Abstract
Purpose
Patients with malignancy are more likely to develop nutritional problems. The Geriatric Nutritional Risk Index (GNRI) is a new prognostic index for evaluating nutritional status. The objective of this study was to assess if preoperative GNRI could be a prognostic factor for patients with pancreatic ductal adenocarcinoma (PDAC) who underwent radical surgery.
Patients and Methods
This study included 282 consecutive patients with incident pancreatic ductal adenocarcinoma who were treated with radical surgery. The Cox regression analysis was performed to calculate the overall survival (OS) and assess the prognostic factors. A nomogram was developed based on the results of the multivariate analysis, and the predictive accuracy of the nomogram was assessed.
Results
Among the 282 patients, there are 117 males and 165 females. The patients had a mean age of 58.7 ±13.5 years, with the median follow-up time of 72.9 months (interquartile range, 0.7 to 115.2 months). They were classified into abnormal (GNRI ≤ 98) and normal (GNRI > 98) GNRI groups, respectively. Multivariate Cox analysis showed that age (HR = 1.023), drinking history (HR = 1.453), tumor grade (HR = 1.633), TNM stage (HR = 1.921), and GNRI (HR = 1.757) were significantly associated with OS. Based on the above variables, the nomogram was established. The concordance index (C-index) and time-dependent receiver operating characteristics curve (tdROC) showed the nomogram was superior to TNM grade and tumor grade in predicting the OS of patients with PDAC.
Conclusion
GNRI could be a useful prognostic indicator in patients with PDAC who received surgery. Based on the GNRI and the other clinical indicators, we developed a nomogram model that can provide an accurate estimation of OS in patients with PDAC after radical surgery.
Keywords: pancreatic ductal adenocarcinoma, the geriatric nutritional risk index, overall survival, nomogram
Introduction
Pancreatic cancer is one of the most common digestive system neoplasms with a poor prognosis. According to the relevant reports, pancreatic ductal adenocarcinoma (PDAC) is the most common histological type, with most patients dying within one year after diagnosis and a less than 6% five-year survival rate.1,2 To date, radical surgical resection is still the main treatment for PDAC, but the prognosis and overall survival (OS) is still not satisfied. Such early mortality burden may be attributed to either surgery-related factors, basic disease, or aggressive nature of the disease. Therefore, to determine the optimal clinical markers for predicting the prognosis of post-surgical PDAC patients is very urgent.
It is well known that malnutrition is a very common complication in cancer patients and is associated not only with postoperative complications but also with long-term outcomes.3–6 According to an epidemiological point of view, malnutrition affects over 80% of patients with upper gastrointestinal cancer and at least 60% of those with lung cancer.7–9 Therefore, it is necessary to predict the prognosis of the disease by assessing the nutritional status of cancer patients. The Geriatric Nutritional Risk Index (GNRI), a new prognostic index of the nutritional status, was proposed and investigated in predicting the risk of nutrition-related complications and mortality in the elderly population.10–12 This index is an objective and simple nutritional assessment tool determined by only serum albumin, height, and body weight. At present, the GNRI has been reported to be an independent risk factor for worse clinical events in patients with heart failure, esophageal squamous cell carcinoma, pathological stage I non-small cell lung cancer and Non-Metastatic Renal Cell Carcinoma.13–16 G. Balzano’s research reported that GNRI grade was an independent predictor of one-year mortality among PDAC patients,17 However, the relationship between the GNRI and overall survival in patients with PDAC, remains unknown. Thus, the aim of our study is to further assess the predictive value of GNRI in overall survival of PDAC patients and to formulate a new prognostic model through developing a nomogram.
Materials and Methods
Participants
We retrospectively analyzed 282 patients with PDAC who underwent curative resection at the First Affiliated Hospital of Wenzhou Medical University between January 1, 2006, and December 31, 2016. The inclusion criteria are list as below: 1) histologically confirmed PDAC; 2) without receiving preoperative anticancer treatment;3) without other malignancies;4) no history of emergent circumstances after surgery, such as intestinal obstruction, perforation, and hemorrhage; 5) complete resection of cancer and laboratory tests were obtained before surgery. Our study was supported by the Ethical Committee and Institutional Review Board of the hospital.
Clinical Information and Laboratory Examinations
Baseline patients clinical and laboratory variables were recorded before the resection of PDAC for patients, including age, gender, smoking history, drinking history, tumor location, tumor size, tumor grade, perineural invasion, vascular invasion, invasion of adjacent tissues, lymph node metastasis, 7th AJCC TNM stage, postoperative chemotherapy, white blood cell count (WBC), platelet count (PLT), albumin levels, hemoglobin levels, carbohydrate antigen 19–9 (CA19-9), carcinoembryonic antigen (CEA). CA19-9 and CEA were determined by UniCel DxI-800 analyzer. WBC and PLT, were tested using a Sysmex XE-2100 analyzer.
Follow-Up
Patients were followed up regularly. Postoperative follow-up included clinical and laboratory examinations every 3 months for the first 2 years, every 6 months from the 3rd to 5th year, annually after the radical resection or until the patient died. OS was defined as the time from surgery to death due to any cause or the last follow-up. Patients who were alive at the last follow-up were suppressed for analysis.
