Abstract
Objectives
Alzheimer’s disease (AD), an overwhelming neurodegenerative disease, has deleterious effects on the brain that consequently causes memory loss and language impairment. This study was intended to investigate the neuroprotective activity of the two essential oils (EOs) from Iranian Pistacia khinjuk (PK) leaves and Allium sativum (AS) cloves against β-Amyloid 25–35 (Aβ25-35) induced elevation of cholinesterase enzymes in AD.
Methods
The EOs of PK (PKEO) and AS (ASEO) were prepared and analyzed in terms of extraction yield, phenolic content, and cholinergic markers in vitro. Moreover, both were administered orally to adult male Wistar rats at concentrations of 1, 2, and 3%. The inhibitory potential of PKEO and ASEO was compared with Donepezil (0.75 mg/kg) against the high activities of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes.
Results
PKEO reached an inhibition rate of 83.6% and 81.4% against AChE and BChE, respectively. ASEO had lower anti-cholinesterase activity (65.4% and 31.5% for the inhibition AChE and BChE). PKEO was found to have more phenolic content than ASEO. A significantly positive correlation was observed between the total phenolics and anti-cholinesterase potential. In rats, both EOs decreased the enzyme activity in a concentration-dependent manner. As compared with Donepezil, the significant difference in the AChE and BChE inhibition occurred as rats were treated with PKEO 3% (p < 0.05).
Conclusion
It could be concluded that PKEO and ASEO are potent inhibitors of AChE and BChE in rats that hold promise to be used for the treatment of AD.
Keywords: alzheimer disease, cholinesterase inhibitor, phenols, wistar rats
1. Introduction
Alzheimer’s disease (AD), a chronic and progressive degenerative disease, is considered as one of the most common causes of dementia in elderly [1]. Bartus et al. suggested that the cholinergic dysfunction in the brain of healthy elderly and dementia people can cause the memory loss and subsequent cognitive damage, thus, the repair of cholinergic activity probably decreases the serious lack of cognitive function [2]. Acetylcholine (ACh) is a critical neurotransmitter used by cholinergic neurons in main physiological processes, including attention, learning, memory, and so forth [3]. The presence of impairment to the cholinergic pathways in the brain has attracted much attention to drug development [4]. Almost all FDA approved drugs consist of acetylcholinesterase (AChE) inhibitors [5] and their effectiveness is attributed to the extent of inhibition of cholinesterase [6]. However, because of serious side effects, such as dizziness, headache, constipation, hepatotoxicity, nausea, diarrhea, and bioavailability complications, these drugs (e.g., tacrine, donepezil and galantamine) are rarely prescribed [7]. Limitation of the available methods emphasizes the importance of natural products containing antioxidant and flavonoid compounds [8, 9]. Essential oils (EOs) are a highly complex mixture of naturally occurring volatile compounds produced by plants in the form of secondary metabolites, and usually extracted by hydro-distillation and cold pressing methods [10]. Many research efforts have been made concerning the cholinesterase inhibition potentials of EOs [11, 12].
Allium sativum (AS, English: Garlic) has been found to have protective effects on learning deficits in mice [13] and to enhance visual memory and attention in healthy human volunteers [14]. Moreover, dietary garlic has shown to elevate the cerebral levels of β-amyloid precursor protein and β-amyloid peptide (sAPPα, sAβ40, sAβ42) [15]. More recently, it has been shown that AS extract using n-Hexane, ethyl acetate, and methanol served to protect the brain owing to high antioxidant phytochemicals and properties of female rats from oxidative stress caused by deltamethrin via attenuating AChE [16]. On the other hand, of the genus Pistacia L. (Anacardiaceae), three species are indigenous to Iran. Recently, it has been reported that the EO of Pistacia khinjuk (PK, Persian: Khinjuk), a wild pistachio with edible and medicinal applications, presents antioxidant activities [17]. This feature is postulated to account for its potential against AD [18]. The ethanol and ethanol–water extracts of the P. terebinthus fruits and PK seeds were observed to strongly inhibit the activities of AChE and butyrylcholinesterase (BChE) [19]. Although there is no report on the effectiveness of PK for AD, some evidence has indicated the promising pharmacological activity of the genus Pistacia L. in AD [19, 20].
Since Iranian AS and PK have not yet been reported for neuroprotective activity despite their broad chemical constituents, the aim of this study is to investigate the inhibition of cholinesterase activity by the EOs obtained from the aerial parts of PK and cloves of AS in both in vitro and in vivo settings. To the best of our knowledge, this is the first study concerning the anti-cholinesterase activity of the EOs from the PK leaves (PKEO) and AS cloves (ASEO).
