Supplemental Digital Content is Available in the Text.
Key Words: pterygium, axial length, etiology, hyperopia, anatomy
Abstract
Purpose:
To test the hypothesis that pterygium presents with both refractive and anatomical changes, especially short axial length.
Methods:
A retrospective, hospital-based cross-sectional study included 521 eyes from 521 patients who were enrolled through a community survey by Shanghai Heping Eye Hospital was conducted. Patients with primary pterygium in at least 1 eye were considered the pterygium group, and those with normal eyes were considered the nonpterygium group. The prevalence and length of pterygium, refractive characteristics including spherical power, astigmatism, corneal curvature, and anatomical parameters including axial length, anterior chamber depth, endothelial cell density, and corneal thickness were compared between groups.
Results:
Five hundred twenty-one eyes of 521 patients (214 men and 307 women) with a mean age of 70.5 ± 7.6 years were included in the study. The prevalence of hyperopia (81.6%, 65.1%, P = 0.001), axial length (23.1 ± 1.2 mm, 24.2 ± 2.4 mm, P < 0.001), anterior chamber depth (2.9 ± 0.3 cm, 3.1 ± 0.4 cm, P = 0.001), flat K value (42.94 ± 2.16 diopters, 43.73 ± 1.48 diopters, P = 0.002), Kmax (51.13 ± 7.74 diopters, 47.49 ± 5.62 diopters, P < 0.001), and spherical power (0.97 ± 2.40 diopters, −0.82 ± 4.40 diopters, P < 0.001) were statistically different between the pterygium and nonpterygium groups. Age (r = −0.21, P = 0.025), corneal astigmatism (r = −0.41, P < 0.001), flat K value (r = −0.39, P < 0.001), and endothelial cell density (r = −0.33, P = 0.001) were all negatively correlated with the length of pterygium. The prevalence of pterygium and severe pterygium over 3 mm were statistically different according to the severity of hyperopia (P < 0.001) and axial length (P < 0.001). Stratified χ2 analysis showed that axial length, rather than hyperopia, was a related factor to pterygium (odds ratio = 5.23, 95% confidence interval: 2.50–10.93).
Conclusions:
We conclude from our study that the prevalence of pterygium is related to small eye size. SDF-1/CXCR4 signaling may play a vital role in pterygium and shorter axial length. Further study focused on SDF-1/CXCR4 signaling will be needed.
Pterygium, one of the most common eye disorders, is an inflammatory and proliferative growth of fibrovascular tissue that extends across the limbus and invades the cornea. With a prevalence ranging from 0.7% to 33% globally,1 this disease may be afflicting as many as 200 million people in the world.2 Symptoms vary from no symptoms to a large mass. In severe cases, pterygium can seriously impair vision quality because of irregular astigmatism and physical occlusion of the visual axis.3
The etiology of pterygium, however, remains poorly understood. Reported risk factors for developing the disease included ultraviolet radiation exposure,4 race,5,6 male gender,5,7 and oncogenic virus infection, for example, human papillomavirus8–10 and herpes simplex virus.11,12In most cases, pterygium affects vision through corneal astigmatism. The relationship between pterygium and astigmatism has been well studied. However, in clinical practice, we have found that pterygium is often accompanied by short axial length or hyperopia. The link between them is still unknown.
In our settings, patients with pterygium do not undergo axial length measurement or refraction test; therefore, there is no evidence to confirm the connection among them. The aim of this study was to determine whether the presence of pterygium in the population is indeed related to hyperopia or short axial length. To the best of our knowledge, this is the first study detailing the correlation between pterygium and axial length.
