The comparison of low-frequency migraine with high-frequency migraine after nitroglycerin administration shows progression in the degree of derangement of spinal nociception processing.
Keywords: Headache, Migraine, Migraine frequency, Temporal summation threshold, Nociceptive withdrawal reflex, Central sensitization, Nitroglycerin
Abstract
The nitric-oxide donor nitroglycerin (NTG) administration induces a facilitation of nociceptive pathways in episodic migraine. This study aims to test the hypothesis that induced spinal sensitization could be more pronounced in patients affected by high-frequency migraine (HF-MIG) with respect to low-frequency migraine (LF-MIG). We enrolled 28 patients with LF-MIG (1-5 migraine days/month), 19 patients with HF-MIG (6-14 migraine days/month), and 21 healthy controls (HCs). Spinal sensitization was evaluated with the neurophysiological recording of the temporal summation threshold (TST) of the nociceptive withdrawal reflex at the lower limb. Temporal summation threshold was recorded at baseline and 30, 60, and 120 minutes after NTG administration (0.9 mg sublingual). Spinal sensitization was detected in LF-MIG at 60 (P = 0.010) and 120 minutes (P = 0.001) and in HF-MIG at 30 (P = 0.008), 60 (P = 0.001), and 120 minutes (P = 0.001) after NTG administration. Temporal summation threshold did not change in HC (P = 0.899). Moreover, TST reduction was more pronounced in HF-MIG with respect to LF-MIG (P = 0.002). The percentage of patients who developed a migraine-like headache after NTG was comparable in the 2 migraine groups (LF-MIG: 53.6%, HF-MIG: 52.6%, P = 0.284), whereas no subjects in the HC group developed a delayed-specific headache. Notably, the latency of headache onset was significantly shorter in the HF-MIG group when compared with the LF-MIG group (P = 0.015). Our data demonstrate a direct relationship between migraine frequency and both neurophysiological and clinical parameters, to suggest an increasing derangement of the nociceptive system control as the disease progresses, probably as a result of the interaction of genetic and environmental factors.
1. Introduction
Systemic administration of the nitric oxide donor nitroglycerin (NTG) is a well-established human experimental model of migraine because it triggers a specific migraine-like headache in 50% to 80% of migraine sufferers, but not in healthy controls (HCs).23 NTG-induced migraine-like headache manifests with a latency of hours after NTG administration and bears clinical and instrumental features that make it undistinguishable from spontaneous migraine attacks: premonitory symptoms, pain-phase-associated symptoms, including allodynia,3,7,11,16,25,29,33 and neuroimaging changes in the very same brain areas that are affected during spontaneous attacks.1,38
A large amount of evidence shows that NTG is also a reliable animal model of migraine. In rodents, NTG activates brain areas that are involved in the transmission and integration of the cephalic pain40 and sensitizes trigeminal3 and spinal second-order neurons, including the wide-dynamic-range (WDR) neurons, which play a pivotal role in physiologic and pathologic nociception.24 Overall, these data strongly support the neurobiological changes induced by NTG as a translational model of migraine to study its neural basis in preclinical and clinical settings.16
Migraine pain is believed to result from abnormal activation and sensitization2,10,21,28 of trigeminal primary afferents innervating dural vasculature and their central projections to the medullary dorsal horn and upper cervical spinal cord, in which WDR neurons are highly represented. The temporal summation threshold (TST) of the nociceptive withdrawal reflex (NWR) at the lower limb represents a robust and sensitive method for the evaluation in humans of the sensitization of spinal nociceptive pathways because it reflects the functional activity of the spinal WDR neurons.4,13,32,36 In a previous study, using this neurophysiological approach, we reported that NTG induces spinal sensitization in low-frequency migraineurs.32 This phenomenon was detected as an early facilitation of the nociceptive spinal temporal processing and it was more pronounced in the subgroup of subjects who developed the migraine-like NTG-induced headache.
In the effort to clarify from a clinical point of view whether the migraine-inducing effect of NTG depended on the frequency of spontaneous migraine attacks, Christiansen et al.15 failed to detect a significant association between NTG-induced headache and migraine frequency, although they noted that the high-frequency group was more prone to develop migraine with respect to the subgroup with infrequent migraine attacks.
In this study, our aim was to investigate the effects of NTG administration on the TST in migraineurs with low or high frequency of attacks to test the hypothesis that spinal sensitization may be more pronounced in patients with a high frequency with respect to those with a low frequency.
2. Materials and Methods
2.1. Subjects
We enrolled 48 subjects suffering from migraine who attended the outpatient clinics of the Headache Science Centre of the IRCCS Mondino Foundation (Pavia, Italy). One patient (low-frequency migraine, female, 32 years old) dropped out because of symptomatic hypotension immediately after NTG administration; therefore, the full data set is formed by 47 patients (37 females and 10 males; mean age 34.1 ± 8.0 years; range 18-55 years). All patients satisfied ICHD-3 criteria for episodic migraine without aura according (code 1.1).22 None of the enrolled subjects complained of interictal allodynia or hyperalgesia.
Inclusion criteria were: males or females aged 18 to 70 years, and migraine without aura for at least 1 year before enrollment. Exclusion criteria were: history of major psychiatric or other neurological conditions; history of chronic migraine; Beck's Depression Inventory score >17; clinically significant medical conditions; chronic pain conditions; alcohol and/or drugs abuse; pregnancy or lactation; previous exposure to NTG administration; and contraindications to NTG administration, namely systolic blood pressure <90 mm Hg, severe hypovolemia, anemia, angle-closure glaucoma, known allergy or intolerance to NTG, concomitant therapy with sildenafil.
All the patients were required to fill in a daily headache diary for at least 2 months before enrollment to reliably assess migraine frequency. Monotherapy with β-blockers, calcium channel blockers, or angiotensin II receptor antagonists was allowed as long as dose and regimen had been stable for at least 2 months before enrollment. Tricyclics, anticonvulsants, and selective serotonin reuptake inhibitors were not allowed because of their potential or known effect on pain threshold.36
According to the number of headache days per month, patients were divided into 2 groups: (1) low-frequency migraine (LF-MIG): from 1 to 5 migraine days/month (n = 28); and (2) moderate- to high-frequency migraine (HF-MIG): from 6 to 14 migraine days/month (n = 19).