2. Methods
2.1. Preparation of Eos
Both plants were purchased from local grocery stores in Tehran, Iran (Geographic coordinate: latitude 35°40′45.5″N and longitude 51°24′02.1″E). But only PK origin was from the slopes of the Zagros, Dallahou, Iran (Geographic coordinate: latitude 34°20′23.3″N and longitude 46°25′23.4″E). They were compared with those species in the Medical Plant Farm, Jahad Daneshgah, Islamic Republic of Iran. Their names have been checked with www.theplantlist.org. The aerial parts of PK and cloves of AS from a single bulb were utilized for the extraction, which was conducted by using hydro-distillation with clevenger apparatus, Electro Mantle model. The final EOs then kept in sealed dark vials at 4°C.
2.2. Selection criteria and outcome assessment
Gas chromatography-mass spectrometry Analysis
The resultant EOs were tested by a gas chromatograph (Agilent Technologies, Wilmington, USA) along with a mass spectrophotometer (Agilent Technologies, Wilmington, USA). An HP-5Ms capillary column (30.0 m × 0.25 mm × 0.25 μm) was utilized for separation. The initial temperature was 50°C maintained for 2 min and then increased to 200°C at a rate of 3.5°C/min. After a two-min stopping at 200°C, it afterwards increased to 280°C in steps of 7°C/min.
Determination of phenolic content
The Folin–Ciocalteu reagent assay was performed to determine the total phenolic content as mg gallic acid equivalents per EOs gram [17]. Briefly, a mixture of 2 N Folin–Ciocalteu reagent and 0.5 mL EOs was prepared. Following 5 min, 2 mL of 75 g/L sodium carbonate was added to the solution. Thereafter, absorbance was recorded at 760 nm.
2.3. Animals
Healthy adult male Wistar rats weighting 300–400 g were provided by the Razi Vaccine and Serum Research Institute. Fifty six rats were randomly assigned into eight groups and then kept separately in laboratory animal room under the standard conditions at 22 ± 2°C and 12–14 hour light (indoor lighting intensity 100 foot-candles). Humidity should be kept within a range of 30–70%. The rats were given the standard diet and water ad libitum. They were also allowed to acclimate to the laboratory conditions for a week before commencement of the study. Experiments were conducted according to local guidelines for the care of laboratory animals of Science and Research Branch, Islamic Azad University, Tehran, Iran. Group 1 was treated with the intra Cerebro Ventricular (ICV) injection of Aβ25-35 peptide (10 μg/rat) (Sigma Aldrich, USA). Group 2 was treated with the ICV injection of Aβ25-35 peptide (10 μL) and then the oral administration (p.o.) of Donepezil (0.75 mg/kg) (positive control). Groups 3, 4, and 5 were treated with the ICV injection of Aβ25-35 peptide (10 μL) and then respectively treated with PK 1, 2, and 3% wt/wt (p.o.). Groups 6, 7, and 8 were treated with the ICV injection of Aβ25-35 peptide (10 μL) and then respectively treated with AS 1, 2, and 3% wt/wt (p.o.). Amnesia was induced by the ICV injection of Aβ25-35 peptide on week 2 following exposure to PK or AS and remained for three weeks.
2.4. Cholinesterase inhibition
In vitro. Enzyme inhibitory activity was determined applying Ellman’s method [21]. Briefly, a total of 50 μL of the EO solution was mixed with 5,5′-dithio-bis-2-nitrobenzoate (DTNB, purity 99%), solution (125 μL), and AChE or BChE solution (25 μL) in Tris–HCl buffer at pH value of 8.0. The final solution was incubated for 15 min at 25°C. With the addition of acetylthiocholine iodide or butyrylthiocholine chloride, the reaction was started. The absorbance was read at 405 nm after 10 min incubation at ambient temperature. The anti-cholinesterase activity was described as the percentage of inhibition. Donepezil was used as standard drug.
In vivo. The rats were decapitated, and then their brains were rapidly removed and transferred into ice-cold saline. Thereafter, frontal cortex, hippocampus, and septum were cut up as rapidly as possible in an ice bath. These tissues were weighed and poured into tubes containing 0.1 M PBS (pH 8) for homogenization. Subsequently, 0.4 mL of the homogenate was completely mixed within a cuvette containing 2.6 mL of PBS and 100 μL of DTNB through bubbling air to measure absorbance at 412 nm by a spectrophotometer. Afterwards, 20 μL of acetylthiocholine iodide or butyrylthiocholine chloride was added and next changes in absorbance were documented [22].
