Abstract
Quality of life (QoL) disturbances are common after aneurysmal subarachnoid hemorrhage (aSAH) both in physical and mental health domains and their causes are not clearly understood. Corticotropin-releasing hormone receptor 1 (CRHR1) is involved in stress reactivity and development of mental health disturbances after negative life-events. We performed a retrospective cohort study of long-term QoL outcomes among 125 surgically treated aSAH patients (2001–2013). QoL was assessed with Short Form Health Survey (SF-36) and compared to an age and gender matched general population. Genotyping of CRHR1 single nucleotide polymorphisms was performed (Rs7209436, Rs110402, Rs242924) and their effect on QoL scores was explored. aSAH patients experienced a reduced quality of life in all domains. CRHR1 minor genotype was associated with higher SF-36 mental health (OR = 1.31–1.6, p < 0.05), role-emotional (OR = 1.57, p = 0.04) and vitality scores (OR = 1.31–1.38, p < 0.05). Association of all studied SNP’s with vitality and Rs242924 with mental health scores remained statistically significant after Bonferroni correction. Mental quality of life scores were associated with physical state of patients, antidepressant history and CRHR1 genotype. Predisposition to mental health disturbances after stressful life-events might be associated with reduced mental QoL after aSAH and selected patients could be provided advanced counselling in the recovery phase.
Subject terms: Predictive markers, Stroke
Introduction
Aneurysmal subarachnoid haemorrhage (aSAH) causes long-term morbidity and leads to reduced quality of life (QoL)1. Incidence of aSAH is around 7.9 per 100 000 patient years and is showing a trend of decrease2,3. More patients survive the subacute phase and in the long-term almost two-thirds of them are functionally independent4. Despite survival rates improving up to 65% and physical disability decreasing among survivors, psychosocial outcomes after aSAH remain to be notably poor since up to 55% of patients report reduced quality of life years after the haemorrhage5. Up to a half of aSAH patients have mental health complaints, including depression and anxiety6,7. Only one third of the patients resume the same work8.
The cause of these changes has not been explored, although genetic background may be involved in the development of psychosocial impairments. Genes that regulate the function of the stress response system are probable moderators of the effect that adverse life events have on development of long-term mental health disturbances9,10. Corticotropin-releasing hormone (CRH) is one of the main stress mediators in the central nervous system and plays a role in the etiology of emotional disorders11,12. Corticotropin-releasing hormone receptor 1 (CRHR1) genotype has been repeatedly associated with emotional disturbances and response to antidepressant treatment13–15. Effect of CRHR1 in major depression is moderated by a history of negative life events16 and CRHR1 genotype is associated with cortisol reactivity to stress17–19. The substantial reduction in mental health related QoL after aSAH associated with neuroendocrine dysfunction has been previously reported20. Emotional health disturbances are connected to quality of life disturbances and explain 23–47% of QOL score reductions in aSAH21.
Few articles have been published on the genetic background of QoL disturbances after aSAH and none have studied the effect of the hypothalamic–pituitary–adrenal (HPA)-system22. We hypothesize that CRHR1 genotype will influence the mental component of quality of life after aSAH.
Methods
We performed a retrospective cohort study of long-term outcome in a group of aSAH survivors (n = 125) surgically treated from January 2001 to November 2013 in a university clinic. All patients diagnosed with aSAH based on medical records during this period (n = 467) were included in the study (spontaneous ICH and other SAH causes were excluded). We identified 185 survivors with available contact information, who were proposed to participate in the study. Exclusion criteria and selection protocol are presented in Fig. 1. Eventually the studied group consisted of 125 patients. Blood samples were collected after the interview by the physician. Informed consent was obtained from all participating individuals and all procedures performed in this study involving human participants were in accordance with the ethical standards and the latest Helsinki declaration. Tartu University ethics committee approval 214/T-2/2012 was given for performing this study.
Figure 1.
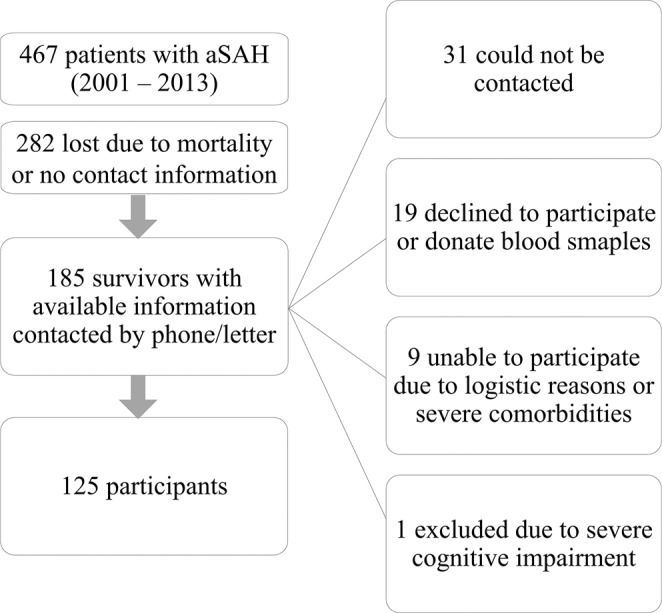
Study Flow Diagram.
