Abstract
An experiment was conducted to quantify the timing and magnitude of the effects of coccidiosis vaccination on the growth performance, apparent ileal digestibility (AID) of nutrients and energy, intestinal morphology, and plasma carotenoids and nitric oxide in broilers. Treatment groups consisted of 3 coccidiosis control methods [unvaccinated, unmedicated (NC), in-feed chemical coccidiostat (PC), and live oocyst vaccination (VAC) at day of hatch] administered to male Cobb broilers reared in floor pens. Body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR) were determined at 12, 16, 20, 28, and 36 d. Blood and ileal digesta were collected from birds in 10 replicate pens of each treatment at 12, 16, 20, and 36 d to evaluate plasma carotenoid and nitric oxide concentrations and determine nutrient AID and IDE. Jejunal samples were taken at 12, 20, and 36 d for morphological measurements. Oocyst shedding in VAC birds was confirmed by increased oocyst counts and decreased carotenoid concentrations (P < 0.05) when compared with PC birds, with no differences (P > 0.05) in nitric oxide concentrations. At 20 d, BWG and FI were lowest (P < 0.05) in VAC birds, intermediate in NC birds, and highest in PC birds, with no differences in FCR (P > 0.05). By 28 and 36 d, FCR was higher (P < 0.05) for VAC and NC birds but BWG and FI of VAC birds were similar (P > 0.05) to PC birds. At d 12, IDE and AID of nitrogen and ether extract were lower (P < 0.05) in VAC birds than PC birds. At d 16, AID of nitrogen was similar (P > 0.05) between PC and VAC birds, whereas AID of ether extract remained lower in VAC birds than PC birds. No differences in AID of nutrients or IDE were observed (P > 0.05) between VAC and PC birds at 20 or 36 d. No differences (P > 0.05) in jejunal morphology were observed at any time point. Overall, VAC elicited a transient reduction in AID and IDE, particularly for lipids, that diminished by d 20.
Keywords: broiler, coccidiosis, vaccination, digestibility, growth performance
Introduction
Coccidiosis, an intestinal parasitic disease caused by protozoa of the genus Eimeria, remains one of the most prevalent diseases in commercial poultry production. Traditionally, coccidiosis has primarily been managed with in-feed administration of anticoccidial ionophores, but in the United States, ionophores are typically not used in antibiotic-free poultry production systems. Although such systems do currently permit the use of chemical anticoccidial drugs, there is a limited number of these compounds and their overuse can quickly lead to emergence of drug resistant Eimeria strains (1, 2). Therefore, this resistance has increased reliance on live oocyst coccidiosis vaccination, which can induce immunity and reintroduce drug-sensitive strains into the rearing facility, as an important control strategy for coccidiosis (3).
Coccidiosis vaccination involves exposure of young chicks, typically at hatch, to small numbers of live Eimeria oocysts to promote immunity and reduce the potential for clinical coccidiosis outbreaks during the later growth periods (3). However, mild infections that occur during the process of vaccinal oocyst cycling, which involves the initial infection, oocyst shedding and sporulation, and reinfection, may compromise broiler growth through reduced feed intake or feed efficiency, and the relatively short lifespan of broilers may be insufficient for compensatory gain (4, 5). Impaired feed efficiency during vaccine cycling is presumably due in part to nutrient malabsorption associated with intestinal damage and inflammation associated with the sub-clinical, vaccine-induced infection (5–7).
Reductions in nutrient and energy digestibility have been reported in cocci-exposed birds, and responses are dependent on diet composition and the type and number of Eimeria species administered in the challenge model (8–12). Increasing the dietary concentration of digestible nutrients for which digestibility is impaired is a potential strategy to support the performance of broilers during coccidial vaccine cycling. Indeed, Adedokun et al. (7) fed increased concentrations of supplemental amino acids to broilers to account for a predicted reduction in amino acid digestibility based on a previous coccidial challenge trial and observed improved feed efficiency of broilers compared those fed a control diet. However, commercial adoption of this approach for floor-reared, vaccinated broilers requires longitudinal characterization of nutrient digestibility to identify appropriate dietary adjustments that may benefit broilers during the critical stages of vaccination.
Most research to characterize the impact of coccidial challenges on nutrient utilization in broilers has been conducted in battery cages equipped with wire flooring, which prevents multiple infections during oocyst cycling (7–9, 11). Additionally, most of these experiments have involved acute challenges that are much more severe and occur later compared with the mild infections that occur within the first 3 weeks of a vaccinated commercial broiler flock. Therefore, the objective of this study was to characterize the timing and magnitude of reductions in growth performance and nutrient utilization in floor-reared broilers throughout different stages of oocyst cycling following live Eimeria vaccination of broilers at day of hatch.
Materials and Methods
General Bird Husbandry and Diets
One thousand, five-hundred Cobb 500 male broiler chicks were obtained from a commercial hatchery on day of hatch. All chicks were group-weighed and distributed to 120 floor pens on clean litter with fresh pine shavings. Each floor pen was equipped with a hanging feeder and a nipple drinker line. To ensure sufficient digesta content, 14 birds were placed (0.07 m2 per bird) in 30 pens pre-selected for the first collection time point at 12 d post-hatch, whereas 12 birds (0.08 m2 per bird) were placed in the other 90 pens to be used for subsequent collections. Birds were provided access to feed and water ad libitum throughout the experiment. The lighting schedule and temperature targets were adjusted according to management guidelines published by the primary breeder (13). Birds were reared up to 36 d post-hatch and fed starter (0–14 d), grower (15–28 d), and finisher (29–36 d) diets based on corn and soybean meal and formulated to meet or exceed published nutrient recommendations (13) (Table 1).
Table 1.
