Supplemental Digital Content is Available in the Text.
Five daily sessions of repetitive transcranial magnetic stimulation with stimulus conditions were ineffective in neuropathic pain relief. Long-term administration should be investigated for clinical use of repetitive transcranial magnetic stimulation in neuropathic pain.
Keywords: Repetitive transcranial magnetic stimulation, Motor cortex stimulation, Neuropathic pain, Randomized controlled trial
Abstract
We conducted a multicenter, randomized, patient- and assessor-blinded, sham-controlled trial to investigate the efficacy of repetitive transcranial magnetic stimulation (rTMS) of the primary motor cortex (M1) in patients with neuropathic pain (NP). Patients were randomly assigned to receive 5 daily sessions of active or sham rTMS of M1 corresponding to the part of the body experiencing the worst pain (500 pulses per session at 5 Hz). Responders were invited to enroll in an open-label continuous trial involving 4 weekly sessions of active rTMS. The primary outcome was a mean decrease in a visual analogue scale of pain intensity (scaled 0-100 mm) measured daily during the daily sessions in an intention-to-treat population. Secondary outcomes were other pain scores, quality-of-life measures, and depression score. One hundred forty-four patients were assigned to the active or sham stimulation groups. The primary outcome, mean visual analogue scale decreases, was not significantly different (P = 0.58) between the active stimulation group (mean, 8.0) and the sham group (9.2) during the daily sessions. The secondary outcomes were not significantly different between 2 groups. The patients enrolled in the continuous weekly rTMS achieved more pain relief in the active stimulation group compared with the sham (P < 0.01). No serious adverse events were observed. Five daily sessions of rTMS with stimulus conditions used in this trial were ineffective in short-term pain relief in the whole study population with various NP. Long-term administration to the responders should be investigated for the clinical use of rTMS on NP in the future trials.
1. Introduction
Neuropathic pain (NP) is defined as “Pain caused by a lesion or disease of the somatosensory nervous system.” by the International Association for the Study of Pain.27 Neuropathic pain can arise from a variety of causes: stroke, spinal cord injury, phantom limb, herpes zoster infection, radiculopathy, diabetic neuropathy, and so on. Characteristic symptoms include spontaneous continuous pain, shooting pain, allodynia, and hyperalgesia with sensory deficits. It is regarded as a distinct clinical entity despite a large variety of causes because of common clinical features and putative pathophysiological mechanisms, which include both peripheral and central sensitization.4 Tricyclic antidepressants, serotonin-noradrenaline reuptake inhibitors, pregabalin, and gabapentin are recommended as first-line treatment in NP.11 Number needed to treat for 50% pain relief, however, ranges from 3.6 to 7.7 on these drugs,11 and no strong recommendation has been made in interventional treatment of NP.6,7 Thus, available treatments are not yet adequate in many patients, and NP still disturbs patients' daily activities and reduces quality of life (QOL).5 There is a critical need for novel therapeutic methods to treat intractable NP. Repetitive transcranial magnetic stimulation (rTMS) of the primary motor cortex (M1) is a noninvasive brain-stimulation method that has garnered interest as an alternative treatment for intractable NP.21 Repetitive transcranial magnetic stimulation can potentially induce therapeutic brain plasticity on the stimulated area and various neural structures related to pain perception.15,18,20 We previously conducted a pilot randomized, patient- and assessor-blinded, sham-controlled, crossover trial to assess the efficacy and safety of 10 daily rTMS treatments (500 pulses per session at 5 Hz) for patients with intractable NP in 7 centers in Japan. The trial results show that daily rTMS of M1 provides transient modest pain relief.16 Recent meta-analyses and therapeutic guidelines report that high-frequency (≥5 Hz) rTMS of M1 is safe and has a transient pain-relieving effect.6,21,25,26,30 Repetitive transcranial magnetic stimulation devices have not been approved in clinical use for treating NP in most of the countries including Japan; nevertheless, many clinical trials reported positive results. It reflects that no well-designed large clinical trials of rTMS for NP have been strictly conducted. There is uncertainty around the previous promising findings derived from the poor quality of evidence. Because clinical use of rTMS requires a regulatory approval in Japan, we planned a large strict clinical trial to obtain a regulatory approval, which was designed based on the results of our previous pilot study.16 This trial investigated the efficacy and safety of 5 daily sessions of rTMS of M1 compared with sham stimulation in patients with intractable NP.
2. Methods
2.1. Trial design
We conducted a multicenter, randomized, patient- and assessor-blinded, sham-controlled, parallel trial at Osaka University Hospital, Hamamatsu University Hospital, and Kindai University Sakai Hospital in Japan. Recruitment and follow-up were conducted from January 2016 through September 2017. This study was an investigator-initiated clinical trial funded by the Japan Agency for Medical Research and Development (AMED; Tokyo, Japan) and Teijin Pharma Limited (Tokyo, Japan) aiming the Japanese regulatory approval for clinical use of medical devices. All data management, monitoring, auditing, and statistical analyses were performed by an independent clinical-research organization (AC Medical, Inc, Tokyo, Japan). Data were captured by an electronic data capture (EDC) system (DATATRAK ONE; DATATRAK International, Inc, Mayfield Heights, OH). The trial was conducted in accordance with Good Clinical Practice (GCP) guidelines and the Declaration of Helsinki. The study protocol strictly based on our previous clinical trial was accepted by the Japanese Regulatory Authority (Pharmaceuticals and Medical Devices Agency) and approved by the institutional review boards of all study sites. All patients provided written informed consent before enrollment. This trial is registered with the University Hospital Medical Information Network Clinical Trials Registry, number UMIN000020291.
2.2. Patients
We enrolled patients aged 20 years or older who had NP (based on International Association for the Study of Pain Terminology27) and met the following inclusion criteria: (1) intractable pain longer than 6 months after pain onset, (2) baseline 30- to 94-mm score using a visual analogue scale (VAS) of pain intensity (scaled 0-100 mm), (3) currently prescribed medication for NP or a history of being prescribed multiple medications for NP without achieving pain control, and (4) continuous pain in upper or lower extremities or face. We did not include patients with ≥95-mm score in VAS because it is unable to adequately evaluate aggravation of pain. Key exclusion criteria were dementia, severe aphasia, severe cognitive dysfunction (mini mental state examination of ≤23), severe mental illness, suicidal thoughts, history of seizures, pregnancy, complete paralysis of the stimulus target site, receiving rTMS within 1 year of consenting, enrolment in any other clinical trials within the past 6 months before obtaining consent, noncompliant with pain medication, and contraindications to rTMS (eg, cardiac pacemaker implantation). We recruited participants from outpatients of departments of neurosurgery and neurology in the study sites and patients referred from the other hospitals or clinics as candidates of this trial.
