Abstract
Purpose
Radioresistance in response to radiotherapy leads to cancer recurrence and poor survival in hypopharyngeal carcinoma patients. Previous studies indicate that ionizing radiation (IR) can induce epithelial–mesenchymal transition (EMT) that promotes the radioresistance, migration and invasiveness of tumors. The aim of this study was to explore the role of Snail in EMT and acquired radioresistance in hypopharyngeal carcinoma.
Methods
Radioresistance human hypopharyngeal carcinoma cells (FaduRR) were previously established from the Fadu cell line. Radiosensitivity was measured by colony forming assay. Western blot and Quantitative real-time PCR were used to detect the expression of EMT phenotypes and AKT/GSK-3β/Snail signaling pathway related proteins in Fadu+4Gy and FaduRR cells. Transwell and wound-healing assays were used to measure cell migration and invasiveness. EMT-related proteins and Snail expression were assessed in 80 hypopharyngeal carcinoma patient samples from radiosensitive and radioresistance groups using immunohistochemistry. Snail was silenced to evaluate its effects on EMT, radioresistance, migration, and invasiveness of FaduRR cells.
Results
The molecular characteristics of EMT were observed following radiation treatment, with migration, invasiveness and radioresistance enhanced in Fadu+4Gy and FaduRR cells. Moreover, we demonstrated that IR-induced EMT by activating the AKT/GSK-3β/Snail signaling pathway and that Snail silencing reversed EMT and attenuated radioresistance in FaduRR cells. Significant differences in EMT-related proteins and Snail expression were observed between radiosensitive and resistant group.
Conclusion
We demonstrate that IR can trigger EMT and enhance the migration, invasiveness, and radioresistance of FaduRR cells through the AKT/GSK-3β/Snail axis. Snail silencing could attenuate these effects and represents a novel therapeutic target for EMT-induced radioresistance in hypopharyngeal carcinoma.
Keywords: hypopharyngeal carcinoma, epithelial-mesenchymal transition, AKT/GSK-3β/Snail, radioresistance, Snail
Introduction
Hypopharyngeal carcinoma is one of the worst prognosis head and neck malignancy with prominent morbidity and mortality. Especially in hypopharyngeal squamous cell carcinoma (HSCC). Commonly, the standard therapy strategy for HSCC is surgery combined with concurrent chemoradiotherapy. IR is used to treat low-grade tumors for larynx preservation, whilst surgery is essential for high-grade HSCC prior to- or following IR.1–5 Although progress has been made in radio-therapeutic strategies for HSCC, distant metastases and local recurrence remain a challenge to HSCC treatment, the 5-year survival rates as low as 25–40% due to tumor acquired radioresistance.6 New strategies to overcome radioresistance in HSCC are therefore urgently required.
EMT is an essential biological process in which epithelial tumor cells lose epithelial polarity, adhesion and motility, and translate to a mesenchymal phenotype. EMT is considered crucial during tumor progression, involving cancer stem cells, wound healing, invasion to distant metastatic disease, and chemotherapeutic resistance.7–12 Recently, IR has been shown to induce EMT leading to acquired radioresistance.13–16 However, the underlying mechanisms governing these effects are largely unknown. Knowledge of the potential mechanisms of EMT during radioresistance are required for the development of more potent anti-HSCC therapeutics.
Snail, as one of the Snail family of zinc finger transcription factors, regulates and induces EMT. Previous studies have shown that Snail contributes to the pathogenesis of malignant tumors, and mediates EMT progression and increases the migratory and invasive behavior.17 A negative correlation between Snail and E-cadherin expression have been reported in an array of cancer types.18 Recently, Snail has been shown to mediate specific features of cancer stem cells shifting them towards chemoresistant and radioresistant phenotypes in ovarian cancer cells.19 EMT regulates a plethora of pro-oncogenic signaling cascades including IL-6/JAK/STAT3,20 and AKT/GSK-3β.21 In addition, a central feature of EMT occurs through the activation of PI3K/AKT/GSK-3β signaling pathway, in which the expression of Snail is regulated by GSK-3β through posttranslational modifications.22 The specific role of Snail in IR-induced EMT and acquired radioresistance is essential in HSCC. These findings highlight the importance of AKT/GSK-3β/Snail signaling pathway in the regulation of EMT.
In this study, we demonstrate that IR can induce EMT and promote radioresistance via the AKT/GSK-3β/Snail axis in HSCC cells. We further found that the depletion of Snail could reverse IR-induced EMT and radioresistance, providing a new therapeutic strategy for much needed anti-HSCC therapeutics.
Materials and Methods
Patients and Tissue Samples
A written informed consent which protected their safety and privacy was obtained from the patients in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Chongqing Medical University. A total of 80 HSCC patients were enrolled from the First Affiliated Hospital of Chongqing Medical University during 2010 to 2019. Inclusion criteria: (1) patients diagnosed with HSCC by hypopharyngeal biopsy and histopathological. (2) Patients received no treatment prior to admission. (3) Patients treated with single modality radiotherapy with curative intent. (4) Clinical data including age, gender, differentiation, CT, and MRI were available. The radiotherapy dose administered was either 70 Gy in 33 fractions or 60 Gy in 33 fractions.
The Response Evaluation Criteria for Solid Tumors (RECIST) version 1.1 was used to estimate treatment responses. Two experienced radiologists were invited to assess the radiographic images, involving magnetic resonance imaging (MRI) and computed tomography (CT). Partial (PR) and complete responses (CR) were included in the radiosensitivity group. Progressive period (PD) and stable periods (SD) were used as the radioresistance group.
Immunohistochemistry (IHC)
IHC was performed on 4 μm sections that were fixed with formalin and paraffin embedded. Sections were dewaxed in xylene and rehydrated in a graded alcohol series. Citrate buffer was used for antigen retrieval at 100°C for 30 min, and slides were pre-processed with Peroxidase Block (ZSGB-BIO, Beijing, China) for 20 min to ensure the quenching of endogenous peroxidase activity. Sections were washed three times in PBS for 5 min. Primary antibodies including E-cadherin, Vimentin, and N-cadherin were obtained from CST. Sections were probed with anti-Snail antibodies (Abcam, London, UK) were applied at 1:100 dilution, placed in 4°C refrigerator overnight. The second day slides were washed twice using cold PBS and labeled with secondary antibodies for 25 min. Sections were stained with DAB (ZSGB-BIO, Beijing, China).
IHC was evaluated by two experienced pathologists and using a double-blind method. According to total area and intensity of the staining, the expression of Snail, Vimentin, N-cadherin and E-cadherin were scored as either negative (< 50% of cells) or positive (> 50% of cells).