General management
All patients were admitted during the acute phase of the disease. The diagnosis of aSAH was confirmed by computed tomography (CT) or lumbar puncture, and aneurysm location was assessed by CT-angiography or digital subtraction angiography. All patients were managed according to general guidelines and our protocol is previously published21. Patients were initially treated in a neurointensive care unit. Almost all patients were acutely operated upon, preferably via a pterional approach, and the aneurysms were clipped using standard microsurgical techniques. Endovascular procedures were preferentially performed in a separate institution during this time and due to this our study includes a series of clipped patients.
Procedure
Clinical variables, including Hunt and Hess grade (HH)23, which is a grading system designed to predict prognosis and outcome in aSAH, were recorded in medical histories during admission. Remaining data was collected during the follow-up evaluation, when patients were interviewed in person with a structured questionnaire. Patient clinical recovery was evaluated according to the modified Rankin Score (mRS)24. Patients were also questioned about treatment for emotional disorders after aSAH, comorbidities, education and social living situation (living with family/someone else or alone). Short Form Health Survey (SF-36) was used to assess the health-related quality of life (HRQoL).
SF-36 is widely used in clinical outcome research and is a validated instrument to assess general QoL. SF-36 consists of 36 questions and measures eight scales: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health25,26. SF-36 scores are transformed by assignment of predefined weights to the different items and calculated separately for each scale. Results can range from 0 (low QoL) to 100 (high QoL). Component scores are separately calculated for mental and physical health. SF-36 questionnaire has been added as Supplementary Material. Data of the study group was compared with corresponding values from an age-matched and gender-matched group of general population (996 subjects) obtained from respondents of a health survey of 1,989 individuals.
Genotyping
The genomic DNA was extracted from venous blood samples in 4 ml EDTA containing vaccuettes by using the standard salting-out method. The EDTA tubes were stored at −20C until DNA extraction. Isolated DNA was dissolved in Tris-EDTA (TE) buffer. The purity and concentrations of the DNA were measured by a spectrophotometer (NanoDrop, ND-1000). The gDNA samples were aliquotted and stored at −80C until usage27. Genotyping of marker single nucleotide polymorphisms (SNP) rs7209436, rs110402, rs242924 and rs242939 was carried out by using TaqMan SNP Genotyping Assay (Applied Biosystems, Foster City, CA, USA), which is a multiplex endpoint assay that detects variants of a single nucleic acid sequence. PCR reactions were run on the ViiA7 instrument (Applied Biosystems, Foster City, CA, USA) by using the following cycling parameters: after the first step at 95C for 10 minutes, 40 cycles of denaturation at 92C for 15 seconds and extension at 60C for 1 minute. Genomic DNA (20 ng/ul) was amplified in a total volume of 10 ul containing 1× Amplification Master Mix (Applied Biosystems, Foster City, CA, USA) and 1× probe. Genotypes were analysed by using the allelic discrimination function of the system (Table 1).
Table 1.
CRHR1 allele distribution (n = 125).
| SNP | Genotype (n) | Minor allele (n) | Major allele (n) | ||
|---|---|---|---|---|---|
| Rs7209436 | C/C (34) | C/T (69) | T/T (22) | T (91) | C (103) |
| Rs110402 | G/G (29) | A/G (68) | A/A (28) | A (96) | G (97) |
| Rs242924 | G/G (31) | G/T (67) | T/T (27) | T (94) | G (98) |
Abbreviations: CRHR1 - corticotropin-releasing hormone receptor 1, SNP– single nucleotide polymorphism.
Statistics
Student’s t-test was used to determine the associations between the subscale scores of the questionnaires and clinical/sociodemographic factors and compare the SF-36 mean scores of the patients with age and gender matched general population. All continuous variables were controlled for normality using Shapiro-Wilk’s W test. Beta-binomial regression analysis was performed to describe the association of CRHR1 genotype with SF-36 scale scores and calculate odds ratios. In the SNP analysis we chose between additive/dominant/recessive model based on the AIC (Akaike information criterion) of the unadjusted model. In the analysis of SF-36 results, an odds ratio (OR) higher than 1, indicates a better quality of life in the respective group (recessive model – minor allele homozygote; dominant model – major allele homozygote; additive model – OR for heterozygotes, which is multiplied in case of minor allele addition); odds ratio lower than 1 indicates a reduced outcome in SF-36 scales. More precisely, OR shows what is the probability of receiving a higher score in the selected scale by 1 point (1 point bring equal to 5 points in physical functioning, vitality, general health scales; 25 points in role-physical scale; about 11 points in pain scale; about 33 points in role-emotional scale, 12,5 points in social functioning scale and about 8 points in emotional wellbeing scale)28,29. Results were considered significant if p < 0.05. The p-values that survived the Bonferroni correction are marked in bold. Pearson’s correlation and multiple logistic regression models were used to study the impact of genotype (frequency of minor alleles), sociodemographic and clinical factors on SF-36 scores. Statistical analysis was performed with Stata 14.2 (StataCorp LLC) and SPSS 24 (IBM).