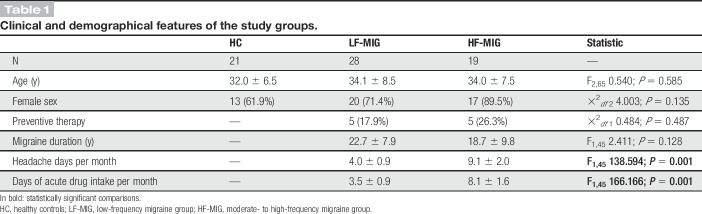
Twenty-one volunteers without personal or family history (first relatives) of neurological disorders (13 females and 8 males; mean age 32.0 ± 6.5 years; range 23-50 years) were enrolled in the study as HCs. The 3 study groups were comparable for sex and age. Migraine duration was 22.7 ± 7.9 years in the LF-MIG group and 18.7 ± 9.8 in the HF-MIG group (P = 0.128). The mean number of headache days per month was 4.0 ± 0.9 in the LF-MIG group and 9.1 ± 2.0 in the HF-MIG group (P = 0.001). Clinical and demographic features of the study groups are presented in Table 1.
Table 1.
Clinical and demographical features of the study groups.
2.2. Nociceptive withdrawal reflex measurements
The NWR was recorded from the right lower limb according to a standardized procedure.19,36 All the examinations started in the morning at 09:00 am in a dedicated room by the same technician (V.G.), who was blinded to the diagnosis of subjects. Subjects were tested while lying comfortably on their back in a standard posture with their ankle flexed at 90° and knee flexed at 130°. The electrical stimuli were delivered at the sural nerve behind the lateral malleolus with a pair of Ag/AgCl surface electrodes.
The electrical stimulation consisted in 5 squared consecutive pulses (1 ms, 200 Hz), randomly delivered every 30 to 60 seconds. The electromyographic response (Synergy, Medelec, United Kingdom) was recorded from the capitis brevis of the homolateral biceps femoris with a pair of Ag/AgCl surface electrodes. The threshold evaluation was achieved with a staircase method, where the intensity was gradually increased by 0.3-mA steps. The recording parameters were: analysis time 300 ms, sensitivity 20 mV, and filter bandpass 3 to 3000 Hz.
The single stimulus reflex threshold of the NWR (RTh) was considered the lowest intensity (mA) able to elicit 3 consecutive stable muscular responses of at least 20 mV and 10 ms. At RTh, we recorded the average area under the curve (AUC − mV × ms) of the 3 muscular responses, and the subjective pain perception on a 0 to 10 point visual analogue scale (VAS).
For the study of TST, we used a train of 5 electrical stimuli as described above at a frequency of 2 Hz. Temporal summation threshold was defined as the lowest intensity (mA) able to elicit 3 consecutive stable muscular responses of at least 20 mV and 10 ms in the fourth and fifth sweeps. At TST, we asked subjects to rate the subjective pain perception of the first (VAS-I) and fifth (VAS-V) stimulus on a 0 to 10 point VAS.
2.3. Experimental procedures and nitroglycerin administration
Before the neurophysiological examination, a neurologist with expertise in the headache field performed a full examination and revised the headache diary to confirm inclusion/exclusion criteria. The neurologist also verified that the patients were headache-free and had been headache-free for the previous 24 hours. For all subjects, use of acute medications for headache in the previous 24 hours and coffee and tea in the morning of the neurophysiological evaluation was not allowed. For female subjects, the NWR recording was performed in the follicular phase of the menstrual cycle to reduce hormonal effect on pain thresholds.18
All subjects underwent a baseline neurophysiological recording that included both RTh and TST evaluation. They subsequently received NTG 0.9 mg sublingually (Acarpia Farmaceutici SRL, Italy), as described by Sances et al.33
Temporal summation threshold evaluation, as an indicator of spinal excitability, was then repeated 30, 60, and 120 minutes after NTG administration.
During the study procedures, the headache onset as well as associated symptoms (namely nausea, photophobia, and phonophobia) was continuously recorded. The headache intensity was rated by the patient on a 0 to 10 VAS.
Vital signs (blood pressure and heart rate in particular) were evaluated at baseline and then every 30 minutes in the absence of any adverse events.
Nitroglycerin induction test was considered “positive” (MIG+) for those subjects who developed a migraine-like attack according to the recently proposed criteria for experimentally induced headache: headache with onset 0 to 12 hours after NTG intake that fulfils ICHD-3 C and D criteria for migraine without aura or that mimics usual migraine attacks and responds to a triptan.6,22,39 In the absence of this response, NTG induction test was considered “negative” (MIG−).
At the end of the neurophysiological evaluation, subjects who developed headache were treated with acute medications (nonsteroidal anti-inflammatory drugs, triptans, and/or antiemetics) according to their needs and preferences. The day after the study procedures, all patients were contacted by phone to collect follow-up information regarding NTG-induced headache (duration, resolution, late onset, and adverse events).
The study was approved by the local ethics committee (14/int/2016), and all subjects signed a written informed consent after a clear and interactive explanation of the study by the investigator.
2.4. Statistical analysis
The sample size was calculated with the Open Source Epidemiologic Statistics for Public Health (www.openepi.com). The primary outcome of the study was to assess the difference in TST percentage modification at 120 minutes after NTG between LF-MIG and HF-MIG. According to previous reports, we considered meaningful a difference between groups of at least a 10% (standard deviation of 10%). To take into account multiple comparisons because of the presence of the HC group, we corrected the level of significance according to Bonferroni method to P = 0.016. Therefore, for the calculation of the sample size for an independent-samples test (t test or Kruskal–Wallis according to data distribution), we used the following parameters: confidence interval (2-sided): 98.4%; power: 80%; and ratio of sample size: 1. The minimum suggested sample size was 42.