Cell Culture, Irradiation and Reagents
The human hypopharyngeal carcinoma cell line Fadu was obtained from the Standard cell bank of the Chinese Academy of Sciences, and a humidified incubator containing DMEM/high glucose medium (Hyclone, Logan, USA) supplemented with 10% FBS (Gibco, Waltham, USA) was used to culture cells at 37°C in a 5% CO2 atmosphere. When cells were grown to approximately 70% confluence, they were irradiated with 2Gy of X-ray in a linear 6 MV X-ray accelerator (UNIQUE, Varian, Palo Alto, CA, USA). Irradiated cells were grown to 70% confluence and fractional doses were gradually aggrandized prior to reaching 10 Gy. The final dose reached 60 Gy. Resistance levels were determined using colony-forming assays and radioresistant cells were termed FaduRR. Cells were established over an 8 month period using 4 and 10 generations for subsequent experiments.
Clonogenic Assays
Clonogenic potency was measured through clonogenic assays. Parental hypopharyngeal cancer cells and radioresistance hypopharyngeal cancer cells in the exponential growth period were seeded in six-well plates (500, 1000, 2000, 3000, 4000 and 5000 per well) and incubated overnight. Cells were treated with irradiation doses (0Gy, 2Gy, 4Gy, 6Gy, 8Gy and 10Gy, respectively) of 6-MV X-ray. Following irradiation, cells were incubated at 37°C in a 5% CO2 incubator for 15 d. After 15 d, the culture medium was discarded, cells were washed with phosphate buffer (PBS), and colonies were fixed in 100% methanol for 25 min, an stained with 0.1% crystal violet. Colony numbers were then counted (Visible colonies were defined as > 50 cells) counted on an inverted microscope. The extrapolation number (N) values, average lethal dosage (D0), quasi-field dosage (Dq), and sensitization enhancement ratio (SER) were assessed. Surviving fractions were estimated as (colony number)/(cell number seeded ×Plating Efficiency) ×100%. A multitarget-single click mathematical model was used to fit the survival curve using GraphPad Prism 5.
Western Blot Analysis
Following irradiation, cells were harvested at the specified time points and lysed in RIPA buffer. BCA assays (Beyotime Institute of Biotechnology, Nantong, China) were used to determine protein concentrations. Equal amounts of total protein were resolved by 10% SDS-PAGE and transferred onto PVDF membranes. Membranes were blocked with 5% non-fat dry milk for 2 h at room temperature, and probed with primary antibodies at 4°C overnight. Membranes were labeled with HRP-conjugated secondary antibodies at room temperature for 1 h and washed three times in PBS. Proteins were visualized using the ECL system (Pierce, Thermo, USA). Antibodies were purchased from CST (Cell Signaling Technology, Beverly, MA) and included: rabbit anti-E-cadherin (catalog no. 3195), rabbit anti-N-cadherin (catalog no. 13116), rabbit anti-Vimentin (catalog no. 5741), rabbit anti-Snail (catalog no. 3879), rabbit anti-GSK-3β(catalog no. 12456), rabbit anti-phosphorylated GSK-3β (p-GSK-3β) (catalog no. 14310), rabbit anti-AKT (catalog no. 4685), rabbit anti-phosphorylated AKT (p-AKT) (catalog no. 12178), rabbit anti-Cleaved caspase 3 (catalog no. 9654), rabbit anti-Bcl-2 (catalog no. 15071), rabbit anti-Bax (catalog no. 14796).
Quantitative Real-Time PCR Assays
Quantitative real-time PCR (Q-PCR) assays were used to assess the relative expression of E-cadherin, N-cadherin, Vimentin and Snail. RNA was extracted in Trizol Reagent (Invitrogen, Carlsbad, USA) and cDNA was synthesized using PrimerScript™ RT Reagent Kits with a gDNA Eraser (Takara, Dalian, China) was performed to reverse transcribe total RNA into cDNA according to the manufacturer’s instructions. A MyiQ thermal cycler (Bio-RAD CFX96, Hercules, CA, USA) and the SYBR Premix Ex Taq (Takara, Dalian, China) were used to perform Q-PCRs. Reactions were performed in triplicate. Primer sequences are listed in Table 1. Data were analyzed using the ΔΔCt method.
Table 1.
The Primer Sequences Used for Real-Time Quantitative Polymerase Chain Reaction
| Name | Primer Sequence |
|---|---|
| E-cadherin | F:5ʹ-GCTCTTCCAGGAACCTCTGTGATG-3ʹ R:5ʹ-AAGCGATGGCGGCATTGTAGG-3’ |
| N-cadherin | F:5ʹ-AAGGTGGATGAAGATGGCATGGTG-3ʹ R:5ʹ-TGCTGACTCCTTCACTGACTCCTC-3’ |
| Vimentin | F:5ʹ-TTGCCGTTGAAGCTGCTAACTACC-3ʹ R:5ʹ-AATCCTGCTCTCTCCTCGCCTTCC-3’ |
| Snail | F:5ʹ-GGCTCCTTCGTCCTTCTCCTCTAC-3ʹ R:5ʹ-CCAGGCTGAGGTATTCCTTGTTGC-3’ |
| GAPDH | F:5ʹ-CCGGGAAACTGTGGCGTGATGG-3ʹ R:5ʹ-AGGTGGAGGAGTGGGTGTCGCTCT-3’ |
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; F, Forward; R, Reverse.
Wound-Healing Assays
Cells treated with IR were seeded into six-well plates and cultured until 80% confluent. A 10 µL pipette tip was used to generate a scratch in the surface of the cells and cells were washed twice in PBS and cultured. Images were obtained at 0 h, 24 h and 48 h intervals on a light microscope.
Transwell Migration and Invasion Assays
An 8 µm chemotaxis chamber (Corning, Acton, MA) was used to measure cell invasiveness and migration. Briefly, to the upper chambers, 2 mg/mL Matrigel (BD Biosciences, Bedford, MA, USA) including 1×105 cells were incubated in 200 µL of culture medium, and the lower chambers were filled with 600 µL of medium containing 10% FBS. Cells were grown at 37°C in a 5% CO2 humidified incubator for 24 h to permit invasion into the lower chamber. Non-migratory cells in the upper surface of the chamber were scraped and washed with PBS. Invaded cells were fixed with paraformaldehyde (Ding guo, Beijing, china), stained with Giemsa and assessed on a light microscope. For the assessment of cell migration, assays were repeated in transwells lacking matrigel.
Cell Transfection
Specific siRNA oligonucleotides (Genepharma, Shanghai, China) were synthesized to silence Snail. The sequence of the siRNA oligonucleotides (si-Snail) were as follows: 5ʹ-CAGGACUCUAAUCCAGAGUTT-3ʹ, negative control group (si-NC): 5ʹ-ACUCUGGAUUAGAGUCCUGTT-3ʹ. Briefly, FaduRR cells were seeded into six-well plates at a density of 1 × 106/well and divided into blank control, si-NC and si-Snail groups. Blank groups were treated with PBS. When the FaduRR cells grew to 70% confluence, cells were transfected with si-NC or si-Snail using Lipofectamine RNA iMAX (Invitrogen, Carlsbad, CA, USA). Q-PCR and Western blot were performed to measure the efficiency of Snail silencing.
