Abstract
Acute lung injury (ALI) is a common clinical disease with high morbidity in both humans and animals. Studies have shown that intestinal microbiota affect the pathology and immune function of respiratory diseases through the "gut–lung axis". The authors investigated the therapeutic effect of fecal microbiota transplantation (FMT) in rats with ALI induced by lipopolysaccharide (LPS). Rats were treated with FMT, and then measured lung wet/dry ratio, PaO2 in artery, proinflammatory marker, and TGF-β1, Smad3, Smad7, and phosphorylated ERK (p-ERK) protein levels, as well as a histopathologic analysis and high-throughput sequencing of intestinal microbiota. FMT significantly reduced lung wet/dry ratio and TNF-α, IL-1β, and IL-6 levels, but increased the levels of PaO2 in artery. In addition, FMT significantly decreased the expression of TGF-β1, Smad3, and p-ERK, while increased the levels of Smad7. Lung histopathological analyses showed that FMT reduced the inflammatory cell infiltration and interstitial lung exudates. High-throughput sequencing of intestinal microbiota analyses showed that FMT reconstructed the structure of intestinal microbiota, and increased the gene abundance of the bacterial community. Therefore, FMT may act on the TGF-β1/Smads/ERK pathway by regulating intestinal microbiota, inhibiting immune inflammation, reducing the production of inflammatory markers in the body and release, and reducing alveolar epithelial damage and repair, thereby improving the endotoxic ALI in rats induced by LPS.
Keywords: Acute lung injury, Lipopolysaccharide, High-throughput sequencing, Intestinal microbiota, Fecal microbiota transplantation, Endotoxic
Introduction
Acute lung injury (ALI) is a common clinical disease with high morbidity in both humans and animals (Yang et al. 2018). Its etiology is diverse, including infection, sepsis, trauma, shock, poisoning, and other factors; it is presented as a kind of pulmonary tissue inflammatory response and increased permeability syndrome dominated by inflammatory cell infiltration; and it is characterized by diffuse alveolar epithelial cells and alveolar capillary endothelial cell injury. The fatality rate of ALI is as high as 30–60% (Toy et al. 2012). Its severe stage is acute respiratory distress syndrome (ARDS) (Chen et al. 2017). In clinical practice, severe infection caused by gram-negative bacteria is the most common cause of ALI. Lipopolysaccharide (LPS), which starts the immune response, is an important component of the cell wall release of gram-negative bacteria. When combined with cell surface receptors, LPS activates a series of immune signaling pathways and secretes a large number of inflammatory factors, eventually leading to uncontrolled inflammatory responses in the body (Durosier et al. 2015). The pathogenesis of ALI is complex and there are no effective therapeutic drugs. The pathogenesis and treatment strategies of ALI have always been an interest for research. In recent years, studies on the effect of fecal microbiota transplantation (FMT) on intestinal and extracorporeal diseases have gradually expanded and developed. Changes in intestinal microbial composition play an important role in the pathogenesis of a variety of intestinal and metabolic diseases, such as inflammatory bowel disease, irritable bowel syndrome, diabetes, and obesity (Sokol et al. 2008). Studies have shown that intestinal microorganisms affect the pathology and immune function of respiratory diseases through the "gut–lung axis" (Marsland et al. 2015). Therefore, this study explores whether FMT affects the progress of ALI through the TGF-1/Smads/ERK pathway, providing new ideas and research directions for the treatment of ALI.
Materials and methods
Animals and reagents
SPF-grade SD male rats (6–8 weeks old), body mass 200 ± 20 g, Animal Experiment Center of Southwest Medical University, animal certificate number: SYSK (Sichuan) 2018-065. All animals were exposed to alternate cycles of 12 h of light and darkness at room temperature (26 °C, 50% humidity, ad libitum access to food and water). All animal experiments were approved and performed in accordance with the guidelines set forth by the Animal Care and Use Committee at the Southwest Medical University. All experimental procedures complied with the Declaration of Helsinki of the World Medical Association. TGF-β1, Smad3, and Smad7 anti-rabbit were purchased from Abcam; TNF-α, IL-1β, and IL-6 ELISA kits were purchased from Kexing Trading Co., LTD (Shanghai, China); ERK and phosphorylated ERK anti-rabbit were purchased from Biyuntian Bio-Technology Co., LTD (Shanghai, China); and LPS was purchased from Sigma (MO, United States).