Composition of experimental diets fed to broilers from 0 to 36 d post-hatcha.
| Ingredient, % as-fed | Starter (0–14 d) | Grower (15–28 d) | Finisher (29–36 d) |
|---|---|---|---|
| Corn | 57.68 | 61.11 | 62.02 |
| Soybean meal (46.8%) | 32.90 | 27.08 | 23.61 |
| DDGS | 4.00 | 6.00 | 8.00 |
| Soybean oil | 1.34 | 2.00 | 2.92 |
| Limestone | 1.25 | 1.22 | 1.17 |
| Dicalcium phosphate | 0.90 | 0.74 | 0.52 |
| Salt | 0.45 | 0.42 | 0.41 |
| DL-methionine | 0.31 | 0.26 | 0.22 |
| L-lysine HCl | 0.24 | 0.24 | 0.22 |
| L-threonine | 0.09 | 0.08 | 0.07 |
| Trace mineral premixb | 0.10 | 0.10 | 0.10 |
| Vitamin premixc | 0.10 | 0.10 | 0.10 |
| Se premixd (0.06%) | 0.02 | 0.02 | 0.02 |
| Choline chloride (60%) | 0.05 | 0.04 | 0.04 |
| Santoquin | 0.02 | 0.02 | 0.02 |
| Phytasee | 0.01 | 0.01 | 0.01 |
| Titanium dioxide | 0.50 | 0.50 | 0.50 |
| Inert fillerf | 0.05 | 0.05 | 0.05 |
| Calculated composition, % unless noted otherwise | |||
| AMEn, kcal/kg | 3,015 | 3,098 | 3,175 |
| CP | 22.01 | 20.00 | 19.00 |
| Digestible lysine | 1.18 | 1.05 | 0.95 |
| Digestible TSAA | 0.89 | 0.80 | 0.74 |
| Digestible threonine | 0.77 | 0.69 | 0.65 |
| Calcium | 0.90 | 0.84 | 0.76 |
| Available P | 0.45 | 0.42 | 0.38 |
| Analyzed composition, % unless noted otherwise | |||
| Gross energy, kcal/kg | 3,991 | 4,027 | 4,059 |
| CP | 22.00 | 19.60 | 19.25 |
| Ether extract | 4.80 | 5.57 | 6.52 |
| Starch | 46.89 | 47.71 | 56.33 |
DDGS, distillers dried grains with solubles; AMEn, nitrogen-corrected apparent metabolizable energy.
Supplied the following per kg of diet: manganese, 100 mg; zinc, 100 mg; copper, 10.0 mg; iodine, 1.0 mg; iron, 50 mg; magnesium, 27 mg.
Supplied the following per kg of diet: vitamin A, 30,863 IU; vitamin D3, 22,045 ICU; vitamin E, 220 IU; vitamin B12, 0.05 mg; menadione, 6.0 mg; riboflavin, 26 mg; d-pantothenic acid, 40 mg; thiamine, 6.2 mg; niacin, 154 mg; pyridoxine, 11 mg; folic acid, 3.5 mg; biotin, 0.33 mg.
Supplied 0.12 mg of selenium per kg of diet.
Optiphos®, (Huvepharma Inc., Peachtree City, GA.) provided 250 FTU/kg of diet.
Clinacox®, (Huvepharma Inc., Peachtree City, GA), provided 1 mg/kg diclazuril to the diet at the expense of the inert filler.
Experimental Treatments
Upon arrival, one-third (500) of the chicks were orally-gavaged with the manufacturer's recommended dose of a live oocyst vaccine (Coccivac®-B52; Merck Animal Health, Intervet Inc. Millsboro, DE, USA). An oral gavage (0.25 mL/bird) was used to provide uniform administration. Throughout the trial, litter was sprayed once daily with water using a handheld garden sprayer to ensure sufficient moisture content for oocyst sporulation. Unvaccinated broilers were fed diets formulated with or without an in-feed chemical anticoccidial drug, resulting in a total of three treatments: (1) unmedicated and unvaccinated (NC), (2) in-feed chemical coccidiostat (Clinacox, Huvepharma) administration (PC), and (3) live oocyst vaccination (VAC) at day of hatch. Each treatment group was represented by 10 replicate pens for each collection time point.
Measurement of Live Performance and Vaccine Cycling
Birds and feeders were weighed at 0, 12, 16, 20, 28, and 36 d post-hatch for calculation of body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR). All dead and culled birds were weighed individually and FCR calculations were adjusted to include the weight gain of dead birds. To assess vaccine cycling, the number of oocysts per gram (OPG) of litter samples collected from each pen was determined before bird placement and at 12, 16, 20, 28, and 36 d post-vaccination. Samples were taken from 3 or 4 different locations within each pen and pooled into airtight plastic bags and kept refrigerated until further analysis. All sample counts were conducted within 1 week of collection. Samples (~150 g) were soaked in water (~1,000 mL) overnight and the solution was vigorously stirred until excreta appeared to be completely solubilized. A 1 ml subsample was further diluted with 9 ml of saturated salt solution and pipetted into the chamber of a McMaster counting slide. Duplicate counts were made for each sample and subsequent calculations based on the following equation:
where the dilution was 10 and the volume of the counting chamber was 0.15 ml.
Digesta, Blood, and Tissue Sampling
To avoid changes in stocking density among time points due to bird sampling, all birds from 10 replicate pens of each treatment were humanely euthanized at 12, 16, 20, and 36 d post-hatch by CO2 inhalation for collection of ileal digesta. Ileal contents from all birds in each pen were collected by gently flushing the distal half of the ileum using deionized water. Digesta samples were pooled within pen and frozen (−20°C) until analysis. At 12, 16, and 36 d post-hatch, 2 birds from the same pens used for digesta collection were randomly selected for blood and jejunal tissue collection and pH determination of duodenal lumen contents. Blood was collected immediately post-mortem via cardiac puncture into tubes containing EDTA, placed on ice, and centrifuged for 15 min at 1,300 × g and 4°C to separate plasma. Plasma from birds within a pen were pooled, aliquoted, and stored at −80°C until further analysis. To determine duodenal pH, a digital pH meter (Mettler-Toledo, UK) with a spear tip piercing pH electrode (Sensorex S175CD) was directly inserted into the digesta in the distal duodenal loop and the pH was recorded. The probe was rinsed with distilled water after each reading and the tip of the pH probe was stored in pH 4 solution when not in use. Jejunal tissue samples (~2 cm in length) were collected at the midpoint of the jejunum between the end of the duodenal loop and the Meckel's diverticulum and rinsed with PBS to remove luminal contents and placed in scintillation vials containing 10% neutral-buffered formalin.
Laboratory Analyses
Frozen digesta samples were lyophilized and ground using an electric coffee grinder to provide an evenly ground sample while avoiding significant loss. Diet and digesta samples were analyzed for dry matter, gross energy, nitrogen, ether extract, and starch content. Dry matter was determined according to AOAC (14) method 934.02. Gross energy was determined with a bomb calorimeter (Parr 6200 bomb calorimeter, Parr Instruments Co., Moline, IL.). Nitrogen was determined using the combustion method (Fisions NA-2000, CE Elantech, Lakewood, NJ) standardized with EDTA [method 990.03, (14)] and ether extract was determined according to AOAC (14) method 920.39. Starch concentrations of feed and digesta samples were measured using the Megazyme Total Starch Assay Kit according to instructions provided by the manufacturer (Megazyme Int. Ireland Ltd., Wicklow, Ireland). Titanium dioxide was included in the feed at 0.5% as an indigestible marker, and diet and digesta TiO2 concentrations were determined in duplicate following the procedures of Short et al. (15). Apparent ileal digestibility (AID) of dry matter, gross energy, ether extract, nitrogen, and starch were calculated using the following equation:
where (X/TiO2) = ratio of nutrient concentration to TiO2 in the diet or ileal digesta. Energy digestibility (%) values obtained from the equation above were multiplied by the gross energy content of the feed to calculate ileal digestible energy (IDE).