2.3. Randomization and blinding
Patients were randomly assigned to either the active or sham stimulation group [1:1]. A minimization method with a determining probability of 80% was used for randomization, and stratification was based on trial site, cause of pain (central or peripheral), and age (<60 vs ≥60 years). Patients were allocated using the allocation function of the EDC system. Knowledge of treatment-group assignments was limited to those administering the intervention. Treatment-group information was stored in a lockable safe for documents and maintained with a password for the EDC system. Patients, assessors, and clinical-research coordinators who assisted assessors were blinded, and assignments were not disclosed during the trial. To ensure blinding, none of the research staff switched his role from blinded to nonblinded or vice versa. The procedure of sham stimulation mimicked that of active stimulation as much as possible (see Interventions section below).
2.4. Trial schedule
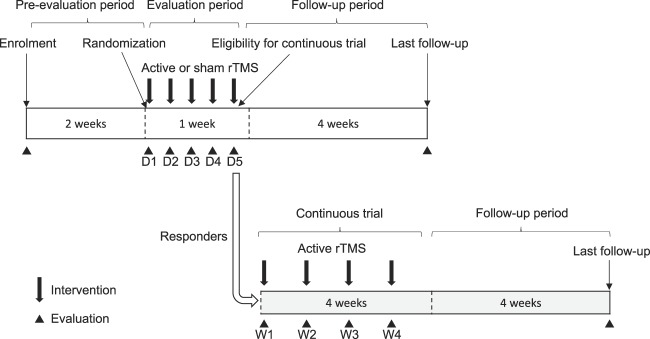
All patients who provided consent were assessed for eligibility by a neurologist or neurosurgeon specialized in chronic pain, including compliance with pain medication for 2 weeks before the intervention. Eligible patients were randomly assigned to a treatment group. Once allocated, patients received 5 daily sessions of active or sham rTMS to evaluate the efficacy and safety of the treatment in out- or in-patient settings (evaluation period). The number of sessions was determined according to minute investigations of results of our pilot study. In addition, responders whose VAS decreased an average of 10 mm or more with daily rTMS could join an open-label continuous trial to test active rTMS administered at least once weekly for 4 weeks in an out-patient setting (continuous-evaluation period). We set 10-mm decrease as a criterion of responders, which corresponds to a minimal importance of change in VAS.8 This extension was primarily designed to collect data of a long-term effect and safety of the active stimulation according to a demand of the regulatory authority. All patients were followed for 4 weeks after completion of the intervention (Fig. 1). Pain medications were not changed, and rehabilitation was held stable during the trial period. Nerve block and acupuncture were prohibited, and spinal cord stimulation was turned off during the trial period.
Figure 1.
Trial schedule. D, day; rTMS, repetitive transcranial magnetic stimulation; W, week.
Current pain intensity was examined before and after each intervention using a VAS and the Japanese version of the short-form McGill pain questionnaire 2 (SF-MPQ2; scaled 0-220, with 4 subscales namely, continuous pain, intermittent pain, NP, and affective descriptors).28 The Patient Global Impression of Change (PGIC), a 7-point scale, ranging from “very much improved” to “very much worse,” was collected on the fifth day and the fourth week for patients in the continuous trial. The EQ-5D-5L (an instrument including an index value scaled 0-1 and a VASEQ-5D scaled 0-100, with higher scores indicating better health status)10 and the Beck Depression Inventory second version (BDI-II; scaled 0-63) were used before intervention on the 1st day, after intervention on the 5th day, and in the 4th week to evaluate health-related QOL and depression. To evaluate blindness, patients were asked to guess which stimulation group they were in after the first and fifth interventions. The last evaluations, including VAS, SF-MPQ2, BDI-II, and mini mental state examination, were conducted in all allocated patients 4 weeks after completion or discontinuation of the intervention. Adverse events were defined as any sign of undesirable or unintended diseased condition or disorder that occurred to the subject, operator, or other personnel during usage of the device. They were categorized according to the severity of adverse event (mild, medium, and severe) and relation to the intervention (related and unrelated), and coded by the MedDRA/J Version 18.0. Adverse events were collected and evaluated by blinded assessors throughout the trial period. The evaluation schedule is provided in Figure 1, and Supplemental Digital Content 1 (available at http://links.lww.com/PAIN/A885).
2.5. Interventions
We used an rTMS system (TEN-P11; Teijin Pharma Limited; Fig. 2) developed in collaboration with Teijin Pharma Limited. The device includes a position-adjusting unit to align TMS coils to the appropriate positions, an efficient eccentric figure-8-coil,37 and equipment for sham stimulation. The eccentric figure-8-coil is basically similar as a conventional concentric figure-8-coil, but can reduce a driving current intensity by approximately 10% to induce comparable neural response to a conventional one.37 The stimulation target was the location in M1 that corresponded to the part of the body experiencing the worst pain (the hand region of M1 for patients with pain in the upper extremity and the foot region for pain in the lower extremity). Interventions were performed by trained neurosurgeons or neurologists.
Figure 2.
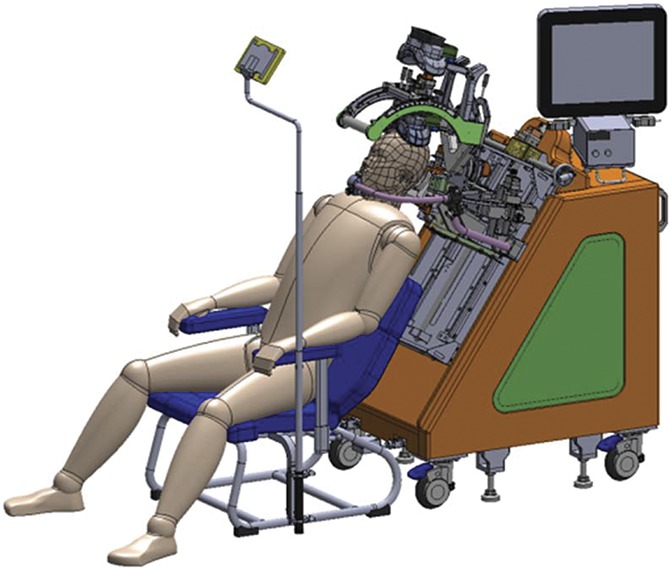
Image of the rTMS equipment. rTMS, repetitive transcranial magnetic stimulation.
