Abstract
Purpose
Excessive bleeding is an acknowledged consequence of cardiac surgery, occurring in up to 10% of adult patients. This clinically important complication leads to poorer patient outcomes. Clinical practice guidelines are available to support best practice however variability in bleeding management practice and related adverse outcomes still exist. This study had two objectives: 1) to gain insight into current bleeding management practice for adult cardiac surgery in Australia and how that compared to guidelines and literature; and 2) to understand perceived difficulties clinicians face implementing improvements in bleeding management.
Methods
A national cross-sectional questionnaire survey was utilized. Perspectives were sought from cardiac surgeons, cardiac anesthesiologists and perfusionists. Thirty-nine closed-ended questions focused on routine bleeding management practices to address pre and intra-operative care. One open-ended question was asked; “What would assist you to improve bleeding management with cardiac surgery patients?” Quantitative data were analysed with SPSS. Qualitative data were categorized into the domains of the Theoretical Domains Framework; the domains were then mapped to the COM-B model.
Results
Survey responses from 159 Anesthesiologists, 39 cardiac surgeons and 86 perfusionists were included (response rate 37%). Four of the recommendations queried in this survey were reported as routinely adhered to < 50% of the time, 9 queried recommendations were adhered to 51–75% of the time and 4 recommendations were routinely followed >76% of the time.
Conclusion
There is a wide variation in peri-operative bleeding management practice among cardiac anaesthesiologists, surgeons and perfusionists in Australian cardiac surgery units. Conceptualizing factors believed necessary to improve practice with the TDF and COM-B model found that bleeding management could be improved with a standardized approach including; point of care diagnostic assays, a bleeding management algorithm, access to concentrated coagulation factors, cardiac surgery specific bleeding management education, multidisciplinary team agreement and support, and an overarching national approach.
Keywords: bleeding, cardiac surgery, clinical practice guidelines, multidisciplinary, theoretical domains framework, implementation
Introduction
Bleeding is an acknowledged consequence of cardiac surgery, with the application of cardiopulmonary bypass differentiating this from other surgical specialities.1,2 Major bleeding occurs in up to 10% of adult patients and is a common, clinically important complication increasing re-exploration surgery, length of stay, resources, cost and allogeneic blood transfusion (ABT).3–5 In Australia, cardiothoracic surgery stands as the largest surgical indication of fresh frozen plasma (FFP) and platelet use; and the second most common surgical indication for red blood cells (RBC).6,7 Major bleeding, blood transfusion, and/or re-exploration surgery have an additive effect of poorer clinical outcomes, including infection, and both early and late mortality.5,8–10 Major bleeding can be influenced by multiple patient and procedural factors including pre-operative anti-platelet and anti-coagulant medication, cardiopulmonary bypass-related dilution/loss/consumption of clotting factors, platelets, fibrinolysis; as well as the contribution of the surgical team.11
Minimising bleeding and blood loss is part of patient blood management (PBM), a World Health Organisation (WHO) supported paradigm that incorporates the implementation of multimodal and multidisciplinary concepts to manage the patient’s own blood: one of the aims being to avoid innappropriate ABT.12 More specifically, many clinical practice guidelines (CPGs) support PBM in cardiac surgery including the 2018 joint European Associations for Cardio-Thoracic Surgery and Cardiothoracic Anaesthesiology, “Guidelines on Patient Blood Management for adult cardiac surgery”.13–18 These guidelines recommend collaboration between cardiothoracic surgeons, anesthesiologists, clinical perfusionists and nurses to: 1) optimise patients pre-operatively and reduce bleeding risk; 2) maintain haemostasis intra-operatively; and 3) provide evidence-based treatment for bleeding complications. Numerous strategies exist to achieve these goals including use of tranexamic acid (TXA); retrograde and antegrade autologous priming; bleeding management treatment algorithms; and haemostatic point of care testing.
Despite existing recommendations, the implementation of interventions to reduce this multifaceted morbidity presents a significant challenge for the surgical team. Literature reports variation in bleeding rates and transfusion, with one study demonstrating a 70% absolute variance in the transfusion of RBCs making it implausible that uniform bleeding management strategies are being applied.1,7,19,20 The ability to provide best practice by individual clinicians, institutions or even wider health-care services does not occur on its own, or even through dissemination of CPGs; CPGs are simply tools used to inform, improve or support behaviour. Practice variation may reflect lack of awareness of evidence and CPGs but may also reflect inadequate emphasis on, or difficulties with, implementation of locally appropriate strategies.20,21
It is likely that confidence, trust and co-operation are required for an effective collaboration between the cardiac surgeon, anesthesiologist and perfusionist to prevent and manage bleeding in cardiac surgery patients. Such strategies also require supporting clinicians, supplementary tools and infrastructure; backed by overarching institutional blood management programs.22 WHO have identified a number of priorities for action for implementing PBM including: 1) setting up multi-disciplinary teams for managing blood effectively; and 2) conducting benchmarking studies to understand and compare practices of blood management-related strategies between clinicians and hospitals.23
Local evidence can elucidate bleeding management care currently provided, and rigorous theoretical constructs can be used to recognise requisites for improvement. Such frameworks and models can provide an explanation of how any improvements or change might successfully occur in complex environments that include clinicians interacting, and operating at different organisational levels.24 The Theoretical Domains Framework (TDF) facilitates understanding of clinical behaviours around evidence-based guidelines and provides a method to categorise behaviour.25 Use of the TDF to identify factors influencing bleeding management can then be mapped into the “Capability, Opportunity and Motivation Model” (COM-B) for designing interventions to improve practice.26 The COM-B model proposes that for any behaviour to take place, a clinician must: 1) have the capability to perform the behaviour; 2) have the physical and social opportunity to do so; and 3) be motivated.27 This combination of theoretical constructs has been used in a variety of settings to understand, explain and improve practice but not before in cardiac surgical bleeding management.28,29
Current intra-operative bleeding management practice in Australian cardiac surgery units is not understood. The barriers and enablers to implementing effective bleeding management strategies, including the relationships between key stakeholders, and institutional support, likely impact on the uptake of evidence-based practice. This study aimed to identify the bleeding management strategies currently used by clinicians in Australian cardiac surgery, and to elucidate what factors influence their ability to deliver best practice.
Materials and Methods
Objectives
The study had two objectives: 1) to gain insight into current bleeding management practice for adult cardiac surgery in Australia and how that compared to guidelines and literature; and 2) to understand perceived difficulties clinicians face implementing improvements in bleeding management.
Design
A national cross-sectional questionnaire survey.
Recruitment and Sample
Perspectives were sought from key clinical stakeholders including cardiac surgeons, cardiac anesthesiologists and perfusionists. To facilitate this, the survey was approved and distributed by the: 1) Australian and New Zealand Society of Cardiothoracic Surgery (ANZSCTS); 2) Australian and New Zealand College of Anaesthetists (ANZCA); and 3) the Australian and New Zealand College of Perfusionists (ANZCP). The surveys were sent via email from the professional bodies on behalf of the researchers, including an invitation, hyperlink and a plain language statement that explained the survey objective. This was followed by one reminder. All ANZSCTS consultant members and all ANZCP clinical perfusionists were included. ANZCA have a large membership and they are conscious to limit survey fatigue for their group; therefore, invitations were only sent to the anesthesiologists belonging to the special interest group that includes cardiac, thoracic, perfusion and vascular anesthesiologists. Responses from vascular and thoracic anesthesiologists were excluded from the analysis, as were responses from consultants practicing in New Zealand. Data collection was undertaken between November 2017 and March 2018.
