Abstract
Background
Several drugs have been tried to obtund the hemodynamic extubation response but all have variable side effects that may affect the quality of short-term recovery.
Objective
Our primary objective was to evaluate the effect of pharmacological agents, such as dexmedetomidine, local anesthetics, and so on, administered for attenuating the extubation response on the quality of extubation, as judged by the presence or absence of cough, sedation, and laryngospasm/bronchospasm in adult patients who had undergone general anesthesia. A secondary objective was to evaluate the effect of these drugs on other immediate post-extubation complications such as respiratory depression, desaturation, bradycardia, hypotension, and nausea and vomiting (PONV).
Methods
This is a systematic review of (randomized controlled trials) RCTs with meta-analysis. The Medical Literature Analysis and Retrieval System Online (MEDLINE), Cumulative Index of Nursing and Allied Health Literature (CINAHL), and Cochrane Central Register of Controlled Trials (CENTRAL) databases were searched for RCTs on the effect of pharmacological agents on both the hemodynamic extubation response as well as the quality of extubation.
Results
Fourteen out of 24 included studies were subjected to a meta-analysis. The risk of cough was less likely in the intervention group as compared to control groups (OR 0.26, 95% CI 0.15 to 0.46, p<0.00001, I2=35%). Sedation, hypotension (OR= 10.47; 95% CI: 1.86, 58.80, p=0.008, I2=0%), and bradycardia (OR= 6.57; 95% CI: 2.09, 20.64, p=0.001, I2=0%) were reported with dexmedetomidine. Only one study reported laryngospasm with dexmedetomidine and two studies with opioids.
Conclusion
Dexmedetomidine 0.4 to 0.5 ug/kg was associated with smooth extubation, minimal coughing, no laryngospasm/ bronchospasm, and with stable hemodynamics, without causing respiratory depression, PONV, and desaturation. However, in higher doses (more than 0.5 ug/kg), it caused bradycardia, hypotension, and sedation. Other pharmacological agents, such as local anesthetics, calcium channel blockers, and opioids, did not attenuate cough associated with extubation.
Keywords: endotracheal extubation, complications, cough, dexmedetomidine, lidocaine, opioids
Introduction
Tracheal extubation following general anesthesia is associated with hemodynamic changes and airway reflexes [1]. The goals of smooth extubation are to avoid hemodynamic changes, minimize airway stimulation, and prevent straining, coughing, breath-holding, and laryngospasm, as well as to ensure continuous oxygen delivery to the lungs. Patients with cardiovascular and/or neurological diseases, active and passive smokers, and those with chronic airway diseases have a higher incidence of complications as related to extubation [1].
Several drugs have been investigated to obtund the hemodynamic extubation response in vulnerable patients. These are narcotics [2-3], local anesthetics [4], calcium channel blockers [5], alpha agonists, and so on [6-7]. All these pharmacological interventions are associated with certain undesirable side effects [4].
The rationale of this systematic review was to determine the effectiveness of the pharmacological agents administered for attenuating the hemodynamic extubation response with minimal effects on the quality of tracheal extubation.
Objectives
Our primary objective was to evaluate the effect of pharmacological agents administered for attenuating the tracheal extubation response on the quality of extubation as judged by the presence or absence of cough and/or sedation and the presence of laryngospasm/bronchospasm in adult patients undergoing general anesthesia. Our secondary objective was to evaluate the effect of these drugs on other, immediate post-extubation complications such as respiratory depression, desaturation, bradycardia, hypotension, and nausea and vomiting.
Materials and methods
Design
A systematic review of randomized controlled trials (RCTs) with a meta-analysis.
Data sources
The Medical Literature Analysis and Retrieval System Online (MEDLINE), Cumulative Index of Nursing and Allied Health Literature (CINAHL), and Cochrane Central Register of Controlled Trials (CENTRAL) databases were systematically searched for articles published between January 1, 1990, and December 31, 2015 (26 years).
The search strategy used and the keywords are provided in the appendix.
A bibliography of relevant articles was searched for additional studies and the search was not restricted by language. Authors of identified publications were not contacted for additional information.
Eligibility criteria
Inclusion Criteria
We included RCTs that studied the effect of pharmacological agents on both the hemodynamic extubation response as well as the quality of extubation. RCTs with both placebo and a drug control group, reporting on adult patients (18 years or above), of any race, either gender, and undergoing elective surgery in the operating room were included.
Studies that reported on any of the following primary or secondary outcomes were included.
Primary outcomes: The primary outcome was the quality of extubation. This was assessed by the presence or absence of cough at the time of extubation (graded from 1 to 5) [8], degree of sedation after extubation (Ramsay scale score of 1 and 2 meaning no sedation) [9-10], and the presence of laryngospasm/ bronchospasm at the time of extubation.
Secondary outcomes: The secondary outcome were respiratory depression (respiratory rate less than 10 breaths per minute), bradycardia (heart rate less than 60 beats per minute), hypotension (blood pressure less than 20% from the baseline), nausea and vomiting, desaturation (peripheral capillary oxygen saturation (SpO2) less than 92%) and any other adverse effects of drugs used for the suppression of the hemodynamic extubation response.
Exclusion Criteria
Studies where different doses of routine anesthetic drugs were used, (induction agents, muscle relaxants or inhalation agents) for attenuating the hemodynamic response to extubation were excluded.
Studies of patients undergoing tracheal extubation outside the operating room were also excluded.
Screening and Study Eligibility
All abstracts were independently screened by two reviewers. The selected articles were again reviewed independently by two reviewers. Any disagreement was referred to the third reviewer. The reasons for the exclusion of studies were also noted.
Data extraction and handling
Data were extracted individually by two reviewers on a predesigned data extraction form.
Assessment of Risk of Bias in Individual Studies
The risk of bias assessment was noted appropriately by the authors according to a standard description for each type of bias based on the Cochrane risk of bias tool [11]. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants (performance bias), blinding of outcome assessment (detection bias), bias of incomplete outcome data (attrition bias), and selective reporting bias (reporting bias) were assessed. After an independent assessment and then comparison, any conflicts were resolved by a discussion with the third reviewer. The studies were categorized into good quality, fair quality, and poor quality according to the thresholds set for converting the Cochrane risk of bias tool to Agency for Health Care Research and Quality (AHRQ) standards [11].
Statistical analysis
Meta-analyses were performed using Review Manager, version 5 software (The Cochrane Collaboration, Oxford, UK). The rate of cough, hypotension, bradycardia and nausea/vomiting of the intervention and control groups were tabulated and presented graphically using forest plots. The Mantel-Haenszel (M-H) analysis method with the random-effects model was used to compute the effect size in terms of the odds ratio for dichotomous outcomes. The chi-square (π2) test and I2 were performed to observe variability in the intervention effect that was due to heterogeneity among studies.