Animal experiments and treatment
Fifteen adult male SD rats were randomly divided into three groups: the NS group, the LPS group, and the LPS + FMT group. The LPS group and the LPS + FMT group were intraperitoneally injected with LPS (5 mg/kg), while the NS group was intraperitoneally injected with a 0.9% NaCl solution. After the modeling, the LPS + FMT group was given fresh fecal bacteria solution by gavage (with reference to the preparation method of professor Zhang Faming (Cui et al. 2015): fresh faeces of 10 g rats in the control group were collected, and 50 mL sterile normal saline was added at 37 °C and stirred. The filtrate was filtered by double-layer sterile gauze and centrifuged again at 6000 r/min for 15 min; the supernatant was then discarded; an equal amount of normal saline was added; it was then suspended, shaken, and mixed well; followed by being centrifuged again. The above operation was repeated three times, and finally the sediment with normal saline was re-suspended to obtain the fecal fungus solution) 10 mL/kg body weight, twice a day for 2 days. The NS group and LPS group were given the same dose of normal 0.9% NaCl solution by gavage. Rats in each group were sacrificed 24 h after the intervention.
The content of PaO2 in artery
After sufficient anesthesia, the abdominal cavity was opened and the aortaventralis was exposed, blood was collected from the aortaventralis using a syringe containing heparin sodium, and 1 mL blood sample was extracted for PaO2 detection on the automatic blood gas analyzer.
Lung wet/dry weight ratio (W/D)
Rats in each group were sacrificed after anesthesia. The chest was opened and lung tissue was taken out. Adhered on the surface of the lungs, with filter paper blot moisture, balance scale was used to measure right pulmonary lung wet weight (W), put the lung tissue at 70 °C oven bake in 72 h to complete dehydration, again in balance scales weighing organization get dry weight (D) of the lung, right lung calculated lung wet/dry weight ratio (W/D).
Histopathological evaluation
The upper lobe of the left lung was taken and washed in normal saline to remove blood stains, fixed with 4%paraformaldehyde, paraffin-embedded and stained with hematoxylin and eosin (HE), and the histopathological changes were observed with a light microscopy. Lung injury was scored according to the indicators of alveolar congestion, hemorrhage, neutrophil infiltration, alveolar septal thickening, and aggregation in the interstitial space or blood vessel wall. Five high-power fields (HPF, X400) were randomly selected, and the mean score of each field was compared between the groups.
High-throughput sequencing of intestinal microbiota
Fresh feces from rats were collected and put into the cryopreservation tube for cryopreservation. The V3 and V4 hypervariable regions of prokaryotic 16S rDNA were selected for amplification and classification analysis. After the end of PCR, agarose electrophoresis was performed on the PCR products, and then DNA purification and recovery were performed. MiSeq control software was used for image analysis.
ELISA for determining TNF-α, IL-1β, and IL-6 in serum
Serum samples from each group were collected 24 h after LPS administration and immediately separated by centrifugation. TNF-α, IL-1β, and IL-6 in serum were determined by ELISA. According to the instructions of ELISA kit, three multiple Wells were set for each sample. The experiment was repeated three times, and the mean value was taken.
ELISA for determining TNF-α, IL-1β, and IL-6 in BALF
Rats in each group were sacrificed after anesthesia. The trachea, ligate trachea, and right lobe were separated, and then a small plastic tube was inserted into the trachea and placed in the left main bronchus. The alveolar lavage solution was recovered and centrifuged in a centrifuge tube at a rate of 3000 r/min (centrifugation radius of 10 cm) for 10 min. The supernatant was taken for cryopreservation. The contents of TNF-α, IL-1β, and IL-6 in BALF were determined by ELISA. According to the instructions of the ELISA kit, three multiple Wells were set for each sample. The experiment was repeated for three times, and the mean value was taken.
Immunohistochemical analysis of TGF-β1, Smad3, and Smad7 protein in lung tissue
After paraffin-embedded sections of the left lung fixed with paraformaldehyde were embedded, immunohistochemical staining was performed using the streptavidin–biotin complex (SABC) method to detect the expressions of TGF-β1, Smad3, and Smad7 in alveolar epithelial cells, and the operation was strictly in accordance with the instructions of the kit. Images were observed and taken under a light microscope. Immunohistochemical staining was analyzed by the image Pro-Plus 6.0 microscope image analysis system. Five high-power fields (×400) were randomly selected from the sections of lung tissue using integrated optical density (IOD).
Western blot analysis
Lung tissue was taken for protein extraction. The protein concentrations were determined using the BCA protein assay kit, SDS-PAGE electrophoresis, membrane transfer, and sealing. The membranes were incubated at 4 °C overnight with primary antibodies against ERK, p-ERK, and GADPH (1:2500 dilutions), with GADPH being used as a control. The membranes were then washed with TBST five times and incubated with the secondary antibodies (1:1000 dilutions) at room temperature for 1 h. After exposure, development, and fixing, the film was scanned. The film was read by the gel image processing system, and semi-quantitative analysis was performed.