The target-stimulation site was determined by identifying the motor hotspot that elicited the most prominent muscle twitch in the affected body part. The resting motor threshold (RMT), defined as the minimum intensity needed to induce one visible muscle twitch, was measured, which corresponded to the RMT measured using motor-evoked potentials (MEPs).13 We did not record MEPs and adopted muscle twitches instead of MEPs because it is more practical on clinical practice. Determination of the stimulation site and RMT was made only on the first day of intervention, and the trial system could reposition the TMS coil in the set position determined on the first day. An active rTMS session involved 10 trains at 90% RMT (50 pulses/train at 5 Hz; intertrain interval, 50 seconds). The maximum rTMS intensity was 67% of the maximum stimulator output. This protocol was developed in accordance with the guidelines for the safe use of rTMS34 and used in our previous trials.14,16,36,38 To match the method of stimulation between active and sham stimulation groups, a pair of electrodes was attached to the scalp near the target-stimulation site in both groups. To generate a realistic sham stimulation,12,16 electrical stimuli at double the intensity of the sensory threshold were simultaneously delivered with magnetic discharges through the sham coil, located approximately 30 cm above the scalp. The sham stimulation produced scalp sensations and sounds similar to active stimulation without M1 stimulation. The coil position and stimulation protocol were the same as those of active stimulation.
2.6. Outcomes
The primary outcome was VAS decrease in the evaluation period, that is, the mean decrease in VAS after each intervention compared with VAS before the first intervention, calculated as below.
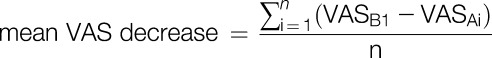 |
where i = day, n = number of interventions during the first to fifth day, VASB1 = VAS before intervention on the first day, and VASAi = VAS after intervention on the day i. We adopted mean values rather than values at a single time point to reduce variation in pain scores.
Key secondary outcomes were short-term VAS decrease (ie, the mean difference in VAS before and after each intervention), SF-MPQ2 decrease, short-term SF-MPQ2 decrease, PGIC score, BDI-II change, and EQ-5D-5L changes in the evaluation period. The rates of change were also calculated. Other outcomes included efficacy ratios, such as ratios of patients with ≥10-mm decrease in VAS to those with <10-mm decrease, and ratios of patients with ≥20-mm decrease to <20-mm decrease. Efficacy thresholds were determined based on the clinical importance of the changes: 10- and 20-mm decreases correspond to minimally and moderately important change in VAS, respectively.8 Considering noninvasive property of rTMS, we set these lower thresholds. Key outcomes of the continuous-evaluation period were decreases in VAS and SF-MPQ2 from the scores at baseline.
2.7. Statistical analysis
In our previous trial, the difference in the mean VAS decrease from the first day to fifth day of the first phase between active and sham stimulation was 4.81, with a same standard deviation of 8.36.16 Therefore, a sample size of 72 patients per group was needed to achieve a 5% two-sided significance level and 90% power while accounting for a 10% dropout rate. The study duration was anticipated to be 2 years.
Main analyses of efficacy were based on the intention-to-treat principle. Missing data were handled without imputation. We used a 2-sample t test to compare change or change rate of outcome measures between active and sham stimulation. We used Wilcoxon rank–sum test for ordered categorical variables, and Fisher exact test for binary variables to compare 2 groups. To evaluate the influence of background factor (age, cause of pain, trial site, sex, and inpatient vs outpatient) on VAS and SF-MPQ2 scores, we used analysis of variance (group [active/sham], patient background, and interaction between group and patient background) without correction for multiple comparisons. We included the following post hoc exploratory analyses: analysis of SF-MPQ2 decrease in the per-protocol population, analysis of decrease in each SF-MPQ2 subscale, and subgroup analyses of decrease in pain scores according to the location experiencing maximum pain and VAS at baseline (≥60 vs <60). In general, a VAS of 60 mm corresponds to a cutoff point between moderate and severe pain.9 Regarding analysis of the continuous-evaluation period, we used a repeated-measures analysis of covariance for efficacy analysis of VAS and SF-MPQ2 (response variable: raw value of a pain score; explanatory variables: group, time, and interaction between group and time), with the baseline measurement as a covariate. To evaluate changes from baseline, we used the Dunnett multiple-comparisons for each group. In all analyses, findings with a 2-sided P value of <0.05 were considered statistically significant. SAS version 9.3 (SAS Institute) was used for statistical analysis.
3. Results
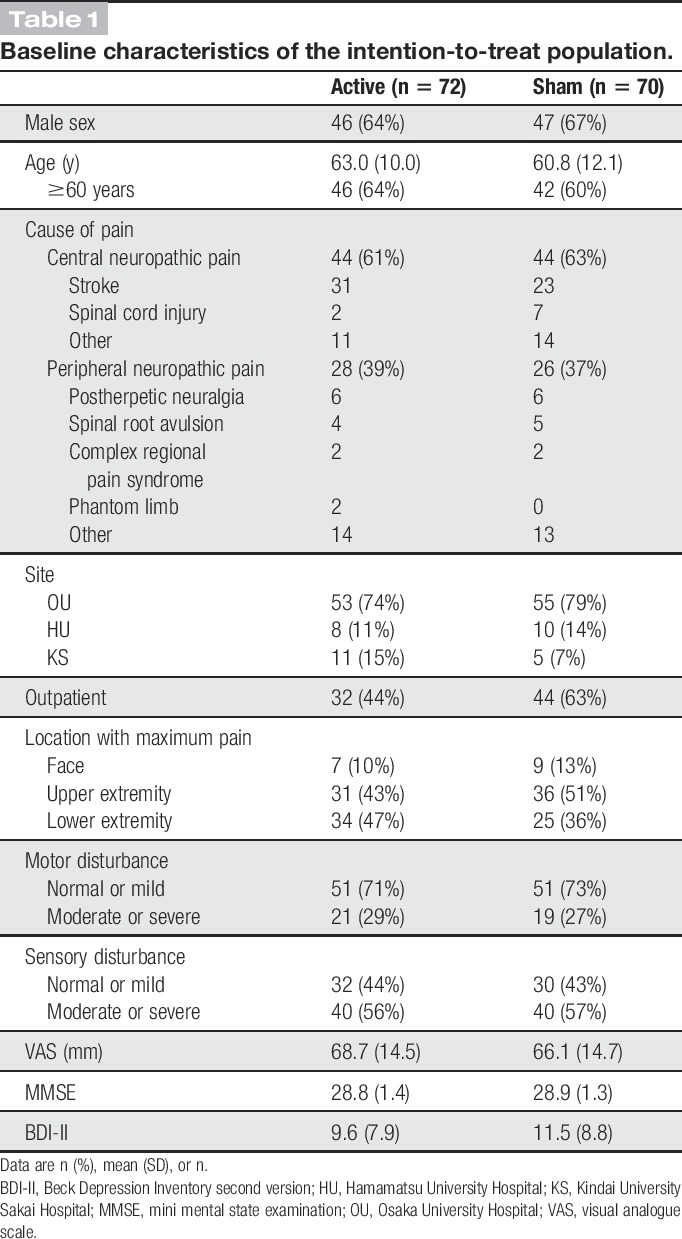
Between January 8, 2016, and June 22, 2017, we enrolled 157 patients, and 144 patients were randomly assigned to 2 groups and received their allocated intervention after 13 patients were excluded from the trial (12 patients did not meet inclusion criteria and one declined to participate). One patient in the active stimulation group was excluded from both safety and intention-to-treat analyses because he withdrew after the first intervention without completing an evaluation. One patient in the sham stimulation group was excluded from the intention-to-treat analysis because he was noncompliant with pain medication during the preevaluation period. Two in the active stimulation group and three in the sham group discontinued intervention during the evaluation study period. Finally, 72 patients in the active stimulation group and 70 in the sham stimulation group were included in the intention-to-treat population, whereas 69 and 65 were, respectively, included in the per-protocol population. Thirty-three patients were enrolled in the continuous trial and one patient was excluded from analyses because of a GCP violation; an invalid informed consent (Fig. 3). Table 1 shows the baseline demographic and clinical characteristics of each group in the intention-to-treat population. The blinding evaluation showed that patients remained blinded throughout the trial (see Table, Supplemental Digital Content 2, available at http://links.lww.com/PAIN/A885).