Results
Study selection
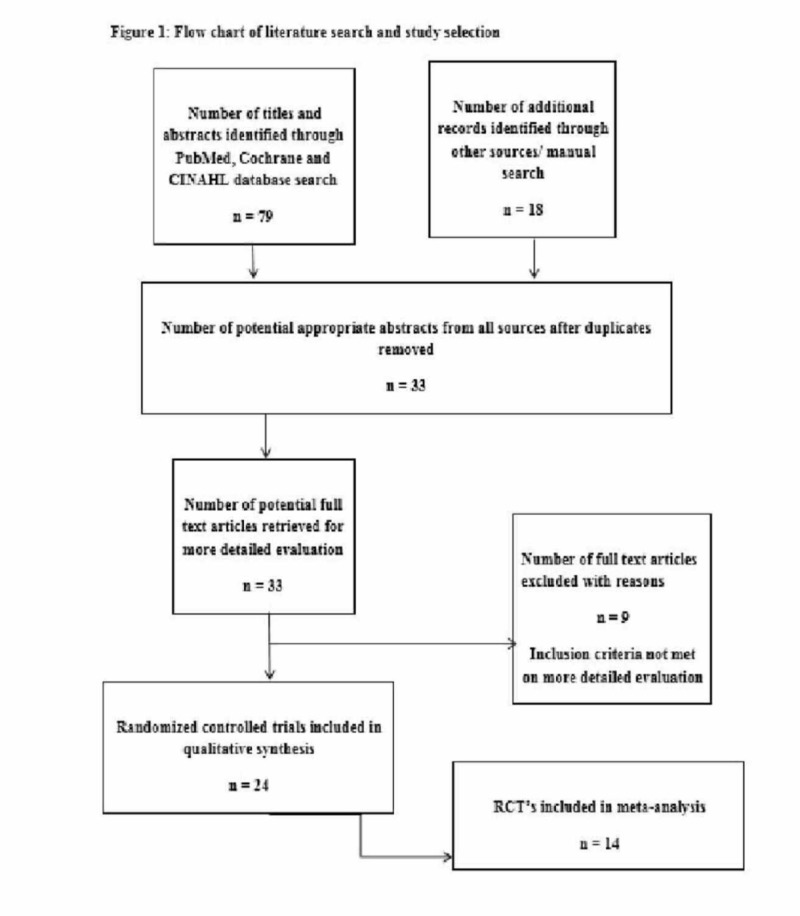
Our literature search identified 33 abstracts through both a database and a manual search. After going through the full texts of the abstracts, nine were excluded, as they did not fulfill our inclusion criteria completely, hence, 24 studies were included in the qualitative analysis (Figure 1).
Figure 1. Flow chart of literature search and study selection.
The data of all the study characteristics are shown in Table 1.
Table 1. Characteristics of included studies.
IV: intravenous; TCM: transcricoid membrane; N/S: normal saline; n: group sample size; N: total sample size; min: minutes
| Author/ Year | N | Study Groups | Dose | Per Group (n) | Route of Administration | Timing | |
| Nishina 1995 [2] | 60 | Saline | 20 | Bolus | At time of peritoneal closure | ||
| Fentanyl | 1 ug/kg | 20 | |||||
| Fentanyl | 2 ug/kg | 20 | |||||
| Aksu 2009 [3] | 40 | Dexmedetomidine | 0.5 ug/kg | 20 | Infusion | Before extubation | |
| Fentanyl | 1 ug/kg | 20 | |||||
| Mistry 2016 [5] | 30 | Verapamil | 0.1mg/kg | 15 | IV Bolus/Infusion | On return of breathing | |
| Dexmedetomidine | 0.3ug/kg | 15 | |||||
| Kim 2015 [6] | 115 | Saline | 0.1 ml/kg/hr | 28(a), 30(b) | Infusion | Drug given after induction | |
| Dexmedetomidine | 0.4 ug/kg/hr | 27(a), 30(b) | Infusion | ||||
| Lidocaine | 1mg/kg | 25 | I.V Bolus/Infusion | ||||
| PG.E | 0.1/mg/kg | 25 | Infusion | ||||
| PG.L | 0.1/mg/kg | 25 | Bolus + Infusion | ||||
| Xiaochun 2014 [7] | 90 | Saline | 30 | IV Bolus | 30 minutes after intubation | ||
| Dexmedetomidine | 0.4 ug/kg | 30 | IV Bolus | ||||
| Dexmedetomidine | 0.8 ug/kg | 30 | IV Bolus | ||||
| Mikawa 1996 [12] | 80 | Saline | 20 | I.V/ Bolus | 3 min after reversal | ||
| Diltiazem | 0.2 mg/kg | 20 | |||||
| Verapamil | 0.5 mg/kg | 20 | |||||
| Verapamil | 0.1 mg/kg | 20 | |||||
| Nishina 1997 [13] | 100 | Saline | 1mg/kg | 25 | I.V | 2 min before extubation | |
| Lidocaine | 1mg/kg | 25 | I.V Bolus/Infusion | ||||
| PG.E | 0.1/mg/kg | 25 | Infusion | ||||
| PG.L | 0.1/mg/kg | 25 | Bolus + Infusion | ||||
| Jee 2002 [14] | 75 | Control | 25 | IV Bolus | 3 to 5 min before extubation | ||
| Lidocaine | 1 mg/kg 2 % | 25 | IV Bolus | ||||
| Lidocaine | 1 mg/kg 2 % | 25 | Intra tracheally | ||||
| Guler 2005 [15] | 60 | Dexmedetomidine | 0.5mg/kg | 30 | I.V bolus | 5 min before end of surgery | |
| Saline | 30 | ||||||
| Mahoori 2014 [16] | 50 | Saline | 25 | Bolus | 90 sec prior to extubation | ||
| Remifentanil | 0.2 ug/kg | 25 | Bolus | ||||
| Andrzejowski 2002 [17] | 40 | Saline | 5ml | 20 | Tube cuff | Insertion of first skin clip | |
| Lidocaine | 2% 5ml | 20 | Tube cuff | ||||
| Lee 2014 [18] | 142 | Saline | 71 | Infusion | After extubation | ||
| Dexmedetomidine | 0.5 ug/kg | 71 | Infusion | ||||
| Shajar 1999 [19] | 40 | Saline | 20 | I.V/ Bolus | At time of last suture | ||
| Remifentanil | 1 ug/kg | 20 | |||||
| Moustafa 2012 [20] | 60 | Lidocaine | 1.0mg/kg | 20 | Bolus | 5 min before extubation | |
| Dexmedetomidine | 1 mg/kg | 20 | |||||
| Dexa +Lidocaine | 0.1 ug/kg + 1 mg/kg | 20 | |||||
| Nho 2009 [21] | 40 | Saline | 20 | Infusion | 4 min post extubation | ||
| Remifentanil | 20 | ||||||
| Aouad 2009 [22] | 60 | Saline | 30 | Infusion | At the end of the surgery | ||
| Remifentanil | 1/10th dose of infusion | 30 | Infusion | ||||
| Qing Fan 2015 [23] | 74 | Sevoflurane-Remifentanil | 0.03 ug/kg/min | 25 | Infusion | 10 min before extubation | |
| Sevoflurane-Dexmedetomide SD5 | 0.5 ug/kg | 24 | |||||
| Sevoflurane-SD7 | 0.7 ug/kg | 25 | |||||
| Dutta 2016 [24] | 45 | Saline | 10 ml | 15 | Endotracheally | After last skin suture | |
| Lidocaine | 1.5 mg/kg | 15 | Endotracheally | ||||
| Dexmedetomidine | 0.3 ug/kg | 15 | IV | ||||
| Turan2008 [25] | 40 | Saline | 20 | Bolus over 60 second | 5 min before end of procedure | ||
| Dexmedetomidine | 0.5 ug/kg | 20 | Bolus over 60 second | ||||
| Sharma 2014 [26] | 60 | Saline | 10 ml | 20 | Bolus | Just before extubation | |
| Lidocaine | 1.5 mg/kg | 20 | Bolus | ||||
| Dexmedetomidine | 0.5 ug/kg | 20 | Bolus | ||||
| Gao 2014 [27] | 70 | Ropivaciane | 20mg | 35 | TCM | Before intubation | |
| Diacine | 20mg | 35 | TCM | ||||
| Kothari 2014 [28] | 50 | Dexmedetomidine | 0.5 ug/kg | 25 | IV bolus | 5 minutes before extubation | |
| Lignocaine | 1.5 mg/kg | 25 | |||||
| Bindu 2013 [29] | 50 | Saline | 100 ml | 25 | I.V infusion | 15 min before extubation | |
| Dexmedetomidine | 0.75 mcg/kg | 25 | I.V infusion | ||||
| Shruthi 2016 [30] | 80 | Saline | 10 ml | 40 | Infusion | Beginning of skin closure | |
| Dexmedetomidine | 0.5 ug/kg | 40 | Infusion | ||||
Hemodynamic changes
The hemodynamic response was reported as blood pressure (BP) and heart rate (HR) change in all trials but the manner of reporting was different among studies. Nine studies documented a change in systolic blood pressure (SBP), diastolic blood pressure (DBP), and HR [2-3,7,12-17] while 12 studies documented the changes in mean arterial pressure (MAP) and HR only [5-6,18-27]. Three studies documented changes in MAP in addition to SBP, DBP, and HR [28-30]. A saline control group was used in 18 studies [2,6-7,12-19,21-22,24-26,29-30]. In four studies, no placebo was used in the control against the study drug [3,20,27-28]. In seven studies, the authors compared two different drugs or the same drug in different doses [3,5,12,20,23,27-28].