Statistical analysis
Statistical analyses were performed using SPSS 17.0, Data were presented as mean ± SEM (X ± S). One-way analysis of variance followed by a S–N–K test was used to determine significant differences between two groups. A value of P < 0.05 was defined as statistically significant.
Results
Histopathological features of lung tissues
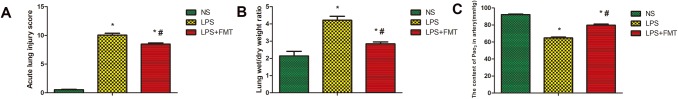
In the NS group, there was no damage to alveolar structure, inflammation, or hemorrhage. For the LPS group, lung tissue was severely damaged, the alveolar septa were markedly oedematous, and thickened, and there was an infiltration of inflammatory cells. Massive hemorrhage in alveolar and alveolar septum, numerous erythrocytes, incomplete alveolar structure, and partial alveolar septal rupture were all present. Compared with the LPS group, the LPS + FMT group significantly reduced the inflammatory cell infiltration, hemorrhage, and lung interstitial swelling in rat lung tissue, with less damage to normal lung tissue structure and significantly lower lung histopathological score (Figs. 1, 2a).
Fig. 1.
HE staining of lung tissue of rats (× 200). In the NS group (a), there was no damage to alveolar structure, inflammation or hemorrhage. In the LPS group (b), lung tissue is severely damaged, massive hemorrhage of alveoli and alveolar septum, numerous erythrocytes. In the LPS + FMT group (c), there were less lung tissue destruction and less inflammatory cell infiltration
Fig. 2.
The effect of FMT on lung histological pathological changes, wet/dry ratio and PaO2 in the artery in LPS-induced rat ALI. Data were expressed as the mean ± SEM (n = 5). a Compared with the NS group, the scores of alveolitis in both the LPS group and the LPS + FMT group showed a significant increase (*P < 0.05); however compared with the LPS group, the scores of alveolitis in LPS + FMT group showed a significant decrease (#P < 0.05). b Compared with the NS group, the lung tissue W/D ratio in both the LPS group and the LPS + FMT group showed a significant increase (*P < 0.05); compared with the LPS group, the lung tissue W/D ratio of LPS + FMT group was significantly decreased (#P < 0.05). c Compared with the NS group, PaO2 in both the LPS group and the LPS + FMT group showed a significant decrease (*P < 0.05); compared with the LPS group, PaO2 in LPS + FMT group was significantly higher than that in LPS group (#P < 0.05)
Lung wet/dry weight (W/D) ratio
Compared with the NS group, the lung tissue W/D ratio of the LPS group was significantly increased. The lung tissue W/D ratio in the LPS + FMT group was lower than that in LPS group, but it was still higher than that of the NS group (Fig. 2b).
The content of PaO2 in artery
Compared with the NS group, PaO2 in the LPS group was significantly decreased. However, PaO2 in the LPS + FMT group was significantly higher than that in LPS group, but still lower than that in the NS group (Fig. 2c).
OTU list generation and comments (default similarity 0.97)
A total of 16 phyla, 24 classes, 39 orders, 62 families, and 128 genera were analyzed. After OTU was produced, the OTU content in each sample and the number of sequences in each OTU were counted, as shown in the Venn diagram. The control group contained 1342 OTUs, the LPS group contained 1216 OTUs, and the LPS + FMT group contained 1567 OTUs. Further, the three groups had 593 OTUs, accounting for 37.3% of the total OTU. The total richness of the three groups was 1588 out (Fig. 3).
Fig. 3.

The Venn diagram. The NS group contained 1342 OTUs, LPS group contained 1216 OTUs, the LPS + FMT group contained 1567 OTUs, and the three groups had 593 OTUs, accounting for 37.3% of the total OTU
Alpha diversity analysis (default similarity 0.97)
Compared with the NS group, the Chao index, ACE index, and Shannon index in the LPS group significantly decreased, while the Simpson index increased. Compared with the LPS group, the Chao index, ACE index, and Shannon index increased in the LPS + FMT group, while the Simpson index decreased (Table 1).
Table 1.