Plasma samples were analyzed to determine carotenoid and nitric oxide concentrations. All blood processing and carotenoid analysis procedures were conducted under yellow light. Plasma carotenoid concentrations were determined by spectrophotometry as previously described by Allen (16). Plasma nitrate (NO3–) + nitrite (NO2–) concentrations were measured to determine total nitric oxide using a colorimetric assay kit (Cayman Chemical CO., Ann Arbor, MI). Prior to nitric oxide analysis, plasma samples were filtered through pre-rinsed centrifugal filters (VWR, Radnor, PA) to remove potentially interfering proteins with a molecular weight >30 kDa.
Jejunal tissue samples were embedded in paraffin, sectioned at 4 μm, set on a glass slide, and stained with hematoxylin and eosin. Photomicrographs of each jejunum sample were acquired using a light microscope (Nikon Eclipse) equipped with a digital camera. Imaging software (Nikon's NIS Elements Basic Research Microscope Imaging) was used for measurement of villus height, crypt depth, and villus width under 4x magnification. For villus height, ~6 intact well-oriented villi per bird were randomly selected and measured. Villus height was measured from the tip of the villus to the villus-crypt junction, whereas crypt depth was defined as the depth of the invagination between adjacent villi. The width of the villus was measured at the basal (crypt-villus junction) and apical ends (17). Apparent jejunal villus surface area was calculated using the following equation published by Iji et al. (17):
Statistical Analysis
Pen was considered the experimental unit with 10 replicate pens per treatment for each collection time point. Treatment groups were arranged in randomized complete block design and the statistical model included pen location as the random blocking factor. Data within each time point were subjected to ANOVA using the MIXED procedure of SAS 9.4 and are presented as least squares means of treatment groups. Jejunal morphology data were transformed (log 10) to meet normality assumptions for the ANOVA. Statistically different treatment means were separated using a Tukey's multiple comparison test. Statistical significance was considered at P < 0.05.
Results
Oocyst Shedding and Plasma Measurements
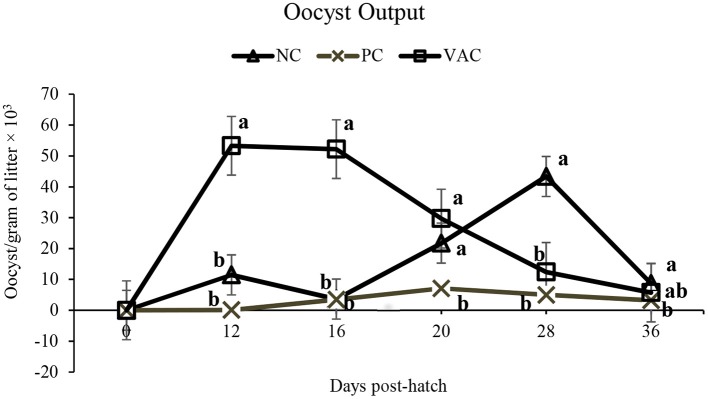
Litter oocyst counts of PC birds remained low throughout the experiment (Figure 1). At 12 and 16 d post hatch, OPG counts from VAC birds were significantly (P < 0.05) higher than those of NC and PC treatments. At 20 d post-hatch, OPG counts in both NC and VAC birds were higher (P < 0.05) than those of PC birds, indicating that NC birds had become inadvertently infected. At 28 d post-hatch, OPG of litter in NC birds was significantly higher (P < 0.05) than both PC and VAC treatments, with no differences (P > 0.05) observed between PC and VAC birds. At 36 d post-hatch, OPG of litter was highest (P < 0.05) in NC birds, intermediate in VAC birds, and lowest in PC birds.
Figure 1.
The effects of coccidiosis vaccination on litter oocyst counts from 0 to 36 d post-hatch. Values are LSMeans of 10 replicate pens. NC, negative control; PC, positive control, birds were given an in-feed anticoccidial drug; VAC, vaccinated, birds were given a commercial dose of vaccine on 0 d. Within each time point, lines that do not share a common superscript are different (P < 0.05).
Plasma carotenoid concentrations and plasma nitric oxide levels are presented in Table 2. Plasma carotenoid concentrations in NC and PC birds were higher (P < 0.05) than VAC birds at both 12 and 16 d post-hatch. At 20 d post-hatch, plasma carotenoid concentrations for NC and VAC birds were lower (P < 0.05) than those of PC birds. At 36 d, no differences (P > 0.05) in plasma carotenoids were observed among the treatments. Plasma nitric oxide concentrations were unaffected (P > 0.05) by any of the treatments, regardless of collection time point.
Table 2.
Effects of coccidiosis vaccination on plasma carotenoid and nitric oxide concentrations from 12 to 36 d post-hatch1,2.
| Item | NC | PC | VAC | SEM | P-value |
|---|---|---|---|---|---|
| 12 d | |||||
| Plasma carotenoids, μg/mL | 2.28a | 2.38a | 1.15b | 0.174 | 0.001 |
| Nitric oxide, μM | 8.41 | 8.40 | 8.18 | 0.483 | 0.933 |
| 16 d | |||||
| Plasma carotenoids, μg/mL | 2.05a | 2.21a | 1.30b | 0.194 | 0.006 |
| Nitric oxide, μM | 9.13 | 8.75 | 8.19 | 0.481 | 0.399 |
| 20 d | |||||
| Plasma carotenoids, μg/mL | 0.54b | 1.29a | 0.48b | 0.134 | 0.001 |
| Nitric oxide, μM | 6.03 | 7.13 | 7.06 | 0.418 | 0.133 |
| 36 d | |||||
| Plasma carotenoids, μg/mL | 3.37 | 4.24 | 3.14 | 0.315 | 0.050 |
| Nitric oxide, μM | 8.26 | 6.99 | 8.24 | 0.630 | 0.279 |
Means within a row that do not share a common superscript are different (P < 0.05).