Hypotension was recorded in three studies [15,29-30] while bradycardia was observed in seven studies (see Table 2) [3,5,15,18,26,29-30].
Table 2. Attenuation of hemodynamic response in the included studies.
HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure
| Study ID Year | Attenuation of Haemodynamic Response | Drug Groups | Comments | |||
| Nishina 1995 [2] | Yes | Fentanyl Saline | HR, SBP, DBP higher in the control group as compared to fentanyl (p<0.05) | |||
| Mikawa 1996 [12] | Yes | Diltiazem, Verapamil, Saline | HR, SBP, DBP. Both drugs attenuated but verapamil 0.1 mg /kg more effective | |||
| Nishina 1997 [13] | Yes | Lidocaine, PGE, PGE, Lidocaine Saline | PGE, Lidocaine combination attenuated SBP, DBP, and HR (p<0.05) | |||
| Shajar 1997 [19] | Yes | Remifentanil Saline | Remi attenuated both MAP, HR in comparison with saline (p<0.01 and 0.05) | |||
| Jee 2002 [14] | Yes | Lidocaine Saline | HR, SBP, DBP were attenuated by Lidocaine sprayed down the ETT immediately after extubation only | |||
| Andrzejowski 2002 [17] | No difference | Lidocaine Saline | No difference between the groups (p>0.05) | |||
| Guler 2005 [15] | Yes | Dex Saline | SAP and DAP were significantly lower in the dex group compared to saline (p<0.05). Episode of bradycardia in 1 and hypotension in 3 patients in the dex group | |||
| Turan 2008 [25] | Yes | Dex Saline | HR and MAP were significantly higher in control as compared to the dex group (p<0.01) | |||
| Aouad 2009 [22] | Yes | Remifentanil Saline | HR and MAP increased in control as compared to remi (p<0.05) | |||
| Nho 2009 [21] | Yes | Remifentanil Saline | HR and MAP were significantly increased in the control group as compared to remi (HR p=0.001 and MAP p=0.002) | |||
| Aksu 2009 [3] | Yes | Dex Fentanyl | HR, SBP, DBP were significantly increased by in fentanyl group as compared to dex (HR p=0.003and SBP p=0.037) | |||
| Moustafa 2012 [20] | Yes | Lidocaine Dex Dex plus lidocaine | Dex+lidocaine combination attenuated HR, MAP, RPP in comparison to the two drugs alone (p<0.05) | |||
| Bindhu 2013 [29] | Yes | Dex Saline | HR, SBP, DBP, and MAP significantly higher in control (p<0.05). Bradycardia and hypotension reported with dex | |||
| Mahoori 2014 [16] | Yes | Dex Saline | HR, SBP, DBP were significantly increased in control (p<0.05) | |||
| Xiachun7 2014 [7] | Yes | Dex Saline | Dexmedetomidine 0.8 ug/kg more effectively attenuated HR, SBP, and DBP | |||
| Sharma 2014 [26] | Yes | Dex Lidocaine Saline | Dexmedetomidine more effective than lignocaine in attenuating HR (p=0.01), MAP. One patient had bradycardia in the dex group | |||
| Lee 2014 [18] | Yes | Dex Saline | HR, MAP were attenuated in the dex group as compared to control. One patient had bradycardia in the dex group | |||
| Kothari 2014 [28] | Yes | Dex Lidocaine | HR, SBP, DBP, MAP were below baseline in the dex group as compared to the lido group (p<0.05) | |||
| Gao 2014 [27] | Yes | Ropivacaine Diacine | HR, MAP Ropivaciane more effective than diacine ( p<0.05) | |||
| Fan 2015 [23] | Yes | Remifentanil Dex | HR, MAP. Dexmedetomidine more effective than remifentanil (p<0.05) | |||
| Kim6 2015 [6] | Yes | Dex Saline | HR was lower in the dex group (p<0.05), no difference in MAP | |||
| Mistry 2016 [5] | Yes | Verapamil Dex | HR, MAP were higher in the verapamil group than in the dex but statistically insignificant | |||
| Shruthi 2016c[30] | Yes | Dex Saline | HR, SBP, DBP, MAP were lower in the dex group but significantly increased in the control (p<0.001) | |||
| Dutta24 2016 [24] | Yes | Lidocaine Dex Saline | HR, MAP. Dexmedetomidine better effect than lignocaine spray (p<0.05) | |||
Surrogate measures used for the quality of extubation and the immediate post-extubation complications
The following outcome measures were used for assessing the quality of extubation and the immediate post-extubation complications. The primary outcome measures were cough, sedation, and laryngospasm/bronchospasm. The secondary outcome measures seen were hypotension, bradycardia, and immediate postoperative nausea and vomiting. The outcome measures are summarized in Table 3.
Table 3. Primary and secondary outcomes reported in the included studies.