Comparison of the Alpha diversity index of intestinal microbiota among the three groups
| Group | n | Chao index | ACE index | Shannon index | Simpson index |
|---|---|---|---|---|---|
| NS | 3 | 1058.04 ± 100.52 | 1099.58 ± 96.73 | 3.94 ± 0.36 | 0.05 ± 0.01 |
| LPS | 3 | 835.37 ± 98.90a | 833.98 ± 77.63a | 2.95 ± 0.69a | 0.2 ± 0.06a |
| LPS + FMT | 3 | 1268.11 ± 39.04ab | 1278.62 ± 85.60ab | 4.56 ± 0.35ab | 0.04 ± 0.01b |
Shannon/Simpson: diversity index; Chao/ACE: richness index; Data were expressed as the mean SEM (n = 3). Compared with NS group aP < 0.05; compared with LPS group bP < 0.05
Single sample species distribution
A total of 16 categories were detected in all rat fecal samples, including Firmicutes, Bacteroides, Actinobacteria, and Proteobacteria. In this experiment, the intestinal microbiota classification and distribution in the LPS group were significantly different from those in the NS group and LPS + FMT group. Compared with the NS group, Firmicutes significantly increased in the LPS group, Bacteroidete and Actinobacteria decreased considerably, while Firmicutes decreased. Bacteroidetes and Actinobacteria increased significantly in intestinal microbiota of the LPS + FMT group after FMT (Fig. 4a). The Bray Tree Plot shows that the intestinal microbiota structure of the LPS group is discernibly different from that of the NS and LPS + FMT groups, which are independent of each other. However, the gut microbiota composition of the NS group and LPS + FMT group was basically similar, which could be divided into another group (Fig. 4b). The principal component analysis (PCA) diagram showed that the distance of the LPS group was far from the NS group and LPS + FMT group, indicating that the intestinal microbiota structure of the ALI model rats was significantly different from that of normal rats. After FMT, the intestinal microbiota structure of the LPS + FMT group of rats was close to that of the NS group, which was between the NS group and LPS group (Fig. 4c).
Fig. 4.
Analysis diagram of intestinal microbiota structure. a Compared with the NS group, Firmicutes increased significantly in LPS group, Bacteroidetes and Actinobacterias decreased significantly, while Firmicutes decreased, Bacteroidetes and Actinobacterias increased significantly in LPS + FMT group. b The intestinal microbiota structure of LPS group (B1, B2, B3) was significantly different from that of NS group (A1, A2, A3) and LPS + FMT group (C1, C2, C3). c The intestinal microbiota structure of LPS group was significantly different from that of NS group and LPS + FMT group, and the intestinal microbiota structure of LPS + FMT group was close to that of NS group
ELISA in serum
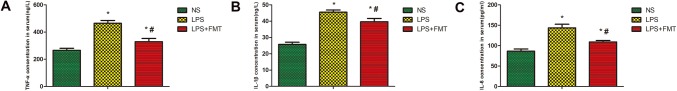
The effects of FMT on the production of cytokines in serum of ALI were measured by an ELISA. The concentrations of TNF-α, IL-1β, and IL-6 in serum were measured using ELISA. As shown in Fig. 5, TNF-α, IL-1β, and IL-6 in serum of LPS group were significantly higher than those of NS group. The serum of TNF-α, IL-1β, and IL-6 in the LPS + FMT group was significantly lower than those in the LPS group, but still higher than those in the NS group. These results revealed that FMT could reduce the release of inflammatory cytokine in serum (Fig. 5).
Fig. 5.
Effects of FMT on the concentrations of TNF-α, IL-1β and IL-6 in Serum in LPS-induced rat ALI. The concentrations of TNF-α, IL-1β and IL-6 in serum were analyzed by ELISA. Values are expressed as the mean ± SEM (n = 5). a Compared with the NS group, the serum of TNF-α in both the LPS group and the LPS + FMT group showed a significant increase (*P < 0.05); compared with the LPS group, the serum of TNF-α in the LPS + FMT group were significantly decreased (#P < 0.05). b Compared with the NS group, the serum of IL-1β in both the LPS group and the LPS + FMT group showed a significant increase (*P < 0.05); compared with the LPS group, the serum of IL-1β in the LPS + FMT group were significantly decreased (#P < 0.05). c Compared with the NS group, the serum of IL-6 in both the LPS group and the LPS + FMT group showed a significant increase (*P < 0.05); compared with the LPS group, the serum of IL-6 in the LPS + FMT group was significantly decreased (#P < 0.05)
ELISA in BALF
The effects of FMT on the production of cytokines in BALF of ALI were measured by an ELISA. The concentrations of TNF-α, IL-1β, and IL-6 in BALF were measured using ELISA. As shown in Fig. 6, TNF-α, IL-1β, and IL-6 in BALF of the LPS group were significantly higher than those in the NS group. The levels of TNF-α, IL-1β, and IL-6 in BALF of the LPS + FMT group were significantly lower than those in the LPS group, but still higher than those in the NS group. These results revealed that FMT could reduce the release of inflammatory cytokine in BALF (Fig. 6).
