Abstract
Background
Plasma apolipoprotein C-III (apoC-III) levels are associated with coronary artery disease (CAD) risk.
Objective
To assess whether lipoprotein-associated apoC-III levels predict risk of CAD events.
Methods
ApoC-III associated with apoB, apoAI, and Lp(a) (apoCIII-apoB, apoCIII-apoAI and apoCIII-Lp(a), respectively) were measured using high-throughput chemiluminescent enzyme-linked immunoassays (ELISA) in 2711 subjects (1879 controls and 832 cases with CAD) in the EPIC-Norfolk prospective population study with 7.4 years of follow-up. These measures were correlated with a variety of lipid measurements and the presence of CAD. The indices of “total apoCIII-apoB” and ‘total apoCIII-apoAI” were derived by multiplying plasma apoB and apoAI, respectively.
Results
ApoCIII-apoB (p=0.001), apoCIII-Lp(a) (p<0.001), apoCIII-apoAI (p=0.005) were higher in cases vs controls, tended to correlate positively with BMI, hsCRP, apoCIII, LDL-C, triglycerides, remnant cholesterol, VLDL, LDL and HDL particle number and VLDL size, but negatively with LDL and HDL particle size (p<0.001 for all). ApoCIII-ApoB, apoCIII-apoAI, apoCIII-Lp(a), total apoCIII-Lp(a) and total apoCIII-apoB were predictors of CAD after adjustment of age, sex, BMI, smoking, diabetes, hypertensive and lipid lowering drug use, but they lost their significance following further adjustment of lipid and lipoprotein variables. In contrast, apoCIII remained a significant risk predictor with multivariable adjustment.
Conclusions
This study suggests that ELISA-measured lipoprotein-associated apoCIII markers reflect atherogenic lipid particles but, as opposed to total plasma apoCIII, do not independently predict risk of CAD events.
Keywords: apoC-III, triglycerides, coronary artery disease, myocardial infarction, risk prediction
Introduction
Low density lipoprotein cholesterol (LDL-C) lowering agents, such as statins, ezetimibe and PCSK9 inhibitors have reduced the incidence of coronary artery disease (CAD).1 However, these successful treatment strategies provide modest absolute risk reductions, even when LDL-C as low as 30 mg/dL is achieved, and as a consequence, significant residual risk of CAD events remains. This residual risk has driven the search for novel and causal therapeutic targets. Genetic studies have shifted the attention from high density lipoprotein cholesterol (HDL-C) to triglyceride-rich lipoproteins (TRLs) as causal risk factors in CAD development.2,3
Apolipoprotein C-III (apoC-III) is circulating apolipoprotein that plays a key role as regulator of TRL metabolism and has therefore been considered a target for therapy. apoC-III inhibits the activation of lipoprotein lipase (LPL) by apolipoprotein C-II (apoC-II), thereby inhibiting triglyceride (TG) hydrolysis.4 It also inhibits TRL uptake by the liver, in an LPL-independent fashion5, mediated by the LDL receptor and LRP1.6 A causal relationship between apoC-III and CAD risk has been demonstrated by genetic studies that show a lower CAD risk for individuals with APOC3 loss-of function mutations.7–9 Furthermore, apoC-III plasma levels in prospective population studies are predictive of CAD risk.10–12 These observations have driven the development of apoC-III lowering drugs, which have been shown to successfully lower apoC-III, TG and TRL particle levels in subjects with hypertriglyceridaemia.5,13–15
One of the unresolved issues regarding apoC-III metabolism is the distribution of apoC-III among circulating lipoproteins and their respective predictive risk compared with apoC-III. Several studies have identified specific subgroups of subjects at increased CAD risk based on the number of apoC-III containing LDL or HDL particles,16,17 but their association with CAD risk has been inconsistent.18 One of the explanations for this inconsistency lies in the complicated methods that are required for these assays. To measure apoC-III content in specific lipoprotein fractions, immunoaffinity chromatography has previously been used, but it is limited in its application in large sample sizes. As a complimentary method, we have recently developed quantitative high-throughput sandwich chemiluminescent enzyme-linked immunoassays (ELISAs) to detect apoC-III on individual lipoproteins in plasma.14
Here we report findings of apoC-III content on apolipoprotein B-100 (apoCIII-apoB), apolipoprotein A-I (apoCIII-apoAI), and lipoprotein(a) (apoCIII-Lp(a)) containing lipoproteins in plasma samples from a large nested case control study in the European Prospective Investigation into Cancer and Nutrition (EPIC-) Norfolk prospective population study. We further derive indices of “total apoCIII-apoB” and “total apoCIII-apoAI” by multiplying these measures with plasma apoB and apoA-I. The aim of this study was to assess the association of lipoprotein-associated apoC-III levels with CAD risk in a prospective manner with long-term follow-up.
Methods
Study design
A nested case-control study was performed in the EPIC-Norfolk cohort study, a prospective population study comprising 25,663 men and women aged 45-79 years living in Norfolk, United Kingdom.19 In short, participants were recruited from general practitioner registries and visited a clinic for nonfasting blood sample collection and completed a detailed health and lifestyle questionnaire for a baseline survey between 1993 and 1997.
During follow up, all participants were flagged for mortality at the UK Office of National Statistics, and vital status was ascertained for the entire cohort. Data on all hospital contacts throughout England and Wales were obtained using National Health Service numbers through linkage with the East Norfolk Health Authority database. Hospital records and death certificates were coded by trained nosologists according to the International Classification of Diseases (ICD) 9th revision.
Participants were identified as having CAD during follow-up if they had a hospital admission and/or died with CAD as the underlying cause, coded as ICD 410–414. These codes encompass the clinical spectrum of CAD, that is, unstable angina, stable angina, and myocardial infarction. The study complies with the Declaration of Helsinki. The Norwich District Health Authority Ethics Committee approved the study and all participants gave written informed consent.
In 2004, we designed a prospective nested case–control study among participants of the EPIC-Norfolk cohort who did not report a history of heart attack or stroke at the baseline clinic visit, as previously described. Cases were people who developed CAD during follow-up through 2003.20 Control participants were apparently healthy study participants who remained free of any cardiovascular disease during 7.4 years of follow-up. Two controls were matched to each case by sex, age (within 5 years), and date of visit (within 3 months).