Values are LSMeans of 10 replicate pens.
NC, negative control; PC, positive control, birds were given an in-feed anticoccidial drug; VAC, birds were given a commercial dose of vaccine on 0 d.
Growth Performance
No differences (P > 0.05) in BWG, FI, or FCR were observed among birds in any of the treatment groups from 0 to 12 d or 0 to 16 d post-hatch (Table 3). From 0 to 20 d post-hatch, BWG and FI were lowest (P < 0.05) in VAC birds, intermediate in NC birds, and highest in PC birds, with no differences (P > 0.05) in FCR. By 28 d post-hatch, BWG in VAC birds did not differ (P > 0.05) from PC and NC birds, whereas NC birds had a lower (P < 0.05) BWG when compared with PC birds. No differences (P > 0.05) in FI were observed among treatments at 28 d post-hatch; however, NC and VAC birds had a higher (P < 0.05) FCR when compared with PC birds. By 36 d post-hatch, FCR remained higher (P < 0.05) in NC and VAC compared with PC birds, whereas BWG of broilers and FI were not influenced (P > 0.05) by treatment.
Table 3.
| Item | NC | PC | VAC | SEM | P-value |
|---|---|---|---|---|---|
| 0–12 d | |||||
| d12 BW, kg/bird | 0.355 | 0.350 | 0.347 | 0.004 | 0.362 |
| BW gain, kg/bird | 0.312 | 0.308 | 0.305 | 0.004 | 0.414 |
| Feed intake, kg/bird | 0.400 | 0.397 | 0.397 | 0.005 | 0.880 |
| FCR | 1.279 | 1.292 | 1.305 | 0.010 | 0.183 |
| 0–16 d | |||||
| d16 BW, kg/bird | 0.544 | 0.548 | 0.531 | 0.006 | 0.133 |
| BW gain, kg/bird | 0.502 | 0.505 | 0.489 | 0.006 | 0.115 |
| Feed intake, kg/bird | 0.736 | 0.742 | 0.724 | 0.007 | 0.185 |
| FCR | 1.468 | 1.473 | 1.488 | 0.011 | 0.440 |
| 0–20 d | |||||
| d20 BW, kg/bird | 0.892ab | 0.909a | 0.877b | 0.008 | 0.038 |
| BW gain, kg/bird | 0.849ab | 0.867a | 0.834b | 0.008 | 0.036 |
| Feed intake, kg/bird | 1.138ab | 1.164a | 1.117b | 0.009 | 0.006 |
| FCR | 1.341 | 1.345 | 1.341 | 0.011 | 0.961 |
| 0–28 d | |||||
| d28, kg/bird | 1.602b | 1.682a | 1.647ab | 0.016 | 0.007 |
| BW gain, kg/bird | 1.560b | 1.639a | 1.605ab | 0.016 | 0.007 |
| Feed intake, kg/bird | 2.334 | 2.375 | 2.393 | 0.019 | 0.096 |
| FCR | 1.501a | 1.454b | 1.493a | 0.007 | 0.001 |
| 0–36 d | |||||
| d36 BW, kg/bird | 2.512 | 2.588 | 2.555 | 0.024 | 0.086 |
| BW gain, kg/bird | 2.469 | 2.545 | 2.513 | 0.024 | 0.087 |
| Feed intake, kg/bird | 3.882 | 3.905 | 3.950 | 0.027 | 0.191 |
| FCR | 1.578a | 1.543b | 1.576a | 0.009 | 0.018 |
Means within a row that do not share a common superscript are different (P < 0.05).
Values are LSMeans of 9 or 10 replicate pens.
NC, negative control; PC, positive control, birds were given an in-feed anticoccidial drug; VAC, vaccinated, birds were given a commercial dose of vaccine on 0 d.
Apparent Ileal Digestibility of Nutrients and IDE
Vaccinated birds had lower (P < 0.05) IDE and AID of nitrogen, ether extract, and starch compared with NC and PC birds at 12 d post-hatch (Table 4). By 16 d post-hatch, no differences (P > 0.05) in AID of nitrogen, starch, or IDE were observed among treatment groups, but AID of ether extract remained lower (P < 0.05) in VAC birds than in NC or PC birds. At 20 d post-hatch, no differences (P > 0.05) in AID or IDE were observed between PC and VAC birds, however, NC birds had lower (P < 0.05) AID of nitrogen, ether extract, and IDE compared with PC and VAC birds. By 36 d post-hatch, no differences (P > 0.05) in IDE and AID of nitrogen or ether extract were observed among treatment groups. At 36 d, AID of starch remained lower (P < 0.05) in NC birds compared with PC birds, with no difference (P > 0.05) in AID of starch between PC and VAC birds.
Table 4.
Effects of coccidiosis vaccination on the apparent ileal digestibility (%) of nutrients and ileal digestible energy (kcal/kg) in broilers to 36 d post-hatch1,2.
| Item | NC | PC | VAC | SEM | P-value |
|---|---|---|---|---|---|
| 12 d | |||||
| Dry matter, % | 70.4a | 71.5a | 66.9b | 0.58 | 0.001 |
| Nitrogen, % | 81.7a | 82.8a | 77.6b | 0.55 | 0.001 |
| Ether extract, % | 88.5a | 89.3a | 79.6b | 1.72 | 0.001 |
| Starch, % | 90.7a | 92.0a | 88.9b | 0.41 | 0.001 |
| IDE, kcal/kg3 | 3,337a | 3,399a | 3,138b | 26 | 0.001 |
| 16 d | |||||
| Dry matter, % | 74.9 | 73.3 | 74.0 | 0.52 | 0.086 |
| Nitrogen, % | 83.9 | 83.4 | 83.4 | 0.45 | 0.706 |
| Ether extract, % | 91.1a | 91.0a | 85.9b | 1.38 | 0.017 |
| Starch, % | 91.0 | 89.7 | 89.7 | 0.76 | 0.380 |
| IDE, kcal/kg3 | 3,587 | 3,542 | 3,543 | 24 | 0.317 |
| 20 d | |||||
| Dry matter, % | 71.1b | 72.6a | 73.2a | 0.43 | 0.005 |
| Nitrogen, % | 79.6b | 82.4a | 81.7a | 0.46 | 0.004 |
| Ether extract, % | 65.1b | 85.9a | 78.6a | 3.53 | 0.001 |
| Starch, % | 90.4b | 91.7a | 91.3ab | 0.34 | 0.028 |
| IDE, kcal/kg3 | 3,326b | 3,469a | 3,447a | 25 | 0.001 |
| 36 d | |||||
| Dry matter, % | 75.5a | 75.6a | 73.7b | 0.41 | 0.005 |
| Nitrogen, % | 83.5 | 83.1 | 83.7 | 0.41 | 0.398 |
| Ether extract, % | 94.0 | 94.1 | 92.5 | 0.88 | 0.271 |
| Starch, % | 89.0b | 90.9a | 89.4ab | 0.48 | 0.023 |
| IDE, kcal/kg3 | 3,629 | 3,642 | 3,605 | 19 | 0.404 |
Means within a row that do not share a common superscript are different (P < 0.05).