Fig. 6.
Effects of FMT on the concentrations of TNF-α, IL-1β and IL-6 in BALF in LPS-induced rat ALI. BALF was collected from different groups of rat lungs. The concentrations of TNF-α, IL-1β and IL-6 in BALF were analyzed by ELISA. Values are expressed as the mean ± SEM (n = 5). a Compared with the NS group, the BALF of TNF-α in both the LPS group and the LPS + FMT group showed a significant increase (*P < 0.05); compared with the LPS group, the BALF of TNF-α in the LPS + FMT group were significantly decreased (#P < 0.05). b Compared with the NS group, the BALF of IL-1β in both the LPS group and the LPS + FMT group showed a significant increase (*P < 0.05); compared with the LPS group, the BALF of IL-1β in the LPS + FMT group were significantly decreased (#P < 0.05). c Compared with the NS group, the BALF of IL-6 in both the LPS group and the LPS + FMT group showed a significant increase (*P < 0.05); compared with the LPS group, the BALF of IL-6 in the LPS + FMT group were significantly decreased (#P < 0.05)
Expression of TGF-β1, Smad3, and Smad7 in lung tissue
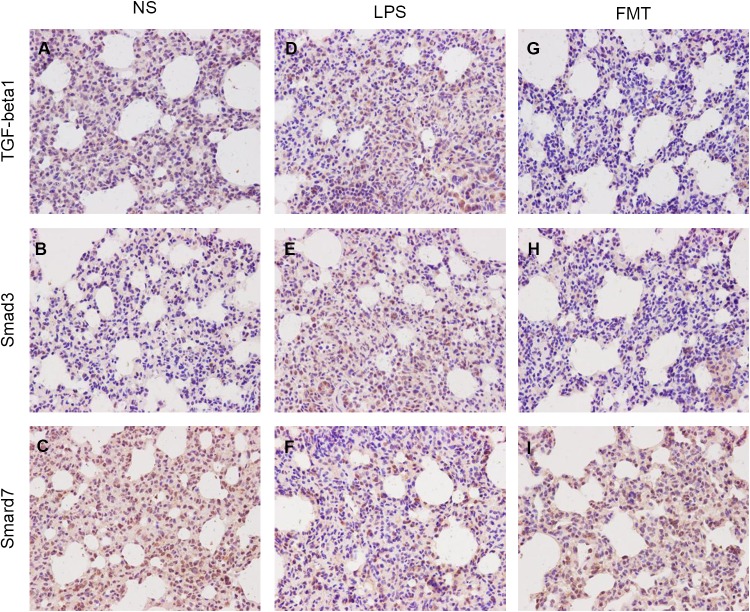
To demonstrate the anti-inflammatory activity of FMT, the levels of TGF-β1, Smad3, and Smad7 were studied in different groups by immunohistochemical analysis. Compared with the NS group, the expression of TGF-β1 and Smad3 in LPS group was significantly increased, and the expression of Smad7 was significantly decreased. Compared with the LPS group, the expressions of TGF-β1 and Smad3 in the LPS + FMT group were significantly decreased, and the expressions of Smad7 were significantly increased (Figs. 7, 8).
Fig. 7.
Immunohistochemical staining for TGF-β1, Smad3 and Smad7 in rat lung tissue (× 400). In the NS group, a, b weak staining, c positive staining was significantly enhanced. In the LPS group, d, e positive staining, f weak staining. In LPS + FMT group, g, h weak staining, i positive staining was significantly enhanced
Fig. 8.

