Abstract
Hemorrhagic shock (HS) is a potential life-threatening condition that may lead to injury to multiple organs, including the lung. The estrogen sulfotransferase (EST, or SULT1E1) is a conjugating enzyme that sulfonates and deactivates estrogens. In this report, we showed that the expression of Est was markedly induced in the liver but not in the lung of female mice subject to HS and resuscitation. Genetic ablation or pharmacological inhibition of Est effectively protected female mice from HS-induced acute lung injury (ALI), including interstitial edema, neutrophil mobilization and infiltration, and inflammation. The pulmonoprotective effect of Est ablation or inhibition was sex-specific, because the HS-induced ALI was not affected in male Est-/- mice. Mechanistically, the pulmonoprotective phenotype in female Est-/- mice was accompanied by increased lung and circulating levels of estrogens, attenuated pulmonary inflammation, and inhibition of neutrophil mobilization from the bone marrow and neutrophil infiltration to the lung, whereas the pulmonoprotective effect was abolished upon ovariectomy, suggesting that the protection was estrogen dependent. The pulmonoprotective effect of Est ablation was also tissue specific, as loss of Est had little effect on HS-induced liver injury. Moreover, transgenic reconstitution of human EST in the liver of global Est-/- mice abolished the pulmonoprotective effect, suggesting that it is the EST in the liver that sensitizes mice to HS-induced ALI. Taken together, our results revealed a sex- and tissue-specific role of EST in HS-induced ALI. Pharmacological inhibition of EST may represent an effective approach to manage HS-induced ALI.
Keywords: hemorrhagic shock, acute lung injury, estrogen, estrogen sulfotransferase, liver
Hemorrhagic shock (HS) can result from a wide range of insults such as trauma, organ transplantation, or other major surgeries. Although acute lethality is rare, HS may lead to the development of multiple organ dysfunction in response to a secondary inflammatory stimulus. Among HS-induced tissue injuries, acute lung injury (ALI) is one of the leading causes of death in surgical and trauma patients with the in-hospital mortality rate as high as 40% (1, 2). The pathogenesis of HS-induced ALI is dynamic, including the deprivation of blood and oxygen supply in the acute phase, followed by blood volume restoration upon resuscitation or re-establishment of the circulation. As a consequence of hemorrhagic shock and resuscitation (HS/R), an exaggerated inflammatory response is initiated and a large number of polymorphonuclear neutrophils (PMNs) are activated and migrate into the lung. The infiltration of PMNs is a hallmark of ALI, and it plays a central role in the development of lung tissue injury (3–5). It is conceivable that a better understanding of the mechanism by which HS induces ALI will help to develop effective strategies for the clinical management of HS in trauma and surgical patients.
Both clinical and animal studies have shown a sex-dependent response to HS. The levels of female sex hormones, especially 17β-estradiol, are believed to be positively associated with a favorable outcome after HS (6, 7). In human studies, it has been reported that the male sex is independently associated with a 40% higher rate of multiple organ failure (MOF), a 25% higher rate of nosocomial infection, and elevated levels of interleukin 6 (IL-6) (8) in trauma patients. In contrast, the female patients tend to have lower levels of systemic inflammatory cytokines, and they are more resistant to MOF (9). However, the role of estrogens in trauma patients is not without controversy. For example, it was reported that elevated serum levels of 17β-estradiol and testosterone in the early time period postinjury have their independent associations with greater risk of MOF and nosocomial infection (10). In animal studies, it was reported that acute estradiol treatment attenuates lung inflammatory responses, such as reducing the basal expression of platelet endothelial cell adhesion molecule-1 and IL-10 levels in male rats subjected to intestinal ischemia (11). Treatment with 17 β-estradiol also attenuated ALI induced by oxidant stresses other than HS such as the herbicide paraquat and acute alveolar anoxia (12). It was noted that most of the reported roles of estrogens in traumatic injury relied on the administration of pharmacological doses of estrogens. It is unclear whether regulation of endogenous estrogen metabolism can affect the clinical outcome of HS-induced tissue injury.
Estrogen sulfotransferase (EST or SULT1E1) is a conjugating enzyme capable of deactivating estrogens by sulfation. Sulfonated estrogens cannot bind to and activate the estrogen receptors, therefore losing their hormonal activities (13). EST is expressed in multiple human tissues such as the liver, intestine, and subcutaneous adipose tissue (14). Low levels of EST expression were also reported in the lung and kidney (15). We have previously reported that the expression of EST is highly inducible in the livers of mouse models of multiple disease, including obesity and type 2 diabetes (16), hepatic ischemia and reperfusion (17), and sepsis (18). The induction of EST by these disease conditions led to attenuated estrogen responses. Reciprocally, the expression and/or activity of EST have major effects on the outcome of these diseases through varied mechanisms and often in a sex- and tissue-specific manner (16–19). A pulmonary function of EST has not been reported. It is unclear whether and how EST plays a role in HS-induced ALI.
In this study, we uncovered a novel function of EST in HS-induced ALI. HS induces the hepatic expression of Est in mice. The HS-responsive induction of Est may have played a pathogenic role in HS-induced ALI because genetic ablation or pharmacological inhibition of Est effectively protected female mice from HS-induced ALI, whereas hepatic reconstitution of EST re-sensitized mice to HS-induced ALI.
Materials and Methods
Chemicals
The EST inhibitor triclosan, 17β-estradiol, estrone, estrone 3-sulfate sodium salt, β-estradiol 3-sulfate sodium salt, and dansyl chloride were purchased from Sigma-Aldrich (St. Louis, MO). N-butyl chloride was purchased from Fisher Scientific (Ottawa, Ontario, Canada). 17β-estradiol-2,4,16,16,17-d5 and 17β-estradiol-2,4,16,16-d4-3-sulfate were purchased from CDN isotopes (Pointe-Claire, Quebec, Canada).
Animals and mouse model of HS/R
Est -/- mice have been described previously (20). KOLE mice are Est-knockout (KO) and Lap-EST (LE) transgenic mice that were generated by cross-breeding Est-/- mice with Lap-tTA/TetRE-EST (Lap-EST) transgenic mice. The Lap-EST transgene overexpresses the human EST exclusively in the liver/hepatocytes under the control of the liver-enriched activator protein (Lap) gene promoter (18). All mice were maintained in a C57/BL6 background. The majority of the experiments were performed on mice 8 weeks of age except those specified for ovariectomy (5 weeks old). Hemorrhagic shock and resuscitation were performed as we have previously described (21), and the serum and tissues were collected 4, 6, or 24 hours after HS. The use of mice in this study has complied with all relevant federal guidelines and institutional policies and has been approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Bronchoalveolar lavage fluid collection
This was performed essentially as described (22). In brief, 4 hours after HS/R, mice were anesthetized by intraperitoneal injections of 150 mg/kg ketamine and 10 mg/kg xylazine. A bronchoalveolar lavage fluid (BALF) catheter was inserted into the trachea and repeatedly injected with 0.5 mL of ice-cold phosphate buffered saline (PBS) (from the “input” syringe filled with 3 mL of PBS), and then BALF was aspirated from the inflated lungs into the initially unfilled “output” syringe via a 3-way stopcock. We typically injected a total of 3 mL and recovered 2.5 mL of BALF in the output syringe. The 3-way stopcock is adjusted after each injection and aspiration round to ensure that BALF was captured in the output syringe. The recovered BALF were centrifuged at 300 g for 5 minutes. The cell-free supernatants of BALF were collected and subjected to the measurement of protein concentration using a BCA protein assay kit from ThermoFisher Scientific (Waltham, MA).