In a recent report,12 we described the relationship of apoC-III levels and incident CAD risk in this same cohort from the EPIC-Norfolk Prospective Population Study. The current analysis is focused on lipoprotein-associated apoC-III levels and their relationship with total apoC-III as predictive variables.
Laboratory measurements
Nonfasting blood samples were taken at baseline and serum levels of total cholesterol, HDL-C and triglycerides were measured on fresh samples with the RA 1000 autoanalyzer (Bayer Diagnostics, Basingstoke, United Kingdom), or stored at -80 °C. LDL-cholesterol levels were calculated with the Friedewald formula. Remnant cholesterol was calculated as total cholesterol minus LDL-Cminus HDL-C, as previously described.21 Lipoprotein subclass particle numbers and size were measured with an automated nuclear magnetic resonance spectroscopic assay as described previously.12, 22
Determination of total apoC-III and lipoprotein-associated apoC-III complex levels: apoCIII-apoB, apoCIII-Lp(a), and apoCIII-apoAI
apoC-III plasma levels were measured at the University of California San Diego, using an in-house chemiluminescent ELISA as previously reported.12
Sensitive and quantitative sandwich-based chemiluminescent ELISA was used to measure apoC-III associated with plasma lipoproteins containing apoB-100 (apoCIII-apoB), Lp(a) (apoCIII-Lp(a)), and apoA-I (apoCIII-apoAI) as previously described in detail.14 Microtiter 96-well plates were coated overnight at 4°C with antibodies: MB47 to bind apoB-100 (MB47 does not detect apoB-48); LPA4 to bind Lp(a); and sheep anti-human apoA-I (The Binding Site, Birmingham, UK) (all at 5 g/mL antigen of 40 L/well). For apoCIII-apoB) and apoA-I (apoCIII-apoAI) conditions were established to ensure that the amount of plasma added was sufficient to provide a saturating and equal amount of apoB and apoAI, respectively (Supplmental Fig. 1). For this reason, the index of “total” apoCIII-apoB and apoCIII-apoAI could be determined by multiplying apoCIII-apoB and apoCIII-apoAI by apoB and apoAI plasma levels, respectively. For the apoCIII-Lp(a) assay, because some patients have unmeasurable or very low Lp(a) levels, at a 1:50 dilution of plasma the microtiter plates cannot be saturated with Lp(a) unless plasma concentration is >~30 mg/dL. Therefore, an index of “total apoCIII-Lp(a)” cannot be determined with this assay. The results are reported as relative light units in 100 milliseconds after the background (TBS/BSA) relative light unit was subtracted. High and low values were added to each 96-well plate as internal controls.
Statistical analysis
Baseline characteristics for CAD cases and controls were reported as mean ±standard deviation for continuous variables with a normal distribution, median (interquartile range) for continuous variables with a non-normal distribution, and percentage (number) for categorical variables. Baseline characteristics were calculated by lipoprotein associated apoC-III quintiles, and then compared using a linear regression model, including lipoprotein associated apoC-III quintiles to test for trend. Owing to a skewed distribution, apoC-III, triglycerides, remnant cholesterol, hsCRP, LPL, Lp(a), and apoA-V levels were logarithmically transformed before statistical analysis. For correlation between apoC-III and other parameters, Spearman correlation coefficients are reported with corresponding P-value.
Mainly due to unavailability of plasma samples, not all study participants could be included for the present study and, as a consequence, the original matching was partially lost. For this reason, we decided to not perform conditional logistic regression using the matching variable in the model, but to perform unconditional logistic regression, incorporating age and sex in the regression model. This model was used to calculate odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs) for CAD risk for each apoC-III quintile, using the lowest quintile as reference. ORs were calculated for model 1 adjusted for age and sex; model 2 plus body mass index (BMI), smoking, diabetes, systolic blood pressure and hypertensive and lipid lowering drug use; model 3 plus LDL-C, TG, and HDL-C; model 4 plus apoAI, apoB, Lp(a); and model 5 plus apoC-III. All statistical analyses were performed using IBM SPSS software, version 22. A P-value < .05 was considered statistically significant.
Results
Baseline characteristics
Baseline plasma samples for lipoprotein-associated apoC-III measurements were available for a total of 2711 subjects, of whom 832 were incident CAD cases and 1879 were CAD event-free controls. Baseline characteristics for cases and controls are shown in Table 1. Age and sex were similar in both groups. Cases were characterized by a higher prevalence of smoking, diabetes, lipid-lowering and antihypertensive drug use, and higher BMI and systolic and diastolic blood pressure levels. Cases had a less favorable lipid profile: higher total cholesterol, LDL-C, triglycerides, apoB and Lp(a) levels and lower HDL-C and apoA-I levels.
Table 1. Baseline characteristics of controls and cases.