Geriatric Nutritional Risk Index
Data on serum albumin, height, and body weight were used to calculate values for the Geriatric Nutritional Risk Index (GNRI), using the following equation: GNRI= [1.489 * Albumin (g/L) + 41.7 * actual body weight/ideal weight]. The ideal weight was calculated from the Lorenz equation, as follows: For males: Height - 100 - [(height - 150)/4]; For females: Height - 100 - [(height - 150)/2.5]. Actual body weight/ideal body weight was set to 1 when the patient’ s body weight exceeded the ideal body weight. From these GNRI values, 4 grades of nutrition-related risk was graded according to previous research:10 major risk (GNRI <82), moderate risk (GNRI 82 to <92), low risk (GNRI 92 to 98), and no risk (GNRI >98) patients with a GNRI > 98 were considered normal, whereas those with a GNRI 98 were at risk of malnutrition. In our study, the patients were divided into two groups based on the GNRI values: GNRI > 98 were considered normal, whereas those with a GNRI ≤ 98 were at risk of malnutrition.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (normally distributed) or median with inter-quartile range (non-normally distributed), and categorical values were expressed by absolute and relative frequencies. Variables with a normal distribution were compared with unpaired Student’s t-test. Variables with a non-normal distribution were compared using the Mann–Whitney U-test. The chi-square test or Fisher’s exact test was used for categorical data, where appropriate. Variables with p < 0.05 in the univariate analysis were progressed to a multivariate cox regression analysis using forward stepwise selection. Hazards ratio (HR) and 95% CI were calculated. A nomogram was formulated based on the results of multivariate analysis, which allowed us to obtain 1-year, 3-year, 5-year overall survival probability estimates. The performance of the nomogram was evaluated by the C-index and it can estimate the probability of concordance between the observed and nomogram-predicted. The time-dependent receiver operating characteristics curve (tdROC) was calculated to measure the discriminatory power of nomogram, TNM stage, and tumor grade.
All analyses were performed by the software statistical package for social sciences version 22.0 (SPSS, Chicago, IL). The nomogram and tdROC were computed with the R version 3.6.1 (http://www.rproject.org/). All statistical tests were two-sided, and P values of less than 0.05 were considered to be statistically significant.
Results
Patients’ Characteristics
Data were obtained from 282 patients in this study cohort, the median survival time was 17.4 months (interquartile range, 0.7–115.2 months). Mortality was 45.4% at 1-year, 73.0% at 3-year and 87.9% at 5-year follow-up. Demographic characteristics as well as laboratory and clinical data are summarized in Table 1.
Table 1.
Baseline Demographics and Clinical Characteristics of Patients
| Variables/Characteristics | N =282 |
|---|---|
| Age (years) | 58.7±13.5 |
| Height (cm) | 163.6±6.9 |
| Weight (kg) | 59.4±10.8 |
| Tumor size (mm) | 40(8–240) |
| CEA (ng/mL) | 3.05(0.2–3555.1) |
| CA19-9 (u/L) | 127.5(0.8–19,860) |
| Albumin (g/L) | 39.7(27–49.4) |
| Hemoglobin (g/L) | 123(53–163) |
| WBC (×109/L) | 6.1(2.7–18.9) |
| PLT (×109/L) | 204(73–808) |
| Sex | |
| Female | 117 (41.5) |
| Male | 165 (58.5) |
| Smoking history | |
| Current/former | 85 (30.1) |
| Never | 197(69.9) |
| Drinking history | |
| Current/former | 73 (25.9) |
| Never | 209 (74.1) |
| Tumor location | |
| Head | 192 (68.1) |
| Body/Tail | 90 (31.9) |
| Tumor size (mm) | |
| ≤40 | 178 (63.1) |
| >40 | 104 (36.9) |
| Tumor grade | |
| Poor (3/4) | 121 (42.9) |
| Well/moderate (1/2) | 161 (57.1) |
| Perineural invasion | |
| Positive | 55 (19.5) |
| Negative | 227 (80.5) |
| Vascular invasion | |
| Positive | 77 (27.3) |
| Negative | 205 (72.7) |
| Invasion of adjacent tissues | |
| Positive | 136 (48.2) |
| Negative | 146 (51.8) |
| Lymph node metastasis | |
| Positive | 93 (33.0) |
| Negative | 189 (67.0) |
| TNM stage | |
| I/II | 227 (80.5) |
| III/IV | 55 (19.5) |
| Postoperative chemotherapy | |
| No | 213 (75.5) |
| Yes | 69 (24.5) |
| GNRI | |
| Normal (GNRI > 98) | 178 (63.1) |
| Abnormal (GNRI ≤ 98) | 104 (36.9) |
Abbreviations: CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19–9; WBC, white blood cell count; PLT, platelet count; GNRI, Geriatric nutritional risk index.
Correlation Between Patients’ Characteristics and Preoperative GNRI
The main relationship was shown in Table 2. Among the 282 patients, 178 patients (63.1%) with GNRI > 98 were regarded as having a normal nutritional risk, whereas the remaining 104 (36.9%) patients were estimated as having an abnormal nutritional risk. Malnutrition indicated by abnormal GNRI was associated with advanced age, carcinoma of head of pancreas, high level of CEA, high level of CA199, hypoalbuminemia and low level of hemoglobin. But without other factors, including sex, smoking status, drinking history, tumor size, tumor grade, perineural invasion, vascular invasion, invasion of adjacent tissues, lymph node metastasis, TNM stage, total bilirubin, WBC, PLT, and postoperative chemotherapy.