Measurement of lung wet/dry ratio
Mice were sacrificed, and lungs were excised 4 hours after the HS/R. Blood was removed by blotting the lungs with filter papers until dry, and the lungs were then weighed for their wet weight. The lungs were subsequently placed in an oven at 65°C for 72 hours, and the dry weight was recorded. The wet/dry weight ratio was calculated to assess tissue edema.
Measurement of IL-6 concentrations in BALF and serum
IL-6 concentrations in BALF and serum were measured using enzyme-linked immunosorbent assay kits from Pierce/Thermo Fisher Scientific (Waltham, MA) according to the manufacturer’s instructions.
Histology, immunohistochemistry, and immunofluorescence
The lungs were freshly harvested and fixed in 10% neutral-buffered formalin for 24 hours. The tissues were histologically processed, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin for general histology. For myeloperoxidase (MPO) immunostaining, deparaffinized sections were incubated with anti-MPO antibody (23) from Abcam (Cambridge, MA) at 1:25 dilution overnight at 4°C. The antibody signal was visualized by peroxidase reaction using 3,3′-diaminobenzidine as the chromogen. Hematoxylin was used as a nuclear counterstain. For estrogen sulfotransferase (EST) immunofluorescence, liver and lung paraffin sections were incubated with anti-estrogen sulfotransferase (24) from Abcam (Cambridge, MA) at 1:100 dilution overnight at 4°C. The secondary antibody used was a cyanine 5 conjugated donkey anti-rabbit polyclonal antibody (25) from MilliporeSigma (Danvers, MA) at 1:500 dilution. DAPI-Fluoromount-G (26) purchased from Southern Biotech, Birmingham, AL, was used as a nuclear counterstain. For immunohistochemistry and immunofluorescence, at least 3 mice were used for each treatment group, and for each sample at least 4 noncontiguous regions were photographed and analyzed.
Real-time PCR and western blotting
Total ribonucleic acid (RNA) was extracted from tissues using TRIzol reagent. Total RNA was treated with ribonuclease-free deoxyribonuclease (RNase-free DNase I) and reverse transcribed into single-stranded complementary deoxyribonucleic acid (cDNA). SYBR Green-based real-time polymerase chain reaction (PCR) was performed with the ABI 7300 real-time PCR system from Applied Biosystems (Foster City, CA). Data were normalized against the housekeeping gene cyclophilin. Relative gene expression was calculated using the ΔΔCT (threshold cycle) method, where fold difference was calculated using the expression 2−ΔΔCT. The primer sequences are provided in Supplementary materials located in a digital research materials repository (27). For western blotting, 30 μg of total proteins for each sample were separated on 13% sodium dodecyl sulfate–polyacrylamide gel. The primary antibodies were polyclonal rabbit anti-SULT1E1 (28) from Proteintech (Rosemont, IL) at 1:200 dilution and monoclonal mouse anti-β-actin (29) from Sigma-Aldrich (St. Louis, MO) at 1:5000 dilution. The secondary antibodies were anti-rabbit antibody (30) and anti-mouse antibody (31) from Cell Signaling Technology (Danvers, MA) at 1:5000 dilution. Detection was achieved by using an ECL system from Amersham (Piscataway, NJ).
Flow cytometry
Tissue dissection and digestion were performed as described (22). Mouse bone marrow neutrophils were isolated and purified as described (32). Flow cytometry was performed as described (3, 22). In brief, 200 μl of suspended cells (~ 2.5 million cells) or 100 ul peripheral blood were stained with anti-CD45 buv395 (33), anti-CD11b-APC antibody (34), anti-Gr-1-PE antibody (35), and anti-CXCR2(CD182)-FITC antibody (36) for the detection of neutrophils. The stained cells were applied for data acquisition on BD STI LSRII cytometer from BD Biosciences (San Jose, CA) and re-analyzed with BD FACSDIVA software from BD Biosciences.
Estradiol extraction and derivatization
Estradiol was extracted by liquid-liquid extraction using n-butyl chloride and then derivatized with dansyl chloride as previously described by Li et al (37). Samples were first spiked with internal standard 20 μl 17β-estradiol-2,4,16,16,17-d5 (1 ng/mL in methanol). 3 mL n-butyl chloride was then added and vortexed for 1 minute. The tubes were then centrifuged at 4770 g at room temperature for 10 minutes, and the organic layer was transferred to salinized culture tubes and dried down under a soft steam of nitrogen at 37°C for 20 minutes. Residues were derivatized in 0.1 mL buffered dansyl chloride solution (a 1:1 mix of acetonitrile: water, pH 10.5), heated at 60°C for 3 minutes, and then transferred to glass vials for ultra performance liquid chromatography–tandem mass spectrometer (UPLC–MS/MS) analysis.
Estradiol sulfate extraction
Estrogen sulfates in lung tissue were extracted in 75% methanol before drying down. Sediment was removed after recovering with 1 mL PBS and centrifuging for 20 minutes (8000 g, 4°C). For both lung tissue samples and serum samples, internal standard 17β-estradiol-2,4,16,16-d4-3-sulfate (100 pg) were added. Solid-phase extraction using Oasiss MCX (3 cc/60) mg extraction cartridges, purchased from Waters (Milford, MA), was performed under gravity. Prior to loading the tissue or serum samples, the cartridges were conditioned with methanol (1 mL), followed by water (1 mL). The sample was loaded and allowed to pass through the cartridges and the eluate discarded. Next, the cartridges were washed with methanol (5% v/v, 3 mL) and again the eluate discarded. Finally, the estrogen sulfates were eluted in methanol (3 mL). Extracts were reduced to dryness under oxygen-free nitrogen at 40°C.
UPLC–MS/MS analysis of estradiol and estradiol sulfate
We used published UPLC–MS/MS methods for the detection of estradiol (37) and estradiol sulfate (18) with minor modifications. Liquid chromatography was performed using an Acquity ultra performance LC autosampler from Waters (Milford, MA). Analytes were separated on a UPLC BEH C-18, 1.7 mm (2.1 × 150 mm) reverse-phase column from Waters. Column temperature was maintained at 55°C. For estradiol detection, mobile phases, delivered at a flow rate of 0.3 mL/min, consisted of (A) acetonitrile and (B) 0.1% formic acid in water at an initial mixture of 50:50 A and B. Mobile phase B was maintained at 50% for 1 minute and then increased to 85% in a linear gradient over 3 minutes, where it remained for 1 minute. This was followed by a linear return to initial conditions over 1.5 minutes. For the detection of estradiol sulfate, mobile phase B was maintained at 80%. Total run time per sample was 6.5 minutes, and all injection volumes were 7.5 μl. Mass spectrometric analysis of analyte formation was performed using a TSQ Quantum Ultra from Thermo Fisher Scientific (San Jose, CA) triple quadrupole mass spectrometer coupled with heated electrospray ionization source operated in negative selective reaction monitoring (SRM) mode with unit resolutions at both Q1 and Q3 set at 0.70 full width at half maximum. Quantification by selected reaction monitoring (SRM) analysis of estradiol and estradiol sulfate was performed by monitoring the m/z transitions.
PMNs isolation from bone marrow
This experiment was performed as described (38). In brief, PMNs were isolated and purified from bone marrow cells using Histopaque-based density gradient centrifugation. 3 mL of Histopaque 1077 (density, 1.077 g/mL) from Sigma-Aldrich (St. Louis, MO) were overlaid on 3 mL of Histopaque 1119 (density, 1.119 g/mL) from Sigma-Aldrich in a 15-mL conical centrifuge tube, and the bone marrow cell suspensions were added on top of the Histopaque 1077 layer. The neutrophils at the interface of the Histopaque 1119 and Histopaque 1077 layers were collected after centrifuging for 30 minutes at 872 × g at room temperature without brake. The collected PMNs were washed twice with RPMI 1640 1X supplemented with 10% FBS and 1% penicillin/streptomycin and centrifuged at 1400 rpm for 7 minutes at 4°C before cell plating.
