Short abstract
Physical exercise has been associated with enhanced memory formation and consolidation in patients with mild cognitive impairment (MCI). This study aimed to investigate the relationship between objective neuropsychological performances and continuously recorded physical activity. A cut-off value of measured physical activity was used to differentiate early-stage and late-stage MCI. Fifty-four patients with MCI were included. The relationship between cognitive function and measures of daily activity measured by continuous three-axis accelerometers in Xiaomi Mi Band, including subject-level average step counts, average distance (kilometers), and average calorie expenditure per day of 7-day activity, was determined. The slope of the receiver operating characteristic curve was used to determine the measures of activity to draw comparisons between early-stage MCI and late-stage MCI. The patients were assessed by using several cognitive tests such as Cognitive Abilities Screening Instrument and Chinese Version Verbal Learning Test. The multivariate linear regression model indicated a significant correlation of higher average step counts per day of 7-day activity (aver-step-counts) with higher score in visual construction (β = 0.355; p = .015). To differentiate patients with late-stage MCI from those with early-stage MCI, the cut-off value of 6,284 steps on aver-step-counts showed an optimal sensitivity and specificity (Youden index = 0.36, area under the curve = 0.651, p = .042). The aver-step-counts showed a significantly better differentiating rate between patients with early-stage and late-stage MCI than average calorie expenditure per day of 7-day activity did (p = .046). Accelerometer-determined measures of activity patterns show as potential measurement to reflect cognitive function.
Keywords: accelerometer, cognition, cognitive dysfunction, exercise, mild cognitive impairment
Introduction
Physical exercise has been associated with enhanced memory formation and consolidation (Fernandes et al., 2016). With regard to individuals with mild cognitive impairment (MCI), two review articles reveal inconclusive results with regard to beneficial effects of exercise on cognitive function (Gates et al., 2013; Ohman et al., 2014), while other two meta-analysis studies show aerobic exercise significantly improves global cognitive ability and memory (Strohle et al., 2015; Zheng et al., 2016). Six months of high-intensity aerobic exercise as measured by heart rate reserve is shown to have favorable effects on executive function in patients with MCI (Baker et al., 2010). Although rigorous controlled training protocols are associated with improvement in cognition, it is not widely acceptable for most elderly people (Geda et al., 2010; Erickson et al., 2011), and it is even inaccessible for some elderly people with cognitive impairment or low education (Baker et al., 2010; Geda et al., 2010).
Using physical exercise questionnaire, a population-based study reported a suggestive association between physical activity and decreased risk of cognitive decline (Geda et al., 2010). Exercise-induced enhancement of neural recruitment in the brain networks (Chirles et al., 2017; Alfini et al., 2019) might be the potential mechanisms underlying the relationship between physical activity and memory improvement in patients with MCI. However, the benefit of physical activity diminishes after discontinuing the aerobic exercise training (Liu-Ambrose et al., 2016). Since adherence to standardized exercise training might be difficult and can be influenced by complicate factors for elderly people with MCI (Hancox et al., 2019), wearable digital technology is one of the choice for patients with MCI to evaluate the effect of real-world physical activity on cognition.
Wearable digital technology has been regularly used and widely adopted by patients and practitioners for physical activity monitoring (Piwek et al., 2016). Wearable activity monitors are able to capture free-living mobility data in a real-world environment, which provide meaningful and accurate information about each participant and facilitate data-driven individualized assessment (Case et al., 2015). Wearable activity trackers such as Jawbone, Fitbit, and Xiaomi Mi Band contain a triaxial accelerometer and global positioning system (GPS) and provide acceptable measurement properties of activity monitors (Straiton et al., 2018). Using proprietary algorithms, day-level data of step counts, distance, and calorie expenditure can be estimated, and Jawbone and Fitbit trackers have been demonstrated to be valid and reliable devices for measuring step counts (Takacs et al., 2014; Kelly et al., 2015) and calorie expenditure (Stahl and Insana, 2014). Xiaomi Mi Band trackers are shown to have similar accuracies of measuring step counts and calorie expenditure to Jawbone and Fitbit trackers (Xie et al., 2018). Moreover, Xiaomi Mi Band has been shown to have high measurement accuracy (96.56%), relatively low variation (coefficient of variation = 5.81), low purchase price ($14), and long battery life (around 3 weeks; El-Amrawy and Nounou, 2015; Xie et al., 2018; Mickova et al., 2019). Due to low cost, accessibility, easy operation, and reliability, Xiaomi Mi Band may help to facilitate mobility monitors, increase physical activity, and provide health promotion at an affordable price for most people (El-Amrawy and Nounou, 2015).
The objectives of this study were to (a) perform a direct relationship analysis between the objective neuropsychological tests of each cognitive domain and the quantified physical activity; (b) explore the cognitive function that average step counts per day of 7-day activity (aver-step-counts), average distance (kilometers) per day of 7-day activity (aver-distance), and average calorie expenditure per day of 7-day activity (aver-calorie-expend) were most associated with; and (c) use a cut-off value of physical activity to differentiate early-stage MCI and late-stage MCI.