Values are LSMeans of 10 replicate pens.
NC, negative control; PC, positive control, birds were given an in-feed anticoccidial drug; VAC, vaccinated, birds were given a commercial dose of vaccine on 0 d.
IDE, ileal digestible energy.
Jejunal Morphology and Duodenal pH
No significant differences (P > 0.05) in intestinal jejunal morphology or duodenal pH were observed among any of the treatment groups at any time point (Table 5). However, at 12 d post-hatch, there was a tendency (P = 0.07) for VAC birds to have deeper jejunal crypts than NC and PC birds. Furthermore, at 36 d post-hatch, there was a tendency (P = 0.07) for VAC birds to have reduced jejunal villus heights compared with NC and PC birds.
Table 5.
Effects of coccidiosis vaccination on jejunal morphology and duodenal pH of broilers in 36 d post-hatch1,2.
| Item | NC | PC | VAC | SEM | P-value3 |
|---|---|---|---|---|---|
| 12 d | |||||
| Villus height, μm | 645 (2.81) | 669 (2.82) | 693 (2.84) | 23.0 (0.015) | 0.392 |
| Crypt depth, μm | 124 (2.08) | 118 (2.82) | 145 (2.84) | 5.7 (0.028) | 0.082 |
| Villus height to crypt depth | 5.81 (0.75) | 6.60 (0.81) | 5.38 (0.71) | 0.372 (0.037) | 0.159 |
| Villus surface area, mm2 | 0.10 (5.01) | 0.10 (5.01) | 0.10 (4.98) | 0.006 (0.025) | 0.772 |
| pH | 5.64 | 5.61 | 5.57 | 0.074 | 0.773 |
| 20 d | |||||
| Villus height, μm | 774 (2.89) | 730 (2.86) | 786 (2.90) | 24.1 (0.014) | 0.189 |
| Crypt depth, μm | 200 (2.28) | 188 (2.26) | 211 (2.31) | 13.4 (0.029) | 0.561 |
| Villus height to crypt depth | 4.58 (0.64) | 4.40 (0.62) | 4.36 (0.62) | 0.352 (0.033) | 0.891 |
| Villus surface area, mm2 | 0.13 (5.10) | 0.13 (5.11) | 0.13 (5.12) | 0.006 (0.021) | 0.800 |
| pH | 6.11 | 6.08 | 6.12 | 0.028 | 0.628 |
| 36 d | |||||
| Villus height, μm | 1,058 (3.02) | 1,100 (3.04) | 1,005 (3.01) | 27.7 (0.012) | 0.071 |
| Crypt depth, μm | 177 (2.23) | 153 (2.17) | 169 (2.21) | 14.4 (0.035) | 0.507 |
| Villus height to crypt depth | 7.10 (0.83) | 8.14 (0.90) | 6.96 (0.82) | 0.672 (0.040) | 0.306 |
| Villus surface area, mm2 | 0.19 (5.28) | 0.20 (5.30) | 0.19 (5.26) | 0.001 (0.029) | 0.653 |
| pH | 6.12 | 6.15 | 6.12 | 0.015 | 0.166 |
Values are LSMeans of 10 replicate pens with transformed data (log10) used for statistical analysis in parentheses.
NC, negative control; PC, positive control, birds were given an in-feed anticoccidial drug; VAC, vaccinated, birds were given a commercial dose of vaccine on 0 d.
P-values represent transformed data (log10).
Discussion
Coccidiosis vaccines can prevent coccidiosis outbreaks in broiler flocks but can induce damage to the intestinal epithelium, impair live performance, and potentially increase susceptibility to other enteric diseases such as necrotic enteritis. The objective of this experiment was to characterize the timing and magnitude by which coccidiosis vaccination at day of hatch influences growth performance and nutrient utilization in floor-reared broilers during the various stages of Eimeria cycling. Litter oocyst counts and plasma carotenoids indicated that in-feed diclazuril administration prevented coccidial infection in PC birds, whereas increased oocyst shedding and decreased plasma carotenoid concentrations reflected cycling of vaccinal oocysts in VAC broilers. This finding aligned with previous reports that plasma carotenoids are a sensitive indicator of coccidial-induced intestinal damage in chickens (18–20) and confirmed that the most commercially-important comparison of the medicated PC group and VAC remained valid throughout the experiment. However, these same measurements indicated an inadvertent infection of the NC group, which likely occurred due to the fact that all treatment groups were distributed evenly in blocks throughout a single experimental facility to minimize environmental or location-related effects. Although the infection of the NC group was unintended, this did provide another time point at which to compare the impact of infection on the responses measured which, as described below, generally aligned with the responses observed in the VAC group at 12 and 16 d post-hatch.
Coccidiosis vaccines can induce coccidiasis, a mild transient form of coccidiosis, usually occurring between 14 and 28 d post-hatch, which can impair broiler performance (5). Coccidiosis vaccination at day of hatch did not impact broiler performance at 12 or 16 d post-hatch in the current experiment, but it did reduce FI and BWG of broilers at 20 d post-hatch, with no effects on FCR. The reductions in BWG and FI for VAC birds, compared with PC birds, had diminished by 28 d. Lehman et al. (5) also reported vaccinated broilers had a reduction in BWG at 21 d of age, although this reduction was a result of impaired FCR and not reduced FI. Furthermore, Silva et al. (21) similarly reported a vaccine-induced reduction in BWG at 21 d of age but observed no differences in BWG between vaccinated and non-vaccinated birds at 36 d of age. Indeed, the goal of coccidiosis vaccination is to provide an early Eimeria exposure to allow sufficient time for the birds to compensate for the minor reduction in weight before the end of the grow-out period (22). The decreased BWG in NC birds relative to PC birds at 28 d post-hatch is in agreement with the litter oocyst counts and further reflects an inadvertent infection on NC birds at this time. However, while VAC and PC birds did not differ in BWG or FI at 28 d, FCR at 28 and 36 d post-hatch remained higher for NC and VAC birds than for PC birds, possibly due to nutrient malabsorption throughout the experiment.