Comparison of TGF-β1, Smad3 and Smad7 immunohistochemical staining IOD of lung tissue of rats in the three groups. Immunohistochemical results were quantified by image-pro Plus 6.0 integral optical density (IOD). Data were expressed as the mean ± SEM (n = 5). Compared with the NS group, the expression of TGF-β1 and Smad3 in both the LPS group and the LPS + FMT group showed a significant increase, and the expression of Smad7 was significantly decreased (*P < 0.05); compared with the LPS group, the expressions of TGF-β1 and Smad3 in LPS + FMT group were significantly decreased, and the expressions of Smad7 were significantly increased (#P < 0.05)
FMT inhibited LPS-induced ERK phosphorylation
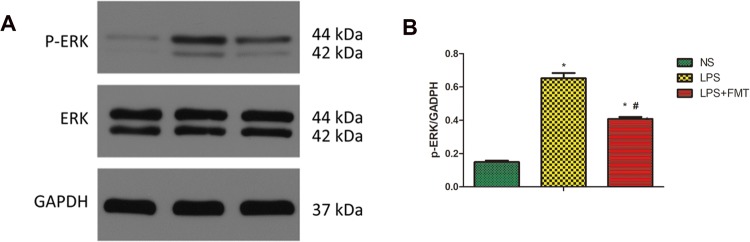
To explore the possible mechanism involved in LPS-induced ALI, the expression level of p-ERK was examined. Compared with the NS group, the expression of p-ERK protein in the lung tissue of the LPS group was significantly increased. Compared with the LPS group, the expression of p-ERK protein in the LPS + FMT group was significantly reduced, indicating that the FMT treatment attenuated this effect of LPS (Fig. 9).
Fig. 9.
Effects of FMT on p-ERK in lung tissue in LPS-induced rat ALI. p-ERK levels in rat lungs were measured by Western blotting. The histogram shows the relative intensity of p-ERK protein bands normalized to the GADPH band. Values are expressed as the mean ± SEM (n = 5). Compared with the NS group, the expression of p-ERK in lung tissue of LPS group was significantly increased (*P < 0.05); compared with the LPS group, the expression of p-ERK in LPS + FMT group was significantly reduced (#P < 0.05)
Discussion
ALI remains a major problem in clinical disease (Zimmermann et al. 2017), which affects the health of most humans. The largest bacterial and endotoxin reservoir in the human body is the intestinal tract. In the physiological state, the complete intestinal mucosa has a barrier effect on the bacteria and endotoxin in the intestinal tract, so a large number of harmful substances are limited to the intestinal tract and will not enter the body. In trauma and infection, such as in the state of stress, the intestinal barrier function is weakened, causing damage, creating a large number of bacteria, and initiating the endotoxin intrusion cycle. This can lead to enterogenous endotoxin blood disease, bacteria translocation, and stimulates the chain reaction of inflammatory mediators in multiple organ dysfunction syndrome. Among them, with the lung as the first organ damaged, enterogenic infection would become a significant cause of ALI, suggesting that the gut–lung is not only physically connected, but also pathologically interacts with each other. Relevant studies also confirmed the existence of this connection between the gut and the lung. That is, rats with ALI caused by LPS would not only exhibit changes in intestinal microbiota, but would also show signs of the lung injury being aggravated further, thus setting in motion a vicious cycle (Li et al. 2014). In recent years, studies on the effects of FMT on respiratory diseases have gradually become more extensive and in-depth. Studies have shown that FMT might be an effective way to prevent and treat chronic respiratory diseases (Liu et al. 2017). Therefore, in the present study, FMT was used as an intervention to rebuild the intestinal microecosystem and to clarify the impact of fecal bacteria transplantation on ALI.
High-throughput sequencing results of intestinal microbiota showed that a total of 16 phyla, 24 classes, 39 orders, 62 families, and 128 genuses were analyzed. The intestinal microbiota structure analysis of rats showed that the intestinal microbiota structure of model rats was significantly different from that of normal rats. Compared with the NS group, the LPS group in the rat intestinal microbiota of Firmicutes noticeably increased, the Bacteroidetes were significantly reduced, Firmicutes/Bacteroidetes increased proportionally, the production of butyrate bacteria increased, and the microbiota of acetic acid and propionic acid decreased. After the intervention of FMT, the intestinal microbiota structure of the LPS + FMT group was similar to the NS group: the Firmicutes/Bacteroidetes proportion decreased, the production of butyrate bacteria was reduced, and the number of produced acetic acid and propionic acid increased. Alpha diversity includes the Chao index, ACE index, Shannon index, and Simpson index. The larger the Chao, ACE, and Shannon indexes are the smaller the Simpson indexes, indicating that species in the sample are more abundant. The Alpha diversity results of this experiment showed that, compared with the NS group, the species abundance of intestinal microbiota of rats in the LPS group decreased, the diversity increased, the heterogeneity increased, and the microbiota structure was relatively unstable, resulting in low Chao, ACE, and Shannon indexes and a high Simpson index. This may be attributable to the acute response of the body after the occurrence of ALI, which resulted in an increase in the diversity of the bacterial community. It should be noted that the specific mechanism of the increase is still unclear. After the intervention of FMT, the gene abundance of the LPS + FMT group was increased, the diversity and heterogeneity of intestinal microbiota were close to that of the NS group, and the microbiota structure was relatively stable, indicating that FMT could correct the imbalance of intestinal microbiota in ALI induced by endotoxins, ultimately improving the intestinal microbiota abundance and diversity. Further, to a certain extent, it could encourage the abnormal intestinal microbiota of ALI rats to change to normal microbiota, as well as increase the production of beneficial bacteria, short-chain fatty acids, and restrain harmful bacteria, playing a positive role in the adjustment of microbiota.