Statistical analysis
Results are expressed as mean plus or minus the standard error of the mean (SEM). Differences between 2 individual groups were determined by Student’s t test. Differences between multiple groups were evaluated using two-way analysis of variance followed by post hoc multiple comparison according to the Tukey test. Statistical significance was accepted at P < 0.05.
Results
HS specifically induces the expression of Est in the liver
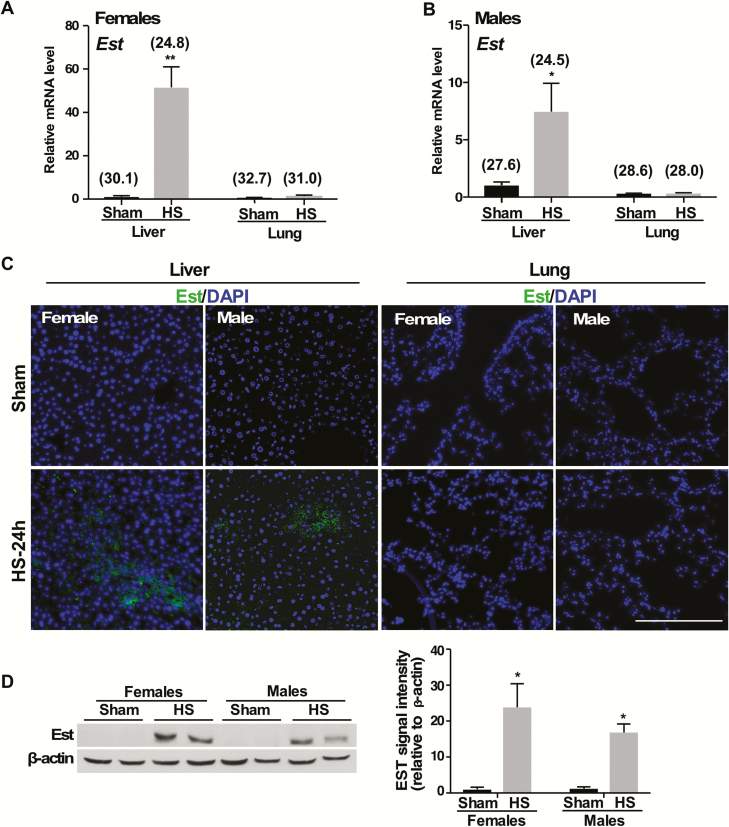
To determine the effect of HS on the expression of Est, we established a mouse model of HS/R, in which mice were subjected to a 2-hour fixed pressure HS followed by R, and the mice were sacrificed 24 hours after the initiation of HS (21). We found the mRNA expression of Est was markedly induced in the liver but not in the lung of female mice subjected to HS/R (Fig. 1A). HS also induced the mRNA expression of Est in male mice, but the fold induction was not as dramatic as in female mice (Fig. 1B). The induction of Est protein in the liver and lack of induction in the lung of female and male mice were further verified by immunofluorescence (Fig. 1C). The expression of Est in the lung was barely detectable, consistent with a previous report (15). The HS/R-responsive inductions of Est protein in the liver of female and male mice were also confirmed by western blotting (Fig. 1D).
Figure 1.
Hemorrhagic shock (HS) specifically induces the expression of Est in the liver. Female and male WT mice were subjected to sham surgery or HS/R. Serum and tissue were harvested at 24 hours after HS. (A and B) The mRNA expression of Est in the liver and lung of female (A) and male (B) mice was measured by real-time PCR. (C) The protein expression of Est in the liver and lung in female and male mice was measured by immunofluorescence staining of DAPI (blue) and Est (green). Bar is 100 μm. (D) The protein expression of Est in the liver of female and male mice was measured by western blotting. Shown to the right is the quantification of the western results. Numbers in parentheses in A and B are average real-time PCR cycle numbers. Results are presented as mean ± SEM, (n = 3 for each group). *P < 0.05; **P < 0.01, compared with the sham groups. Abbreviations: mRNA, messenger ribonucleic acid; PCR, polymerase chain reaction; R, resuscitation; SEM, standard error of the mean; WT, wild-type.
Genetic ablation or pharmacological inhibition of Est protects female but not male mice from HS-induced ALI
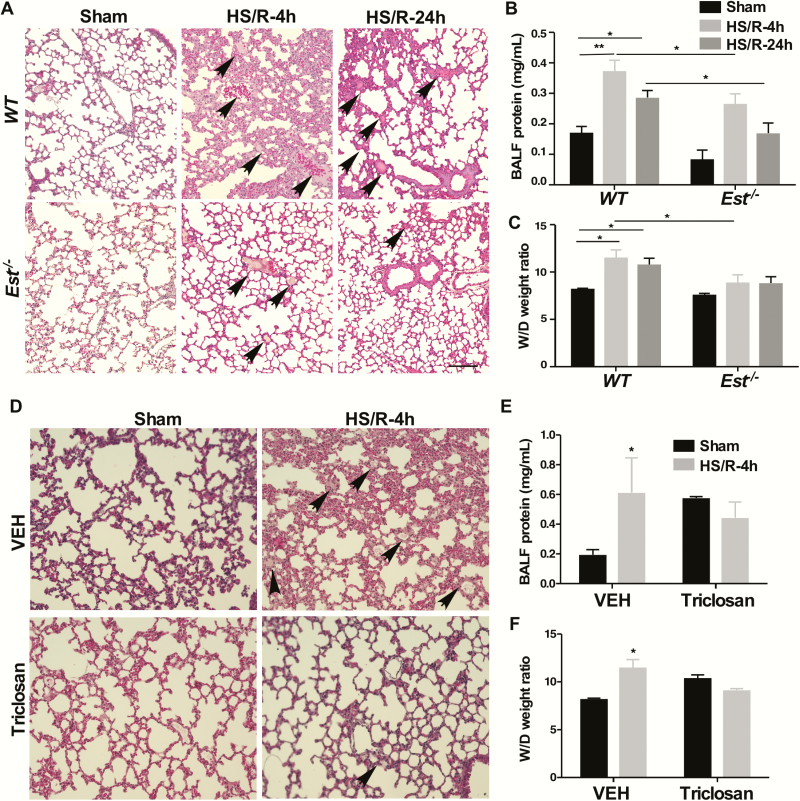
To determine the functional relevance of Est induction in HS-induced ALI, we first subjected female wild-type (WT) and Est-/- mice to HS/R surgery, and mice were sacrificed either 4 or 24 hours after HS. The liver expression of Est was efficiently induced at both time points (27). When ALI was evaluated, female Est-/- mice were found to be protected from HS-induced ALI at both time points, including decreased pulmonary interstitial edema and infiltration of cells into the interstitium and alveolar spaces at the histological level (Fig. 2A), decreased BALF protein concentrations (Fig. 2B), and decreased lung wet to dry (W/D) ratio (Fig. 2C). In an independent model of pharmacological inhibition of Est, treatment of female WT mice with the EST inhibitor triclosan (5-chloro-2(2,4-dichlorophenoxy)-phenol) (39) also inhibited the 4-hour HS-induced ALI, as shown by histology (Fig. 2D) and measurements of BALF protein concentration (Fig. 2E) and W/D ratio (Fig. 2F). Interestingly, we noticed triclosan treatment itself tended to induce an increase in BALF protein and W/D ratio in the sham condition (Fig. 2E and F). It has been reported that triclosan can trigger colonic inflammation in mice (27), so it is possible triclosan may have also induced mild inflammation in the lung and caused a mild induction of the total BALF protein level in the sham group. However, the inhibition of EST by triclosan, which results in the elevation of estrogens overrides the proinflammatory activity of triclosan, leading to an overall protective effect, including decreased total protein in BALF.