Figure 3.
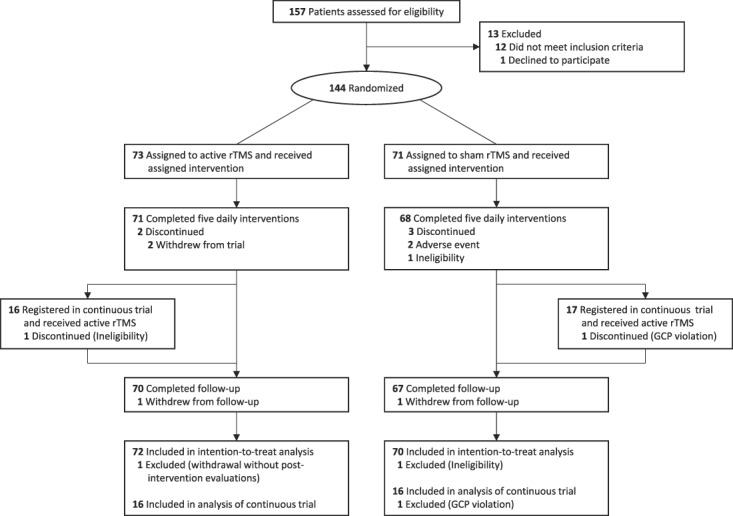
Flow diagram. GCP, good clinical practice; rTMS, repetitive transcranial magnetic stimulation.
Table 1.
Baseline characteristics of the intention-to-treat population.
3.1. Primary outcome
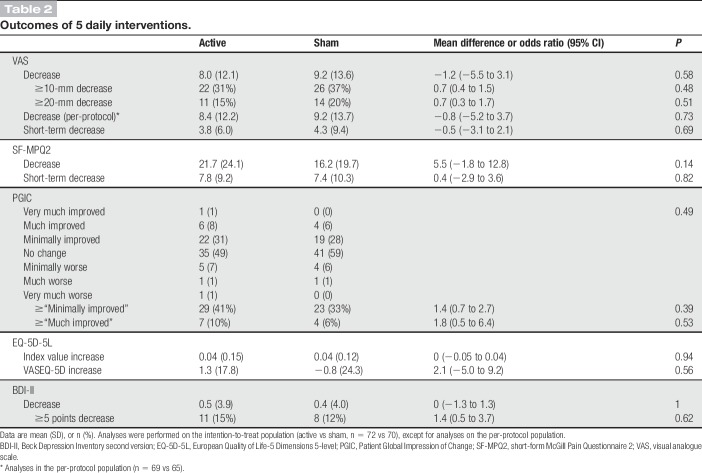
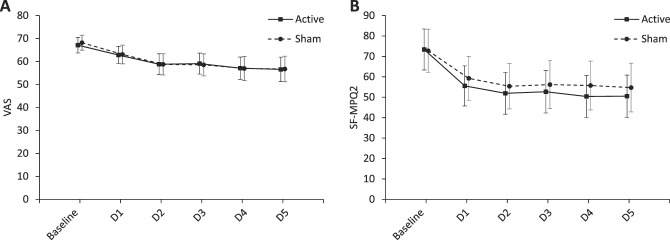
The primary outcome, the mean VAS decrease, was not significantly different between groups in the intention-to-treat analysis. The mean VAS decreases were 8.0 (SD, 12.1) in the active stimulation group and 9.2 (13.6) in the sham group (mean difference, −1.2; 95% confidence interval [CI], −5.5 to 3.1; P = 0.58). The ratios of patients with ≥10- and ≥20-mm decrease in VAS in the intention-to-treat population and the VAS decrease in the per-protocol population were not significantly different between groups (Table 2). Figure 4A shows the time course of VAS in the intention-to-treat population.
Table 2.
Outcomes of 5 daily interventions.
Figure 4.
Time course of pain scores during the evaluation period in the intention-to-treat population. The mean and 95% CI are shown for (A) visual analogue scale (VAS) and (B) short-form McGill pain questionnaire 2 (SF-MPQ2) at baseline, that is, before the 1st-day intervention, and after each intervention in the evaluation period. CI, confidence interval; D, day.
3.2. Secondary outcomes
None of the predefined secondary outcome analyses, including pain intensity, QOL, and depression, were significantly different between groups (Table 2). The mean SF-MPQ2 decreases were 21.7 (SD, 24.1) in the active stimulation group and 16.2 (19.7) in the sham group (mean difference, 5.5; 95% CI, −1.8 to 12.8; P = 0.14). Figure 4B shows the time course of SF-MPQ2 in the intention-to-treat population. The short-term decreases in VAS and SF-MPQ2 were not significantly different between groups. Twenty-nine (41%) patients in the active stimulation group and 23 (33%) patients in the sham group reported “minimally improved” or more. In addition, 7 (10%) patients in the active and 4 (6%) patients in the sham reported “much improved” or “very much improved.” No significant between-group differences in PGIC, EQ-5D-5L, and BDI-II were noted. Analyses using change rates did not show significant between-group differences (see Table, Supplemental Digital Content 3, available at http://links.lww.com/PAIN/A885). In the final evaluation at the last follow-up, there were no significant differences in score changes between 2 groups (see Table, Supplemental Digital Content 4, available at http://links.lww.com/PAIN/A885). In all these secondary outcome measures, positive values indicate improvements in scores.