| Controls (n=1,879) | Cases (n=832) | p-value | ||
|---|---|---|---|---|
| Age, years | 65.3 ±7.7 | 65.6 ±7.7 | 0.46 | |
| Sex, male | 62.5% (1174) | 62.5% (520) | 0.99 | |
| Body mass index, kg/m2 | 26.3 ±3.5 | 27.3 ±3.8 | <0.001 | |
| Current smoking | 8.4% (157) | 15.4% (127) | <0.001 | |
| Systolic blood pressure, mmHg | 139 ±18 | 144 ±19 | <0.001 | |
| Diastolic blood pressure, mmHg | 84 ±11 | 86 ±12 | <0.001 | |
| Diabetes mellitus | 1.8% (33) | 6.9% (57) | <0.001 | |
| Lipid-lowering drugs | 1.1% (20) | 3.7% (31) | <0.001 | |
| Anti-hypertensive drugs | 17.5% (329) | 42.3% (352) | <0.001 | |
| ApoC-III-apoB, RLU | 10,026 (5,945-16,068) | 11,080 (6,553-17,604) | 0.001 | |
| ApoC-III-Lp(a), RLU | 7,385 (4,756-11,199) | 8,325 (5,280-14,137) | <0.001 | |
| ApoC-III-apoAI, RLU | 6,423 (4,011-9,414) | 6,749 (4,487-9,847) | 0.005 | |
| Total plasma apoC-III, mg/dL | 4.8 (3.5-6.9) | 5.5 (3.8-7.9) | <0.001 | |
| Total cholesterol, mmol/L | 6.3 ±1.2 | 6.5 ±1.2 | <0.001 | |
| LDL cholesterol, mmol/L | 4.1 ±1.0 | 4.3 ±1.0 | <0.001 | |
| HDL cholesterol, mmol/L | 1.4 ±0.4 | 1.3 ±0.4 | <0.001 | |
| Remnant cholesterol, mmol/L | 0.7 (0.5-1.0) | 0.8 (0.6-1.2) | <0.001 | |
| Triglycerides, mmol/L | 1.7 (1.2-2.3) | 2.0 (1.4-2.8) | <0.001 | |
| Apolipoprotein A-I, mg/dL | 162.2 ±28.9 | 154.6 ±29.3 | <0.001 | |
| Apolipoprotein B, mg/dL | 129.1 ±31.2 | 137.6 ±32.7 | <0.001 | |
| Lipoprotein(a), mg/dL | 8.4 (6.4-13.6) | 9.5 (6.9-24.7) | <0.001 | |
apoC-III, apolipoprotein C-III; RLU, relative light units; LDL, low-density lipoprotein; HDL, high-density lipoprotein; apoCIII-apoAI, apoC-III content on apolipoprotein A-I containing lipoproteins; apoCIII-apoB, apoC-III content on apolipoprotein B-100 containing lipoproteins; apoCIII-Lp(a), apoC-III content on apolipoprotein(a).
Continuous variables with a normal distribution are reported as mean ± standard deviation.
Continuous variables with a non-normal distribution are reported as median (interquartile range).
Dichotomous variables are reported as percentage of total cohort (number).
P-values are for student’ s T-test for continuous variables and Χ2 test for dichotomous variables.
Triglycerides, remnant cholesterol and apoC-III parameters were log-transformed before analysis.
RLU = relative light units.
Baseline characteristics according to quintiles of apoCIII-apoAI, apoCIII-B, apoCIII-Lp(a), total apoCIII-apoAI and total apoCIII-apoB are shown in Supplemental tables 1-5. Age was similar for all quintiles and there was a trend toward more females in the higher quintile groups. BMI, systolic and diastolic blood pressure, as well as antihypertensive and lipid-lowering drug use were higher in the highest quintiles. Subjects with high apoCIII-apoB, apoCIII-apoAI and apoCIII-Lp(a) tended to have unfavorable baseline characteristics and lipid profiles, including higher levels of apoC-III, BMI, systolic and diastolic blood pressure, large very low density lipoprotein (VLDL), triglycerides, LDL particle number, small dense LDL, apoB, hsCRP, LCAT and apoA-V concentrations, and lower levels of HDL-C and LPL levels.
Lipoprotein-associated apoC-III levels and CAD risk
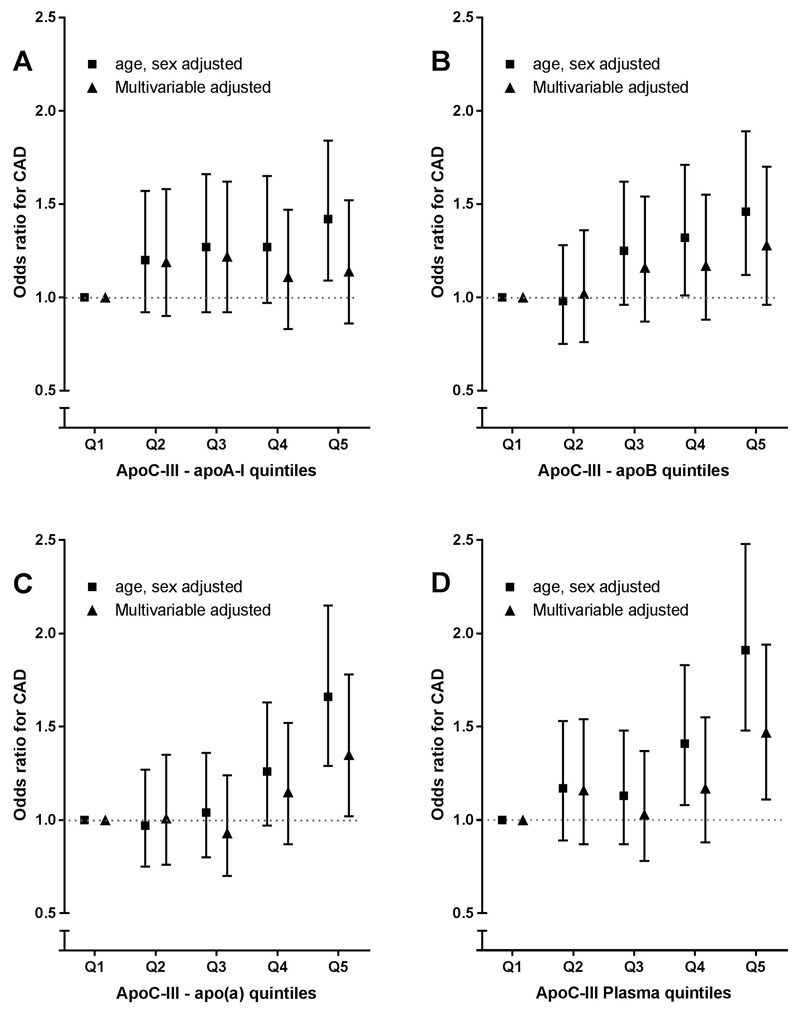
Figure 1A-C shows the ORs of CAD risk for quintiles of lipoprotein-associated apoC-III. apoCIII-apoAI was significantly associated with CAD risk in age and sex adjusted analysis (OR 1.42, 95% CI: 1.09-1.84, for highest compared with lowest quintile) but significance was lost (OR 1.14, 95%CI: 0.86-1.52) after adjustment for CAD risk factors (BMI, current smoking, diabetes mellitus, systolic blood pressure, lipid-lowering and antihypertensive drug use (Fig. 1A). Additional adjustments did not substantially change the association (Table 2).