Figure 2.
Genetic ablation or pharmacological inhibition of Est protects female, but not male mice from HS-induced ALI. (A-C) Female WT and Est-/- mice were subjected to HS/R. The mice were sacrificed 4 or 24 hours after the initiation of HS. Shown are H&E staining of the lung sections (A, bar is 100 μm). The section shows interstitial edema and infiltrated blood cells in the lung tissue (arrowhead). Protein concentrations in the cell-free bronchoalveolar lavage fluid (BALF) (B), and wet/dry (W/D) weight ratio of the lung tissues (C) were also shown. (D-F) Female WT mice were subcutaneously injected daily with either dimethyl sulfoxide as vehicle (VEH) or triclosan (10 mg/kg) beginning 3 days before HS/R surgery. Mice were sacrificed 4 hours after the initiation of HS. Shown are H&E staining of the lung sections (D, original magnification 200x), BALF protein concentrations (E), and lung W/D ratio (F). Results are presented as mean ± SEM, (n = 4–5 for each group). *P < 0.05; **P < 0.01, with the comparisons among groups as labeled (B, C, E, and F).
Abbreviations: ALI, acute lung injury; H&E, hematoxylin and eosin; HS, hemorrhagic shock; R, resuscitation; WT, wild-type
Interestingly, the pulmonoprotective effect of Est ablation was female specific, because the male Est-/- mice were equally sensitive to HS-induced ALI as their WT counterparts (27). Treatment of WT male mice with triclosan also had little effect on HS-induced ALI (27). The effect of Est ablation on HS-induced organ injury was also tissue specific, as Est ablation in female mice had little effect on 24-hour HS-induced liver injury, as evidenced by the liver histology (27) and serum level of alanine aminotransferase (27).
The pulmonoprotective effect of Est ablation is estrogen dependent
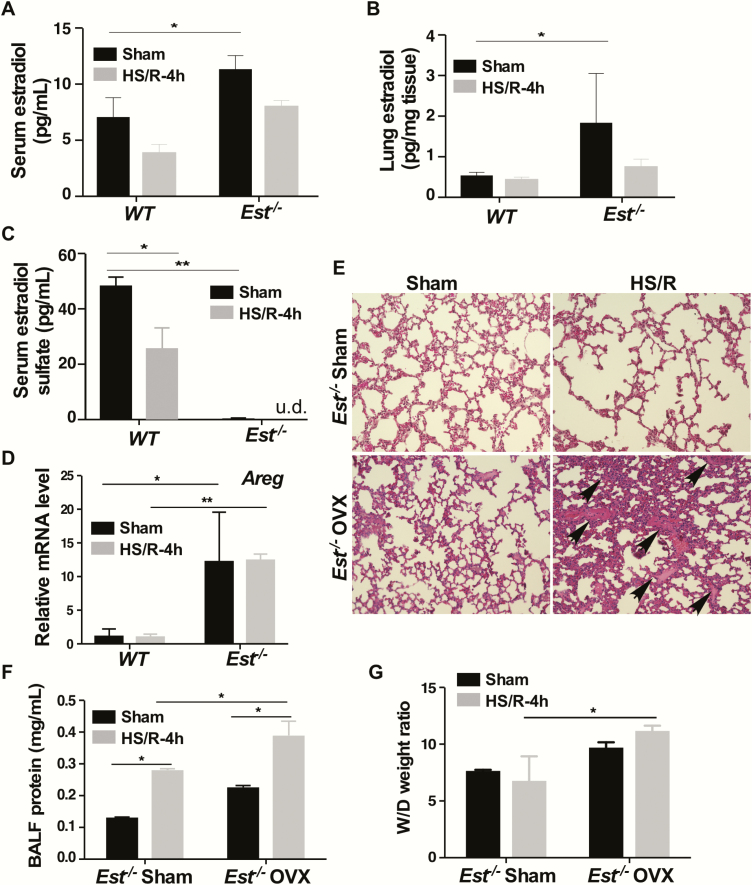
Consistent with the notion that a primary function of EST is to sulfonate and deactivate estrogens, we found the circulating (Fig. 3A) and lung tissue (Fig. 3B) levels of estradiol were elevated in sham-treated female Est-/-mice. In contrast, the circulating level of estradiol sulfate was decreased in sham-treated female Est-/- mice (Fig. 3C). The dropped levels of estradiol and estradiol sulfate in 4-hour HS-treated Est-/- mice were likely accounted for by the withdrawal of a large volume of blood during HS and insufficient time to replenish estrogens within 4 hours before the mice were sacrificed. Consistent with the increased lung tissue concentration of estradiol, the pulmonary expression of the estrogen-responsive gene Areg, encoding the amphiregulin protein (40), was increased in Est-/- mice subjected to sham surgery (Fig. 3D). Since estrogens are known for their pulmonoprotective effect (11), we speculated that the elevated circulating and lung tissue estrogen levels might have accounted for the pulmonoprotective phenotype in the female Est-/- mice. To evaluate the estrogen dependence, we performed ovariectomy on female Est-/- mice before subjecting them to HS/R. Indeed, ovariectomy attenuated the pulmonoprotective phenotype as shown by histology (Fig. 3E), and measurements of BALF protein concentration (Fig. 3F) and lung W/D ratio (Fig. 3G), suggesting that the pulmonoprotective effect of Est ablation was estrogen dependent. Interestingly, treatment of female Est-/- mice with fulvestrant, an estrogen receptor (ER) antagonist that destabilizes ER (41), failed to abolish the pulmonoprotective effect as shown by histology (27), measurements of BALF protein concentration, (27) and W/D ratio (27). As expected, the pulmonary mRNA expression of Areg was inhibited by fulvestrant (27). These results suggested that although the protective effect of Est ablation was estrogen dependent, it might be ER independent. HS had little effect on the pulmonary expression of other enzymes involved in the local estrogen homeostasis such as aromatase (Cyp19a1) (27), 17β-hydroxysteroid dehydrogenase 17β1 (Hsd17b1) (27), and steroid sulfatase (27) regardless of the Est genotypes.
Figure 3.
The pulmonoprotective effect of Est ablation is estrogen dependent. (A-D) Mice are the same 4-hour HS/R mice described in Fig. 2A. Shown are serum estradiol levels (A), lung estradiol levels (B), serum estradiol sulfate levels (C), and pulmonary mRNA expression of Areg (D). (E-G) Female Est-/- mice were subjected to sham surgery or ovariectomy (OVX) at 5 weeks of age before being subjected to sham surgery or HS/R when the mice were 8 weeks old. Mice were sacrificed 4 hours after the initiation of HS. Shown are H&E staining of the lung sections (E, original magnification 200x). The section shows interstitial edema and infiltrated blood cells in the lung tissue (arrowhead). BALF protein concentrations (F) and lung W/D ratio (G) were also shown. Results are presented as mean ± SEM, (n = 4–5 for each group). *P < 0.05; **P < 0.01, with the comparisons labeled.
Abbreviations: BALF, bronchoalveolar lavage fluid; H&E, hematoxylin and eosin; HS, hemorrhagic shock; mRNA, messenger ribonucleic acid; R, resuscitation; SEM, standard error of the mean; W/D, wet/dry.
