Abstract
The neuropathology associated with cognitive decline in military personnel exposed to traumatic brain injury (TBI) and chronic stress is incompletely understood. Few studies have examined clinicopathologic correlations between phosphorylated-tau neurofibrillary tangles, β-amyloid neuritic plaques, neuroinflammation, or white matter (WM) lesions, and neuropsychiatric disorders in veterans. We describe clinicopathologic findings in 4 military veterans with early-onset dementia (EOD) who had varying histories of blunt- and blast-TBI, cognitive decline, behavioral abnormalities, post-traumatic stress disorder, suicidal ideation, and suicide. We found that pathologic lesions in these military-EOD cases could not be categorized as classic Alzheimer’s disease (AD), chronic traumatic encephalopathy, traumatic axonal injury, or other well-characterized clinicopathologic entities. Rather, we observed a mixture of polypathology with unusual patterns compared with pathologies found in AD or other dementias. Also, ultrahigh resolution ex vivo MRI in 2 of these 4 brains revealed unusual patterns of periventricular WM injury. These findings suggest that military-EOD cases are associated with atypical combinations of brain lesions and distribution rarely seen in nonmilitary populations. Future prospective studies that acquire neuropsychiatric data before and after deployments, as well as genetic and environmental exposure data, are needed to further elucidate clinicopathologic correlations in military-EOD.
Keywords: Brain co-occurring pathologies, Chronic stress, Combat-TBI, Histologic distribution, Short- and long-terms neuropsychiatric manifestations in veterans, War settings
INTRODUCTION
Military personnel who develop early-onset dementia (EOD), or early cognitive decline associated with behavioral changes, may present with atypical neurologic and psychiatric phenotypes due to combat exposures to blunt- and blast-traumatic brain injury (TBI) in the context of chronic psychologic stress. Moreover, chronic physical and psychologic stress during intensive military training might represent potential risk factors for early or accelerated cognitive decline or behavioral abnormalities. Civilian populations may similarly be at a higher risk for EOD or accelerated cognitive/behavioral decline if exposed to comparable levels of chronic psychologic stress and TBI.
A key question in neuropathologic studies of EOD in war veterans with a history of TBI and battlefield stress is whether there are specific molecular changes and histopathologic patterns that are distinct from those observed in other cognitive and behavioral disorders such as Alzheimer’s disease (AD), frontotemporal dementia (FTD), dementia with Lewy bodies, and chronic traumatic encephalopathy (CTE). Moreover, it remains to be determined whether the pathophysiologic processes underlying EOD in military personnel exposed to combat-TBI and chronic stress are implicated in the pathogenesis of psychiatric manifestations such as post-traumatic stress disorder (PTSD), personality changes, and suicidal ideation (1–3).
Here, we describe clinical, cognitive, behavioral, neuroimaging, genetic, and neuropathologic data in 4 military veterans with EOD. The goal of this preliminary investigation was to perform a cliniconeuropathologic characterization of the distribution of brain pathologies across 15 regions of the cerebral hemispheres and brainstem in these military-EOD cases. We used a comprehensive panel of antibodies to identify different types of brain pathologies, including typical neurodegenerative lesions (e.g. hyperphosphorylated-tau tangles [pTau], β-amyloid neuritic plaques [βA-NP], α-synuclein [α-syn]-positive Lewy bodies), astrogliosis, microglial activation, white matter (WM) abnormalities, and vascular lesions. We also performed ex vivo ultrahigh resolution MRI for 2 of the cases to complement the histopathologic analysis. We demonstrate that military-EOD cases can result in uncommon combinations and distributions of brain lesions, which are associated with unusual neuropsychiatric features compared with nonmilitary age-matched subjects. These neuropsychiatric features include early-onset cognitive decline and non-AD dementias that may be associated with combat-PTSD, severe behavioral changes, suicidal ideation, and suicide, as documented in the 4 military-EOD cases described here.
Clinical History
We present the clinical histories of a series of 4 military-EOD subjects whose brains were consecutively received as donations for our military brain tissue repository (BTR) (https://www.researchbraininjury.org).
Case 1
The main clinical, cognitive, and neuropathologic features of this subject have been previously described (4). However, in the present study, we extend the neuropathologic analyses of this brain by including new aspects not previously described. Briefly, the patient was a retired military officer with no family history of neuropsychiatric diseases. He had a mild TBI with brief loss of consciousness (LOC) during combat training in his second decade of life. At age 46, he experienced a second TBI due to a motor vehicle accident with LOC of unknown duration. A head computed tomography (CT) scan was negative for intracranial abnormalities. He was hospitalized for 12 days and had post-traumatic amnesia for 18 days.
One month postinjury, he continued to experience cognitive impairment and a brain MRI showed a focus of increased signal intensity in the left periventricular WM. Over the next 18 months, he made a near-complete cognitive recovery except for persistent dysnomia, irritability, and diminished short-term memory. Twenty-one months after injury, he performed average on a comprehensive neuropsychologic examination, but mild deficits in graphomotor skills and working memory persisted. At age 51, he retired from the military and worked in the private sector until the age 59, when he began to complain of fatigue and memory difficulties. He experienced depression, irritability, agitation, fatigability, deficits of memory, visuospatial, calculations, executive functions, and had recurrent episodes of hyperventilation with confusion not associated with epileptiform activity. At age 63, he recalled 0/3 items at 5 minutes and scored 20/30 on the Mini-Mental State Examination (MMSE). At age 67, his MMSE decreased to 11/30. A brain MRI at age 67 showed global volume loss and multifocal hyperintense signals in the subcortical and periventricular WM with no change from prior scans. His agitation increased and included public outbursts of violence requiring intervention. He was moved to a secure nursing facility for his safety and care. He died at age 72 with a diagnosis of severe dementia (not otherwise specified), 25 years after his last TBI, and 12 years after his cognitive decline was clinically assessed. During his progressive neurocognitive decline, he was variably diagnosed with “TBI-related FTD”, probable AD, or “cortical dementia”.
Case 2
The subject joined the Marines Corps at age 17 and deployed to Vietnam in 1968, where he reported a blunt-TBI without LOC. This event was described by the family as a major head trauma. His medical history was also notable for chronic heavy smoking and alcohol abuse (40 years), pulmonary emphysema, and hypertension. He had no family history of major neurologic or psychiatric diseases.
At age 59, he suffered a fall causing right scapula and rib fractures (TBI or LOC was not reported). At age 60, the subject carried a diagnosis of depression without psychosis by Veterans Affairs (VA) psychiatrists. At age 65, he suffered multiple episodes of “ministrokes” (neuroimaging not available) and stopped smoking. Family members reported that he frequently had nightmares, especially during the last 2–3 years of his life, as well as memory difficulties. When questioned by family members about his nightmares, the patient preferred to avoid talking about them. No sleep disorders (e.g. sleepwalking or REM sleep behavioral disorder) were reported in this subject or in his family. Cognitive decline and behavioral disorders were assessed multiple times at his local VA medical center. The subject never received a diagnosis of possible or probable AD (5) and he was diagnosed as having early cognitive decline associated with possible PTSD, psychiatric abnormalities, and alcoholism.
A week before committing suicide (age 66), he awakened his mother at 3 am to tell her that “he killed a kid in Vietnam but that he was ordered and he didn’t want to, and that he could not sleep since this episode was bothering him”. The day he committed suicide, he informed his family that “he was told by the VA that he killed 22 people when he was in Vietnam but that they were wrong and he did not do it and that for that reason he was going to kill himself”. After this call, he attempted suicide by hanging himself and, when found, he was transported to the local emergency department for resuscitation. A CT scan of the head was performed and was notable for the absence of acute lesions or edema and preservation of grey-WM differentiation. Mild generalized volume loss was observed along with chronic lacunar infarcts and subcortical hypodensities consistent with chronic microangiopathic changes. After a series of resuscitation procedures for more than 30 minutes, the patient continued to decline and he was declared dead 4 hours after his arrival at the emergency department.
Case 3
The subject joined the British Army at age 18 and deployed in Northern Ireland (1980), Sierra Leone (1991), and Bosnia (1993). He experienced at least 3 life-threatening attacks with explosives. In 1980, he reported having been exposed to blasts with resulting shrapnel wounds to his legs. Additional TBIs occurred during the Balkans conflict in 1993 when his armored vehicle was damaged by a roadside bomb. No LOC or post-traumatic amnesia were reported. During conflicts he was exposed to the corpses of murdered women and children, witnessed the death of his interpreter killed next to him, civilian rape in orphanages, and tortures. No family history of major neurologic or psychiatric diseases were reported.
At age 52, his wife reported that he was having significant memory difficulties. At age 57, he experienced further cognitive decline with “patchy” memory and problems with time orientation, speech, reading, writing, attention, spatial awareness, and anger outbursts. At age 59, he experienced dissociative episodes “acting as if he was in another environment and situation, and states of high emotional arousal, agitation, aggression and distress”. The same year, he was diagnosed with PTSD by a psychiatrist. He was then referred to the local mental health services where he was diagnosed with probable mixed Alzheimer/vascular dementia. Neuropsychologic testing confirmed severe impairment of memory, word finding, frontal lobe functioning, visuospatial, and object perception.
At age 60, a military psychiatrist reported: “The subject was able to describe incidents in Bosnia and Sierra Leone. He struggles with the incident of the rape of children in Bosnia which caused him much guilt and shame. He tends to ruminate over these experiences and was distressed talking about them. The ruminations that he re-experiences seem to have been released by his cognitive decline. Problems with his memory—for example he couldn’t remember his service number—or much of his military career. It is unclear whether he had true intrusive thoughts or how the traumatic memories were manifest in his current cognitive situation”. A brain MRI showed WM changes, enlarged lateral ventricles, reduced hippocampal volumes, and marked bilateral cortical atrophy with loss of volume in the parietal and occipital lobes.