Among post hoc analyses, the total SF-MPQ2 score in the per-protocol population was not significantly different between groups (mean, 22.3 [SD 24.3] in active vs 15.3 [19.8] in sham; mean difference, 7.0 [95% CI −0.6 to 14.6], P = 0.07). The results of analysis of the SF-MPQ2 subscales are presented in the supplemental table (see Table, Supplemental Digital Content 5, available at http://links.lww.com/PAIN/A885). Subgroup analyses of VAS decrease suggested that patients receiving outpatient interventions tended to have better outcomes (see Figure, Supplemental Digital Content 6, available at http://links.lww.com/PAIN/A885). The results of the subgroup analyses of SF-MPQ2 decrease are presented in the supplemental table (see Figure, Supplemental Digital Content 7, available at http://links.lww.com/PAIN/A885).
3.3. Continuous trial
The repeated-measures analysis of covariance of VAS and SF-MPQ2 scores showed significant effects on active vs sham groups (P = 0.001 and P < 0.001, respectively) and time (P < 0.001 and P = 0.003, respectively), but no effect on their interaction (P = 0.47 and P = 0.08, respectively). Significant VAS changes from baseline appeared from the second day in the active stimulation group, whereas the sham group showed the significant changes in VAS from the third day with less pain relief. Significant SF-MPQ2 changes from baseline appeared from the first day in the active stimulation group, but no significant changes in the sham. The mean VAS decrease rates after the last intervention were 53.5% (SD, 24.6) in the active stimulation group and 38.7% (27.5) in the sham group (mean difference, 14.9%; 95% CI, −4.3 to 34.1; P = 0.12). The mean SF-MPQ2 decrease rates after the last intervention were 73.1% (SD, 21.3) in the active stimulation group and 46.5% (38.4) in the sham group (mean difference, 26.6%; 95% CI, 3.6-49.6; P = 0.03). Patients enrolled from the active stimulation group achieved more pain relief with continuous weekly rTMS compared with those from the sham group (Fig. 5).
Figure 5.
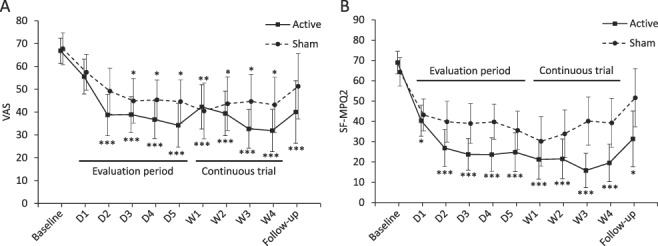
Time course of pain scores in patients enrolled in the continuous trial. The means and 95% CI are shown for (A) visual analogue scale (VAS) and (B) short-form McGill pain questionnaire 2 (SF-MPQ2) at baseline, after each intervention and at the last follow-up. Significant changes from the baseline are indicated as *P < 0.05; **P < 0.01; ***P < 0.001. CI, confidence interval; D, day; W, week.
3.4. Adverse events
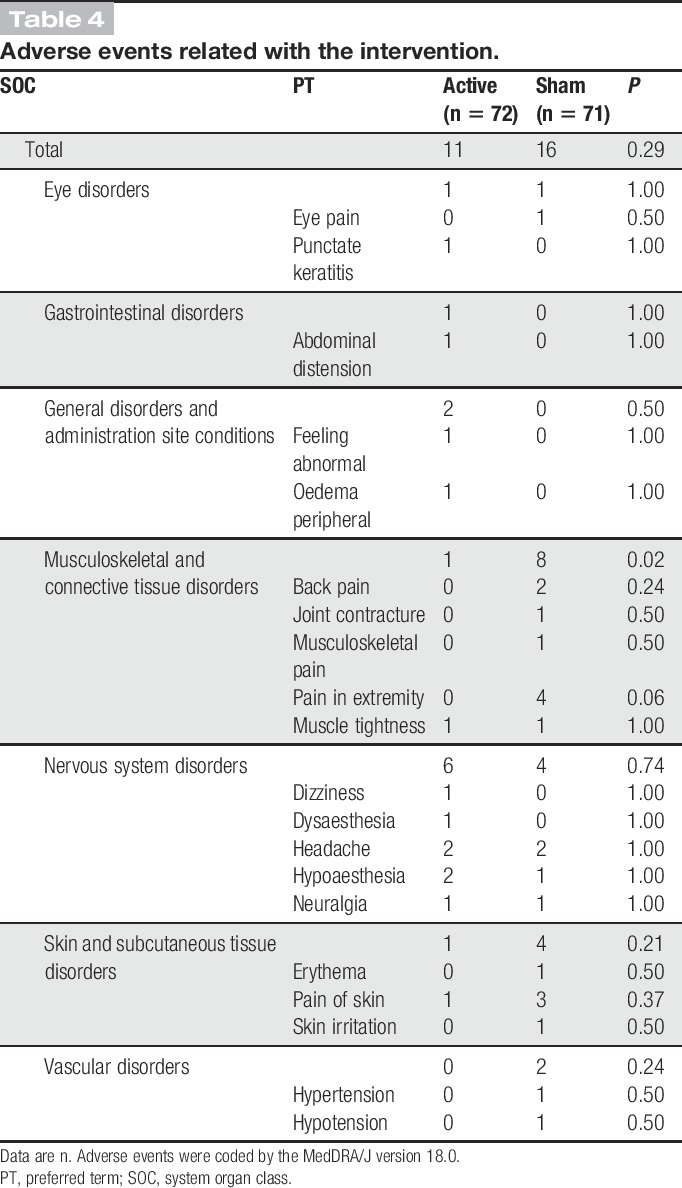
No serious adverse events were observed in this trial. There were no significant between-group differences in adverse event frequency or severity during the trial period. The numbers of adverse events related to the intervention did not differ between groups (Tables 3 and 4).
Table 3.
Adverse events in the safety-analysis population.
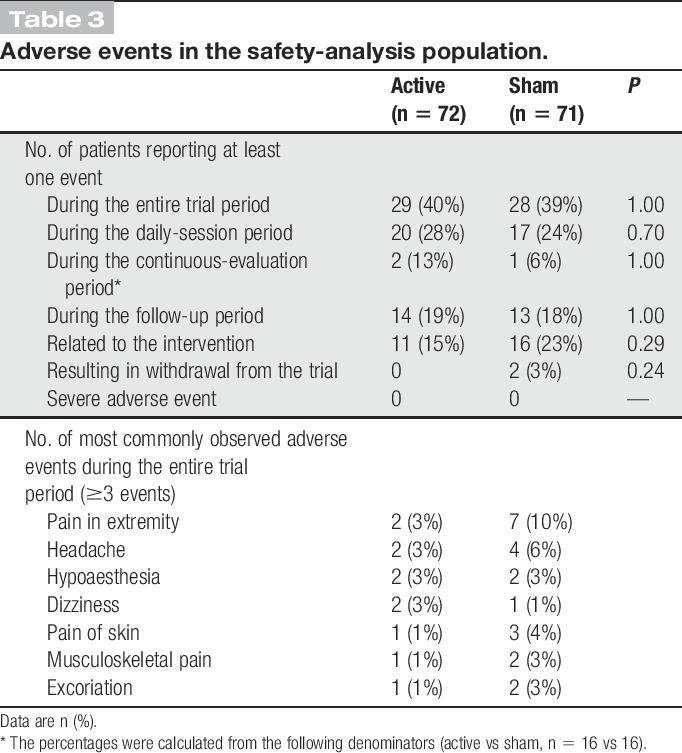
Table 4.