PATIENTS AND METHODS
Study Population
This retrospective, hospital-based cross-sectional study included 521 patients who were enrolled through a community survey by Shanghai Heping Eye Hospital between January 2018 and September 2018. Patients with primary pterygium in at least 1 eye were considered the pterygium group, and those without pterygium were considered the nonpterygium group. The affected eye in patients with unilateral pterygium, the right eye in patients with bilateral pterygium, and the right eye in nonpterygium group were analyzed. All eligible patients were identified and included in our study consecutively. Patients were excluded if any of the following conditions were met:
Patients with pseudopterygium, uveitis, keratitis, or glaucoma in either eye
Patients without biometry data
Patients with a history of any ocular surgery or trauma
Data Collection
Demographic data including age, sex, onset of pterygium, and medical history were recorded. The demographics were recorded with detailed eye examination findings. Optometric and anatomic data including the prevalence of hyperopia, corneal astigmatism, flat K value (FK), steep K value (SK), Kmax, visual acuity, best-corrected visual acuity, spherical power, cylindrical power, axial length, anterior chamber depth, endothelial cell density, and corneal thickness were also recorded.
For every study subject, visual acuity, best-corrected visual acuity, manifest refraction, and slit-lamp examination were conducted. Standardized slit-lamp examination was performed by trained ophthalmologists to examine the anterior segment. Pterygium was defined as an extension of the conjunctiva onto the clear cornea for which there was no alternative explanation, for example, pseudopterygium, pinguecula, tumor, Terrien marginal corneal degeneration, or trauma. Length of pterygium, defined as the distance from the limbus to the edge of pterygium, was also recorded. Central corneal curvature of each eye was acquired using corneal topography (Sirius System; C.S.O. Srl, Florence, Italy). Astigmatism and axial length were obtained using the Zeiss IOLmaster 500 (Carl Zeiss Meditec AG, Jena, Germany). Corneal endothelial cell density was calculated using a specular microscope (specular microscope SP-2000P; Topcon).
Statistical Analysis
A chi-square test was used to compare the prevalence of hyperopia among patients in the pterygium group and nonpterygium group. Refractive and anatomical characteristics were compared using a Mann–Whitney U test. Correlation between refractive or anatomical characteristics and length of pterygium was based on the Spearman correlation coefficient. The prevalence of pterygium among different severities of hyperopia and spherical power was analyzed using a trend χ2 test. A stratified χ2 test was performed to evaluate the effect of hyperopia and axial length on the prevalence of pterygium after controlling confounding factors. All data analyses were performed using IBM SPSS statistical software package version 22 (IBM Co, Chicago).
RESULTS
Demographics in Study Population
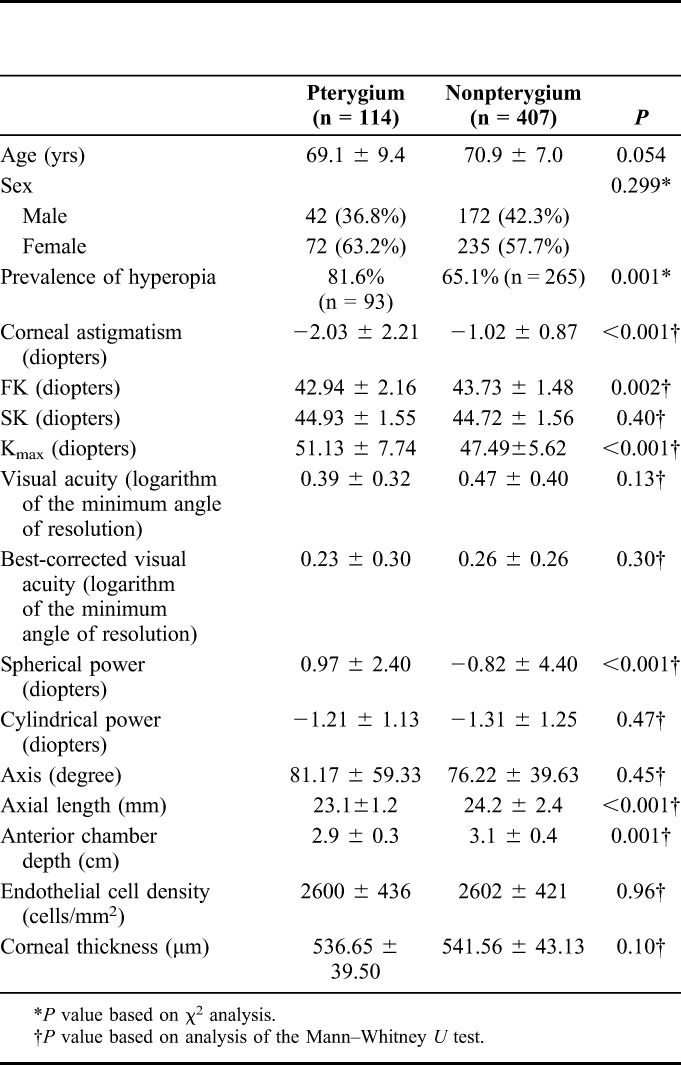
A total of 521 eyes from 521 subjects were used. The overall prevalence of hyperopia was 68.7% (n = 358), of which 282 had binocular hyperopia (54.1%), and pterygium was seen in 21.9% (n = 114). The overall mean age ± SD was 70.5 ± 7.6 years with a male:female ratio of 214:307 (Table 1).