2.5. Statistical Analysis
All measurements were performed in three replicates. Data was described as mean ± standard deviation (SD). The Kolmogorov–Smirnov tests were performed to ensure data normal distribution. Considering normal distribution, Pearson correlation coefficient, one-way ANOVA and Duncan’s multiple range tests were initially conducted to determine any significant difference at P-values < 0.05 (SPSS 19.0 software Package, IBM Inc., Chicago IL, USA). If normal distribution evaded, non-parametric equivalents were used.
3. Results and Discussion
In the present study, the two plants extensively used for both cooking and medicinal purposes among Iranian were investigated for anti-cholinesterase activity in vitro and in vivo. There have been various pharmaceutical and biological applications for garlic mainly due to its anti-oxidative features that culminate in protective effects against oxidative damage as well as reductions in the risk of biomolecular impairment. These beneficial outcomes can also prevent the onset and progression of brain aging and neurodegenerative diseases to varying extent [23, 24]. In mice, it has been reported that aged garlic extract restore atrophic changes in the frontal brain, increase learning abilities and memory retention, and augment longevity in an accelerated senescence process [25, 26]. When it comes to PK, evidence has been shown its suitability for edible and medicinal applications [19, 27]. Moreover, it was found with the significant potential to induce inhibition of fungal and bacterial strains [28]. Despite considerable improvements in AD symptoms upon the administration of several cholinesterase inhibitors, including tacrine, rivastigmine, and donepezil, the undesirable outcomes associated with these medications have become increasingly noticeable [29, 30]. Thus, plant-based new drug discovery has garnered a great deal of attention and should identify the candidates with dual function and lesser negative effects for AD patients [9].
The extraction yield and phenolic content were 2.2% vol/wt and 50.5 ± 1.1 mg GAEs/g extract for PKEO and 0.1% vol/wt and 258.6 ± 4.3 mg GAEs/g extract for ASEO. As shown in Table 2, the major three constituents of PKEO were phellandrene (53.14%), α-Pinene (15.58%), and octadecanoic acid (6.04%). There have been three published data about PKEO. Abolghasemi et al. prepared the EO from PK leaves collected from an orchard in Rafsanjan, Iran (Geographic coordinate: latitude 30°25′29.0″N and longitude 56°00′12.8″E). They identified 40 compounds out of 48 total compounds in PKEO, with myrcene (18.7%), α-eudesmol (12.3%), β-eudesmol (9.3%), 1,7-di-epi-β-cedrene (7.3%), bicyclogermacrene (5.6%) and δ-eudesmol (4.9%) being major constituents [31]. Their documented PKEO composition was different from ours. Moreover, the mean yield was 1.82 ± 0.12 mg/kg dry mass in their study [31], which was slightly lower from our result. Likewise, Gha-semi Pirbalouti and Aghaee reported consistent results (phellandrene 52.33%, α-Pinene 15.28% and Δ-limonene 5.08%) [27], whereas a completely different composition was determined by Taran et al. (Spathulenol 20.87%, Germacrene B 9.53% and Aromadendrone<dehydro> 8.80%) [32]. Such disparities could be related to the collection time, environmental conditions, geographic locations, and extraction method employed in these studies [32, 33]. Several studies have also revealed the presence of phenolic constituents in P. khinjuk [17, 32]. Phenolic compounds in plants are responsible for a range of antibacterial, antioxidant, or free radicals scavenger properties [34].
Table 2.
