Abstract
Background: Toxoplasma gondii infects millions of individuals worldwide. This protozoan is food and water-borne transmitted but blood transfusion and organ transplantation constitute alternative forms for transmission. However, the influence of IgG anti-T. gondii antibodies in molecular analysis carried out in peripheral blood still remain unclear. This study aimed to investigate the serum IgG anti-T. gondii antibody concentrations correlate Nested PCR results in blood donors.
Methods: 750 blood donors were enrolled. IgM and IgG anti-T. gondii antibodies were assessed by ELISA (DiaSorin, Italy). Nested PCR was performed with primers JW62/JW63 (288 bp) and B22/B23 (115 bp) of the T. gondii B1 gene. The mean values of IgG concentration were compared for PCR positive and PCR Negative blood donors using the t-test or Mann-Whitney according to the normal distribution (p-value ≤ 0.05).
Results: 361 (48.1%) blood donors presented positive serology as follow: IgM+/IgG−: 5 (0.6%); IgM+/IgG+: 21 (2.8%); IgM−/IgG+: 335 (44.7%) and 389 (51.9%), negative serology. From 353 blood donors with positive serology tested, the Nested PCR was positive in 38 (10.8%) and negative in 315 (89.2%). There were no differences statistically significant between the mean values of serum IgG anti-T. gondii antibody concentrations and the Nested PCR results.
Conclusions: In conclusion, our data show that variations in the serum IgG anti-T. gondii antibody concentrations do not correlate T. gondii parasitemia detected by Nested PCR in chronically infected healthy blood donors.
Keywords: Toxoplasma gondii, serology, molecular diagnosis, Nested PCR, serology assay, transfusion, blood donation and transfusion, blood donnors
Background
The infection by Toxoplasma gondii is frequent around the world and its prevalence range from <30% to more than 60% (Pappas et al., 2009; Dubey et al., 2012; Wallon and Peyron, 2018; Greigert et al., 2019). Different clinical forms of toxoplasmosis resulting from the infection by this Apicomplexan parasite arises and drawn attention especially for pregnant women, newborns and other immunosuppressed patients (Robert-Gangneux and Dardé, 2012; Neu et al., 2015; Rostami et al., 2019; Vidal, 2019).
T. gondii infection is a subject of social, epidemiological, clinical and scientific interest in Brazil. The rates of seroprevalence and the great genomic diversity of this parasite are high around the country (Dubey et al., 2012). Among the different ways to transmit T. gondii infection, the transfusion of blood products has been less explored. Two studies carried out in the past reported transmission of T. gondii by transfusion of leucocytes and platelets but the authors reached their conclusions after exclude other potential ways by which this parasite could be transmitted (Siegel et al., 1971; Nelson et al., 1989). Even so, this matter still represents a challenge for contemporaneous transfusion medicine (Foroutan et al., 2018; La Hoz et al., 2019).
The diagnosis of infection by T. gondii is essentially serological but there are number of published papers demonstrating that the high sensitivity of molecular methods can offer more accurate results on the investigation of infection by this parasite (Mattos et al., 2011; Brenier-Pinchart et al., 2015; Robert-Gangneux et al., 2015; Camilo et al., 2017; Murata et al., 2017; Roux et al., 2018; Greigert et al., 2019; Lévêque et al., 2019; Pleyer et al., 2019). The combination of serology and molecular methods has been used to improve the diagnosis of infection by T. gondii. One of them reported that IgG anti-T. gondii antibody low avidity correlates positive PCR in pregnant women (Yamada et al., 2011; Murata et al., 2016, 2017; Olariu et al., 2019). The other one also showed that IgG anti-T. gondii antibody low avidity correlate positive PCR among patients with ocular toxoplasmosis (Costa-Silva et al., 2008; Mattos et al., 2011; Tsirouki et al., 2018; Cortés et al., 2019; Greigert et al., 2019; Rahimi Esboei et al., 2019). However, correlations between serum anti-T. gondii antibody concentrations and molecular diagnosis of T. gondii infection among blood donors are scarce in the literature.
Evaluation of the serum IgG anti-T. gondii antibody concentrations could contribute to the understanding of the importance of these antibodies as risk biomarkers for transfusional purposes in respect to T. gondii transfusional transmission. The aim of this study is to test the hypothesis that low serum concentrations of IgG anti-T. gondii antibodies correlate T. gondii parasitemia.
Methods
Ethics Considerations
This study was approved by Research Ethics Committee from Faculdade de Medicina de São José do Rio Preto (case 006/2011). All blood donors received information about the objectives of the study and gave their informed consent.