TABLE 1.
Demographic Characteristics

Comparison of Refractive and Anatomical Characteristics
No difference was found in age (P = 0.054) and sex ratio (P = 0.299) between pterygium and nonpterygium groups. The prevalence of hyperopia in the pterygium group was higher than that in the nonpterygium group (81.6%, 65.1%, P = 0.001). Axial length (23.1 ± 1.2 mm, 24.2 ± 2.4 mm, P < 0.001) and anterior chamber depth (2.9 ± 0.3 cm, 3.1 ± 0.4 cm, P = 0.001), which indicate the size of the eye, were shorter in the pterygium group. FK (P = 0.002), Kmax (P < 0.001), and spherical power (P < 0.001) were different between groups. No difference was found in SK (P = 0.40), visual acuity (P = 0.13), best-corrected visual acuity (P = 0.30), cylindrical power (P = 0.47), axis (P = 0.45), endothelial cell density (P = 0.96), or corneal thickness (P = 0.10) between groups (Table 2).
TABLE 2.
Comparison of Refractive and Anatomical Characteristics
Analysis of Correlation Between Refractive or Anatomical Parameter and Pterygium
Age (Spearman correlation coefficient, r = −0.21, P = 0.025), corneal astigmatism (Spearman correlation coefficient, r = −0.41, P < 0.001), FK (Spearman correlation coefficient, r = −0.39, P < 0.001), and endothelial cell density (Spearman correlation coefficient, r = −0.33, P = 0.001) were all negatively correlated with the length of pterygium (see Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/ICO/A924).
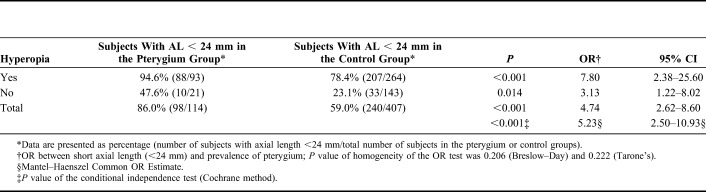
No difference was found in age and sex ratio among subjects with hyperopia and those without hyperopia. Interestingly, an increasing trend was found in the prevalence of pterygium with the increasing number of eyes with hyperopia using the χ2 test for trend (Mantel–Haenszel test, Table 3). When the subjects were divided according to axial length, the prevalence of any pterygium and the severe pterygium over 3 mm increased with decreasing of axial length (P < 0.001, Mantel–Haenszel test, Table 4).
TABLE 3.
Demographics and Characteristics of Pterygium in the Population With Hyperopia

TABLE 4.