Chemical compositions of PKEO and ASEO in percent
| No | Compound | (%) (w/w) | Compound | (%) (w/w) |
|---|---|---|---|---|
|
| ||||
| PKEO | ASEO | |||
| 1 | α-pinene | 15.58 | Methyl 2-propanol disulfide | 2.86 |
| 2 | Sabinene | 0.8 | Dimethyl trisulfide | 1.32 |
| 3 | Phellandrene | 53.14 | Allyl methyl disulfide | 7.00 |
| 4 | Δ-limonene | 4.56 | 2-Ethylidene[1,3]ditiane | 2.54 |
| 5 | 1,3,6-octatriene | 1.50 | Diallyl disulfide | 10.11 |
| 6 | γ-terpinene | 1.30 | Allyl methyl trisulfide | 8.67 |
| 7 | α-terpinolene | 1.02 | 3-Vinyl-1,2-ditiocyclohex-5-ene | 6.43 |
| 8 | (Z)-4,8-dimetyl-1,3,7 nonatriene | 1.43 | Di-2-propenyl trisulfide (isomer) | <0.02 |
| 9 | L-linalool | 1.69 | Diallyl trisulfide | 23.03 |
| 10 | Thujopsene | 1.40 | Diallyl tetrasulfide | 6.91 |
| 11 | Caryophyllene oxide | 1.65 | Benzeneacetaldehyde | 5.62 |
| 12 | Hexadecanoic acid | 1.10 | - | - |
| 13 | Octadecanoic acid | 6.04 | - | - |
| 14 | 9-Octadecanoic acid | 1.21 | - | - |
| 15 | Ethyl oleate | 1.37 | - | - |
On the other hand, ASEO in the present study mainly consisted of the three components of diallyl trisulfide (23.03%), diallyl disulfide (10.11%), and allyl methyl trisulfide (8.67%) (Table 2). Owing to the lack of published data on the EO from AS cloves, it is impossible to compare the ASEO composition with other findings from different Iran regions. The extraction yield of ASEO in this study was virtually comparable to that reported by Khadri et al. (0.09%) [35]. Nonetheless, this yield represents one fourth of the yield calculated in the study by Lawrence and Lawrence (0.4%) [36]. Our findings on the chemical profile of ASEO was relatively similar to that documented by Mnayer et al. [37], whose study, however, showed diallyl disulfide (37.90%), diallyl trisulfide (28.06%), and allyl methyl trisulfide (7.26%) as the three major constituents. Khadri et al. [35] found that allyl methyl trisulfide (34.61%), diallyl disulfide (31.65%), and allyl methyl disulfide (9.27%) were rather the main compounds of ASEO. There are still other reports indicating diallyl disulfide and diallyl trisulfide as the two major constituents of ASEO [38, 39]. There has been no study available that reported the total phenol content of ASEO. The previous work by Azimi et al. showed the same amount of phenolics for PKEO [17]. The findings of the present study revealed that ASEO possessed significantly higher phenolic content than PKEO. Indeed, EOs consist of secondary metabolites of plants, particularly the active lipophilic compounds, since solvents with a marked polarity can afford to extract higher amounts of phenolic compounds [40].
Table 1.
Extraction yield and total phenolic content of PKEO and ASEO (mean ± SD)
| Samples | Extraction yield (%) | Total phenol content (mg GAEs/g extract) |
|---|---|---|
| ASEO | 0.1 | 258.6 ± 4.3a |
| PKEO | 2.2 | 50.5 ± 1.1b |
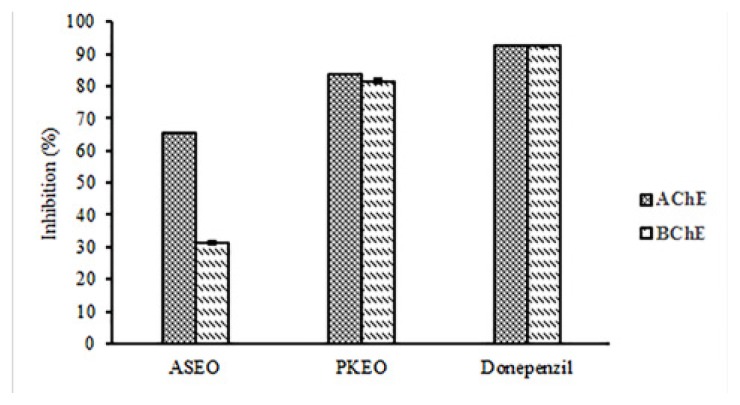
The PKEO and ASEO were tested for their inhibitory potential on AChE and BChE in vitro. As indicated in Fig. 1, PKEO reached an inhibition rate of 83.6% and 81.4% against AChE and BChE, respectively. In this regard, ASEO had a lower anti-cholinesterase activity (65.4% and 31.5% for the inhibition of AChE and BChE) compared to PKEO. Therefore, both EOs had AChE and BChE inhibitory activities, with PKEO showing more strength. Noteworthy, our results showed that ASEO appeared to be a weak inhibitor of BChE; that is, the inhibition rate of BChE (31.5%) was half of that achieved against AChE (65.4%). Table 3 summarizes the in vivo activity of both enzymes in the presence of PKEO and ASEO. The inhibition rate of AChE at three different concentrations of PKEO was reported: Group 3 (PKEO 1%): 38.5 ± 0.1%; Group 4 (PKEO 2%): 56.4 ± 0.2%; Group 5 (PKEO 3%): 62.2 ± 0.3%. Treatment of the rats with 1%, 2%, and 3% of ASEO could improve Aβ-induced increases in the AChE activity by 29.5 ± 0.5%, 36.4 ± 0.2%, and 40.2 ± 0.2%, respectively. In relation to Donepezil, PKEO 3% and ASEO 1% respectively expressed the highest (62.2 ± 0.3%) and lowest (29.5 ± 0.5%) rates of the AChE inhibition, which were statistically significant as compared with group 1 (p < 0.05). Both EOs decreased the enzyme activity in a concentration-dependent manner. PKEO suppressed the AChE and BChE activity to the almost same degree, while on the contrary, ASEO showed more potential to inhibit the activity of AChE than BChE. In comparison with Donepezil, the significant difference in the BChE inhibition occurred as rats were treated with PKEO 1%, ASEO 2%, PKEO 3%, and ASEO 3% (p < 0.05).