Selection of Blood Donors
We selected a total of 750 blood donors from both genders able to donate at Regional Blood Center from São José do Rio Preto. All of them were seronegative for other infectious diseases as required by Brazilian policy for blood donation—B and C hepatitis, HIV, Chagas, syphilis, HTLV I/II (Ministério da Saúde, 2011).
Blood Sampling
Two blood samples were obtained from each blood donor by venipuncture from peripheral blood. One of them was collected with EDTA as anticoagulant and used to DNA extraction. The other one was collected without anticoagulant and stored at −20°C until used for detection of IgM and IgG anti-T. gondii antibodies.
Serology Assays for IgM and IgG Anti-T. gondii Antibodies
Serological tests for specific IgM and IgG antibodies anti-T. gondii were carried out by a commercial immunoenzymatic assay kit (DiaSorin, Italy). IgG anti-T. gondii antibody concentrations were defined according to the calibrators representing the cut-off values. All the manufacturer's instructions were precisely followed.
DNA Extraction
Genomic DNA of buffy coat from 5 mL of blood samples collected with EDTA was extracted using PureLink Genomic DNA Kits (Invitrogen, Carlsbad, CA), as previously described (Mattos et al., 2011).
PCR Nested Molecular Analysis
Nested PCR was performed using the B1 gene (accession numbers: B1 gene T. gondii = GenBank: KR559682.1) of T. gondii genomic DNA was carried out according to the protocol published by Okay and colleagues (Okay et al., 2009).
The first PCR reaction used the set of primers JW62 (Anti-sense: 5′-TTCTCGCCTCATTTCTGGGTCTAC-3′) and JW63 (Sense: 5′-GCACCTTTCGGACCTCAACAACCG-3′) to amplify a fragment of 288 base pairs. The composition of the mix for each reaction with 25 μL of final volume was: 0.2 μL of each primer, 100 ng of genomic DNA and 1× of Go Taq Green Master Mix (Promega, USA). The conditions of amplification were: 1× initial denaturation at 95°C: 5 min, 40× (denaturation at 95°C: 45 s, annealing at 55°C: 45 s, extension at 72°C: 45 s), 1× final extension at 72°C: 5 min, final at 4°C: 30 min. The amplified fragments were electrophoresed in 2% agarose gel stained with ethidium bromide under UV light.
The second PCR reaction used the set of primers B22 (Sense: 5′-AACGGGCGAGTAGCACCTGAGGAGA-3′) and B23 (Anti-sense: 5′-TGGGTCTACGTCGATGGCATGACAACT-3′) to amplify a fragment of 115 base pairs. The composition of the mix for each reaction with 25 μL of final volume was: 1.2 μM of each primer, 0.5 μL of pre-amplified DNA and 1× of Go Taq Green Master Mix (Promega, USA). The conditions of amplification were: 1× initial denaturation at 95°C: 5 min, 45× (denaturation at 95°C: 45 s, annealing at 55°C: 45 s, extension at 72°C: 45 s), 1× final extension at 72°C: 5 min, final at 4°C: 30 min. The amplified fragments were electrophoresed in 2% agarose gel stained with ethidium bromide under UV light.
Statistical Analysis
The mean values of IgG concentration were compared for blood donors with positive and negative PCR using the t-test or Mann-Whitney according to the normal distribution. The level of significance was set at 5% (p-value ≤ 0.05). The GraphPad Instat® (GraphPad Software Inc., USA) computer program version 3.06 was used for all analyses.
Results
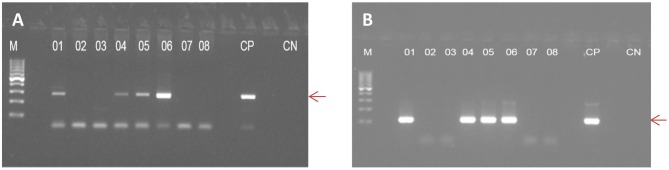
The serology results and their interpretation are shown in Table 1. From the overall blood donors able to donate (n = 750), 244 were female (mean age: 32.7 ± 10.7 years), and 506 were male (mean age: 34.7 ± 11.6) (p = 0.097). The IgM+/IgG+ serology was more frequent in males than in females (p = 0.0308). We performed the Nested PCR in 353 blood donors carrying serum IgG antibodies (IgM+/IgG+ and IgM−/IgG+). Figure 1 shows the amplified fragment from the genomic DNA of T. gondii extracted from peripheral blood carrying 288 and 115 bp, respectively.