Studies have shown that LPS can stimulate the body to produce a large number of inflammatory cytokines, among which TNF-α, IL-1β, and IL-6 play an important role in the pathogenesis of lung injury (Mokra and Kosutova 2015; Wang et al. 2015; Wu et al. 2015). TNF-α is a key pro-inflammatory immune molecule in post-traumatic inflammation, potentially causing ALI through aggregation and activation of neutrophils. However, IL-1β and IL-6, together with TNF-α, can cause alveolar cell destruction in the early stages of inflammation and promote the occurrence and development of ALI. Relevant studies have shown that intestinal microbiota induces the secretion of macrophage proinflammatory cytokines such as IL-1β and IL-6, as well as promote the occurrence of immune inflammatory diseases, indicating that intestinal microbiota play an important role in establishing pro-inflammatory and regulating immune responses (Messemaker et al. 2015). In the present study, TNF-α, IL-1β, and IL-6 inflammatory cytokines were significantly increased in serum and BALF of the LPS group compared with the NS group. However, the levels of TNF-α, IL-1β, and IL-6 inflammatory cytokines in the lung serum and BALF of the LPS + FMT group decreased after the intervention of FMT, signifying that FMT can regulate the immune inflammatory response, reduce the production of pro-inflammatory cytokines, and play a protective role in ALI by regulating intestinal microbiota.
TGF-β1 was activated early in ALI, and it was closely related to the subsequent formation of pulmonary edema and the release of pro-inflammatory cytokines (Gao et al. 2010). Smads is an important signal transduction molecule of TGF-β1 in the cytoplasm, and the multiple functions of TGF-β1 depend on the signal transduction and regulation of the Smads protein (Zhou 2011). The key roles are Smad3 and Smad7. Among which, Smad3 is a receptor activated Smads, playing a positive regulatory role in TGF-β1 signal transduction, while Smad7 has a negative regulatory role in TGF-β1 signal transduction. The TGF-β/Smad3 signaling pathway is involved in the regulation of the immune response (Delisle et al. 2013), inhibiting the release of extracellular collagenase and promoting the repair of damaged tissue by inducing the synthesis of extracellular matrix collagen and mucin (Lampropoulos et al. 2012). ERK is an important member of the mitogen-activated protein kinase system and plays an important role in mediating the inflammatory response and regulating the production of inflammatory factors, as well as inhibiting the apoptosis of intestinal epithelial cells. p-ERK is transposed from cytoplasm into the nucleus, and then mediates the activation of a variety of transcription factors and participates in a number of cellular biological responses. In the present study, it was revealed that the expressions of TGF-β1, Smad3, and p-ERK in lung tissues of the LPS group were significantly increased, while the expressions of Smad7 were significantly decreased. However, the lung injury in the LPS + FMT group was significantly alleviated after the intervention of FMT, and the expressions of TGF-β1, Smad3, and p-ERK in lung tissues were decreased, while the expression of Smad7 was increased. This indicates that FMT can inhibit the activation of Smads and ERK signaling pathways by inhibiting the expression of TGF-β1, thereby reducing the release of TNF-α, IL-1β, IL-6, and other inflammatory factors, essentially alleviating the inflammatory response to a certain extent and improving the progression of ALI.
In conclusion, the research conducted by the authors demonstrated that FMT antagonizes LPS-induced ALI in rats, reduces the release of inflammatory cytokine, decreases the expression of TGF-β1 and Smad3, and increases the expression of Smad7. Its mechanism may be related to the remodeling of intestinal microbiota and the down-regulation of the activation of TGF-β1/Smads/ERK signaling pathway and the interaction of cytokines. This study provides a theoretical basis for the clinical application of FMT in the treatment of ALI. Further, FMT might be considered as a promising new strategy in the treatment of ALI. However, this experiment did not further reveal the content of short-chain fatty acids in intestinal microbiota. Whether FMT also acts upon ALI through other targets remains to be further explored.
Acknowledgements
The authors acknowledge Mr. Yi Liao for providing linguistic assistance in the preparation of the manuscript.