At age 61, he was admitted to a psychiatric hospital (UK), with a diagnosis of “anxiety and depressive disorder, cognitive impairment, possible diagnosis of postconcussional syndrome and atypical PTSD”. After discharge from the hospital he became very irritable, going to a neighbor’s house and saying that his wife was drug-dealing in the house. He used to grab and push his wife down into a chair, have his hands around her throat, then stop and run away. He often went to a neighbor’s house who brought him back, but upon returning home, he would again attack his wife and try to strangle and hit her. During another of these episodes, he suddenly ripped the computer from the cables and stamped on it saying “I know what I’m doing”. After these episodes, he received a diagnosis of “pseudodementia”, as referred by a dementia specialist who concluded that he most likely had AD with PTSD.
At age 62, he and his wife went to Greece where the subject disappeared for 24 hours. He was found by the Greek military many miles from where he was last seen, having traveled on foot overnight through very rough terrain and when found, he was confused. After 2 months, he went missing again from home and was found 8 hours later, wet and confused, saying that he was expecting to be put in a truck to go back to camp. At age 64, he was admitted under a medical hold following another attempt to strangle his wife, and there were a number of incidents reported in which he pushed care workers trying to help him. After a few months, the subject was admitted to a general hospital with a high temperature and stomach cramps where he was diagnosed with a perforated bowel. Reparative surgery was performed but the subject died postoperatively.
Case 4
The subject, a Vietnam War veteran, died at age 63 with a diagnosis of probable FTD (6), which had been diagnosed at age 50. The diagnosis of probable FTD was based on episodes of spatial disorientation and socially inappropriate behaviors. After returning from the war, family members reported that he acted “weird” and he was not “like before”. His medical history was characterized by a motor vehicle accident at age 37 for which he was hospitalized for 1 week and discharged with a diagnosis of moderate TBI with LOC (during the postmortem interview with his family members, his son, and daughter suspected that this motor vehicle accident could have been his first episode of attempted suicide). At age 38, the subject retired from active duty service and ran a private business until age 51. At age 38, there was a suicide attempt, which was witnessed by his daughter. After this last suicide attempt, the family recognized that the subject abused alcohol on a daily basis. Around age 40, the patient started to suffer episodes of memory loss. The cognitive impairment was progressive and he was moved to an assisted living center at age 55. From age 58 until his death he became progressively aphasic but still capable of self-feeding until the age of 61.
No family history of dementia or other major neurologic or psychiatric conditions, blast exposure, single or repetitive TBI episodes, or contact sports practices were reported in his medical records. An interview with his daughter and son confirmed that the subject was experiencing spatial disorientation for at least 16 years before death. Both daughter and son confirmed the absence of other family members affected by any genetic, neurologic, or psychiatric disorders.
MATERIALS AND METHODS
Whole formalin-fixed brains from these 4 male military veterans who had sustained nonblast or blast-TBI in early-mid adulthood were consecutively received as part of our brain donation program and stored in our brain bank (http://www.researchbraininjury.org) for neuropathologic assessment and research studies. All 4 veterans were clinically diagnosed with early cognitive decline and psychiatric disorders between their fifth and sixth decade of life. All subjects had a history of military life plus at least 2 of the following: Impact-TBI or blast-TBI, life-threatening war experiences, exposure to highly stressful military settings including training and deployments in war zones. Subjects occupied different levels of the military hierarchy. Table 1 summarizes the relevant demographic, neurologic, psychiatric, APOE genotype, and TBI history for each case.
TABLE 1.
Main Demographic, Neurologic, Psychiatric, APOE Genotype, and TBI History of 4 Military-EOD Cases
| Case ID | Sex | Race | APOE | Impact TBI | Blast TBI | War Experiences/Combat Settings/Military Training | Contact Sport Practice | Dementia onset (Age) | Age at Death | Neurologic Diagnoses | Psychiatric Diagnoses | Family History for Neurologic or Psychiatric Conditions | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | M | W | E3/E3 | Yes (LOC during training; MVA at 46) | Unknown/possible | Yes | Yes (not professional, boxing at the Naval academy) | 59 (formal diagnosis) 48 (initial deficits) | 72 | Dementia (atypical AD) | Not reported | Not reported | Cardio-respiratory arrest |
| #2 | M | W | E3/E4 | Yes (Vietnam war; major trauma at 59) | Unknown/possible | Yes (Vietnam) | No | ∼60 | 66 | Alcoholism | Depression; possible maniac episodes | Not reported | Suicide |
| #3 | M | W | E3/E3 | Yes (during each of the 3 deployment periods) | Yes (3 episodes reported) | Yes (Northern Ireland 1980, Sierra Leone 1990, Balkans 1993) | No | 52 | 64 | Dementia (mixed probable AD/vascular dementia) | PTSD | Not reported | Bowel perforation; Multi-organ failure; sepsis, |
| #4 | M | W | E3/E3 | Yes (MVA at 35) | No | Yes (Vietnam) | No | 50 | 63 | Dementia (possible bv FTD [Pick disease]; atypical AD) | Suicide attempt (age 66) | Not reported | Cardio-respiratory arrest |
| 52.5±5.2 | 66.0±3.5 |
The table summarizes the main demographic, APOE genotype, and clinical data of 4 early-onset dementia (EOD) cases among military and war veterans.
LOC, loss of consciousness; MVA, motor vehicle accident; PTSD, post-traumatic stress disorder; AD, Alzheimer’s disease; bv-FTD, behavioral variant-frontotemporal dementia; M, male; W, white; E3, APOE3; E4, APOE4.
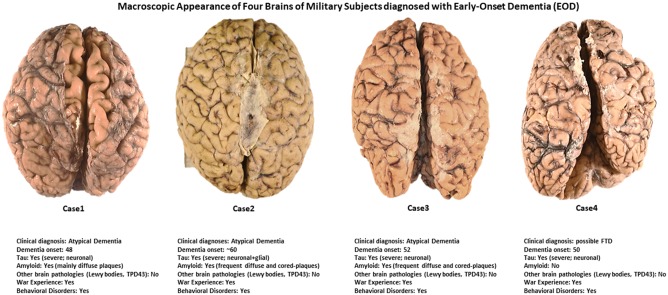
For each brain, the decedents’ next-of-kin or legal representative provided an institutional IRB-approved written consent to donate the specimen for diagnostic and research purposes. Three brains (Cases 1–3) are part of the BTR and Neuropathology Core, CNRM (https://www.usuhs.edu/cnrm), Uniformed Services University (USU), Bethesda, Maryland. Case 4 is part of the Chronic Effects of Neurotrauma Consortium (CENC) (https://www.cencstudy.org) brain collection stored at BTR on behalf of the Department of Pathology, F. Edward Hébert School of Medicine, Bethesda, Maryland. The external macroscopic appearances and a limited set of clinical data of these 4 military-EOD brains are shown in Figure 1.
FIGURE 1.
Macroscopic appearance of 4 brains of military subjects diagnosed with early onset dementia. All brains looked relatively normal except for Case 4 (see right cerebral hemisphere at level of the frontal pole) and Case 1 in which a moderate level of diffuse cortical atrophy is evident (see intersulcal enlargements).
Ex Vivo MRI Acquisition and Processing
For 2 of 4 cases studied (Cases 1 and 3), we performed ex vivo ultrahigh resolution MRI on the formalin-fixed brains. The ex vivo MRI image acquisitions were performed at the Athinoula A. Martinos Center for Biomedical Imaging, Boston, Massachusetts (https://www.nmr.mgh.harvard.edu). The ex vivo MRI protocol included a 7-Tesla scan (∼18.5 hours) that utilized a multiecho FLASH (MEF) sequence acquired with different flip angles at 200 µm spatial resolution. Acquisition parameters and data processing procedures for the 7-Tesla ex vivo MEF sequence have been previously described (7). Upon completion of data processing, parameter maps (T1, T2*, and proton density) computed from the MEF dataset were analyzed for pathoanatomic lesions, which were then correlated with histopathologic data. No in vivo MRI images were available for 3 of the 4 cases described.
Neuropathology Assessment and Procedures
Brains were immersed in 10% buffered formalin for tissue fixation for 3–5 weeks. At gross examination, each brain was assessed to identify externally visible macroscopic lesions or abnormalities. A symmetric brain cutting procedure was applied to all 4 specimens (8). To keep consistency across all brain cuttings, a human-adapted 3D printed brain mold cutter was employed (9). For each brain, 15 regions from the left and right cerebral hemisphere and brainstem were sampled, resulting in a total of 30 regions/tissue blocks for each brain.
The sampled regions from each hemisphere were olfactory bulb (OB), middle frontal gyrus (MFG), orbito-frontal gyrus/gyrus rectus (OFG), middle temporal cortex (MTC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), anterior insular cortex (AInsC), occipital cortex (BA17 and 18) (OC), amygdala (AMY), basal ganglia (BGMY), mammillary bodies + hypothalamus (MAM+HYPO), posterior hippocampus (at CGL) including entorhinal cortex and inf. temp cortex (PH+EC+ITC), mesencephalon (including substantia nigra) (MESSN), pons (including locus coeruleus) (PONS), medulla oblongata (including dorsal motor of Vagus) (MObl).