| Author/Year | Study Groups | Per Group (n) | Primary Outcome (Event/n) | Secondary Outcome (Event/n) | ||||||||
| Cough | Sedation | Laryngospasm | Hypotension | Desaturation | Bradycardia | Nausea/ vomiting | Respiratory depression | |||||
| Nishina 1995 [2] | Saline | 20 | 20/20 | Zero | Zero | Zero | Zero | Zero | 16/20 | Zero | ||
| Fentanyl | 20 | 19/20 | Zero | Zero | Zero | Zero | Zero | 19/20 | Zero | |||
| Fentanyl | 20 | 17/20 | Zero | Zero | Zero | Zero | Zero | 19/20 | Zero | |||
| Mikawa 1996 [12] | Saline | 20 | 3/20 | NR | Zero | Zero | NR | Zero | NR | NR | ||
| Diltiazem | 20 | 3/20 | NR | Zero | Zero | NR | Zero | NR | NR | |||
| Verapamil | 20 | 3/20 | NR | Zero | Zero | NR | Zero | NR | NR | |||
| Verapamil | 20 | 3/20 | NR | Zero | Zero | NR | Zero | NR | NR | |||
| Nishina 1997 [13] | Saline | 25 | 25/25 | NR | Zero | Zero | NR | Zero | NR | NR | ||
| Lidocaine | 25 | 13/25 | NR | Zero | Zero | NR | Zero | NR | NR | |||
| PG.E | 25 | 25/25 | NR | Zero | Zero | NR | Zero | NR | NR | |||
| PG.L | 25 | 14/25 | NR | Zero | Zero | NR | Zero | NR | NR | |||
| Shajar 1999 [19] | Saline | 20 | 11/20 | 10/20 | NR | zero | NR | Zero | 1 | Zero | ||
| Remifentanil | 20 | 9/20 | 3/20 | NR | zero | NR | Zero | 2 | Zero | |||
| Jee 2002 [14] | Saline | 14/25 | NR | Zero | NR | NR | NR | NR | NR | |||
| Lidocaine | 25 | 10/25 | NR | Zero | NR | NR | NR | NR | NR | |||
| Lidocaine | 25 | 11/25 | NR | Zero | NR | NR | Non | NR | NR | |||
| Andrzejowski 2002 [17] | Saline | 20 | Zero | NR | NR | Zero | NR | Zero | NR | NR | ||
| Lidocaine | 20 | Zero | NR | NR | Zero | NR | Zero | NR | NR | |||
| Guler 2005 [15] | Dexmedetomidine | 30 | 3 | NR | Zero | 3 | Zero | 1 | NR | Zero | ||
| Saline | 30 | 8 | NR | Zero | Zero | Zero | Zero | NR | Zero | |||
| Turan 2008 [25] | Saline | 20 | 4/20 | NR | Zero | Zero | Zero | Zero | Zero | Zero | ||
| Dexmedetomidine | 20 | 0/20 | NR | Zero | Zero | Zero | Zero | Zero | Zero | |||
| Aouad 2009 [22] | Saline | 30 | 0/30 | NR | Zero | Zero | Zero | Zero | NR | Zero | ||
| Remifentanil | 30 | 2/30 | NR | Zero | Zero | Zero | Zero | NR | Zero | |||
| Nho 2009 [21] | Saline | 20 | 8/20 | NR | NR | Zero | Zero | Zero | 3/20 | Zero | ||
| Remifentanil | 20 | 0/20 | NR | NR | Zero | Zero | Zero | 0/20 | Zero | |||
| Aksu 2009 [3] | Dexmedetomidine | 20 | 1 (5%) | 1 | Zero | Zero | Zero | 2/20 | 2/20 | NR | ||
| Fentanyl | 20 | 4 (20%) | 2 | 1 | Zero | Zero | 2/20 | 3/20 | NR | |||
| Moustafa 2012 [20] | Lidocaine | 20 | 5 | NR | NR | Zero | Zero | Zero | NR | NR | ||
| Dexmedetomidine | 20 | 14 | NR | NR | NR | Zero | NR | NR | NR | |||
| Dexmedetomidine +Lidocaine | 20 | 5 | NR | NR | NR | Zero | NR | NR | NR | |||
| Bindu 2013 [29] | Saline | 25 | 21/25 | 5/25 | Zero | 0/25 | Zero | 2/25 | 2/25 | Zero | ||
| Dexmedetomidine | 25 | 4/25 | 21/25 | Zero | 2/25 | Zero | 13/25 | 1/25 | Zero | |||
| Mahoori 2014 [16] | Saline | 25 | 11/25 | NR | 1 | Zero | Zero | Zero | NR | NR | ||
| Remifentanil | 25 | 6/25 | NR | Zero | Zero | Zero | Zero | NR | NR | |||
| Xiaochun 2014 [7] | Saline | 30 | Zero | Zero | NR | Zero | Zero | NR | Zero | NR | ||
| Dexmedetomidine | 30 | Zero | Zero | NR | Zero | NR | NR | Zero | NR | |||
| Dexmedetomidine | 30 | Zero | Zero | NR | Zero | NR | NR | Zero | NR | |||
| Sharma 2014 [26] | Saline | 20 | 2/20 | Zero | Zero | Zero | Zero | 0/20 | NR | NR | ||
| Lidocaine | 20 | 0/20 | Zero | Zero | Zero | Zero | 0/20 | NR | NR | |||
| Dexmedetomidine | 20 | 0/20 | Zero | Zero | Zero | Zero | 1/20 | NR | NR | |||
| Lee 2014 [18] | Saline | 71 | 14/71 | 3 | Zero | Zero | Zero | Zero | Zero | Zero | ||
| Dexmedetomidine | 70 | 5/70 | 3 | Zero | Zero | Zero | 1/70 | Zero | Zero | |||
| Kothari 2014 [28] | Dexmedetomidine | 25 | Zero | 18/25 | Zero | Zero | Zero | Zero | NR | Zero | ||
| Lignocaine | 25 | 5 | Zero | Zero | Zero | Zero | Zero | NR | Zero | |||
| Gao 2014 [27] | Ropivaciane | 35 | 0/35 | NR | NR | Zero | Zero | Zero | 2/35 | Zero | ||
| Diacine | 35 | 4/35 | NR | NR | Zero | Zero | Zero | 3/35 | Zero | |||
| Qing Fan 2015 [23] | Sevoflurane-Remifentanil | 25 | Zero | Zero | Zero | NR | Zero | NR | 12/25 | NR | ||
| Sevoflurane-Dexmedetomide SD5 | 24 | Zero | Zero | 1 (4.2) | Zero | 1 (4.2) | NR | 4/25 | NR | |||
| Sevoflurane-SD7 | 25 | Zero | Zero | Zero | Zero | Zero | NR | 4/25 | NR | |||
| Kim 2015 [6] | Saline | 28(a), 30(b) | NR | 1(a) 6(b) | NR | Zero | NR | Zero | 11/28 (a) 3/30 (b) | NR | ||
| Dexmedetomidine | 27(a), 30(b) | NR | 13(a) 11(b) | NR | Zero | NR | Zero | 9/27 (a) 2/30 (b) | NR | |||
| Mistry 2016 [5] | Verapamil | 15 | Zero | Zero | Zero | Zero | NR | Zero | NR | NR | ||
| Dexmedetomidine | 15 | Zero | Zero | Zero | Zero | NR | 1/15 | NR | NR | |||
| Shruthi 2016 [30] | Saline | 40 | 12/40 | Zero | Zero | 0/40 | Zero | 0/40 | NR | NR | ||
| Dexmedetomidine | 40 | 2/40 | Zero | Zero | 9/40 | Zero | 2/40 | NR | NR | |||
| Dutta 2016 [24] | Saline | 15 | Zero | Zero | Zero | Zero | NR | Zero | NR | NR | ||
| Lidocaine | 15 | Zero | Zero | Zero | Zero | NR | Zero | NR | NR | |||
| Dexmedetomidine | 15 | Zero | Zero | Zero | Zero | NR | Zero | NR | NR | |||
Cough
Cough was observed in 13 placebo-controlled studies [2,13-16,18-19,21-22,25-26,29-30], and all these studies were included in the meta-analysis. Overall, cough developed in 23.2% of patients in the intervention group and 42.6% in the control group. The risk of cough was less likely in the intervention group as compared to the control group (OR 0.26, 95% CI 0.15 to 0.46, p=0.00001, I2=35%) (Figure 2).
Figure 2. Comparison of incidence of cough between interventions vs. placebo.
The odds ratio was calculated in the following subgroups.
Local Anesthetics Versus Placebo
Three studies compared lidocaine with placebo and were subjected to a meta-analysis [13-14,26]. There were 95 patients in each group, 34 developed a cough in the intervention group and 55 in the control group. The odds ratio was found to be 0.33 (95% CI 0.10 - 1.06) p=0.06) I2 =46%.
Alpha Agonist Versus Placebo
Six studies compared the alpha agonist with the placebo [15,18,25-26,29-30]. The incidence of cough was significantly reduced with alpha agonists. The odds ratio was 0.16 (95% CI: 0.07, 0.33) p< 0.00001) I2= 19%.
Opioids Versus Placebo
In five studies, the authors compared opioids with the placebo [2,16,19,21-22]. There was no statistical significance in the incidence of cough [OR=0.42 95%CI: 0.16, 1.09; p=0.08, I2=24].