For the statistical analysis, we used the Statistical Package for the Social Sciences (SPSS) for Windows, version 21.0. The Kolmogorov–Smirnov test confirmed a normal distribution of the data. Quantitative variables are presented as: mean ± SD. Univariate exploratory analysis was performed with a one-way analysis of variance (ANOVA), followed by a Bonferroni's correction for intergroup comparison and with t test for paired samples for intragroup comparison. The main study analysis was performed with a 2-factor ANOVA for repeated measures (factor “TIME” with 4 levels: baseline, 30, 60, and 120 minutes after NTG; factor “GROUP” with 3 levels: LF-MIG, HF-MIG, and HC), followed by a post hoc analysis with Bonferroni's correction. Subgroups analysis, in particular to assess intragroup differences between MIG+ and MIG− subjects, were performed with a 3-factor ANOVA for repeated measures (factor “TIME” with 4 levels: baseline, 30, 60, and 120 minutes after NTG; factor “GROUP” with 2 levels: LF-MIG and HF-MIG; factor HEADACHE with 2 levels: MIG+ and MIG−), followed by a post hoc analysis with Bonferroni's correction.
In all cases, additional analyses were performed only if the main factorial ANOVA was significant and, in particular, when a significant difference between factors interaction was found.
Qualitative variables were plotted in crossed tables and are presented as absolute values (percentage). Statistical association between variables was performed with Pearson χ2 test or Fisher exact test, where appropriate.
In migraine patients, correlations between percentage modification of TST and clinical–demographical variables were performed using the bivariate Pearson test.
The level of significance α was set at 0.05 (corrected for multiple comparisons if necessary).
3. Results
3.1. Clinical response to NTG administration
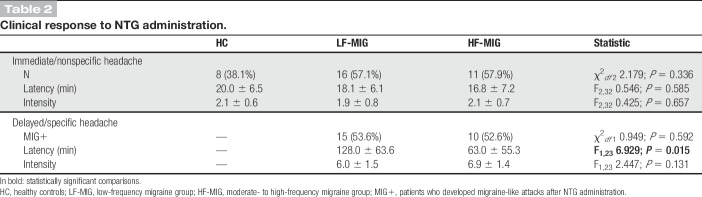
As expected, NTG induced 2 types of headache response: (1) an immediate, nonspecific, mild, and short-lasting headache and (2) a delayed migraine-like headache. The percentage of patients who developed headache (immediate/nonspecific, delayed/specific, or both) was lower in the HC groups (38.1%) with respect to migraine patients (LF-MIG 82.1%, HF-MIG 84.2%) (χ2df 2 13.721; P = 0.001). The proportion of subjects who developed the immediate/nonspecific headache after NTG administration was comparable in all study groups (HC 38.1%, LF-MIG 57.1%, HF-MIG 57.9%; χ2df 2 2.179; P = 0.336). Also, the latency of onset and the average intensity of this immediate/nonspecific headache were comparable among study groups (Table 2).
Table 2.
Clinical response to NTG administration.
No subjects in the HC group developed a headache that qualified positive for the NTG induction test. At variance, a positive provocative test (MIG+) was present in 15/28 (53.6%) patients in the LF-MIG group and in 10/19 (52.6%) patients in the HF-MIG group (P = 0.592). The intensity of the MIG+ response was comparable between LF-MIG and HF-MIG groups (P = 0.131), whereas the latency of onset was instead significantly shorter in the HF-MIG group when compared with the LF-MIG group (63.0 ± 55.3 and 128.0 ± 63.6 minutes, respectively; P = 0.015) (Table 2).
We did not find any significant difference in clinical and demographical features when comparing MIG+ (72.0% female; age 34.0 ± 7.6 years; migraine duration 21.6 ± 8.1 years; headache days per month 6.2 ± 2.9; days of drug intake per month 5.5 ± 2.6) and MIG- groups (86.4% female; age 34.1 ± 8.6 years; migraine duration 20.5 ± 9.8 years; headache days per month 5.9 ± 2.9; days of drug intake per month 5.3 ± 2.5) (P > 0.05 for all comparisons).
3.2. Baseline neurophysiological and psychophysical evaluation
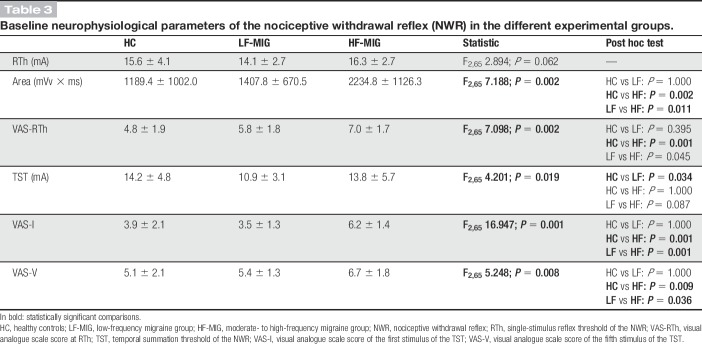
RTh was comparable in all groups (P = 0.062) (Table 3), although the AUC was significantly higher in the HF-MIG group with respect to LF-MIG (P = 0.011) and HC (P = 0.002) groups, and VAS score recorded at RTh was significantly higher in the HF-MIG group with respect to HCs (P = 0.001).
Table 3.
Baseline neurophysiological parameters of the nociceptive withdrawal reflex (NWR) in the different experimental groups.
Temporal summation threshold was significantly lower in the LF-MIG group with respect to HC (P = 0.034) (Table 3). The increase of VAS-V with respect to VAS-I was statistically significant in all groups. However, both VAS-I and VAS-V values were significantly higher in HF-MIG when compared with either LF-MIG (P = 0.036) or HC groups (P = 0.009). The neurophysiological parameters are summarized in Table 3.