Comparison of Characteristics of Pterygium Among Different Axial Length

Effect of Axial Length or Hyperopia on Pterygium After Controlling Confounding Factors
The prevalence of hyperopia in the pterygium group was different from that in the nonpterygium group (P = 0.001). Hyperopia seems to be associated with pterygium with an odds ratio (OR) value of 2.25 (95% confidence interval [CI]: 1.34–3.78). However, the difference was no longer significant when stratified χ2 analysis was performed with 24 mm of axial length as the cutoff point (P = 0.477, AL < 24 mm, OR = 1.31, 95% CI: 0.62–2.79; P = 0.332, AL ≥ 24 mm, OR = 0.53, 95% CI: 0.14–1.96). After correcting the effect of the confounding factor of axial length, the corrected P value was 0.924, with an OR value of 1.03 (95% CI: 0.55–1.93) (Table 5).
TABLE 5.
Effect of Hyperopia on the Prevalence of Pterygium After Controlling the Axial Length (Stratified χ2 Analysis)
The percentage of subjects with an axial length less than 24 mm in the pterygium group was different from that in the nonpterygium group (P < 0.001). Short axial length (<24 mm) was related to pterygium with an OR value of 4.74 (95% CI: 2.62–8.60). The difference, however, was still significant when stratified χ2 analysis was performed with hyperopia as a cofounding factor (P < 0.001, with hyperopia, OR = 7.80, 95% CI: 2.38–25.60; P = 0.014, without hyperopia, OR = 3.13, 95% CI: 1.22–8.02). After correcting the effect of the confounding factor of hyperopia, the corrected P value was still less than 0.001, with an OR value of 5.228 (95% CI: 2.50–10.93) (Table 6).
TABLE 6.
Effect of Axial Length on the Prevalence of Pterygium After Controlling the Prevalence of Hyperopia (Stratified χ2 Analysis)
DISCUSSION
This is the first study to link pterygium to hyperopia and short axial length. The effect of pterygium on corneal astigmatism and optical irregularity is well known. The results of the current study demonstrated that pterygium had an influence on astigmatism, and this influence was dependent on the size of pterygium, which means that the larger the size of pterygium, the more astigmatism it exerts to the ocular surface. At the anterior corneal surface, a more flattened FK was found in the pterygium group. However, the difference in SK was not significant between the 2 groups, as shown by recent studies.13,14
In this study, an increasing trend was found in the prevalence of pterygium with the severity of hyperopia, which has a connection with short axial length.15,16To avoid the effect of corneal curvature exerts on refractive status, axial length and anterior chamber depth were deliberately measured to directly reflect the size of the eye, which was shorter than that in patients without pterygium. Interestingly, hyperopia seemed to have an association with pterygium; however, stratified chi-square analysis showed that hyperopia was not a related factor; instead, it was just a consequence of short axial length.
Results from some research studies indicated that a high proportion of the scleral cell population were myofibroblasts in treeshrew and guinea pig models with shorter axial length,17,18 suggesting that the reduction in axial length may have been caused by activation of a contractile mechanism involving scleral myofibroblasts. Myofibroblasts have been reported to be present in the sclera of humans and monkeys.19 Myofibroblasts, just as fibroblasts, are responsible for the normal renewal of collagen and are likely to act as scaffolds until a new matrix is synthesized to prevent rapid expanding of tissue on degrading the matrix with matrix metalloproteinases.18,20,21 Excessive activation of myofibroblasts can lead to an increase in the transmission of the force generated by myofibroblasts into the surrounding matrix, which may cause myofibroblasts to maintain high tension for a long period of time, resulting in slow tissue growth and short axial length. Stromal myofibroblasts or fibroblasts also play a vital role in pterygium progression. SDF-1, probably produced by resident fibroblasts, can recruit circulating cells expressing CXCR4 to the surface of the eye where pterygium is generated. CXCR4-positive cells can be activated and transformed into myofibroblasts through SDF-1/CXCR4 signaling.18 Unoccupied CXCR4-positive cells may lose their phenotype over time and become inactive CXCR4-negative resident stromal fibroblasts. Then, some of these cells may be eventually transformed into myofibroblasts through a non-SDF-1–dependent pathway.22