Histology and Immunohistochemistry Procedures
All 30 tissue blocks from each brain were uniformly processed using an automated tissue processor (ASP 6025, Leica Biosystems, Nussloch, Germany). After tissue processing, each tissue block was embedded in paraffin and cut in a series of 20 5-µm-thick consecutive sections. The first 3 sections were selected for hematoxylin and eosin (H&E), Luxol fast blue (LFB), and cresyl violet stains, while the remaining sections were available for immunohistochemistry procedures. Immunohistochemistry procedures for each antibody on all dissected brain regions were performed using a Leica Bond III automated immunostainer with a diaminobenzidine chromogen detection system (DS9800, Leica Biosystems, Buffalo Grove, IL). The following antibodies were used: antiphosphorylated tau (pTau) (AT8, mouse antihuman monoclonal antibody, dilution 1:2000 epitope retrieval time 10 minutes, MN1020; ThermoScientific, Waltham, MA); antiphosphorylated tau (CP13, mouse antihuman monoclonal antibody, dilution 1: 2000, epitope retrieval time 10 minutes; this antibody was kindly donated by Dr Peter Davies, Albert Einstein College of Medicine, New York, NY); all-forms tau (HT7, mouse antihuman monoclonal antibody, dilution 1: 150, epitope retrieval time 10 minutes, MN1000; ThermoScientific); anti-1–42 β-amyloid (1–42 βA) (4G8; mouse antihuman monoclonal antibody, dilution 1: 500, epitope retrieval time 10 minutes, SIG-39220; Covance/BiolLegend, San Diego, CA); antiamyloid precursor protein ([APP]; mouse antihuman monoclonal antibody clone 22c11, dilution 1: 10, epitope retrieval time 10 minutes, MAB348; EMD Millipore, Burlington); antiphosphorylated α-syn (pα-syn; rabbit monoclonal antiphosphor S129, dilution 1:100, epitope retrieval time 10 minutes, Abcam 51253; Abcam, Cambridge, UK); antiglial fibrillary acidic protein ([GFAP]; mouse antihuman monoclonal antibody GA5, with bond heat-induced epitope retrieval, epitope retrieval time 10 minutes, PA0026; Leica Biosystems, Wetzlar, Germany); anti-ionized calcium-binding adapter molecule 1 ([Iba-1]; rabbit polyclonal, dilution 1: 100, epitope retrieval time 10 minutes, Wako 016-20001; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan); antitransactive response DNA binding protein 43 kDa ([TDP43]; rabbit monoclonal anti-TDP43, dilution 1: 100, epitope retrieval time 10 minutes; Abcam 109535; Abcam); antiubiquitin ([anti-Ubiq]; mouse monoclonal, dilution 1: 100, epitope retrieval time 10 minutes, Abcam 7254; Abcam); antinucleoporin p62 ([p62]; mouse monoclonal, dilution 1: 100, epitope retrieval time 10 minutes; #610833; BD Biosciences, San Jose, CA); anti-CD68 (CD68, mouse antihuman monoclonal antibody clone 514H12, epitope retrieval time 20 minutes, PA0273; Leica Biosystems).
All stained sections were scanned by Aperio scanner system (Aperio AT2 - High Volume, Digital whole slide scanning scanner, Leica Biosystems, Inc., Richmond, IL) and stored in Biolucida, a hub for 2D and 3D image data (version 2017, MBF Bioscience, Williston, VT), for further assessment and analyses. A preliminary neuropathologic assessment for each section was performed using Aperio ImageScope (Aperio ImageScope, version 2016, Leica Biosystems, Inc.) to verify the positive immunoreactivity for each antibody as well as localization and pathologic severity, to identify possible differences between left and right hemispheres in each brain, and across all 4 brains. After a preliminary ImageScope inspection (max 20×), a Zeiss Imager A2 (ImagerA2 microscope, Zeiss, Munich, Germany) bright-field microscope with higher magnification lenses (×40, ×63 oil-immersion) was used to identify and photograph histologic and pathologic details as needed.
RESULTS
Gross and main neuropathologic diagnoses are summarized in Table 2. Detailed neuropathologic findings are separately described for each case as follows and summarized in Tables 3–5 and Supplementary DataTables S1–S3.
TABLE 2.
Main Brain Autopsy and Neuropathologic Data of 4 Military-EOD Cases
| CaseID | BW (g) | PMI (hours) | Cortical Atrophy | AD Pathology | PART | ARTAG | CTE | Lewy Body Pathology | Pigmented Loss SNc/LC | Vascular Pathology (Ischemia/Hypoxia/Hemorrhage) | Myelin Loss (LFB) | Astrogliosis (GFAP) | Microgliosis (Iba-1) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CERAD | Braak | ABC | Level of AD neuropathologic changes | |||||||||||||
| #1 | 920.0 | N/A | Yes | C | VI | A3B3C3 | High | No | No | Yes | No | No/No | Yes | Yes | Yes | Yes |
| #2 | 1242.18 | N/A | No | C | VI | A2B3C3 | Intermediate | No | Yes | Yes | No | No/No | No | No | Yes | Yes |
| #3 | 1182.59 | N/A | Yes | C | VI | A2B3C3 | Intermediate | No | No | No | No | No/No | No | No | Yes | Yes |
| #4 | 827.0 | N/A | Yes | 0 | VI | A0B3C0 | Low | Yes | Yes | No | No | No/No | No | No | Yes | Yes |
| 1042.9±200.7 | ||||||||||||||||
The table summarizes the main neuropathologic data of each EOD case analyzed in this study.
BW, brain weight (in grams); PMI, postmortem interval (in hours); AD-pathology, Alzheimer’s disease pathology; PART, primary aging-related tauopathy; ARTAG, aging-related tau astrogliopathy; CTE, chronic traumatic encephalopathy; LFB, Luxol fast blue stain; GFAP, antiglial fibrillary acidic protein antibody; Iba-1, anti-ionized calcium binding adaptor molecule 1 antibody; SNc, substantia nigra pars compacta; LC, locus coeruleus.
TABLE 3.
Vascular Pathology in Military Early-Onset Dementia Cases
| Case #1 |
Case #2 |
Case #3 |
Case #4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Bi-hem/Sym | Left | Right | Bi-hem/Sym | Left | Right | Bi-hem/Sym | Left | Right | Bi-hem/Sym | |
| OB | Neuronal loss | Neuronal loss | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | |
| MFG | Perisulcal neuronal loss | Perisulcal neuronal loss | Yes/Yes | Neg (arteriosclerosis) | Neg (arteriosclerosis) | Yes/Yes | Spongiform tissue (superficial cortical layers) | Spongiform tissue (superficial cortical layers) | Yes/Yes | Spongiform tissue (superficial cortical layers) | Spongiform tissue (superficial cortical layers) | Yes/Yes |
| OFG | Superficial layers atrophy; tissue rarefaction as per ischemia in the white matter | Superficial layers atrophy; tissue rarefaction as per ischemia in the white matter | Yes/Yes | Neg | Neg (arteriosclerosis) | Yes/No | Neg | Neg | Yes/Yes | Spongiform tissue (superficial cortical layers) | Spongiform tissue (superficial cortical layers) | Yes/Yes |
| MTC | Perivascular space enlargements with cellular loss | Perivascular space enlargements with cellular loss | Yes/Yes | Neg | Neg | Yes/Yes | Spongiform tissue (superficial cortical layers) | Spongiform tissue (superficial cortical layers) | Yes/Yes | Spongiform tissue (superficial cortical layers) | Spongiform tissue (superficial cortical layers) | Yes/Yes |
| ACC | Perivascular space enlargements with cellular loss (thinner corpus callosum) | Perivascular space enlargements with cellular loss (thinner corpus callosum) | Yes/Yes | Perivascular enlargement in WM; diffuse arteriosclerosis | Perivascular enlargement in WM; diffuse arteriosclerosis | Yes/Yes | Neg | Neg | Yes/Yes | Spongiform tissue (superficial cortical layers) | Spongiform tissue (superficial cortical layers) | Yes/Yes |
| PCC | Perivascular space enlargements with cellular loss (thinner corpus callosum with marked cellular loss) | Perivascular space enlargements with cellular loss (thinner corpus callosum with marked cellular loss) | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | Area of tissue loss (Corpus Callosum) | Neg | No/No |
| AInsC | Perivascular space enlargements with cellular loss) | Perivascular space enlargements with cellular loss) | Yes/Yes | Perivascular enlargement in WM; diffuse arteriosclerosis | Perivascular enlargement in WM; diffuse arteriosclerosis | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes |
| OC | Perivascular space enlargements with cellular loss; multiple ischemic lesions | Perivascular space enlargements with cellular loss; multiple ischemic lesions | Yes/Yes | Neg (arteriosclerosis) | Neg (arteriosclerosis) | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes |
| AMY | Tissue rarefaction, corpora amylacea | Tissue rarefaction, corpora amylacea | Yes/Yes | Perivascular enlargement; diffuse arteriosclerosis | Perivascular enlargement; diffuse arteriosclerosis | Yes/Yes | Neg | Neg | Yes/Yes | Neg (some perivascular enlargements) | Neg | Yes/Yes |
| BGMY | Perivascular space enlargements with cellular loss; | Perivascular space enlargements with cellular loss; | Yes/Yes | Perivascular enlargement; diffuse arteriosclerosis | Perivascular enlargement; diffuse arteriosclerosis | Yes/Yes | Neg | Neg | Yes/Yes | Neg (some perivascular enlargements) | Neg (some perivascular enlargements) | Yes/Yes |
| MAM+HYPO | Perivascular space enlargements with cellular loss; | Perivascular space enlargements with cellular loss; | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | Neg (some perivascular enlargements; microbleeds) | Neg (some perivascular enlargements; microbleeds) | Yes/Yes |
| PH+EC+ITC | Perivascular space enlargements with cellular loss; multiple ischemic lesions | Perivascular space enlargements with cellular loss; multiple ischemic lesions | Yes/Yes | Neg | Neg | Yes/Yes | Spongiform tissue superficial cortical layers | Spongiform tissue superficial cortical layers | Yes/Yes | Neg (some perivascular enlargements) | Neg (some perivascular enlargements; microbleeds) | Yes/Yes |
| MESSN | Perivascular space enlargements with cellular loss; multiple ischemic lesions | Perivascular space enlargements with cellular loss; multiple ischemic lesions | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes |
| PONS | Perivascular space enlargements with cellular loss; multiple ischemic lesions | Perivascular space enlargements with cellular loss; multiple ischemic lesions | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes |
| MObl | Perivascular space enlargements with cellular loss; multiple ischemic lesions | Perivascular space enlargements with cellular loss; multiple ischemic lesions | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes |
The table describes the vascular pathology findings observed in each early-onset dementia (EOD) military case. The neuropathologic diagnoses were performed on 15 different neuroanatomical regions from both left and right hemisphere.