Statistical Analysis
SPSS version 22.0 was used for data analysis. Datas were presented as mean ± SD. Group differences were assessed using a Student’s t-test. Differences in clinical characteristics were evaluated using a Chi-square test or Fisher’s exact test. Statistical significance was considered as P-values< 0.05 (*P< 0.05; **P < 0.01; ***P < 0.001).
Results
EMT-Related Proteins and Snail Expression Predicted Radioresistance in Patients with Hypopharyngeal Carcinoma
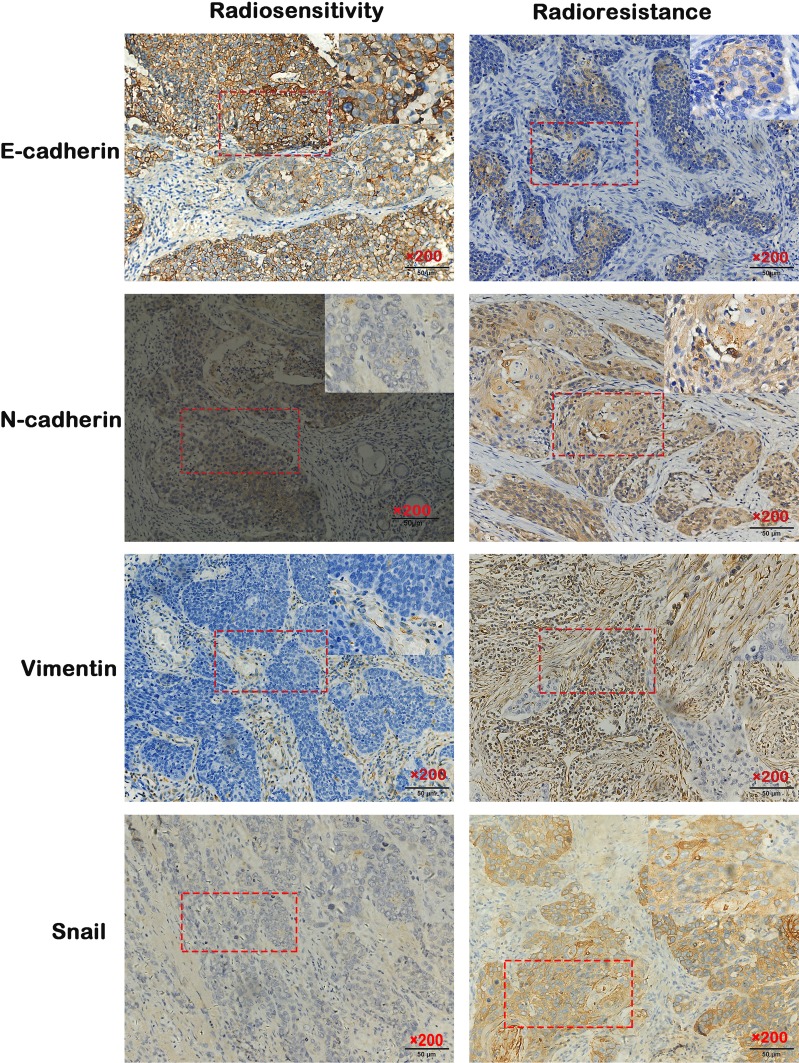
Of the patients with hypopharyngeal carcinoma, 46 were divided into the radiosensitivity group and the remaining 34 patients were assigned to the radioresistance group. Compared to the radiosensitivity group, epithelial biomarker E-cadherin expression was downregulated (p<0.006, 63.04% vs 32.35%), however, the mesenchymal biomarkers Vimentin, N-cadherin and Snail were upregulated in the radioresistance group (p<0.005, p<0.002, p<0.011, 30.43% vs 61.76%, 39.13% vs 73.52%, 45.65% vs 73.52%) (Figure 1). The relationship between clinicopathologic features and EMT expression are shown in Tables 2 and 3. The results indicated that patients with low E-cadherin expression and high N-cadherin, Vimentin and Snail expression had higher rates of radioresistance compared to those with high E-cadherin expression and low N-cadherin, Vimentin and Snail expression. The high Snail expression showed a negatively correlation with the expression of E-cadherin in the radioresistance group, the high expression of Snail was positively correlated with N-cadherin and Vimentin expression in the radioresistance group. Moreover, the expression of E-cadherin, Vimentin, N-cadherin and Snail in hypopharyngeal carcinoma samples showed significant differences between advanced pathological stages (T3–T4) and early pathological stages (T1–T2). However, there are no significant differences between the expression of E-cadherin, N-cadherin, Vimentin and Snail with regard to gender, age and differentiation. These data suggested that radiotherapy resistant patients exhibit EMT. Importantly, the high Snail expression foreboded the radioresistance in patients with HSCC.
Figure 1.
Expression of EMT surface marker proteins in HSCC tissue detected by immunohistochemistry (IHC) (200× magnification).
Abbreviation: EMT, epithelial mesenchymal transformation.
Table 2.
Clinical Characteristics Associated with the Expression of E-Cadherin and N-Cadherin
| Clinicopathologic Factors | n | E-Cadherin | p | N-Cadherin | p | ||
|---|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | Positive (%) | Negative (%) | ||||
| Gender | |||||||
| Male | 75 | 39(52.00) | 36(48.00) | 0.179 | 41(54.66) | 34(45.34) | 0.428 |
| Female | 5 | 1(20.00) | 4(80.00) | 2(40.00) | 3(60.00) | ||
| Age | |||||||
| ≥60 | 54 | 30(55.55) | 24(44.45) | 0.833 | 30(55.55) | 24(44.45) | 0.409 |
| <60 | 26 | 10(38.46) | 16(61.36) | 13(50.00) | 13(50.00) | ||
| Differentiation | |||||||
| Well and moderate | 67 | 35(52.23) | 32(47.77) | 0.273 | 31(46.62) | 36(63.38) | 0.112 |
| Poor | 13 | 5(38.46) | 8(61.52) | 9(69.23) | 4(30.76) | ||
| Tumor stage | |||||||
| Ⅰ+Ⅱ | 51 | 31(60.79) | 20(39.21) | 0.021* | 24(47.05) | 27(52.95) | 0.004* |
| Ⅲ+Ⅳ | 29 | 10(34.49) | 19(65.51) | 23(79.31) | 6(20.68) | ||
| Response | |||||||
| Radiosensitive | 46 | 29(63.04) | 17(36.96) | 0.006* | 18(39.13) | 28(60.87) | 0.002* |
| Radioresistance | 34 | 11(32.35) | 23(67.65) | 25(73.52) | 9(26.48) | ||
Note: *p<0.05.
Table 3.