Table 2.
Preoperative GNRI by Patient Characteristics
| Variable | Normal GNRI (n =178) |
Abnormal GNRI (n =104) |
p-value |
|---|---|---|---|
| Sex | 0.590 | ||
| Male | 76(42.7) | 41(39.4) | |
| Female | 102(57.3) | 63(60.6) | |
| Age (years) | 56.8±13.9 | 62.1±11.9 | 0.001 |
| Smoking history | 0.476 | ||
| Current/former | 51(28.7) | 34(32.7) | |
| Never | 127(71.3) | 70(67.3) | |
| Drinking history | 0.982 | ||
| Current/former | 46(25.8) | 27(26.0) | |
| Never | 132(74.2) | 77(74.0) | |
| Tumor location | 0.003 | ||
| Head | 110(61.8) | 82(78.8) | |
| Body/Tail | 68(38.2) | 22(21.2) | |
| Tumor size (mm) | 0.265 | ||
| ≤40 | 108(60.7) | 70(67.3) | |
| >40 | 70(39.3) | 34(32.7) | |
| Tumor grade | 0.554 | ||
| Poor (3/4) | 74(41.6) | 47(45.2) | |
| Well/moderate (1/2) | 104(58.4) | 57(54.8) | |
| Perineural invasion | 0.306 | ||
| Positive | 38(21.3) | 17(16.3) | |
| Negative | 140(78.7) | 87(83.7) | |
| Vascular invasion | 0.318 | ||
| Positive | 45(25.3) | 32(30.8) | |
| Negative | 133(74.7) | 72(69.2) | |
| Invasion of adjacent tissues | 0.969 | ||
| Positive | 86(48.3) | 50(48.1) | |
| Negative | 92(51.7) | 54(51.9) | |
| Lymph node metastasis | 0.387 | ||
| Positive | 62(34.8) | 31(29.8) | |
| Negative | 116(65.2) | 73(70.2) | |
| TNM stage | 0.477 | ||
| I/II | 141(79.2) | 86(82.7) | |
| III/IV | 37(20.8) | 18(17.3) | |
| Postoperative chemotherapy | 0.678 | ||
| No | 133(74.7) | 80(76.9) | |
| Yes | 45(25.3) | 24(23.1) | |
| CEA (ng/mL) | 2.5(0.2–3555.1) | 3.7(0.3–280) | 0.009 |
| CA199 (u/L) | 96.3(0.8–15,722.6) | 174.75(0.8–19,860) | 0.024 |
| Albumin (g/L) | 41.85(36–49.4) | 35.5(27–42.4) | < 0.001 |
| Total bilirubin (μmol/L) | 13.0(5–536) | 56.5(5–455) | 0.108 |
| Hemoglobin (g/L) | 126(84–163) | 115.5(53–152) | < 0.001 |
| WBC (×109/L) | 6.125(3.1–18.9) | 6.085(2.66–14.2) | 0.320 |
| PLT (×109/L) | 196(89–808) | 218.5(73–650) | 0.098 |
Abbreviations: CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19–9; WBC, white blood cell count; PLT, platelet count; GNRI, Geriatric nutritional risk index.
Prognostic Factors in Patients with PDAC
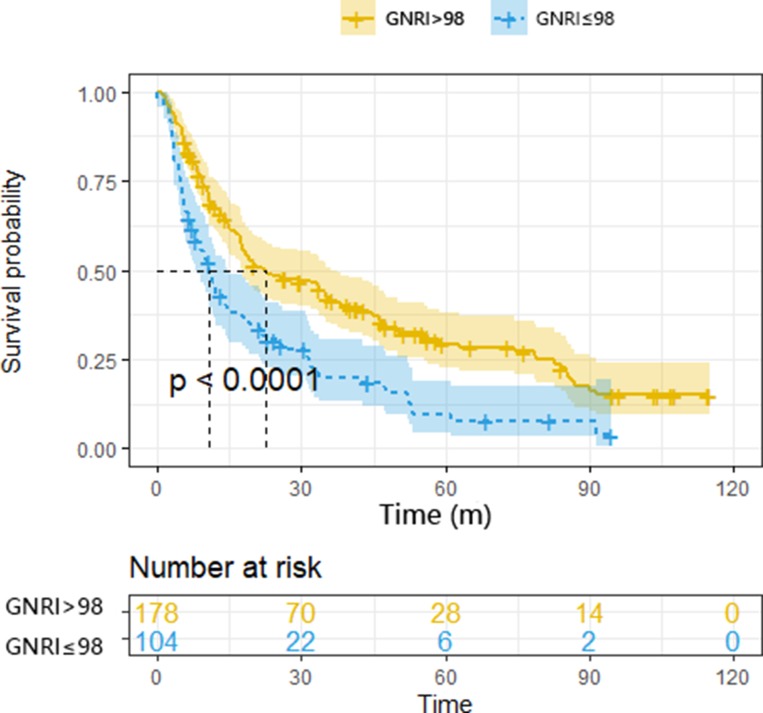
In Kaplan-Meier analysis, the survival curves were significantly stratified by preoperative GNRI categories (Figure 1). The median survival of the normal GNRI group was two and a half times longer than that of the patients with abnormal nutritional (22.4 vs 10.8 months). Cox-regression univariate analysis showed that the sex (HR, 1.815, 95% CI, 1.366–2.411, p <0.001), age (HR, 1.826, 95% CI, 1.375–2.4251, p <0.001), smoking history (HR, 1.478, 95% CI, 1.110–1.968, p =0.008), drinking history (HR, 1.568, 95% CI, 1.167–2.106, p =0.003), tumor grade (HR, 1.742, 95% CI, 1.326–2.288, p <0.001), TNM stage (HR, 2.009, 95% CI, 1.442–2.798, p <0.001), lymph node metastasis (HR, 