OB, Olfactory bulb; MFG, middle frontal gyrus; OFG, orbito-frontal gyrus/gyrus rectus; MTC, middle temporal cortex; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; AInsC, anterior insular cortex; OC, occipital cortex (BA17 and 18); AMY, amygdala; BGMY, basal ganglia; MAM+HYPO, mammillary bodies+hypothalamus; PH+EC+ITC, posterior hippocampus (at CGL) including entorhinal cortex and inf. temp cortex; MESSN, mesencephalon (including substantia nigra); PONS, pons (including locus coeruleus); MObl, medulla oblongata (including dorsal motor of vagus).
Neuropathology Assessment
Vascular pathology for all cases is summarized in Table 3.
Case 1
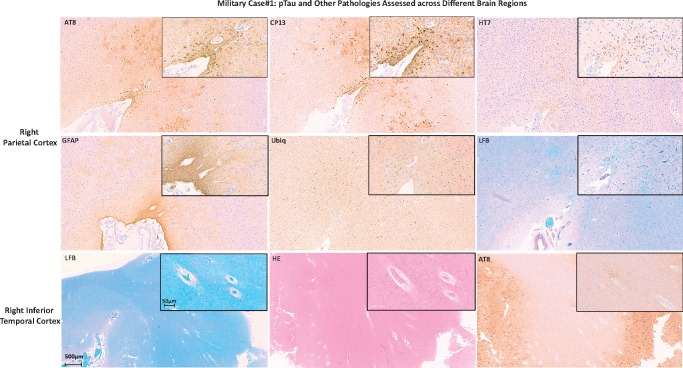
pTau lesions (pTau-NFTs, pTau neurites) and 1–42 β-amyloid plaques (neuritic β-amyloid plaques [1–42 βA-NP]) were frequently observed across all examined regions of the brain (see Tables 4 and 5, respectively). Additionally, both pTau and β-amyloid lesions showed a bihemispheric symmetrical distribution across the examined regions. A more detailed assessment for pTau immunoreactivity using AT8, CP13, and HT7 antibodies showed that pTau levels of severity and distribution could vary across the same anatomical region. In fact, while both AT8 and CP13 showed comparable levels of immunoreactivity in the right parietal cortex (Fig. 2, upper row) with pTau lesions localized in either deeper (AD pathology-like) and more superficial (CTE-like) cortical layers (10), immunoreactivity to HT7 was positive mainly where pTau CTE-like lesions were found (Fig. 2, upper row, AT8, CP13, HT7). Additionally, anti-Ubiq stain evidenced different levels of immunoreactivity between AD-like versus CTE-like pTau pathology with much more Ubiq-positive lesions (e.g. neurites) associated with AD-like pTau lesions (Fig. 2, middle row, Ubiq). Moreover, GFAP stain showed a much more intense reactivity when colocalized with CTE-like pTau than AD-like lesions (Fig. 2, middle row, GFAP). The LFB stain showed areas of myelin loss across multiple regions such as middle and inferior temporal cortex and hippocampal area with marked perivascular space enlargements (Fig. 2, lower row, LFB). CD68, APP, α-syn, and TDP43 immunoreactivity did not show any evidence of traumatic axonal injury (TAI) or intracellular pathology (e.g. Lewy bodies, TDP43 intraneuronal inclusions), or recent ischemic damage. Based on the NIA-AA neuropathologic guidelines for the assessment of AD (11), the level of AD neuropathologic changes was high with an ABC score equal to A3B3C3. However, the distribution and amount of Tau-pathology was not typical for “classic” definite AD (e.g. pTau-NFT mainly distributed in the deeper layers of the cortex versus the most superficial layers usually observed in AD, even advanced AD cases). In summary, this subject was diagnosed with dementia with atypical pTau and β-amyloid pathology distribution mixed with isolated CTE lesions and focal WM loss (10).
TABLE 4.
pTau Pathology (AT8 and CP13) in Military Early-Onset Dementia Cases
| Case #1 |
Case #2 |
Case #3 |
Case #4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Bi-hem/Sym | Left | Right | Bi-hem/Sym | Left | Right | Bi-hem/Sym | Left | Right | Bi-hem/Sym | |
| OB | Freq NFTs | Freq NFTs | Yes/Yes | Rare NFTs | Mod NFTs | Yes/No | Freq NFTs | Isolated cluster NFTs | Yes/No | Mod NFTs | Freq NFTs | Yes/No |
| MFG | Freq NFTs | Freq NFTs | Yes/Yes | Rare NFTs; sparse granular/fuzzy astrocytes in GM and WM) | Rare NFTs; Mod granular/fuzzy astrocytes in GM and WM) | Yes/No | Freq NFTs | Freq NFTs | Yes/Yes | Freq (++) NFTs (all cortical layers and WM glia-tau/ARTAG?) | Freq NFTs (all cortical layers and WM glia-tau/ARTAG?) | Yes/No |
| OFG | Freq NFTs | Freq NFTs | Yes/Yes | Sparse NFTs; sparse granular/fuzzy astrocytes in WM; CTE | Sparse NFTs; sparse granular/fuzzy astrocytes in WM; perivascular-CTE | Yes/Yes sulcal-CTENo/No perivascular-CTE | Freq NFTs; Mod Tau-plaques (superficial cortical layers) | Freq NFTs; Mod Tau-plaques (superficial cortical layers) | Yes/Yes | Freq NFTs (all cortical layers and WM glia-tau/ARTAG?) | Freq NFTs (all cortical layers and WM glia-tau/ARTAG?) | Yes/Yes |
| MTC | Freq NFTs | Freq NFTs | Yes/Yes | Freq NFTs across all cortical layers; Freq granular/fuzzy astrocytes in GM and WM); Isolated CTE | Freq NFTs across all cortical layers; frequent granular/fuzzy astrocytes in GM and WM) | Yes/Yes | Freq NFTs; Mod Tau-plaques (all cortical layers) | Freq NFTs; Mod Tau-plaques (all cortical layers) | Yes/Yes | Freq (+) NFTs (all cortical layers and WM glia-tau/ARTAG?) | Freq NFTs (all cortical layers and WM glia-tau/ARTAG?) | Yes/No |
| ACC | Freq NFTs | Freq NFTs | Yes/Yes | Sparse NFTs; rare granular/fuzzy astrocytes in GM; cluster of granular/fuzzy astrocytes in WM); isolated perivascular lesion (CTE) | Sparse NFTs; rare granular/fuzzy astrocytes in GM and WM) | Yes/No | Freq NFTs | Freq NFTs | Yes/Yes | Freq (+) NFTs (all cortical layers and WM glia-tau/ARTAG?) | Freq (+) NFTs (all cortical layers and WM glia-tau/ARTAG?) | Yes/Yes |
| PCC | Freq NFTs | Freq NFTs | Yes/Yes | No NFTs; very rare granular/fuzzy astrocytes in GM) | No NFTs; rare granular/fuzzy astrocytes in GM) | Yes/Yes | Freq NFTs; Mod Tau-plaques (superficial cortical layers) | Freq NFTs; Mod Tau-plaques (superficial cortical layers) | Yes/Yes | Freq (+) NFTs (all cortical layers and WM glia-tau/ARTAG?) | Freq (+) NFTs (all cortical layers and WM glia-tau/ARTAG?) | Yes/Yes |
| AInsC | Freq NFTs | Freq NFTs | Yes/Yes | Freq NFTs across all cortical layers; Freq granular/fuzzy astrocytes in GM and WM) | Freq NFTs across all cortical layers; Mod granular/fuzzy astrocytes in GM and WM) | Yes/No | Freq NFTs; Mod Tau-plaques (superficial cortical layers) | Freq NFTs; Mod Tau-plaques (superficial cortical layers) | Yes/Yes | Freq (+) NFTs (all cortical layers and WM glia-tau/ARTAG?) | Mod NFTs (all cortical layers) and WM Freq glia-tau/ARTAG?) | Yes/No |
| OC | Freq NFTs | Freq NFTs | Yes/Yes | Neg | Neg | Yes/Yes | Freq NFTs; Mod Tau-plaques (all cortical layers) | Freq NFTs; Mod Tau-plaques (all cortical layers) | Yes/Yes | Neg | Neg | Yes/Yes |
| AMY | Freq NFTs | Freq NFTs | Yes/Yes | Freq NFTs; Freq granular/fuzzy astrocytes) | Freq NFTs; Freq granular/fuzzy astrocytes) | Yes/Yes | Freq NFTs; Mod Tau-plaques | Freq NFTs; Mod Tau-plaques | Yes/Yes | Freq (+) NFTs | Freq (+) NFTs | Yes/Yes |
| BGMY | Freq NFTs | Freq NFTs | Yes/Yes | Freq NFTs; Freq granular/fuzzy astrocytes) | Freq NFTs; Freq granular/fuzzy astrocytes) | Yes/Yes | Neg | Neg | Yes/Yes | Freq in Neurons and Glia cells | Freq in Neurons and Glia cells | Yes/Yes |
| MAM+HYPO | Freq NFTs | Freq NFTs | Yes/Yes | Freq NFTs; Freq granular/fuzzy astrocytes); CTE | Freq NFTs; Freq granular/fuzzy astrocytes); CTE | Yes/Yes | Freq NFTs | Freq NFTs | Yes/Yes | Freq in Neurons and Glia cells | Freq (+) in Neurons and Glia cells | Yes/No |
| PH+EC+ITC | Freq NFTs | Freq NFTs | Yes/Yes | Freq NFTs; Freq granular/fuzzy astrocytes) | Freq NFTs; Freq granular/fuzzy astrocytes) | Yes/No | Freq NFTs; Mod Tau-plaques (all cortical layers) | Freq NFTs; Mod Tau-plaques (all cortical layers) | Yes/Yes | Freq (pick bodies) in neurons and glia cells | Freq (pick bodies) in neurons and glia cells | Yes/Yes |
| MESSN | Freq NFTs | Freq NFTs | Yes/Yes | Sparse NFTs | Rare NFTs | Yes/No | Mod NFTs (in SN) | Mod NFTs (in SN) | Yes/Yes | Freq (pick bodies) | Freq (pick bodies) | Yes/Yes |
| PONS | Freq NFTs | Freq NFTs | Yes/Yes | Sparse NFTs | Rare NFTs | Yes/No | Mod NFTs (in LC and raphei nuclei) | Mod NFTs (in LC and raphei nuclei) | Yes/Yes | Freq (pick bodies) | Freq (pick bodies) | Yes/Yes |
| MObl | Freq NFTs | Freq NFTs | Yes/Yes | Sparse NFTs | Rare NFTs | Yes/No | Sparse NFTs (in LC and raphei nuclei) | Sparse NFTs (in LC and raphei nuclei) | Yes/Yes | Freq (pick bodies) | Freq (pick bodies) | Yes/Yes |
The table describes the tau pathology findings for each early-onset dementia (EOD) case as assessed by the 2 antibodies for pTau used in this study (AT8 and CP13). The neuropathologic diagnoses were performed on 15 different neuroanatomical regions from both left and right hemisphere.