The recruitment email provided a web-link to the participant information sheet and online survey. Consent was implied by participants moving through the eligibility criteria, participation involvement requirements and rights. The survey was designed to be completed on-line; however, a printable version was made available on request. Estimated time for completion of the survey was 10–15 minutes. The study approved by the Human Research Ethics Committees from The Prince Charles Hospital (IRB No. HREC/17/QPCH/340), Griffith University (IRB No. 2017/590) and University of Sunshine Coast (IRB No. S171105); and carried out in accordance with the principles of the Declaration of Helsinki.
Survey Development
The 40-item self-administered anonymous open questionnaire was developed within the web-based platform, LimeSurvey.30 The questionnaire was developed from survey instruments previously validated for blood management and aligned with bleeding management strategies from literature, CPGs and discussion among authors.31,32 Thirty-nine closed-ended questions focused on routine bleeding management practices by individual clinicians to address pre-operative risk management and intra-operative care. Answers were based on CPG best practice with an “Other” check box that allowed text data to be entered if respondents wished to add additional comments on their practice; this was added to accommodate the heterogeneity with cardiac surgery procedures. Survey questions covered topics of: 1) demographics including occupation, hospital type and post code (n=6); 2) supporting committees and staff (n=2); 3) pre-operative management (n=6); and 4) intra-operative management (n=25). There was one open-ended question at the end of the survey; “What would assist you to improve bleeding management with cardiac surgery patients?” This question aimed to gather insight into participants’ perceptions of barriers or facilitators that impact their ability to manage bleeding or implement improvement strategies in their current environment.
The survey was tested for face and content validity with a convenience sample of 10 expert clinicians and researchers not involved in the survey development. Based on feedback, some modifications were made primarily regarding the use of language, with a final review by the authors prior to distribution.
Analysis
Close Ended Survey Questions
Survey data were imported into SPSS (v.25.0) for coding and statistical analysis.33 Descriptive statistics were used to summarise the data collected in terms of demographic and clinical characteristics of the cardiac surgery units, their patient cohorts and reported bleeding management strategies. Results were excluded from analysis if respondents completed less than 50% of the survey, as they did not contain enough data to meet the study objectives.
Open-Ended Question
Initially, responses to the open-ended question were read to achieve an understanding of the data and to separate statements that contained more than one theme. Data were imported into NVivo 12 and coded into the 14 TDF domains with a recursive process following a thematic analysis approach.24,25 Statements were also identified as barriers or facilitators that influence behaviours and finally, the statements were mapped into the COM-B model. The entire analytic process was achieved by two of the authors (BP, SK) and discrepancies resolved through discussion.
Results
Demographics
Surveys were sent to 761 members (131 cardiac surgeons; 476 anesthesiologists and; 154 perfusionists). Of these, 351 responses were received (46% response). Excluded responses comprised; clinicians not practicing in Australia (n=5); and if less than 50% of the survey was complete (n=62). A total of 284 survey responses were included in the final analysis. Response rate by participants from the Australian and New Zealand Society of Cardiothoracic Surgery was 30% (39/131); 2. Australian and New Zealand College of anaesthetists was 33% (159/476); and, 3. the Australian and New Zealand College of Perfusionists 56% (86/154). Survey respondents were predominately anesthesiologists (56%), working primarily in metropolitan public hospitals (64%), in NSW (40%) (Table 1).
Table 1.
Demographic Characteristics of the Survey Sample (n=284)
| Specialty | |
| Anesthesiologist | 159 (56%) |
| Perfusionist | 86 (30.3%) |
| Cardiac Surgeon | 39 (13.7%) |
| Type of hospital setting | |
| Metropolitan Public Hospital | 183 (64.4%) |
| Metropolitan Private Hospital | 59 (20.8%) |
| Regional Public Hospital | 27 (9.5%) |
| Regional Private Hospital | 15 (5.3%) |
| Heart/Lung Transplant service | |
| Yes | 60 (21.1%) |
| No | 224 (78.9%) |
| ECMO and/or VAD | |
| Extracorporeal Membrane Oxygenation (ECMO) | 165 (58%) |
| Ventricular Assist Device (VAD) | 2 (0.7%) |
| Both | 72 (25.4%) |
| Participating State the answers refer to | |
| New South Wales | 113 (39.8%) |
| Victoria | 73 (25.7%) |
| Queensland | 58 (20.4%) |
| Western Australia | 20 (7%) |
| South Australia | 17 (6%) |
| Tasmania | 3 (1.1%) |
Routine Use of CPG Recommended Bleeding Management Strategies
Blood Management Committees and Blood Management Nurses
Most (71%) participants reported having a blood management committee at their institution, however only 42% reported having a blood management nurse at their institution; the remainder did not have or were unaware of the existence of a role. CPGs recommend committees and dedicated blood management positions to support best practice15,16,18 In the “Other comments” text box for this question, many participants stated that blood management would be improved with additional clinical support such as: “blood management nurse onsite”, “transfusion nurse onsite”, “properly trained transfusion expert” and “more blood management nurses” (Table 2). Finally, participants perceived issues related to hospital resources and processes were needed for successful bleeding management at the bedside: “convince executive to purchase equipment and fund support staff” and “Patient Blood Management program (our previous program was scrapped in transition to a new hospital)”.
Table 2.
Respondent Perceptions of Requirements to Improve Bleeding Management Practice
| COM-B | TDF Domains | Themes | Example Quotations | Frequency Out of 99 |
|---|---|---|---|---|
| Capability | Behavioural Regulation | Standardization | “A more structured approach to bleeding” A “Blood product availability should not be unit dependent for institutions within the same state or territory. Every blood bank at every hospital has different process for providing blood products” P “Need consistency in approach between different surgeons and ICU clinicians” A “Our local algorithm included in an app to make it easy to follow” A “Minimise haemodilution in the first place, is the most important strategy” S |
16 |
| Knowledge | Cardiac surgery specific bleeding management education/guidelines | “Online CPD courses specifically looking at bleeding in cardiac surgical patients” A “National guidelines which set a standard for blood product administration based on a single ROTEM algorithm accepted nationally rather than unit based” P “Some evidence-based bleeding management guidelines would be helpful” P “National guidelines would allow us to push past local funding and political problems” A “More and more education” A |
13 | |
| Opportunity | Environmental Context and Resources | Access to point of care diagnostic assays (ROTEM/TEG & Multiplate) |
“Better POCCT availability (i.e. rapid)” P “ROTEM ± Multiplate in MOT” A “I believe we should be using point of care thromboelastography with a panel of tests” A “Easy point of care access to TEG/ROTEM” “ROTEM, Multiplate and vWF assays” S “Better availability of Multiplate analysis” A “Better point of care assessment - rapid response time” A “Need Multiplate, ROTEM” P “The availability of TEG has changed our management” A “ROTEM or equivalent in theatre itself” S |
20 |
| Dedicated blood management support clinicians | “I expect it would not be a viable business model, but it would be novel and helpful to have a separate and properly trained transfusion expert to manage massive bleeding” A “Blood management nurse support” A “Transfusion nurse onsite” A “More blood management nurses” S |
15 | ||
| Decision support tool for bleeding management (algorithm/app) | “Standardized ROTEM guided algorithm” A “Algorithms” P “Decisions guided by an algorithm/app approved and adopted nationally” A “ROTEM/Multiplate interpretation app” S “An agreed protocol for the bleeding patient would be useful” A “App would be useful, including doses, (on/kg basis)” A |
9 | ||
| Access to fibrinogen concentrate | “Fibrinogen concentrate” A “Fibrinogen concentrate more readily available” A “Availability of more specific factors such as fibrinogen concentrate” S “Availability of FC” A |
6 | ||
| Social Influences | Multidisciplinary team contribution | “Combined cardiac surgery, cardiac anaesthesia, intensive care and haematology consensus on management with emphasis on point of care testing” P “An awareness by cardiologists re their impact on potential post op bleeding in acute patients” S “Needs to be multi-disciplinary to work” P “I would like the intensivists to be more interested” A “We have a good response at this hospital, with good advice from Haematology” A “If the various departments (cardiac, surgery, anaesthesia, ICU and blood bank) could actually work together” A “Haematologist with experience in acute bleeding” A “More awareness from everyone involved - ICU, surgeons, hospital administration” S “Need a co-ordinated blood management program” A “A haematologist that answers the phone” S |
12 | |
| Motivation |
Beliefs about capability | Control (or lack of) over ability to effectively manage bleeding | “We have the best available tools and agents available to us for routine use” A “Too many times tests are performed, and the results are either ignored or not considered in the management of post-operative bleeding” P “We have a documented strategy for dealing with bleeding that includes administration of platelets. cryoprecipitate and desmopressin. Factor VII is only used when surgical bleeding continues beyond reasonable use of blood products” P “Better blood bank support for peripheral hospitals” S “It is frequent to have a normal ROTEM in the bleeding post-op patient, but blood products are given anyway as opposed to discussion about surgical intervention” A |
8 |
Abbreviations: S, Surgeon; A, Anesthesiologist; P, Perfusionist.