Figure 1.
Cholinergic markers of PKEO and ASEO in vitro
Table 3.
Enzyme inhibitory activity of positive control (group 2) and different concentrations of PKEO and ASEO in vivo.
| Samples | AChE (Inhibition %) | BChE (Inhibition %) |
|---|---|---|
| PKEO 1% | 38.5 ± 0.1 | 30.2 ± 0.0* |
| PKEO 2% | 56.4 ± 0.2 | 51.2 ± 0.1 |
| PKEO 3% | 62.2 ± 0.3* | 58.7 ± 0.5* |
| ASEO 1% | 29.5 ± 0.5* | ND |
| ASEO 2% | 36.4 ± 0.2 | 12.9 ± 0.0* |
| ASEO 3% | 40.2 ± 0.2 | 28.1 ± 0.4* |
| Donepenzil | 45.5 ± 0.6 | 43.6 ± 0.7 |
Asterisks in each column indicating significant difference from group 1 (P < 0.05).
There has some evidence confirming the anti-cholinesterase activity of AS or its family, Amaryllidaceae. Ncir et al. reported that AS extracts could successfully mitigate the brain and serum AChE activity in rats [16]. Borek [41] highlighted the potential of aged garlic extract in the reduction of the risk of dementia and AD through the brain protection against neurodegenerative conditions. Moreover, garlic is considered as an effective substance with great antimicrobial, cardiovascular, anti-inflammatory, anticancer, and immunomodulatory activities. However, it may cause some degree of toxicity at high doses [42]. The plant family is rich in alkaloid structures ascribed to their biogenesis from norbelladine, known as the common amino acid-derived precursor [43]. Galantamine, a natural product with considerable anti-cholinesterase properties, was originally extracted from snowdrop belonging to the Amaryllidaceae family [44]. Recently, it has been given an approval for clinical application and has become an effective therapeutic strategy to impede neurological degeneration in AD [11]. In another study by Okello et al., the cholinesterase inhibitory action of flower oil obtained from Narcissus poeticus L. was reported that belongs to the family Amaryllidaceae [12]. The GC-MS analysis indicated the three major constituents, such as benzyl benzoate (19.0%), phenylethyl alcohol (17.5%), and benzyl alcohol (11.0%). With 0.1 mg/mL concentration, an inhibition rate of 39% was achieved [12]. Despite the lack of galanthamine in AS, more recently, it has been shown that allicin has an ability to inhibit cholinesterase enzymes, and restore cognitive function and memory loss in AD [45]. Differently, some studies have highlighted the cholinesterase inhibitory activity of the plant species from the family Anacardiaceae [46, 47]. Hacıbekiroğlu et al. revealed that the ethanol and ethanol–water extracts of the P. terebinthus fruits and PK seeds could strongly inhibit the activity of AChE and BChE [19]. Of the family Anacardiaceae, Elufioye et al. reported that Spondias mombin with three main compounds, including botulin, campesterol and phytol, was active against AChE and BChE [46].
There was a strong correlation between the phenol content and the inhibition rate of AChE and BChE for ASEO and PKEO (r > 0.7, p < 0.05) (Table 4). Ali Reza et al. confirmed the positive correlation between the total phenolics and inhibition of AChE and BChE by the methanolic extract of Elatostema papillosum rich in phenolic content [48]. Bone and Mills [49] highlighted that the polyphenols, tannins, and flavonoids as the major constituents of the biological material have unraveled remarkable cholinesterase inhibitory effects. This finding increases the importance of phytochemistry in the anti-cholinesterase activity of the plants. Moreover, this implies that the higher the phenolics the higher the enzyme inhibitory effects. The cholinesterase inhibitory activity of these plants has not previously studied elsewhere. Thus, this investigation showed data on the enzyme inhibition activity of PKEO and ASEO for the first time.
Table 4.