1.638, 95% CI, 1.231–2.181, p = 0.001), perineural invasion (HR, 1.538, 95% CI, 1.105–2.141, p =0.011), vascular invasion (HR, 1.589, 95% CI, 1.176–2.147, p =0.003), invasion of adjacent tissues (HR, 1.715, 95% CI, 1.301–2.262, p <0.001), CA19-9 level (HR, 1.000, 95% CI, 1.000–1.000, p =0.024), albumin level (HR, 0.929, 95% CI, 0.904–0.955, p <0.001), total bilirubin level (HR, 1.001, 95% CI, 1.000–1.002, p = 0.046) and GNRI (HR, 1.850, 95% CI, 1.401–2.443, p <0.001) were significantly associated with overall survival (Table 3). The significantly associated variables above were included to perform multivariate analysis by using the cox proportional hazards model. Multivariate analysis showed that age (HR, 1.023, 95% CI, 1.011–1.035, p <0.001), drinking history (HR, 1.453, 95% CI, 1.080–1.954, p =0.014), tumor grade (HR, 1.633, 95% CI, 1.239–2.152, p <0.001), TNM stage (HR, 1.921, 95% CI, 1.363–2.709, p < 0.001), and GNRI (HR, 1.757, 95% CI, 1.318–2.341, p <0.001) were confirmed to be independent prognostic factors for overall survival. (Table 4).
Figure 1.
Kaplan – Meier survival curves showing patient overall survival stratified by GNRI categories.
Table 3.
Univariate Analysis of Factors Associated with Overall Survival
| Variable | Univariate Analysis | p-value |
|---|---|---|
| HR (95% CI) | ||
| Sex (male vs female) | 1.815 (1.366–2.411) | <0.001 |
| Age (years) | 1.826 (1.375–2.425) | <0.001 |
| Smoking history (current/former vs never) | 1.478 (1.110–1.968) | 0.008 |
| Drinking history (current/former vs never) | 1.568 (1.167–2.106) | 0.003 |
| Tumor grade (poor vs well/moderate) | 1.742 (1.326–2.288) | <0.001 |
| TNM stage (III/IV vs I/II) | 2.009 (1.442–2.798) | <0.001 |
| Tumor location (body/tail vs head) | 0.813 (0.604–1.095) | 0.173 |
| Tumor size (mm) (≤40 vs >40) | 0.983 (0.743–1.300) | 0.902 |
| Lymph node metastasis (positive vs negative) | 1.638 (1.231–2.181) | 0.001 |
| Perineural invasion (positive vs negative) | 1.538 (1.105–2.141) | 0.011 |
| Vascular invasion (positive vs negative) | 1.589 (1.176–2.147) | 0.003 |
| Invasion of adjacent tissues (positive vs negative) | 1.715 (1.301–2.262) | <0.001 |
| CEA (ng/mL) | 0.999 (0.997–1.001) | 0.392 |
| CA199 (u/L) | 1.000 (1.000–1.000) | 0.024 |
| WBC (×109/L) | 0.999 (0.945–1.056) | 0.965 |
| Hemoglobin (g/L) | 0.996 (0.988–1.003) | 0.264 |
| Albumin (g/L) | 0.929 (0.904–0.955) | <0.001 |
| Total bilirubin (μmol/L) | 1.001 (1.000–1.002) | 0.046 |
| PLT (×109/L) | 0.999 (0.998–1.001) | 0.381 |
| Postoperative chemotherapy (yes vs no) | 0.909 (0.658–1.255) | 0.561 |
| GNRI (abnormal vs normal) | 1.850 (1.401–2.443) | <0.001 |
Abbreviations: CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19–9; WBC, white blood cell count; PLT, platelet count; GNRI, Geriatric nutritional risk index.
Table 4.