Results
Patient and aSAH characteristics are presented in Table 2. Most were female (70%, n = 88) and the mean age at the time of the hemorrhage was 54 years (SD ± 13; range 24–82 years). The mean time between initial admission and the study was 4 years (SD ± 2.8; range 1–13 years). 51 patients (41%) were evaluated later than 3 years from ictus. Most of the patients (78%) had more than 10-years of education. Only 1 patient had a previous diagnosis of depression based on the medical histories available from 2009. The mean age of the patients at the time of follow-up was 58 years (SD ± 12, range 26–82). 78% (n = 97) of the patients were living with family or somebody else. 41% (n = 51) of the patients required daily help. The most common comorbidities were hypertension (67%), joint pain (14%) and diabetes (7%). 24% (n = 30) saw a psychologist or psychiatrist and 38% (n = 30) used antidepressants during recovery. There was no statistically significant difference between minor and major CRHR1 genotypes and sociodemographic characteristics.
Table 2.
Patient (n = 125) and aSAH characteristics.
| Characteristic | N | % | |
|---|---|---|---|
| Male | 37 | 30 | |
| Female | 88 | 70 | |
| Hunt Hess score | 1 | 17 | 14 |
| 2 | 66 | 53 | |
| 3 | 23 | 18 | |
| 4 | 14 | 11 | |
| 5 | 5 | 4 | |
| Aneurysm location | ICA | 40 | 32 |
| AcomA | 44 | 35 | |
| MCA | 22 | 18 | |
| ACA | 8 | 6 | |
| BA | 9 | 7 | |
| VA | 2 | 2 | |
| Intracerebral haemorrhage | 22 | 18 | |
| Symptomatic vasospasm | 34 | 27 | |
| Hydrocephalus | acute | 43 | 34 |
| chronic | 14* | 11 | |
| Modified Rankin Score | 0 | 4 | 3 |
| 1 | 7 | 6 | |
| 2 | 57 | 46 | |
| 3 | 49 | 39 | |
| 4 | 8 | 6 |
Abbreviations: ICA internal carotid artery, AcomA anterior communicating artery, MCA middle cerebral artery, ACA anterior cerebral artery, BA basilar artery, VA vertebral artery, aSAH aneurysmal subarachnoid haemorrhage. *These 14 patients required ventriculoperitoneal shunting after aSAH.
Of the patients, 55% (n = 68) had a mRS score of 0–2 and 38% (n = 48) had a score of 3. mRS score was worse among women (2.5 (SD = 0.8) vs 2.1 (SD = 0.8), p = 0.019) and those requiring daily help (3.1 (SD = 0.5) vs 2.0 (SD = 0.7), p < 0.001).
Quality of life scores
Quality of life scores of patients measured with SF-36 were significantly lower than the general population scores on all scales, except mental health (Table 3). Physical health scores and role limitation scores due to physical and emotional problems were affected the most. The mean SF-36 summary measures were: physical health component score (PCS-36) - 43 (SD ± 9.6) and mental health component score (MCS-36) - 48.6 (SD ± 9.4).
Table 3.
Short Form Health Survey 36 results among patients and gender/age matched general population.
| SF-36 scales | Mean aSAH (n = 125) | SD aSAH | Mean population (n = 996) | SD population | p |
|---|---|---|---|---|---|
| Physical Functioning | 62.8 | 25.9 | 79 | 25.8 | <0.001 |
| Role-Physical | 38 | 41.4 | 71.4 | 39.1 | <0.001 |
| Bodily Pain | 66.2 | 27.7 | 72.6 | 26.4 | 0.008 |
| General Health | 48.6 | 21.4 | 56.3 | 19.2 | <0.001 |
| Vitality | 51.3 | 20 | 55 | 18.9 | 0.03 |
| Social Functioning | 72.1 | 24.5 | 77.4 | 28.6 | 0.01 |
| Mental Health | 67.7 | 17.9 | 69.4 | 17.8 | 0.31 |
| Role-Emotional | 53.1 | 42 | 76.3 | 36.8 | <0.001 |
Abbreviations: SF-36 - Short Form Health Survey 36, aSAH – aneurysmal subarachnoid haemorrhage, SD - standard deviation, p – P value.
Being older than 55 years old at ictus was associated with a worse physical functioning score (mean 54 (SD ± 25.4) vs 68 (SD ± 25), p = 0.003) and a worse PCS-36 score (mean 40.6 (SD ± 9.4) vs 44.6 (SD ± 9.5), p = 0.024).
Being female was associated with a worse physical functioning score (mean 56.9 (SD ± 26.4) vs 76.6 (SD ± 19.1), p < 0.001); role-physical score (mean 32.4 (SD ± 38.6) vs 51.4 (SD ± 45.6), p = 0.019); mental health score (mean 64.9 (SD ± 18.9) vs 74.1 (SD ± 13.7), p = 0.08) and a worse PCS-36 score (mean 41.6 (SD ± 9.2) vs 46.8 (SD ± 9.5), p = 0.005).
Having more than 3 years from aSAH to evaluation was associated with a worse physical functioning score (mean 58.3 (SD ± 26.7) vs 67.4 (SD ± 24.5), p = 0.048); a worse general health score (mean 43 (SD ± 20.5) vs 54.2 (SD ± 21.1), p = 0.003); and a worse PCS-36 score (mean 40.8 (SD ± 8.8) vs 45.4 (SD ± 9.9), p = 0.008). The difference in physical functioning score became statistically insignificant after adjustment for age.