Clinical Characteristics Associated with the Expression of Vimentin and Snail
| Clinicopathologic Factors | n | Vimentin | p | Snail | p | ||
|---|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | Positive (%) | Negative (%) | ||||
| Gender | |||||||
| Male | 75 | 32(42.66) | 43(57.34) | 0.381 | 44(58.66) | 31(41.33) | 0.358 |
| Female | 5 | 3(60.00) | 2(40.00) | 2(40.00) | 3(60.00) | ||
| Age | |||||||
| ≥60 | 54 | 29(53.70) | 25(46.30) | 0.338 | 33(61.11) | 21(38.88) | 0.090 |
| <60 | 26 | 16(61.53) | 10(38.47) | 11(42.30) | 15(57.70) | ||
| Differentiation | |||||||
| Well and moderate | 67 | 27(40.29) | 40(59.70) | 0.270 | 29(43.28) | 38(56.72) | 0.542 |
| Poor | 13 | 8(61.53) | 5(38.47) | 6(46.15) | 7(53.84) | ||
| Tumor stage | |||||||
| Ⅰ+Ⅱ | 51 | 19(37.25) | 32(62.74) | 0.000* | 20(39.21) | 31(60.79) | 0.000* |
| Ⅲ+Ⅳ | 29 | 10(34.49) | 19(65.51) | 21(72.41) | 8(27.59) | ||
| Response | |||||||
| Radiosensitive | 46 | 14(30.43) | 32(69.57) | 0.005* | 21(45.65) | 25(54.34) | 0.011* |
| Radioresistance | 34 | 21(61.76) | 13(38.23) | 26(76.47) | 8(23.52) | ||
Note: *p<0.05.
EMT Phenotypes are Induced by IR in Fadu Cells
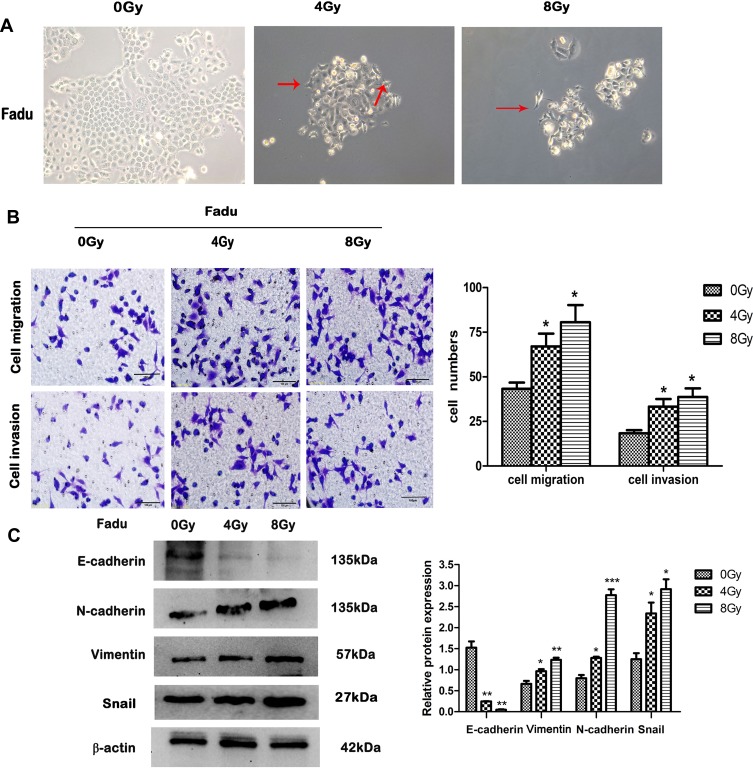
Fadu cells under 0 Gy were brick shaped and with tight cell-cell adhesion. The morphology of the cells changed however after treatment with 4 Gy and 8 Gy IR, in which cells possessed a mesenchymal phenotype with an irregular and narrow phenotype, and a loss of cell polarity and adhesion (Figure 2A). Morphological changes of Fadu were observed at increasing irradiation doses (4, 8Gy) which were similar to the typical morphological changes associated with EMT. To investigate whether these morphological transformations occurred in cells treated with IR and were consistent with EMT, transwell assays were performed to assess the effects of IR on the migratory and invasive capabilities of Fadu cells. Transwell assays showed enhanced migration and invasive capacity of Fadu cells after treatment with IR (Figure 2B). We detected the expression of EMT-related proteins using Western blot analysis. The results (Figure 2C) demonstrated that the expression of N-cadherin, Vimentin and Snail were enhanced, whilst the expression of E-cadherin declined in Fadu cells exposed to IR compared to parental cells. These results indicate that EMT is induced by IR in Fadu cells.
Figure 2.
EMT phenotypes. (A) Representative morphological changes in IR-treated Fadu cells (200× magnification). (B) Transwell assays to assess cell migration and invasion (left), and their quantitative analysis (right) (200× magnification after 24 h). (C) Relative expression of the EMT biomarkers: E-cadherin, N-cadherin, Vimentin and Snail detected by Western blotting under different doses of IR. β-actin was used as an internal control. Data are the mean ± SD. n=3. *P<0.05, **P<0.01, ***P<0.001 vs control group. The red arrows indicate spindle-cell morphology formed and pseudopodia were stretched out.
Abbreviation: EMT, epithelial mesenchymal transformation.
EMT Phenotypes are Acquired in FaduRR Cells
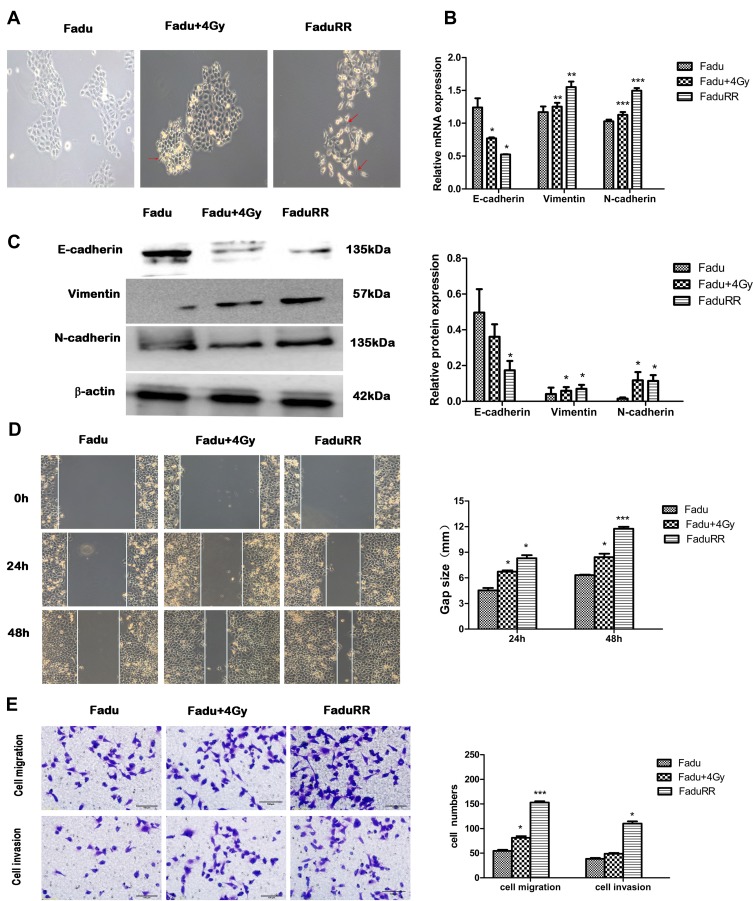
As shown in Figure 3A, FaduRR cells showed a mesenchymal morphology. The morphologies of FaduRR and Fadu cells after treatment with 4Gy (Fadu+4Gy) were long and narrow, spindle-shaped, and extended with pseudopodia and increased cell spaces, whilst the morphology of Fadu cells were round and pebble-shaped, with tight cell connections. Compared to parental cells, the morphological changes of FaduRR were similar to the typical cellular morphological changes of EMT. To assess whether EMT was induced by IR in radioresistance FaduRR cells, we measured the expression of EMT-related proteins using Western blot and Q-PCR assays. As shown in Figure 3B and C, compared to Fadu cells, the expression of the EMT marker protein E-cadherin decreased, whilst the expression of the mesenchymal marker protein N-cadherin and Vimentin were enhanced in Fadu+4Gy and FaduRR. These findings validated that IR can induce EMT in FaduRR cells.