Multivariate Analysis of Factors Associated with Overall Survival
| Variable | Multivariate Analysis | p-value |
|---|---|---|
| HR (95% CI) | ||
| Age (years) | 1.023 (1.011–1.035) | <0.001 |
| Drinking history (current/former vs never) | 1.453 (1.080–1.954) | 0.014 |
| Tumor grade (poor vs well/moderate) | 1.633 (1.239–2.152) | <0.001 |
| TNM stage (III/IV vs I/II) | 1.921 (1.363–2.709) | <0.001 |
| GNRI (abnormal vs normal) | 1.757 (1.318–2.341) | <0.001 |
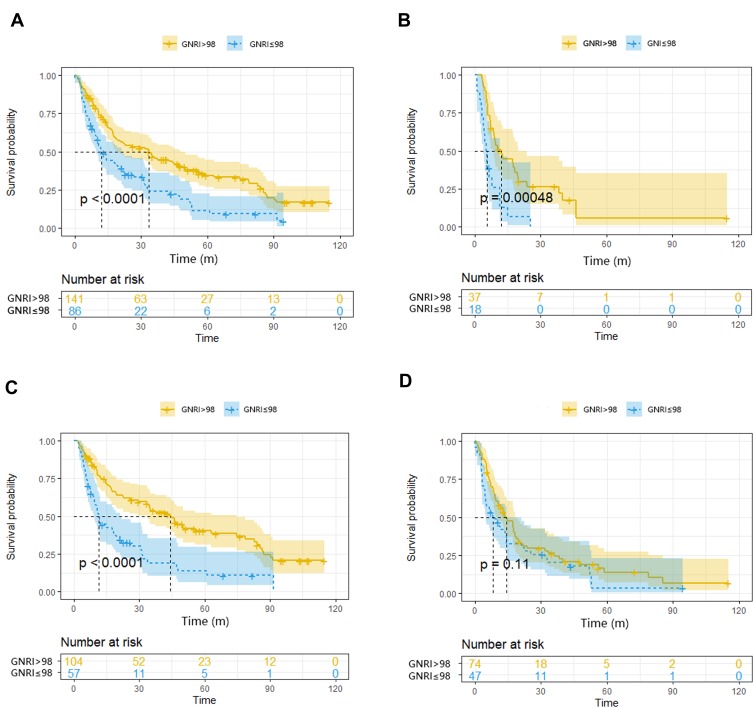
TNM staging system and tumor grade were widely used to predict survival. Therefore, based on different TNM stage and tumor grade, we further made subgroup analysis of GNRI. When compared with abnormal GNRI, normal GNRI had a better prognosis for OS in TNM stage I/II, TNM stage III/IV and tumor grade 1/2. However, in tumor grade 3/4, there was no statistical significance of survival between normal and abnormal GNRI groups. (P>0.05). (Figure 2).
Figure 2.
Kaplan-Meier curves of overall survival in (A) stage I/II (B) stage III/IV by TNM stage, (C) Well/moderate (D) Poor by tumor grade, and categorized according to the GNRI.
The Nomogram and Its Performance
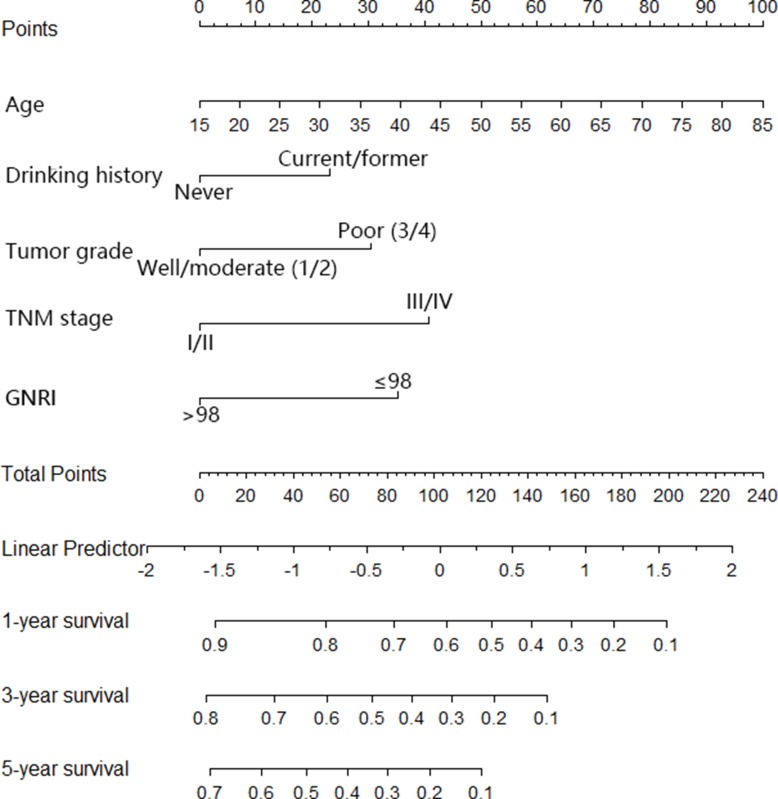
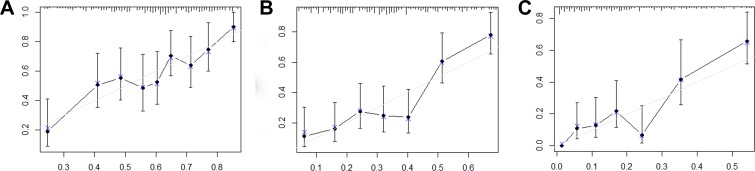
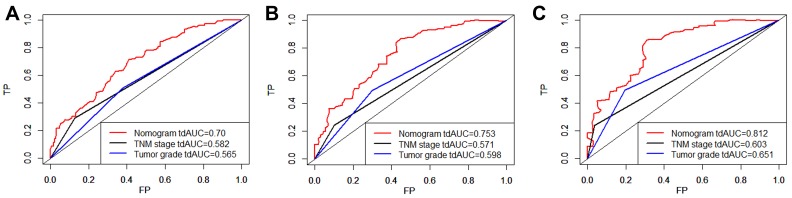
The nomogram was constructed based upon the five independent risk factors described above (Figure 3). In the nomogram model, each predictor was ascribed a total point value or a weighted point total (top scale), which implied the probability of 1-year, 3-yer, 5-year survival rates (bottom scale). In addition, the nomogram scoring system could be used for a more precise calculation of the 1-year, 3-yer, 5-year survival rate predictions than drawing lines on the nomogram (Table 5). The C-index of model for overall survival prediction was 0.661 (95% CI, 0.621–0.701), while TNM stage and tumor stage were 0.555 (95% CI, 0.527–0.583) and 0.575 (95% CI, 0.539–0.611). The calibration plot of the nomogram was subsequently developed. As illustrated in Figure 4, calibration curves were used to verify the performance of the model in predicting OS. To further validate the performance of the model for evaluating the prognosis of PDAC, we plotted the tdROC curve for the nomogram, TNM stage, and tumor grade. In 1-year, 3-years and 5-years OS, the nomogram achieved a tdAUC of 0.70, 0.753 and 0.812, respectively. While TNM stage and tumor grade are only obtain 0.582, 0.571, 0.603 and 0.565, 0.598, 0.651, respectively. (Figure 5).