Table 1.
Serology results and the interpretation of the serological profiles and Nested PCR for blood donors.
| Serology | Interpretation* | Male | Female | OR | CI 95% | p** | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||||
| IgM+/IgG− | 5 | 0.6 | Recent infection | 3 | 0.6 | 2 | 0.8 | 0.721 | 0.119–4.349 | 0.662 |
| IgM+/IgG+ | 21 | 2.8 | Recent infection | 19 | 3.7 | 2 | 0.8 | 4.721 | 1.090–20.440 | 0.030 |
| IgM−/IgG+ | 335 | 44.7 | Chronic infection | 233 | 46.0 | 102 | 41.8 | 1.188 | 0.872–1.618 | 0.308 |
| IgM−/IgG− | 389 | 51.9 | Non-immunized | 251 | 49.7 | 138 | 56.6 | 0.756 | 0.556–1.028 | 0.086 |
| Total | 750 | 100.0 | 506 | 244 | ||||||
According to Montoya (2002).
Calculated by exact Fisher's test.
Figure 1.
Electrophoretic profile of fragments of B1 gene from T. gondii genomic DNA extracted from peripheral blood of blood donors, amplified by Nested PCR. In (A), the first amplification is showing a fragment of 288 base pairs; in (B), the second amplification is showing a fragment of 115 base pairs. M, molecular marker; PC, Positive Control; CN, Negative Control.
There were no differences statistically significant between the mean serum IgG anti-T. gondii antibody concentrations and positive or negative Nested PCR results even when the comparisons were made by gender. Table 2 shows the data from male and female blood donors with positive and negative Nested PCR and serum IgG anti-T. gondii antibody concentrations.
Table 2.
Mean age, median, range, normal distribution, IgG anti-T. gondii antibodies concentrations according to Nested PCR Positive and Negative in male and female healthy blood donors.
| Values | Nested PCR (N = 353) | |||
|---|---|---|---|---|
| Positive (n = 38) | Negative (n = 315) | |||
| Male (n = 31) | Female (n = 7) | Male (n = 221) | Female (n = 94) | |
| Mean age ± SD | 37.7 ± 11.2 | 38.7 ± 11.9 | 37.9 ± 10.9 | 35.0 ± 10.5 |
| Median | 38 | 33 | 37 | 34 |
| Range | 19–60 | 23–52 | 18–65 | 18–59 |
| 25th Percentile (range) | 28 (19–37) | 23 (23–32) | 28 (18–36) | 27 (18–33) |
| 75th Percentile (range) | 47 (39–60) | 51 (50–52) | 46 (38–65) | 43 (34–59) |
| Normal distribution | Yes | Yes | Yes | No |
| IgG+ | ||||
| IgG ± SD (UI/mL) | 196.0 ± 35.2 | 165.6 ± 62.7 | 204.0 ± 33.2 | 196.6 ± 39.5 |
| Median | 205.6 | 186.8 | 208.3 | 205.9 |
| Range | 66.1–232.1 | 36.1–222.8 | 21.4–257.3 | 55.2–244.7 |
| 25th Percentile (range) | 171.9 (66.1–202.1) | 145.1 (36.1–168.9) | 180.1 (21.4–208.2) | 169.6 (55.2–205.8) |
| 75th Percentile (range) | 219.5 (206.0–232.1) | 212.5 (186.9–222.8) | 227.9 (208.4–257.3) | 223.6 (205.9–244.7) |
| Normal distribution | No | Yes | No | No |
OBS: Male-PCR+ vs. Male-PCR–: p = 0.2252 (Mann–Whitney U′ = 3,887.0); Female-PCR+ vs. Female-PCR–: p = 0.0960 (Mann–Whitney U′ = 454.00); Male-PCR+ vs. Female-PCR+: p = 0.1065 (Mann–Whitney U′ = 152.00); Male-PCR– vs. Female-PCR–: p = 0.2486 (Mann–Whitney U′ = 11,241.00).
Discussion
The aim of this study was to test the hypothesis that serum concentrations of IgG anti-T. gondii antibodies correlate T. gondii parasitemia in healthy blood donors. As the screening for anti-T. gondii antibodies is not compulsory for blood donors in Brazil (Ministério da Saúde, 2011) we performed serological tests to detect IgM and IgG anti-T. gondii antibodies as well as Nested PCR targeting B1 gene from T. gondii to detect parasitemia.