Funding
The project was supported by Luzhou Municipal people's government-Southwest Medical University Science and Technology Strategic Cooperation Project (2019LZXNYDJ04).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval and consent to participate
All animal experiments were approved and performed in accordance with the guidelines set forth by the Animal Care and Use Committee at the Southwest Medical University, and all experimental procedures complied with the Declaration of Helsinki of the World Medical Association.
References
- Chen X, Tang L, Feng J, Wang Y. Downregulation of Paralemmin-3 ameliorates lipopolysaccharide-induced acute lung injury in rats by regulating inflammatory response and inhibiting formation of TLR4/MyD88 and TLR4/TRIF complexes. Inflammation. 2017;40:1983–1999. doi: 10.1007/s10753-017-0639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B, Feng Q, Wang H, Wang M. Fecal microbiota transplantation through mid-gut for refractory Crohn's disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol. 2015;30:51–58. doi: 10.1111/jgh.12727. [DOI] [PubMed] [Google Scholar]
- Delisle JS, Giroux M, Boucher G, Landry JR. The TGF-β-Smad3 pathway inhibits CD28-dependent cell growth and proliferation of CD4 T cells. Genes Immun. 2013;14:115–126. doi: 10.1038/gene.2012.63. [DOI] [PubMed] [Google Scholar]
- Durosier LD, Herry CL, Cortes M, Cao M. Does heart rate variability reflect the systemic inflammatory response in a fetal sheep model of lipopolysaccharide-induced sepsis. PhysiolMeas. 2015;36:2089–2102. doi: 10.1088/0967-3334/36/10/2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Zhao WX, Xue FS, Zhou LJ. Early administration of propofol protects against endotoxin-induced acute lung injury in rats by inhibiting the TGF-beta1-Smad2 dependent pathway. Inflamm Res. 2010;59:491–501. doi: 10.1007/s00011-009-0110-y. [DOI] [PubMed] [Google Scholar]
- Lampropoulos P, Zizi-Sermpetzoglou A, Rizos S, Kostakis A. TGF-beta signalling in colon carcinogenesis. Cancer Lett. 2012;314:1–7. doi: 10.1016/j.canlet.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu XY, Ma MM, Qi ZJ. Changes in intestinal microbiota in rats with acute respiratory distress syndrome. World J Gastroenterol. 2014;20:5849–5858. doi: 10.3748/wjg.v20.i19.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Yang Z, Zhang X, Han N. 16S rDNA analysis of the effect of fecal microbiota transplantation on pulmonary and intestinal microbiota. 3 Biotech. 2017;7(6):370. doi: 10.1007/s13205-017-0997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland BJ, Trompette A, Gollwitzer ES. The gut–lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12(Suppl 2):S150–S156. doi: 10.1513/AnnalsATS. [DOI] [PubMed] [Google Scholar]
- Messemaker TC, Huizinga TW, Kurreeman F. Immunogenetics of rheumatoid arthritis: understanding functional implications. J Autoimmun. 2015;64:74–81. doi: 10.1016/j.jaut.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Mokra D, Kosutova P. Biomarkers in acute lung injury. Respir Physiol Neurobiol. 2015;209:52–58. doi: 10.1016/j.resp.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Sokol H, Pigneur B, Watterlot L, Lakhdari O. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy P, Gajic O, Bacchetti P, Looney MR. Transfusion related acute lung injury: incidence and risk factors. Blood. 2012;119:1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Qiu XG, Ren HL. Inhibition of acute lung injury by rubri-flordilactone in LPS-induced rat model through suppression of inflammatory factor expression. Int J Clin Exp Pathol. 2015;8:15954–15959. doi: 10.1016/j.resp.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GM, Mou M, Mo LQ, Liu L. Penehyclidine hydrochloride postconditioning on lipopolysaccharide-induced acute lung injury by inhibition of inflammatory factors in a rodent model. J Surg Res. 2015;195:219–227. doi: 10.1016/j.jss.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Yang J, Li S, Wang L, Du F. Ginsenoside Rg3 attenuates lipopolysaccharide-induced acute lung injury via MerTK-dependent activation of the PI3K/AKT/mTOR pathway. Front Pharmacol. 2018;9:850. doi: 10.3389/fphar.2018.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. TGF-β regulates β-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J Cell Biochem. 2011;112:1651–1660. doi: 10.1002/jcb.23079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann KK, Spassov SG, Strosing KM, Ihle PM. Hydrogen sulfide exerts anti-oxidative and anti-inflammatory effects in acute lung injury. Inflammation. 2017;41:249–259. doi: 10.1007/s10753-017-0684-4. [DOI] [PubMed] [Google Scholar]