Hypertension was associated with a worse physical functioning score (mean 58.9 (SD ± 25) vs 70.9 (SD ± 26.3), p = 0.018). Diabetes was also associated with a worse physical functioning score (mean 40 (SD ± 24.1) vs 64.6 (SD ± 25.4), p = 0.016). Having joint pains or rheumatoid arthritis was associated with a worse role-physical score (mean 18.1 (SD ± 26.9) vs 41.5 (SD ± 42.8), p = 0.004).
mRS score was negatively correlated to all SF-36 scales with −0.62 for physical functioning, −0.47 for role-physical, −0.45 for general health and −0.45 for role-emotional (p < 0.001). Correlations with mental health, vitality, social functioning scales and pain were below −0.4 (p < 0.001).
Association of CRHR1 genotype with SF-36 outcomes
In beta-binomial regression analysis we explored the association of CRHR1 genotype with SF-36 quality of life scores (Table 4). CRHR1 minor alleles (rs7209436, rs110402, and rs242924) were associated with higher mental health scores (OR = 1.31 − 1.6, p < 0.05) in additive and recessive models; and higher vitality scores (OR = 1.31 − 1.38, p < 0.05) in the additive model. Rs7209436 minor alleles were associated with a higher role-emotional score (OR = 1.57, 95% CI, 1.01–2.44, p = 0.044) in the additive model and rs110402 major alleles with a lower role-emotional score (OR = 0.43, 95% CI, 0.21–0.87, p = 0.019) in the dominant model. The results remained statistically significant after adjustment for gender, neurological state at admission (HH), patient age and time from aSAH to evaluation.
Table 4.
Association of genotype with Short Form Health Survey 36 scales (only statistically significant results are reported).
| SNP | Allele | Model | OR | 95% CI | p | OR* | 95% CI* | p* |
|---|---|---|---|---|---|---|---|---|
| Mental health | ||||||||
| Rs7209436 | Minor | Additive | 1.31 | 1.07–1.6 | 0.009 | 1.31 | 1.07–1.6 | 0.009 |
| Rs110402 | Minor | Additive | 1.29 | 1.06–1.57 | 0.011 | 1.26 | 1.04–1.54 | 0.019 |
| Rs242924 | Minor | Recessive | 1.6 | 1.14–2.24 | 0.006 | 1.59 | 1.14–2.22 | 0.007 |
| Vitality | ||||||||
| Rs7209436 | Minor | Additive | 1.38 | 1.13–1.7 | 0.002 | 1.38 | 1.13–1.69 | 0.002 |
| Rs110402 | Minor | Additive | 1.31 | 1.07–1.6 | 0.008 | 1.31 | 1.07–1.6 | 0.009 |
| Rs242924 | Minor | Additive | 1.33 | 1.09–1.62 | 0.005 | 1.32 | 1.08–1.62 | 0.006 |
| Role-emotional | ||||||||
| Rs7209436 | Minor | Additive | 1.57 | 1.01–2.44 | 0.044 | 1.53 | 0.98–2.4 | 0.063 |
| Rs110402 | Major | Dominant | 0.43 | 0.21–0.87 | 0.019 | 0.44 | 0.22–0.91 | 0.026 |
Abbreviations: SNP – single nucleotide polymorphism, OR-odds ratio, CI-confidence interval, p –P-value. *-values adjusted for sex, Hunt Hess score, patient age and time of evaluation from aSAH. P-values that survived the Bonferroni correction are marked with bold.
Effect of CRHR1 genotype on mental health (Rs242924) and vitality scales (all SNP’s) remained statistically significant after Bonferroni correction for multiple comparisons. Homozygotes for Rs242924 minor allele had higher mental health scores (OR = 1.6, 95% CI, 1.14–2.24, p = 0.007). Homozygotes for minor alleles of Rs7209436 (OR = 1.9, p = 0.002), Rs110402 (OR = 1.69, p = 0.008) and Rs242924 (OR = 1.77, p = 0.005) had higher vitality scores.
Patients with more minor alleles of rs7209436, rs110402, and rs242924 had higher mental QoL scores. Homozygotes for minor allele of Rs242924 had a higher mental health score compared to major allele carriers - 76 vs 66. Homozygotes for minor alleles of Rs7209436, Rs110402 and Rs242924 had higher vitality scores (60 vs 43; 58 vs 44; and 58 vs 44, respectively). Same trend is observable for other alleles in Table 5. rs242939 did not show a statistically significant association with SF-36 scores. TAT-haplotype, formed by the three minor alleles, did not show any statistically significant effect on the quality of life scores.
Table 5.