Reconstitution of EST to the liver abolishes the pulmonoprotective effect of Est ablation
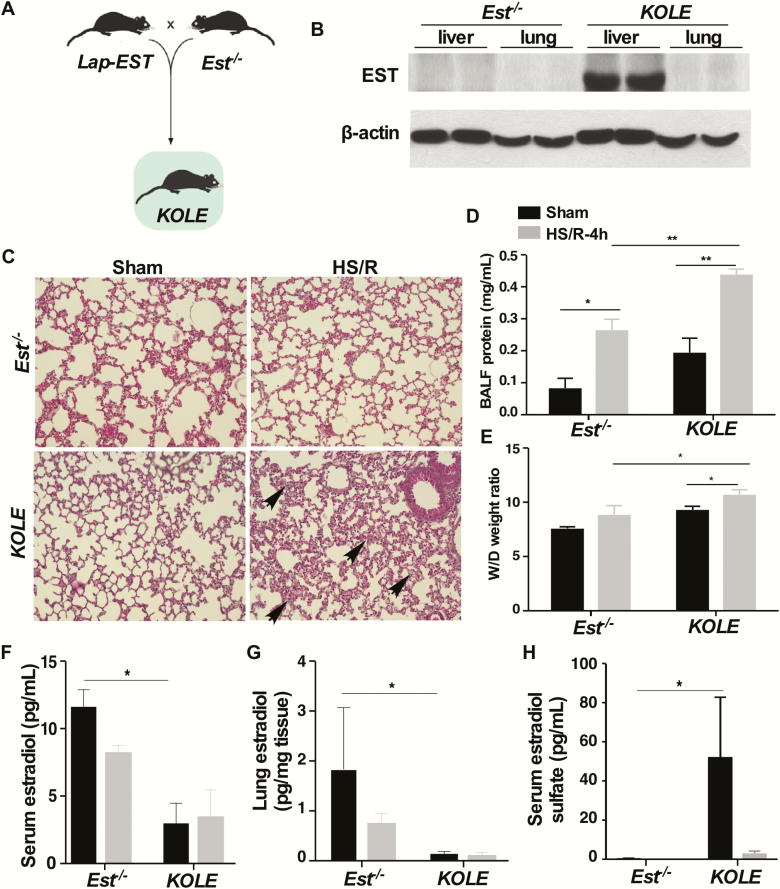
Since the expression of Est in the lung was barely detectable, and it was not inducible by HS/R, we speculated that the phenotype in female Est-/- mice may have resulted from the loss of Est in an extrapulmonary tissue. Knowing that hepatic expression of Est was induced by HS/R in WT mice, we wanted to determine whether EST in the liver played a pathogenic role in HS-induced ALI. For this purpose, we generated the KOLE mice that are Est KO and LE transgenic. The KOLE mice were generated by breeding the liver-specific LE transgene (18) into the Est-/- background as outlined in Fig. 4A. The LE transgenic mice express the human EST/SULT1E1 exclusively in the liver under the control of the Lap gene promoter (18). The reconstitution of EST in the liver, but not in the lung, was verified by western blotting (Fig. 4B). Compared to the ALI resistant female Est-/- mice, the female KOLE mice showed sensitivity to HS/R-induced ALI, as evidence by their increased pulmonary interstitial edema (Fig. 4C), BALF protein contents (Fig. 4D), and lung W/D ratio (Fig. 4E). Consistent with their reconstitution of EST, the sham-treated female KOLE mice showed decreased levels of serum (Fig. 4F) and lung tissue (Fig. 4G) estradiol and an increased circulating level of estradiol sulfate (Fig. 4H) compared with their Est-/- counterparts.
Figure 4.
Reconstitution of EST to the liver abolishes the pulmonoprotective effect of Est ablation. (A) Schematic representation of the creation of the KOLE transgenic mice. (B) Expression of EST in the liver and lung as measured by western blotting. (C-H) Female Est-/- and KOLE mice were subjected to the sham surgery or HS/R. Mice were sacrificed 4 hours after the initiation of HS. Shown are H&E staining of the lung sections (C, original magnification 200x). The section shows interstitial edema and infiltrated blood cells in the lung tissue (arrowhead). BALF protein concentrations (D), lung W/D ratio (E), serum estradiol levels (F), lung estradiol levels (G), and serum estradiol sulfate levels (H) were also shown. Data of estradiol and estradiol sulfate levels in Est-/- mice are from the same mice in Fig. 3. Results are presented as mean ± SEM, (n = 4–5 for each group). *P < 0.05; **P < 0.01, with the comparisons labeled.
Abbreviations: BALF, bronchoalveolar lavage fluid; H&E, hematoxylin and eosin; HS, hemorrhagic shock; R, resuscitation; SEM, standard error of the mean; W/D, wet/dry.
Est ablation attenuates, whereas liver reconstitution of EST restores HS-induced lung local and systemic inflammation
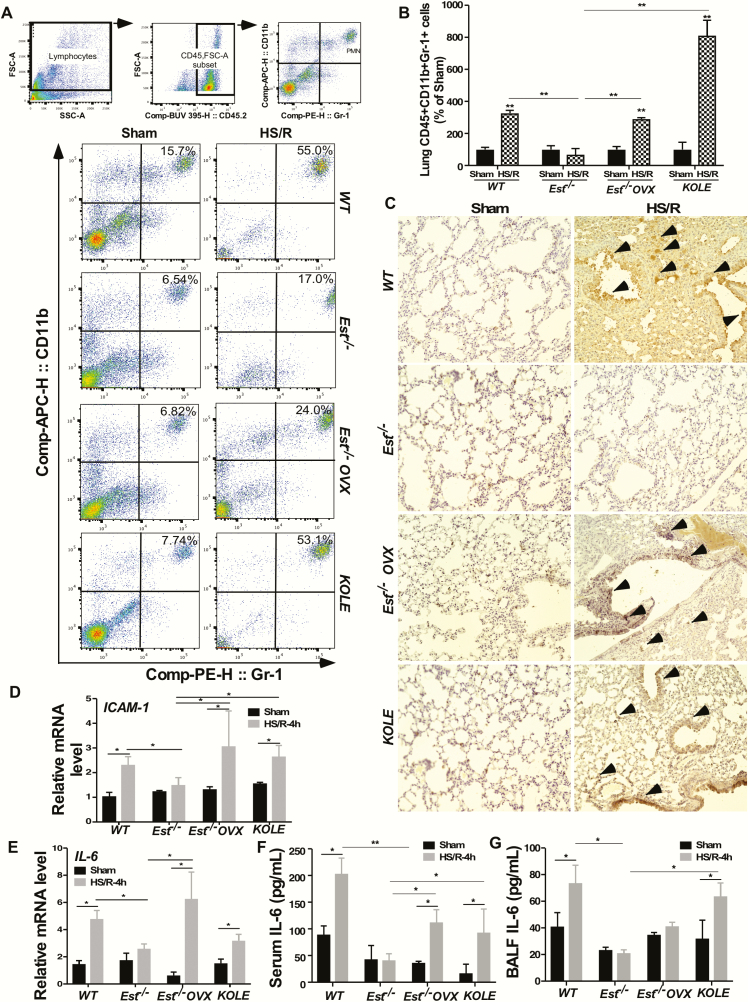
Estrogens are known for their anti-inflammatory activities. To determine whether the inhibition of HS-induced ALI in female Est-/- mice was accompanied by the attenuation of inflammation, we evaluated pulmonary infiltration of PMNs, another hallmark of ALI (42), by measuring the number of lung PMNs by flow cytometry and the level of MPO by immunohistochemistry. MPO is a widely used index of PMN sequestration that reflects the infiltration of lung parenchymal phagocytes (43). Compared to HS-treated female WT mice, HS-treated female Est-/- mice showed a reduced level of lung CD45+/CD11b+/Gr-1+ cells as shown by flow cytometry (Fig. 5A) and its quantification (Fig. 5B), as well as MPO immunostaining (Fig. 5C), and these effects were abolished in ovariectomized Est-/- mice and intact KOLE mice (Fig. 5A–C). Consistent with the results of flow cytometry and histology, the reductions in the pulmonary mRNA expression of ICAM-1 (Fig. 5D) and IL-6 (Fig. 5E), and in the serum (Fig. 5F) and BALF (Fig. 5G) levels of IL-6 observed in HS-treated female Est-/- mice were attenuated or abolished in their ovariectomized counterparts or in intact KOLE mice. ICAM-1 contributes to the firm adhesion and emigration of PMNs (44), whereas IL-6 is known to regulate neutrophil trafficking and function (45). One exception is that ovariectomy did not increase the BALF IL-6 level, suggesting that there might be other yet-to-be identified substrates or metabolites of Est that may have also contributed to the protective effect Est ablation.