Correlation coefficients between the total phenolic contents and cholinesterase inhibitory activity of ASEO and PKEO
| Variables | Total phenol content | |
|---|---|---|
|
| ||
| PKEO | ASEO | |
|
|
||
| AChE inhibitory activity | 0.978* | 0.891* |
| BChE inhibitory activity | 0.862* | 0.762* |
Asterisks in each column indicating significance (P < 0.05: *).
4. Conclusion
In conclusion, the findings of the present study showed that the EO of Iranian P. khinjuk leaves and A. sativum cloves ameliorates amyloid β induced disturbance in cholinesterase enzymes in rats using cholinergic markers. This anti-cholinesterase activity might be correlated with the phenolic content. Considering the antioxidant properties of both plant essential oils, for future studies, it will be of interest to investigate their effect on oxidative stress in AD as one of the major cause of neurotoxicity.
Footnotes
This paper meets the requirements of KS X ISO 9706, ISO 9706-1994 and ANSI/NISO Z39.48-1992 (Permanence of Paper).
Conflicts of Interest
The authors declare that there is no conflict of interest concerning the publication of this article.
References
- 1.Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer’s disease. Archives of medical research. 2012;43(8):600–8. doi: 10.1016/j.arcmed.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Bartus RT, Dean Rr, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 3.Du X, Wang X, Geng M. Alzheimer’s disease hypothesis and related therapies. Translational Neurodegeneration. 2018;7:2. doi: 10.1186/s40035-018-0107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houghton PJ, Howes MJ. Natural products and derivatives affecting neurotransmission relevant to Alzheimer’s and Parkinson’s disease. Neurosignals. 2005;14(1–2):6–22. doi: 10.1159/000085382. [DOI] [PubMed] [Google Scholar]
- 5.Heinrich M, Lee Teoh H. Galanthamine from snowdrop-the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. Journal of ethnopharmacology. 2004;92(2–3):147–62. doi: 10.1016/j.jep.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Grutzendler J, Morris JC. Cholinesterase inhibitors for Alzheimer’s disease. Drugs. 2001;61(1):41–52. doi: 10.2165/00003495-200161010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Singh A, Ekavali A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67(2):195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Essa MM, Vijayan RK, Castellano-Gonzalez G, Memon MA, Braidy N, Guillemin GJ. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochemical research. 2012;37(9):1829–42. doi: 10.1007/s11064-012-0799-9. [DOI] [PubMed] [Google Scholar]
- 9.Ebrahimpour S, Fazeli M, Mehri S, Taherianfard M, Hosseinzadeh H. Boswellic Acid Improves Cognitive Function in a Rat Model Through Its Antioxidant Activity: - Neuroprotective effect of Boswellic acid. J Pharmacopuncture. 2017;20(1):10–7. doi: 10.3831/KPI.2017.20.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytotherapy research: PTR. 2007;21(4):308–23. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 11.Ayaz M, Junaid M, Ullah F, Sadiq A, Khan MA, Ahmad W, et al. Comparative chemical profiling, cholinesterase inhibitions and anti-radicals properties of essential oils from Polygonum hydropiper L: A Preliminary anti- Alzheimer’s study. Lipids in Health and Disease. 2015;14:141. doi: 10.1186/s12944-015-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okello E, Dimaki C, Howes M, Houghton P, Perry E. In vitro inhibition of human acetyl-and butyryl-cholinesterase by Narcissus poeticus L. (Amaryllidaceae) flower absolute. Int J Essent Oil Ther. 2008;2:105–10. [Google Scholar]
- 13.Nishiyama N, Moriguchi T, Morihara N, Saito H. Ameliorative effect of S-allylcysteine, a major thioallyl constituent in aged garlic extract, on learning deficits in senescence-accelerated mice. The Journal of nutrition. 2001;131(3):1093S–5S. doi: 10.1093/jn/131.3.1093S. [DOI] [PubMed] [Google Scholar]
- 14.Tasnim S, Haque PS, Bari MS, Hossain MM, Islam SMA, Shahriar M, et al. Allium sativum L. improves visual memory and attention in healthy human volunteers. Evidence-Based Complementary and Alternative Medicine. 2015. p. 2015. [DOI] [PMC free article] [PubMed]
