google Scholar]
- 164. Fukumoto H, Cheung BS, Hyman BT, et al. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol 2002;59:1381–9 [DOI] [PubMed] [Google Scholar]
- 165. Yang LB, Lindholm K, Yan R, et al. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med 2003;9:3–4 [DOI] [PubMed] [Google Scholar]
- 166. Ahmed RR, Holler CJ, Webb RL, et al. BACE1 and BACE2 enzymatic activities in Alzheimer's disease. J Neurochem 2010;112:1045–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Gannon PJ, Akay-Espinoza C, Yee AC, et al. HIV protease inhibitors alter amyloid precursor protein processing via beta-site amyloid precursor protein cleaving enzyme-1 translational up-regulation. Am J Pathol 2017;187:91–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Stern AL, Ghura S, Gannon PJ, et al. BACE1 mediates HIV-associated and excitotoxic neuronal damage through an APP-dependent mechanism. J Neurosci 2018;38:4288–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ohno M, Cole SL, Yasvoina M, et al. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol Dis 2007;26:134–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mouton-Liger F, Paquet C, Dumurgier J, et al. Oxidative stress increases BACE1 protein levels through activation of the PKR-eIF2alpha pathway. Biochim Biophys Acta 2012;1822:885–96 [DOI] [PubMed] [Google Scholar]
- 171. O'Connor T, Sadleir KR, Maus E, et al. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron 2008;60:988–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gabr AA, Lee HJ, Onphachanh X, et al. Ethanol-induced PGE2 up-regulates Aβ production through PKA/CREB signaling pathway. Biochim Biophys Acta Mol Basis Dis 2017;1863:2942–53 [DOI] [PubMed] [Google Scholar]
- 173. Devi L, Ohno M.. Mechanisms that lessen benefits of beta-secretase reduction in a mouse model of Alzheimer's disease. Transl Psychiatry 2013;3:e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ill-Raga G, Palomer E, Wozniak MA, et al. Activation of PKR causes amyloid ss-peptide accumulation via de-repression of BACE1 expression. PLoS One 2011;6:e21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Bullido MJ, Martinez-Garcia A, Tenorio R, et al. Double stranded RNA activated EIF2 alpha kinase (EIF2AK2; PKR) is associated with Alzheimer's disease. Neurobiol Aging 2008;29:1160–6 [DOI] [PubMed] [Google Scholar]
- 176. Dumurgier J, Mouton-Liger F, Lapalus P, et al. Cerebrospinal fluid PKR level predicts cognitive decline in Alzheimer's disease. PLoS One 2013;8:e53587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Mouton-Liger F, Paquet C, Dumurgier J, et al. Increased cerebrospinal fluid levels of double-stranded RNA-dependant protein kinase in Alzheimer’s disease. Biol Psychiatry 2012;71:829–35 [DOI] [PubMed] [Google Scholar]
- 178. Mouton-Liger F, Wallon D, Troussiere AC, et al. Impact of cerebro-spinal fluid biomarkers of Alzheimer's disease in clinical practice: A multicentric study. J Neurol 2014;261:144–51 [DOI] [PubMed] [Google Scholar]
- 179. Carret-Rebillat AS, Pace C, Gourmaud S, et al. Neuroinflammation and Abeta accumulation linked to systemic inflammation are decreased by genetic PKR down-regulation. Sci Rep 2015;5:8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Mouton-Liger F, Rebillat AS, Gourmaud S, et al. PKR downregulation prevents neurodegeneration and beta-amyloid production in a thiamine-deficient model. Cell Death Dis 2015;6:e1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Tible M, Mouton Liger F, Schmitt J, et al. PKR knockout in the 5xFAD model of Alzheimer's disease reveals beneficial effects on spatial memory and brain lesions. Aging Cell 2019;18:e12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ill-Raga G, Tajes M, Busquets-Garcia A, et al. Physiological control of nitric oxide in neuronal BACE1 translation by heme-regulated eIF2ɑ kinase HRI induces synaptogenesis. Antioxid Redox Signal 2015;22:1295–307 [DOI] [PubMed] [Google Scholar]
- 183. Devi L, Ohno M.. Deletion of the eIF2alpha kinase GCN2 fails to rescue the memory decline associated with Alzheimer's disease. PLoS One 2013;8:e77335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lourenco MV, Clarke JR, Frozza RL, et al. TNF-alpha mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer's beta-amyloid oligomers in mice and monkeys. Cell Metab 2013;18:831–43 [DOI] [PubMed] [Google Scholar]
- 185. Imbimbo BP, Watling M.. Investigational BACE inhibitors for the treatment of Alzheimer's disease. Expert Opin Investig Drugs 2019;28:967–75 [DOI] [PubMed] [Google Scholar]