Sedation
Sedation was reported in 13 studies using the Ramsay scale [2-3,5-7,18-19,23-24,26,28-30]. These studies compared dexmedetomidine with remifentanil, verapamil, fentanyl, and lidocaine.
Alpha Agonists
Dexmedetomidine in different doses was compared to saline in seven studies [6-7,15,18,25,29-30]. The doses used were 0.4 ug/kg [6-7,18], 0.5 ug/kg [15,25,30], 0.75 ug/kg [29], and 0.8 ug/kg [7]. All the authors reported significantly higher sedation in the patient groups who were administered dexmedetomidine. Dexmedetomidine 0.1 ug/kg resulted in a higher degree of sedation as compared to verapamil 0.3 ug/kg [5], but patients who received verapamil were anxious, agitated, and restless. The results were equivocal in studies that compared dexmedetomidine with lidocaine [24,26]. Dexmedetomidine in a dose of 0.3 and 0.5 ug/kg as compared to lidocaine did not show a significant difference in sedation [24,26]. Two studies compared dexmedetomidine with opioids [3,23]. Dexmedetomidine 0.5 ug/kg was compared with fentanyl 1 ug/kg. One patient in the dexmedetomidine group and two in the fentanyl group were not arousable [3]. Remifentanil 0.03 ug/kg/min was compared with dexmedetomidine 0.5 ug/kg and 0.7 ug/kg [23]. Time to awakening was comparable in all the groups p=0.24.
Opioids
Two studies compared opioids with the placebo [2,19]. Remifentanil 1 ug/kg was compared with saline. Only three patients were sedated in the remifentanil group as compared to saline where 10 patients had sedation, p=0.056 [19]. Two doses of fentanyl 1 ug/kg and 2 ug/kg were compared with saline and none of the patients were moderately or severely sedated in any group [2].
Laryngospasm/ bronchospasm
Laryngospasm/bronchospasm was reported in three studies [3,16,23]. One study looked at the effect of dexmedetomidine 0.5 ug/kg and fentanyl 1 ug/kg before extubation and reported one episode of laryngospasm in the fentanyl group [3]. Another study compared the effect of remifentanil 0.2 ug/kg with saline and reported one episode of laryngospasm in the saline group [16]. Two different doses of dexmedetomidine 0.5 and 0.7 ug/kg were compared with remifentanil 0.03 ug/kg/min in another study, resulting in one episode of laryngospasm in the 0.5 ug/kg dexmedetomidine group [23].
Hypotension
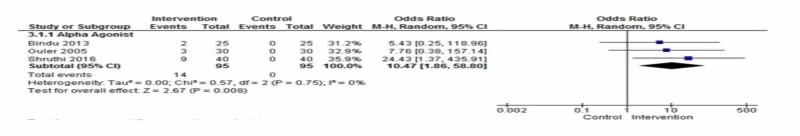
Hypotension was observed in three studies using alpha agonists [15,29-30]. Fourteen out of 95 patients had hypotension in the intervention group as compared to none in the control group. The odds ratio was 10.47 (CI: 1.86-58.80) with a p-value of 0.008, I2=0% (Figure 3).
Figure 3. Comparison of the incidence of hypotension between interventions vs. placebo.
Bradycardia
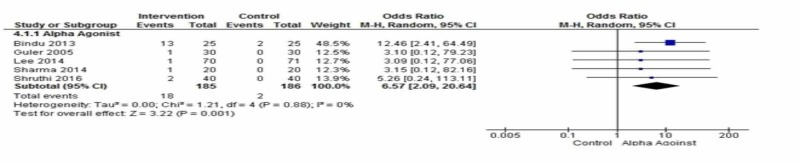
Five placebo-controlled studies using dexmedetomidine reported on bradycardia at extubation [15,18,26,29-,30]. All reported bradycardia with dexmedetomidine. Eighteen events of bradycardia occurred in the intervention group as compared to two in the control group. The risk of bradycardia was about seven times more likely in the intervention group as compared to the control group [OR= 6.57; 95% CI: 2.09, 20.64, p=0.001, I2=0%] (Figure 4).
Figure 4. Comparison of the incidence of bradycardia between interventions vs. placebo.
Nausea and vomiting
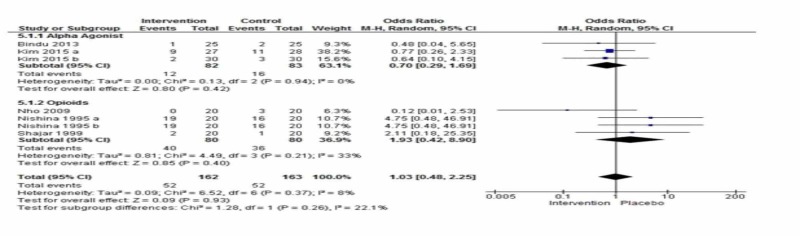
Five studies reported nausea and vomiting in the immediate postoperative period [2,6,19,21,29]. The combined effect was not statistically significant between groups [OR= 1.03; 95% CI: 0.48, 2.25, p=0.37, I2=8%] (Figure 5).
Figure 5. Comparison of the Incidence of Nausea or Vomiting Between Interventions vs. Placebo.
Subgroup Analysis for Alpha Agonist and Opioid with Control
Nausea and vomiting were observed and reported in two studies using alpha agonists [3,6] and three studies with opioids [2,19,21].
In the subgroup analysis, the effect was not statistically significant between groups [OR= 0.70; 95% CI: 0.29, 1.68, p=0.42, I2=0%] and [OR= 1.93; 95% CI: 0.42, 8.90; p=0.40, I2=33%].
Studies Not Subjected to the Meta-Analysis
Descriptive results
Studies with Local Anesthetics
Ropivacaine 1% was compared with diacine 1% via a transcricoid membrane injection. In the ropivacaine group, 91.9% (95%CI = 85.2-98.7%) patients did not experience any cough versus 46% (95% CI=34.4-59.2%) patients in the diacine group (P<0.05) [27].
The efficacy of 2% lidocaine administered through the tracheal tube in attenuating the extubation response in patients who were beta blocked with propranolol 1 mg/kg was compared with placebo, resulting in no difference between lidocaine and placebo in the degree of cough (p-value 0.4) [17].
Studies with Prostaglandins
Intravenous lidocaine 1 mg /kg, prostaglandin E 0.1 ug/kg, and a combination of lidocaine and prostaglandin E in the same dose were compared with placebo. Cough was reported in 52% of patients treated with lidocaine alone, 56% with lidocaine prostaglandin E combination while in all patients in prostaglandin E group and placebo [13].
Studies with Alpha Agonists
No patient experienced cough with 0.8 ug/kg dexmedetomidine as compared to 3.3% of patients treated with 0.4 ug/kg dexmedetomidine. This study was in Chinese, and we were not able to get it translated into English; hence, the information presented here is taken from the abstract [7].
Three studies compared dexmedetomidine with lidocaine [24,26,28], the effect of intravenous dexmedetomidine 0.1 ug/ kg was compared with lidocaine 1 mg/kg or their combination in the same dose [20]. Twenty-five percent of patients in the dexmedetomidine group and 5% in both the lidocaine and lidocaine with dexmedetomidine groups developed a severe cough.
Dexmedetomidine 0.3 ug/kg was compared with lidocaine 1.5 mg/kg. The number of patients with no cough was 86.6% in the dexmedetomidine group compared to 60% in the lidocaine group (P=0.0087) [23].
Dexmedetomidine 0.5 ug/kg was compared with lidocaine 1.5 mg/kg. Five patients (20%) had a cough during extubation in the lidocaine group as compared to none in the dexmedetomidine group (p=<0.05) [28].