Figure 3.
Nomogram for predicting overall survival after curative resection of PDAC.
Table 5.
Nomogram Scoring System
| Age | Points | Drink History | Points | Tumor Grade | Points | TNM Stage | Points | GNRI | Points |
|---|---|---|---|---|---|---|---|---|---|
| 30 | 21 | No | 0 | Well/moderate | 0 | I/II | 0 | Normal | 0 |
| 50 | 50 | Yes | 23 | Poor | 31 | III/IV | 41 | Abnormal | 35 |
| 60 | 64 | ||||||||
| 70 | 79 | ||||||||
| 80 | 93 | ||||||||
| 85 | 100 | ||||||||
| Total points | 1-year survival probability | Total points | 3-year survival probability | Total points | 3-year survival probability | ||||
| 7 | 0.9 | 3 | 0.8 | 4 | 0.7 | ||||
| 54 | 0.8 | 32 | 0.7 | 27 | 0.6 | ||||
| 83 | 0.7 | 55 | 0.6 | 46 | 0.5 | ||||
| 105 | 0.6 | 74 | 0.5 | 63 | 0.4 | ||||
| 124 | 0.5 | 91 | 0.4 | 80 | 0.3 | ||||
| 142 | 0.4 | 108 | 0.3 | 98 | 0.2 | ||||
| 159 | 0.3 | 126 | 0.2 | 121 | 0.1 | ||||
| 177 | 0.2 | 148 | 0.1 | ||||||
| 199 | 0.1 | ||||||||
Figure 4.
Calibration curves for predicting overall survival probability by the nomogram.
Notes: Calibration curves of the prognostic nomogram for 1-year overall survival (A), 3-year overall survival (B) and 5-year overall survival (C).
Figure 5.
The tdROC curve of the prognostic nomogram, TNM stage and tumor grade.
Notes: The tdROC curve for 1-year survival (A), 3-year survival (B), and 5-year survival (C).
Discussion
Pancreatic carcinoma frequently leads to malnutrition. This is caused by tumor progression and decreased oral intake as a result of gastrointestinal obstruction, biliary obstruction, concomitant pancreatitis and cancerous pain.18–20 Poor nutritional status is associated with poor clinical outcomes and poor survival, for instance, higher infection rates, impaired wound healing and mortality as well as longer hospital stay, leading to increased overall costs.19,21,22 Hence, early identification of high-risk patients is crucial for patient-centered quality of care.23
Although a number of screening tools have been developed to assess the nutritional status of patients. Such as SGA, NRS, and NRI, etc. However, these tools are regarded as subjective tools, which require patients to provide recent changes in weight, even changes in eating conditions, and the elderly cannot accurately remember these due to memory loss, so the nutritional screening results will often exist in deviation.24 In addition, there are still no consensus concerning criteria suitable for predicting the nutrition-related risk in patients. Considering these findings, GNRI was proposed to evaluate the nutrition-related risk, since this criterion is simple and objective, and has been shown to be associated with mortality not only in elderly patients in various health care settings, but also in patients with a wide variety of pathological conditions and at different ages.10,13,25–28
To the best of our knowledge, this study is the first attempt to evaluate the impact of GNRI on the overall survival of PDAC patients treated with radical resection. In our study, we confirm that GNRI is capable of discriminating survival for PDAC patients and patients with different TNM stages. Meanwhile, multivariate cox analysis also showed that GNRI is an independent risk factor of overall survival for PDAC patients. GNRI ≤ 98 often means a worse prognosis in PDAC patients. Regarding the indifference in OS between two groups of GNRI at the tumor grade of 3/4, we believe that it may be related to the small sample size at this level. Finally, we built a nomogram model by combining other independent risk factors and compared with the TNM stage and tumor grade in predictive ability. Research finding showed that the nomogram model had a higher value whether on tdAUC or C-index. which indicated that combines multiple variables is much better than a single predictor in discriminatory ability.