Materials and Methods
Participants
Fifty-four patients with MCI were enrolled from the Department of Neurology of Chang Gung Memorial Hospital. MCI patients had a subjective memory complaint, objective memory loss as indicated by scores on the memory tests (1.5 standard deviations [SDs] below the education-adjusted normative mean; C. C. Chang et al., 2010), a global Clinical Dementia Rating (CDR) score of 0.5 and a score ≥ 0.5 on the memory box of the CDR, and an absence of dementia (Y. L. Chang et al., 2011). Participants with coronary artery disease or congestive heart failure, stroke, chronic obstructive pulmonary disease, or renal failure were excluded. In this study, 5 subjects had diabetes mellitus (Seino et al., 2010), 11 subjects had hypertension, and 17 subjects had hyperlipidemia. All of these participants were under stable treatment for 3 months before inclusion (Karr, 2017; Cuspidi et al., 2018).
Study Design
Neurobehavioral testing and activity monitoring were all performed within duration of 4 weeks. All participants were asked to do activity normally as they usually did before and after the study.
Details of Neurobehavioral Examinations
Participants were evaluated of general cognitive performance using CDR (Morris, 1993) and Cognitive Abilities Screening Instrument (CASI; Liu et al., 1994). In addition to general cognitive performance, CASI was used to evaluate specific domains, including short-term memory, attention and concentration, abstraction, visual construction, language, and list-generating fluency (Meguro et al., 2001). Verbal memory was evaluated by using CASI-Short-term memory and the Chinese Version Verbal Learning Test (CVVLT; C. C. Chang et al., 2010), assessing free recall of number of items retrieved over four learning trials of a nine-word list after 30 s (CVVLT-30 s) and after 10 min (CVVLT-10 min). CVVLT-30 s and CVVLT-10 min were used to evaluate immediate and delayed free recalls (Lezak, 2004). The Visual Object and Space Perception (VOSP; Warrington and James, 1991), Rey–Osterrieth complex figure copy (Boone, 2000), and CASI-Visual construction were used to assess the visual-spatial abilities. Attention was assessed with Digital Span Forward (Weintraub et al., 2009), CASI-Attention, and CASI-Concentration. The participants’ executive function was evaluated by using Trail Making Test B (TMB; corrected; Reitan, 1955) and Stroop interference test (Amieva et al., 2004). As the calculation ability depends on executive function (Jogi and Kikas, 2016), calculation ability was evaluated to reflect part of executive function. Our calculation tests comprised two additions, two subtractions, and one multiplication. Each addition and each subtraction consisted of one and two items, respectively, and the multiplication is composed of a multiplicand of greater than 100 and a multiplier of less than 100 (e.g., 214 × 35). Abstract reasoning was evaluated by using similarities (conceptualization) in frontal assessment battery-Similarities (Dubois et al., 2000) and CASI-Abstraction. Boston Naming Test and CASI-Language ability were used to evaluate language ability in naming (Kaplan et al., 1983). CASI-List-generating fluency, animal naming, fruit naming transportation naming, and town naming tests were used to evaluate semantic category fluency (Morris et al., 1989).
Activity Monitoring and Data Collection
Xiaomi Mi Band devices (https://www.mi.com/global/miband/) were given to participants directly with instructions on activation and authorization of data uploading. Xiaomi Mi Band is a GPS-enabled Health Band with allowing for phone-free running while tracking essential metrics like step counts, distance (kilometers), and calorie expenditure (https://www.mi.com/global/miband/). The device contains a three-axis accelerometer that determines threshold of steps based on motion patterns that are most indicative of people walking. The device has wireless automatic syncing features compatible with smartphone and has a battery life of approximately 14 days. In addition, the app primarily functions as a command center for Xiaomi Mi Band like account creation (sign up and login) with allowing Bluetooth pairing. This authentication granted research personnel permission to access their real-time device data. The device automatically transmits activity data to Xiaomi Mi servers when in proximity of the subject’s Bluetooth®-enabled smartphone. Participants were asked to wear the devices all day long for 7 days. Daily information was collected from all participants using the Xiaomi Mi Band device: (a) activation/authentication of the device, (b) number of steps taken per day (step counts), (c) distance per day (kilometers), and (d) calorie expenditure per day. The smartwatch adherence was assessed by checking the records on the mobile app. Each subject’s heart rate was monitored every 5 min, and the plots of continuous heart rate were recorded on the app. If the subjects had any noncompliance episode with the records showing heart rate as low as 0, he or she would be requested to wear the devices for further several days to achieve the goal of 7-day physical activity record.
Data Processing and Transformation
Initial reviews were performed to examine data density, outliers, and unreasonable values. First, day-level physical activity data (step counts, distance, and calorie expenditure) were obtained for 54 participants in the study. Activity data from the first and last day were removed as these data points may be reflective of a partial day (e.g., participant connects a device the first time on the second half of the day) or might not completely get synchronized or uploaded for the last day. Second, subject-level aver-step-counts, aver-distance, and aver-calorie-expend were computed, and the data were used to create a subject-level activity measures. For our secondary data analyses, subject-level average activity measures (aver-step-counts, aver-distance-kilometers, and aver-calorie-expend for entire 7 days) were used as a proxy for overall physical activity.