Adverse events related with the intervention.
4. Discussion
This randomized controlled trial (RCT) investigating rTMS of M1 for NP and using a strictly conducted trial system had the largest number of patients of any similar trial to date. The sham procedure, which included simultaneous electrical stimuli on the scalp, was so rigorous that patients could not determine their intervention assignment. A Cochrane review evaluating therapeutic uses of rTMS for chronic pain suggests there was a short-term, small effect for single-session, high-frequency rTMS of M1 on NP. Meanwhile, results from multiple-session studies were heterogeneous and showed no evidence of an effect. Most studies included in this meta-analysis had unclear risk of bias, especially with respect to participant blinding, and sham treatments did not mimic stimulus sensation on the scalp.30 The positive effects seen in the previous studies may result from a function of bias.
Our previous multicenter RCT, which implemented similar trial methods (including the sham stimulation) and was used for the sample size calculation for this trial, yielded a positive effect.16 The mean short-term VAS decrease rate in the active stimulation group was approximately the same for both our trials (6.3% in this trial vs 6.5% in the previous one). Nevertheless, the results of this trial suggest that rTMS of M1 was ineffective for the whole population with various types of NP. One possible reason why we were unable to demonstrate the same results as the pilot study could be the larger improvement in the sham group compared with that of the pilot study. An improvement in a placebo group reflects a placebo effect and other factors, such as natural history, regression to the mean, and a positive psychosocial impact to being enrolled in a trial.39 The present trial was designed to obtain regulatory approval, and the study design was so strict that the improvements in the sham could have been amplified by increased patient expectations, the formal conduct of the trial, and the increased interactions with health care providers and trial staff (eg, frequent and courteous dealings of clinical research coordinators who were not involved in the pilot studies). Moreover, patients with mild pain (VAS of <40 mm),8 who tend to have a high and variable placebo response,9 were included in this trial.
There are several potential reasons for the modest pain relief observed after active stimulation in this trial. First, it could be the result of the suboptimal stimulus procedure. The 5 Hz frequency and 500 pulses/session used in this trial were relatively low and small, respectively, compared with the 10 to 20 Hz and 2000 to 3000 pulses/session used in recent studies that reported positive results.2,3,17,29,30 Although the most optimal stimulus frequency and number of pulses per session have not yet been established, it is likely that 10 to 20 Hz and 2000 to 3000 pulses/session is more efficacious. A rigorous trial with these higher doses should be conducted. Second, stimulation of the M1 foot area seemed to have a lesser effect on pain than that of the M1 hand area. Our pilot trial also reported similar results.16 The M1 foot area is located in a deep region of the brain that is difficult to stimulate. Actually, patients with lower limb pain often have a high RMT,35 and these kinds of patients could not receive rTMS with a sufficient intensity because of the maximum limit of rTMS intensity in this trial. In line with this anatomical issue, deep rTMS with an H-coil provided better pain relief for lower-limb pain compared to rTMS with a figure-8-coil.38 Various sites of the M1 were stimulated in the previous studies, and more than half of these studies adopted the M1 hand area for pain outside the hand.1–3,16,23,29 The M1 hand area may be the optimal stimulation target within the M1 for even face and lower-limb pain. The stimulus protocol, including a stimulus frequency, number of pulses, site, and intensity, should be adjusted in future trials to improve the efficacy of rTMS. Third, a recent increase in the availability of NP medications in Japan (pregabalin, duloxetine, and tramadol) could have altered the patient background and influenced the results of this trial. Taken together, the findings of this trial could be affected by these artefacts and bias. More suitable patient groups and evaluation methods should be considered for future studies.
Few previous RCTs investigated an effect on pain relief by long-term administration of rTMS that lasted up to several weeks in patients with NP. An open-label study of weekly stimulation for 3 months to 1 year reported continuous pain relief in patients with central poststroke pain during the study period.19 A prospective observational study of multiple rTMS sessions each separated by several weeks reported cumulative pain relief in patients with central NP for more than 1 year. They selected responders by a series of 4 sessions separated from each other by a 3- to 4-week interval. Then, rTMS sessions were repeated in responders with intervals between sessions that were adapted to the patient and to the duration of the analgesic effect.31,32 Interestingly, the infrequent sessions seemed to be enough to make cumulative pain relief and to select responders in the early phase of the long-term rTMS. Furthermore, the same group presented the higher percentage of pain relief after the 4 active rTMS sessions separated by 2 to 3 weeks compared with sham in a randomized crossover trial .33 These long-lasting and cumulative effects are thought to result from cortical excitability changes that were reported to reflect long-term potentiation of excitatory synapses and long-term depression of inhibitory synaptic strength in some animal studies.24 A longer period of interleaved rTMS may improve the efficacy. In line with these previous trials, the results of our open-label continuous trial suggested that patients with long-term rTMS could benefit from an additional weekly session. A weekly or monthly maintenance administration after selection of responder could be a promising protocol for the clinical use of rTMS.
Minor or transient side effects were reported to be associated with active rTMS and sham stimulation in previous studies on chronic pain. In line with the previous trials, no severe adverse events were induced with either the 5 daily or 4 weekly rTMS interventions in this trial. The noninvasive property of rTMS is one of its major clinical advantages.
4.1. Limitations
This trial has several limitations. First, we cannot draw strong conclusions from the results of the post hoc analysis and subgroup analysis. Second, we recruited patients with various types of pharmacoresistant NP; therefore, we cannot generalize the noneffectiveness of rTMS to a specific NP patient population. Third, there might be a positive effect of electrical stimulation of the sham. We set the intensity of electrical stimulation to double of the sensory threshold, and a mean intensity of actual delivered electrical stimulations was 7.8 (SD, 3.4) mA, which was much higher than 2 mA often used for transcranial direct current stimulation.22
5. Conclusions
In conclusion, 5 daily sessions of rTMS with 500 pulses/session at 5 Hz were ineffective in short-term pain relief than sham stimulations in the whole study population with various types of NP. Responders to daily active rTMS could benefit from additional weekly sessions. The findings of this study suggest the importance of further optimization of enrolled subjects, trial protocol, and stimulus procedure in future trials to determine whether rTMS of M1 for NP could be an effective treatment. Long-term administration to the responders should be also investigated for the clinical use of rTMS on NP in the future studies.