Anti-Platelet Medication
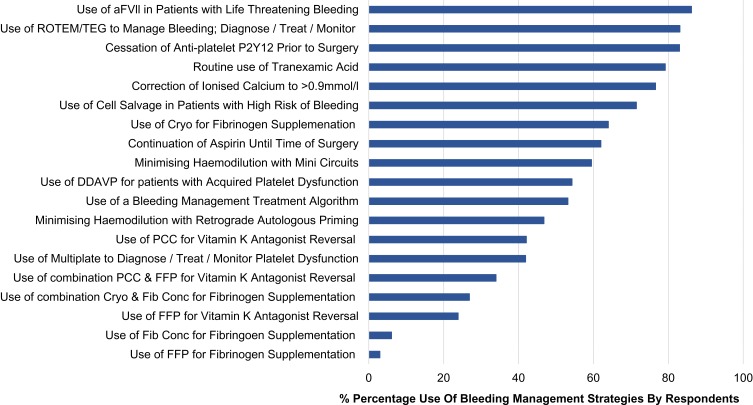
Participants reported it was a routine practice to cease P2Y12 receptor inhibitors (83%) for elective surgery consistent with CPG recommendations.13,14,16,17 Sixty-two percent of respondents reported that it was a routine practice to continue aspirin therapy (62%) prior to elective cardiac surgery (Figure 1) consistent with guidelines recommending there is sufficient evidence for continuation.13,14,16,17 In the text field for aspirin cessation, surgeons commented that these decisions were patient specific for example: “not for redo cases”, “case dependent”, and “dependent of stability and lesion type”. Anesthesiologist comments were related to the practice being “surgeon dependent”.
Figure 1.
Frequency of routinely applied bleeding management strategies in cardiac surgery.
Abbreviations: aFVll, activated factor seven; FFP, fresh frozen plasma; PCC, prothrombin complex concentrate; Cryo, Cryoprecipitate; Fib Conc, Fibrinogen concentrate; ROTEM, Rotational Thromboelastometry; TEG, Thromboelastography; DDAVP, D-Deamino Arginine Vasopressin.
Tranexamic Acid (TXA)
Routine use of TXA was reported by 79% of anesthesiologists and 74% of cardiac surgeons (perfusionist responses were excluded) consistent with CPGs.13–16,18 Mode of delivery and dosing by anesthesiologists and cardiac surgeons are reported in Table 3. Surgeon responses were excluded from “dosing” as they were largely unaware of doses of TXA: “unsure”, “no idea”, and “don’t know”. Dosing by anesthesiologists varied (Table 3) with comments in the text field for TXA use, including: “generic 500mg/hr”, “4.5mg/kg/hr as per the info pamphlet”, “I follow the ATACAS trial recommendations”, “up to 4.5g depending on eGFR”, “algorithm from product info based on weight and GFR” and “most get 2g infused over 2–3 hrs”. Four percent of anesthesiologists mentioned the ATACAS trial results in the field for additional comments.34
Table 3.
Routine Use of Tranexamic Acid by Anesthesiologists and Surgeons
| Mode of Delivery (n=181) | |||||
| Bolus only | Continuous only during the surgical procedure | Bolus + Continuous | Not sure | Other | |
| Anesthesiologist (n=142) | 17.6% | 9.2% | 67.6% | 0% | 5.6% |
| Surgeon (n=39) | 43.6% | 7.7% | 38.4% | 10.3% | 0% |
| Dose (n=142) | |||||
| Bolus only | Continuous only during surgical procedure | Bolus | + Continuous | ||
| Anesthesiologist | 1–2g total or 15–50mg/kg | 1g/hr for 2–3 hrs, or over the entire surgical procedure, or 2–15mg/kg/hr | 1–2g or 10mg/kg up to 50mg/kg | 2–15mg/kg/hr | |
Minimising Haemodilution
Reported routine use of retrograde autologous priming (RAP) varied by: 1) surgeons (59%), 2) anesthesiologists (49%), and 3) perfusion (40%). Variation was also recorded in the routine use of mini circuits by 1) surgeons (72%), 2) anesthesiologists (63%) and 3) Perfusion (48%). Limiting haemodilution is recommended to reduce bleeding.13,15 Perfusionists recorded detailed responses to RAP and mini circuits: mini-prime circuits for all patients (500–800mls prime), “more stringent use of low prime”, “RAP” and “RAP/VAP”. Anesthesiologists comments included: “occasionally with additional bleeding risk factors” and “depends on surgeon”. However, the surgeons commented with recognition of an overarching need to avoid haemodilution: “minimise haemodilution in the first place, is the most important strategy”.
Bleeding Management Treatment Algorithm and Point of Care Diagnostic Assays (ROTEM/TEG and Multiplate)
Just over half of the respondents (54%) reported using a bleeding management algorithm, which is recommended by multiple CPGs.13,14,17 Rotational Thromboelastometry ([ROTEM], Werfen® Bedford, MA) or rotational thromboelastography ([TEG®]; Haemonetics, Braintree, MA) were reportedly used routinely by 83% of respondents.13,14 However, only 42% reported using platelet function testing, with remainder reporting they did not use it at all, or that it was not available at their institution (Figure 1).
Diagnosis and Treatment of Fibrinogen Deficiency
There was a wide variation in participant responses regarding the diagnosis and routine treatment of fibrinogen deficiency (Table 4). Recent recommendations state that in patients with excessive bleeding: 1) consider the use of fibrinogen concentrate, 2) there is insufficient literature to evaluate the intraoperative or postoperative transfusion of cryoprecipitate to manage actual or potential coagulopathy, and 3) FFP may not provide an adequate increase in plasma fibrinogen concentrations to impact bleeding.13,14,17
Table 4.