NFT, pTau-positive neurofibrillary tangles; Freq, frequent lesions (>6 per microscopic field at 20×); Mod, moderate lesions (2–6 per microscopic field at 20×); Sparse, sparse lesions (1–2 per microscopic field at 20×); Rare, rare lesions (1 per microscopic field at 20×); GM, gray matter; WM, white matter; ARTAG, aging-related tau astrogliopathy; CTE, chronic traumatic encephalopathy; OB, olfactory bulb; MFG, middle frontal gyrus; OFG, orbito-frontal gyrus/gyrus rectus; MTC, middle temporal cortex; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; AInsC, anterior insular cortex; OC, occipital cortex (BA17 and 18); AMY, amygdala; BGMY, basal ganglia; MAM+HYPO, mammillary bodies+hypothalamus; PH+EC+ITC, posterior hippocampus (at CGL) including entorhinal cortex and inf. temp cortex; MESSN, mesencephalon (including substantia nigra); PONS, pons (including locus coeruleus [LC]).
TABLE 5.
β-Amyloid Pathology (4G8) in Military Early-Onset Dementia Cases
| Case #1 |
Case #2 |
Case #3 |
Case #4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Bi-hem/Sym | Left | Right | Bi-hem/Sym | Left | Right | Bi-hem/Sym | Left | Right | Bi-hem/Sym | |
| OB | N/A | N/A | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | |
| MFG | Freq diffuse- and cored-plaques | Freq diffuse- and cored-plaques | Yes/Yes | Freq diffuse- and cored-plaques | Freq diffuse- and cored-plaques | Yes/Yes | Freq diffuse-plaques (including MW) | Freq diffuse-plaques (including WM) | Yes/Yes | Neg | Neg | Yes/Yes |
| OFG | Freq diffuse- and cored-plaques | Freq diffuse- and cored-plaques | Yes/Yes | Freq diffuse- and Mod cored-plaques | Freq diffuse- and Mod cored-plaques | Yes/Yes | Freq diffuse-plaques (including MW) | Freq diffuse-plaques (including WM) | Yes/Yes | Neg | Neg | Yes/Yes |
| MTC | Freq diffuse- and cored-plaques | Freq diffuse- and cored-plaques | Yes/Yes | Freq diffuse- and rare cored-plaques | Freq diffuse- and rare cored-plaques | Yes/Yes | Freq diffuse-plaques (including MW) | Freq diffuse-plaques (including WM) | Yes/Yes | Neg | Neg | Yes/Yes |
| ACC | Freq diffuse- and cored-plaques | Freq diffuse- and cored-plaques | Yes/Yes | Freq diffuse- and rare cored-plaques | Freq diffuse- and rare cored-plaques | Yes/Yes | Freq diffuse-plaques | Freq diffuse-plaques | Yes/Yes | Neg | Neg | Yes/Yes |
| PCC | Freq diffuse- and cored-plaques | Freq diffuse- and cored-plaques | Yes/Yes | Freq diffuse- and rare cored-plaques | Freq diffuse- and rare cored-plaques | Yes/Yes | Freq diffuse-plaques | Freq diffuse-plaques | Yes/Yes | Neg | Neg | Yes/Yes |
| AInsC | Freq diffuse- and cored-plaques | Freq diffuse- and cored-plaques | Yes/Yes | Freq diffuse- and rare cored-plaques | Freq diffuse- and rare cored-plaques | Yes/Yes | Freq diffuse-plaques | Freq diffuse-plaques | Yes/Yes | Neg | Neg | Yes/Yes |
| OC | Freq diffuse-plaques | Freq diffuse-plaques | Yes/Yes | Freq diffuse-plaques | Freq diffuse-plaques | Yes/Yes | Freq diffuse- and rare cored-plaques | Freq diffuse- rare cored-plaques | Yes/Yes | Neg | Neg | Yes/Yes |
| AMY | Freq diffuse-plaques | Freq diffuse-plaques | Yes/Yes | Freq diffuse-plaques | Freq diffuse-plaques | Yes/Yes | Freq diffuse-plaques | Freq diffuse-plaques | Yes/Yes | Neg | Neg | Yes/Yes |
| BGMY | Mod diffuse-plaques | Mod diffuse-plaques | Mod diffuse-plaques | Mod diffuse-plaques | Yes/Yes | Freq diffuse-plaques | Freq diffuse-plaques | Yes/Yes | Neg | Neg | Yes/Yes | |
| MAM+HYPO | Sparse diffuse-plaques | Sparse diffuse-plaques | Yes/Yes | Rare diffuse-plaques | Rare diffuse-plaques | Yes/Yes | Freq diffuse-plaques | Freq diffuse-plaques | Yes/Yes | Neg | Neg | Yes/Yes |
| PH+EC+ITC | Freq diffuse- and rare cored-plaques | Freq diffuse- and rare cored-plaques | Yes/Yes | Freq diffuse- and rare cored-plaques | Freq diffuse- and rare cored-plaques | Yes/Yes | Freq diffuse-plaques | Freq diffuse-plaques | Yes/Yes | Neg | Neg | Yes/Yes |
| MESSN | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | Rare diffuse-plaques | Rare diffuse-plaques | Yes/Yes | Neg | Neg | Yes/Yes |
| PONS | Neg | Neg | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | |
| MObl | Neg | Neg | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | Neg | Neg | Yes/Yes | |
The table describes the β-amyloid (βA) pathology findings for each early-onset dementia (EOD) case as assessed by the antibody for 1–42 βA used in this study (4G8). The neuropathologic diagnoses were performed on 15 different neuroanatomical regions from both left and right hemisphere. Freq, frequent lesions (>6 per microscopic field at 20×); Mod, moderate lesions (2–6 per microscopic field at 20×); Sparse, sparse lesions (1–2 per microscopic field at 20×); Rare, rare lesions (1 per microscopic field at 20×).
OB, olfactory bulb; MFG, middle frontal gyrus; OFG, orbito-frontal gyrus/gyrus rectus; MTC, middle temporal cortex; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; AInsC, anterior insular cortex; OC, occipital cortex (BA17 and 18); AMY, amygdala; BGMY, basal ganglia; MAM+HYPO, mammillary bodies+hypothalamus; PH+EC+ITC, posterior hippocampus (at CGL) including entorhinal cortex and inf. temp cortex; MESSN, Mesencephalon (including substantia nigra); PONS, pons (including locus coeruleus [LC]); MObl, medulla oblongata (including dorsal motor of vagus).
FIGURE 2.
Military Case 1: pTau and other pathologies assessed across different brain regions. The figure shows images of AT8 and CP13 (pTau), HT7 (all Tau), GFAP, Ubiq, LFB staining of the right parietal cortex (2 upper rows of images), and LFB, H&E and AT8 staining of the right inferior temporal cortex of Case 1. Each image contains a larger image at lower magnification and an inset (upper right corner of each image) of the same cortical area at higher magnification.