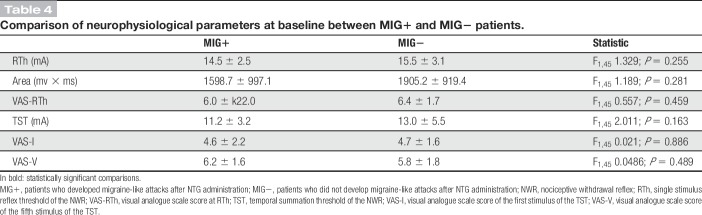
When comparing MIG+ to MIG− patients, we did not find any statistically significant difference in baseline neurophysiological parameters (Table 4).
Table 4.
Comparison of neurophysiological parameters at baseline between MIG+ and MIG− patients.
3.3. Neurophysiological modifications after nitroglycerin administration
Temporal summation threshold reduction after NTG administration was significant in the LF-MIG and HF-MIG groups with respect to the HC group (P = 0.007 and P = 0.001, respectively), being more pronounced in the HF-MIG group respect to the LF-MIG group (P = 0.002) (Fig. 1).
Figure 1.
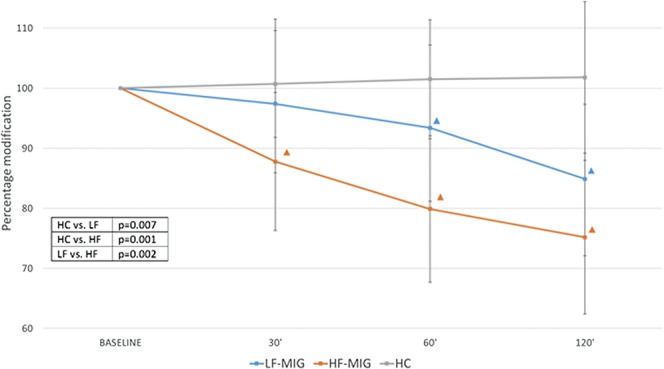
Percent change of the temporal summation threshold after NTG administration in the experimental groups. HC, healthy controls; LF-MIG, low-frequency migraine group; HF-MIG, moderate- to high-frequency migraine group. ▲ intragroup analysis: time point vs baseline P < 0.05. In table: ANOVA intergroup post hoc comparisons. ANOVA, analysis of variance.
The intragroup analysis demonstrated that the reduction in TST was significant at 60 and 120 minutes after NTG administration in the LF-MIG group (P = 0.010 and P = 0.001 vs baseline, respectively), whereas it was significant at 30, 60, and 120 minutes in the HF-MIG group (P = 0.008 for 30′, and P = 0.001 for 60′ and 120′) (Fig. 1). Nitroglycerin administration did not change TST values in the HC group.
When migraine-like headache was taken into account (factor HEADACHE aimed to assess differences between MIG+ and MIG− patients), we found a significant interaction GROUP × HEADACHE. To further investigate the meaning of the interaction GROUP × HEADACHE, a post hoc analysis was performed in the LF-MIG and HF-MIG groups separately. In the LF-MIG group, TST reduction was more pronounced in MIG+ patients with respect to MIG− patients at 120 minutes (P = 0.001). At variance, in the HF-MIG group, we did not find differences between MIG+ and MIG− patients (P = 0.285) (Fig. 2).
Figure 2.
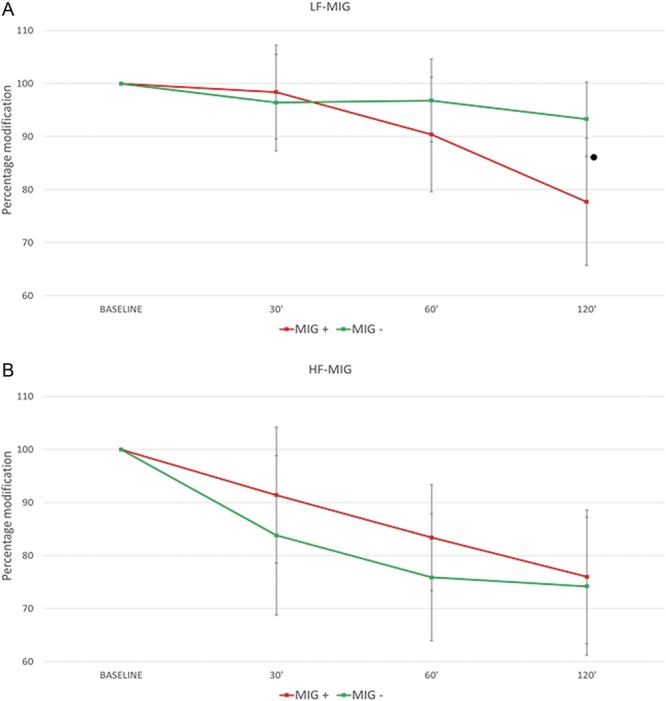
Percent change of the temporal summation threshold after NTG administration: comparison between MIG+ and MIG− patients. MIG+: patients who developed migraine-like attacks after NTG administration. MIG−: patients who did not develop migraine-like attacks after NTG administration. Panel A: MIG+ vs MIG− comparison in the LF-MIG group (low-frequency migraine). Panel B: MIG+ vs MIG− comparison in the HF-MIG group (moderate- to high-frequency migraine). ● MIG + vs MIG— P < 0.05.
3.4. Psychophysical modifications after nitroglycerin administration
Visual analogue scale-I was not significantly modified by NTG administration across time points (P = 0.150) in any of the experimental groups (P = 0.235) (Fig. 3A).
Figure 3.