Participant Responses for Diagnosis and Correction of Fibrinogen Deficiency
| Diagnosing the cause of fibrinogen deficiency (n=158) | |||
| Point of care diagnostic assays | Standard laboratory tests | Combination of point of care diagnostic assays and standard laboratory tests | Empirical diagnosis |
| 68.2% | 25.8% | 4.5% | 1.5% |
| Correction of fibrinogen deficiency (n=39) | |||
| Cryoprecipitate | Combination of cryoprecipitate and fibrinogen concentrate | Fibrinogen concentrate | FFP |
| 64% | 26.9% | 6.1% | 3% |
Vitamin K Antagonists (VKAs)
Participants reported routinely correcting post-bypass bleeding associated with warfarin therapy with: 1) prothrombin complex concentrate (PCC) (42%), 2) a combination of FFP and PCC (34%) and, 3) FFP (24%) (Figure 1). The recommendation for bleeding associated with warfarin is PCC or FFP if PCC is not available.13,14,17,35 Additional text comments for this question included: “should have gatekeeper access to FFP, its outside guidelines”.
D-Deamino Arginine Vasopressin (DDAVP)
The use of DDAVP for post-operative bleeding associated with impaired platelet function or decreased platelet count was reported by 54% of surgeons and anesthesiologist respondents, which follows current guideline recommendations (Figure 1).13,14,17 Additional anesthesiologists comments included: “for VADs and uraemic patients”, “renal imp” (airment), “renal disease” and “frequent surgeon request”.
Activated Factor Vll (FVlla)
The use of FVlla as a rescue therapy in the setting of excessive life-threatening bleeding was reported by 86% of surgeons and anesthesiologist respondents; the remaining respondents stating they did not use it at all (Figure 1). Responses in the optional text field included: “only as a last resort, almost never”, “only when certain surgeons stamp their feet”, and “rarely”.
Cell Salvage
Cell salvage was reportedly used routinely by 39% of all respondents, 33% reported use in patients with a high risk of blood loss and post-operative bleeding only, and the remainder reporting no use of cell salvage (Figure 1). CPGs recommend postoperative cell salvage may be considered to reduce transfusions in patients with bleeding.13,14,17
Residual Pump Blood (RPB)
Routine re-infusion of RPB varied with 69% stating that they would return the blood to the patient unwashed, 18% washed the RPB in a cell saver, the remaining using various combinations of washed or unwashed related to Hb and time on pump (Figure 1). Participants responded with additional comments including: “half unwashed, half washed”, “combined with cell saver volume”, “unwashed <2hr washed >2 hr run”, “either unwashed or cell-salvaged depending on the duration of CPB and also volume status” and “unwashed<1hr washed >1 hr”.
Ionised Calcium
The correction of ionised calcium to a level >0.9 mmol/l was reportedly practiced by 77% of respondents (Figure 1), with additional comments: “1.2 −1.3 mmol/l ionized”, “no specific target”, “it’s not for coagulation!! it’s for myocardial function!!! If low enough so stop clotting pt is dead”, “> 1 mmol/l” and “1.2 mmol/”. Surgeons commented they were “not sure” or “> 1 mmol/l”; there were no comments from perfusionists.
Barriers and Enablers to Implementing Bleeding Management
A total of 69 (25%) respondents answered the open-ended question at the end of the survey; “What would assist you to improve bleeding management for cardiac surgery patients”? (Table 2). Responses were received from 41 anaesthesiologists, 14 cardiac surgeons and 14 perfusionists. Many responses contained more than one belief; in total there were 99 individual belief statements which were coded into themes with the TDF (Table 2). Five domains from the TDF contained content relevant to the study objectives, including: knowledge; social influences; beliefs about capabilities; environmental context and resources; and behavioural regulation (Table 2).
Once the TDF domains were mapped into the COM-B model, opportunity was identified as the biggest influence to implementing optimal bleeding management (Table 2). This included: 1) access to point of care diagnostic assays (ROTEM/TEG and Multiplate); 2) dedicated blood management clinicians; 3) decision support tool for bleeding management (algorithm/app); 4) access to fibrinogen concentrate and; 5) multidisciplinary team contribution. Motivation to improve bleeding management was influenced by the individuals’ perceptions regarding their ability to effectively manage bleeding. Respondents identified the capability to manage bleeding effectively as influenced by: 1) standardization of practice; and 2) cardiac surgery specific, bleeding management education/guidelines.
Discussion
To our knowledge, this is the first study that has aimed to gain insight into current practices for managing bleeding risk, and actual bleeding during adult cardiac surgery in Australia, as reported by clinicians reflecting on their practice. We also aimed to understand what clinicians perceived as barriers and enablers to improving their bleeding management practice. Importantly, these aims were explored from the perspective of key stakeholders in the peri-operative phase of surgery, using a recognised theoretical approach (TDF and COM-B model). Our study demonstrated wide variation and incomplete implementation of the queried CPG bleeding management recommendations. Four of the recommendations queried in this survey were reported as routinely adhered to less than 50% of the time, 9 queried recommendations were adhered to 51–75% of the time and only 4 recommendations were routinely followed greater than 76% of the time.
Participants consistently stated that a key requirement for effective bleeding management was a supportive multidisciplinary approach to blood management; however, one-third of respondents did not have, or were not aware of, a blood management committee at their institution. Various guidelines including the Society of Thoracic Surgeons/Society of Cardiovascular Anesthesiologists Blood Conservation CPGs recommend the establishment of blood management committees.
A multi-disciplinary approach involving multiple stakeholders, institutional support, enforceable transfusion algorithms supplemented with point-of-care testing, and all of the already mentioned efficacious blood conservation interventions limits blood transfusion and provides optimal blood conservation for cardiac operations. (Class I, Level of evidence, A)15,16
Furthermore, a significant body of literature supports comprehensive programmes with jurisdictional, executive support and clinical leadership, a multidisciplinary approach, multimodal strategies, education, communication and feedback as essential to facilitate effective bleeding management.21,22,36–38 An Australian study of four hospitals found this approach reduced mortality, length of hospital stay, blood product use, and costs across 4 hospitals.22 Data from this study show that while the need for support at the front line is essential, support is necessary at multiple organisational levels. A motivated and well-informed clinician is often not sufficient to implement change if relevant departments or the wider organisation does not support practice improvement. This is likely one of the most significant variables influencing clinicians’ ability to implement all other bleeding management strategies. This was highlighted in the results from participants' comments demonstrating a lack of an “opportunity” (COM-B) to improve practice.
The responses recorded a low level of concordance for the continuation of aspirin through the peri-operative period.13,14,16,39 A 2017 meta-analysis on patients undergoing cardiac surgery observed that: 1) any dose of aspirin confers benefit in decreasing mortality and post-operative acute renal failure (mainly in CABG patients); 2) low-dose (≤160 mg) aspirin prior to CABG surgery is associated with a reduced incidence of MI; and 3) low dose (<160mg) preoperative aspirin did not increase the incidence of RBC transfusion or re-operation for bleeding.40 Poor compliance with this strategy is likely a result of the level of evidence (Class lla, Level C) and lack of clarity on whether preoperative aspirin is beneficial in isolated valve surgery or other non-CABG cardiac surgery procedures.13 However, there was a good agreement for stopping anti-platelet P2Y12 receptor inhibitors (83%) prior to elective surgery, where evidence is stronger.13–16,39
There was also good compliance for the routine use of TXA (recommended to reduce ABT in patients undergoing CPB, Class 1, Level of evidence, A).13,15–17 However, there was significant variation in the method of administration and dosing. This may reflect heterogeneity of dose and mode of delivery (bolus, bolus + infusion and infusion) in studies investigating TXA in cardiac surgery.34,41,42 Few anesthesiologists mentioned the ATACAS trial in the text field on TXA prescribing. This was an Australian multi-national trial (the largest trial to date (n=4,631)) that investigated TXA use in patients at increased risk of perioperative complications undergoing on, or off-pump CABG, or a combination of CABG/Valve/other procedures.34 Compared to placebo, TXA use was associated with a decreased incidence of ABT; consistent with results from meta-analyses.43,44 Additionally, there was a reduction in re-operation surgery and no difference in the incidence of death or thromboembolic complications. There was, however, an increase in seizures, and dosing was reduced during the trial with emerging evidence of seizure risk with high doses. Currently, the clinical significance is unclear and previous prospective studies have reported seizures as self-limiting and not associated with increased morbidity or mortality.45
Despite the ATACAS conclusions, clinicians conceivably remain concerned about patient-important outcomes related to the optimal dose that balances the risk of seizures and bleeding and this was reflected in the small number of responses using ATACAS dosing. The recorded responses exemplify known barriers to evidence-based practice including lack of agreement and outcome expectancy with research, as well as knowledge and awareness of guidelines.46
There was reported variation for routine use of strategies to minimise haemodilution (RAP and mini circuits). On the other hand, there was agreement in the comments across all three disciplines that managing haemodilution in cardiac surgery was imperative. It is likely the results reflect underpowered RCTs and lack of strong recommendations from CPGs (Class lIa, Level of evidence, B) resulting in uncertainty regarding specific strategies.13–15,47 This is supported by participants’ common beliefs that standardization, cardiac surgery specific bleeding management education, and national guidelines could improve bleeding management.