Statistical Analyses
All data were expressed as mean ± SD. We used partial correlation to analyze the relationship between each subject-level average activity measures and each cognitive function score in all MCI patients. Stepwise regression analyses were carried out to determine the cognitive function that aver-step-counts, aver-distance-kilometers, and aver-calorie-expend were most associated with. Age (years), education (years), and sex were included as covariates.
We calculated the area under the curve (AUC) of the receiver operating characteristics (ROC) curves as a measure of the predictive value of the measures of activity. Predictive accuracy was assessed by calculating the sensitivity and specificity for the threshold that yielded the highest Youden index (Youden index = sensitivity – [1 – specificity]). The slope of the ROC curve was used to determine the measures of activity to draw comparisons between early-stage MCI, as indicated by scores on the memory tests being 1.5 SDs below the education-adjusted normative mean, and late-stage MCI, as indicated by scores on the memory tests being 2 SDs below the education-adjusted normative mean (C. C. Chang et al., 2010; Ye et al., 2014).
All of the statistical analyses were conducted using the Statistical Package for Social Sciences software (version 18 for Windows®, SPSS Inc., Chicago, IL), and a p value <.05 (two-tailed) was considered statistically significant.
Ethical Guidelines
This study was approved by the Institutional Review Committee on Human Research of Chang Gung Memorial Hospital (IRB 201601664B0C601, 201601664B0), and all of the participants were provided written informed consent. All procedures were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
Demographic and Clinical Characteristics
Fifty-four participants completed the study. Their demographic, activity, and cognitive variables are shown in Table 1. All participants had CDR of 0.5. The aver-step-counts, aver-distance-kilometers, and aver-calorie-expend were 6,935.9 ± 3,902.0 counts, 4.6 ± 2.8 km, and 114.0 ± 73.0 cal. In these participants, education (years) was significantly correlated with the aver-calorie-expend (ρ = 0.273, p = .045) per day, but not with aver-step-counts or aver-distance-kilometers (p > .05). Age (years) was not correlated with any measure of activity (p > .05). Table S1 in Supplementary Material shows the correlation of cognitive performances with age (years) and education (years). Moreover, men of this study had higher aver-calorie-expend than women of this study—t(53) = 2.162, p = .035. Therefore, in the correlation and regression model, age (years), education (years), and sex were adjusted for the relationship analyses between cognitive function scores and measures of activity.
Table 1.
General Characteristics of the Mild Cognitive Impairment Participants.
| Clinical and demographic characteristics | Mean ± standard deviation |
|---|---|
| Sample size (n) | 54 |
| Age (years) | 69.7 ± 8.0 |
| Sex (Female/Male) | 31/23 |
| Education (years) | 8.7 ± 5.0 |
| Memory tasks | |
| CASI-Short-term memory | 9.3 ± 9.4 |
| CVVLT-30 s (immediate free recalls) | 6.0 ± 2.1 |
| CVVLT-10 min (delayed free recalls) | 5.1 ± 3.0 |
| CVVLT-cued (memory in cued response) | 5.4 ± 2.7 |
| Abstract reasoning | |
| CASI-Abstraction | 9.4 ± 2.5 |
| FAB-Similarity (conceptualization) | 1.6 ± 1.0 |
| Attention | |
| CASI-Concentration | 7.3 ± 2.6 |
| CASI-Attention | 6.9 ± 0.9 |
| Digital Forward Span | 7.6 ± 1.2 |
| Executive task scores | |
| TMB (corrected) | 10.6 ± 4.2 |
| Stroop interference test | 33.6 ± 12.4 |
| Calculation | 4.2 ± 1.1 |
| Visuospatial task scores | |
| VOSP | 8.0 ± 2.6 |
| Modified ROCF copy | 15.3 ± 3.3 |
| CASI-Visual construction | 9.3 ± 1.5 |
| Language ability | |
| Boston naming test | 14.2 ± 2.6 |
| CASI-Language ability | 9.1 ± 1.2 |
| Semantic category fluency | |
| CASI-List-generating | 6.6 ± 2.4 |
| Animal naming | 13.5 ± 5.2 |
| Fruit naming | 11.6 ± 3.0 |
| Transportation naming | 8.5 ± 2.7 |
| Town naming | 12.1 ± 4.2 |
Note. Parametric continuous variables presented as mean ± standard deviation. CASI = Cognitive Abilities Screening Instrument; CVVLT = Chinese version of the Verbal Learning Test (CVVLT-30 s: free recall after 30 s; CVVLT-10 min: free recall after 10 min); FAB-Similarities = similarities (conceptualization) in frontal assessment battery; ROCF = Rey–Osterrieth complex figure; TMB = Trail Making Test B; VOSP = Visual Object and Space Perception.
Correlates of Physical Activity With Cognitive Function
The correlations between cognitive function scores and measures of activity are shown in Figure 1 and Table S2 in Supplementary Material. Left panel of Figure 1 demonstrates the relationship between each cognitive score and each measure of activity by using Spearman correlation tests. Middle and right panel of Figure 1, respectively, demonstrates the relationship between each cognitive score and each measure of activity after controlling age (years) and sex, and that after controlling for age (years), sex, and education (years).
Figure 1.