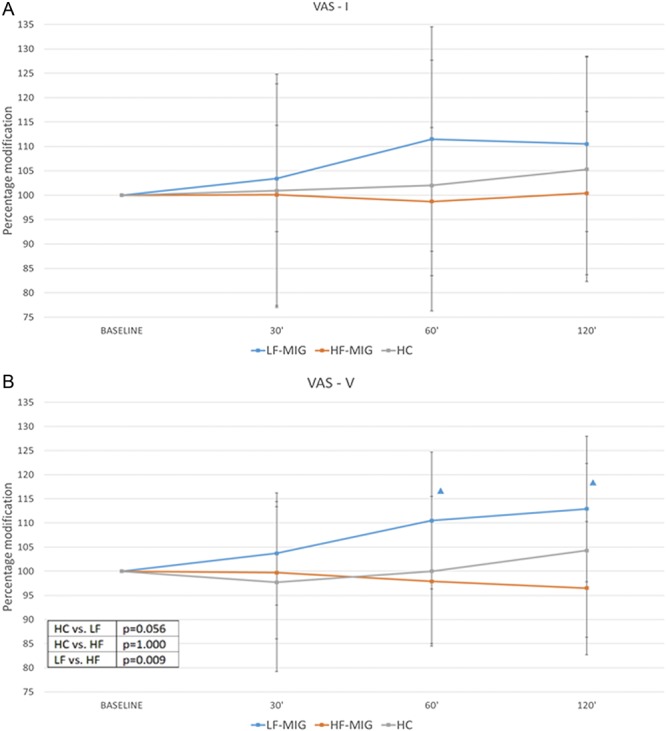
Percent change of VAS of temporal summation after NTG administration in the experimental groups. Panel A: VAS-I: visual analogue scale score recorded for the first stimulus of the temporal summation. Panel B: VAS-V: visual analogue scale score recorded for the fifth stimulus of the temporal summation. ▲ intragroup analysis: time point vs baseline P < 0.05. In table: ANOVA intergroup post hoc comparisons. ANOVA, analysis of variance; HC, healthy controls; HF-MIG, moderate- to high-frequency migraine group; LF-MIG, low-frequency migraine group.
Regarding VAS-V of temporal summation, we found a significant effect of factor GROUP (P = 0.006) and interaction TIME × GROUP (P = 0.024). The post hoc analysis showed that the percentage increase of VAS-V was significantly higher in the LF-MIG group with respect to the HF-MIG group (P = 0.009).
In the intragroup analysis, VAS-V was not significantly modified over time in the HF-MIG (P = 0.729) and HC groups (P = 0.483) groups. In the LF-MIG group, VAS-V score significantly increased over time, in particular at 60 and 120 minutes (P = 0.003 and P = 0.001, respectively) (Fig. 3B).
Finally, we did not find any significant differences in MIG+ patients when compared to MIG− patients for both VAS-I (P = 0.950) and VAS-V (P = 0.789).
3.5. Correlations between clinical–demographical and neurophysiological variables
We did not find significant correlations between the percentage reduction of TST at 30 minutes after NTG administration and clinical–demographical variables (namely age, sex, migraine duration, headache days per month, and days of drug intake per month).
The percentage reduction of TST at 60 and 120 minutes after NTG administration positively correlated with headache days per month (Pearson −0.491; P = 0.001 and Pearson −0.332; P = 0.023, respectively) and days of drug intake per month (Pearson −0.516; P = 0.001 and Pearson −0.333; P = 0.022, respectively) (Fig. 4), but not with age, sex, and migraine duration.
Figure 4.
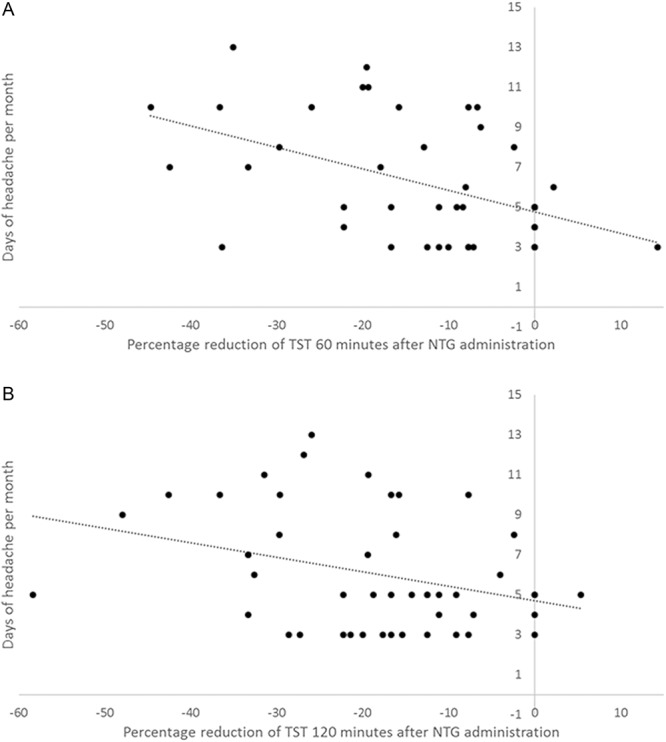
Correlation between percentage modification of TST after NTG administration and days of headache per month. TST, temporal summation threshold of the NWR. Dotted line represents linear regression. Panel A: correlation between percentage reduction of TST at 60 minutes after NTG administration and days of headache per month: Pearson −0.491; P = 0.001. Panel B: correlation between percentage reduction of TST at 120 minutes after NTG administration and days of headache per month: Pearson −0.332; P = 0.023. NWR, nociceptive withdrawal reflex.
4. Discussion
Our study confirms previous findings that showed a temporal facilitation of nociceptive pathways at spinal levels in LF-MIG subjects 120 minutes after oral exposure to NTG,32 a response that is specific for migraine patients because it was not present in HCs. Expanding on those findings, we show that there is a progression in the degree of derangement of nociception processing when comparing LF-MIG with HF-MIG. Indeed, we observed that TST reduction was detected as early as at 30 minutes after NTG administration in the HF-MIG group. At 60 and 120 minutes, both migraine groups experienced a significant reduction in TST, but still the entity of reduction was more pronounced in the HF-MIG group.