Average scores of SF-36 QoL scales associated with CRHR1 genotype according to major and minor alleles.
| Genotype | Mental health | Vitality | Role-emotional |
|---|---|---|---|
| Rs7209436 | |||
| MM | 63.5 ± 24.0 | 43.4 ± 15.3 | 41.2 ± 40.5 |
| mM | 67.2 ± 18.5 | 52.5 ± 21.4 | 55.6 ± 43.1 |
| mm | 75.5 ± 15.4 | 59.5 ± 17.6 | 63.6 ± 36.1 |
| Rs110402 | |||
| MM | 63.3 ± 17.4 | 44.0 ± 15.1 | 36.8 ± 39.5 |
| mM | 66.6 ± 18.5 | 51.7 ± 22.1 | 55.9 ± 42.6 |
| mm | 74.7 ± 14.9 | 57.9 ± 16.3 | 63.1 ± 38.2 |
| Rs242924 | |||
| MM | 65.9 ± 17.9 | 43.7 ± 15.3 | n/a |
| mM | 65.3 ± 18.3 | 52.0 ± 21.9 | n/a |
| mm | 75.7 ± 14.2 | 58.1 ± 16.6 | n/a |
Abbreviations: n/a – not associated. MM – homozygote for major allele, mM – heterozygote, mm – homozygote for minor allele.
Factors influencing mental QoL
The best multiple regression analysis models for SF-36 scales associated with CRHR1 genotype (Table 6) included mRS score, physical health component score, antidepressant usage history and CRHR1 genotype (number of minor alleles), with R2 = 0.36 for role-emotional, R2 = 0.32 for vitality and R2 = 0.3 for mental health score, all p < 0.001 (Table 6). Minor allele count of Rs110402 was associated with role-emotional score (β = 0.15, p = 0.044) and of Rs7209436 with vitality score (β = 0.23, p = 0.003). PCS-36 score was associated with role-emotional (β = 0.45, p < 0.001), vitality (β = 0.5, p < 0.001) and mental health scores (β = 0.26, p = 0.005). mRS score was associated with role-emotional (β = −0.19, p = 0.032) and mental health scores (β = −0.23, p = 0.013). Antidepressant usage history was associated with mental health score (β = −0.23, p = 0.007). Sociodemographic factors, aneurysm location and comorbidities were not associated with SF-36 mental scale outcomes in multiple regression analysis.
Table 6.
Multiple regression for SF-36 scales associated with CRHR1 genotype.
| Variables | B | SE | β | p-value | R2 |
|---|---|---|---|---|---|
| Role-Emotional | |||||
| PCS-36 | 1.96 | 0.37 | 0.45 | <0.001 | 0.36 |
| mRS | −15.56 | 7.17 | −0.19 | 0.032 | |
| Rs110402 | 9.43 | 4.64 | 0.15 | 0.044 | |
| Vitality | |||||
| PCS-36 | 1.04 | 0.16 | 0.5 | <0.001 | 0.32 |
| Rs7209436 | 6.85 | 2.3 | 0.23 | 0.003 | |
| Mental Health | |||||
| PCS-36 | 0.48 | 0.17 | 0.26 | 0.005 | 0.3 |
| Antidepressants | −8.41 | 3.06 | −0.23 | 0.007 | |
| mRS | −8.13 | 3.23 | −0.23 | 0.013 | |
Discussion
In this retrospective cohort study, we describe the effect of CRHR1 gene polymorphisms on long-term quality of life outcomes after aSAH measured with SF-36 questionnaire. Beta-binomial regression analysis showed that CRHR1 genotype (rs7209436, rs110402, and rs242924) significantly affected mental quality of life outcomes after aSAH in studied patients. CRHR1 minor genotype carriers had higher quality of life scores in mental health, role-emotional and vitality scales, compared to the more common alleles. Rs110402 major genotype increased the risk of a worse score in role-emotional scale. Results remained statistically significant after adjustment for gender, neurological state at admission (HH), patient age and time from aSAH to evaluation. Effect of CRHR1 genotype on mental health (Rs242924) and vitality scales (all SNP’s) remained statistically significant after Bonferroni correction.
In our study, aSAH patients scored significantly lower on all SF-36 scales when compared to age and gender matched general population, except mental health where the difference did not reach statistical significance. We describe a more pronounced effect of aSAH on the physical component of QoL. Age, female gender, time from aSAH and comorbidities were associated with worse physical scale and general health scores. Female gender was associated with worse mental health. All SF-36 scales were negatively correlated to mRS score. Compared to a Swedish population studied with SF-36 after ischemic stroke, aSAH patients scored higher on physical function, but lower in mental health scales30. When compared to a group of 4-year myocardial infarction survivors aged under 65 years the biggest difference presented in role-limitation scores, with aSAH patients scoring lower31. Our patient group scored somewhat lower in the mental health scale of SF-36 than the age and gender matched general population, but the difference did not reach statistical significance. Elsewhere we have shown that when studied with a validated emotional health survey (EST-Q), which is based on DSMIV and ICD-10 diagnostic criteria, the same patient group exhibited an almost 3 times higher prevalence of depression and anxiety symptoms as an age and gender matched population. SF-36 mental health scale score consists of five questions (24–26, 28, 30). The shortage of statistical difference in detailed analysis of mental health scale depended on a better result of the study population in question 30 (amount of time spent being happy in the last 4 weeks). Patients scored higher than the general population on this sole question – 51 (SD ± 25.3) vs 46.4 (SD ± 27.8), p = 0.03. When question 30 was excluded and the remaining four questions averaged, the patient group scored significantly lower than the general population – 71.8 (SD ± 19.5) vs 75.2 (SD ± 18.4), p = 0.03.