Correlation between each measure of activity and each cognitive score. The relationship between each cognitive score and each measure of activity (left panel), after controlling age (years) and sex (middle panel), and after controlling for age (years), sex, and education (years) (right panel). CASI = Cognitive Abilities Screening Instrument; FAB = frontal assessment battery; ROCF = Rey–Osterrieth complex figure; TMB = Trail Making Test B; VOSP = Visual Object and Space Perception.
The details of the measures of physical activity and cognitive performances are shown in Table S2 in Supplementary Material.
The aver-step-counts were correlated with scores in CASI-Short-term memory, TMB (corrected), CASI-Visual construction, and animal naming (p < .05). Higher score in CASI-Visual construction was independently correlated with higher aver-step-counts (β = 0.355, p = .015; Table S3 in Supplementary Material).
The aver-distance-kilometers were correlated with score in TMB (corrected), CASI-Attention, and CASI-Visual construction (p < .05). Higher score in CASI-Visual construction was independently correlated with higher aver-distance-kilometers (β = 0.346, p = .018; Table S4 in Supplementary Material).
The aver-calorie-expend was correlated with TMB (corrected), CASI-Visual construction, and animal naming (p < .05). Higher scores in animal naming (β = 0.392, p = .009) and in CASI-Visual construction (β = 0.342, p = .017) were independently correlated with higher aver-calorie-expend (Table S5 in Supplementary Material).
Using AUC in Measures of Activity for Detecting Late-Stage MCI
Participants with MCI were categorized into 30 individuals with early-stage MCI and 24 individuals with late-stage MCI.
When compared with participants with late-stage MCI, participants with early-stage MCI did not have significant higher or lower aver-step-counts, aver-distance-kilometers, and aver-calorie-expend (p > .05) than those with late-stage MCI.
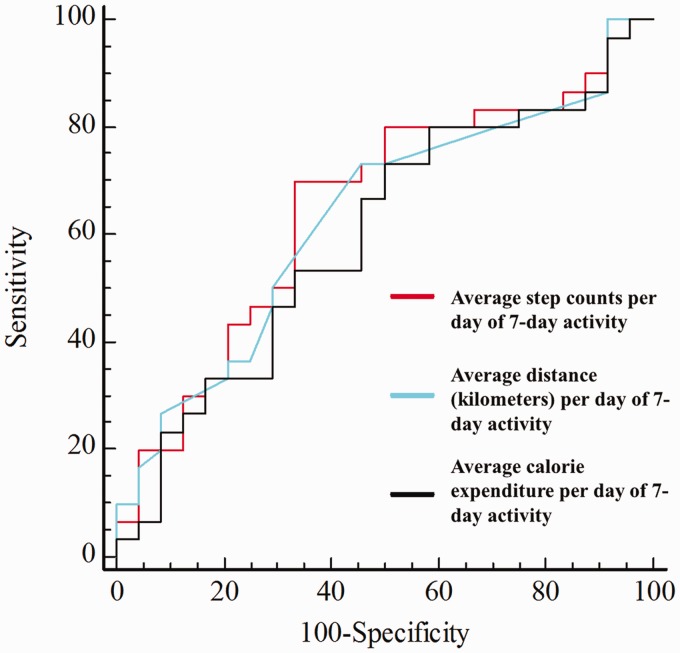
An ROC analysis was used to evaluate and compare the potential of measures of activity to differentiate between participants with early-stage MCI and those with late-stage MCI. A summary of the comparison of the AUC for the two MCI groups is shown in Figure 2. The optimal cut-off values of 6,284 steps on the aver-step-counts (Youden index = 0.36, AUC = 0.651, p = .042), 3.7 km on the aver-distance-kilometers (Youden index = 0.28, AUC = 0.632, p = .081), and 79 kcal on the aver-calorie-expend (Youden index = 0.23, AUC = 0.597, p = .209) were calculated to differentiate between early-stage and late-stage MCI. Using a cut-off value of 6,284 steps on the aver-step-counts showed a significantly better differentiating rate between participants with early-stage and late-stage MCI than using a cut-off value of 79 kcal on the aver-calorie-expend did (p = .046).
Figure 2.
Receiver operating characteristic curve comparing the utility of different measures of activity for discriminating between early- and late-stage mild cognitive impairment participants.
Discussion
Main Findings
In this study, we have several main findings. First, after adequately controlling for the covariates, there was a direct relationship between cognitive performances and the measures of activity. The average steps per day were correlated with score in CASI-Short-term memory, CASI-Visual construction, animal naming, and TMB (corrected). The average distance (kilometers) per day was correlated with score in CASI-Attention, TMB (corrected), and CASI-Visual construction. The average calorie expenditure per day was correlated with score in TMB (corrected) and animal naming. Second, among the cognition-activity relationship, CASI-Visual construction function was independently associated with average step per day, average distance (kilometers) per day, and average calorie expenditure per day. In addition, score in animal naming was independently associated with average calorie expenditure per day. Third, to differentiate early- and late-stage MCI participants, average step per day showed an optimal sensitivity and specificity, but average distance (kilometers) per day and average calorie expenditure per day did not. The performance of average steps per day was significantly superior to that of average calorie expenditure per day.