A clear finding from this study was the contribution of point of care diagnostic assays (ROTEM/TEG) to bleeding management, with 83% of respondents reporting routine use. There is a significant body of literature in cardiac surgery demonstrating a move away from use of SLTs, an increased use of pont of care diagnostic assays and reduced ABT in situations that require rapid assessment and treatment of bleeding.36,48–50 The key advantage of these assays is short result turnaround time, allowing clinicians to diagnose and treat bleeding promptly, as well as track treatment response. This is supported by a Cochrane review (2018) which states there is “growing evidence that application of TEG‐ or ROTEM‐guided transfusion strategies may reduce the need for blood products, and improve morbidity in patients with bleeding”.51 However, less than half of respondents reported access to the point of care diagnostic assay to assess platelet function likely because the implications of platelet function testing intra-operatively including testing intra and postoperatively are not well understood. However, pre-operative platelet function testing may guide decision-making regarding proceeding or cancelling surgery where possible.52–54 Participants uniformly identified access to point of care diagnostic assays was critical to improving bleeding management; with access (or lack of) these instruments reflecting resource issues but also issues of support (or lack of) from external disciplines and departments (haematology, blood bank).
The results from point of care diagnostic assays are routinely used in conjunction with bleeding management treatment algorithms.50 However, reported use of algorithms was low, despite recommendations by CPGs;
We recommend the application of intervention algorithms incorporating pre-defined triggers and targets based on VHA coagulation monitoring to guide individualised haemostatic intervention in the case of perioperative bleeding. (Grade 1C)13,14,17
This is notable because the incidence of coagulopathy and bleeding in cardiac surgery is high,1,50,55 and use of algorithms has been shown to independently improve standardization and reduce morbidities, including reductions ABT.49,50,56 A cited barrier was the lack of multi-disciplinary agreement required for the development and use of algorithms. Implementation literature regarding uptake of CPGs identified decision support systems and social interactions as successful strategies for practice improvement. Importantly though, literature demonstrates successful implementation is related to a planning with a multi-layered approach addressing context-specific barriers.21,22,36,46
Variation in use of therapies to replete fibrinogen deficits was also identified. The last decade has seen increased interest in supplementing low plasma fibrinogen with fibrinogen concentrate (FC) in the post-operative bleeding scenario.57–63 While several single-centre RCTs and observational studies have demonstrated reduced bleeding and ABT associated with FC supplementation, a multi-national, multi-center, randomized, double-blind, placebo-controlled trial comparing FC with placebo, (152 patients, from 34 centers in 11 countries), found that FC use was associated with an increase in administration of blood products55,59,60,62–64 The post hoc analysis suggested that the unexpected outcomes with FC were a result of a combination of poor adherence to the transfusion algorithm, inclusion of patients with higher than expected pre-treatment fibrinogen levels, and investigators who lacked familiarity with implementing the protocol.57
Despite these inconsistencies in the literature, participants of this survey consistently cited access to FC as an important factor in improving bleeding management. A recent pragmatic, multi-center, randomized, non-inferiority Phase 3 trial (FIBRES) in adult cardiac surgical patients found FC was noninferior to cryoprecipitate when considering the number of ABTs in the 24 hrs post bypass.65 However, surprisingly, time to treat for FC and cryoprecipitate was the same, negating a major advantage of using a product that is lyophilized, allowing for easy storage, reconstitution, and administration compared to cryoprecipitate that is retained in a frozen state and must be thawed and pooled prior to administration. Furthermore, FC has a standardized concentration, is purified and pathogen reduced which mitigates risks associated with ABT and is a central tenet of blood management.12 It is likely that the licensed indications for FC, as well as cost and logistical issues will continue to be barriers to the use of FC despite recommendations in guidelines and clinician call for increasing access to the product.14
Results also demonstrated similar variation in reported therapy to reverse bleeding in cardiac surgery associated with the use of VKAs, possibly as a result of the disparities in guidelines (Table 5). Consistency across guidelines as well as quality and clarity are important features for successful uptake of evidence.46 In the absence of cohort-specific national guidelines, disparities such as those seen in Table 5, make a case for institution-specific implementation of evidence by quality improvement teams of clinicians to ensure local input, supported by senior leadership and peer review.22
Table 5.