Figure 1.
Association of lipoprotein-associated apoC-III quintiles and coronary artery diseaserisk. Data are odds ratios and corresponding 95% confidence intervals. (A) apoCIII-apoAI quintiles, (B) apoCIII-apoB quintiles, (C) apoCIII-Lp(a) quintiles. Model 1 is matched for age and sex, the multivariable model is adjusted for age, sex, BMI, current smoking, diabetes mellitus, and use of blood pressure–lowering and lipid-lowering drugs. apoC-III, apolipoprotein C-III; apoCIII-apoAI, apoC-III content on apolipoprotein A-I containing lipoproteins; apoCIII-apoB, apoC-III content on apolipoprotein B-100 containing lipoproteins; apoCIII-Lp(a), apoC-III content on apolipoprotein(a).
Table 2. Odds ratios for coronary artery disease risk according to lipoprotein-associated apoC-III levels.
| Variable | Q1 | Q2 | Q3 | Q4 | Q5 |
|---|---|---|---|---|---|
| apoCIII-apoAI | |||||
| Model 1: adjusted for age, sex | 1.00 | 1.20 (0.92–1.57) | 1.27 (0.98–1.66) | 1.27 (0.97–1.65) | 1.42 (1.09–1.84) |
| Model 2: model 1 + BMI, smoking, diabetes, hypertensive and lipid-lowering drug use. | 1.00 | 1.19 (0.90–1.58) | 1.22 (0.92–1.62) | 1.11 (0.83–1.47) | 1.14 (0.86–1.52) |
| Model 3: model 2 + LDL-C, TG, and HDL-C | 1.00 | 1.12 (0.84–1.50) | 1.02 (0.75–1.37) | 0.88 (0.64–1.20) | 0.88 (0.64–1.21) |
| apoCIII-apoB | |||||
| Model 1: adjusted for age, sex | 1.00 | 0.98 (0.75–1.28) | 1.25 (0.96–1.62) | 1.32 (1.01–1.71) | 1.46 (1.12–1.89) |
| Model 2: model 1 + BMI, smoking, diabetes, hypertensive and lipid-lowering drug use. | 1.00 | 1.02 (0.76–1.36) | 1.16 (0.87–1.54) | 1.17 (0.88–1.55) | 1.28 (0.96–1.70) |
| Model 3: model 2 + LDL-C, TG, and HDL-C | 1.00 | 0.90 (0.67–1.22) | 0.97 (0.71–1.31) | 0.94 (0.68–1.29) | 0.96 (0.68–1.35) |
| apoCIII-Lp(a) | |||||
| Model 1: adjusted for age, sex | 1.00 | 0.97 (0.75–1.27) | 1.04 (0.80–1.36) | 1.26 (0.97–1.63) | 1.66 (1.29–2.15) |
| Model 2: model 1 + BMI, smoking, diabetes, hypertensive and lipid-lowering drug use. | 1.00 | 1.01 (0.76–1.35) | 0.93 (0.70–1.24) | 1.15 (0.87–1.52) | 1.35 (1.02–1.78) |
| Model 3: model 2 + LDL-C, TG, and HDL-C | 1.00 | 0.94 (0.70–1.26) | 0.82 (0.61–1.12) | 1.02 (0.75–1.38) | 0.99 (0.71–1.39) |
| Total apoCIII-apoB | |||||
| Model 1: adjusted for age, sex | 1.00 | 1.30 (0.98–1.73) | 1.04 (0.78–1.39) | 1.19 (0.89–1.59) | 1.22 (0.92–1.63) |
| Model 2: model 1 + BMI smoking, diabetes, hypertensive and lipid-lowering drug use. | 1.00 | 1.31 (0.96–1.78) | 0.99 (0.72–1.36) | 1.08 (0.79–1.47) | 1.03 (0.76–1.42) |
| Model 3: model 2 + LDL-C, TG, and HDL-C | 1.00 | 1.19 (0.86–1.63) | 0.84 (0.60–1.17) | 0.91 (0.64–1.28) | 0.86 (0.60–1.22) |
| Total apoCIII-apoAI | |||||
| Model 1: adjusted for age, sex | 1.00 | 1.30 (0.98–1.73) | 1.04 (0.78–1.39) | 1.19 (0.89–1.59) | 1.22 (0.92–1.63) |
| Model 2: model 1 + BMI smoking, diabetes, hypertensive and lipid-lowering drug use. | 1.00 | 1.31 (0.96–1.78) | 0.99 (0.72–1.36) | 1.08 (0.79–1.47) | 1.03 (0.76–1.42) |
| Model 3: model 2 + LDL-C, TG, and HDL-C | 1.00 | 1.19 (0.86–1.63) | 0.84 (0.60–1.17) | 0.91 (0.64–1.28) | 0.86 (0.60–1.22) |
| apoC-III | |||||
| Model 1: adjusted for age, sex | 1.00 | 1.18 (0.89–1.55) | 1.12 (0.86–1.47) | 1.42 (1.09–1.85) | 1.93 (1.49–2.51) |
| Model 2: model 1 + BMI, smoking, diabetes, hypertensive and lipid-lowering drug use. | 1.00 | 1.19 (0.89–1.58) | 1.02 (0.77–1.36) | 1.20 (0.90–1.59) | 1.50 (1.13–1.98) |
| Model 3: model 2 + LDL-C, TG, and HDL-C | 1.00 | 1.07 (0.80–1.43) | 0.91 (0.68–1.23) | 1.03 (0.76–1.39) | 1.20 (0.87–1.64) |
apoC-III, apolipoprotein C-III; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; apoCIII-apoAI, apoC-III content on apolipoprotein A-I containing lipoproteins; apoCIII-apoB, apoC-III content on apolipoprotein B-100 containing lipoproteins; apoCIII-Lp(a), apoC-III content on apolipoprotein(a).