Serum IgM and IgG anti-T. gondii antibodies have been investigated in blood donors aiming to determine the prevalence of infection in different countries as reviewed by Foroutan-Rad and colleagues (Foroutan-Rad et al., 2016; Foroutan et al., 2018) as well as to estimate the risk of transfusional transmission of this parasite (Siransy et al., 2016; Ferreira et al., 2017; Botein et al., 2019; El-Tantawy et al., 2019). However, correlations between serum IgG anti-T. gondii antibody concentrations and the parasitemia determined by molecular methods have not been explored in healthy blood donors. A correlation between high serum IgG anti-T. gondii concentrations and negative PCR could be explored as an indicator for low risk of transfusional transmission of this parasite by blood products.
In this study, we observed that none of the IgM+/IgG− blood donors were positive for Nested PCR. Only one of the IgM+/IgG+ presented positive Nested PCR. Moreover, male and female blood donors with positive Nested PCR presented the mean values of serum IgG anti-T. gondii antibody concentrations lower in comparison to their counterpart with negative Nested PCR. However, the differences were not statistically significant. Therefore, low or high serum IgG anti-T. gondii antibody concentrations do not correlate the result of molecular analysis by Nested PCR aiming to detect genomic DNA from T. gondii in peripheral blood from healthy blood donors.
Molecular methods, such as conventional PCR, Nested PCR, and real-time PCR have been used either in isolation or in association, to detect T. gondii parasitemia in acute and chronically infected individuals since they show high sensitivity (Brenier-Pinchart et al., 2015; Dard et al., 2016; Camilo et al., 2017; Roux et al., 2018; Botein et al., 2019; Greigert et al., 2019). In this study, we used the Nested PCR to target the B1 gene which is one of the most used tests in the literature for detecting T. gondii parasitemia (Okay et al., 2009; Mattos et al., 2011; Teixeira et al., 2013; Roux et al., 2018). Moreover, it has been demonstrated that the B1 gene might be targeted for molecular detection of T. gondii parasitemia in Brazilian samples, especially when the investigation is limited to one gene (Okay et al., 2009; Teixeira et al., 2013).
It would be desirable to obtain T. gondii isolates from the blood donor or the donate samples (blood bags). However, due to the short length of parasitemia, which is apparently restricted to the acute phase of infection, is difficult to obtain viable parasites from blood samples. Maybe, an alternative would be to isolate parasite's mRNA from the donated blood but this procedure could interfere in the routine process in blood banks and contaminate the blood bags. Due to these difficulties, the studies that explored molecular approaches to detect the infection by this parasite among blood donors carry some limitations. Molecular methods aiming to detect T. gondii genomic DNA in the peripheral blood, such as conventional and Nested PCR are unable to distinguish live or dead parasites as well as residual DNA. They can only give a measure of the risk of transmission as well as overestimate the presence of the parasite in the peripheral blood (Rousseau et al., 2018).
The data presented here are supported by other reports. Three Iranian studies detected T. gondii infection only in blood donors carrying IgM anti-T. gondii antibodies by real-time PCR (Mahmoudvand et al., 2015) and Nested PCR (Sadooghian et al., 2017; Saki et al., 2019). In fact, there is a strong correlation between the IgM anti-T. gondii antibodies and parasitemia. However, parasitemia cannot be discharged in immunocompetent individuals (potential blood donors) carrying circulating IgG anti-T. gondii antibodies (Mattos et al., 2011; Park, 2012), especially when these antibodies present low avidity (Mattos et al., 2011; Yamada et al., 2011; Saki et al., 2019). All these studies did not correlate the serum concentration of anti-T. gondii antibodies to the PCR results.
The role of the host's immune response is crucial to protect chronically infected individuals (Coombes and Hunter, 2015). On the one hand, the cellular immune response led by macrophages, T CD8 lymphocytes, Natural Killer cells and cytokines as Interferon gama (IFN-ɤ) protects against the intracellular forms of the parasite. On the other hand, humoral immune response, which is thought to play a minor role in the immune protection of the host, seems to be effective against T. gondii extracellular forms, such as tachyzoites (Cohen and Denkers, 2015). The anamnestic immune response against T. gondii is characterized by the expression of IgG antibodies with high avidity and this class of immunoglobulins is effective at least in three immune events: opsonization and phagocytosis, Complement activation and Antibody-Dependent Cytotoxicity (ADCC) by Natural Killer cells and other white blood cells (Pleass and Woof, 2001; Filisetti and Candolfi, 2004; Ortiz-Alegría et al., 2010).