- 15.Chauhan NB. Anti-amyloidogenic effect of Allium sativum in Alzheimer’s transgenic model Tg2576. Journal of herbal pharmacotherapy. 2003;3(1):95–107. doi: 10.1080/J157v03n01_05. [DOI] [PubMed] [Google Scholar]
- 16.Ncir M, Saoudi M, Sellami H, Rahmouni F, Lahyani A, Makni Ayadi F, et al. In vitro and in vivo studies of Allium sativum extract against deltamethrin-induced oxidative stress in rats brain and kidney. Arch Physiol Biochem. 2018;124(3):207–17. doi: 10.1080/13813455.2017.1376335. [DOI] [PubMed] [Google Scholar]
- 17.Azimi M, Sharifan A, Ghiasi Tarzi B. The Use of Pistacia khinjuk Essential Oil to Modulate Shelf-Life and Organoleptic Traits of Mechanically Deboned Chicken Meat. J Food Process Preserv. 2016 [Google Scholar]
- 18.Asha H. A Review: Natural Compounds as Anti-Alzheimer´s Disease Agents. Current Nutrition & Food Science. 2017;13(4):247–54. [Google Scholar]
- 19.Hacıbekiroğlu Iı, Yılmaz PK, Haşimi N, Kılınç E, Tolan V, Kolak U. In vitro biological activities and fatty acid profiles of Pistacia terebinthus fruits and Pistacia khinjuk seeds. Natural product research. 2015;29(5):444–6. doi: 10.1080/14786419.2014.947492. [DOI] [PubMed] [Google Scholar]
- 20.Rauf A, Patel S. Pistagremic acid as a broad spectrum natural inhibitor from Pistacia integerrima Stewart. Natural product research. 2017;31(4):367–8. doi: 10.1080/14786419.2016.1188099. [DOI] [PubMed] [Google Scholar]
- 21.Aktumsek A, Zengin G, Guler GO, Cakmak YS, Duran A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2013;55:290–6. doi: 10.1016/j.fct.2013.01.018. Epub 2013/01/30. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Prakash A, Dogra S. Centella asiatica attenuates D-galactose-induced cognitive impairment, oxidative and mitochondrial dysfunction in mice. International journal of Alzheimer’s disease. 2011;2011:1–9. doi: 10.4061/2011/347569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borek C. Antioxidant and cancer. Sci Med. 1997;4:51–62. [Google Scholar]
- 24.Gutteridge JM. Free radicals in disease processes: a compilation of cause and consequence. Free radical research communications. 1993;19(3):141–58. doi: 10.3109/10715769309111598. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 25.Moriguchi T, Saito H, Nishiyama N. Anti-ageing effect of aged garlic extract in the inbred brain atrophy mouse model. Clinical and experimental pharmacology & physiology. 1997;24(3–4):235–42. doi: 10.1111/j.1440-1681.1997.tb01813.x. Epub 1997/03/01. [DOI] [PubMed] [Google Scholar]
- 26.Nishiyama N, Moriguchi T, Saito H. Beneficial effects of aged garlic extract on learning and memory impairment in the senescence-accelerated mouse. Experimental gerontology. 1997;32(1–2):149–60. doi: 10.1016/S0531-5565(96)00062-9. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 27.Ghasemi Pirbalouti A, Aghaee K. Chemical composition of essential oil of Pistacia khinjuk stocks grown in Bakhtiari Zagross Mountains, Iran. Electronic Journal of Biology. 2011;7(4):67–9. [Google Scholar]
- 28.Taran M, Sharifi M, Azizi E, Khanahmadi M. Antimicrobial activity of the leaves of Pistacia khinjuk. Journal of Medicinal Plants. 2010;1(33):81–5. [Google Scholar]
- 29.Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. International journal of clinical practice Supplement. 2002;(127):45–63. [PubMed] [Google Scholar]
- 30.Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clinical pharmacokinetics. 2002;41(10):719–39. doi: 10.2165/00003088-200241100-00003. [DOI] [PubMed] [Google Scholar]
- 31.Abolghasemi A, Shojaaddini M, Tajabadipour A, Sefidkon F. Composition of Pistacia khinjuk (Anacardiaceae) Leaf Essential Oil and its Insecticidal Activity on Common Pistachio Psyllid, Agonoscena pistaciae (Hem., Psylloidea) Journal of Essential Oil Bearing Plants. 2018;21(3):796–802. doi: 10.1080/0972060X.2018.1498397. [DOI] [Google Scholar]
- 32.Taran M, Sharifi M, Azizi E, Khanahmadi M. Antimicrobial activity of the leaves of Pistacia khinjuk. J Medicin Plant. 2010;1(33):81–5. [Google Scholar]
- 33.De Pooter H, Schamp N, Aboutabl E, El Tohamy S, Doss S. Essential oils from the leaves of three Pistacia species grown in Egypt. Flavour and Fragrance journal. 1991;6(3):229–32. doi: 10.1002/ffj.2730060313. [DOI] [Google Scholar]