Role-emotional and vitality scores were substantially lower among aSAH patients and were significantly affected by CRHR1 genotype. Role-emotional scale shows the subjective limitations people add to daily activities due to perceived mental problems. Vitality scale is a measure of energy/fatigue and it is often reduced after severe illness32. Fatigue could present in patients due to a state of mental exhaustion from having to deal with the processes of rehabilitation and adaptation to a new situation.
The three scales of SF-36, that reflect on the mental component of quality of life, were affected by CRHR1 genotype. Other factors that influenced the results, based on the multiple regression analysis, were modified Rankin scale, PCS-36 score and history of antidepressant usage. Two thirds of the variables affecting mental QoL scores remained unknown. It was previously reported that depression and fatigue have a significant role in mental QoL score reductions measured with SF-36, explaining 42% to 47% of the variance21. Involvement of CRHR1 genotype in the present study might be explained by its association with mental health disease. CRHR1 receptor is important in regulation of HPA-axis reactivity to stress and its genotype moderates the risk of mental health disorders after stressful life events16,33, including severe illness and intensive care treatment. CRHR1 (rs1876831) genotype has been associated with post-traumatic stress disorder and depression symptoms in critical illness survivors34. This warrants for further research and separate evaluation of specific QoL modalities in patients after aSAH with focus on mental health.
Despite advances in diagnosis and early management of aSAH, outcomes for most patients remain suboptimal and long-term sequelae of the disease may be unrecognized. Quality of life in aSAH patients has been linked to both physical and mental health disturbances in the long-term5,35–39. Variations exist in the capacity and timing of recovery in different components of quality of life after aSAH, with emotional health requiring more time to improve40. It was reported that productivity losses related to aSAH reached £278.9 million in the UK in 200541. Neuropsychological disturbances can affect the ability of patients to return to work42,43, but proper rehabilitation can lead to reintegration in the long term44. We suggest that role of CRHR1 genotype warrants more research as it could help provide optimal rehabilitation and neuro-psychological support for aSAH patients.
Study limitations
Our study consisted of 125 subjects and was a retrospective design. A selection bias due to loss of follow up exists and is related to the longevity of the study, social factors and patient participation. Although, we describe an association of CRHR1 genotype with mental QoL, our study might be underpowered to draw certain conclusions. Our findings require replication in a larger cohort. It is unknown whether the studied SNP-s are functional, or they are in linkage disequilibrium with other regions. Despite mental health diagnosis being extractable from the national database, we do not know the full extent of previous emotional problems in our patient group. We lack information regarding the cognitive profile of the patients, but none of them had severe disabilities when interviewed. SF-36 is a generic questionnaire that measures multiple modalities of quality of life. It has a correlation around 0.7 with other QoL assessment tools45–47. Differences exist in results among QoL questionnaires and subscales exhibit ceiling and floor effects30,48. Cultural factors can also influence outcomes.
Conclusion
Results of our study suggest that CRHR1 genotype could influence mental quality of life outcomes after aSAH. rs7209436, rs110402, and rs242924 minor genotype significantly increased SF-36 mental health, role emotional and vitality scale scores This effect might be explained by CRHR1 role in the pathogenesis of emotional disorders and stress reactivity. New and improved biomarkers are needed to predict, diagnose and treat the long-term consequences of aSAH. Such biomarkers could help identify patients who would benefit from early neuropsychological rehabilitation.
Supplementary information
Author contributions
A.V. contributed to conception and design of the study, drafted the manuscript, prepared the tables and figures and revised the manuscript. E.P. performed the PCR analysis, wrote the genetic analysis section and revised the manuscript. S.K. contributed in organisation and revised the manuscript. T.R. contributed to conception and design of the study and revised the manuscript. T.A. contributed to conception and design of the study, contributed in organisation and revised the manuscript. All authors approved the submitted version.
Competing interests
We declare that the authors have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper. Three of the authors (E.P., S.K., T.A.) received grants from the Estonian Ministry of Education and Research. Other authors (A.V., T.R.) have no financial disclosure. E.P., S.K.: This work was supported by institutional research grants IUT20-46 of the Estonian Ministry of Education and Research. TA: This work was supported by institutional research grants IUT2-4 of the Estonian Ministry of Education and Research.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-57527-4.