Erbe et al. (1991) demonstrated that human myeloid and lymphoid cells to kill T. gondii tachyzoites. These authors concluded that opsonization allows the binding of Fc IgG portion to Fc receptors (FcɤR) on phagocytic cells and significantly enhances the killing of tachyzoites coated by IgG anti-T. gondii antibodies. Additionally, Costa-Silva et al. (2012) reported that high levels of IgG from chronically infected mice decreases T. gondii RH strain parasitemia in comparison to those from naive mice. Exploring an experimental model, Seeber (2000) demonstrated the lytic activity mediated by Complement against T. gondii tachyzoites (Seeber, 2000). Other experimental study demonstrated the ability of IgG anti-excreted-secreted antigens from T. gondii to agglutinate tachyzoites and kill them by Complement lysis in a mouse model (Costa-Silva et al., 2008). Pleass and Woof (2001) reported that NK cells activated by IFN-γ display Fc receptor for Fc IgG portion which binds IgG anti-T. gondii antibodies and kills tachyzoites through ADCC (Pleass and Woof, 2001). All these observations suggest that IgG anti-T. gondii antibodies are effective and promote the clearance of parasitemia in chronically infected healthy blood donors.
Despite the limitations of this study which evaluated only the B1 gene and did not determine the IgG avidity, our data confirm the potential parasitemia in blood donors with circulating IgG anti-T. gondii antibodies, and demonstrate that the mean values of serum concentration of these antibodies do not correlate the results of Nested PCR. Also, it supports the view that blood products collected from chronically infected blood donors constitute a risk for transfusional transmission of T. gondii. In conclusion, our data show that variations in the serum IgG anti-T. gondii antibody concentrations do not correlate T. gondii parasitemia detected by Nested PCR in chronically infected healthy blood donors. Therefore, the use of serum IgG anti-T. gondii antibody concentrations to estimate the risk of transfusional transmission of this parasite does not constitute a potential biomarker for transfusional purposes.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethics Committee from Faculdade de Medicina de São José do Rio Preto (case 006/2011). All blood donors received information about the objectives of the study and gave their informed consent. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
FN, CB, VP-C, and LM designed the study and wrote the manuscript. FN and FM performed the molecular tests. OR selected the blood donors. VP, NP, MM, and SL collected the blood samples from blood donors and performed the serology tests.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo/São Paulo Research Foundation (FAPESP 2011/13939-8 to VP-C; 2012/07716-9 to LM; 2012/07750-2 to FN; 2013/15879-8 to FM; 2014/00900-4 to VP). The opinions, assumptions, and conclusions or recommendations expressed in this material are the responsibility of the authors and do not necessarily reflect the views of the FAPESP.
References
- Botein E. F., Darwish A., El-Tantawy N. L., EL-baz R., Eid M. I., Shaltot A. M., et al. (2019). Serological and molecular screening of umbilical cord blood for Toxoplasma gondii infection. Transpl. Infect. Dis. 21:e13117. 10.1111/tid.13117 [DOI] [PubMed] [Google Scholar]
- Brenier-Pinchart M. P., Capderou E., Bertini R. L., Bailly S., Fricker-Hidalgo H., Varlet-Marie E., et al. (2015). Molecular diagnosis of toxoplasmosis: value of the buffy coat for the detection of circulating Toxoplasma gondii. Diagn. Microbiol. Infect. Dis. 82, 289–291. 10.1016/j.diagmicrobio.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Camilo L. M., Pereira-Chioccola V. L., Gava R., Meira-Strejevitch C. D. S., Vidal J. E., Brandão de Mattos C. C., et al. (2017). Molecular diagnosis of symptomatic toxoplasmosis: a 9-year retrospective and prospective study in a referral laboratory in São Paulo, Brazil. Braz. J. Infect. Dis. 21, 638–647. 10.1016/j.bjid.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. B., Denkers E. Y. (2015). The gut mucosal immune response to Toxoplasma gondii. Parasite Immunol. 37, 108–117. 10.1111/pim.12164 [DOI] [PubMed] [Google Scholar]