Average Step Counts per Day and Cognition
In this study, participants with MCI had an average of 6,935.9 (SD = 3,902.0) steps per day. Interestingly, another study (Chen et al., 2019) shows that cognitively normal older adults with an average age of 69.9 (SD = 5.0) years, which is similar to our MCI participants with an average age of 69.7 (SD = 8.0) years, have an average of 7,454.6 (SD = 3,404.4) steps per day. Although we did not include normal controls (NCs) in this study, our results, taken together with the previous study (Chen et al., 2019), suggested that there was a significant difference in average step counts between NCs and participants with MCI. Subsequently, we demonstrated that greater average steps per day were associated with higher scores in memory, visuospatial function, semantic category fluency, and executive function. The findings are consistent with rich literatures suggesting that greater engagement in physical activity is associated with increased cerebral blood perfusion (Alfini et al., 2019), changes in brain networks (Chirles et al., 2017), modulation of brain plasticity (Fernandes et al., 2017), and activation of noradrenergic system (Segal et al., 2012). Through these mechanisms, physical activity is associated with better memory (Fernandes et al., 2017), executive function (Suzuki et al., 2012), verbal fluency (Suzuki et al., 2012), and visuospatial function (Suzuki et al., 2012).
Interestingly, we suggested that more average step counts per day instead of longer walking distance or higher burned calories were associated with better cognition. The finding of importance of step counts is in consistent one recent study demonstrating that physical activity of steps measured by pedometer is associated with amyloid-related cognitive decline and neurodegeneration (Rabin et al., 2019).
Regarding the importance of average steps per day, the study shows that more step counts per day preferentially attenuated cortical thinning in the entorhinal cortex, lateral temporal cortex, and medial parietal regions (Rabin et al., 2019). These regions have been implicated in memory (Rabin et al., 2019). When we further used ROC analysis to differentiate between participants with early-stage MCI and those with late-stage MCI, we found the optimal cut-off values of average step counts per day significantly differentiated early-stage MCI participants from late-stage MCI participants. Because the risk of Alzheimer’s disease (AD) conversion is higher in late-stage MCI than early-stage MCI (Ye et al., 2013), early-stage MCI participants with more average step counts per day may be associated with less risk of AD conversion.
Average Calorie Expenditure per Day and Cognition
Although much literature suggests the association between exercise training and cognition (Geda et al., 2010; Nagamatsu et al., 2012; Nagamatsu et al., 2013), still two review articles reveal inconclusive effects of exercise on cognition (Gates et al., 2013; Ohman et al., 2014). Among the studies showing no effect of exercise on cognition, the effectiveness of exercise interventions is assessed by reporting whether there are positive effects of moderate or vigorous exercise on cognition or not. The activity intensity of moderate or vigorous exercise is estimated by heart rate during the activity (Karvonen et al., 1957), which has been associated with calorie expenditure. In this study, we suggested a lack of association between calorie expenditure per day and most of cognitive function. This observation combined with the context of these previous literatures about inconclusive reports on benefit effect of moderate or vigorous exercise on cognition, we suggested that the relationship between calorie expenditure per day and cognition is less prominent than the relationship between average step per day and cognition.
Comparing the Ability of Each Measure of Activity to Differentiate Late-Stage MCI From Early-Stage MCI
Late-stage MCI has been suggested a transitional state between early-stage MCI and AD (Ye et al., 2014). The pattern of cortical thinning in late-stage MCI patients is more widespread than early-stage MCI patients and similar to cortical thinning in AD patients (Dickerson et al., 2009). To differentiate late-stage MCI from early-stage MCI (C. C. Chang et al., 2010; Ye et al., 2014), we found that average step per day yielded a better sensitivity and specificity than average calorie expenditure per day did, while we found a comparable AUC between average distance (kilometers) per day and average step per day or between average distance (kilometers) per day and average calorie expenditure per day. As a whole, among the three measures of activity, only average step counts per day showed an optimal cut-off value to differentiate late-stage MCI participants from early-stage MCI participants.
The impact of average step per day on the stage of MCI might be associated with increased blood flow and functional connectivity of the brain, which can be induced by walking. Previously, exercise of treadmill walking has been found to increase cerebral blood flow in the left insula (Alfini et al., 2019) and to increased functional connectivity between precuneus and parietal lobe (Chirles et al., 2017). Moreover, a study provides evidences that exercise capacity is associated with memory function (Pedrinolla et al., 2019). A longitudinal study further demonstrates that physical activity can slow down the usual cognitive worsening (Fonte et al., 2019). Our present results are in agreement with the positive effect of physical activity on cognition and further suggest that the step counts rather the calorie expenditure of the treadmill walking play a key role in mediating the benefit effects of exercise on cognition.
Although the results of this study support the finding of that average step counts per day may be an important factor in cognitive performances, further studies are necessary to investigate the effect of similar measurement of physical activity on cognition in cognitively normal elderly subjects. One previous study has reported that the percentage of improvement in memory function is associated with the percentage of increase in daily steps in cognitively normal older adults (Nishiguchi et al., 2015). Our result is consistent with the association between daily steps and cognitive functions. The mechanisms might be involved with the influence of exercise training on neural efficiency of prefrontal cortex (Nishiguchi et al., 2015).