Figure 5.
Est ablation attenuates, whereas liver reconstitution of EST restores HS-induced lung local and systemic inflammation. (A and B) Polymorphonuclear neutrophils (PMN) were quantified by flow cytometry in lung collected from female WT, Est-/-, Est-/- OVX, and KOLE mice subjected to the sham surgery or HS/R. Mice were sacrificed 4 hours after the initiation of HS. Shown are gating strategy (upper 3 panels) and representative density plots (bottom panels) of flow cytometry (A) and quantification of lung CD45+/CD11b+/Gr-1+ PMN cells normalized by PMNs in Sham group (B). (C) Myeloperoxidase (MPO) immunohistochemical staining on lung paraffin sections. Arrowheads indicate positive MPO staining. Original magnification 200x. (D and E) The mRNA expression of ICAM-1 (D) and IL-6 (E) was measured by real-time PCR. (F and G) The serum (F) and BALF (G) levels of IL-6 were measured by ELISA. Results are presented as mean ± SEM, (n = 4~5 for each group). * P< 0.05; **P < 0.01, with the comparisons labeled.
Abbreviations: BALF, bronchoalveolar lavage fluid; ELISA, enzyme-linked immunosorbent assay; HS, hemorrhagic shock; OVX, ovariectomy; PCR, polymerase chain reaction; R, resuscitation.
Est ablation attenuates HS-induced PMN mobilization from the bone marrow
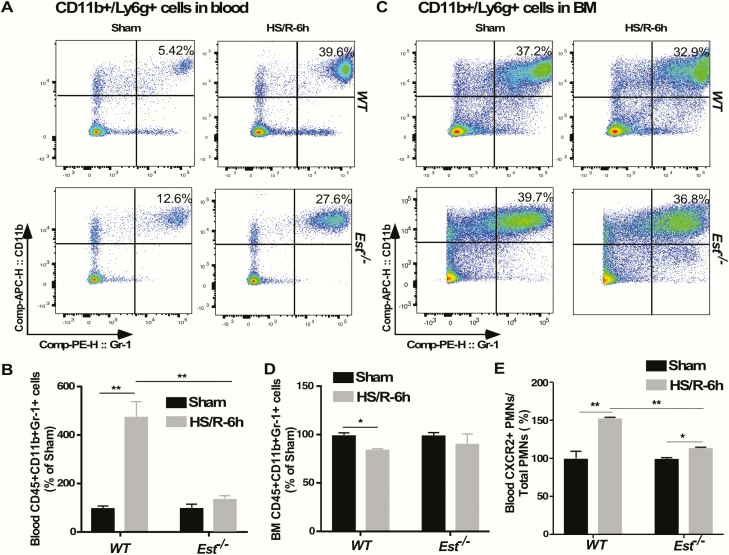
Besides the PMN migration to the lung, we also wondered whether Est ablation also affected HS-responsive mobilization of PMNs from the bone marrow (BM) to the circulation by flow cytometry measurement of the numbers of PMNs in peripheral blood and BM. Consistent with their attenuation of ALI, HS-treated Est-/- mice showed reduced PMN mobilization as shown by decreased PMN induction in peripheral blood (Fig. 6A and B) and attenuated PMN reduction in BM (Fig. 6C and D) as compared with WT mice at 6 hours after HS. We observed a slightly higher level of blood PMNs in Est-/- mice than WT mice in the sham group. As reported by others, there is an unresolved paradox with respect to the immunomodulating role of estrogens, either suppression of inflammation during trauma and sepsis or the proinflammatory effects in some chronic autoimmune diseases in humans. It has been suggested that E2 can stimulate the expression and secretion of IL-1β and IL-6 in certain cell or tissue types (35). Future studies are necessary to further define the role of EST and estradiol in HS-induced lung inflammation. The C-X-C chemokine receptor type 2 (CXCR2) is an important PMN surface receptor that contributes to PMN release from BM (46). There was a 5-fold induction of CXCR2 positive PMNs in the peripheral blood of HS-treated WT mice, but this induction was abolished in HS-treated Est-/- mice (Fig. 6E), further suggesting that Est ablation inhibited the mobilization of PMNs from BM.
Figure 6.
Est ablation attenuates HS-induced PMN mobilization from the bone marrow. (A-D) PMNs in peripheral blood and bone marrow (BM) of female WT and Est-/- mice subjected to HS/R are quantified by flow cytometry. Mice were sacrificed 6 hours after the initiation of HS. Shown are representative density plots (A) and quantification of CD45+/CD11b+/Gr-1+ PMN cells normalized by PMNs in Sham group (B) in peripheral blood, as well as representative density plots (C) and quantification of CD45+/CD11b+/Gr-1+ PMN cells normalized by PMNs in Sham group (D) in BM. (E) Quantification of CXCR2+ PMNs in peripheral blood by flow cytometry. Results are presented as mean ± SEM, (n = 3 for each group). *P < 0.05; **P < 0.01, with the comparisons labeled.
Abbreviations: HS, hemorrhagic shock; PMN, polymorphonuclear neutrophils; R, resuscitation; SEM, standard error of the mean; WT, wild-type.
Discussion
HS remains a major health concern largely due to the secondary tissue and organ injuries. A better understanding of the pathophysiology of HS and the mechanisms by which HS induces tissue injury, including lung injury, will help to develop better therapeutic strategies to reduce the morbidity and mortality of HS. In this study, we have uncovered a novel function of liver EST in HS-induced ALI.
One of our interesting findings was the estrogen dependence and sex-specific effect of Est inhibition on HS-induced ALI. Sex-specific differences in pulmonary morbidity in humans have been well documented. The mortality of ALI was higher in male patients compared with female patients (6, 7). The female protection was postulated to be due to the protective effect of estrogens in premenopausal patients (6, 8, 47), a notion consistent with our finding that the female Est-/- mice were protected from HS-induced ALI, and this protection was estrogen dependent. Knowing the ER mediates many of the estrogen functions, we were surprised to find that treatment with the ER antagonist fulvestrant had little effect on the pulmonoprotective effect of Est ablation, suggesting the protection was ER independent. Estrogens are known to exert their functions through multiple pathways in either an ER-dependent or ER-independent manner (48, 49). Future use of ER α- and/or β-KO mice will further clarify the role of ER in mediating the EST effect. Most of the reported roles of estrogens in traumatic injury relied on the administration of pharmacological doses of estrogens. Our results suggested that the benefit of estrogens in organ protection can also be achieved through the regulation of endogenous estrogen homeostasis, such as that mediated by EST and its regulation. Modulation of estrogen activity by regulating the metabolism of endogenous estrogens has the potential benefit of avoiding side effects associated with pharmacological estrogen therapies (47).