Dexmedetomidine 0.3 ug/kg, when compared with verapamil 0.1 mg/kg, [5] resulted in 12 (80%) patients in the dexmedetomidine group with no cough while 9 (60 %) in the verapamil group had minimal coughing (P<0.0029).
Two studies compared dexmedetomidine with remifentanil and fentanyl [3,23]. Fan et al. compared two different doses of dexmedetomidine, 0.5 and 0.7 ug/kg, with remifentanil 0.03 ug/kg/min. Only two patients had moderate cough in the remifentanil group, four had moderate, and two had severe cough in the dexmedetomidine 0.5 ug/kg group while none had moderate to severe cough in the dexmedetomidine 0.7 ug/kg group. One patient had laryngospasm in the dexmedetomidine 0.5 group [23]. Aksu et al. studied the effect of dexmedetomidine 0.5 ug/kg and fentanyl 1 ug/kg. No patient had severe cough in the dexmedetomidine group while four had in the fentanyl group. Only one patient (5%) had moderate cough in the dexmedetomidine group in contrast to four (20%) in the fentanyl group (p= 0.003). One patient developed laryngospasm in the fentanyl group [3].
Studies with Narcotics
Nho et al. studied the effect of remifentanil infusion maintained at a target organ concentration of 1.5 ng/ml during emergence. Coughing was less frequent in the remifentanil group than in the control group. They did not give the numbers of patients who experienced cough neither the grade of cough [21].
Studies with Calcium Channel Blockers
Mikawa et al. studied the effect of two different doses of verapamil 0.05 ug/kg and 0.1 ug/kg with diltiazem 0.2 ug/kg and saline. They reported that all patients coughed with the extubation quality scores (median 3, range 2-5) being the same in all the four groups. No patient developed laryngospasm, hypotension, and bradycardia [12].
Risk of bias across studies
The quality of each study was assessed using the Cochrane risk of bias tool for RCTs [27]. This information is given in Table 4.
Table 4. Quality assessment of selected studies.
| Risk of Bias Assessment |
| Study ID | Year | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | Quality of Studies |
| Nishina [2] | 1995 | unclear | low | low | low | low | low | fair |
| Mikawa [12] | 1996 | low | low | low | low | low | low | good |
| Nishina [13] | 1997 | unclear | unclear | low | unclear | high | low | poor |
| Shajar [19] | 1999 | low | low | low | low | low | low | good |
| Jee [14] | 2002 | high | unclear | low | low | low | low | fair |
| Andrzejowski [17] | 2002 | unclear | low | low | low | high | low | poor |
| Guler [15] | 2005 | unclear | low | low | low | low | low | fair |
| Turan [25] | 2008 | unclear | low | low | low | low | low | fair |
| Aouad [22] | 2009 | low | low | low | low | low | low | good |
| Nho [21] | 2009 | low | low | low | low | low | low | good |
| Aksu [3] | 2009 | unclear | low | low | low | low | low | fair |
| Moustafa [20] | 2012 | unclear | unclear | low | low | low | Low | fair |
| Bindu [29] | 2013 | low | unclear | low | low | low | low | fair |
| Mahoori [16] | 2014 | low | unclear | low | low | unclear | low | poor |
| Zhoo Xiaochun [7] | 2014 | low | low | low | low | low | low | good |
| Sharma [26] | 2014 | Low | low | low | low | low | low | good |
| Lee [18] | 2014 | low | low | low | low | low | low | good |
| Kothari [28] | 2014 | Low | low | low | low | low | low | good |
| Gao [27] | 2014 | low | unclear | low | low | high | low | poor |
| Qing Fan [23] | 2015 | low | low | low | low | low | low | good |
| Kim [6] | 2015 | low | unclear | low | unclear | low | Low | fair |
| Mistry [5] | 2016 | low | low | low | low | low | low | good |
| Shruthi [30] | 2016 | low | low | low | low | low | low | good |
| Dutta [24] | 2016 | unclear | low | low | low | low | low | fair |
Discussion
The main findings of this review are that at tracheal extubation, dexmedetomidine significantly reduced the incidence of cough but caused hypotension and bradycardia. Local anesthetics and opioids did not cause hypotension and bradycardia at extubation but their effect on cough was equivocal. Nausea and vomiting were observed with opioids, but this was not statistically significant in comparison to saline. Patients who received dexmedetomidine had a higher Ramsay score in recovery when compared to local anesthetics while the results of opioids on sedation were equivocal.
Tracheal extubation is associated with cardiovascular as well as respiratory complications. Hemodynamic complications, such as hypertension may lead to an increase in intraocular and intracranial pressure, tachycardia, and dysrhythmias [12,28]. This can be hazardous in high-risk patients who have hypertension, coronary artery, and /or cerebrovascular disease due to an increase in myocardial oxygen demand, which can lead to further myocardial ischemia and infarction, pulmonary edema, and cerebrovascular hemorrhage [4,6]. Various drugs like beta-blockers, calcium channel blockers, vasodilators, lidocaine, and opioids have been used to attenuate this reflex sympathetic stimulation to extubation, with equivocal results and undesirable side effects like sedation, hypotension, bradycardia, nausea, and vomiting [26,29]. An ideal agent is the one that keeps blood pressure and heart rate stable and has no undesirable side effects. Hemodynamic response was attenuated significantly by all drugs used in all included studies.
Sedation, respiratory depression, agitation, and nausea and vomiting are not desirable during and after extubation. Excessive sedation can lead to respiratory depression and increases morbidity and length of stay in PACU [29]. Similarly, agitation in the postoperative period can be very unpleasant for the patient and can lead to hemodynamic compromise. The aim is to have a calm patient with stable hemodynamics in the recovery room.
Extubation can stimulate unwanted airway responses due to laryngeal and tracheal irritation leading to cough, laryngospasm, and bronchospasm. These airway and circulatory responses on extubation can lead to surgical bleeding, cardiovascular instability, and respiratory compromise [1]. The incidence of post-extubation coughing reported in different studies was between 76% and 96% [1,4,6]. Dexmedetomidine 0.5 ug/kg showed a significant reduction in the incidence of cough after intraocular [15], intracranial [17], and spinal surgeries [24], hence improving the quality of extubation when compared to placebo. It also decreased the need for postoperative analgesia without increasing the duration of stay in recovery [21]. It may cause bradycardia and hypotension in a dose-dependent manner but without other side effects. The dose of dexmedetomidine most commonly used in studies was 0.5 ug/kg but favorable results were seen with doses as low as 0.3 ug/kg [5]. Doses higher than 0.5 ug/kg resulted in higher sedation scores when compared to placebo [7].
Lidocaine alone, given intravenously or intratracheally, failed to produce a favorable outcome on the quality of extubation [24,28]. Combination with other drugs, such as prostaglandin E1 and dexmedetomidine, gave better results. Intravenous lidocaine 1 mg/kg, when used in combination with prostaglandin E1, resulted in good-quality extubation with minimal cough or strain [13]. Laryngotracheal instillation with 2% lidocaine did not produce any difference in the degree of coughing [17]. Only one study reported the use of 2% lidocaine 1 mg/kg spray down the endotracheal tube, which attenuated the airway circulatory reflexes when compared to lidocaine given intravenously in the same dose [14]. Dexmedetomidine and lidocaine in combination when administered intravenously resulted in a favorable quality of extubation when compared with dexmedetomidine 0.1 ug/kg alone [20].