Case 2
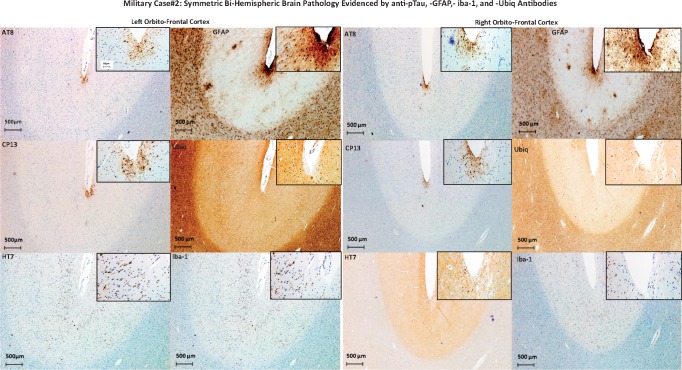
The brain was positive for diffuse and cored 1–42 βA-plaques and pTau lesions across all different examined cerebral regions. Levels of severity and frequencies are described in Tables 4 and 5. In particular, isolated CTE lesions were found in the left and right OFG, left MTC, and left ACC (Table 4). Apart from the CTE lesions, the comparison between pTau lesions detected using AT8 versus CP13 did not distinguish any consistent differences either in terms of severity across all examined regions in each hemisphere or between the 2 hemispheres (Table 4; Fig. 3). In contrast, HT7 immunoreactivity was positive in the deeper cortical layers. In addition, frequent pTau-glia cells of fuzzy type in the WM and gray matter (GM) as per aging-related tau astrogliopathy (ARTAG) were observed (12). An ARTAG pathology did not show any specific predilection for a specific cortical layer in the GM of any examined region. Intriguingly, while HT7-stained cells were positive in both GM and WM (although with lower immunoreactivity in comparison to AT8 and CP13) across multiple regions, they were positive in the deeper cortical layers. Isolated CTE lesions were present in the OFG of both hemispheres and ACC in the left hemisphere only (Fig. 3). Moreover, anti-Ubiq immunoreactivity showed severe immunoreactivity in the WM versus GM. No Ubiq intraneuronal inclusions were found in the areas with CTE. No LB pathology, TDP43, or p62 intraneuronal inclusions were detected. The GFAP and Iba-1 immunoreactivity showed variable levels of astrocytic reactivity and microglial response (based on astrocytic and microglia cells nuclear changes rather than an increased cell number). Both GFAP and Iba-1 immunoreactivity were overlapping with pTau and β-amyloid pathology. An APP stain did not show TAI in any examined region of both hemispheres. For details on APP findings, see Supplementary DataTable S3. Based on the NIA-AA neuropathologic guidelines for the assessment of AD (11), the level of AD neuropathologic changes was intermediate with an ABC score equal to A2B3C3.
FIGURE 3.
Military Case 2: Symmetric bihemispheric brain pathology evidenced by anti-pTau, -GFAP, -Iba-1, and -Ubiq antibodies. The figure shows left and right orbito-frontal cortical areas of Case 2 brain stained for pTau (AT8, CP13), all Tau (HT7), astroglial cells (GFAP), ubiquitin (Ubiq), and microglia (Iba-1). Each image contains a larger image at lower magnification and an inset (upper right corner of each image) of the same cortical area at higher magnification.
Case 3
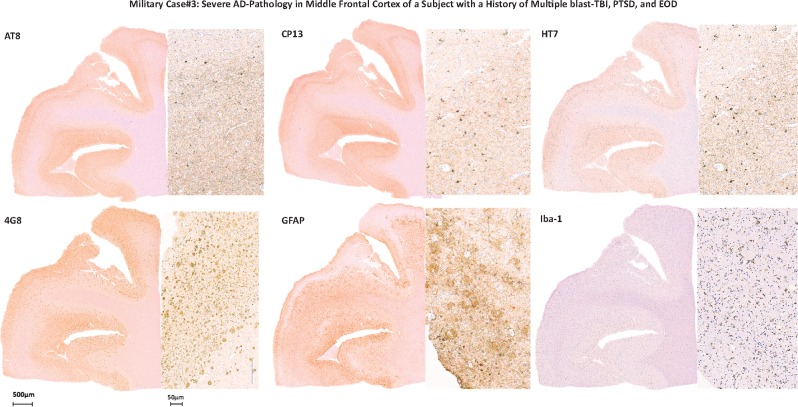
The brain of this subject was positive for 1–42 βA-plaques, which were mainly diffuse and, more rarely, cored plaques (Fig. 4), as well as pTau lesions across almost all examined brain regions (Tables 4 and 5). The comparison between pTau lesions detected using AT8 versus CP13 did not distinguish consistent differences in terms of pTau pathology severity, histologic distribution, or type of lesion in any of the examined regions of each hemisphere, or between the 2 hemispheres (Table 4). No ARTAG or other co-occurring pathologies such as α-syn-positive lesions or TDP43 intraneuronal inclusions (as for behavioral variant of FTD [bvFTD]) were detected (12). The GFAP and Iba-1 immunoreactivity showed variable levels of astrocytic and microglial reactivity mainly overlapping with β-amyloid and pTau lesions distribution. An APP stain did not show any TAI as per recent acute TBI event (for details, see Tables 3–5; Supplementary DataTables S1–S3).
FIGURE 4.
Military Case 3: Severe AD pathology in middle frontal cortex of a subject with a history of multiple blast-TBI, PTSD, and EOD. The figure shows positivity for pTau (AT8, CP13), all tau (HT7), β-amyloid lesions, and immunoreactivity for GFAP (astroglial cells) and Iba-1 (microglial cells) in Case 3.
Based on the NIA-AA neuropathologic guidelines for the assessment of AD (11), the level of AD neuropathologic changes was high with an ABC score equal to A3B3C3. Of note, the subject was evaluated by multiple neurologists and psychiatrists and he never received a firm diagnosis of possible or probable AD (5), mainly due to the atypical aspects of cognitive manifestations and psychiatric symptomatology, which included a diagnosis of PTSD, multiple episodes of violence and social disinhibition that might have masked or confounded the subjacent AD pathology and its cognitive outcomes. It is striking to observe such a severe level of β-amyloid and pTau pathology across so many cerebral regions in a relatively young subject in the absence of a known genetic mutation or family risk factor such as APOE4 allele, for example.
Case 4
The brain of this subject was negative for 1–42 βA-plaques (Table 5). In contrast, this brain showed severe levels of pTau lesions across nearly all examined regions (Table 4; Fig. 5). The comparison between pTau pathology detected using AT8 versus CP13 did not distinguish consistent differences in terms of pTau lesion severity, histologic distribution, or type of lesion in any of the examined regions of each hemisphere, or between the 2 cerebral hemispheres. pTau lesions in the hippocampus were characteristic for Pick bodies (Fig. 6). In addition, pTau lesions were present in the glial cells of the WM in almost all regions except in the occipital cortex, which is now classified as diffuse ARTAG pathology (12). No other co-occurring pathologies such as α-syn lesions or TDP43 intraneuronal inclusions were detected. GFAP and Iba-1 immunoreactivity showed variable levels of astrocytic and microglial reactivity mainly overlapping with β-amyloid and pTau lesions. An APP stain did not show any TAI as per recent TBI event (for details, see Tables 3–5; Supplementary DataTables S1–S3).
FIGURE 5.
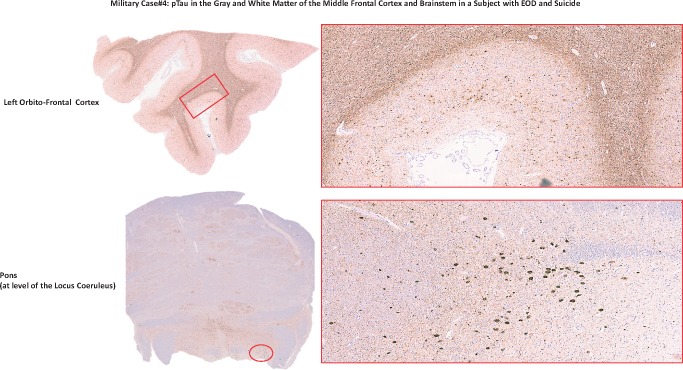
pTau in the gray and white matter of the middle frontal cortex and brainstem in a subject with EOD and suicide. The figure shows left orbito-frontal cortex and pons area of the Case 4 brain stained for pTau (AT8). Photographs were taken at lower (left) and higher (right) magnification. The red rectangle and circle on the lower magnification photographs correspond to the area on the right photographed at higher magnification.
FIGURE 6.
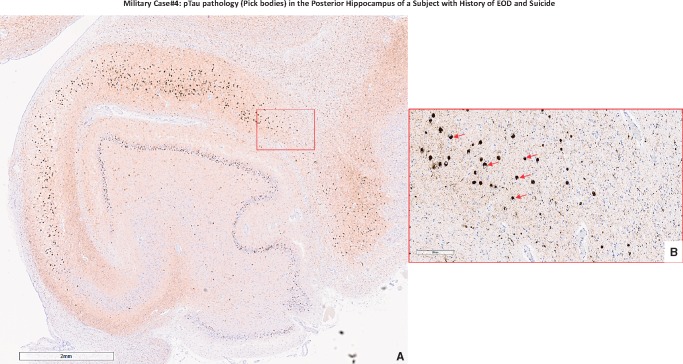
Military Case 4: pTau pathology (Pick bodies) in the posterior hippocampus of a subject with EOD and suicide. (A) The frequency and distribution of rounded pTau lesions (Pick bodies) across the entire posterior hippocampus region (CA subregions and dentate gyrus; left hemisphere) as observed at a lower magnification (×2; scale bar in lower left corner equal to 2 mm distance); (B) Rounded intracytoplasmic pTau-positive lesions (Pick bodies; red arrows) as observed at a higher magnification (×20; scale bar in lower left corner equal to 200 μm distance) localized in the CA1 region of the posterior hippocampus of Case 4 (red square in A).
Based on the NIA-AA neuropathologic guidelines for the assessment of AD (11), the level of AD neuropathologic changes was low with an ABC score equal to A0B3C0. We also hypothesized that the chronic abuse of alcohol for ∼40 years could have severely contributed to the neuropathology (pTau pathology) of this subject, as previously hypothesized (13).