The inhibition of PMN mobilization from BM by Est ablation was also interesting. The HS-responsive mobilization of PMNs from BM was at least in part contributed to by the induction of CXCR2, a chemokine receptor important for PMN release from BM. The HS-responsive induction of CXCR2-positive PMNs was abolished in Est-/- mice, which may have contributed to the inhibition of PMN mobilization. The effect of Est on the number of CXCR2-positive PMNs was unlikely intrinsic because PMNs isolated from BM had no detectable expression of Est (data not shown). Rather, the induction of CXCR2-positive PMNs may have been explained by the induction of hepatic Est and decreased circulating level of estrogens because CXCR2 is known to be negatively regulated by estradiol (50, 51).
Interestingly, ablation of Est had little effect on HS-induced ALI in male mice. The mechanism for the lack of pulmonoprotective effect in male Est-/- mice remains to be understood. Est ablation had little detectable effect on the circulating and lung tissue levels of estrogens in male mice, which may be due to the extremely low levels of endogenous estrogens that were beyond the detections by UPLC-MS/MS (data not shown). Nevertheless, the sex-specific effect of EST was not uncommon. Sex-specific effects of Est ablation were also observed in our previous studies of metabolic disease and liver ischemia/reperfusion-induced liver injury. We reported that Est ablation protected female ob/ob (leptin deficient, or Lepob) mice from obesity and type 2 diabetes but sensitized male ob/ob mice to metabolic syndrome (16). In a mouse model of liver ischemia-reperfusion, Est ablation conferred hepatoprotection to female mice, whereas the male Est-/- mice were further sensitized (17). Interestingly, unlike in the ob/ob mice or in the liver ischemia/reperfusion models, Est ablation did not sensitize male mice to HS-induced ALI.
Another interesting finding was the evidence of organ crosstalk and the tissue-specific effect of EST on HS-induced ALI. Although HS-responsive induction of Est was liver specific, Est ablation surprisingly had little effect on HS-induced liver injury. The lack of Est KO effect on HS-induced liver injury was reminiscent of the lack of effect of Est ablation on obesity and type 2 diabetes despite a marked hepatic induction of this enzyme in metabolic disease (16, 19). An even more interesting finding is that the hepatic EST can distantly sensitize mice to HS-induced ALI, which was supported by 2 key pieces of evidence: (1) There was no appreciable expression or induction of Est in the lung in response to HS; and (2) Reconstitution of EST to the liver of KOLE mice was sufficient to re-sensitize female Est-/- mice to HS-induced ALI. The distal effect of hepatic EST on HS-induced ALI was likely mediated by hepatic metabolism of estrogens, which led to decreased circulating and lung tissue levels of active estrogens. The lack of HS effect on the pulmonary expression of other enzymes involved in local estrogen homeostasis suggested that the increased estrogen activity in the lung was independent of local estrogen production.
In summary, we have uncovered a novel contribution of liver EST to HS-induced ALI. EST has been shown to affect the structure and function of several tissues including the male and female reproductive tissues, liver, and adipose tissue. To our best knowledge, the current study represents the first report on the pulmonary function of EST, and the pulmonary effect of EST was achieved via the liver-to-lung organ crosstalk. Our results suggested that pharmacological inhibition of EST, at least in females, might represent a novel therapeutic approach to manage HS-induced ALI.
Acknowledgments
Financial Support: This work was supported in part by the US National Instiutes of Health grants ES023438 and ES030429 (to W.X.). W.X. was also supported in part by the Joseph Koslow Endowed Professorship from the University of Pittsburgh School of Pharmacy. We thank Junyi Li for her assistance in the analysis of estrogens.
Author Contributions: Participated in research design: Y. Xie and W. Xie; conducted experiments: Y. Xie, A. Barbosa, M. Xu, P. Oberly, S. Ren; performed data analysis: Y. Xie and W. Xie; contributed key reagents: W. Song; wrote or contributed to writing of the manuscript: Y. Xie, R.B.G., S.M.P., W. Song, J. Fan, and W. Xie; acquired funding: W. Xie.
The guarantors are Y.X. and W.X. and, as such, have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Glossary
Abbreviations:
- ALI
acute lung injury
- BALF
bronchoalveolar lavage fluid
- BM
bone marrow
- CXCR2
C-X-C chemokine receptor type 2
- ER
estrogen receptor
- EST/Est
estrogen sulfotransferase
- HS
hemorrhagic shock
- IL
interleukin
- KO
knockout
- Lap
liver-enriched activator protein
- LE
Lap-EST
- MOF
multiple organ failure
- MPO
myeloperoxidase
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PMN
polymorphonuclear neutrophil
- R
resuscitation
- RNA
ribonucleic acid
- SULTIE1
estrogen sulfotransferase
- UPLC–MS/MS
ultra performance liquid chromatography–tandem mass spectrometer
- W/D
wet to dry
- WT
wild-type
Additional Information
Disclosure Summary: No conflict of interest for any of the authors.
References
- 1. Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. [DOI] [PubMed] [Google Scholar]
- 2. Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care. 2014;20(1):3–9. [DOI] [PubMed] [Google Scholar]
- 3. Liu Y, Yuan Y, Li Y, et al. Interacting neuroendocrine and innate and acquired immune pathways regulate neutrophil mobilization from bone marrow following hemorrhagic shock. J Immunol. 2009;182(1):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wen Z, Fan L, Li Y, et al. Neutrophils counteract autophagy-mediated anti-inflammatory mechanisms in alveolar macrophage: role in posthemorrhagic shock acute lung inflammation. J Immunol. 2014;193(9):4623–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu J, Guardado J, Hoffman R, et al. IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: a reverse translation study from a human cohort to a mouse trauma model. PLoS Med. 2017;14(7):e1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang KC, Zhou MJ, Sperry JL, et al. Significant sex-based outcome differences in severely injured Chinese trauma patients. Shock. 2014;42(1):11–15. [DOI] [PubMed] [Google Scholar]
- 7. Sperry JL, Vodovotz Y, Ferrell RE, et al. Racial disparities and sex-based outcomes differences after severe injury. J Am Coll Surg. 2012;214(6):973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sperry JL, Friese RS, Frankel HL, et al. ; Inflammation and the Host Response to Injury Investigators . Male gender is associated with excessive IL-6 expression following severe injury. J Trauma. 2008;64(3):572–578; discussion 578. [DOI] [PubMed] [Google Scholar]
- 9. Frink M, Pape HC, van Griensven M, Krettek C, Chaudry IH, Hildebrand F. Influence of sex and age on mods and cytokines after multiple injuries. Shock. 2007;27(2):151–156. [DOI] [PubMed] [Google Scholar]
- 10. Zolin SJ, Vodovotz Y, Forsythe RM, et al. The early evolving sex hormone environment is associated with significant outcome and inflammatory response differences after injury. J Trauma Acute Care Surg. 2015;78(3):451–457; discussion 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breithaupt-Faloppa AC, Thais Fantozzi E, Romero DC, et al. Acute effects of estradiol on lung inflammation due to intestinal ischemic insult in male rats. Shock. 2014;41(3):208–213. [DOI] [PubMed] [Google Scholar]