Yet, the origin of pterygium fibroblasts has been a matter of debate. It is worth mentioning that in a previous study which first proved the presence of myofibroblasts in pterygium, myofibroblasts were found in the fibrous adipose tissue near the nasal conjunctiva around the orbit, rather than in pterygium.23 Recently, mesenchymal progenitor cells from which profibrotic myofibroblasts can also arise through endothelia–mesenchymal transition have been isolated from human orbital adipose tissue.24
Several studies have shown that children who spend more time outdoors have a lower incidence of myopia and less axial length elongation.25–29 It also should be noted that outdoor time and sunlight exposure are related to pterygium. One likely biological explanation for this association is that the retina responds to high levels of light by releasing dopamine, which inhibits axial length growth.30,31 Interestingly, CXCL12, formerly known as stromal cell-derived factor one alpha (SDF-1α), is one of the few chemokines located in the central nervous system and can modulate dopamine transmission through activation of its receptor CXCR4.32,33 CXCL12 injected into the substantia nigra enhances extracellular dopamine in the dorsal striatum in a CXCR4 receptor-dependent way.34,35 In the retina, which is part of the central nervous system, CXCR4 is required for survival of embryonic ganglion cells36 and functions as a morphogen in modulating retinal vasculogenesis.37 Its interaction with dopamine in the retinal field, however, remains unclear. Another possible molecular mechanism is through vitamin D. Although the connection between sunlight exposure and serum 25-hydroxyvitamin D (25[OH]D) is clear, a large birth cohort study that enrolled 2666 children suggested that serum 25(OH)D levels were inversely associated with axial length even after adjusting for time spent outdoors.38 This effect appeared independent of outdoor exposure and may suggest a more direct role for 25(OH)D in axial length. It is also worth mentioning that 25(OH)D upregulates eosinophil surface expression of CXCR4 in a concentration-dependent manner.39
The Spearman correlation test showed a negative relationship between pterygium invasion and endothelial cell density. Several retrospective studies have confirmed that pterygium is related to a decrease in corneal endothelial cell density.40 However, these studies ignore the fact that corneal endothelial density decreases with age, and most studies have not ruled out the influence of age to draw this conclusion. Therefore, it cannot be concluded that the decrease in corneal endothelial density is necessarily related to pterygium. In this study, corneal endothelial density was not different between pterygium and nonpterygium groups, but negatively correlated with the length of pterygium, which may be because of the influence exerted by older age. Previous reports revealed controversial findings regarding age in the presence of pterygium. Most studies found that increasing age was associated with any pterygium.6,41 The mean age reported in previous studies was 35 to 60 years old according to different regions.6,41,42 Patients in this study were enrolled through a community survey that examined all residents in an area, not only those with pterygium were screened. Therefore, the average age was different from most previous studies. This explains the result that age was not correlated with the prevalence of pterygium. Interestingly, decreasing age was related to the length of pterygium. This may be because the younger the age, the more viable the fibroblasts, and the more easily they are activated, the faster the pterygium grows.
There are some drawbacks in this study. Usually, retrospective study designs are inferior to prospective study designs because they rely on the accuracy of written records. Furthermore, the number of patients may not be sufficient. A larger sample size is needed to obtain more generalized findings. Time and intensity of exposure to ultraviolet radiation were not recorded in this study, and therefore, it could not directly demonstrate the relationship between outdoor exposure and axial length.
To our knowledge, this study of the relationship between anatomic data and prevalence of pterygium is the only one of its kind. Our results show that the prevalence of pterygium is related to short axial length and anterior chamber depth. SDF-1/CXCR4 signaling may play a vital role in pterygium and shorter axial length. Further study focused on SDF-1/CXCR4 signaling will be needed.
Supplementary Material
Footnotes
The authors have no conflicts of interest to disclose.
L. M. Zhang and Y. Lu contributed equally to this work and should be considered co-first authors.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.corneajrnl.com).
This is a retrospective study. All data were from medical history. Institutional Review Board approval is not required under our country's regulations.