Limitations
An advantage to this study is physical exercise was recorded continuously over a week period. However, there are several limitations to this study. First, only a small size in this study may increase the likelihood of a Type II error. Further study of population-based study using wearable digital technology to evaluate the effect of real-world physical activity on cognition may provide further evidence of how exercise improves cognitive function in individuals with MCI. Second, we included individuals with MCI on the bases of clinical assessments. Further neurobiochemical or in vivo pathological evaluation will be needed to investigate the pathophysiological effect of the continuously recorded physical exercise on cognition and dementia. Third, this study aimed to use objective measures to record real-world physical activity. Further studies will be needed to compare the accuracy in differentiating early-stage and late-stage MCI between objective and subjective measures, such as Sedentary Behaviour Questionnaire and International Physical Activity Questionnaire, of fitness. Moreover, other objective measures such as VO2max and heart rate are necessarily included in the further studies to enrich the investigation of the effects of physical activity on cognition. Fourth, it was an exploratory study and the analyses were not adjusted for multiple comparisons. Additional dedicated studies will be needed to confirm the results. Fifth, cognitively normal elderly subjects were not included in this study. Further studies are necessary to investigate the effect of each measure of physical activity on cognition in normal aging.
Conclusion
To differentiate early- and late-stage MCI participants, average step per day showed an optimal sensitivity and specificity with cut-off value of 6,284 steps. Accelerometer-determined measures of activity patterns show as potential measurement to reflect cognitive function.
Supplemental Material
Supplemental material, ASN901182 Supplemental material for Physical Activity and Cognitive Function in Mild Cognitive Impairment by Ya-Ting Chang in ASN Neuro
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by grants from Ministry of Science and Technology (NMRPG8G0351, MOST106-2314-B-182A-169). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ORCID iD
Ya-Ting Chang https://orcid.org/0000-0002-7560-7224
Supplemental Material
Supplemental material for this article is available online.
References
- Alfini A. J., Weiss L. R., Nielson K. A., Verber M. D., Smith J. C. (2019). Resting cerebral blood flow after exercise training in mild cognitive impairment. J Alzheimers Dis, 67, 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieva H., Lafont S., Rouch-Leroyer I., Rainville C., Dartigues J. F., Orgogozo J. M., Fabrigoule C. (2004). Evidencing inhibitory deficits in Alzheimer’s disease through interference effects and shifting disabilities in the Stroop test. Arch Clin Neuropsychol, 19, 791–803. [DOI] [PubMed] [Google Scholar]
- Baker L. D., Frank L. L., Foster-Schubert K., Green P. S., Wilkinson C. W., McTiernan A., Plymate S. R., Fishel M. A., Watson G. S., Cholerton B. A., Duncan G. E., Mehta P. D., Craft S. (2010). Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol, 67, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone K. B. (2000). The Boston Qualitative Scoring System for the Rey-Osterrieth complex figure. J Clin Exp Neuropsychol, 22, 430–434. [DOI] [PubMed] [Google Scholar]
- Case M. A., Burwick H. A., Volpp K. G., Patel M. S. (2015). Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA, 313, 625–626. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Kramer J. H., Lin K. N., Chang W. N., Wang Y. L., Huang C. W., Lin Y. T., Chen C., Wang P. N. (2010). Validating the Chinese version of the Verbal Learning Test for screening Alzheimer’s disease. J Int Neuropsychol Soc, 16, 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. L., Bondi M. W., McEvoy L. K., Fennema-Notestine C., Salmon D. P., Galasko D., Hagler D. J., Jr, Dale A. M. (2011). Global clinical dementia rating of 0.5 in MCI masks variability related to level of function. Neurology, 76, 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. I., Hsueh M. C., Rutherford R., Park J. H., Liao Y. (2019). The associations between neighborhood walkability attributes and objectively measured physical activity in older adults. PLoS One, 14, e0222268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirles T. J., Reiter K., Weiss L. R., Alfini A. J., Nielson K. A., Smith J. C. (2017). Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J Alzheimers Dis, 57, 845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuspidi C., Tadic M., Grassi G., Mancia G. (2018). Treatment of hypertension: The ESH/ESC guidelines recommendations. Pharmacol Res, 128, 315–321. [DOI] [PubMed] [Google Scholar]
- Dickerson B. C., Bakkour A., Salat D. H., Feczko E., Pacheco J., Greve D. N., Grodstein F., Wright C. I., Blacker D., Rosas H. D., Sperling R. A., Atri A., Growdon J. H., Hyman B. T., Morris J. C., Fischl B., Buckner R. L. (2009). The cortical signature of Alzheimer’s disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex, 19, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B., Slachevsky A., Litvan I., Pillon B. (2000). The FAB: A Frontal Assessment Battery at bedside. Neurology, 55, 1621–1626. [DOI] [PubMed] [Google Scholar]