Figure 3.
EMT phenotypes in radioresistant FaduRR and Fadu cells after 4Gy radiotherapy. (A) Fadu cells after 4Gy radiotherapy and radioresistant FaduRR cells showed morphologic changes consistent with EMT (200× magnification). (B, C) Expression of EMT related proteins detected by quantitative real-time PCR and Western blot analysis. Quantification relative to β-actin expression. (D, E) Wound-healing assays (100× magnification) and Transwell assays (200× magnification) to measure migration and invasive abilities. Data are the Mean ± SD. n=3. *P<0.05, **P<0.01, ***P<0.001 vs control group. The red arrows indicate spindle-cell morphology formed and pseudopodia were stretched out.
Abbreviation: EMT, epithelial mesenchymal transformation.
IR Enhances Migration, Invasiveness and Activates AKT/GSK-3β/Snail Pathway in FaduRR Cells
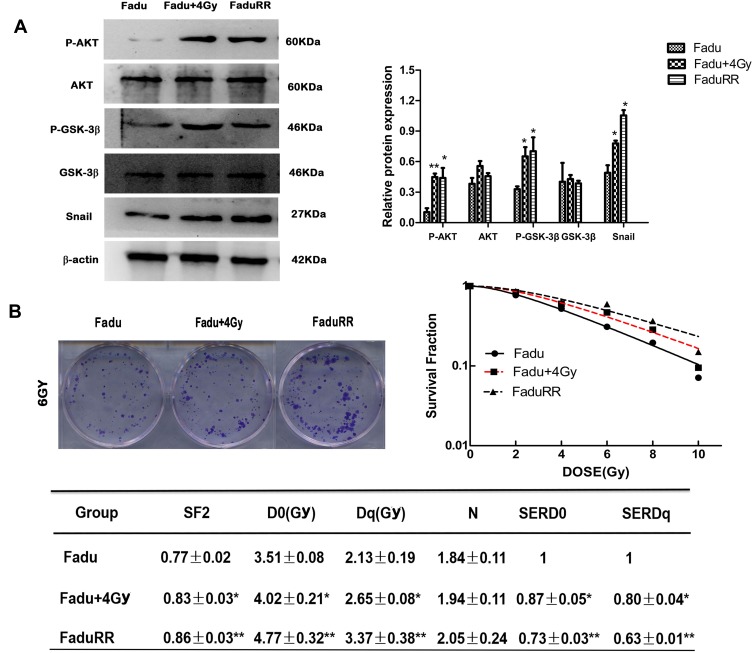
Many studies have demonstrated that EMT plays a vital role in migration and invasiveness, our results have showed that IR can induce EMT in FaduRR cells. The effects of IR on migration and invasiveness were explored in FaduRR cells using wound-healing and transwell assays. The results of wound-healing assay showed that FaduRR cells have a stronger ability for migration at 24h and 48h after exposed to IR compared to Fadu cells (Figure 3D). Cells exposed to IR for 24h showed enhanced invasive and migratory capability in transwell assay compared to Fadu cells (Figure 3E). To determine the mechanism of EMT induced by IR in Fadu and FaduRR cells, AKT/GSK-3β/Snail signaling pathway was investigated through Western blot assays. The levels of phosphorylated GSK-3β, AKT and Snail were higher in Fadu cells treated with 4Gy (Fadu+4Gy) and FaduRR cells. In contrast, no significant differences between GSK-3β and AKT were observed (Figure 4A). These results demonstrated that FaduRR cells have higher EMT level and the invasiveness and migration capacity compared to its parental cells. Importantly, IR-induced EMT by activating AKT/GSK-3β/Snail signaling pathway in FaduRR cells.
Figure 4.
Acquired resistance evaluation and activated AKT/GSK-3β/Snail signaling pathway. (A) AKT, p-AKT, GSK-3β, p-GSK-3β and Snail expression detected by Western blot analysis. (left), and their quantitative analysis (right). (B) Radiation survival curves and the clonogenic figures of Fadu and FaduRR cells after irradiation with 4Gy. Data are the mean ± SD. n=3. *P<0.05, **P<0.01 vs control group.
EMT Is Accompanied by Radioresistant Acquisition in FaduRR Cells
Colony-forming assays were used to confirm that FaduRR cells acquired radioresistance. The results demonstrated that radiobiology parameters (D0, Dq, N and SF2) and the surviving fraction of Fadu cells after treatment with 4 Gy (Fadu+4Gy) and untreated FaduRR cells were upregulated relative to Fadu cells. The enhanced radiosensitivity ratios (SERDq and SERD0) were downregulated (Figure 4B). This confirmed that Fadu+4Gy and FaduRR cells acquired radioresistance. Fadu+4Gy and FaduRR cells displayed an EMT phenotype (Figure 3) confirming that radioresistance cells underwent EMT.