Nomogram is a simple graphical representation of a statistical prediction model that has been regarded by scholars as useful and efficient methods for predicting tumor prognosis in recent years.29–32 Our nomogram contained five factors in which age, sex, TNM stage, and tumor grade were consistent with those of previous research.33–35 The GNRI is based on three important nutritional indices including serum albumin level, body weight, and height. Serum albumin is one of the most relevant malnutrition indicators. Hendifar et al recently reported that Low preoperative Serum albumin was associated with worse disease-free survival and OS in patients with resected PDAC.36 Gupta et al also reported that high serum albumin concentration was associated with better survival of cancer patients.37 The body mass index (BMI) consists of body weight and height, which are included in GNRI calculations. Several studies have reported that low BMI is considered a poor prognostic factor of pancreatic cancer.38,39 Particularly, Umegaki et al indicated that a low BMI was associated with an increased risk of death with pancreatic cancer, Okura et al suggested that extremely low BMI was significantly associated with a higher risk of mortality in pancreatectomy patients.
There were several limitations to this study. First, this study was a retrospective study from a single institution with a relatively small sample size. A multicenter prospective study and larger sample clinical analysis is required for further study. Second, this study only included PDAC patients who received pancreatectomy, the results may not represent a general population of PDAC patients undergoing other therapy methods (such as palliative therapy and targeted therapy and so on). Third, there are some additional limitations for this study, such as residual confounders, potential information bias.
Conclusion
In conclusion, the present study suggests that the GNRI could be a simple and effective tool to predict the overall survival in PDAC patients treated with curative resection. The nomogram based on GNRI and other variables in our study can provide an individualized risk estimation of OS in patients with PDAC, and may offered to clinicians to improve their prognostication for patients and strengthen prognosis-based decision making for each patient.
Ethics Approval and Consent to Participate
This study was approved by the institutional review boards of the First Affiliated Hospital of Wenzhou Medical University and consent was obtained from every patient. This study was conducted in accordance with the Declaration of Helsinki. Patients’ records were anonymized and de-identified prior to analysis.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yadav D, Lowenfels A. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–1261. doi: 10.1053/j.gastro.2013.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.v127:12 [DOI] [PubMed] [Google Scholar]
- 3.Barao K, Abe Vicente Cavagnari M, Silva Fucuta P, Manoukian Forones N. Association between nutrition status and survival in elderly patients with colorectal cancer. Nutr Clin Pract. 2017;32(5):658–663. doi: 10.1177/0884533617706894 [DOI] [PubMed] [Google Scholar]
- 4.Lien Y, Hsieh C, Wu Y, et al. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. 2004;8(8):1041–1048. doi: 10.1016/j.gassur.2004.09.033 [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa T, Toyazaki T, Chiba N, Ueda Y, Gotoh M. Prognostic value of body mass index and change in body weight in postoperative outcomes of lung cancer surgery. Interact Cardiovasc Thorac Surg. 2016;23(4):560–566. doi: 10.1093/icvts/ivw175 [DOI] [PubMed] [Google Scholar]
- 6.Schwegler I, von Holzen A, Gutzwiller J, Schlumpf R, Mühlebach S, Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. 2010;97(1):92–97. doi: 10.1002/bjs.6805 [DOI] [PubMed] [Google Scholar]
- 7.MacDonald N, Easson A, Mazurak V, Dunn G, Baracos V. Understanding and managing cancer cachexia. J Am Coll Surg. 2003;197(1):143–161. doi: 10.1016/S1072-7515(03)00382-X [DOI] [PubMed] [Google Scholar]
- 8.Muscaritoli M, Anker S, Argilés J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29(2):154–159. doi: 10.1016/j.clnu.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 9.Paccagnella A, Morassutti I, Rosti G. Nutritional intervention for improving treatment tolerance in cancer patients. Curr Opin Oncol. 2011;23(4):322–330. doi: 10.1097/CCO.0b013e3283479c66 [DOI] [PubMed] [Google Scholar]
- 10.Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783. doi: 10.1093/ajcn/82.4.777 [DOI] [PubMed] [Google Scholar]
- 11.Cereda E, Pedrolli C, Zagami A, et al. Nutritional risk, functional status and mortality in newly institutionalised elderly. Br J Nutr. 2013;110(10):1903–1909. doi: 10.1017/S0007114513001062 [DOI] [PubMed] [Google Scholar]
- 12.Cereda E, Zagami A, Vanotti A, Piffer S, Pedrolli C. Geriatric Nutritional Risk Index and overall-cause mortality prediction in institutionalised elderly: a 3-year survival analysis. Clin Nutr. 2008;27(5):717–723. doi: 10.1016/j.clnu.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 13.Miyake H, Tei H, Fujisawa M. Geriatric nutrition risk index is an important predictor of cancer-specific survival, but not recurrence-free survival, in patients undergoing surgical resection for non-metastatic renal cell carcinoma. Curr Urol. 2017;10(1):26–31. doi: 10.1159/000447147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoji F, Matsubara T, Kozuma Y, et al. Preoperative Geriatric Nutritional Risk Index: a predictive and prognostic factor in patients with pathological stage I non-small cell lung cancer. Surg Oncol. 2017;26(4):483–488. doi: 10.1016/j.suronc.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 15.Wada H, Dohi T, Miyauchi K, et al. Prognostic impact of the geriatric nutritional risk index on long-term outcomes in patients who underwent percutaneous coronary intervention. Am J Cardiol. 2017;119(11):1740–1745. doi: 10.1016/j.amjcard.2017.02.051 [DOI] [PubMed] [Google Scholar]