References
- 1.Rinkel GJE, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10:349–356. doi: 10.1016/S1474-4422(11)70017-5. [DOI] [PubMed] [Google Scholar]
- 2.Etminan N, et al. Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population. JAMA Neurol. 2019;76:588–597. doi: 10.1001/jamaneurol.2019.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korja M, Lehto H, Juvela S, Kaprio J. Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology. 2016;87:1118–1123. doi: 10.1212/WNL.0000000000003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieuwkamp DJ, et al. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8:635–642. doi: 10.1016/S1474-4422(09)70126-7. [DOI] [PubMed] [Google Scholar]
- 5.Noble AJ, Schenk T. Which variables help explain the poor health-related quality of life after subarachnoid hemorrhage? A meta-analysis. Neurosurgery. 2010;66:772–783. doi: 10.1227/01.NEU.0000367548.63164.B2. [DOI] [PubMed] [Google Scholar]
- 6.Kreiter KT, et al. Depressed mood and quality of life after subarachnoid hemorrhage. J. Neurol. Sci. 2013;335:64–71. doi: 10.1016/j.jns.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Von-Vogelsang AC, Forsberg C, Svensson M, Wengström Y. Patients Experience High Levels of Anxiety 2 Years Following Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2015;83:1090–1097. doi: 10.1016/j.wneu.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Passier PECA, Visser-Meily JMA, Rinkel GJE, Lindeman E, Post MWM. Life Satisfaction and Return to Work After Aneurysmal Subarachnoid Hemorrhage. J. Stroke Cerebrovasc. Dis. 2011;20:324–329. doi: 10.1016/j.jstrokecerebrovasdis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Gerritsen L, et al. HPA Axis Genes, and Their Interaction with Childhood Maltreatment, are Related to Cortisol Levels and Stress-Related Phenotypes. Neuropsychopharmacology. 2017;42:2446–2455. doi: 10.1038/npp.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry. 2015;20:32–47. doi: 10.1038/mp.2014.163. [DOI] [PubMed] [Google Scholar]
- 11.Wasserman D, Wasserman J, Sokolowski M. Genetics of HPA-axis, depression and suicidality. Eur. Psychiatry. 2010;25:278–280. doi: 10.1016/j.eurpsy.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Naughton M, Dinan TG, Scott LV. Corticotropin-releasing hormone and the hypothalamic–pituitary–adrenal axis in psychiatric disease. Handb. Clin. Neurol. 2014;124:69–91. doi: 10.1016/B978-0-444-59602-4.00005-8. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, et al. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci. Lett. 2007;414:155–158. doi: 10.1016/j.neulet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Ishitobi Y, et al. Association of CRHR1 and CRHR2 with major depressive disorder and panic disorder in a Japanese population. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2012;159B:429–436. doi: 10.1002/ajmg.b.32046. [DOI] [PubMed] [Google Scholar]
- 15.White S, et al. Association of CRHR1 variants and posttraumatic stress symptoms in hurricane exposed adults. J. Anxiety Disord. 2013;27:678–83. doi: 10.1016/j.janxdis.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, et al. Negative life events and corticotropin-releasing-hormone receptor1 gene in recurrent major depressive disorder. Sci. Rep. 2013;3:1–5. doi: 10.1038/srep01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyrka AR, Price LH, Gelernter J, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with corticotropin-releasing hormone receptor gene: effect on HPA axis reactivity. Biol. Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahon PB. Genetic Association of FKBP5 and CRHR1 with Cortisol Response to Acute Psychosocial Stress in Healthy Adults. Psychopharmacol. 2013;227:231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumner JA, McLaughlin KA, Walsh K, Sheridan MA, Koenen KC. CRHR1 genotype and history of maltreatment predict cortisol reactivity to stress in adolescents. Psychoneuroendocrinology. 2014;43:71–80. doi: 10.1016/j.psyneuen.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreitschmann-Andermahr I, et al. Quality of life and psychiatric sequelae following aneurysmal subarachnoid haemorrhage: Does neuroendocrine dysfunction play a role? Clin. Endocrinol. (Oxf). 2007;66:833–837. doi: 10.1111/j.1365-2265.2007.02821.x. [DOI] [PubMed] [Google Scholar]
- 21.Vetkas A, Lepik T, Eilat T, Rätsep T, Asser T. Emotional health and quality of life after aneurysmal subarachnoid hemorrhage. Acta Neurochir. (Wien). 2013;155:1107–1114. doi: 10.1007/s00701-013-1683-3. [DOI] [PubMed] [Google Scholar]
- 22.Alfieri A, et al. Psychosocial and neurocognitive performance after spontaneous nonaneurysmal subarachnoid hemorrhage related to the APOE- ε 4 genotype: a prospective 5-year follow-up study. J. Neurosurg. 2008;109:1019–1026. doi: 10.3171/JNS.2008.109.12.1019. [DOI] [PubMed] [Google Scholar]
- 23.Hunt WE, Hess RM. Surgical Risk as Related to Time of Intervention in the Repair of Intracranial Aneurysms. J. Neurosurg. 1968;28:14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 24.Van-Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7. doi: 10.1161/01.STR.19.5.604. [DOI] [PubMed] [Google Scholar]