Conflict of interest statement
K. Hosomi, K. Sugiyama, Y. Nakamura, T. Shimokawa, and Y. Saitoh had grants from AMED during the conduct of the study. Y. Saitoh received consultancy fees from Teijin Pharma Limited during the conduct of the study. Y. Saitoh has an issued patent about TMS coils. K. Hosomi, Y. Goto, T. Mano, T. Shimizu, and Y. Saitoh belong to the Department of Neuromodulation and Neurosurgery, Osaka University Graduate School of Medicine, which is a joint research department established with sponsorship by Teijin Pharma Limited. The remaining authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A885.
Supplementary Material
Acknowledgements
TEN-P11-01 investigators: Osaka University Hospital: Y. Saitoh (chair), S. Oshino (local principal investigator), K. Hosomi (co-principal investigator), Haruhiko Kishima, Masayuki Hirata, Naoki Kagawa, Hajime Nakamura, Yuichiro Ohnishi, T. Yanagisawa, Hideyuki Arita, Koshi Ninomiya, Kohtaroh Edakawa, Tomohiko Ozaki, Y. Goto, Katsunori Asai, Maki Kobayashi, Koji Takano, Shogo Fukuya, Yoshinori Kadono, Daisuke Eino, Masataka Tanaka, Tomoaki Murakami, T. Shimizu, Takanori Fukunaga, Fumiaki Yoshida, Hiroaki Hashimoto, Takeo Nishida, Hideki Mochizuki, Masahito Mihara, Kuni Konaka, Masahiko Shibata, Hironobu Uematsu, Hiroyuki Tanaka, Takahiro Makino, Sho Fujiwara, Ryohei Takaha, Chisato Yokota, Tomoo Mano, Tomoki Kidani, Takamune Achiha, Shota Yamamoto, and Takashi Moriwaki.
Hamamatsu University Hospital: K. Sugiyama (local principal investigator), Hiroki Namba, Tsutomu Tokuyama, Takao Nozaki, Hiroshi Kawaji, Tomohiro Yamasaki, Shinichiro Koizumi, Takashi Mizushima, Katsuya Yamauchi, Tetsuyuki Nagafusa, Makoto Hasui, Nao Takahashi, Chisato Yasuda, Machiko Sakai, and Kouji Watanabe.
Kindai University Sakai Hospital (closed at the end of March 2018): Yusaku Nakamura (local principal investigator), Makito Hirano, Hikaru Sakamoto, Shuichi Ueno, Yukihiro Hamada, Tomoaki Higashizawa, Takuya Uchiyama, Masaharu Miyauchi, Yasuhiro Ohno, Mika Fujimoto, Youichi Tanaka, Koji Kinoshita, Taeko Yumoto, Hideki Shimadzu, Ryuuta Haraguchi, Sho Saeki, and Hiromasa Yoshioka.
Wakayama Medical University: Toshio Shimokawa (lead statistician).
Additional Contributions: The authors thank the participating patients, clinical trial assistance teams at the Department of Medical Innovation, Osaka University Hospital, and the Center for Clinical Research, Hamamatsu University Hospital, and administrative and clinical staff at all trial sites.
Sakai Hospital Kindai University was closed at the end of March 2018, which was denoted at the affiliation for the author Y. Nakamura
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Members are listed in the Acknowledgment section.
References
- [1].Andre-Obadia N, Magnin M, Simon E, Garcia-Larrea L. Somatotopic effects of rTMS in neuropathic pain? A comparison between stimulation over hand and face motor areas. Eur J Pain 2018;22:707–15. [DOI] [PubMed] [Google Scholar]
- [2].Attal N, Ayache SS, Ciampi De Andrade D, Mhalla A, Baudic S, Jazat F, Ahdab R, Neves DO, Sorel M, Lefaucheur JP, Bouhassira D. Repetitive transcranial magnetic stimulation and transcranial direct-current stimulation in neuropathic pain due to radiculopathy: a randomized sham-controlled comparative study. PAIN 2016;157:1224–31. [DOI] [PubMed] [Google Scholar]
- [3].Ayache SS, Ahdab R, Chalah MA, Farhat WH, Mylius V, Goujon C, Sorel M, Lefaucheur JP. Analgesic effects of navigated motor cortex rTMS in patients with chronic neuropathic pain. Eur J Pain 2016;20:1413–22. [DOI] [PubMed] [Google Scholar]
- [4].Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–19. [DOI] [PubMed] [Google Scholar]
- [5].Cruccu G, Aziz TZ, Garcia-Larrea L, Hansson P, Jensen TS, Lefaucheur JP, Simpson BA, Taylor RS. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol 2007;14:952–70. [DOI] [PubMed] [Google Scholar]
- [6].Cruccu G, Garcia-Larrea L, Hansson P, Keindl M, Lefaucheur JP, Paulus W, Taylor R, Tronnier V, Truini A, Attal N. EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur J Neurol 2016;23:1489–99. [DOI] [PubMed] [Google Scholar]
- [7].Dworkin RH, O'Connor AB, Kent J, Mackey SC, Raja SN, Stacey BR, Levy RM, Backonja M, Baron R, Harke H, Loeser JD, Treede RD, Turk DC, Wells CD. Interventional management of neuropathic pain: NeuPSIG recommendations. PAIN 2013;154:2249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105–21. [DOI] [PubMed] [Google Scholar]
- [9].European Medicines Agency. Guideline on the clinical development of medicinal products intended for the treatment of pain. London, United Kingdom: European Medicines Agency, 2016. [Google Scholar]
- [10].EuroQol G. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- [11].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hamada M, Ugawa Y, Tsuji S. High-frequency rTMS over the supplementary motor area for treatment of Parkinson's disease. Mov Disord 2008;23:1524–31. [DOI] [PubMed] [Google Scholar]
- [13].Hanajima R, Wang R, Nakatani-Enomoto S, Hamada M, Terao Y, Furubayashi T, Okabe S, Inomata-Terada S, Yugeta A, Rothwell JC, Ugawa Y. Comparison of different methods for estimating motor threshold with transcranial magnetic stimulation. Clin Neurophysiol 2007;118:2120–2. [DOI] [PubMed] [Google Scholar]
- [14].Hirayama A, Saitoh Y, Kishima H, Shimokawa T, Oshino S, Hirata M, Kato A, Yoshimine T. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. PAIN 2006;122:22–7. [DOI] [PubMed] [Google Scholar]
- [15].Hosomi K, Seymour B, Saitoh Y. Modulating the pain network—neurostimulation for central poststroke pain. Nat Rev Neurol 2015;11:290–9. [DOI] [PubMed] [Google Scholar]