- Coombes J. L., Hunter C. A. (2015). Immunity to Toxoplasma gondii–into the 21st century. Parasite Immunol. 37, 105–107. 10.1111/pim.12177 [DOI] [PubMed] [Google Scholar]
- Cortés J. A., Roncancio Á., Uribe L. G., Cortés-Luna C. F., Montoya J. G. (2019). Approach to ocular toxoplasmosis including pregnant women. Curr. Opin. Infect. Dis. 32, 426–434. 10.1097/QCO.0000000000000577 [DOI] [PubMed] [Google Scholar]
- Costa-Silva T. A., Borges M. M., Galhardo C. S., Pereira-Chioccola V. L. (2012). Immunization with excreted/secreted proteins in AS/n mice activating cellular and humoral response against Toxoplasma gondii infection. Acta Trop. 124, 203–209. 10.1016/j.actatropica.2012.08.013 [DOI] [PubMed] [Google Scholar]
- Costa-Silva T. A., Meira C. S., Ferreira I. M., Hiramoto R. M., Pereira-Chioccola V. L. (2008). Evaluation of immunization with tachyzoite excreted–secreted proteins in a novel susceptible mouse model (A/Sn) for Toxoplasma gondii. Exp. Parasitol. 120, 227–234. 10.1016/j.exppara.2008.07.015 [DOI] [PubMed] [Google Scholar]
- Dard C., Fricker-Hidalgo H., Brenier-Pinchart M. P., Pelloux H. (2016). Relevance of and new developments in serology for toxoplasmosis. Trends Parasitol. 32, 492–506. 10.1016/j.pt.2016.04.001 [DOI] [PubMed] [Google Scholar]
- Dubey J. P., Lago E. G., Gennari S. M., Su C., Jones J. L. (2012). Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology 139, 1375–1424. 10.1017/S0031182012000765 [DOI] [PubMed] [Google Scholar]
- El-Tantawy N., Darwish A., Eissa E. (2019). Seroprevalence of Toxoplasma gondii infection among B-thalassemia major pediatric population: implications for transfusion transmissible toxoplasmosis. Pediatr. Infect. Dis. J. 38, 236–240. 10.1097/INF.0000000000002111 [DOI] [PubMed] [Google Scholar]
- Erbe D. V., Pfefferkorn E. R., Fanger M. W. (1991). Functions of the various IgG Fc receptors in mediating killing of Toxoplasma gondii. J. Immunol. 146, 3145–3151. [PubMed] [Google Scholar]
- Ferreira M. N., Bonini-Domingos C. R., Fonseca Estevão I., De Castro Lobo C. L., Souza Carrocini G. C., Silveira-Carvalho A. P., et al. (2017). Anti-Toxoplasma gondii antibodies in patients with beta-hemoglobinopathies: the first report in the Americas. BMC Res. Notes 1:211 10.1186/s13104-017-2535-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filisetti D., Candolfi E. (2004). Immune response to Toxoplasma gondii. Ann. Ist. Super Sanita. 40, 71–80. [PubMed] [Google Scholar]
- Foroutan M., Rostami A., Majidiani H., Riahi S. M., Khazaei S., Badri M., et al. (2018). A systematic review and meta-analysis of the prevalence of toxoplasmosis in hemodialysis patients in Iran. Epidemiol. Health 40:e2018016. 10.4178/epih.e2018016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroutan-Rad M., Majidiani H., Dalvand S., Daryani A., Kooti W., Saki J., et al. (2016). Toxoplasmosis in blood donors: a systematic review and meta-analysis. Transfus. Med. Rev. 30, 116–122. 10.1016/j.tmrv.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Greigert V., Di Foggia E., Filisetti D., Villard O., Pfaff A. W., Sauer A., et al. (2019). When biology supports clinical diagnosis: review of techniques to diagnose ocular toxoplasmosis. Br. J. Ophthalmol. 103, 1008–1012. 10.1136/bjophthalmol-2019-313884 [DOI] [PubMed] [Google Scholar]
- La Hoz R. M., Morris M. I., Infectious Diseases Community of Practice of the American Society of Transplantation (2019). Tissue and blood protozoa including toxoplasmosis, chagas disease, leishmaniasis, babesia, acanthamoeba, balamuthia, and naegleria in solid organ transplant recipients—guidelines from the American Society of Transplantation. Clin. Transplant. 33:e13546 10.1111/ctr.13546 [DOI] [PubMed] [Google Scholar]