- El-Amrawy F., Nounou M. I. (2015). Are currently available wearable devices for activity tracking and heart rate monitoring accurate, precise, and medically beneficial? Healthc Inform Res, 21, 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. I., Voss M. W., Prakash R. S., Basak C., Szabo A., Chaddock L., Kim J. S., Heo S., Alves H., White S. M., Wojcicki T. R., Mailey E., Vieira V. J., Martin S. A., Pence B. D., Woods J. A., McAuley E., Kramer A. F. (2011). Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A, 108, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J., Arida R. M., Gomez-Pinilla F. (2017). Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci Biobehav Rev, 80, 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J., Soares J. C., do Amaral Baliego L. G., Arida R. M. (2016). A single bout of resistance exercise improves memory consolidation and increases the expression of synaptic proteins in the hippocampus. Hippocampus, 26, 1096–1103. [DOI] [PubMed] [Google Scholar]
- Fonte C., Smania N., Pedrinolla A., Munari D., Gandolfi M., Picelli A., Varalta V., Benetti M. V., Brugnera A., Federico A., Muti E., Tamburin S., Schena F., Venturelli M. (2019). Comparison between physical and cognitive treatment in patients with MCI and Alzheimer’s disease. Aging (Albany NY), 11, 3138–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates N., Fiatarone Singh M. A., Sachdev P. S., Valenzuela M. (2013). The effect of exercise training on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. Am J Geriatr Psychiatry, 21, 1086–1097. [DOI] [PubMed] [Google Scholar]
- Geda Y. E., Roberts R. O., Knopman D. S., Christianson T. J., Pankratz V. S., Ivnik R. J., Boeve B. F., Tangalos E. G., Petersen R. C., Rocca W. A. (2010). Physical exercise, aging, and mild cognitive impairment: A population-based study. Arch Neurol, 67, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancox J. E., van der Wardt V., Pollock K., Booth V., Vedhara K., Harwood R. H. (2019). Factors influencing adherence to home-based strength and balance exercises among older adults with mild cognitive impairment and early dementia: Promoting Activity, Independence and Stability in Early Dementia (PrAISED). PLoS One, 14, e0217387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogi A. L., Kikas E. (2016). Calculation and word problem-solving skills in primary grades—Impact of cognitive abilities and longitudinal interrelations with task-persistent behavior. Br J Educ Psychol, 86, 165–181. [DOI] [PubMed] [Google Scholar]
- Kaplan E. F., Goodglass H., Weintraub S. (1983). The Boston naming test. Philadelphia, PA: Lea & Febiger. [Google Scholar]
- Karr S. (2017). Epidemiology and management of hyperlipidemia. Am J Manag Care, 23, S139–S148. [PubMed] [Google Scholar]
- Karvonen M. J., Kentala E., Mustala O. (1957). The effects of training on heart rate: A longitudinal study. Ann Med Exp Biol Fenn, 35, 307–315. [PubMed] [Google Scholar]
- Kelly R. E., Michelle M. G., Robert D. F. (2015). Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Activity, 12, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M. D. (2004). Neuropsychological assessment (4th ed.) New York, NY: Oxford University Press. [Google Scholar]
- Liu H. C., Chou P., Lin K. N., Wang S. J., Fuh J. L., Lin H. C., Liu C. Y., Wu G. S., Larson E. B., White L. R., Graves A. B. (1994). Assessing cognitive abilities and dementia in a predominantly illiterate population of older individuals in Kinmen. Psychol Med, 24, 763–770. [DOI] [PubMed] [Google Scholar]
- Liu-Ambrose T., Best J. R., Davis J. C., Eng J. J., Lee P. E., Jacova C., Boyd L. A., Brasher P. M., Munkacsy M., Cheung W., Hsiung G. R. (2016). Aerobic exercise and vascular cognitive impairment: A randomized controlled trial. Neurology, 87, 2082–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro K., Shimada M., Yamaguchi S., Ishizaki J., Ishii H., Shimada Y., Sato M., Yamadori A., Sekita Y. (2001). Cognitive function and frontal lobe atrophy in normal elderly adults: Implications for dementia not as aging-related disorders and the reserve hypothesis. Psychiatry Clin Neurosci, 55, 565–572. [DOI] [PubMed] [Google Scholar]
- Mickova E., Machova K., Dadova K., Svobodova I. (2019). Does dog ownership affect physical activity, sleep, and self-reported health in older adults? Int J Environ Res Public Health, 16(18), 3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. C. (1993). The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology, 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- Morris J. C., Heyman A., Mohs R. C., Hughes J. P., van Belle G., Fillenbaum G., Mellits E. D., Clark C. (1989). The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology, 39, 1159–1165. [DOI] [PubMed] [Google Scholar]
- Nagamatsu L. S., Chan A., Davis J. C., Beattie B. L., Graf P., Voss M. W., Sharma D., Liu-Ambrose T. (2013). Physical activity improves verbal and spatial memory in older adults with probable mild cognitive impairment: A 6-month randomized controlled trial. J Aging Res, 2013, 861893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu L. S., Handy T. C., Hsu C. L., Voss M., Liu-Ambrose T. (2012). Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med, 172, 666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi S., Yamada M., Tanigawa T., Sekiyama K., Kawagoe T., Suzuki M., Yoshikawa S., Abe N., Otsuka Y., Nakai R., Aoyama T., Tsuboyama T. (2015). A 12-week physical and cognitive exercise program can improve cognitive function and neural efficiency in community-dwelling older adults: A randomized controlled trial. J Am Geriatr Soc, 63, 1355–1363. [DOI] [PubMed] [Google Scholar]