Ex Vivo MRI
Analysis of the ex vivo 7-Tesla MEF data revealed structural abnormalities shared by Cases 1 and 3: Thinning of the corpus callosum, rarefaction of the parietal WM, and hippocampal atrophy (Figs. 7 and 8, Case 1; Fig. 9, Case 3). All of these findings were more prominent in Case 1. These large periventricular WM lesions are challenging to visualize in totality using histologic methods unless specifically investigated. Although it is not possible to make generalizations about WM lesions in military-EOD from these 2 cases, the ex vivo MRI data are hypothesis-generating for future studies of periventricular WM injury as a potential feature of military-EOD. These WM lesions may be more conspicuous using ultrahigh resolution ex vivo MRI scans, which can be performed over much longer periods of time than in vivo MRI scans. Unfortunately, in vivo MRI data from these 2 subjects were not available for ante and postmortem comparative analyses.
FIGURE 7.
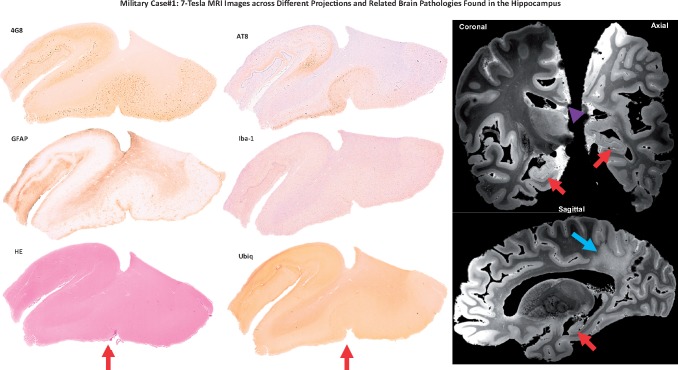
A 7-Tesla MRI data detect hippocampal and white matter pathologies. The figure shows immunohistochemistry images of the hippocampal area (right hemisphere) of Case 1 stained for amyloid (4G8), pTau (AT8), astrocytes (GFAP), microglia (Iba-1), intraneuronal inclsuions (Ubiq), and possible vascular lesions (H&E). Right panel shows coronal, axial, and sagittal images from the ex vivo 7-Tesla MRI dataset at the level of the hippocampal area analyzed in the neuropathologic analyses. The ex vivo MRI data reveal hippocampal atrophy (red arrows), as well as thinning of the corpus callosum (purple arrow head) and rarefaction of the parietal periventricular white matter (blue arrow).
FIGURE 9.
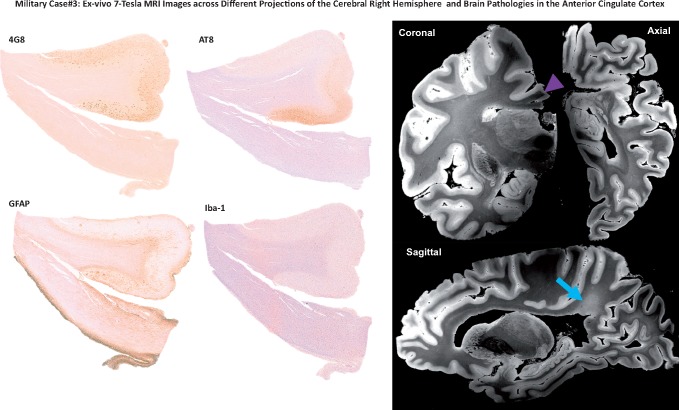
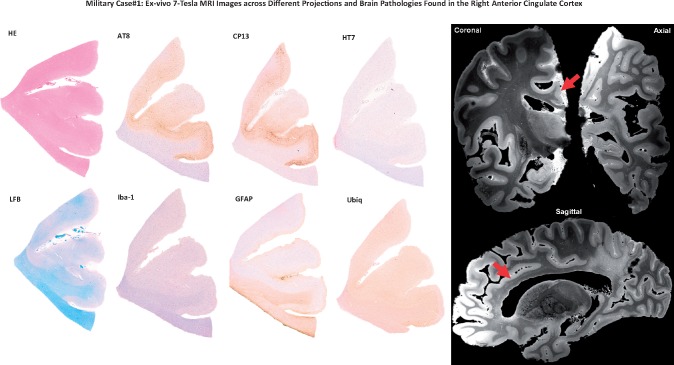
A 7-Tesla MRI investigation of pathology in the right cerebral hemisphere and the anterior cingulate cortex. Left panel shows immunohistochemistry images of the anterior cingulate cortex (right hemisphere) of Case 3 stained with 4G8 (β-amyloid), AT8 (pTau), GFAP (astroglial cells), and Iba-1 (microglial cells). Right panel shows ex vivo MRI results obtained from the 7 Tesla scan in coronal, axial, and sagittal projections of the right anterior cingulate cortex. The region of the anterior cingulate cortex analyzed in the immunohistochemistry images is indicated by the purple arrow. The ex vivo MRI data demonstrate rarefaction of the parietal periventricular white matter (blue arrow).
FIGURE 8.
A 7-Tesla MRI investigation of pathology in the right anterior cingulate cortex. Immunohistochemistry images of the anterior cingulate cortex (right hemisphere) of Case 1 stained with H&E, AT8 and CP13 (pTau), HT7 (all Tau), LFB (myelin), Iba-1 (microglia), astrocytes (GFAP), and possible intracytoplasmic inclusions (Ubiq). Right panel shows ex vivo MRI results from the 7 Tesla scan in coronal, axial, and sagittal projections of the right anterior cingulate cortex (red arrows) that included the anterior cingulate cortical area used for the neuropathologic analyses. In this region, ex vivo MRI does not reveal the burden of pathology seen with immunohistochemistry.
DISCUSSION
We report on 4 rare cases of EOD in military veterans who experienced TBI, combat stress, and life-threatening situations during different periods of their life. Neuropathologic lesions observed in these military-EOD cases were atypical, particularly with respect to their variable combinations. None of the examined cases could be clinically categorized as typical AD, bvFTD, dementia with Lewy bodies or other well-defined neuro-psychiatric illnesses. Rather, all shared a common neuropathologic feature, the abundant accumulation of pTau across different brain regions representing the prevalent brain pathology when compared, for example, to the extracellular accumulation of β-amyloid. Moreover, no Lewy bodies or TPD43-positive lesions were observed. In addition, except for Case 2, a possible contribution of vascular pathology was excluded.
The distribution and burden of pTau pathology in these veterans with EOD, together with variable levels of astroglial activation and microglial response, as well as WM lesions (in 2 of 4 cases), suggest that atypical histopathologic patterns, or combination of them, may be associated with neuropsychiatric disorders in military subjects exposed to combat-TBI. Specifically, the pTau lesions were localized in the frontal cortex (Case 3) and hippocampus (Case 4), which could be associated with behavioral abnormalities and memory deficits manifested in 2 those subjects, respectively. In Case 2, the pTau lesions and neuroinflammatory response in the raphe nuclei and locus coeruleus (pons) (14) could explain the major mood and depressive disorders reported in that subject.
It is also notable that the mean age of onset for cognitive and behavioral decline was 52.5 ± 5.2 years. The burden of histopathologic lesions (especially pTau pathology) found in these brains appears to be even greater than those found in genetically determined EOD cases (15–17). This observation suggests that other factors such as genetic predisposition or unrecognized environmental factors could be involved in the pathogenesis of the neuropathologic lesions found in these military-EOD cases. The possibility of a genetic contribution to EOD pathogenesis cannot be ruled out based on the absence of clinical and family history of genetic disorders in this cohort of 4 subjects.
The main limitation of this study is the small number of cases analyzed. Nevertheless, we provide evidence that the neuropathology of EOD in veterans with a history of TBI and chronic stress differs from that of dementias following nonmilitary TBI or contact sports TBI (i.e. CTE) cases. It is also important to consider that despite recent concerns about brain health in military personnel with combat-TBI, systematic clinicopathologic studies of military-EOD cases are rare. Importantly, while the histopathologic and ex vivo MRI findings reported here may be particularly relevant to cognitive deficits and neuropsychiatric syndromes observed in veterans of Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) (18–20), these findings may also be relevant to civilian populations exposed to TBI and chronic stress in war zones.
One of the possible pathophysiologic explanations for the unusual cognitive, behavioral, and neuropathologic findings observed in veterans of recent Middle East conflicts could be the use of more effective body protection systems. On the one hand, they reduce combat mortality rates (21, 22), but, on the other hand, may have led to longer-term effects on different organ systems, including the brain. As a result, an increased number of “rarely observed” neurologic and psychiatric sequelae among war veterans has now been described (23–25). Among the “newer” neuropsychiatric phenomena observed in OEF/OIF/OND veterans, there is a higher incidence of suicidal ideation and completed suicide, as well as persistent combat-PTSD, and pervasive changes in personality that profoundly affect the lives of veterans and their families (26, 27).
We could not directly relate any of these events (e.g. suicide) to a specific brain region or brain pathology, but we speculate that the variable combination and accumulation of neuroinflammation, WM lesions, and cortical lesions could contribute to the neuropsychiatric illnesses that ultimately led to suicide (28, 29).
As a newer approach for analyzing these complex military-EOD TBI brains, we combined ex vivo 7 Tesla MRI analyses with postmortem histopathologic analyses when possible. We developed this approach to provide pathophysiologic information that could guide and complement brain autopsy procedures before brain dissections (30). In the current study, ex vivo MRI data (available for Cases 1 and 3) provided a 3D view of regional atrophy patterns. The ex vivo MRI data also revealed an unusual pattern of parietal periventricular WM pathology in the absence of obvious diffuse vascular lesions or DAI lesions. Importantly, these imaging observations would have not been appreciated using standard 2D histopathologic analyses. Thus, ex vivo MRI may indeed play an important role in identifying pathologic lesions before a brain dissection, especially in unusual neurologic and psychiatric disorders.