As a point of novelty, in this study, we describe a different behavior in the HF-MIG group: an earlier and more marked TST reduction after NTG administration both in MIG+ and MIG− patients, irrespective of the response to the provocative test. When considering the fact that NTG-induced migraine-like headache is the result of multiple events leading to a hyperalgesic condition,3,16,26 this finding is consistent with the occurrence in HF-MIG subjects of a spinal sensitization of the nociceptive pathways detectable with our neurophysiological approach, without the need of the prohyperalgesic steps leading to the migraine-like attack. Along this line, we speculate that the exposure to a higher number of migraine attacks per month in the HF-MIG group makes these subjects more prone to develop an early facilitation in the temporal processing of pain stimuli, irrespective of their NTG phenotype, at least from a neurophysiological point of view. Indeed, in terms of clinical response, the proportion of patients who developed a specific migraine-like headache was similar in LF-MIG and HF-MIG. It is, however, worth noting that HF-MIG subjects developed a MIG+ response with a shorter latency than the LF-MIG group. This could mean that although at the basis of the clinical response to NTG there is a specific trait of a subgroup of migraine sufferers, when the spinal sensitization is considered, NTG sensitivity clearly permits to discern HF-MIG from LF-MIG. We speculate that, as observed in other pain conditions, a stimulation overload (in our case a high frequency of migraine attacks) could drive some functional and structural changes in the excitability of spinal cord dorsal neurons, with an enhanced synaptic strength or prolonged alteration in the basic membrane potential that, in turn, leads to an earlier and more pronounced NTG effect. In this sense, the observed clinical (reduced latency of the delayed-specific headache) and neurophysiological (early reduction in TST of the NWR) temporal gradient of involvement from LF-MIG to HF-MIG could therefore represent an objective evidence of a prechronic condition. This result is reinforced by the detection of a significant correlation between the TST percent reduction observed 60 and 120 minutes after NTG and the number of headache days and days of drug intake per month in the overall migraine population.
This hypothesis is in line with the current theory that recognizes the increasing frequency of headache days, along with the increasing intake of acute medication, as the main risk factors for migraine progression and for migraine chronification.14 Specifically, it seems that patients with HF-MIG are 4 times more at risk of chronification as compared to LF-MIG, with a proposed cutoff of 5 or more headache days per month.8,9,37 The more pronounced temporal facilitation in pain processing observed in our group of patients with moderate to HF-MIG may represent one of the pathophysiological mechanisms underlying migraine progression.
Of note, distinctive neurophysiological traits were already evident at baseline in migraine patients: (1) the AUC at RTh (single stimuli recording methodology of NWR) was significantly higher in migraine patients, and more so in HF-MIG; (2) the psychophysical subjective pain perception at Rth and TST (either for the first stimulus and the fifth stimulus) was higher in HF-MIG patients; and (3) the TST was lower in migraine patients with respect to HC. Taken together, these findings suggest a propensity of migraine patients towards an interictal spinal sensitization, leading to a facilitation of related pain perception.
Our present findings highlight an apparent discrepancy of pattern at baseline, when they show that the TST threshold was significantly reduced in the LF-MIG group, but not in the HF-MIG group, whereas the psychophysical subjective pain perception was higher in both HF-MIG and LF-MIG groups when compared to HC; however, the difference reached a statistically significant level only in the HF-MIG group. When considering our previous data on chronic migraine, we would have expected a significantly lower TST of the NWR also in the HF-MIG group.30,31,34–36 We could attribute these discrepancies to the intrinsic degree of variability of subjective psychophysical measures of pain such as VAS on one side and to an intersubjective variability of endogenous control of pain between low frequency, prechronic, and chronic migraine subjects on the other. We speculate that in high-frequency/prechronic migraine, the temporal processing of pain, which reflects the functional activity of the spinal WDR, could be less homogenous when compared with both low-frequency and chronic migraine, thus yielding the conflicting results observed. It is, however, worth noting that this study was not powered to test the hypothesis that HF-MIG patients presented lower TST when compared with a LF-MIG and to HCs. Furthermore, a single-session NWR neurophysiological evaluation, performed interictally, may not be sensitive enough to distinguish different phenotypes across episodic migraine. This hypothesis supports once more the importance of experimental and translational models of migraine because these are able to explore and unveil important features not otherwise detectable.
Along with the neurophysiological evidence of an enhanced spinal sensitization in both HF-MIG and LF-MIG after NTG administration, patients reported higher psychophysical perception of pain only in the LF-MIG group. It is noteworthy that the HF-MIG group of patients reported significant higher values of subjective pain perception already at baseline. We think that in HF-MIG patients, the sensitization in central nervous system leads to an altered psychophysical pain perception already in the interictal phase. In this altered situation, spinal nociceptive processing is still facilitated by NTG administration, but the patients may not report higher VAS values because of a possible “ceiling-like” effect. In other words, subjective psychophysical pain responses such as VAS are in nature maximal and poorly sensitive to changes when the nociceptive system is sensitized. This could explain the scarce difference we observed in psychophysical measures of pain before and after NTG administration in the HF-MIG group.
From a clinical point of view, our results are in line with previous data from literature, with about 80% of migraine patients developing headache (immediate/nonspecific, delayed/specific, or both) after NTG exposure.33 Although comparable with our previous experience,32 the percentage of patients with a positive provocative test was 53.6% in LF-MIG and 52.6% in HF-MIG, slightly less than expected.7,29,33 This discrepancy is likely related to the new definition of the response to the NTG provocative test according to the most recent version of diagnostic criteria for migraine-like attacks in experimental studies,6 which are more restrictive as compared to our previous observation.33 Indeed, when we calculated the positive responses to NTG provocative test according to the old criteria, the percentage of positive responses raised to 71.4% for LF-MIG and 73.7% for HF-MIG.
In agreement with Christiansen et al.,15 the percentage of patients who developed a migraine-like attack after NTG was comparable in the LF-MIG group with respect to the HF-MIG group.
In our study, we found a significant shorter latency of migraine-like attack onset in HF-MIG when compared with LF-MIG (63.0 ± 55.3 and 128.0 ± 63.6 minutes respectively; P = 0.015). This clinical feature reinforces our neurophysiological findings pointing towards a more pronounced NTG sensitivity as the headache frequency increases. This shorter latency is not entirely new, as it was previously reported.29 In addition, it is similar to the latency reported for cluster headache patients evaluated in their active phases.33 Thus, it is possible that the shorter latency may represent a distinctive feature of primary headaches in their more active/aggressive phases.