Data represent odds ratios ± 95% CI, in different models with different adjustments.
Similarly, apoCIII-apoB was associated with CAD risk in age- and sex-adjusted analysis when comparing highest with lowest quintile (OR 1.46, 95% CI 1.12-1.89), but significance was lost after adjustments for CAD risk factors (OR 1.28, 95%CI 0.96-1.70) (Fig. 1B and Table 2).
apoCIII-Lp(a) was associated with CAD risk in age- and sex- adjusted analysis (OR 1.66, 95% CI 1.29-2.15). apoCIII-Lp(a) remained significant after additional adjusting for CAD risk factors (OR 1.35, 95% CI 1.02-1.78), but this significance was lost after adding lipid variables and apoC-III (Fig. 1C and Table 2).
Similar results were found for lipoprotein-associated apoC-III and total apoC-III as continuous variables (data not shown).
Total apoC-III in apoAI and apoB-100 pools and CAD risk
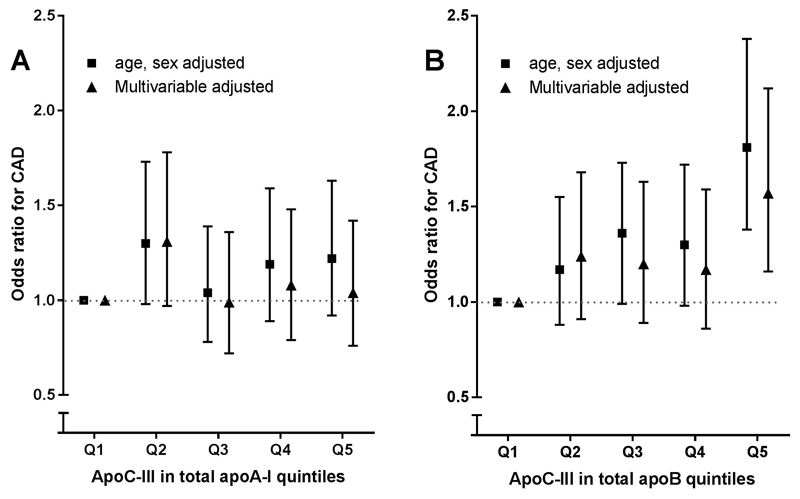
Next, we calculated an index of the amount of apoC-III in the total apoA-I and apoB pool by multiplying average apoCIII-apoAI or apoCIII-apoB with total apoA-I and apoB levels, respectively. apoC-III content in apoA-I containing lipoproteins was not associated with CAD risk in age- and sex- adjusted analysis (OR 1.22, 95% CI 0.92-1.63, Fig. 2A). By contrast, apoC-III content in apoB containing lipoproteins was significantly associated with CAD risk in age and sex (OR 1.81 95% CI 1.38-2.38) and in model 2 (OR 1.57 95% CI 1.16-2.12) for highest compared with lowest quintile, Figure 2B. However, with further adjustment for LDL-C, TG, and Lp(a), as well as apoC-III, significance was no longer present (Table 2).
Figure 2.
ApoC-III in total apoA-I and apoB and CAD risk. apoC-III in total apoAI and apoB and coronary artery disease risk. apoC-III in total apoAI and apoB was calculated by multiplying average apoC-III per lipoprotein with total lipoprotein concentration. Association of apoC-III in total apoAI and apoB quintiles and coronary artery disease risk are shown in Figure 3. Data are odds ratios and corresponding 95% confidence intervals. Model 1 is matched for age and sex, the multivariable model is adjusted for age, sex, BMI, current smoking, diabetes mellitus, and use of blood pressure–lowering and lipid-lowering drugs. apoC-III, apolipoprotein C-III.
Correlations of lipoprotein-associated apoC-III levels and lipid metabolism related parameters
Spearman correlations of lipoprotein-associated apoC-III levels and lipid and nonlipid-related parameters are shown in Table 3. The strongest correlations were found for levels of triglycerides, remnant cholesterol, LDL-C, and apoB. There was a weak correlation with apoA-I levels and no correlation with Lp(a) levels. For nuclear magnetic resonance-related parameters, all lipoprotein associated apoC-III levels were correlated with total VLDL particles concentration and size, total LDL particles concentration and smaller particle size, and total HDL particles concentration and smaller particle size. Overall the correlations were strikingly similar, and there was no large difference between apoCIII-apoAI, apoCIII-apoB or apoCIII-apo(a)-associated apoC-III.
Table 3. Spearman correlation coefficients with lipid and non-lipid parameters.