- 186. Egan MF, Mukai Y, Voss T, et al. Further analyses of the safety of verubecestat in the phase 3 EPOCH trial of mild-to-moderate Alzheimer's disease. Alzheimers Res Ther 2019;11:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Doggrell SA. Lessons that can be learnt from the failure of verubecestat in Alzheimer's disease. Expert Opin Pharmacother 2019;20:2095–9 [DOI] [PubMed] [Google Scholar]
- 188. Barao S, Moechars D, Lichtenthaler SF, et al. BACE1 physiological functions may limit its use as therapeutic target for Alzheimer's disease. Trends Neurosci 2016;39:158–69 [DOI] [PubMed] [Google Scholar]
- 189. Moussa CE. Beta-secretase inhibitors in phase I and phase II clinical trials for Alzheimer's disease. Expert Opin Investig Drugs 2017;26:1131–6 [DOI] [PubMed] [Google Scholar]
- 190. Koelsch G. BACE1 function and inhibition: Implications of intervention in the amyloid pathway of Alzheimer's disease pathology. Molecules 2017;22:1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Liu L, Lauro BM, Ding L, et al. Multiple BACE1 inhibitors abnormally increase the BACE1 protein level in neurons by prolonging its half-life. Alzheimers Dement 2019;15:1183–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Yan R, Vassar R.. Targeting the beta secretase BACE1 for Alzheimer's disease therapy. Lancet Neurol 2014;13:319–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Pettus LH, Bourbeau MP, Bradley J, et al. Discovery of AM-6494: A potent and orally efficacious beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitor with in vivo selectivity over BACE2. J Med Chem 2019;10.1021/acs.jmedchem.9b01034 [DOI] [PubMed] [Google Scholar]
- 194. Read J, Suphioglu C.. Identification of a BACE1 binding peptide candidate for the prevention of amyloid beta in Alzheimer's disease. Cell Physiol Biochem 2019;53:413–28 [DOI] [PubMed] [Google Scholar]
- 195. Bejanin A, Schonhaut DR, La Joie R, et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer's disease. Brain 2017;140:3286–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Stutzbach LD, Xie SX, Naj AC, et al. The unfolded protein response is activated in disease-affected brain regions in progressive supranuclear palsy and Alzheimer's disease. Acta Neuropathol Commun 2013;1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Unterberger U, Hoftberger R, Gelpi E, et al. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J Neuropathol Exp Neurol 2006;65:348–57 [DOI] [PubMed] [Google Scholar]
- 198. Bose A, Mouton-Liger F, Paquet C, et al. Modulation of tau phosphorylation by the kinase PKR: Implications in Alzheimer's disease. Brain Pathol 2011;21:189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Fu ZQ, Yang Y, Song J, et al. LiCl attenuates thapsigargin-induced tau hyperphosphorylation by inhibiting GSK-3beta in vivo and in vitro. J Alzheimers Dis 2010;21:1107–17 [DOI] [PubMed] [Google Scholar]
- 200. Ho YS, Yang X, Lau JC, et al. Endoplasmic reticulum stress induces tau pathology and forms a vicious cycle: Implication in Alzheimer's disease pathogenesis. J Alzheimers Dis 2012;28:839–54 [DOI] [PubMed] [Google Scholar]
- 201. Lin L, Yang SS, Chu J, et al. Region-specific expression of tau, amyloid-beta protein precursor, and synaptic proteins at physiological condition or under endoplasmic reticulum stress in rats. J Alzheimers Dis 2014;41:1149–63 [DOI] [PubMed] [Google Scholar]
- 202. Radford H, Moreno JA, Verity N, et al. PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia. Acta Neuropathol 2015;130:633–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Qu L, Huang S, Baltzis D, et al. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3beta. Genes Dev 2004;18:261–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Baltzis D, Pluquet O, Papadakis AI, et al. The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J Biol Chem 2007;282:31675–87 [DOI] [PubMed] [Google Scholar]
- 205. Bruch J, Xu H, Rosler TW, et al. PERK activation mitigates tau pathology in vitro and in vivo. EMBO Mol Med 2017;9:371–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Jo C, Gundemir S, Pritchard S, et al. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun 2014;5:3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Jiang P, Gan M, Ebrahim AS, et al. ER stress response plays an important role in aggregation of alpha-synuclein. Mol Neurodegener 2010;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Tronel C, Page G, Bodard S, et al. The specific PKR inhibitor C16 prevents apoptosis and IL-1beta production in an acute excitotoxic rat model with a neuroinflammatory component. Neurochem Int 2014;64:73–83 [DOI] [PubMed] [Google Scholar]