5. Limitations of the study
To fully interpret the results, some limitations must be acknowledged. First, we lack a placebo control for the test. This choice was dictated by the need to preserve study feasibility, when considering the fact that NTG-induced headache and the associated serial neurophysiological evaluations may have a relevant burden on patients and be time-consuming for the physician, respectively. In addition, we had previously demonstrated the activity of NTG against placebo in the induction of the MIG+ response in migraineurs.25 For this reason, we decided to adopt a control group formed by HCs, rather than exposing migraine patients to a double session with placebo and NTG. Another factor that may have theoretically influenced our results is the small percentage of patients who were on a stable preventive therapy. It must be, however, noted that the preventive drugs allowed had not shown in the past competing interaction with the NTG provocative test.36,41 Furthermore, we considered unethical to stop or to avoid a preventive therapy in patients with high disability correlated to migraine.20 Finally, preclinical reports suggest that repeated exposure to acute antimigraine drugs may be associated with sensitization phenomena and our HF-MIG subjects were taking acute medications more frequently than the LF-MIG group.12,17,27 Presently, we cannot exclude with certainty a possible role of acute medications because all our HF-MIG subjects were using acute drugs with a frequency very similar to their migraine days. Future, specifically targeted studies are needed to separate the role of acute medications intake from the role of migraine frequency.
6. Conclusions
So far, NTG-provoked headache has been considered as a distinctive, mainly genetic, trait of migraine patients. This hypothesis is supported by the demonstration of the poor provoking potency of NTG in nonvascular primary headaches5,16,33 and by the lack of data about the association between headache severity and NTG response.15 Our data demonstrate for the first time a clear relationship between migraine frequency, and both neurophysiological (spinal sensitization) and clinical (latency of onset) parameters. It is conceivable that the genetic predisposition of migraine patients to NTG plays a major role, on which environmental factors may act as positive or negative modulators. Negative environmental factors, namely the increase in the number of headache attacks, but probably a lot more (stress, depression, high use of symptomatic drugs, and so on), could also force those migraine patients without an intrinsic additional sensitivity to nitric oxide32 towards a similar phenotype.
Conflict of interest statement
C. Tassorelli received honoraria for the participation in advisory boards or for oral presentations from: Allergan, ElectroCore, Eli-Lilly, Novartis, and Teva. C. Tassorelli has no ownership interest and does not own stocks of any pharmaceutical company. C. Tassorelli serves as Chief Section Editor of Frontiers in Neurology—Section Headache Medicine and Facial Pain and on the editorial board of The Journal of Headache and Pain. The remaining authors have no conflicts of interest to declare.
Acknowledgements
The authors thank the Research Nurse Team for their precious assistance in all of the activities of the Headache Science Centre of the IRCCS Mondino Foundation.
This study was funded by the Ministry of Health (RF-2013-02355704).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RSJ, Goadsby PJ. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 2005;128:932–9. [DOI] [PubMed] [Google Scholar]
- [2].Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 2011;12:570–84. [DOI] [PubMed] [Google Scholar]
- [3].Akerman S, Karsan N, Bose P, Hoffmann J, Holland PR, Romero-Reyes M, Goadsby PJ. Nitroglyceryn triggers triptan-responsive cranial allodynia and trigeminal neuronal hypersensitivity. Brain 2019;42:103–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Arendt-Nielsen L, Brennum J, Sindrup S, Bak P. Physiology and Occupational Physiology Electrophysiological and psychophysical quantification of temporal summation in the human nociceptive system. Eur J Appl Physiol 1994;68:266–73. [DOI] [PubMed] [Google Scholar]
- [5].Ashina M, Bendtsen L, Jensen R, Olesen J. Nitric oxide-induced headache in patients with chronic tension-type headache. Brain 2000;123:1830–7. [DOI] [PubMed] [Google Scholar]
- [6].Ashina M, Hansen JM, Á Dunga BO, Olesen J. Human models of migraine-short-Term pain for long-Term gain. Nat Rev Neurol 2017;13:713–24. [DOI] [PubMed] [Google Scholar]
- [7].Ashina M, Hansen JM, Olesen J. Pearls and pitfalls in human pharmacological models of migraine: 30 Years' experience. Cephalalgia 2013;33:540–53. [DOI] [PubMed] [Google Scholar]
- [8].Ashina S, Serrano D, Lipton RB, Maizels M, Manack AN, Turkel CC, Reed ML, Buse DC. Depression and risk of transformation of episodic to chronic migraine. J Headache Pain 2012;13:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache 2008;48:1157–68. [DOI] [PubMed] [Google Scholar]
- [10].Burstein R. Deconstructing migraine headache into peripheral and central sensitization. PAIN 2001;89:107–10. [DOI] [PubMed] [Google Scholar]
- [11].Burstein R. The development of cutaneous allodynia during a migraine attack Clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 2000;123:1703–9. [DOI] [PubMed] [Google Scholar]
- [12].Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol 2004;55:19–26. [DOI] [PubMed] [Google Scholar]
- [13].Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 2010;68:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Buse DC, Greisman JD, Baigi K, Lipton RB. Migraine progression: a systematic review. Headache 2019;59:306–38. [DOI] [PubMed] [Google Scholar]
- [15].Christiansen I, Daugaard D, Thomsen LL, Olesen J. Glyceryl trinitrate induced headache in migraineurs—relation to attack frequency. Eur J Neurol 2000;7:405–11. [DOI] [PubMed] [Google Scholar]