| apoCIII-ApoAI | P-value | apoCIII-apoB | P-value | apoCIII-Lp(a) | P-value | Total ApoCIII-apoA1 | P-value | Total ApoCIII-apoB | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total plasma ApoCIII, mg/dL | 0.34 | <0.001 | 0.35 | <0.001 | 0.41 | <0.001 | 0.32 | <0.001 | 0.37 | <0.001 |
| Triglycerides, mmol/L | 0.45 | <0.001 | 0.56 | <0.001 | 0.52 | <0.001 | 0.36 | <0.001 | 0.62 | <0.001 |
| Remnant Cholesterol, mmol/L | 0.43 | <0.001 | 0.53 | <0.001 | 0.48 | <0.001 | 0.34 | <0.001 | 0.60 | <0.001 |
| Total Cholesterol, mmol/L | 0.30 | <0.001 | 0.30 | <0.001 | 0.32 | <0.001 | 0.34 | <0.001 | 0.46 | <0.001 |
| LDL-Cholesterol, mmol/L | 0.19 | <0.001 | 0.14 | <0.001 | 0.18 | <0.001 | 0.18 | <0.001 | 0.34 | <0.001 |
| HDL-Cholesterol, mmol/L | -0.06 | 0.001 | -0.07 | 0.001 | -0.05 | 0.02 | 0.15 | <0.001 | -0.11 | <0.001 |
| Apolipoprotein A-I, mg/dL | 0.07 | <0.001 | 0.05 | 0.02 | -0.01 | 0.73 | 0.33 | <0.001 | 0.04 | 0.08 |
| Apolipoprotein B, mg/dL | 0.35 | <0.001 | 0.32 | <0.001 | 0.31 | <0.001 | 0.32 | <0.001 | 0.55 | <0.001 |
| Lipoprotein(a), mg/dL | 0.01 | 0.45 | -0.03 | 0.06 | 0.01 | 0.71 | 0.02 | 0.36 | 0.01 | 0.61 |
| VLDL particles (NMR), nmol/L | 0.32 | <0.001 | 0.37 | <0.001 | 0.35 | <0.001 | 0.27 | <0.001 | 0.51 | <0.001 |
| VLDL size (NMR), nm | 0.27 | <0.001 | 0.36 | <0.001 | 0.35 | <0.001 | 0.20 | <0.001 | 0.34 | <0.001 |
| LDL particles (NMR), nmol/L | 0.41 | <0.001 | 0.40 | <0.001 | 0.32 | <0.001 | 0.33 | <0.001 | 0.57 | <0.001 |
| LDL size (NMR), nm | -0.28 | <0.001 | -0.32 | <0.001 | -0.24 | <0.001 | -0.15 | <0.001 | -0.35 | <0.001 |
| HDL particles (NMR), nmol/L | 0.33 | <0.001 | 0.35 | <0.001 | 0.34 | <0.001 | 0.42 | <0.001 | 0.32 | <0.001 |
| HDL size (NMR), nm | -0.18 | <0.001 | -0.23 | <0.001 | -0.28 | <0.001 | -0.02 | 0.43 | -0.31 | <0.001 |
| BMI, kg/m2 | 0.14 | <0.001 | 0.17 | <0.001 | 0.18 | <0.001 | 0.09 | <0.001 | 0.19 | <0.001 |
| hsCRP, mg/L | 0.09 | <0.001 | 0.06 | <0.01 | 0.08 | <0.001 | 0.060 | 0.005 | 0.08 | <0.001 |
apoC-III, apolipoprotein C-III; LDL, low-density lipoprotein; HDL, high-density lipoprotein; NMR, nuclear magnetic resonance; VLDL, very low density lipoprotein.
Distribution of plasma total apoC-III levels and lipoprotein-associated apoC-III levels
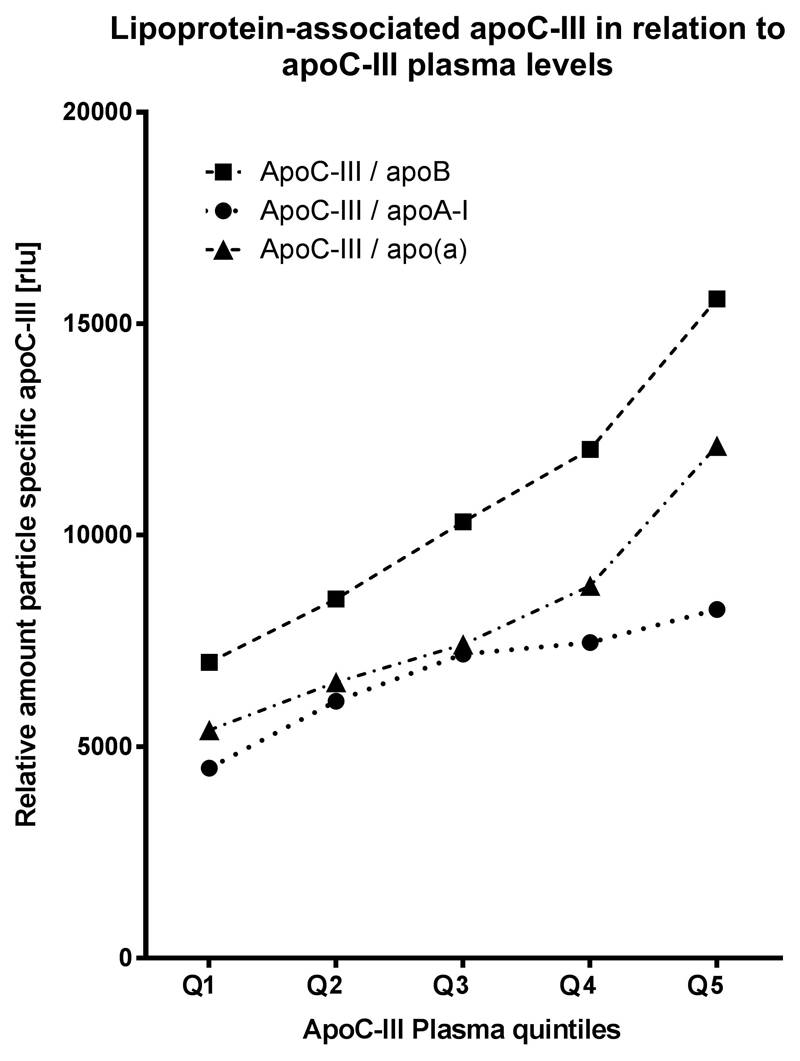
Finally, we show the distribution of lipoprotein-associated apoC-III levels per quintiles of total plasma apoC-III (Fig. 3). The baseline values are higher for apoCIII-apoB and the relative amount of all lipoprotein-associated apoC-III levels are significantly associated with increasing plasma apoC-III quintiles (P for trend <.001).
Figure 3.
Levels of lipoprotein-associated apoC-III for quintiles of total plasma apoC-III. Figure shows median apoCIII-apoAI, apoCIII-apoB, and apoCIII-Lp(a) levels for quintiles of plasma total apoC-III. apoC-III, apolipoprotein C-III; apoCIII-apoAI, apoC-III content on apolipoprotein A-I containing lipoproteins; apoCIII-apoB, apoC-III content on apolipoprotein B-100 containing lipoproteins; apoCIII-Lp(a), apoC-III content on apolipoprotein(a).