- Lévêque M. F., Chiffré D., Galtier C., Albaba S., Ravel C., Lachaud L., et al. (2019). Molecular diagnosis of toxoplasmosis at the onset of symptomatic primary infection: a straightforward alternative to serological examinations. Int. J. Infect. Dis. 79, 131–133. 10.1016/j.ijid.2018.11.368 [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H., Saedi Dezaki E., Soleimani S., Baneshi M. R. R., Kheirandish F., Ezatpour B., et al. (2015). Seroprevalence and risk factors of Toxoplasma gondii infection among healthy blood donors in south-east of Iran. Parasite Immunol. 37, 362–367. 10.1111/pim.12198 [DOI] [PubMed] [Google Scholar]
- Mattos C. C., Meira C. S., Ferreira A. I., Frederico F. B., Hiramoto R. M., Almeida G. D., Jr., et al. (2011). Contribution of laboratory methods in diagnosing clinically suspected ocular toxoplasmosis in Brazilian patients. Diagn. Microbiol. Infect. Dis. 70, 362–366. 10.1016/j.diagmicrobio.2011.02.002 [DOI] [PubMed] [Google Scholar]
- Ministério da Saúde (2011). Portaria no 1353, de 13 de Junho de 2011. Ministério da Saúde. [Google Scholar]
- Montoya J. G. (2002). Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J. Infect. Dis. 185, S73–S82. 10.1086/338827 [DOI] [PubMed] [Google Scholar]
- Murata F. H., Ferreira M. N., Camargo N. S., Santos G. S., Spegiorin L. C., Silveira-Carvalho A. P., et al. (2016). Frequency of anti-Toxoplasma gondii IgA, IgM, and IgG antibodies in high-risk pregnancies, in Brazil. Rev. Soc. Bras. Med. Trop. 49, 512–514. 10.1590/0037-8682-0046-2016 [DOI] [PubMed] [Google Scholar]
- Murata F. H. A., Ferreira M. N., Pereira-Chioccola V. L., Spegiorin L. C. J. F., Meira-Strejevitch C. D. S., Gava R., et al. (2017). Evaluation of serological and molecular tests used to identify Toxoplasma gondii infection in pregnant women attended in a public health service in São Paulo state, Brazil. Diagn. Microbiol. Infect. Dis. 89, 13–19. 10.1016/j.diagmicrobio.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Nelson J. C., Kauffmann D. J., Ciavarella D., Senisi W. J. (1989). Acquired toxoplasmic retinochoroiditis after platelet transfusions. Ann. Ophthalmol. 21, 253–254. [PubMed] [Google Scholar]
- Neu N., Duchon J., Zachariah P. (2015). TORCH infections. Clin. Perinatol. 42, 77–103. 10.1016/j.clp.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Okay T. S., Yamamoto L., Oliveira L. C., Manuli E. R., Andrade Junior H. F., Del Negro G. M., et al. (2009). Significant performance variation among PCR systems in diagnosing congenital toxoplasmosis in São Paulo, Brazil: analysis of 467 amniotic fluid samples. Clinics 64, 171–176. 10.1590/S1807-59322009000300004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olariu T. R., Blackburn B. G., Press C., Talucod J., Remington J. S., Montoya J. G. (2019). Role of toxoplasma IgA as part of a reference panel for the diagnosis of acute toxoplasmosis during pregnancy. J. Clin. Microbiol. 57, e01357–e01318. 10.1128/JCM.01357-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Alegría L. B., Caballero-Ortega H., Cañedo-Solares I., Rico-Torres C. P., Sahagún-Ruiz A., Medina-Escutia M. E., et al. (2010). Congenital toxoplasmosis: candidate host immune genes relevant for vertical transmission and pathogenesis. Genes Immun. 11, 363–373. 10.1038/gene.2010.21 [DOI] [PubMed] [Google Scholar]
- Pappas G., Roussos N., Falagas M. E. (2009). Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 39, 1385–1394. 10.1016/j.ijpara.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Park Y. H. (2012). Toxoplasma gondii in the peripheral blood of patients with ocular toxoplasmosis. Br. J. Ophthalmol. 96:766. 10.1136/bjophthalmol-2011-301068 [DOI] [PubMed] [Google Scholar]
- Pleass R. J., Woof J. M. (2001). Fc receptors and immunity to parasites. Trends Parasitol. 17, 545–551. 10.1016/S1471-4922(01)02086-4 [DOI] [PubMed] [Google Scholar]
- Pleyer U., Gross U., Schlüter D., Wilking H. H., Seeber F., Groß U., et al. (2019). Toxoplasmosis in Germany. Dtsch. Arztebl. Int. 116, 435–444. 10.3238/arztebl.2019.0435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi Esboei B., Kazemi B., Zarei M., Mohebali M., Keshavarz Valian H., Shojaee S., et al. (2019). Evaluation of RE and B1 genes as targets for detection of Toxoplasma gondii by Nested PCR in blood samples of patients with ocular toxoplasmosis. Acta Parasitol. 64, 384–389. 10.2478/s11686-019-00056-6 [DOI] [PubMed] [Google Scholar]