The greatest impacts of vaccination on nutrient and energy digestibility were observed at 12 d post-hatch, which likely corresponds with the second Eimeria life cycle (23). Specifically, vaccination decreased IDE by 261 kcal/kg and AID of nitrogen, ether extract, and starch by 5.2, 9.7, and 3.1 percentage units, respectively (6.3, 10.9, and 3.4 percentage reduction, respectively) compared with PC birds at 12 d post-hatch. Reduced nitrogen digestibility leads to an increased amount of protein in the terminal ileum, which can be fermented to produce toxic compounds such as biogenic amines (24, 25). While differences in nitrogen digestibility between VAC and PC birds had diminished by 16 d post-hatch, the impaired 12 d digestibility may onset early intestinal bacteria overgrowth to predispose the birds to secondary infections, such as necrotic enteritis, that typically manifests between the second and fifth week of age (26).
Caloric costs due to coccidiosis vaccination at 12 d post-hatch, as reflected by reduced IDE, were associated with reductions in ether extract and starch digestibility. However, no differences in starch or IDE were observed between VAC and PC birds by 16 d post-hatch. On the other hand, ether extract digestibility remained 5.1 percentage units lower (6% reduction) in VAC birds than in PC birds at 16 post-hatch. Moreover, the severe impact of a coccidial challenge on lipid digestibility was also observed with the inadvertent infection of NC birds at 20 d post-hatch, whereby digestibility of ether extract was 20.8 percentage units lower (24% reduction) in NC birds compared to PC birds. The variation in ether extract digestibility was also higher at 20 d post-hatch than at earlier time points, and as such, the numerical reduction in ether extract digestibility of 7.3 percentage units (8% reduction) in VAC birds compared with PC birds was not statistically different. The relative impacts on starch and lipid digestibility observed in the current experiment are in agreement with findings by Amerah and Ravindran (10), who reported broilers subjected to a mixed species challenge had an 18.8% reduction in starch and 96% reduction in lipid digestibility at 7 d post-challenge compared with non-challenged broilers. Starch provides a greater relative contribution to the overall energy content of the diet, but has a lower caloric value than that of lipids. Therefore, the impact of overall energy utilization on starch and lipid was determined by multiplying the FI per bird by the analyzed concentration of either starch or lipid, multiplied by the assumed caloric value, either 4 or 9.5 kcal/kg for starch and lipid, respectively. As such, the VAC-induced reductions in starch and lipid digestibility resulted in a similar caloric cost.
Carotenoids are fat-soluble components (27), and the marked reductions in plasma carotenoids observed in the current experiment were likely associated with the observed reductions in lipid digestibility. The profound effect of coccidia on lipid digestibility can subsequently impair the absorption of other fat-soluble nutrients, including vitamin D, which can consequently impair the absorption of calcium and phosphorus, negatively impacting bone development (28–30). Increased digesta lipid content may also reduce the absorption of calcium via intestinal soap formation, and excess calcium in the intestinal lumen may be another predisposing factor for necrotic enteritis (31). However, since lipid digestion and absorption are relatively complex processes, it is currently unknown which of these processes are most impacted during coccidia-exposure. Sharma and Fernando (32) observed an accumulation of lipid globules within the duodenal villus epithelial cells of E. acervulina infected birds, indicating that intracellular lipid processing or transport across the basolateral cell membrane of the enterocyte may be compromised. Furthermore, Adams et al. (33) reported coccidiosis challenged birds had an improvement in lipid digestion when supplemented with cholic acid, indicating that bile salt synthesis or secretion may be impaired during an infection.
In the current experiment, jejunum villi height was not influenced by coccidiosis vaccination at 12 d post-hatch when the greatest impacts of vaccination on nutrient and energy digestibility were observed. Although severe coccidiosis can cause morphologic damage to the intestinal mucosa, as indicated by increased crypt cell depth and shortened villi (30, 34), other authors have similarly reported a lack of effects of coccidiosis vaccines on intestinal morphology when administered at commercially recommended doses (35, 36). There was a tendency for VAC birds to have deeper crypts in the jejunum at 12 d post-hatch in the current experiment, and this may be reflective of increased cellular proliferation to maintain villi structure during periods of increased enterocyte turnover associated with vaccine-induced coccidiasis. Indeed, Luquetti et al. (36) reported that coccidiosis vaccination did not affect duodenum, jejunum, or ileum villi heights but did increase jejunum and ileum crypt depths of broilers at 14 d post-hatch. Although increased cellular turnover appears to be sufficient to maintain villus structure under these conditions, it also likely increases intestinal maintenance costs for nutrients and energy (34, 37). Furthermore, rapid enterocyte turnover may reduce nutrient transporter expression and brush border enzyme activity, which would consequently reduce the digestion and absorption of nutrients (38).
It has been suggested that Eimeria-induced pH reductions can cause intestinal pH to fall below the optima efficiency for digestive enzyme activity (39). To our knowledge, no published work had evaluated pH in a model that mimics field relevant conditions of coccidiosis vaccination. However, no differences in pH of the duodenum, where pancreatic enzymes are secreted and the majority of enzymatic digestion occurs, were observed in the current experiment. Therefore, the lack of differences observed in histology or pH in coccidiosis vaccinated broilers suggests that these factors alone are not primary contributors to the observed reductions in nutrient digestibility.
Intestinal inflammation may also contribute to the transient reductions in nutrient digestibility experienced by coccidiosis-vaccinated broilers. Nitric oxide is produced by macrophages during the inflammatory response to Eimeria infection via the enzyme nitric oxide synthase, and Allen (40) reported that an E. maxima infection induced nitric oxide production in the mucosa of the infected intestinal area, as well as in the blood. Recently, Rochell et al. (41) reported that a 40% reduction in dietary arginine, the key substrate for nitric oxide, did not limit the marked increase in plasma nitric oxide elicited by E. acervulina infection, indicating a high prioritization of arginine for nitric oxide synthesis during a coccidial challenge. In the current experiment, coccidiosis vaccination did not increase nitric oxide in the plasma, which is in agreement with the findings of Perez-Carbajal et al. (42). Local nitric oxide production in the mucosa was not measured in the current experiment. Nonetheless, it appears that the transient coccidiosis elicited by vaccination does not induce nitric oxide production in the periphery as do more severe challenges.