Discussion
This study demonstrates that lipoprotein-associated apoC-III levels, measured by immunoassays, predict risk of CAD in age- and sex- adjusted analyses, as well as in multivariable analyses additionally adjusting for plus BMI, smoking, diabetes, systolic blood pressure, and hypertensive and lipid-lowering drug use. However, these associations become nonsignificant with further adjustment of lipid variables. These data suggest that measurement of apoC-III on individual lipoproteins using ELISA techniques does not provide additional predictive information in fully adjusted multivariable models including HDL-C, LDL-C, and triglycerides.
Previous measurements of lipoprotein-associated apoC-III levels used immunoaffinity chromatography techniques that are labor-intensive, which limits the sample size of study groups.16 A significant association has been reported for LDL or VLDL fractions containing apoC-III with CHD risk after multivariable analyses compared with LDL or VLDL without apoC-III or total plasma apoC-III levels.16 In the same cohort HDL fractions containing were associated with CHD, but this was borderline significant (P = .05) with multivariable analysis including triglyceride levels.17 In addition, total apoC-III was not associated with CHD after multivariable analysis. In a more recent analysis that is consistent with our data using an apoCIII-apoAI ELISA similar to the one we first described,14 no association in a quintile analysis was present with CHD in both the MESA and Danish diet, Cancer and Heart Study, although significance was noted in the Danish Diet, Cancer and Heart Study by analysis per standard deviation.23 In the same study in 4 cohorts combined in a total of 2997 incident cases, the hazard ratio for HDL fractions containing apoC-III was 1.09 (1.01–1.18) when adjusting for age, sex, prevalent diabetes mellitus, treatment with antihypertensive medication, race/ethnicity in MESA, systolic blood pressure, smoking, and total cholesterol. However, when LDL-C and triglycerides were added, it was no longer significant (hazard ratio 1.05 [0.96–1.14]). Overall, this suggests that various lipoprotein fractions containing apoC-III, either by ELISA or by immunoaffinity chromatography techniques, have modest to no predictive value when full adjustment of data is performed. Total apoC-III is predictive,18 but also loses it predictive value after adjustment. As we have recently shown in a mediation analysis in the EPIC-Norfolk study, it appears that the risk of apoC-III can be explained by TRLs, small dense LDL and inflammatory mediators.12Because these lipoproteins have modest correlations with each other, independent associations are difficult to document statistically.
To unravel the potentially adverse impact of apoC-III on particular lipoproteins we developed a technique enabling us to quantify the average content of apoC-III particles within a specific, total lipoprotein fraction, in contrast to the quantification of total apoC-III content within a specific lipoprotein fraction. apoC-III is not evenly distributed among lipoproteins, for example, 40%-60% of VLDL (up to 100 apoC-III proteins per lipoprotein) and 10%-20% of LDL particles and up to 15% of HDL particles carry apoC-III.23 This reflected in our study with higher relative readings for apoCIII-apoB relative to the other lipoprotein-associated measures. Our study shows that measurement of average apoC-III content in individual lipoprotein fractions does not provide more predictive information compared with total plasma apoC-III measurement.
Interestingly, the present study does uncover a gap in our current understanding of the processes regulating the distribution of apoC-III on lipoproteins. This is best appreciated from the apoCIII-Lp(a) measurement, in which the content of apoC-III on Lp(a) was not a function of the Lp(a) level, but a function of plasma apoC-III level. Thus, in people with higher total plasma apoC-III, apoCIII-Lp(a) is higher, while Lp(a) is similar, hence, apoC-III content on Lp(a) appears to be controlled by plasma apoC-III levels, and not by plasma Lp(a) levels. Results from apoC-III lowering trials with volanesorsen, an antisense drug that targets APOC3 mRNA, resulted in robust reductions of total plasma apoC-III and lipoprotein-associated apo-CIII on apoB, apoAI and Lp(a) in hypertriglyceridemic subjects.14 It is interesting to note that inhibition of apoC-III production results in reductions of lipoprotein-associated apoC-III levels that are virtually the same for all lipoproteins that were measured, for example, 12 weeks 300 mg volanesorsen treatment resulted in 81±14%, 82±12% and 81±16% reductions in apoCIII-apoAI, apoCIII-apoB, and apoCIII-Lp(a), respectively. How this relates to a strict separation in apoC-III containing lipoproteins, which have increased atherogenic potential and lipoproteins without apoC-III, requires further study.23
Our study has several limitations. Intrinsic to our methodology we did not directly measure total apoC-III content in a specific lipoprotein pool. We also did not measure the ratio of apoC-III-positive and -negative lipoproteins and cannot rule out that this discrimination might be an even stronger CAD risk factor.
In summary, these data suggest that measurement of apoC-III on individual lipoproteins may not provide additional predictive data in patients in the primary care setting. Further study is required to assess if these biomarkers predict future risk in individuals with established CVD.
Supplementary Material
Highlights.
High-throughput ELISAs were developed to measure lipoprotein-associated apoCIII levels
ApoCIII-apoB, apoCIII-Lp(a) and apoCIII-apoAI were measured in EPIC-Norfolk
ApoCIII-apoB, apoCIII-Lp(a) and apoCIII-apoAI reflect atherogenic lipid particles
As opposed to total apoCIII, they do not independently predict risk of CAD events
Their role in patients with prior history of CAD needs to be evaluated
Acknowledgements
The authors wish to thank the participants and staff of the EPIC-Norfolk prospective population study. Authors' contributions: J.C.v.C. and R.V. helped in data analysis and interpretation; S-R.L. helped in data acquisition; J.J.P.K., N.J.W., E.S.G.S., G.K.H., K-T.K., S.M.B., and J.L.W. participated in revising the article critically for intellectual content; S.T. helped in conception, design, analysis, and interpretation of data for the work. All authors have approved the final version of the article.
Sources of funding
The EPIC-Norfolk Study is funded by Cancer Research UK grant number 14136 and the Medical Research Council grant number G1000143. J.J.K. is a recipient of the Dutch Heart Foundation Lifetime Achievement Award (2010) #2010T082. G.K.H. is holder of a Vidi grant [016.156.445] from the Netherlands Organisation for Scientific Research (NWO). S.T. and J.L.W. are supported by NIH grants R01-HL119828, P01-HL088093, P01 HL055798, R01-HL106579, R01-HL078610, and R01-HL124174.