- 16.Yamana I, Takeno S, Shimaoka H, et al. Geriatric Nutritional Risk Index as a prognostic factor in patients with esophageal squamous cell carcinoma -retrospective cohort study. Int J Surg. 2018;56:44–48. doi: 10.1016/j.ijsu.2018.03.052 [DOI] [PubMed] [Google Scholar]
- 17.Balzano G, Dugnani E, Crippa S, et al. A preoperative score to predict early death after pancreatic cancer resection. Dig Liver Dis. 2017;49(9):1050–1056. doi: 10.1016/j.dld.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 18.Goonetilleke KS, Siriwardena AK. Systematic review of peri-operative nutritional supplementation in patients undergoing pancreaticoduodenectomy. Jop. 2006;7(1):5–13. [PubMed] [Google Scholar]
- 19.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98(2):268–274. doi: 10.1002/bjs.v98.2 [DOI] [PubMed] [Google Scholar]
- 20.Schrader H, Menge B, Belyaev O, Uhl W, Schmidt W, Meier J. Amino acid malnutrition in patients with chronic pancreatitis and pancreatic carcinoma. Pancreas. 2009;38(4):416–421. doi: 10.1097/MPA.0b013e318194fc7a [DOI] [PubMed] [Google Scholar]
- 21.Mourão F, Amado D, Ravasco P, Vidal P, Camilo M. Nutritional risk and status assessment in surgical patients: a challenge amidst plenty. Nutr Hosp. 2004;19(2):83–88. [PubMed] [Google Scholar]
- 22.Okumura S, Kaido T, Hamaguchi Y, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157(6):1088–1098. doi: 10.1016/j.surg.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 23.O’Flynn J, Peake H, Hickson M, Foster D, Frost G. The prevalence of malnutrition in hospitals can be reduced: results from three consecutive cross-sectional studies. Clin Nutr. 2005;24(6):1078–1088. doi: 10.1016/j.clnu.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 24.Poulia K, Yannakoulia M, Karageorgou D, et al. Evaluation of the efficacy of six nutritional screening tools to predict malnutrition in the elderly. Clin Nutr. 2012;31(3):378–385. doi: 10.1016/j.clnu.2011.11.017 [DOI] [PubMed] [Google Scholar]
- 25.Jung Y, You G, Shin H, Rim H. Relationship between Geriatric Nutritional Risk Index and total lymphocyte count and mortality of hemodialysis patients. Hemodial Int. 2014;18(1):104–112. doi: 10.1111/hdi.2014.18.issue-1 [DOI] [PubMed] [Google Scholar]
- 26.Kinugasa Y, Kato M, Sugihara S, et al. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J. 2013;77(3):705–711. doi: 10.1253/circj.CJ-12-1091 [DOI] [PubMed] [Google Scholar]
- 27.Yamada K, Furuya R, Takita T, et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. 2008;87(1):106–113. doi: 10.1093/ajcn/87.1.106 [DOI] [PubMed] [Google Scholar]
- 28.Yamana I, Takeno S, Shibata R, et al. Is the geriatric nutritional risk index a significant predictor of postoperative complications in patients with esophageal cancer undergoing esophagectomy? Eur Surg Res. 2015;55(1–2):35–42. doi: 10.1159/000376610 [DOI] [PubMed] [Google Scholar]
- 29.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi: 10.1200/JCO.2007.12.9791 [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Li X, Wen X, et al. Prognostic nomogram integrated baseline serum lipids for patients with non-esophageal squamous cell carcinoma. Ann Transl Med. 2019;7(20):548. doi: 10.21037/atm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rho SY, Lee SG, Park M, et al. Developing a preoperative serum metabolome-based recurrence-predicting nomogram for patients with resected pancreatic ductal adenocarcinoma. Sci Rep. 2019;9(1):18634. doi: 10.1038/s41598-019-55016-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao Z, Zheng Z, Ke W, et al. Prognostic nomogram for bladder cancer with brain metastases: a national cancer database analysis. J Transl Med. 2019;17(1):411. doi: 10.1186/s12967-019-2109-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 34.Kaaja R, Are K. Myocardial LDH isoenzyme patterns in rats exposed to cold and/or hypobaric hypoxia. Acta Med Scand Suppl. 1982;668:136–142. doi: 10.1111/j.0954-6820.1982.tb08536.x [DOI] [PubMed] [Google Scholar]
- 35.van Roessel S, Kasumova GG, Verheij J, et al. International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system in patients with resected pancreatic cancer. JAMA Surg. 2018;153(12):e183617. doi: 10.1001/jamasurg.2018.3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendifar A, Osipov A, Khanuja J, et al. Influence of body mass index and albumin on perioperative morbidity and clinical outcomes in resected pancreatic adenocarcinoma. PLoS ONE. 2016;11(3):e0152172. doi: 10.1371/journal.pone.0152172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta D, Lis C. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56(4):918–928. doi: 10.1002/(ISSN)1097-0142 [DOI] [PubMed] [Google Scholar]
- 39.Umegaki T, Kunisawa S, Kotsuka M, Yamaki S, Kamibayashi T, Imanaka Y. The impact of low body mass index on postoperative outcomes in pancreatectomy patients: a retrospective analysis of Japanese administrative data. J Anesth. 2018;32:624–631. doi: 10.1007/s00540-018-2527-3 [DOI] [PubMed] [Google Scholar]