- Robert-Gangneux F., Dardé M. L. (2012). Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25, 264–296. 10.1128/CMR.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Gangneux F., Sterkers Y., Yera H., Accoceberry I., Menotti J., Cassaing S., et al. (2015). Molecular diagnosis of toxoplasmosis in immunocompromised patients: a 3-year multicenter retrospective study. J. Clin. Microbiol. 53, 1677–1684. 10.1128/JCM.03282-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami A., Riahi S. M., Contopoulos-Ioannidis D. G., Gamble H. R., Fakhri Y., Shiadeh M. N., et al. (2019). Acute Toxoplasma infection in pregnant women worldwide: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 13:e0007807. 10.1371/journal.pntd.0007807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau A., La Carbona S., Dumètre A., Robertson L. J., Gargala G., Escotte-Binet S., et al. (2018). Assessing viability and infectivity of foodborne and waterborne stages (cysts/oocysts) of Giardia duodenalis, Cryptosporidium spp., and Toxoplasma gondii: a review of methods. Parasite 25:14. 10.1051/parasite/2018009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux G., Varlet-Marie E., Bastien P., Sterkers Y. (2018). Evolution of Toxoplasma-PCR methods and practices: a French national survey and proposal for technical guidelines. Int. J. Parasitol. 48, 701–707. 10.1016/j.ijpara.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Sadooghian S., Mahmoudvand H., Mohammadi M. A., Nazari Sarcheshmeh N., Tavakoli Kareshk A., Kamiabi H., et al. (2017). Prevalence of Toxoplasma gondii Infection among healthy blood donors in Northeast of Iran. Iran. J. Parasitol. 12, 554–562. [PMC free article] [PubMed] [Google Scholar]
- Saki J., Foroutan M., Khodkar I., Khodadadi A., Nazari L. (2019). Seroprevalence and molecular detection of Toxoplasma gondii in healthy blood donors in southwest Iran. Transfus. Apher. Sci. 58, 79–82. 10.1016/j.transci.2018.12.003 [DOI] [PubMed] [Google Scholar]
- Seeber F. (2000). An enzyme-release assay for the assessment of the lytic activities of complement or antimicrobial peptides on extracellular Toxoplasma gondii. J. Microbiol. Methods 39, 189–196. 10.1016/S0167-7012(99)00117-7 [DOI] [PubMed] [Google Scholar]
- Siegel S. E., Lunde M. N., Gelderman A. H., Halterman R. H., Brown J. A., Levine A. S., et al. (1971). Transmission of toxoplasmosis by leukocyte transfusion. Blood 37, 388–394. 10.1182/blood.V37.4.388.388 [DOI] [PubMed] [Google Scholar]
- Siransy L., Dasse S. R., Dou Gonat S. P., Legbedji A., N'guessan K., Kouacou P. A., et al. (2016). Immunity status of blood donors regarding Toxoplasma gondii infection in a low-income district of Abidjan, côte d'ivoire, West Africa. J. Immunol. Res. 2016, 1–7. 10.1155/2016/6830895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira L. E., Kanunfre K. A., Shimokawa P. T., Targa L. S., Rodrigues J. C., Domingues W., et al. (2013). The performance of four molecular methods for the laboratory diagnosis of congenital toxoplasmosis in amniotic fluid samples. Rev. Soc. Bras. Med. Trop. 46, 584–588. 10.1590/0037-8682-0095-2013 [DOI] [PubMed] [Google Scholar]
- Tsirouki T., Dastiridou A., Symeonidis C., Tounakaki O., Brazitikou I., Kalogeropoulos C., et al. (2018). A focus on the epidemiology of uveitis. Ocul. Immunol. Inflamm. 26, 2–16. 10.1080/09273948.2016.1196713 [DOI] [PubMed] [Google Scholar]
- Vidal J. E. (2019). HIV-related cerebral toxoplasmosis revisited: current concepts and controversies of an old disease. J. Int. Assoc. Provid. AIDS Care 18:232595821986731. 10.1177/2325958219867315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallon M., Peyron F. (2018). Congenital toxoplasmosis: a plea for a neglected disease. Pathogens 7:25. 10.3390/pathogens7010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Nishikawa A., Yamamoto T., Mizue Y., Yamada T., Morizane M., et al. (2011). Prospective study of congenital toxoplasmosis screening with use of IgG avidity and multiplex Nested PCR methods. J. Clin. Microbiol. 49, 2552–2556. 10.1128/JCM.02092-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.