Calcium channel blocker was not found to be effective in the attenuation of cough reflex irrespective of dose and drug used [12]. Short-acting opioids like remifentanil and fentanyl have been used for the suppression of cough reflex, with remifentanil having more favorable effects. Remifentanil infusion resulted in suppressing the cough reflex better than the placebo [21]. Remifentanil infusion has also been effectively used to blunt the cough reflexes after thyroidectomies and nasal surgeries [21-22]. When used in patients undergoing abdominal surgery, remifentanil had no significant difference compared to placebo [16]. This variation can be due to the difference in the type of surgery as well as the use of bolus versus infusion. Fentanyl in a 1 ug/kg dose failed to suppress the cough reflex when compared with 0.5 ug/kg dexmedetomidine [3]. When given in a dose of 2 ug/kg, fentanyl resulted in a lesser incidence of cough compared to 1 ug/kg but that was not statistically significant [2]. Nausea and vomiting were not significantly increased with any of the drugs used in the included studies. The majority of the included studies in this review were of good or fair quality with a low risk of bias. Only three studies had one or more criteria for a high risk of bias.
This review has some limitations. First, not all studies were placebo-controlled. There was heterogeneity among the studies (I2 for cough = 60%). Another limitation was that the population included in most studies was the American Society of Anesthesiologists (ASA) I and II. Only three studies included ASA III patients and only one mentioned the associated co-morbidity present in the patients. The results, therefore, may not be extrapolated to patients with co-morbidity who are those actually at risk of having complications. Further work needs to be done with different doses of dexmedetomidine to recommend a dose attenuating the cough reflex but resulting in stable hemodynamics and a calm patient.
Conclusions
This meta-analysis results show that dexmedetomidine 0.4-0.5 ug/kg is associated with good-quality smooth extubation, minimal coughing, no laryngospasm/ bronchospasm, and a calm patient, with stable hemodynamics, without causing respiratory depression, nausea and vomiting, and desaturation. However, in higher doses of more than 0.5 ug/kg, it can cause bradycardia, hypotension, and sedation. More studies are needed to find out the ideal dose to be used for the attenuation of extubation response without causing any untoward circulatory depression. Other pharmacological agents, such as local anesthetics, opioids, and calcium channel blockers, did not attenuate cough.
Appendices
PubMed search
(extubation) OR (tracheal extubation) OR (airway extubation) OR (endotracheal extubation) OR (intratracheal extubation)) AND ((beta blocking drug*) OR (esmolol) OR (labetalol) OR (metoprolol) OR (propranolol) OR (local anesthetic) OR (lidocaine) OR (lignocaine) OR (xylocaine) OR (alpha 2 adrenergic receptor agonists) OR (dexmedetomidine) OR (clonidine) OR (calcium channel blockers) OR (calcium channel antagonist) OR (nicardipine) OR (diltiazem) OR (verapamil) OR (magnesium sulphate)) AND ((cough) OR (dyspnea) OR (apnea) OR (bronchospasm) OR (bronchial spasm) OR (bronchial hyper reactivity) OR (laryngospasm) OR (laryngismus) OR (vocal cord dysfunction) AND (breath holding) OR (propofol) OR (airway obstruction) OR (hypoventilation) OR (hypoxia) OR (hypoxemia) OR (respiratory depression))) AND (Randomized Controlled Trial [ptyp] AND ("1990/01/01"[PDAT]: "2016/12/31"[PDAT]) AND Humans [Mesh] AND adult [Mesh]) (77 items)
CINAHL search
Extubation, airway extubation, Esmolol or labetalol or metoprolol, or propranolol, or lidaocaine, or lignocaine, or xylocaine, or dexmedetomidine, or clonidine, or calcium channel antagonist or nicardipine, or diltiazem, or verapamil, or magnesium sulphate, or dyspnea, or bronchospasm, or laryngospasm or vocal cord dysfunction, or propofol or hypoventilation or hypoxemia, or respiratory depression.
Cochrane database search
Extubation, airway extubation, Esmolol or labetalol or metoprolol, or propranolol, or lidocaine, or lignocaine, or xylocaine, or dexmedetomidine, or clonidine, or calcium channel antagonist or nicardipine, or diltiazem, or verapamil, or magnesium sulfate, or dyspnea, or bronchospasm, or laryngospasm or vocal cord dysfunction, or propofol or hypoventilation or hypoxemia, or respiratory depression.
Cough
1=no coughing
2=smooth extubation, minimal coughing
3=moderate coughing
4=severe coughing
5=poor extubation, very uncomfortable (laryngospasm and coughing 10 times)
1 and 2 mean no coughing.
3, 4, and 5 are yes.
Sedation score
Ramsay Scale
1=anxious or agitated and restless or both
2=cooperative, oriented and tranquil
3=drowsy but responds to commands
4=asleep and brisk response to light glabella tap or loud auditory stimulus
5=asleep, sluggish response to light glabella tap or loud auditory stimulus
6=asleep and unarguable
1 and 2 mean no sedation.
3, 4, 5, and 6 mean yes.
Laryngospasm/ bronchospasm
1=no spasm
2=spasm present
Respiratory depression: respiratory rate of less than 10 breaths per minute
Bradycardia: heart rate of less than 60 b per minute
Hypotension: blood pressure of less than 20 % of baseline
Nausea/vomiting
1=no nausea / vomiting
2=nausea and vomiting present
Desaturation=oxygen saturation of less than 92% immediately after extubation.
Thresholds for converting the Cochrane risk of bias tool to AHRQ Standards (good, fair, and poor)
Good quality: All criteria met (i.e. low for each domain)
Fair quality: One criterion not met (i.e. high risk of bias for one domain) or two criteria unclear, and the assessment that this was unlikely to have biased the outcome, and there is no known important limitation that could invalidate the results
Poor quality: One criterion not met (i.e. high risk of bias for one domain) or two criteria unclear, and the assessment that this was likely to have biased the outcome, and there are important limitations that could invalidate the results
Poor quality: Two or more criteria listed as high or unclear risk of bias
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Effect on postoperative sore throat of spraying the endotracheal tube cuff with benzydamine hydrochloride, 10% lidocaine, and 2% lidocaine. Hung NK, Wu CT, Chan SM, et al. https://www.ncbi.nlm.nih.gov/pubmed/20304980. Anesth Analg. 2010;111:882–886. doi: 10.1213/ANE.0b013e3181d4854e. [DOI] [PubMed] [Google Scholar]
- 2.Fentanyl attenuates cardiovascular responses to tracheal extubation. Nishina K, Mikawa K, Maekawa N, Obara H. Acta Anaesthesiol Scand. 1995;39:85–89. doi: 10.1111/j.1399-6576.1995.tb05597.x. [DOI] [PubMed] [Google Scholar]
- 3.Comparison of the effects of dexmedetomidine versus fentanyl on airway reflexes and hemodynamic responses to tracheal extubation during rhinoplasty: a double-blind, randomized, controlled study. Aksu R, Akin A, Biçer C, Esmaoğlu A, Tosun Z, Boyaci A. Curr Ther Res. 2009;70:209–220. doi: 10.1016/j.curtheres.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Local airway anesthesia attenuates hemodynamic responses to intubation and extubation in hypertensive surgical patients. Meng Y-F, Cui G-X, Gao W, Li Z-W. Med Sci Monit Int Med J Exp Clin Res. 2014;20:1518. doi: 10.12659/MSM.890703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attenuation of extubation responses: comparison of prior treatment with verapamil and dexmedetomidine. Mistry T, Purohit S, Arora G, Gill N, Sharma J. J Neuroanaesth Crit Care. 2016;3:33–39. [Google Scholar]