- 12. Hamidi SA, Dickman KG, Berisha H, Said SI. 17β-estradiol protects the lung against acute injury: possible mediation by vasoactive intestinal polypeptide. Endocrinology. 2011;152(12): 4729–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbosa ACS, Feng Y, Yu C, Huang M, Xie W. Estrogen sulfotransferase in the metabolism of estrogenic drugs and in the pathogenesis of diseases. Expert Opin Drug Metab Toxicol. 2019;15(4):329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garbacz WG, Jiang M, Xie W. Sex-dependent role of estrogen sulfotransferase and steroid sulfatase in metabolic homeostasis. Adv Exp Med Biol. 2017;1043:455–469. [DOI] [PubMed] [Google Scholar]
- 15. Riches Z, Stanley EL, Bloomer JC, Coughtrie MW. Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: the SULT “pie”. Drug Metab Dispos. 2009;37(11):2255–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao J, He J, Shi X, et al. Sex-specific effect of estrogen sulfotransferase on mouse models of type 2 diabetes. Diabetes. 2012;61(6):1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo Y, Hu B, Huang H, et al. Estrogen sulfotransferase is an oxidative stress-responsive gene that gender-specifically affects liver ischemia/reperfusion injury. J Biol Chem. 2015;290(23):14754–14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chai X, Guo Y, Jiang M, et al. Oestrogen sulfotransferase ablation sensitizes mice to sepsis. Nat Commun. 2015;6:7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garbacz WG, Jiang M, Xu M, Yamauchi J, Dong HH, Xie W. Sex- and tissue-specific role of estrogen sulfotransferase in energy homeostasis and insulin sensitivity. Endocrinology. 2017;158(11):4093–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qian YM, Sun XJ, Tong MH, Li XP, Richa J, Song WC. Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology. 2001;142(12):5342–5350. [DOI] [PubMed] [Google Scholar]
- 21. Xie Y, Xu M, Deng M, et al. Activation of pregnane X receptor sensitizes mice to hemorrhagic shock-induced liver injury. Hepatology. 2019;70(3):995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jhingran A, Kasahara S, Hohl TM. Flow cytometry of lung and bronchoalveolar lavage fluid cells from mice challenged with fluorescent aspergillus reporter (FLARE) conidia. Bio Protoc. 2016;6(18):e1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. RRID: AB_9353 R. https://www.abcam.com/myeloperoxidase-antibody-ab9535.html. Accessed December 29, 2019. [Google Scholar]
- 24. RRID: AB_197674. https://www.abcam.com/estrogen-sulfotransferase-antibody-ab197674.html. Accessed December 29, 2019. [Google Scholar]
- 25. RRID: AP182SA6MI. https://www.fishersci.com/shop/products/donkey-anti-rabbit-igg-antibod/ap182sa6mi. Accessed December 29, 2019. [Google Scholar]
- 26. RRID: 0100-20. https://www.southernbiotech.com/?catno=0100-20&type=Other#&panel1-1&panel2-1. Accessed December 29, 2019. [Google Scholar]
- 27. Xie Y, Barbosa ACS, Xu M, et al. Data from: hepatic estrogen sulfotransferase distantly sensitizes mice to hemorrhagic shock-induced acute lung injury. Dryad Digital Repository. Deposited 4 Decmber 2019. 10.5061/dryad.00000000j. Accessed December 29, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. RRID: 12522-1-AP. https://www.ptglab.com/products/SULT1E1-Antibody-12522-1-AP.htm. Accessed December 29, 2019. [Google Scholar]
- 29. RRID: A1978. https://www.sigmaaldrich.com/catalog/product/sigma/a1978?lang=en®ion=US. Accessed December 29, 2019. [Google Scholar]
- 30. RRID: 7074S. https://www.cellsignal.com/products/secondary-antibodies/anti-rabbit-igg-hrp-linked-antibody/7074. Accessed December 29, 2019. [Google Scholar]
- 31. RRID: 7076S. https://www.cellsignal.com/products/secondary-antibodies/anti-rabbit-igg-hrp-linked-antibody/7076. Accessed December 29, 2019. [Google Scholar]
- 32. Swamydas M, Lionakis MS. Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. J Vis Exp. 2013;(77):e50586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. RRID: 564279. https://www.bdbiosciences.com/us/reagents/research/antibodies-buffers/immunology-reagents/anti-mouse-antibodies/cell-surface-antigens/buv395-rat-anti-mouse-cd45-30-f11/p/564279. Accessed December 29, 2019. [Google Scholar]
- 34. RRID: 101212. https://www.biolegend.com/en-us/products/apc-anti-mouse-human-cd11b-antibody-345. Accessed December 29, 2019. [Google Scholar]
- 35. RRID: 108407. https://www.biolegend.com/en-us/products/pe-anti-mouse-ly-6g-ly-6c-gr-1-antibody-460. Accessed December 29, 2019. [Google Scholar]
- 36. RRID: 149309. https://www.biolegend.com/en-us/products/fitc-anti-mouse-cd182-cxcr2-antibody-12299. Accessed December 29, 2019. [Google Scholar]
- 37. Li J, Oberly PJ, Poloyac SM, Gibbs RB. A microsomal based method to detect aromatase activity in different brain regions of the rat using ultra performance liquid chromatography-mass spectrometry. J Steroid Biochem Mol Biol. 2016;163:113–120. [DOI] [PubMed] [Google Scholar]
- 38. Swamydas M, Luo Y, Dorf ME, Lionakis MS. Isolation of mouse neutrophils. Curr Protoc Immunol. 2015;110:3.20.1–3.20.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang LQ, Falany CN, James MO. Triclosan as a substrate and inhibitor of 3’-phosphoadenosine 5’-phosphosulfate-sulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab Dispos. 2004;32(10):1162–1169. [DOI] [PubMed] [Google Scholar]
- 40. Deroo BJ, Hewitt SC, Collins JB, Grissom SF, Hamilton KJ, Korach KS. Profile of estrogen-responsive genes in an estrogen-specific mammary gland outgrowth model. Mol Reprod Dev. 2009;76(8):733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Win S, Min RW, Chen CQ, et al. Expression of mitochondrial membrane-linked SAB determines severity of sex-dependent acute liver injury. J Clin Invest. 2019;129(12):5278–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L379–L399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78(3):206–209. [DOI] [PubMed] [Google Scholar]
- 44. Bullard DC, Qin L, Lorenzo I, et al. P-selectin/ICAM-1 double mutant mice: acute emigration of neutrophils into the peritoneum is completely absent but is normal into pulmonary alveoli. J Clin Invest. 1995;95(4):1782–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fielding CA, McLoughlin RM, McLeod L, et al. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181(3):2189–2195. [DOI] [PubMed] [Google Scholar]
- 46. Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120(7):2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Staren ED, Omer S. Hormone replacement therapy in postmenopausal women. Am J Surg. 2004;188(2):136–149. [DOI] [PubMed] [Google Scholar]
- 48. Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78(2):161–170. [DOI] [PubMed] [Google Scholar]
- 49. Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276(40):36869–36872. [DOI] [PubMed] [Google Scholar]
- 50. Lei ZB, Fu XJ, Lu ZT, Wang BC, Liu XL, You NZ. Effect of estradiol on chemokine receptor CXCR2 expression in rats: implications for atherosclerosis. Acta Pharmacol Sin. 2003;24(7):670–674. [PubMed] [Google Scholar]
- 51. Lasarte S, Samaniego R, Salinas-Muñoz L, et al. Sex hormones coordinate neutrophil immunity in the vagina by controlling chemokine gradients. J Infect Dis. 2016;213(3):476–484. [DOI] [PubMed] [Google Scholar]