Abbreviations
- ApoC-II
apolipoprotein C-II
- ApoC-III
apolipoprotein C-III
- ApoCIII-apoAI
apoC-III content on apolipoprotein A-I containing lipoproteins
- ApoCIII-apoB
apoC-III content on apolipoprotein B-100 containing lipoproteins
- ApoCIII-Lp(a)
apoC-III content on apolipoprotein(a)
- CAD
coronary artery disease
- CETP
cholesteryl ester transfer protein
- hsCRP
high sensitivity C-reactive protein
- LCAT
lecithin–cholesterol acyltransferase
- Lp(a)
ipoprotein(a)
- LPL
lipoprotein lipase
- NMR
nuclear magnetic resonance
- RLU
relative light unit
- TRL
triglyceride rich lipoprotein
Footnotes
Disclosures
J.C.v.C., S-R.L., R.V., N.J.W., K-T.K. and S.M.B. have nothing to disclose; J.J.P.K. reports personal fees from CSL Behring, Regeneron, Staten Biotech, Madrigal, the Medicines Company, Kowa, li Lilly, Esperion, Gemphire, Ionis Pharmaceuticals and Akcea Therapeutics; E.S.G.S. reports that his institution has received lecture/consulting fees from Akcea Therapeutics, Amgen, Sanofi, AstraZeneca, and Novartis; J.L.W. is a consultant to Ionis Pharmaceuticals. S.T. and J.L.W. are coinventors and receive royalties from patents owned by the University of California San Diego on oxidation-specific antibodies. S.T. currently has a dual appointment at UCSD and as an employee of Ionis Pharmaceuticals.
References
- 1.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 2.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–1352. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginsberg HN, Le NA, Goldberg IJ, et al. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest. 1986;78:1287–1295. doi: 10.1172/JCI112713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaudet D, Brisson D, Tremblay K, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371:2200–2206. doi: 10.1056/NEJMoa1400284. [DOI] [PubMed] [Google Scholar]
- 6.Gordts PL, Nock R, Son NH, et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest. 2016;126:2855–2866. doi: 10.1172/JCI86610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natarajan P, Kohli P, Baber U, et al. Association of APOC3 Loss-of-function mutations with plasma lipids and subclinical atherosclerosis: The Multi-Ethnic BioImage Study. J Am Coll Cardiol. 2015;66:2053–2055. doi: 10.1016/j.jacc.2015.08.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and Risk of ischemic vascular disease. N Engl J Med. 2014 doi: 10.1056/NEJMoa1308027. 0:null. [DOI] [PubMed] [Google Scholar]
- 9.HDL Working Group of the Exome Sequencing Project NHL, Blood I. Crosby J, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheffer PG, Teerlink T, Dekker JM, et al. Increased plasma apolipoprotein C-III concentration independently predicts cardiovascular mortality: the Hoorn Study. Clin Chem. 2008;54:1325–1330. doi: 10.1373/clinchem.2008.103234. [DOI] [PubMed] [Google Scholar]
- 11.Pechlaner R, Tsimikas S, Yin X, et al. Very-low-density lipoprotein-associated apolipoproteins predict cardiovascular events and are lowered by inhibition of APOC-III. J Am Coll Cardiol. 2017;69:789–800. doi: 10.1016/j.jacc.2016.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Capelleveen JC, Bernelot Moens SJ, Yang X, et al. Apolipoprotein C-III levels and incident coronary artery disease risk: The EPIC-Norfolk Prospective Population Study. Arterioscler Thromb Vasc Biol. 2017;37:1206–1212. doi: 10.1161/ATVBAHA.117.309007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaudet D, Alexander VJ, Baker BF, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373:438–447. doi: 10.1056/NEJMoa1400283. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Lee SR, Choi YS, et al. Reduction in lipoprotein-associated apoC-III levels following volanesorsen therapy: phase 2 randomized trial results. J Lipid Res. 2016;57:706–713. doi: 10.1194/jlr.M066399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khetarpal SA, Zeng X, Millar JS, et al. A human APOC3 missense variant and monoclonal antibody accelerate apoC-III clearance and lower triglyceride-rich lipoprotein levels. Nat Med. 2017;23:1086–1094. doi: 10.1038/nm.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendivil CO, Rimm EB, Furtado J, Chiuve SE, Sacks FM. Low-density lipoproteins containing apolipoprotein C-III and the risk of coronary heart disease. Circulation. 2011;124:2065–2072. doi: 10.1161/CIRCULATIONAHA.111.056986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen MK, Rimm EB, Furtado JD, Sacks FM. Apolipoprotein C-III as a potential modulator of the association between hdl-cholesterol and incident coronary heart disease. Journal of the American Heart Association. 2012;1 doi: 10.1161/JAHA.111.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyler von Ballmoos MC, Haring B, Sacks FM. The risk of cardiovascular events with increased apolipoprotein CIII: A systematic review and meta-analysis. J Clin Lipidol. 2015;9:498–510. doi: 10.1016/j.jacl.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Day N, Oakes S, Luben R, et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer. 1999;80(Suppl 1):95–103. [PubMed] [Google Scholar]
- 20.Boekholdt SM, Peters RJ, Day NE, et al. Macrophage migration inhibitory factor and the risk of myocardial infarction or death due to coronary artery disease in adults without prior myocardial infarction or stroke: the EPIC-Norfolk Prospective Population study. Am J Med. 2004;117:390–397. doi: 10.1016/j.amjmed.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Jepsen AM, Langsted A, Varbo A, Bang LE, Kamstrup PR, Nordestgaard BG. Increased Remnant cholesterol explains part of residual risk of all-cause mortality in 5414 patients with ischemic heart disease. Clin Chem. 2016;62:593–604. doi: 10.1373/clinchem.2015.253757. [DOI] [PubMed] [Google Scholar]
- 22.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Kawakami A, Aikawa M, Libby P, Alcaide P, Luscinskas FW, Sacks FM. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation. 2006;113:691–700. doi: 10.1161/CIRCULATIONAHA.105.591743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.