Summary of Guideline Recommendations for the Immediate Reversal of VKAs
| Guideline | Recommendation | Class/Level of Evidence/Grade |
|---|---|---|
| 2017 EACTS/EACTA Guidelines on PBM for adult cardiac surgery | The use of PCC or FFP may be considered to reverse the action of VKAs13 | Class llb Level of Evidence B |
| 2016 Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology | In bleeding patients where VKA-induced coagulopathy is considered a contributing factor, we recommend the administration of PCC, If PCC is not available, we recommend the transfusion of plasma14 | Grade 1 B Grade 1C |
| 2013 Australasian Society of Thrombosis and Haemostasis | For immediate reversal, PCCs are preferred over FFP. FFP is not routinely needed in combination with PCC. FFP can be used when PCC is unavailable35 | Grade 2C |
| 2011 Update to The Society of Thoracic Surgeons and the Society of Cardiovascular Anaesthesiologists Blood Conservation Clinical Practice Guidelines | For urgent warfarin reversal, administration of PCC is preferred, but FFP is reasonable when adequate levels of Factor VII are not present in PCC15 | Class lla Level of evidence B |
Half of the respondents reported routine use of DDAVP when patients were suffering with intractable bleeding and known platelet dysfunction or decreased platelet count. This is likely to be a reflection of the non-specific nature of the recommendation, as well as the class and level of evidence; “prophylactic use of DDAVP to reduce bleeding is not recommended, but it’s administration might be beneficial in patients who have inherited or acquired bleeding disorders or platelet dysfunction" (Class lla, Level of Evidence C).13 This is further compounded by a Cochrane review (2017) that concluded, “It is possible that people who are more vulnerable to bleeding, such as those taking antiplatelet agents, may gain more benefit from DDAVP”.66
Consistent with CPG recommendations, 86% of surgeons and anesthesiologists only infused factor Vlla as rescue therapy in the setting of excessive life-threatening bleeding with uncontrollable bleeding that cannot be managed by other procoagulant interventions.13–16
In this survey, more than 70% of participants reported using cell salvage for patients at high risk of bleeding. This is consistent with recommendations and the last Cochrane meta-analysis (2010) demonstrating the use of washed cell salvage in cardiac surgery was associated with a 34% relative risk reduction in exposure to RBCs (RR 0.66; 95% CI =0.55 to 0.80).13–15,18,47,67,68 A more recent meta-analysis of cardiac surgery patients including twenty-one studies showed an overall reduction in exposure to RBCs, and a decrease in RBC units per patient with no effect on infection or mortality.69 Nearly 30% of respondents in this survey reported that they did not routinely use this strategy. This may be related to issues of staffing, training and funding as reported by a recent survey by the UK cell salvage action group.68
Again, non-specific guidelines and a paucity of evidence may be associated with variation in the return of residual pump blood (RPB) from the reservoir and circuit at the end of CPB. Nearly 70% respondents stated they would routinely return RPB the patient unwashed, nearly 18% washing the RPB in a cell saver, and the remainder using various other combinations. The 2011 Society for Thoracic Surgeons guidelines non-specifically recommend the reinfusion of RPB (Class IIa/level of evidence C).15
Lastly, good agreement was reported for the correction of ionised calcium to a level >0.9 mmol/l despite the reported variance in target levels for correction, as well as clinician statements regarding the impact on haemostasis. This could be related to the knowledge that while calcium is an important cofactor to several physiological components of haemostasis, hypocalcemia in acute bleeding is common, and treatment with ABT may worsen this with chelation by citrate additive.70 It remains that calcium supplementation an important principle of bleeding management with a grade 1B recommendation “ calcium should be administered during massive transfusion if calcium concentration is low, to preserve normocalaemia (>0.9 mmol/l)“.14
Strengths and Limitations
This study is limited by factors inherent to the survey method. We did not directly observe clinical practice and self-reported data may not reflect true behaviour. Also, as participation was voluntary, those agreeing to report potentially more likely to have an interest in bleeding management. As a result, our findings may over-represent clinicians with significant knowledge and compliance with guideline recommendations. While our study design could not provide details on the underlying reasons for treatment choices, the textual data offered explanatory data on the thoughts and beliefs about the consequences and outcomes of bleeding management. Furthermore, overall accuracy would have been enhanced by a greater response rate, although 37% is a common response rate for professional surveys.31
Despite these limitations, this study provides important information about current bleeding management practice in Australia and documents the issues clinicians face to improve the implementation of therapies to manage this complex morbidity. The use of guideline recommendations for bleeding management in cardiac surgery support: 1) evidence-based care, 2) reductions in practice variation and, 3) a stimulus for organisational redesign to support best practice. The wide variation in reported practice in our study suggests that these goals may not always be achieved. Our findings mirror previous studies investigating the uptake of recommendations from CPGs in blood management.7,31,32 Literature identifies low level of strength of evidence as a contributor to poor uptake of guideline recommendations. Many recommendations include phrases such as; “it may be considered”, “it is not unreasonable”, or “it is not recommended however …”. This type of phrasing can convey an ambiguous message on an individual level and not enough authority to support organisational change. Given the complexity of managing bleeding in the cardiac surgical cohort, and the multi-disciplinary input not only from front line clinicians but also from ICU, cardiology, haematology and blood banks; an overarching co-ordinated national approach may be required for change.
This is supported by participant beliefs developed with the TDF and COM-B model proposing that institutions and professional bodies need to facilitate clinicians to improve: 1) their “capability” to manage bleeding more effectively with a well-documented and structured approach to bleeding, supported by cardiac surgery specific bleeding management education and a national guideline; 2) the “opportunity” to manage bleeding more effectively with improved access to point of care coagulation assays, bleeding management algorithms, dedicated blood management clinicians, access to fibrinogen concentrate and collaboration with the multi-disciplinary team; and 3) “motivation” to manage bleeding more effectively with improved confidence in their peers to adhere to agreed bleeding management processes.
Conclusion
In conclusion, there is a wide variation in peri-operative bleeding management practice among surgeons, anesthesiologists and perfusionists in Australian cardiac surgery units. Conceptualizing factors believed necessary to improve practice with the TDF and COM-B model found that bleeding management could be improved with a standardized approach including; point of care diagnostic assays, a bleeding management algorithm, access to concentrated coagulation factors, cardiac surgery specific bleeding management education, multidisciplinary team agreement and support, and an overarching national approach.
Acknowledgments
The authors would like to acknowledge the contribution of the clinicians who participated in this research project. This study received funding from the Australian National Blood Authority Research and Development program. Griffith University has received unrestricted investigator-initiated research or educational grants on CMR’s behalf from: 3M, Angiodynamics; Baxter; BD-Bard; Cardinal Health, Medtronic; Smiths Medical. Griffith University has received consultancy payments on CMR’s behalf from: 3M, BBraun, BD-Bard, ResQDevices, Smiths Medical.
Data Sharing Statement
The survey tool is available as an online supplementary file. The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Disclosure
SK’s current (Queensland University of Technology) and previous employer (Griffith University) have received monies on her behalf from 3M and BD for research and educational consultancies, investigator-initiated grants and unrestricted grants in aid. The authors report no other conflicts of interest in this work.
References
- 1.Biancari F, Kinnunen EM, Kiviniemi T, et al. Meta-analysis of the sources of bleeding after adult cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32(4):1618–1624. doi: 10.1053/j.jvca.2017.12.024 [DOI] [PubMed] [Google Scholar]
- 2.Colson PH, Gaudard P, Fellahi JL, et al. Active bleeding after cardiac surgery: a prospective observational multicenter study. PLoS One. 2016;11(9):1–14. doi: 10.1371/journal.pone.0162396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruseckaite R, McQuilten ZK, Oldroyd JC, et al. Descriptive characteristics and in-hospital mortality of critically bleeding patients requiring massive transfusion: results from the Australian and New Zealand massive transfusion registry. Vox Sang. 2017;112(3):240–248. doi: 10.1111/vox.2017.112.issue-3 [DOI] [PubMed] [Google Scholar]
- 4.Al-Attar N, Johnston S, Jamous N, et al. Impact of bleeding complications on length of stay and critical care utilization in cardiac surgery patients in England. J Cardiothorac Surg. 2019;14(1):1–10. doi: 10.1186/s13019-019-0881-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali JM, Wallwork K, Moorjani N. Do patients who require re-exploration for bleeding have inferior outcomes following cardiac surgery? Interact Cardiovasc Thorac Surg. 2019;28(4):613–618. doi: 10.1093/icvts/ivy285 [DOI] [PubMed] [Google Scholar]
- 6.Shortt J, Polizzotto MN, Waters N, et al. Assessment of the urgency and deferability of transfusion to inform emergency blood planning and triage: the Bloodhound prospective audit of red blood cell use. Transfusion. 2009;49(11):2296–2303. doi: 10.1111/trf.2009.49.issue-11 [DOI] [PubMed] [Google Scholar]
- 7.McQuilten ZK, Andrianopoulos N, Wood EM, et al. Transfusion practice varies widely in cardiac surgery: results from a national registry. J Thorac Cardiovasc Surg. 2014;147(5):1684–1690. doi: 10.1016/j.jtcvs.2013.10.051 [DOI] [PubMed] [Google Scholar]
- 8.Fröjd V, Jeppsson, A. Reexploration for bleeding and its association with mortality after cardiac surgery. Ann Thorac Surg. 2016;102(1):109–117. doi: 10.1016/j.athoracsur.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 9.Ranucci M, Baryshnikova E, Castelvecchio S, Pelissero G; Surgical and Clinical Outcome Research (SCORE) Group. Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Ann Thorac Surg. 2013;96(2):478–485. doi: 10.1016/j.athoracsur.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 10.Cutrell JB, Barros N, McBroom M, et al. Risk factors for deep sternal wound infection after cardiac surgery: influence of red blood cell transfusions and chronic infection. Am J Infect Control. 2016;44(11):1302–1309. doi: 10.1016/j.ajic.2016.03.027 [DOI] [PubMed] [Google Scholar]
- 11.Davidson S. State of the art - How I manage coagulopathy in cardiac surgery patients. Br J Haematol. 2014;164(6):779–789. doi: 10.1111/bjh.12746 [DOI] [PubMed] [Google Scholar]
- 12.WHO. Availability, safety and quality of blood products: World Health Organisation (WHO). Sixty-third World Health Assembly, Resolution WHA63.12. 2010; Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R12-en.pdf. Accessed November, 2014.