- 209. Stone S, Yue Y, Stanojlovic M, et al. Neuron-specific PERK inactivation exacerbates neurodegeneration during experimental autoimmune encephalomyelitis. JCI Insight 2019;4:e124232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Lu Z, Wang Z, Yu L, et al. GCN2 reduces inflammation by p-eIF2alpha/ATF4 pathway after intracerebral hemorrhage in mice. Exp Neurol 2019;313:16–25 [DOI] [PubMed] [Google Scholar]
- 211. Keil M, Sonner JK, Lanz TV, et al. General control non-derepressible 2 (GCN2) in T cells controls disease progression of autoimmune neuroinflammation. J Neuroimmunol 2016;297:117–26 [DOI] [PubMed] [Google Scholar]
- 212. Axten JM. Protein kinase R(PKR)-like endoplasmic reticulum kinase (PERK) inhibitors: A patent review (2010-2015). Expert Opin Ther Pat 2017;27:37–48 [DOI] [PubMed] [Google Scholar]
- 213. Axten JM, Medina JR, Feng Y, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2, 3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2, 3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem 2012;55:7193–207 [DOI] [PubMed] [Google Scholar]
- 214. Axten JM, Romeril SP, Shu A, et al. Discovery of GSK2656157: An optimized PERK inhibitor selected for preclinical development. ACS Med Chem Lett 2013;4:964–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Mercado G, Castillo V, Soto P, et al. Targeting PERK signaling with the small molecule GSK2606414 prevents neurodegeneration in a model of Parkinson's disease. Neurobiol Dis 2018;112:136–48 [DOI] [PubMed] [Google Scholar]
- 216. Zhao Q, Che X, Zhang H, et al. Thioredoxin-interacting protein links endoplasmic reticulum stress to inflammatory brain injury and apoptosis after subarachnoid haemorrhage. J Neuroinflammation 2017;14:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Wang H, Blais J, Ron D, et al. Structural determinants of PERK inhibitor potency and selectivity. Chem Biol Drug Des 2010;76:480–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Lederkremer GZ, Leitman J, Eiger H, et al. Perk inhibitors and uses thereof in treating diseases associated with aggregation-prone proteins. US patent application. US 20190010130, 2019
- 219. Jammi NV, Whitby LR, Beal PA.. Small molecule inhibitors of the RNA-dependent protein kinase. Biochem Biophys Res Commun 2003;308:50–7 [DOI] [PubMed] [Google Scholar]
- 220. Shimazawa M, Hara H.. Inhibitor of double stranded RNA-dependent protein kinase protects against cell damage induced by ER stress. Neurosci Lett 2006;409:192–5 [DOI] [PubMed] [Google Scholar]
- 221. Ingrand S, Barrier L, Lafay-Chebassier C, et al. The oxindole/imidazole derivative C16 reduces in vivo brain PKR activation. FEBS Lett 2007;581:4473–8 [DOI] [PubMed] [Google Scholar]
- 222. Boyce M, Bryant KF, Jousse C, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 2005;307:935–9 [DOI] [PubMed] [Google Scholar]
- 223. Wu L, Luo N, Zhao HR, et al. Salubrinal protects against rotenone-induced SH-SY5Y cell death via ATF4-parkin pathway. Brain Res 2014;1549:52–62 [DOI] [PubMed] [Google Scholar]
- 224. Vaccaro A, Patten SA, Aggad D, et al. Pharmacological reduction of ER stress protects against TDP-43 neuronal toxicity in vivo. Neurobiol Dis 2013;55:64–75 [DOI] [PubMed] [Google Scholar]
- 225. Huang X, Chen Y, Zhang H, et al. Salubrinal attenuates β-amyloid-induced neuronal death and microglial activation by inhibition of the NF-κB pathway. Neurobiol Aging 2012;33:1007.e9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Rubovitch V, Barak S, Rachmany L, et al. The neuroprotective effect of salubrinal in a mouse model of traumatic brain injury. Neuromol Med 2015;17:58–70 [DOI] [PubMed] [Google Scholar]
- 227. Lucke-Wold BP, Logsdon AF, Turner RC, et al. Endoplasmic reticulum stress modulation as a target for ameliorating effects of blast induced traumatic brain injury. J Neurotrauma 2017;34:S62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Chen Y, Podojil JR, Kunjamma RB, et al. Sephin1, which prolongs the integrated stress response, is a promising therapeutic for multiple sclerosis. Brain 2019;142:344–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Jiang HQ, Ren M, Jiang HZ, et al. Guanabenz delays the onset of disease symptoms, extends lifespan, improves motor performance and attenuates motor neuron loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neuroscience 2014;277:132–8 [DOI] [PubMed] [Google Scholar]