- [16].Demartini C, Greco R, Zanaboni AM, Sances G, De Icco R, Borsook D, Tassorelli C. Nitroglycerin as a comparative experimental model of migraine pain: from animal to human and back. Prog Neurobiol 2019;177:15–32. [DOI] [PubMed] [Google Scholar]
- [17].De Felice M, Ossipov MH, Wang R, Lai J, Chichorro J, Meng I, Dodick DW, Vanderah TW, Dussor G, Porreca F. Triptan-induced latent sensitization a possible basis for medication overuse headache. Ann Neurol 2010;67:325–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].De Icco R, Cucinella L, De Paoli I, Martella S, Sances G, Bitetto V, Sandrini G, Nappi G, Tassorelli C, Nappi RE. Modulation of nociceptive threshold by combined hormonal contraceptives in women with oestrogen-withdrawal migraine attacks: a pilot study. J Headache Pain 2016;17:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].De Icco R, Martinelli D, Bitetto V, Fresia M, Liebler E, Sandrini G, Tassorelli C. Peripheral vagal nerve stimulation modulates the nociceptive withdrawal reflex in healthy subjects: a randomized, cross-over, sham-controlled study. Cephalalgia 2018;38:1658–64. [DOI] [PubMed] [Google Scholar]
- [20].GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:459–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017;97:553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Headache Classification Committee of the International Headache Society (IHS). Headache classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- [23].Iversen HK, Olesen J, Tfelt-Hansen P. Intravenous nitroglycerin as an experimental model of vascular headache: basic characteristics. PAIN 1989;38:17–24. [DOI] [PubMed] [Google Scholar]
- [24].Lin Q, Peng YB, Cui M, Willis WD, Wu J, Bo Peng Y. Nitric oxide-mediated spinal disinhibition contributes to the sensitization of primate spinothalamic tract neurons. J Neurophysiol 1999;81:1086–94. [DOI] [PubMed] [Google Scholar]
- [25].Lipton RB, Munjal S, Buse DC, Bennett A, Fanning KM, Burstein R, Reed ML. Allodynia is associated with initial and sustained response to acute migraine treatment: results from the American Migraine Prevalence and Prevention Study. Headache 2017;57:1026–40. [DOI] [PubMed] [Google Scholar]
- [26].Marone IM, De Logu F, Nassini R, De Carvalho Goncalves M, Benemei S, Ferreira J, Jain P, Li Puma S, Bunnett NW, Geppetti P, Materazzi S. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain 2018;141:2312–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nation KM, Dodick DW, Navratilova E, Porreca F. Sustained exposure to acute migraine medications combined with repeated noxious stimulation dysregulates descending pain modulatory circuits: relevance to medication overuse headache. Cephalalgia 2019;39:617–25. [DOI] [PubMed] [Google Scholar]
- [28].Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. PAIN 2013;154:S44–53. [DOI] [PubMed] [Google Scholar]
- [29].Olesen J, Iversen HK, Thomsen LL. Nitric oxide supersensitivity: a possible molecular mechanism of migraine pain. Neuroreport 1993;4:1027–30. [DOI] [PubMed] [Google Scholar]
- [30].Perrotta A, Arce-Leal N, Tassorelli C, Gasperi V, Sances G, Blandini F, Serrao M, Bolla M, Pierelli F, Nappi G, MacCarrone M, Sandrini G. Acute reduction of anandamide-hydrolase (FAAH) activity is coupled with a reduction of nociceptive pathways facilitation in medication-overuse headache subjects after withdrawal treatment. Headache 2012;52:1350–61. [DOI] [PubMed] [Google Scholar]
- [31].Perrotta A, Serrao M, Sandrini G, Burstein R, Sances G, Rossi P, Bartolo M, Pierelli F, Nappi G. Sensitisation of spinal cord pain processing in medication overuse headache involves supraspinal pain control. Cephalalgia 2010;30:272–84. [DOI] [PubMed] [Google Scholar]
- [32].Perrotta A, Serrao M, Tassorelli C, Arce-Leal N, Guaschino E, Sances G, Rossi P, Bartolo M, Pierelli F, Sandrini G, Nappi G. Oral nitric-oxide donor glyceryl-trinitrate induces sensitization in spinal cord pain processing in migraineurs: a double-blind, placebo-controlled, cross-over study. Eur J Pain 2011;15:482–90. [DOI] [PubMed] [Google Scholar]
- [33].Sances G, Tassorelli C, Pucci E, Ghiotto N, Sandrini G, Nappi G. Reliability of the nitroglycerin provocative test in the diagnosis of neurovascular headaches. Cephalalgia 2004;24:110–19. [DOI] [PubMed] [Google Scholar]
- [34].Sandrini G, Arrigo A, Bono G, Nappi G. The nociceptive flexion reflex as a tool for exploring pain control systems in headache and other pain syndromes. Cephalalgia 1993;13:21–7. [DOI] [PubMed] [Google Scholar]
- [35].Sandrini G, Martignoni E, Micieli G, Alfonsi E, Sances G, Nappi G. Pain reflexes in the clinical assessment of migraine syndromes. Funct Neurol 1986;1:423–9. [PubMed] [Google Scholar]
- [36].Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Prog Neurobiol 2005;77:353–95. [DOI] [PubMed] [Google Scholar]
- [37].Scher AI, Buse DC, Fanning KM, Kelly AM, Franznick DA, Adams AM, Lipton RB. Comorbid pain and migraine chronicity: the Chronic Migraine Epidemiology and Outcomes Study. Neurology 2017;89:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schwedt TJ, Larson-Prior L, Coalson RS, Nolan T, Mar S, Ances BM, Benzinger T, Schlaggar BL. Allodynia and descending pain modulation in migraine: a resting state functional connectivity analysis. Pain Med 2014;15:154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schytz HW. Investigation of carbachol and PACAP38 in a human model of migraine. Dan Med Bull 2010;57:B4223. [PubMed] [Google Scholar]
- [40].Tassorelli C, Joseph SA. Systemic nitroglycerin induces Fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res 1995;682:167–81. [DOI] [PubMed] [Google Scholar]
- [41].Tvedskov JF, Thomsen LL, Thomsen LL, Iversen HK, Williams P, Gibson A, Jenkins K, Peck R, Olesen J. The effect of propranolol on glyceryltrinitrate-induced headache and arterial response. Cephalalgia 2004;24:1076–87. [DOI] [PubMed] [Google Scholar]