REFERENCES
- 1.Droutsas K, Sekundo W. Epidemiology of pterygium. A review [in German]. Ophthalmologe. 2010;107:511. [DOI] [PubMed] [Google Scholar]
- 2.Lucas RM, McMichael AJ, Armstrong BK, et al. Estimating the global disease burden due to ultraviolet radiation exposure. Int J Epidemiol. 2008;37:654–667. [DOI] [PubMed] [Google Scholar]
- 3.Gazzard G, Saw SM, Farook M, et al. Pterygium in Indonesia: prevalence, severity and risk factors. Br J Ophthalmol. 2002;86:1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asokan R, Venkatasubbu RS, Velumuri L, et al. Prevalence and associated factors for pterygium and pinguecula in a South Indian population. Ophthalmic Physiol Opt. 2011;32:39–44. [DOI] [PubMed] [Google Scholar]
- 5.Waldmann H, Graca L, Adams E, et al. Prevalence and racial differences in pterygium: a cross-sectional study in Han and Uygur adults in Xinjiang, China. Invest Ophthalmol Vis Sci. 2015;56:1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang M, Li X, Wong W, et al. Prevalence of and racial differences in pterygium: a multiethnic population study in Asians. Ophthalmology. 2012;119:1509–1515. [DOI] [PubMed] [Google Scholar]
- 7.Lei L, Wu J, Jin G, et al. Geographical prevalence and risk factors for pterygium: a systematic review and meta-analysis. Bmj Open. 2013;3:e003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamedazzam S, Edison N, Briscoe D, et al. Identification of human papillomavirus in pterygium. Acta Ophthalmol. 2016;94:e195–e197. [DOI] [PubMed] [Google Scholar]
- 9.Chalkia AK, Spandidos DA, Detorakis ET. Viral involvement in the pathogenesis and clinical features of ophthalmic pterygium (review). Int J Mol Med. 2013;32:539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woods M, Chow S, Heng B, et al. Detecting human papillomavirus in ocular surface diseases. Invest Ophthalmol Vis Sci. 2013;54:8069–8078. [DOI] [PubMed] [Google Scholar]
- 11.Detorakis ET, Sourvinos G, Spandidos DA. Detection of herpes simplex virus and human papilloma virus in ophthalmic pterygium. Cornea. 2001;20:164–167. [DOI] [PubMed] [Google Scholar]
- 12.Lan W, Petznick A, Heryati S, et al. Nuclear Factor-κB: central regulator in ocular surface inflammation and diseases. Ocul Surf. 2012;10:137–148. [DOI] [PubMed] [Google Scholar]
- 13.Vanathi M, Goel S, Ganger A, et al. Corneal tomography and biomechanics in primary pterygium. Int Ophthalmol. 2018;38:1–9. [DOI] [PubMed] [Google Scholar]
- 14.Avisar R, Loya N, Yassur Y, et al. Pterygium-induced corneal astigmatism. Clin Exp Optom. 2001;84:200.12366316 [Google Scholar]
- 15.Cheng CY, Yen MY, Lin HY, et al. Association of ocular dominance and anisometropic myopia. Invest Ophthalmol Vis Sci. 2004;45:2856–2860. [DOI] [PubMed] [Google Scholar]
- 16.Takafumi M, Yukinori S, Ichiro M, et al. Subfoveal choroidal thickness and axial length in preschool children with hyperopic anisometropic amblyopia. Curr Eye Res. 2015;40:954–961. [DOI] [PubMed] [Google Scholar]
- 17.Phillips JR, McBrien NA. Pressure-induced changes in axial eye length of chick and tree shrew: significance of myofibroblasts in the sclera. Invest Ophthalmol Vis Sci. 2004;45:758–763. [DOI] [PubMed] [Google Scholar]
- 18.Simon B, Phillips JR. Effect of induced myopia on scleral myofibroblasts and in vivo ocular biomechanical compliance in the Guinea pig. Invest Ophthalmol Vis Sci. 2010;51:6162–6171. [DOI] [PubMed] [Google Scholar]
- 19.Poukens V, Glasgow BJ, Demer JL. Nonvascular contractile cells in sclera and choroid of humans and monkeys. Invest Ophthalmol Vis Sci. 1998;39:1765–1774. [PubMed] [Google Scholar]
- 20.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. [DOI] [PubMed] [Google Scholar]