- 230. Vieira FG, Ping Q, Moreno AJ, et al. Guanabenz treatment accelerates disease in a mutant SOD1 mouse model of ALS. PLoS One 2015;10:e0135570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Fardghassemi Y, Tauffenberger A, Gosselin S, et al. Rescue of ATXN3 neuronal toxicity in Caenorhabditis elegans by chemical modification of endoplasmic reticulum stress. Dis Model Mech 2017;10:1465–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Sun X, Aime P, Dai D, et al. Guanabenz promotes neuronal survival via enhancement of ATF4 and parkin expression in models of Parkinson disease. Exp Neurol 2018;303:95–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. De Gassart A, Bujisic B, Zaffalon L, et al. An inhibitor of HIV-1 protease modulates constitutive eIF2alpha dephosphorylation to trigger a specific integrated stress response. Proc Natl Acad Sci U S A 2016;113:E117–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Akay C, Cooper M, Odeleye A, et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol 2014;20:39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Shah A, Gangwani MR, Chaudhari NS, et al. Neurotoxicity in the post-HAART Era: Caution for the antiretroviral therapeutics. Neurotox Res 2016;30:677–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Lewerenz J, Maher P.. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009;284:1106–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Stockwell SR, Platt G, Barrie SE, et al. Mechanism-based screen for G1/S checkpoint activators identifies a selective activator of EIF2AK3/PERK signalling. PLoS One 2012;7:e28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Sidrauski C, Acosta-Alvear D, Khoutorsky A, et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife 2013;2:e00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Sekine Y, Zyryanova A, Crespillo-Casado A, et al. Mutations in a translation initiation factor identify the target of a memory-enhancing compound. Science 2015;348:1027–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Tsai JC, Miller-Vedam LE, Anand AA, et al. Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule. Science 2018;359:eaaq0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Zyryanova AF, Weis F, Faille A, et al. Binding of ISRIB reveals a regulatory site in the nucleotide exchange factor eIF2B. Science 2018;359:1533–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Sidrauski C, McGeachy AM, Ingolia NT, et al. The small molecule ISRIB reverses the effects of eIF2alpha phosphorylation on translation and stress granule assembly. Elife 2015;4:e05033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Halliday M, Radford H, Sekine Y, et al. Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis 2015;6:e1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Chou A, Krukowski K, Jopson T, et al. Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury. Proc Natl Acad Sci U S A 2017;114:E6420–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Briggs DI, Defensor E, Memar Ardestani P, et al. Role of endoplasmic reticulum stress in learning and memory impairment and Alzheimer's disease-like neuropathology in the PS19 and APP(Swe) mouse models of tauopathy and amyloidosis. eNeuro 2017;4:ENEURO.0025-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Hosoi T, Kakimoto M, Tanaka K, et al. Unique pharmacological property of ISRIB in inhibition of Abeta-induced neuronal cell death. J Pharmacol Sci 2016;131:292–5 [DOI] [PubMed] [Google Scholar]
- 247. Stern AL, Lee RN, Panvelker N, et al. Differential effects of antiretroviral drugs on neurons in vitro: Roles for oxidative stress and integrated stress response. J Neuroimmune Pharmacol 2018;13:64–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Rabouw HH, Langereis MA, Anand AA, et al. Small molecule ISRIB suppresses the integrated stress response within a defined window of activation. Proc Natl Acad Sci U S A 2019;116:2097–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Wong YL, LeBon L, Basso AM, et al. eIF2B activator prevents neurological defects caused by a chronic integrated stress response. eLife 2019;8:e42940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Wong YL, LeBon L, Edalji R, et al. The small molecule ISRIB rescues the stability and activity of Vanishing White Matter Disease eIF2B mutant complexes. eLife 2018;7:e32733 [DOI] [PMC free article] [PubMed] [Google Scholar]