- 25.McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 26.McHorney CA, Ware JE, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Najera-Zuloaga J, Lee D-J, Arostegui I. Comparison of beta-binomial regression model approaches to analyze health-related quality of life data. Stat. Methods Med. Res. 2018;27:2989–3009. doi: 10.1177/0962280217690413. [DOI] [PubMed] [Google Scholar]
- 29.Arostegui I, Núñez-Antón V, Quintana JM. Analysis of the short form-36 (SF-36): the beta-binomial distribution approach. Stat. Med. 2007;26:1318–1342. doi: 10.1002/sim.2612. [DOI] [PubMed] [Google Scholar]
- 30.Almborg A, Berg S. Quality of life among Swedish patients after stroke: Psychometric evaluation of SF-36. J. Rehabil. Med. 2009;41:48–53. doi: 10.2340/16501977-0287. [DOI] [PubMed] [Google Scholar]
- 31.Brown N, et al. Quality of life four years after acute myocardial infarction: short form 36 scores compared with a normal population. Heart. 1999;81:352–8. doi: 10.1136/hrt.81.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cumming TB, Packer M, Kramer SF, English C. The prevalence of fatigue after stroke: A systematic review and meta-analysis. Int. J. Stroke. 2016;11:968–977. doi: 10.1177/1747493016669861. [DOI] [PubMed] [Google Scholar]
- 33.Bradley RG, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch. Gen. Psychiatry. 2008;65:190. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davydow DS, et al. A pilot investigation of the association of genetic polymorphisms regulating corticotrophin-releasing hormone with posttraumatic stress and depressive symptoms in medical-surgical intensive care unit survivors. J. Crit. Care. 2014;29:101–106. doi: 10.1016/j.jcrc.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visser-Meily JMA, et al. Post-Traumatic stress disorder in patients 3 years after aneurysmal subarachnoid haemorrhage. Cerebrovasc. Dis. 2013;36:126–130. doi: 10.1159/000353642. [DOI] [PubMed] [Google Scholar]
- 36.Hedlund M, Zetterling M, Ronne-Engström E, Carlsson M, Ekselius L. Depression and post-traumatic stress disorder after aneurysmal subarachnoid haemorrhage in relation to lifetime psychiatric morbidity. Br. J. Neurosurg. 2011;25:693–700. doi: 10.3109/02688697.2011.578769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Passier PECA, Visser-Meily JMA, Rinkel GJE, Lindeman E, Post MWM. Determinants of health-related quality of life after aneurysmal subarachnoid hemorrhage: A systematic review. Qual. Life Res. 2013;22:1027–1043. doi: 10.1007/s11136-012-0236-1. [DOI] [PubMed] [Google Scholar]
- 38.Meyer B, et al. Health-Related Quality of Life in Patients with Subarachnoid Haemorrhage. Cerebrovasc. Dis. 2010;30:423–431. doi: 10.1159/000317078. [DOI] [PubMed] [Google Scholar]
- 39.Persson HC, Carlsson L, Sunnerhagen KS. Life situation 5 years after subarachnoid haemorrhage. Acta Neurol. Scand. 2018;137:99–104. doi: 10.1111/ane.12815. [DOI] [PubMed] [Google Scholar]
- 40.He S, Mack WJ. Health-Related Quality of Life After Aneurysmal Subarachnoid Hemorrhage: Interplay Between Physical, Cognitive, and Emotional Factors. World Neurosurg. 2014;81:37–39. doi: 10.1016/j.wneu.2013.01.066. [DOI] [PubMed] [Google Scholar]
- 41.Rivero-Arias O, Gray A, Wolstenholme J. Burden of disease and costs of aneurysmal subarachnoid haemorrhage (aSAH) in the United Kingdom. Cost Eff. Resour. Alloc. 2010;8:6. doi: 10.1186/1478-7547-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crago EA, et al. Impaired Work Productivity After Aneurysmal Subarachnoid Hemorrhage. J. Neurosci. Nurs. 2016;48:260–268. doi: 10.1097/JNN.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Yassin A, Ouyang B, Temes R. Depression and Anxiety Following Aneurysmal Subarachnoid Hemorrhage Are Associated With Higher Six-Month Unemployment Rates. J. Neuropsychiatry Clin. Neurosci. 2017;29:67–69. doi: 10.1176/appi.neuropsych.15070171. [DOI] [PubMed] [Google Scholar]
- 44.Sonesson B, Kronvall E, Säveland H, Brandt L, Nilsson OG. Long-term reintegration and quality of life in patients with subarachnoid hemorrhage and a good neurological outcome: findings after more than 20 years. J. Neurosurg. 2018;128:785–792. doi: 10.3171/2016.11.JNS16805. [DOI] [PubMed] [Google Scholar]
- 45.Richardson J, Iezzi A, Khan MA, Chen G, Maxwell A. Measuring the Sensitivity and Construct Validity of 6 Utility Instruments in 7 Disease Areas. Med. Decis. Mak. 2016;36:147–159. doi: 10.1177/0272989X15613522. [DOI] [PubMed] [Google Scholar]
- 46.Linde L, Sørensen J, Ostergaard M, Hørslev-Petersen K, Hetland ML. Health-related quality of life: validity, reliability, and responsiveness of SF-36, 15D, EQ-5D [corrected] RAQoL, and HAQ in patients with rheumatoid arthritis. J. Rheumatol. 2008;35:1528–37. [PubMed] [Google Scholar]
- 47.McDonough CM, et al. Comparison of EQ-5D, HUI, and SF-36-derived societal health state values among spine patient outcomes research trial (SPORT) participants. Qual. Life Res. 2005;14:1321–32. doi: 10.1007/s11136-004-5743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rautalin M, et al. Health-related quality of life in different states of breast cancer – comparing different instruments. Acta Oncol. (Madr). 2018;57:622–628. doi: 10.1080/0284186X.2017.1400683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