- 21.McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cel Biol. 2007;39:666–671. [DOI] [PubMed] [Google Scholar]
- 22.Kyoung Woo K, Soo Hyun P, Seung Hoon L, et al. Upregulated stromal cell-derived factor 1 (SDF-1) expression and its interaction with CXCR4 contribute to the pathogenesis of severe pterygia. Invest Ophthalmol Vis Sci. 2013;54:7198–7206. [DOI] [PubMed] [Google Scholar]
- 23.Touhami A, Di PMT, Valle MD, et al. Characterisation of myofibroblasts in fibrovascular tissues of primary and recurrent pterygia. Br J Ophthalmol. 2005;89:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szu-Yu C, Megha M, Elan H, et al. Isolation and characterization of mesenchymal progenitor cells from human orbital adipose tissue. Invest Ophthalmol Vis Sci. 2014;55:4842–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu PC, Tsai CL, Wu HL, et al. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120:1080–1085. [DOI] [PubMed] [Google Scholar]
- 26.Jones LA, Sinnott LT, Mutti DO, et al. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48:3524–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. Jama-J Am Med Assoc. 2015;314:1142–1148. [DOI] [PubMed] [Google Scholar]
- 28.Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285. [DOI] [PubMed] [Google Scholar]
- 29.Wu PC, Chen CT, Lin KK, et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018;125:1239–1250. [DOI] [PubMed] [Google Scholar]
- 30.Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51:5247–5253. [DOI] [PubMed] [Google Scholar]
- 31.Cohen Y, Peleg E, Belkin M, et al. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012;103:33–40. [DOI] [PubMed] [Google Scholar]
- 32.Ghazal B, Philippe F, France H, et al. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2015;16:1661–1671. [DOI] [PubMed] [Google Scholar]
- 33.Tissir F, Wang CE, Goffinet AM. Expression of the chemokine receptor Cxcr4 mRNA during mouse brain development. Brain Res Dev Brain Res. 2004;149:63–71. [DOI] [PubMed] [Google Scholar]
- 34.Skrzydelski D, Guyon A, Daug V, et al. The chemokine stromal cell-derived factor-1/CXCL12 activates the nigrostriatal dopamine system . J Neurochem. 2010;102:1175–1183. [DOI] [PubMed] [Google Scholar]
- 35.Guyon A. CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Front Cel Neurosci. 2014;8:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalasani SH, Baribaud F, Coughlan CM, et al. The chemokine stromal cell-derived factor-1 promotes the survival of embryonic retinal ganglion cells. J Neurosci. 2003;23:4601–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mcleod DS, Hasegawa T, Prow T, et al. The initial fetal human retinal vasculature develops by vasculogenesis. Dev Dynam. 2010;235:3336–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tideman JW, Polling JR, Voortman T, et al. Low serum vitamin D is associated with axial length and risk of myopia in young children. Eur J Epidemiol. 2016;31:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiraguchi Y, Tanida H, Sugimoto M, et al. 1,25-Dihydroxyvitamin D3 upregulates functional C-x-C chemokine receptor type 4 expression in human eosinophils. Int Arch Allergy Imm. 2012;158:51–57. [DOI] [PubMed] [Google Scholar]
- 40.Hsu MY, Lee HN, Liang CY, et al. Pterygium is related to a decrease in corneal endothelial cell density. Cornea. 2014;33:712–715. [DOI] [PubMed] [Google Scholar]
- 41.Ting C, Lin D, Guangliang S, et al. Prevalence and racial differences in pterygium: a cross-sectional study in Han and Uygur adults in Xinjiang, China. Invest Ophthalmol Vis Sci. 2015;56:1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malekifar P, Esfandiari H, Behnaz N, et al. Risk factors for pterygium in ilam province, Iran. J Ophthalmic Vis Res. 2017;12:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.