- 34.Rahnama M, Najimi M, Ali S. Antibacterial effects of Myristica fragrans, Zataria multiflora Boiss, Syzygium aromaticum, and Zingiber officinale Rosci essential oils, alone and in combination with nisin on Listeria monocytogenes. Comp Clin Pathol. 2012;21(6):1313–6. doi: 10.1007/s00580-011-1287-3. [DOI] [Google Scholar]
- 35.Khadri S, Boutefnouchet N, Dekhil M. Antibacterial activity evaluation of Allium sativum essential oil compared to different Pseudomonas aeruginosa strains in Eastern Algeria. Scientific Study & Research. 2010;11(4):421–8. [Google Scholar]
- 36.Lawrence R, Lawrence K. Antioxidant activity of garlic essential oil (Allium sativum) grown in north Indian plains. Asian Pac J Trop Biomed. 2011;1(Suppl 1):S51–S54. doi: 10.1016/S2221-1691(11)60122-6. [DOI] [Google Scholar]
- 37.Mnayer D, Fabiano-Tixier AS, Petitcolas E, Hamieh T, Nehme N, Ferrant C, et al. Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules (Basel, Switzerland) 2014;19(12):20034–53. doi: 10.3390/molecules191220034. Epub 2014/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casella S, Leonardi M, Melai B, Fratini F, Pistelli L. The role of diallyl sulfides and dipropyl sulfides in the in vitro antimicrobial activity of the essential oil of garlic, Allium sativum L., and leek, Allium porrum L. Phytotherapy Research. 2013;27(3):380–3. doi: 10.1002/ptr.4725. [DOI] [PubMed] [Google Scholar]
- 39.Corzo-Martínez M, Corzo N, Villamiel M. Biological properties of onions and garlic. Trends in food science & technology. 2007;18(12):609–25. doi: 10.1016/j.tifs.2007.07.011. [DOI] [Google Scholar]
- 40.Sultana B, Anwar F, Przybylski R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chemistry. 2007;104(3):1106–14. doi: 10.1016/j.foodchem.2007.01.019. [DOI] [Google Scholar]
- 41.Borek C. Garlic reduces dementia and heart-disease risk. J Nutr. 2006;136(3):810S–2S. doi: 10.1093/jn/136.3.810S. [DOI] [PubMed] [Google Scholar]
- 42.Mikaili P, Maadirad S, Moloudizargari M, Aghajanshakeri S, Sarahroodi S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iranian journal of basic medical sciences. 2013;16(10):1031–48. Epub 2014/01/01. [PMC free article] [PubMed] [Google Scholar]
- 43.Cortes N, Posada-Duque RA, Alvarez R, Alzate F, Berkov S, Cardona-Gómez GP, et al. Neuroprotective activity and acetylcholinesterase inhibition of five Amaryllidaceae species: A comparative study. Life sciences. 2015;122:42–50. doi: 10.1016/j.lfs.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Heinrich M, Teoh HL. Galanthamine from snowdrop—the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. Journal of ethnopharmacology. 2004;92(2–3):147–62. doi: 10.1016/j.jep.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Kumar S. Dual inhibition of acetylcholinesterase and butyrylcholinesterase enzymes by allicin. Indian journal of pharmacology. 2015;47(4):444–6. doi: 10.4103/0253-7613.161274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elufioye TO, Obuotor EM, Agbedahunsi JM, Adesanya SA. Anticholinesterase constituents from the leaves of Spondias mombin L. (Anacardiaceae) Biologics: Targets & Therapy. 2017;11:107–14. doi: 10.2147/BTT.S136011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moyo M, Ndhlala AR, Finnie JF, Van Staden J. Phenolic composition, antioxidant and acetylcholinesterase inhibitory activities of Sclerocarya birrea and Harpephyllum caffrum (Anacardiaceae) extracts. Food Chemistry. 2010;123(1):69–76. doi: 10.1016/j.foodchem.2010.03.130. [DOI] [Google Scholar]
- 48.Ali Reza ASM, Hossain MS, Akhter S, Rahman MR, Nasrin MS, Uddin MJ, et al. In vitro antioxidant and cholinesterase inhibitory activities of Elatostema papillosum leaves and correlation with their phytochemical profiles: a study relevant to the treatment of Alzheimer’s disease. BMC complementary and alternative medicine. 2018;18:123. doi: 10.1186/s12906-018-2182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bone K, Mills S. Principles and practice of phytotherapy: modern herbal medicine. London, UK: Elsevier Health Sciences; 2013. [Google Scholar]