- 13.Task Force on Patient Blood Management for Adult Cardiac Surgery of the European Association for Cardio-Thoracic S, The European Association of Cardiothoracic A; Boer C, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32(1):88–120. doi: 10.1053/j.jvca.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 14.Kozek-Langenecker SA, Ahmed AB, Afshari A, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol. 2017;34(6):332–395. doi: 10.1097/EJA.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 15.Society of Thoracic Surgeons Blood Conservation Guideline Task Force; Ferraris VA, Brown JR, et al. 2011 update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–982. doi: 10.1016/j.athoracsur.2010.11.078 [DOI] [PubMed] [Google Scholar]
- 16.Australian National Blood Authority. Patient blood management guidelines: module 2 perioperative: Australian National Blood Authority. 2012; Available from: http://www.blood.gov.au/pbm-module-2. Accessed December17, 2014..
- 17.American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management an updated report by the American society of anesthesiologists task force on perioperative blood management. Anesthesiology. 2015;122(2):241–275. doi: 10.1097/ALN.0000000000000463 [DOI] [PubMed] [Google Scholar]
- 18.NHS. National blood transfusion committee's patient blood management recommendations: NHS. 2014; Available from: http://www.transfusionguidelines.org.uk/uk-transfusion-committees/national-blood-transfusion-committee/patient-blood-management. Accessed February03 2015.
- 19.Dixon B, Reid D, Collins M, et al. The operating surgeon is an independent predictor of chest tube drainage following cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28(2):242–246. doi: 10.1053/j.jvca.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 20.Jin R, Zelinka ES, McDonald J, et al. Effect of hospital culture on blood transfusion in cardiac procedures. Ann Thorac Surg. 2013;95(4):1269–1274. doi: 10.1016/j.athoracsur.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 21.Pearse BL, Rickard CM, Keogh S, Lin Fung Y. A retrospective explanatory case study of the implementation of a bleeding management quality initiative, in an Australian cardiac surgery unit. Aust Crit Care. 2019;32(2):92–99. doi: 10.1016/j.aucc.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 22.Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57(6):1347–1358. doi: 10.1111/trf.2017.57.issue-6 [DOI] [PubMed] [Google Scholar]
- 23.WHO. Global Forum for Blood Safety: Patient Blood Management - Priorities for Action. Dubai, UAE: World Health Organisation; 2011. [Google Scholar]
- 24.Nilsen P. Making sense of implementation theories, models and frameworks. Implementation Sci. 2015;10:1–12. doi: 10.1186/s13012-015-0242-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michie S, Johnston M, Abraham C, et al. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care. 2005;14(1):26–33. doi: 10.1136/qshc.2004.011155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implementation Sci. 2012;7(37):1–17. doi: 10.1186/1748-5908-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation Sci. 2011;6(42):1–11. doi: 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sargent L, McCullough A, Del Mar C, Lowe J. Using theory to explore facilitators and barriers to delayed prescribing in Australia: a qualitative study using the theoretical domains framework and the behaviour change wheel. BMC Fam Pract. 2017;18(1):1–13. doi: 10.1186/s12875-017-0589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassidy C, Bishop A, Steenbeek A, Langille D, Martin-Misener R, Curran J. Barriers and enablers to sexual health service use among university students: a qualitative descriptive study using the theoretical domains framework and COM-B model. BMC Health Serv Res. 2018;18(1):581. doi: 10.1186/s12913-018-3379-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LimeSurvey: an Open Source survey tool LimeSurvey GmbH. Available from: http://www.limesurvey.org.Accessed December16, 2019.
- 31.Likosky DS, FitzGerald DC, Groom RC, et al. The effect of the perioperative blood transfusion and blood conservation in cardiac surgery clinical practice guidelines of the society of thoracic surgeons and the society of cardiovascular anesthesiologists upon clinical practices. Anesth Analg. 2010;111(2):316–323. doi: 10.1213/ANE.0b013e3181e329f1 [DOI] [PubMed] [Google Scholar]
- 32.So-Osman C, Van der Wal DE, Allard S. Patient blood management initiatives on a global level: the results of an international society of blood transfusion survey. ISBT Sci Ser. 2017;12:327–335. doi: 10.1111/voxs.2017.12.issue-3 [DOI] [Google Scholar]
- 33.SPSS IBM. Statistics for Windows Version 25.0. IBM Corp.; 2016. [Google Scholar]
- 34.Myles PS, Smith JA, Forbes A, et al. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017;376(2):136–148. doi: 10.1056/NEJMoa1606424 [DOI] [PubMed] [Google Scholar]
- 35.Tran HA, Chunilal SD, Harper PL, Tran H, Wood EM, Gallus AS. An update of consensus guidelines for warfarin reversal. MJA. 2013;198(4):198–199. doi: 10.5694/mja12.10614 [DOI] [PubMed] [Google Scholar]
- 36.Pearse BL, Smith I, Faulke D, et al. Protocol guided bleeding management improves cardiac surgery patient outcomes. Vox Sang. 2015;109(3):267–279. doi: 10.1111/vox.2015.109.issue-3 [DOI] [PubMed] [Google Scholar]
- 37.Verdecchia NM, Wisniewski MK, Waters JH, Triulzi DJ, Alarcon LH, Yazer MH. Changes in blood product utilization in a seven-hospital system after the implementation of a patient blood management program: a 9-year follow-up. Hematology. 2016;21(8):490–499. doi: 10.1080/10245332.2015.1112496 [DOI] [PubMed] [Google Scholar]
- 38.ACSQHC. National-safety-and-quality-health-service-standards 2nd In: Standards HC,editor. Sydney: Australian Commission of Safety and Qualtiy in Health Care; 2017. [Google Scholar]
- 39.Sousa-Uva M, Head SJ, Milojevic M, et al. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5–33. doi: 10.1093/ejcts/ezx314 [DOI] [PubMed] [Google Scholar]
- 40.Aboul-Hassan SS, Stankowski T, Marczak J, et al. The use of preoperative aspirin in cardiac surgery: a systematic review and meta-analysis. J Card Surg. 2017;32(12):758–774. doi: 10.1111/jocs.v32.12 [DOI] [PubMed] [Google Scholar]
- 41.Couture P, Lebon JS, Laliberte E, et al. Low-dose versus high-dose tranexamic acid reduces the risk of nonischemic seizures after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2017;31(5):1611–1617. doi: 10.1053/j.jvca.2017.04.026 [DOI] [PubMed] [Google Scholar]
- 42.Khair S, Perelman I, Yates J, et al. Exclusion criteria and adverse events in perioperative trials of tranexamic acid in cardiac surgery: a systematic review and meta-analysis. Can J Anaesth. 2019;66:1240–1250. doi: 10.1007/s12630-019-01393-w [DOI] [PubMed] [Google Scholar]
- 43.Ngaage DL, Bland JM. Lessons from aprotinin: is the routine use and inconsistent dosing of tranexamic acid prudent? Meta-analysis of randomised and large matched observational studies. Eur J Cardiothorac Surg. 2010;37(6):1375–1383. doi: 10.1016/j.ejcts.2009.11.055 [DOI] [PubMed] [Google Scholar]
- 44.Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. doi: 10.1136/bmj.e3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goton TE, Chu MWA, Norton L, et al. A prospective observational study of seizures after cardiac surgery using continuous EEG monitoring. Neurocrit Care. 2014;21:220–227. doi: 10.1007/s12028-014-9967-x [DOI] [PubMed] [Google Scholar]
- 46.Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and strategies in guideline implementation-a scoping review. Healthcare (Basel). 2016;4(36):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.American Society of ExtraCorporeal Technology. American society of extracorporeal technology standards and guidelines for perfusion practice In. Available from: http://www.amsect.org/p/cm/ld/fid=16172017.Accessed December16, 2019.