- [16].Hosomi K, Shimokawa T, Ikoma K, Nakamura Y, Sugiyama K, Ugawa Y, Uozumi T, Yamamoto T, Saitoh Y. Daily repetitive transcranial magnetic stimulation of primary motor cortex for neuropathic pain: a randomized, multicenter, double-blind, crossover, sham-controlled trial. PAIN 2013;154:1065–72. [DOI] [PubMed] [Google Scholar]
- [17].Khedr EM, Kotb HI, Mostafa MG, Mohamad MF, Amr SA, Ahmed MA, Karim AA, Kamal SM. Repetitive transcranial magnetic stimulation in neuropathic pain secondary to malignancy: a randomized clinical trial. Eur J Pain 2015;19:519–27. [DOI] [PubMed] [Google Scholar]
- [18].Klein MM, Treister R, Raij T, Pascual-Leone A, Park L, Nurmikko T, Lenz F, Lefaucheur JP, Lang M, Hallett M, Fox M, Cudkowicz M, Costello A, Carr DB, Ayache SS, Oaklander AL. Transcranial magnetic stimulation of the brain: guidelines for pain treatment research. PAIN 2015;156:1601–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kobayashi M, Fujimaki T, Mihara B, Ohira T. Repetitive transcranial magnetic stimulation once a week induces sustainable long-term relief of central poststroke pain. Neuromodulation 2015;18:249–54. [DOI] [PubMed] [Google Scholar]
- [20].Lefaucheur JP. The use of repetitive transcranial magnetic stimulation (rTMS) in chronic neuropathic pain. Neurophysiol Clin 2006;36:117–24. [DOI] [PubMed] [Google Scholar]
- [21].Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipovic SR, Hummel FC, Jaaskelainen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini PM, Rothwell JC, Schonfeldt-Lecuona C, Siebner HR, Slotema CW, Stagg CJ, Valls-Sole J, Ziemann U, Paulus W, Garcia-Larrea L. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014;125:2150–206. [DOI] [PubMed] [Google Scholar]
- [22].Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B, Marangolo P, Mylius V, Nitsche MA, Padberg F, Palm U, Poulet E, Priori A, Rossi S, Schecklmann M, Vanneste S, Ziemann U, Garcia-Larrea L, Paulus W. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 2017;128:56–92. [DOI] [PubMed] [Google Scholar]
- [23].Lefaucheur JP, Hatem S, Nineb A, Menard-Lefaucheur I, Wendling S, Keravel Y, Nguyen JP. Somatotopic organization of the analgesic effects of motor cortex rTMS in neuropathic pain. Neurology 2006;67:1998–2004. [DOI] [PubMed] [Google Scholar]
- [24].Lenz M, Vlachos A. Releasing the cortical brake by non-invasive electromagnetic stimulation? rTMS induces LTD of GABAergic neurotransmission. Front Neural Circuits 2016;10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Leung A, Donohue M, Xu R, Lee R, Lefaucheur JP, Khedr EM, Saitoh Y, Andre-Obadia N, Rollnik J, Wallace M, Chen R. rTMS for suppressing neuropathic pain: a meta-analysis. J Pain 2009;10:1205–16. [DOI] [PubMed] [Google Scholar]
- [26].Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology 2008;70:2329–37. [DOI] [PubMed] [Google Scholar]
- [27].Loeser JD, Treede RD. The kyoto protocol of IASP basic pain terminology. PAIN 2008;137:473–7. [DOI] [PubMed] [Google Scholar]
- [28].Maruo T, Nakae A, Maeda L, Shi K, Takahashi K, Morris S, Hosomi K, Kanatani H, Matsuzaki T, Saitoh Y. Validity, reliability, and assessment sensitivity of the Japanese version of the short-form McGill pain questionnaire 2 in Japanese patients with neuropathic and non-neuropathic pain. Pain Med 2014;15:1930–7. [DOI] [PubMed] [Google Scholar]
- [29].Nurmikko T, MacIver K, Bresnahan R, Hird E, Nelson A, Sacco P. Motor cortex reorganization and repetitive transcranial magnetic stimulation for pain: a methodological study. Neuromodulation 2016;19:669–78. [DOI] [PubMed] [Google Scholar]
- [30].O'Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev 2018;4:CD008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pommier B, Creac'h C, Beauvieux V, Nuti C, Vassal F, Peyron R. Robot-guided neuronavigated rTMS as an alternative therapy for central (neuropathic) pain: clinical experience and long-term follow-up. Eur J Pain 2016;20:907–16. [DOI] [PubMed] [Google Scholar]
- [32].Quesada C, Pommier B, Fauchon C, Bradley C, Creac'h C, Vassal F, Peyron R. Robot-Guided neuronavigated repetitive transcranial magnetic stimulation (rTMS) in central neuropathic pain. Arch Phys Med Rehabil 2018;99:2203.e2201–15.e2201. [DOI] [PubMed] [Google Scholar]
- [33].Quesada C, Pommier B, Fauchon C, Créaćh C, Vassal F, Peyron R. Neuronavigated 20 Hz rTMS efficiency in patients with central pain. A placebo controlled randomized cross-over study. Proceedings, 17th World Congress on Pain, Boston, September 14, 2018. [Google Scholar]
- [34].Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120:2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application: an updated report from an I.F.C.N. Committee. Clin Neurophysiol 2015;126:1071–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Saitoh Y, Hirayama A, Kishima H, Shimokawa T, Oshino S, Hirata M, Tani N, Kato A, Yoshimine T. Reduction of intractable deafferentation pain due to spinal cord or peripheral lesion by high-frequency repetitive transcranial magnetic stimulation of the primary motor cortex. J Neurosurg 2007;107:555–9. [DOI] [PubMed] [Google Scholar]
- [37].Sekino M, Ohsaki H, Takiyama Y, Yamamoto K, Matsuzaki T, Yasumuro Y, Nishikawa A, Maruo T, Hosomi K, Saitoh Y. Eccentric figure-eight coils for transcranial magnetic stimulation. Bioelectromagnetics 2015;36:55–65. [DOI] [PubMed] [Google Scholar]
- [38].Shimizu T, Hosomi K, Maruo T, Goto Y, Yokoe M, Kageyama Y, Shimokawa T, Yoshimine T, Saitoh Y. Efficacy of deep rTMS for neuropathic pain in the lower limb: a randomized, double-blind crossover trial of an H-coil and figure-8 coil. J Neurosurg 2017;127:1172–80. [DOI] [PubMed] [Google Scholar]
- [39].Wager TD, Fields HL. Placebo analgesia. In: McMahon SB, Koltzenburg M, Tracey I, Turk DC, editors. Wall and Melzack's textbook of pain. Philadelphia: Elsevier Saunders, 2013. pp. 362–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A885.