- Ohman H., Savikko N., Strandberg T. E., Pitkala K. H. (2014). Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: A systematic review. Dement Geriatr Cogn Disord, 38, 347–365. [DOI] [PubMed] [Google Scholar]
- Pedrinolla A., Venturelli M., Tamburin S., Fonte C., Stabile A. M., Galazzo I. B., Ghinassi B., Venneri M. A., Pizzini F. B., Muti E., Smania N., Di Baldassarre A., Naro F., Rende M., Schena F. (2019). Non-Abeta-dependent factors associated with global cognitive and physical function in Alzheimer’s disease: A pilot multivariate analysis. J Clin Med, 8, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwek L., Ellis D. A., Andrews S., Joinson A. (2016). The rise of consumer health wearables: Promises and barriers. PLoS Med, 13, e1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin J. S., Klein H., Kirn D. R., Schultz A. P., Yang H. S., Hampton O., Jiang S., Buckley R. F., Viswanathan A., Hedden T., Pruzin J., Yau W. W., Guzman-Velez E., Quiroz Y. T., Properzi M., Marshall G. A., Rentz D. M., Johnson K. A., Sperling R. A., Chhatwal J. P. (2019). Associations of physical activity and beta-amyloid with longitudinal cognition and neurodegeneration in clinically normal older adults. JAMA Neurol, 76(10), 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. M. (1955). The relation of the trail making test to organic brain damage. J Consult Psychol, 19, 393–394. [DOI] [PubMed] [Google Scholar]
- Segal S. K., Cotman C. W., Cahill L. F. (2012). Exercise-induced noradrenergic activation enhances memory consolidation in both normal aging and patients with amnestic mild cognitive impairment. J Alzheimers Dis, 32, 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y., Nanjo K., Tajima N., Kadowaki T., Kashiwagi A., Araki E., Ito C., Inagaki N., Iwamoto Y., Kasuga M., Hanafusa T., Haneda M., Ueki K. (2010). Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig, 1, 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S. T., Insana S. P. (2014). Caloric expenditure assessment among older adults: Criterion validity of a novel accelerometry device. J Health Psychol, 19, 1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiton N., Alharbi M., Bauman A., Neubeck L., Gullick J., Bhindi R., Gallagher R. (2018). The validity and reliability of consumer-grade activity trackers in older, community-dwelling adults: A systematic review. Maturitas, 112, 85–93. [DOI] [PubMed] [Google Scholar]
- Strohle A., Schmidt D. K., Schultz F., Fricke N., Staden T., Hellweg R., Priller J., Rapp M. A., Rieckmann N. (2015). Drug and exercise treatment of Alzheimer disease and mild cognitive impairment: A systematic review and meta-analysis of effects on cognition in randomized controlled trials. Am J Geriatr Psychiatry, 23, 1234–1249. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Shimada H., Makizako H., Doi T., Yoshida D., Tsutsumimoto K., Anan Y., Uemura K., Lee S., Park H. (2012). Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: A randomized controlled trial. BMC Neurol, 12, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs J., Pollock C. L., Guenther J. R., Bahar M., Napier C., Hunt M. A. (2014). Validation of the Fitbit One activity monitor device during treadmill walking. J Sci Med Sport, 17, 496–500. [DOI] [PubMed] [Google Scholar]
- Warrington E. K., James M. (1991). The visual object and space perception battery. Bury St Edmunds, England: Thames Valley Test Company. [Google Scholar]
- Weintraub S., Salmon D., Mercaldo N., Ferris S., Graff-Radford N. R., Chui H., Cummings J., DeCarli C., Foster N. L., Galasko D., Peskind E., Dietrich W., Beekly D. L., Kukull W. A., Morris J. C. (2009). The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord, 23, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Wen D., Liang L., Jia Y., Gao L., Lei J. (2018). Evaluating the validity of current mainstream wearable devices in fitness tracking under various physical activities: Comparative study. JMIR Mhealth Uhealth, 6, e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B. S., Seo S. W., Cho H., Kim S. Y., Lee J. S., Kim E. J., Lee Y., Back J. H., Hong C. H., Choi S. H., Park K. W., Ku B. D., Moon S. Y., Kim S., Han S. H., Lee J. H., Cheong H. K., Na D. L. (2013). Effects of education on the progression of early- versus late-stage mild cognitive impairment. Int Psychogeriatr, 25, 597–606. [DOI] [PubMed] [Google Scholar]
- Ye B. S., Seo S. W., Yang J. J., Kim H. J., Kim Y. J., Yoon C. W., Cho H., Noh Y., Kim G. H., Chin J., Kim J. H., Jeon S., Lee J. M., Na D. L. (2014). Comparison of cortical thickness in patients with early-stage versus late-stage amnestic mild cognitive impairment. Eur J Neurol, 21, 86–92. [DOI] [PubMed] [Google Scholar]
- Zheng G., Xia R., Zhou W., Tao J., Chen L. (2016). Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med, 50, 1443–1450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, ASN901182 Supplemental material for Physical Activity and Cognitive Function in Mild Cognitive Impairment by Ya-Ting Chang in ASN Neuro