An important limitation of this study is the retrospective nature of its analyses. We cannot establish a mechanistic link between the combat exposures, neuropsychiatric symptoms, and brain lesions observed in these 4 veterans. However, these data are consistent with previous studies showing that chronic stress is a risk factor for AD, and dementia more generally (31, 32). We consider these new clinicopathologic findings in military-EOD cases to be hypothesis-generating for future studies about the relationships between brain polypathology and neuropsychiatric manifestations in military as well as civilian populations exposed to combat-TBI. Our findings will need to be confirmed by longitudinal studies of pathogenetic interactions among chronic psychologic stress, combat-TBI (including blast-TBI), and blunt-TBI (33, 34). While the described clinicopathologic correlations may not be exclusive to war veterans, the neuropsychiatric manifestations in these 4 veterans with EOD appear to be associated with atypical neuropathologic lesions and ex vivo MRI features that differ from those typically seen in populations not exposed to military or battlefield TBI (35–37).
Supplementary Material
ACKNOWLEDGMENTS
We thank the subjects’ families that consented for brain donations for the better understanding of early dementia and TBI consequences. We also thank Paul Gegbeh for his technical work. We are grateful to Mrs Stacey Gentile, Mrs Deona Cooper, and Mr Harold Kramer Anderson for their administrative and writing assistance. The opinions expressed herein are those of the authors and not necessarily representative of those of the Uniformed Services University of the Health Sciences (USUSH), the Department of Defense (DOD), VA, NIH or any other US government agency.
This study was supported by the Brain Tissue Repository and Neuropathology Core, CNRM, USU award no. 308049-4.01-60855 and CENC grant award W81XWH-13-2-0095. Support was also provided by the National Institute of Neurological Disorders and Stroke (K23NS094538, U01NS086625), National Institute of Child Health and Development (U01NS086625; K01HD074651), and the James S. McDonnell Foundation. This research also utilized resources provided by National Institutes of Health shared instrumentation grants 1S10RR023401, 1S10RR019307, and 1S10RR023043. Additional support was provided in part by the National Institute for Biomedical Imaging and Bioengineering (P41EB015896, 1R01EB023281, R01EB006758, R21EB018907, R01EB019956), the National Institute on Aging (5R01AG008122, R01AG016495), the National Institute of Diabetes and Digestive and Kidney Diseases (1-R21-DK-108277-01), the National Institute for Neurological Disorders and Stroke (R01NS0525851, R21NS072652, R01NS070963, R01NS083534, 5U01NS086625), and by the NIH Blueprint for Neuroscience Research (5U01-MH093765), part of the multi-institutional Human Connectome Project.
None of the authors has a conflicting financial interest. Dr Fischl has financial interest in CorticoMetrics, a company whose medical pursuits focus on brain imaging and measurement technologies. His interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Ursano RJ, Kessler RC, Stein MB, et al. Suicide attempts in the US Army during the wars in Afghanistan and Iraq, 2004 to 2009. JAMA Psychiatry 2015;72:917–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ursano RJ, Kessler RC, Naifeh JA, et al. Frequency of improvised explosive devices and suicide attempts in the U.S. Army. Mil Med 2017;182:e1697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry 2018;75:1022–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kenney K, Iacono D, Edlow BL, et al. Dementia after moderate-severe traumatic brain injury: Coexistence of multiple proteinopathies. J Neuropathol Exp Neurol 2018;77:50–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 1998;51:1546–54 [DOI] [PubMed] [Google Scholar]
- 7. Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004;23(Suppl 1):S69–84 [DOI] [PubMed] [Google Scholar]
- 8. Iacono D, Geraci-Erck M, Peng H, et al. Symmetric bihemispheric postmortem brain cutting to study healthy and pathological brain conditions in humans. J Vis Exp 2016;54602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacobowitz DM, Liacouras PC, Singh VK, et al. 3-D printing of a non-human primate brain slicer for accurate, high resolution sampling of specific regions of fresh or frozen brain. J Primatol 2015;04:125 [Google Scholar]
- 10. McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016;131:75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kovacs GG, Ferrer I, Grinberg LT, et al. Aging-related tau astrogliopathy (ARTAG): Harmonized evaluation strategy. Acta Neuropathol 2016;131:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de la Monte SM, Kril JJ.. Human alcohol-related neuropathology. Acta Neuropathol 2014;127:71–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teissier A, Chemiakine A, Inbar B, et al. Activity of Raphé serotonergic neurons controls emotional behaviors. Cell Rep 2015;13:1965–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krüger J, Moilanen V, Majamaa K, et al. Molecular genetic analysis of the APP, PSEN1, and PSEN2 genes in Finnish patients with early-onset Alzheimer disease and frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord 2012;26:272–6 [DOI] [PubMed] [Google Scholar]
- 16. Sassi C, Guerreiro R, Gibbs R, et al. Exome sequencing identifies 2 novel presenilin 1 mutations (p.L166V and p.S230R) in British early-onset Alzheimer's disease. Neurobiol Aging 2014;35:2422e13–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong TH, Seelaar H, Melhem S, et al. Genetic screening in early-onset Alzheimer's disease identified three novel presenilin mutations. Neurobiol Aging 2019;29; pii:S0197-4580(19)30029-6 [DOI] [PubMed] [Google Scholar]
- 18. Chard KM, Schumm JA, Owens GP, et al. A comparison of OEF and OIF veterans and Vietnam veterans receiving cognitive processing therapy. J Trauma Stress 2010;23:25–32 [DOI] [PubMed] [Google Scholar]
- 19. Pugh MJ, Orman JA, Jaramillo CA, et al. The prevalence of epilepsy and association with traumatic brain injury in veterans of the Afghanistan and Iraq wars. J Head Trauma Rehabil 2015;30:29–37 [DOI] [PubMed] [Google Scholar]
- 20. Pagulayan KF, Rau H, Madathil R, et al. Retrospective and prospective memory among OEF/OIF/OND Veterans with a self-reported history of blast-related mTBI. J Int Neuropsychol Soc 2018;24:324–34 [DOI] [PubMed] [Google Scholar]
- 21. Brennan J. Head and neck trauma in Iraq and Afghanistan: Different war, different surgery, lessons learned. Laryngoscope 2013;123:2411–7 [DOI] [PubMed] [Google Scholar]
- 22. Breeze J, Combes JG, DuBose J, et al. How are we currently training and maintaining clinical readiness of US and UK military surgeons responsible for managing head, face and neck wounds on deployment? J R Army Med Corps 2018;164:183–5 [DOI] [PubMed] [Google Scholar]
- 23. Rosenfeld JV, McFarlane AC, Bragge P, et al. Blast-related traumatic brain injury. Lancet Neurol 2013;12:882–93 [DOI] [PubMed] [Google Scholar]
- 24. Finley EP, Bollinger M, Noël PH, et al. A national cohort study of the association between the polytrauma clinical triad and suicide-related behavior among US Veterans who served in Iraq and Afghanistan. Am J Public Health 2015;105:380–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lippa SM, Fonda JR, Fortier CB, et al. Deployment-related psychiatric and behavioral conditions and their association with functional disability in OEF/OIF/OND Veterans. J Traumatic Stress 2015;28:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams JL, McDevitt-Murphy ME, Murphy JG, et al. Postconcussive symptoms, PTSD, and medical disease burden in treatment-seeking OEF/OIF/OND Veterans. Mil Med 2017;182:e1645–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bourn LE, Sexton MB, Raggio GA, et al. Posttraumatic stress disorder and somatic complaints: Contrasting Vietnam and OIF/OEF Veterans' experiences. J Psychosom Res 2016;82:35–40 [DOI] [PubMed] [Google Scholar]
- 28. Courtet P, Giner L, Seneque M, et al. Neuroinflammation in suicide: Toward a comprehensive model. World J Biol Psychiatry 2016;17:564–86 [DOI] [PubMed] [Google Scholar]
- 29. Suzuki H, Ohgidani M, Kuwano N, et al. Suicide and microglia: Recent findings and future perspectives based on human studies. Front Cell Neurosci 2019;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edlow BL, Keene CD, Perl DP, et al. Multimodal characterization of the late effects of traumatic brain injury: A methodological overview of the late effects of traumatic brain injury project. J Neurotrauma 2018;35:1604–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Escher CM, Sannemann L, Jessen F.. Stress and Alzheimer’s disease. J Neural Transm 2019;126:1155–61 [DOI] [PubMed] [Google Scholar]
- 32. Snyder HM, Carare RO, DeKosky ST, et al. Military-related risk factors for dementia. Alzheimers Dement 2018;14:1651–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tripathy A, Shade A, Erskine B, et al. No evidence of increased chronic traumatic encephalopathy pathology of neurodegenerative proteinopathy in former military service members: A preliminary study. JAD 2019;67:1277–89 [DOI] [PubMed] [Google Scholar]
- 34. Washington PM, Villapol S, Burns MP.. Polypathology and dementia after brain trauma: Does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Exp Neurol 2016;275:381–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leonard BE. HPA and immune axes in stress: Involvement of the serotonergic system. Neuroimmunomodulation 2006;13:268–76 [DOI] [PubMed] [Google Scholar]
- 36. Picard C, Pasquier F, Martinaud O, et al. Early onset dementia: Characteristics in a large cohort from academic memory clinics. Alzheimer Dis Assoc Disord 2011;25:203–5 [DOI] [PubMed] [Google Scholar]
- 37. Masellis M, Sherborn K, Neto P, et al. Early-onset dementias: Diagnostic and etiological considerations. Alzheimers Res Ther 2013;5:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.