- 6.Effects of dexmedetomidine on smooth emergence from anaesthesia in elderly patients undergoing orthopaedic surgery. Kim DJ, Kim SH, So KY, Jung KT. BMC Anesthesiol. 2015;15:139. doi: 10.1186/s12871-015-0127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Effects of different doses of dexmedetomidine on the recovery quality from general anesthesia undergoing thyroidectomy [Article in Chinese] Zhao X, Tong D, Long B, Wu X. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26:239–243. doi: 10.3760/cma.j.issn.2095-4352.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Efficacy of endotracheal lidocaine administration with continuous infusion of remifentanil for attenuating tube-induced coughing during emergence from total intravenous anesthesia. Yamasaki H, Takahashi K, Yamamoto S, Yamamoto Y, Miyata Y, Terai T. J Anesth. 2013;27:822–826. doi: 10.1007/s00540-013-1627-3. [DOI] [PubMed] [Google Scholar]
- 9.Teaching pain recognition through art: the Ramsay-Caravaggio sedation scale. Poropat F, Cozzi G, Magnolato A, et al. Ital J Pediatr. 2018;44:20. doi: 10.1186/s13052-018-0453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Effect of dexmedetomidine on haemodynamic and recovery responses during tracheal extubation: a randomized comparative study. Devi Vankayalapati S, Ramsali V M, Dumpala S, Pasupuleti S. J Evol Med Dent Sci. 2016;5:2880–2883. [Google Scholar]
- 11.Higgins JPT, Altman DG, Sterne JAC. Cochrane Handbook for Systematic Reviews of Interventions. 2008. Assessing Risk of Bias in Included Studies; pp. 187–241. [Google Scholar]
- 12.Attenuation of cardiovascular responses to tracheal extubation: verapamil versus diltiazem. Mikawa K1, Nishina K, Maekawa N, Obara H. https://www.ncbi.nlm.nih.gov/pubmed/8638792. Anesth Analg. 1996;82:1205–1210. doi: 10.1097/00000539-199606000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Prostaglandin E 1, lidocaine, and prostaglandin E 1-lidocaine combination for attenuating cardiovascular responses to extubation. Nishina K, Mikawa K, Takao Y, Shiga M, Maekawa N, Obara H. Can J Anaesth. 1997;44:1211–1214. doi: 10.1007/BF03013348. [DOI] [PubMed] [Google Scholar]
- 14.Lidocaine sprayed down the endotracheal tube attenuates the airway-circulatory reflexes by local anesthesia during emergence and extubation. Jee D, Park SY. Anesth Analg. 2003;96:293–297. doi: 10.1097/00000539-200301000-00058. [DOI] [PubMed] [Google Scholar]
- 15.Single‐dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Guler G, Akin A, Tosun Z, Eskitascoglu E, Mizrak A, Boyaci A. Acta Anaesthesiol Scand. 2005;49:1088–1091. doi: 10.1111/j.1399-6576.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 16.The effect of low-dose remifentanil on the hemodynamic responses of endotracheal extubation. Mahoori A, Noroozinia H, Hasani E, Karami N, Pashaei N, Hatami S. http://acta.tums.ac.ir/index.php/acta/article/view/4536. Acta Med Iran. 2014;52:844–847. [PubMed] [Google Scholar]
- 17.The efficacy of lidocaine administered via the LITATM tracheal tube in attenuating the extubation response in beta‐blocked patients following craniotomy. Andrzejowski J, Francis G. Anaesthesia. 2002;57:387–403. doi: 10.1046/j.1365-2044.2002.2466_4.x. [DOI] [PubMed] [Google Scholar]
- 18.Efficacy of a single dose of dexmedetomidine for cough suppression during anesthetic emergence: a randomized controlled trial. Lee JS, Choi SH, Kang YR, Kim Y, Shim YH. Can J Anesth. 2015;62:392–398. doi: 10.1007/s12630-014-0295-6. [DOI] [PubMed] [Google Scholar]
- 19.Effect of a remifentanil bolus dose on the cardiovascular response to emergence from anaesthesia and tracheal extubation. Shajar MA, Thompson JP, Hall AP, Leslie NAP, Fox AJ. Br J Anaest. 1999;83:654–656. doi: 10.1093/bja/83.4.654. [DOI] [PubMed] [Google Scholar]
- 20.Comparison of dexmedetomidine, lidocaine, and their combination in attenuation of cardiovascular and catecholamine responses to tracheal extubation and anesthesia emergence in hypertensive patients. Moustafa AM, Atalla H, Koptan HM. http://roaic.eg.net/article.asp?issn=2356-9115;year=2015;volume=2;issue=2;spage=1;epage=6;aulast=Moustafa Res Opin Anesth Intensive Care. 2015;2:1–6. [Google Scholar]
- 21.Effects of maintaining a remifentanil infusion on the recovery profiles during emergence from anaesthesia and tracheal extubation. Nho JS, Lee SY, Kang JM, et al. Br J Anaesth. 2009;103:817–821. doi: 10.1093/bja/aep307. [DOI] [PubMed] [Google Scholar]
- 22.The effect of low-dose remifentanil on responses to the endotracheal tube during emergence from general anesthesia. Aouad MT, Al-Alami AA, Nasr VG, Souki FG, Zbeidy RA, Siddik-Sayyid SM. Anesth Analg. 2009;108:1157–1160. doi: 10.1213/ane.0b013e31819b03d8. [DOI] [PubMed] [Google Scholar]
- 23.Dexmedetomidine for tracheal extubation in deeply anesthetized adult patients after otologic surgery: a comparison with remifentanil. Fan Q, Hu C, Ye M, Shen X. BMC Anesthesiol. 2015;15:106. doi: 10.1186/s12871-015-0088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comparison of the effect of intravenous dexmedetomidine and lignocaine spray instilled into the endotracheal tube on extubation response in patients undergoing spine surgery. Dutta D, Godara M, Purohit S, Kalra P, Sharma S, Gill N. J Neuroanaesth Crit Care. 2016;3:239–244. [Google Scholar]
- 25.Advantageous effects of dexmedetomidine on haemodynamic and recovery responses during extubation for intracranial surgery. Turan G, Ozgultekin A, Turan C, Dincer E, Yuksel G. Eur J Anaesthesiol. 2008Oct;25:816–820. doi: 10.1017/S0265021508004201. [DOI] [PubMed] [Google Scholar]
- 26.Comparison of dexmedetomidine and lignocaine on attenuation of airway and pressor responses during tracheal extubation. Sharma VB, Prabhakar H, Rath GP, Bithal PK. J Neuroanaesthesiol Crit Care. 2014;1:50–55. [Google Scholar]
- 27.Ropivacaine via trans-cricothyroid membrane injection inhibits the extubation response in patients undergoing surgery for maxillary and mandibular fractures. Gao W, Xi JH, Ju NY, Cui GX. https://www.ncbi.nlm.nih.gov/pubmed/24668638. Genet Mol Res. 2014;13:1635–1642. doi: 10.4238/2014.March.12.16. [DOI] [PubMed] [Google Scholar]
- 28.Attenuation of circulatory and airway responses to endotracheal extubation in craniotomies for intracerebral space occupying lesions: dexmedetomidine versus lignocaine. Kothari D, Tandon N, Singh M, Kumar A. Anesth Essays Res. 2014;8:78–82. doi: 10.4103/0259-1162.128916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.A double blind, randomized, controlled trial to study the effect of dexmedetomidine on hemodynamic and recovery responses during tracheal extubation. Bindu B, Pasupuleti S, Gowd UP, Gorre V, Murthy RR, Laxmi MB. J Anaesthesiol Clin Pharmacol. 2013;29:162–167. doi: 10.4103/0970-9185.111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Effect of dexmedetomidine on hemodynamic parameters during extubation. A prospective randomized double blind study. Shruthi AH, Nethra SS, Sudheesh K, Devika DR, Raghavendra RSR. https://www.ncbi.nlm.nih.gov/pubmed/27382816. Middle East J Anaesthesiol. 2016;23:457–463. [PubMed] [Google Scholar]