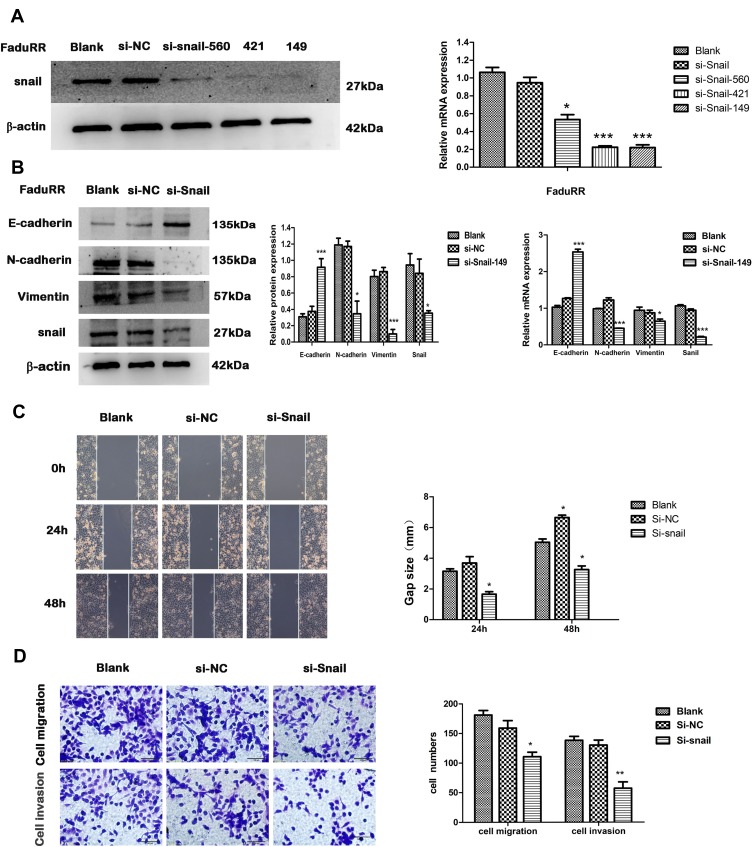
Snail Silencing Reverses EMT and Reduces the Invasion and Migration Ability of FaduRR Cells
IR could induce EMT and increase the invasive and migratory abilities of FaduRR cells through AKT/GSK-3β/Snail signaling. Due to FaduRR cells with a high expression of Snail, We therefore explored whether EMT and the invasiveness and migration of FaduRR cells could be reversed through Snail silencing. Q-PCR and Western blot analysis confirmed efficient Snail silencing in transfected cells (Figure 5A). The results showed Snail expression of FaduRR cells which transfected with si-Snail-149 was significantly downregulated at both protein and mRNA levels compared with Blank group, si-NC, si-Snail-560 and si-Snail-421. Therefore, FaduRR cells with si-Snail-149 were determined as the experimental group. The results showed that the silencing of Snail led to an enhancement of E-cadherin expression and a suppression of Vimentin and N-cadherin expression in FaduRR cells compared with Blank group and si-NC group (Figure 5B). Furthermore, the migratory and invasive capacities of FaduRR cells were impaired by Snail depletion compared with Blank group and si-NC group (Figure 5C and D). These data suggest that silencing Snail could reverse EMT and reduce the migratory and invasive capabilities of FaduRR cells.
Figure 5.
EMT reversion by Snail silencing. (A) Snail expression detected by Real Time PCR and Western blot analysis. (B) mRNA expression of EMT related proteins assessed by quantitative real-time PCR and quantitative analyses of GAPDH expression. The expression of EMT related proteins detected by Western blot. (C, D) Wound-healing (100× magnification) and Transwell assays (200× magnification) were used to measure migration and invasion abilities after Snail silencing in FaduRR cells. Data are the Mean ± SD. n=3. *P<0.05, **P<0.01, ***p<0.001 vs si-NC group.
Abbreviations: EMT, epithelial mesenchymal transformation; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; si-NC, negative control siRNA; si-Snail, Snail-specific siRNA.
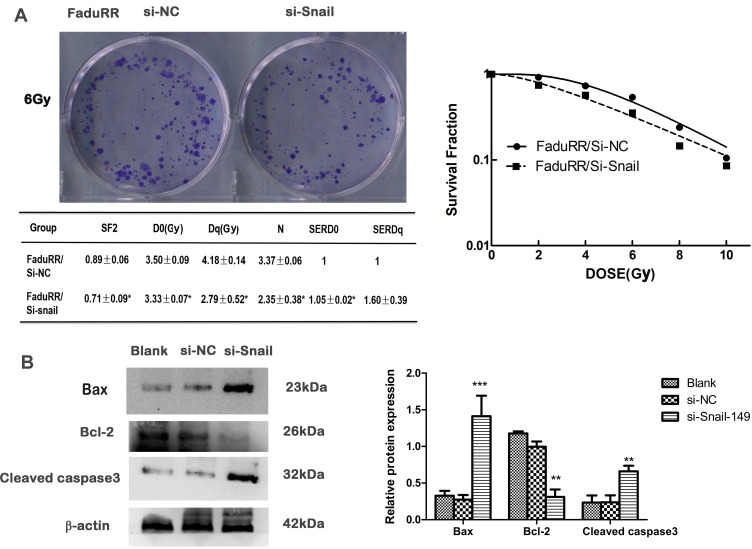
Snail Silencing Reverses Acquired Radioresistance by Promoting Apoptosis in FaduRR Cells
The data thus far suggested that the AKT/GSK-3β/Snail axis is key to the occurrence and development of EMT. Along with the reversion of EMT, we predicted that the radiosensitivity of FaduRR cells would be increased by Snail silencing. Colony-formation assays showed that radiobiology parameters (D0, Dq, N and SF2) and the surviving fraction of FaduRR/si-Snail cells significantly increased compared to FaduRR/si-NC cells and that the enhanced radiosensitivity enhancement ratios (SERDq and SERD0) were reduced (Figure 6A). The results displayed that Snail silencing could increase the radiosensitivity of FaduRR cells. Snail silencing was found to promote FaduRR cells apoptosis using Western blot analysis. It showed enhanced levels of cleaved caspase3 expression and Bax expression and reduced levels of Bcl-2 expression compared with FaduRR/si-NC cells and Blank groups (Figure 6B). These results demonstrated that the restoring radiosensitivity and reversion of IR-induced EMT following Snail silencing could be attributed to promoting apoptosis.
Figure 6.
Acquired radioresistance is reversed by Snail silencing. (A) Radiation cell survival curves and clonogenic assays in si-NC group and si-Snail group. (B) Expression of apoptosis related proteins assessed by Western blot (left) and quantitated relative to β-actin. Data are the mean ± SD. n=3. *P<0.05, **P<0.01, ***p<0.001 vs si-NC group.
Abbreviations: si-NC, negative control siRNA; si-Snail, Snail-specific siRNA.
Discussion
Radiotherapy is a common and effective treatment in HSCC. However, radioresistance is a major challenge to successful treatment.23 Currently, radioresistance is divided into acquired and intrinsic resistance. Intrinsic radioresistance is related to several factors, including DNA repair capability, decreased apoptosis, cell cycle status and cell signaling.24 The mechanisms of acquired radioresistance are less well understood. In this study, the expression of EMT-related proteins and Snail were investigated in radioresistant HSCC patients. Furthermore, EMT-related phenotypes and AKT/GSK-3β/Snail signaling pathway were investigated in the radioresistance human HSCC cell line FaduRR when compared with its parental cells.
Accumulating evidences show that IR-induced EMT accelerates the malignant characteristics of radiation-treated tumors, leading to increased invasion, migration, radioresistance and chemoresistance, leading to tumor recurrence and treatment failure in esophagus, cervical, breast, lung cancer cell lines.14,15,25–28 To explore whether the radioresistant patients with HSCC undergo EMT phenotypes. Tissue samples of hypopharyngeal were detected by IHC assay. The results demonstrated that the expression of N-cadherin, Vimentin and Snail in radioresistant group of HSCC were significantly higher compared to the radiosensitive group. Nevertheless, the expression of E-cadherin in radiosensitive samples of HSCC was higher than that in the radioresistant samples. Furthermore, we found that the expression of EMT-related proteins and Snail were closely correlated to TNM stage, but were not related to gender, age, and differentiation. Consistently, we observed that IR induced EMT and increased the ability of invasion and migration of Fadu cells. Importantly, EMT phenotypes were also observed in FaduRR cells with long, narrow, spindle-shapes and a loss of cell adhesion and the capacity of invasiveness and migration were enhanced. Further Q-PCR and Western blot analysis demonstrated an obvious increase in Vimentin and N-cadherin expression and a loss of E-cadherin expression. The results above confirmed EMT exist both in vivo and vitro. Thus, we speculated that EMT was associated with the acquisition of radioresistance of FaduRR cells during segment radiotherapy.