In conclusion, results reported herein indicate that coccidiosis vaccination had no significant impact on overall BWG and FI of VAC birds, although overall FCR was impaired by vaccination. Coccidiosis vaccination elicited a transient reduction in digestibility of energy and nutrients that was most apparent at 12 post-hatch, particularly for lipids, but VAC birds were able to recover from these reductions by 20 d post-hatch. These results indicate that impaired nutrient digestibility during coccidiosis vaccination may be attributed to a combination of effects, since jejunal morphology and duodenal pH were both not drastically impacted. Furthermore, the prolonged reduction of lipid digestibility in VAC broilers suggests VAC birds have an impaired ability to utilize dietary lipids throughout the various stages of vaccinal oocyst cycling. Therefore, further research is needed to determine the effects of undigested lipids on broiler gastrointestinal health, as well as practical nutrition strategies to ameliorate these effects.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by University of Arkansas.
Author Contributions
SR and JL: designed the experiment. AG: performed the experiment, data analysis, and wrote the paper. AG and PM: laboratory analysis. AG and SR: paper revisions and final approval.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This project was supported by a grant from the US Poultry and Egg Association.
References
- 1.Chapman HD. Use of anticoccidial drugs in broiler chickens in the USA: analysis for the years 1995–1999. Poult Sci. (2001) 80:572–80. 10.1093/ps/80.5.572 [DOI] [PubMed] [Google Scholar]
- 2.Kitandu A, Juranova R. Progress in control measures for chicken coccidiosis. Acta Vet Brno. (2006) 75:265–76. 10.2754/avb200675020265 [DOI] [Google Scholar]
- 3.Chapman H, Cherry T, Danforth H, Richards G, Shirley M, Williams R. Sustainable coccidiosis control in poultry production: the role of live vaccines. Int J Parasitol. (2002) 32:617–29. 10.1016/S0020-7519(01)00362-9 [DOI] [PubMed] [Google Scholar]
- 4.Williams RB. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int J Parasitol. (1998) 28:1089–98. 10.1016/S0020-7519(98)00066-6 [DOI] [PubMed] [Google Scholar]
- 5.Lehman R, Moran ET, Hess JB. Response of coccidiostat- versus vaccination-protected broilers to gelatin inclusion in high and low crude protein diets. Poult Sci. (2009) 88:984–93. 10.3382/ps.2008-00469 [DOI] [PubMed] [Google Scholar]
- 6.Lee JT, Eckert NH, Ameiss KA, Stevens SM, Anderson PN, Caldwell DJ, et al. The effect of dietary protein level on performance characteristics of coccidiosis vaccinated and nonvaccinated broilers following mixed-species Eimeria challenge. Poult Sci. (2011) 90:1916–25. 10.3382/ps.2011-01362 [DOI] [PubMed] [Google Scholar]
- 7.Adedokun SA, Helmbrecht A, Applegate TJ. Investigation of the effect of coccidial vaccine challenge on apparent and standardized ileal amino acid digestibility in grower and finisher broilers and its evaluation in 21-day-old broilers. Poult Sci. (2016) 95:1825–35. 10.3382/ps/pew066 [DOI] [PubMed] [Google Scholar]
- 8.Persia ME, Young EL, Utterback PL, Parsons CM. Effects of dietary ingredients and Eimeria acervulina infection on chick performance, apparent metabolizable energy, and amino acid digestibility. Poult Sci. (2006) 85:48–55. 10.1093/ps/85.1.48 [DOI] [PubMed] [Google Scholar]
- 9.Parker J, Oviedo-Rondon EO, Clack BA, Clemente-Hernandez S, Osborne J, Pierson EM. Enzymes as feed additive to aid in responses against Eimeria species in coccidia-vaccinated broilers fed corn-soybean meal diets with different protein levels. Poult Sci. (2007) 86:643–53. 10.1093/ps/86.4.643 [DOI] [PubMed] [Google Scholar]
- 10.Amerah AM, Ravindran V. Effect of coccidia challenge and natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal level of dietary methionine. Poult Sci. (2015) 94:673–80. 10.3382/ps/pev022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rochell SJ, Parsons CM, Dilger RN. Effects of Eimeria acervulina infection severity on growth performance, apparent ileal amino acid digestibility, and plasma concentrations of amino acids, carotenoids, and α;1-acid glycoprotein in broilers. Poult Sci. (2016) 95:1573–81. 10.3382/ps/pew035 [DOI] [PubMed] [Google Scholar]
- 12.Adedokun SA, Adeola O. The response in jejunal and ileal nutrient and energy digestibility and the expression of markers of intestinal inflammation in broiler chickens to coccidial vaccine challenge and phytase supplementation. Can J Anim Sci. (2017) 97:258–67. 10.1139/CJAS-2016-0093 [DOI] [Google Scholar]
- 13.Cobb-Vantress Broiler Management Guide Cobb 500. Siloam Springs, AR: Cobb-Vantress Inc; (2015). [Google Scholar]
- 14.AOAC International. Official Methods of Analysis. 18th ed. Gaithersburg, MD: AOAC International; (2006). [Google Scholar]
- 15.Short FJ, Gorton P, Wiseman J, Boorman KN. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim Feed Sci Tech. (1996) 59:215–21. 10.1016/0377-8401(95)00916-7 [DOI] [Google Scholar]
- 16.Allen PC. Physiological responses of chicken gut tissue to coccidial infection: comparative effects of Eimeria acervulina and Eimeria mitis on mucosal mass, carotenoid content, and brush border enzyme activity. Poult Sci. (1987) 66:1306–15. 10.3382/ps.0661306 [DOI] [PubMed] [Google Scholar]
- 17.Iji PA, Saki A, Tivey DR. Body and intestinal growth of broiler chicks on a commercial starter diet. Intestinal weight and mucosal development. Br Poult Sci. (2001) 42:505–13. 10.1080/00071660120073151 [DOI] [PubMed] [Google Scholar]
- 18.Conway DP, Sasai K, Gaafar SM, Smothers CD. Effects of different levels of oocyst inocula of Eimeria acervulina, E. tenella, E. maxima on plasma constituents, packed cell volume, lesion scores, and performance in chickens. Avian Dis. (1993) 37:118–23. 10.2307/1591464 [DOI] [PubMed] [Google Scholar]