- 48.Karkouti K, Callum J, Wijeysundera DN, et al. Point-of-care hemostatic testing in cardiac surgery: a stepped-wedge clustered randomized controlled trial. Circulation. 2016;134(16):1152–1162. doi: 10.1161/CIRCULATIONAHA.116.023956 [DOI] [PubMed] [Google Scholar]
- 49.Kuiper G, van Egmond LT, Henskens YMC, et al. Shifts of transfusion demand in cardiac surgery after implementation of rotational thromboelastometry-guided transfusion protocols: analysis of the HEROES-CS (HEmostasis Registry of patiEntS in Cardiac Surgery) observational, prospective open cohort database. J Cardiothorac Vasc Anesth. 2019;33(2):307–317. doi: 10.1053/j.jvca.2018.08.203 [DOI] [PubMed] [Google Scholar]
- 50.Gorlinger K, Perez-Ferrer A, Dirkmann D, et al. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J Anesthesiol. 2019;72(4):297–322. doi: 10.4097/kja.19169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wikkelso A, Wetterslev J, Moller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Sys Rev. 2016;(8):CD007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petricevic M, Konosic S, Biocina B, et al. Bleeding risk assessment in patients undergoing elective cardiac surgery using ROTEM((R)) platelet and Multiplate((R)) impedance aggregometry. Anaesthesia. 2016;71(6):636–647. doi: 10.1111/anae.13303 [DOI] [PubMed] [Google Scholar]
- 53.Kong R, Trimmings A, Hutchinson N, et al. Consensus recommendations for using the Multiplate((R)) for platelet function monitoring before cardiac surgery. Int J Lab Hematol. 2015;37(2):143–147. doi: 10.1111/ijlh.12279 [DOI] [PubMed] [Google Scholar]
- 54.Corredor C, Wasowicz M, Karkouti K, Sharma V. The role of point-of-care platelet function testing in predicting postoperative bleeding following cardiac surgery: a systematic review and meta-analysis. Anaesthesia. 2015;70(6):715–731. doi: 10.1111/anae.13083 [DOI] [PubMed] [Google Scholar]
- 55.Shams Hakimi C, Singh S, Hesse C, Jeppsson A. Effects of fibrinogen and platelet transfusion on coagulation and platelet function in bleeding cardiac surgery patients. Acta Anaesthesiol Scand. 2019;63(4):475–482. doi: 10.1111/aas.2019.63.issue-4 [DOI] [PubMed] [Google Scholar]
- 56.Spath N, Lala HM, Robinson SC. Introduction of a simple algorithm improves thromboelastography-guided blood product use during cardiac surgery. Anaesth Intensive Care. 2017;45(1):122–123. [PubMed] [Google Scholar]
- 57.Rahe-Meyer N, Levy JH, Mazer CD, et al. Randomized evaluation of fibrinogen versus placebo in complex cardiovascular surgery: post hoc analysis and interpretation of Phase III results. Interact Cardiovasc Thorac Surg. 2019;28(4):566–574. doi: 10.1093/icvts/ivy302 [DOI] [PubMed] [Google Scholar]
- 58.He C, Pearse B, Tesar P, Faulke D. Fibrinogen concentrate dosing in complex cardiac surgery. Heart Lung Circ. 2017;26. doi: 10.1016/j.hlc.2017.03.051 [DOI] [Google Scholar]
- 59.Ranucci M, Baryshnikova E. Fibrinogen supplementation after cardiac surgery: insights from the Zero-Plasma trial (ZEPLAST). Br J Anaesth. 2016;116(5):618–623. doi: 10.1093/bja/aev539 [DOI] [PubMed] [Google Scholar]
- 60.Miceli A, Ranucci M, Glauber M. Fibrinogen concentrate as first-line hemostatic treatment for the management of bleeding in complex cardiac surgery. J Thorac Cardiovasc Surg. 2016;151(2):383–384. doi: 10.1016/j.jtcvs.2015.09.023 [DOI] [PubMed] [Google Scholar]
- 61.Solomon C, Groner A, Ye J, Pendrak I. Safety of fibrinogen concentrate: analysis of more than 27 years of pharmacovigilance data. Thromb Haemost. 2015;113(4):759–771. doi: 10.1160/TH14-06-0514 [DOI] [PubMed] [Google Scholar]
- 62.Ranucci M, Baryshnikova E, Crapelli GB, Rahe-Meyer N, Menicanti L, Frigiola A. Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J Am Heart Assoc. 2015;4(6). doi: 10.1161/JAHA.115.002066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahe-Meyer N, Solomon C, Hanke A, et al. Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery: a randomized, placebo-controlled trial. Anesthesiology. 2013;118(1):40–50. doi: 10.1097/ALN.0b013e3182715d4d [DOI] [PubMed] [Google Scholar]
- 64.Rahe-Meyer N, Levy JH, Mazer CD, et al. Randomized evaluation of fibrinogen vs placebo in complex cardiovascular surgery (REPLACE): a double-blind phase III study of haemostatic therapy. Br J Anaesth. 2016;117(1):41–51. doi: 10.1093/bja/aew169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Callum J, Farkouh ME, Scales DC, et al. Effect of fibrinogen concentrate vs cryoprecipitate on blood component transfusion after cardiac surgery: the FIBRES randomized clinical trial. JAMA. 2019;102:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desborough MJ, Oakland K, Brierley C, et al. Desmopressin use for minimising perioperative blood transfusion. Cochrane Database Sys Rev. 2017;7:CD001884. doi: 10.1002/14651858.CD003881.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carless PA, Henry DA, Moxey AJ, O’Connell D, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Lib. 2010;(4):CD001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein AA, Bailey CR, Charlton AJ, et al. Association of Anaesthetists guidelines: cell salvage for peri-operative blood conservation 2018. Anaesthesia. 2018;73(9):1141–1150. doi: 10.1111/anae.14331 [DOI] [PubMed] [Google Scholar]
- 69.Meybohm P, Choorapoikayil S, Wessels A, Herrmann E, Zacharowski K, Spahn DR. Washed cell salvage in surgical patients: a review and meta-analysis of prospective randomized trials under PRISMA. Medicine (Baltimore). 2016;95(31):e4490. doi: 10.1097/MD.0000000000004490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Webster S, Todd S, Redhead J, Wright C. Ionised calcium levels in major trauma patients who received blood in the emergency department. Emerg Med J. 2016;33(8):569–572. doi: 10.1136/emermed-2015-205096 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- WHO. Availability, safety and quality of blood products: World Health Organisation (WHO). Sixty-third World Health Assembly, Resolution WHA63.12. 2010; Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R12-en.pdf. Accessed November, 2014.