AKT can induce the inactivation (phosphorylation) of GSK-3β, and stabilize Snail, a key transcriptional repressor of E-cadherin in radioresistant cancer cells.29,30 Li et al demonstrated that EMT can be reversed through the AKT/GSK-3β/Snail signaling pathway.31 In this study, we confirmed that the expression of phosphorylation of GSK-3β, AKT and Snail were up-regulated in radioresistant hypopharyngeal cancer FaduRR cells. This suggested that IR induced EMT and acquired radioresistance via activating AKT/GSK-3β/Snail signaling pathway in FaduRR cells. These results led us to explore the possibility that inhibiting AKT/GSK-3β/Snail signaling pathway can reverse EMT and EMT-associated radioresistance.
A crucial stage in the EMT process is the downregulation of E-cadherin mediated through the elementary helix–loop–helix transcription factor, Twist, SIP-1/ZEB-2 and zinc-finger transcriptional repressors (Slug and Snail). Snail is a central mediator of EMT that promotes invasion and migration behavior in multiple malignant tumors.32,33 Moreover, Kurrey and colleagues exhibited that overexpression of Snail is associated with the acquisition of radioresistance and increases cell survival in ovarian cancer cells.19 The activation of Snail is a potential mechanism of EMT development and radioresistance in FaduRR cells.
To further demonstrate our hypothesis that EMT reversion leads to the reduction of radioresistance, we explored the sensitivity of radioresistant cancer cells to irradiation when EMT is reversed by silencing Snail expression. Silencing Snail expression induced MET (mesenchymal-epithelial transition) and led to a loss of stem cell-like phenotypes in gastric carcinoma cells.34 Moreover, the depletion of Snail remarkably reversed EMT and led to a recovery of drug sensitivity in our cisplatin resistant cell lines.35 Snail expression was down-regulation by si-RNA and used to investigate the EMT phenotype in FaduRR cells. Our results showed that the down-regulation of Snail by si-RNA increased the expression of epithelial biomarkers and decreased mesenchymal biomarker expression. Moreover the invasion and migration ability of HSCC cells was weakened. In addition, the radiosensitivity of FaduRR cells was enhanced. Recent studies have shown that IR stimulates various transcription factors, including upregulating the expression of Snail, thus accelerating EMT via PI3K/AKT signaling pathway in NPC patients.30 Numerous studies demonstrate that Snail participates in the regulation of apoptosis related genes.36 In this study, we found that silencing Snail expression promoted apoptosis in FaduRR cells. PTEN plays a key role in IR-induced EMT through activating AKT signaling pathway in human esophageal cancer cells.37 The possible mechanism is through which Snail mediates the effects of PTEN on IR generated EMT and the radioresistance acquired by activating AKT signaling pathway requires further investigation in future studies.
We also confirmed that irradiation increases cancer cell migration and invasiveness in HSCC. Studies have shown that the migratory and invasiveness behavior of tumor cells is enhanced by sub-lethal doses of irradiation. Similarly, radiation-enhanced invasiveness contributes to enhanced MMP-9 expression in hepatocellular carcinoma. Increasing evidence has confirmed that IR triggered EMT enhances the invasiveness and migration of cancer cells through IL-6/STAT3/TWIST signaling pathway.38 The molecular mechanisms of these effects should now be explored in more detail and the precise radiation doses that promote EMT should be verified.
Conclusions
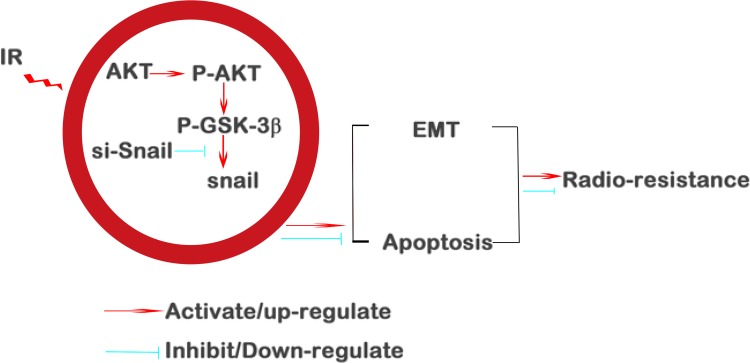
In summary, we demonstrate that IR could generate EMT and radioresistance in HSCC via AKT/GSK-3β/Snail signaling pathway. More importantly, this pathway was highlighted as a potential therapeutic target. The depletion of Snail attenuates EMT (summarized in Figure 7) which might prove to be an efficacious strategy to reverse radioresistance during HSCC therapy.
Figure 7.
Summary of the mechanisms of Snail mediated cancer cell resistance.