- 19.Holdsworth PA, Conway DP, McKenzie ME, Dayton AD, Chapman HD, Williams RB. World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet Parasitol. (2004) 121:189–212. 10.1016/j.vetpar.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 20.Hernández-Velasco XD, Chapman H, Owens CM, Kuttappan VA, Fuente-Martínez B, Tellez G. Absorption and deposition of xanthophylls in broilers challenged with three dosages of Eimeria acervulina oocysts. Br Poult Sci. (2014) 55:167–73. 10.1080/00071668.2013.879095 [DOI] [PubMed] [Google Scholar]
- 21.Silva ICM, Ribeiro AML, Canal CW, Pinheiro CC, de Vieira MM, Lacerda L. Broiler chicken responses to immunological stimuli as mediated by different levels of vitamin E in the diet. J Appl Poult Res. (2009) 18:752–60. 10.3382/japr.2009-00055 [DOI] [Google Scholar]
- 22.Chapman HD, Roberts B, Shirley MW, Williams RB. Guidelines for evaluating the efficacy and safety of live anticoccidial vaccines, and obtaining approval for their use in chickens and turkeys. Avian Pathol. (2005) 34:279–90. 10.1080/03079450500178378 [DOI] [PubMed] [Google Scholar]
- 23.Hammond D. Life cycles and development of coccidia. Coccidia. (1973) 1:45–79. [Google Scholar]
- 24.Sander JE, Cai T, Dale N, Bennett LW. Development of biogenic amines during fermentation of poultry carcasses. J Appl Poult Res. (1996) 5:161–6. 10.1093/japr/5.2.161 [DOI] [Google Scholar]
- 25.Tamim NM, Bennett LW, Shellem TA, Doerr JA. High-performance liquid chromatographic determination of biogenic amines in poultry carcasses. J Agric Food Chem. (2002) 50:5012–5. 10.1021/jf020015b [DOI] [PubMed] [Google Scholar]
- 26.Timbermont L, Haesebrouck F, Ducatelle R, Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. (2011) 40:341–7. 10.1080/03079457.2011.590967 [DOI] [PubMed] [Google Scholar]
- 27.Yonekura L, Nagao A. Intestinal absorption of dietary carotenoids. Mol Nutr Food Res. (2007) 51:107–15 10.1002/mnfr.200600145 [DOI] [PubMed] [Google Scholar]
- 28.Morris A, Shanmugasundaram R, McDonald J, Selvaraj RK. Effect of in vitro and in vivo 25-hydroxyvitamin D treatment on macrophages, T cells, and layer chickens during a coccidia challenge. J Anim Sci. (2015) 93:2894–903. 10.2527/jas.2014-8866 [DOI] [PubMed] [Google Scholar]
- 29.Swiatkiewicz S, Arczewska-Wlosek A, Bederska-Lojewska D, Józefiak D. Efficacy of dietary vitamin D and its metabolites in poultry—review and implications of the recent studies. Worlds Poult Sci J. (2017) 73:57–68. 10.1017/S0043933916001057 [DOI] [Google Scholar]
- 30.Oikeh I, Sakkas P, Blake DP, Kyriazakis I. Interactions between dietary calcium and phosphorus level, and vitamin D source on bone mineralization, performance, and intestinal morphology of coccidia-infected broilers. Poult Sci. (2019) 98:5679–90. 10.3382/ps/pez350 [DOI] [PubMed] [Google Scholar]
- 31.Titball R, Naylor C, Basak A. The Clostridium perfringens alpha-toxin. Anaerobe. (1999) 5:51–64. 10.1006/anae.1999.0191 [DOI] [PubMed] [Google Scholar]
- 32.Sharma VD, Fernando MA. Effect of Eimeria acervulina infection on nutrient retention with special reference to fat malabsorption in chickens. Can J Comp Med. (1975) 39:146–54. [PMC free article] [PubMed] [Google Scholar]
- 33.Adams C, Vahl HA, Veldman A. Interaction between nutrition and Eimeria acervulina infection in broiler chickens: diet compositions that improve fat digestion during E. acervulina infection. Br J Nutr. (1996) 75:875–80. 10.1079/BJN19960193 [DOI] [PubMed] [Google Scholar]
- 34.Fernando MA, McCraw BM. Mucosal morphology and cellular renewal in the intestine of chickens following a single infection of Eimeria acervulina. J Parasitol. (1973) 59:493–501. 10.2307/3278782 [DOI] [PubMed] [Google Scholar]
- 35.Alfaro DM, Silva AVF, Borges SA, Maiorka FA, Vargas S, Santin E. Use of Yucca schidigera extract in broiler diets and its effect on performance results obtained with different coccidiosis control methods. J Appl Poult Res. (2007) 16:248–54. 10.1093/japr/16.2.248 [DOI] [Google Scholar]
- 36.Luquetti BC, Alarcon MFF, Lunedo RDMB, Furlan RL, Macari M. Effects of glutamine on performance and intestinal mucosa morphometry of broiler. Sci Agric. (2016) 73:322–7. 10.1590/0103-9016-2015-0114 [DOI] [Google Scholar]
- 37.Teeter RG, Beker A, Brown C, Broussard C, Fitz-Coy S, Radu J, et al. Transforming coccidiosis-mediated lesion scores into production and calorific cost. In: Proceedings of 23rd World's Poultry Congress. Brisbane, QLD (2008). p. 18–21. [Google Scholar]
- 38.Paris NE, Wong EA. Expression of digestive enzymes and nutrient transporters in the intestine of Eimeria maxima-infected chickens. Poult Sci. (2013) 92:1331–5. 10.3382/ps.2012-02966 [DOI] [PubMed] [Google Scholar]
- 39.Major JR, Ruff MD. Eimeria Spp: influence of coccidia on digestion (amylolytic activity) in broiler chickens. Exp Parasitol. (1978) 45:234–40. 10.1016/0014-4894(78)90064-4 [DOI] [PubMed] [Google Scholar]
- 40.Allen PC. Production of free radical species during Eimeria maxima infections in chickens. Poultry Sci. (1997) 76:814–21. 10.1093/ps/76.6.814 [DOI] [PubMed] [Google Scholar]
- 41.Rochell SJ, Helmbrecht A, Parsons CM, Dilger RN. Interactive effects of dietary arginine and Eimeria acervulina infections on broiler growth performance and metabolism. Poult Sci. (2017) 96:659–66. 10.3382/ps/pew295 [DOI] [PubMed] [Google Scholar]
- 42.Perez-Carbajal C, Caldwell D, Farnell M, Stringfellow K, Pohl S, Casco G, et al. Immune response of broiler chickens fed different levels of arginine and vitamin E to a coccidiosis vaccine and Eimeria challenge. Poult Sci. (2010) 89:1870–7. 10.3382/ps.2010-00753 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.