Acknowledgments
We thank Center for Molecular Medicine and Cancer Research, Chongqing Medical University, China for providing technical assistance and the experiment platform.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhou J, Li Y, Wei D, et al. Overall survival with and without laryngeal function preservation in 580 patients with hypopharyngeal squamous cell carcinoma. Oncol Rep. 2015;34(6):3196–3202. doi: 10.3892/or.2015.4313 [DOI] [PubMed] [Google Scholar]
- 2.Gourin CG, Johnson JT. A contemporary review of indications for primary surgical care of patients with squamous cell carcinoma of the head and neck. Laryngoscope. 2009;119(11):2124–2134. doi: 10.1002/lary.20619 [DOI] [PubMed] [Google Scholar]
- 3.Kuo P, Sosa JA, Burtness BA, et al. Treatment trends and survival effects of chemotherapy for hypopharyngeal cancer: analysis of the national cancer data base. Cancer. 2016;122(12):1853–1860. doi: 10.1002/cncr.29962 [DOI] [PubMed] [Google Scholar]
- 4.Vandenbrouck C, Eschwege F, De la Rochefordiere A, et al. Squamous cell carcinoma of the pyriform sinus: retrospective study of 351 cases treated at the Institut GustaveRoussy. Head Neck Surg. 1987;10(1):4–13. doi: 10.1002/hed.2890100103 [DOI] [PubMed] [Google Scholar]
- 5.Shiotani A, Tomifuji M, Araki K. Transoral videolaryngoscopic surgery for en bloc resection of supraglottic and hypopharyngeal cancers. Otolaryngol Head Neck Surg. 2011;144(2):288–289. doi: 10.1177/0194599810392148 [DOI] [PubMed] [Google Scholar]
- 6.Wycliffe ND, Grover RS, Kim PD, et al. Hypopharyngeal cancer. Top Magn Reson Imaging. 2007;18(4):243–258. doi: 10.1097/RMR.0b013e3181570c3f [DOI] [PubMed] [Google Scholar]
- 7.Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233(3):706–720. doi: 10.1002/(ISSN)1097-0177 [DOI] [PubMed] [Google Scholar]
- 8.Xia YY, Yin L, Jiang N, et al. Downregulating HMGA2 attenuates epithelial-mesenchymal transition-induced invasion and migration in nasopharyngeal cancer cells. Biochem Biophys Res Commun. 2015;174:175–183. [DOI] [PubMed] [Google Scholar]
- 9.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822 [DOI] [PubMed] [Google Scholar]
- 10.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu YD, Zhou BHP. Snail more than EMT. Cell Adh Migr. 2009;4:199–203. doi: 10.4161/cam.4.2.10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou YC, Liu JY, Li J, et al. Ionizing radiation promotes migration and invasion of cancer cells through transforming growth factor-beta-mediated epithelial-mesenchymal transition. Inte J Radiat Oncol. 2011;81:1530–1537. doi: 10.1016/j.ijrobp.2011.06.1956 [DOI] [PubMed] [Google Scholar]
- 14.Jung J-W, Hwang S-Y, Hwang J-S, et al. Ionising radiation induces changes associated with epithelial-mesenchymal transdifferentiation and increased cell motility of A549 lung epithelial cells. Eur J Cancer. 2007;43:1214–1224. doi: 10.1016/j.ejca.2007.01.034 [DOI] [PubMed] [Google Scholar]
- 15.Theys J, Jutten B, Habets R, et al. E-Cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother Oncol. 2011;99(3):392–397. doi: 10.1016/j.radonc.2011.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasser M, Shaikh R, Chilakapati MK, Teni T. Raman spectroscopic study of radioresistant oral cancer sublines established by fractionated ionizing radiation. PLoS One. 2014;9(5):e97777. doi: 10.1371/journal.pone.0097777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrallo-Gimeno A, Nieto MA. The Snail as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907 [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama K, Kamata N, Hayashi E, et al. Reverse correlation of E-cadherin and Snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol. 2001;37(1):65–71. doi: 10.1016/S1368-8375(00)00059-2 [DOI] [PubMed] [Google Scholar]
- 19.Kurrey NK, Jalgaonkar SP, Joglekar AV, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27(9):2059–2068. doi: 10.1002/stem.v27:9 [DOI] [PubMed] [Google Scholar]
- 20.Sullivan NJ, Sasser AK, Axel AE, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28(33):2940–2947. doi: 10.1038/onc.2009.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Wang HS, Zhou BH, et al. Epithelial-mesenchymal transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS One. 2013;8:e56664. doi: 10.1371/journal.pone.0056664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maseki S, Ijichi K, Tanaka H, et al. Acquisition of EMT phenotype in the gefitinib-resistant cells of a head and neck squamous cell carcinoma cell line through Akt/GSK-3b/snail signalling pathway. Br J Cancer. 2013;106:1196–1204. doi: 10.1038/bjc.2012.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukusumi T, Ishii H, Konno M, et al. CD10 as a novel marker of therapeutic resistance and cancer stem cells in head and neck squamous cell carcinoma. Br J Cancer. 2014;111(3):506–514. doi: 10.1038/bjc.2014.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J, Cao S, Li W, et al. Time-course differential lncRNA and mRNA expressions in radioresistant hypopharyngeal cancer cells. Oncotarget. 2017;8(25):40994–41010. doi: 10.18632/oncotarget.17343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SY, Jeong EK, Ju MK, et al. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo M, Wu C, Guo E, et al. FOXO3a knockdown promotes radioresistance in nasopharyngeal carcinoma by inducing epithelial-mesenchymal transition and the Wnt/β-catenin signaling pathway. Cancer Lett. 2019;455:26–35. doi: 10.1016/j.canlet.2019.04.019 [DOI] [PubMed] [Google Scholar]
- 27.Kim RK, Cui YH, Yoo KC, et al. Radiation promotes malignant phenotypes through SRC in breast cancer cells. Cancer Sci. 2015;106(1):78–85. doi: 10.1111/cas.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su H, Jin X, Zhang X, et al. FH535 increases the radiosensitivity and reverses epithelial-to-mesenchymal transition of radioresistant esophageal cancer cell line KYSE-150R. J Transl Med. 2015;13:104. doi: 10.1186/s12967-015-0464-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu W, Huang YJ, Liu C, et al. Inhibition of TBK1 Attenuates radiation-induced epithelial-mesenchymal transition of A549 human lung cancer cells via activation of GSK-3beta and repression of ZEB1. Lab Invest. 2014;94(4):362–370. doi: 10.1038/labinvest.2013.153 [DOI] [PubMed] [Google Scholar]
- 30.Viniegra JG, Martínez N, Modirassari P, et al. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J Biol Chem. 2005;280(6):4029–4036. doi: 10.1074/jbc.M410344200 [DOI] [PubMed] [Google Scholar]
- 31.Yuan L, Zhou M, Huang D, et al. Resveratrol inhibits the invasion and metastasis of colon cancer through reversal of epithelial-mesenchymal transition via the AKT/GSK-3β/Snail signaling pathway. Mol Med Rep. 2019;20(3):2783–2795. doi: 10.3892/mmr.2019.10528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batlle E, Sancho C, Franci D, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034 [DOI] [PubMed] [Google Scholar]
- 33.Zhou BP, Deng J, Xia W, et al. Dual regulation of Snail by GSK-3beta mediated phosphorylation in control of epithelial mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173 [DOI] [PubMed] [Google Scholar]
- 34.Yang YJ, Li ZB, Zhang GR, et al. Snail-induced epithelial-mesenchymal transition in gastric carcinoma cells and generation of cancer stem cell characteristics. Genet Mol Res. 2016;15(3). doi: 10.4238/gmr.15038510 [DOI] [PubMed] [Google Scholar]
- 35.Haslehurst AM, Koti M, Dharsee M, et al. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer. 2012;12:91. doi: 10.1186/1471-2407-12-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun CC, Li SJ, Yuan ZP, Li DJ. MicroRNA-346 facilitates cell growth and metastasis, and suppresses cell apoptosis in human non-small cell lung cancer by regulation of XPC/ERK/Snail/E-cadherin pathway. Aging. 2016;8(10):2509–2524. doi: 10.18632/aging.101080 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.He E, Pan F, Li G. Fractionated ionizing radiation promotes epithelial-mesenchymal transition in human esophageal cancer cells through PTEN deficiency-mediated Akt activation. PLoS One. 2015;10(5):e0126149. doi: 10.1371/journal.pone.0126149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zang C, Liu X, Bing L, et al. IL-6/STAT3/TWIST inhibition reverses ionizing radiationinduced EMT and radioresistance in esophageal squamousCarcinoma. Oncotarget. 2017;8(7):11228–11238. doi: 10.18632/oncotarget.14495 [DOI] [PMC free article] [PubMed] [Google Scholar]