Abstract
Background
In contrast to vitamin K antagonists (VKA), direct oral anticoagulants (DOAC's) are not strictly monitored and dose titrated by anticoagulation clinics in the Netherlands. This may affect drug persistence of atrial fibrillation (AF) patients, whom often require lifelong treatment.
Objectives
To assess persistence of DOACs and of VKAs in patients with AF.
Methods
Dispensing data from the Dutch Foundation of Pharmaceutical Statistics were used to monitor persistence of AF patients to DOAC from 1 January 2012‐1 April 2016. In addition, we estimated the persistence of AF patients to VKA between 1 January 2004 and 1 January 2012 in data from the Anticoagulation Clinic Leiden. Non‐persistence was defined as the cumulative incidence of patients who completely stopped DOAC, switched to another oral anticoagulant or stopped their VKA.
Results
DOAC users (n = 77 333) were younger than VKA users (n = 10 079; 70 vs 73 years). Non‐Persistence to DOAC (ie stopping with any oral anticoagulant) was 34% at 1 and 64% at 4 years, compared to 22% at one and 36% at 4 years for VKA. Approximately a Twenty‐five percent of those who had stopped their initial DOAC switched to another anticoagulant (VKA or another DOAC). Multivariable analyses revealed that young age, female sex, no concomitant drug use and non‐adherence were predictors for non‐persistence of DOAC.
Conclusions
Persistence to DOAC was low and in line with other observational studies, and higher for VKA. Our results show a clear correlation between age <60 years and worse persistence, as well as with female and non‐adherence to DOAC.
Keywords: atrial fibrillation, direct oral anticoagulants, oral anticoagulants, Persistence, vitamin K antagonist
Essentials.
Persistence of DOACs in every day clinical practice may be lower than reported in trials.
In this cohort study of atrial fibrillation patients 34% stopped their DOAC within 12 months.
Young age, female sex and non‐adherence to DOAC were predictors of non‐persistence to DOAC.
Clinicians should be aware of the low persistence to DOAC in patients with atrial fibrillation.
1. INTRODUCTION
In the Netherlands, approximately 300 000 people receive oral anticoagulant drugs (ie, 1.8% of the population) for the prevention of embolic stroke in atrial fibrillation1. Currently, the majority of these patients is using vitamin K antagonist (VKA) treatment, but the proportion of direct oral anticoagulant (DOAC) users is steadily rising2. DOACs are registered as drugs that can be taken in fixed doses which do not require routine testing to evaluate the anticoagulation effect. This is an important advantage over VKAs which need to be monitored and titrated on a regular basis. Although the efficacy of DOACs has been proven, the discontinuation or persistence rate will determine its successfulness, especially because the efficacy can be affected by even one delayed or missed dose3, 4, 5, 6, 7. A recently published nationwide observational study from New Zealand showed that persistence was poor in DOAC users (n = 43 339 dabigatran users), with as many as 41% of atrial fibrillation patients who discontinued dabigatran over a 2 year follow‐up period8. In addition, in a study from the United States, 32% of patients with atrial fibrillation discontinued with dabigatran at 6 months, increasing to nearly 50% by 1 year9. This is in stark contrast to the discontinuation rate in the clinical trials where only 21%–25% of patients with atrial fibrillation were non‐persistent to DOAC treatment at 2 years of follow‐up10, 11, 12. In a recent observational study, including 25 976 patients with atrial fibrillation, Jackevicius et al13 found an association between non‐persistence of DOAC and adverse cardiovascular outcomes, with a 4‐6 fold increased risk of stroke/transient ischaemic attack (TIA) when patients were non‐persistent to dabigatran or rivaroxaban. Another study suggested that the lower discontinuation rate of DOACs in clinical trials compared with the daily clinical situation can be explained by efforts in such trials to minimize non‐persistence by means of telephone or face to face contact14. Such intensive patient care practice resembles the routine care for VKA users by anticoagulation clinics such as in the Netherlands, which has been proven to be efficient for other preventive cardiovascular drugs as well, like clopidogrel, beta blockers and statins15. Obviously, for the same level of efficacy of DOACs in phase III trials to be translated into clinical practice, the same level of DOAC persistence is required.
We therefore aimed to explore the persistence to DOACs in community dwelling patients with atrial fibrillation from 1 April 2012 to 1 April 2016 in the Netherlands. We contrasted these findings to a cohort of patients with atrial fibrillation who were treated at the Leiden Anticoagulation Clinic, the Netherlands, between 2004 and 2011 (ie at a time when only VKA was available and patients could not switch to DOAC). Of note, VKA was only included as a reference cohort and not to directly compare between DOAC or VKA users as such comparisons would be confounded (by indication)16.
2. METHODS
2.1. Population description
Patterns of drug use can be studied from pharmacy dispensing data17. In the Netherlands, the Foundation for Pharmaceutical Statistics (SFK) gathers pharmacy dispensing data from >95% of community pharmacies to monitor medication prescriptions and does not contain information about clinical indication or outcome18. SFK data provides detailed information on the drugs dispensed, including the codes from the Anatomic–Therapeutic–Chemical (ATC) system of the World Health Organization, the prescribed dose, and the amount dispensed. In the current study, data on DOAC use (by ATC code), with the DOAC dose, number of tablets dispensed, date of dispensing, patient's sex, age, any concomitant medical therapy, and if a patient used VKA prior to DOAC initiation or switched to VKA during follow‐up, were collected19. Four digit postal codes of the patients were also provided by SFK, which allowed us to characterize neighbourhood socioeconomic status. The latter information was retrieved by using information from the Netherlands Institute of Social Research, which keeps record of neighbourhood socioeconomic status by use of 4‐digit postcodes20.
As a comparison, we also included a cohort of patients with atrial fibrillation who received anticoagulant treatment with VKAs at the Anticoagulation Clinic in Leiden, the Netherlands. In this cohort, age at VKA initiation, sex and indication for which the VKA was prescribed (atrial fibrillation) were provided.
2.2. Inclusion criteria
We included all patients who had at least one dispensing of the DOAC agents dabigatran and rivaroxaban from 1st of January 2012 until 1st of April 2016. The DOAC apixaban was registered in the Netherlands in April 2013 for the prevention of systemic embolism in atrial fibrillation. Therefore, all available patient information on apixaban use ranged between 1 April 2013 and 1 April 2016. The DOAC edoxaban was not included since it was not yet approved in the Netherlands during the time period studied. Only incident (first time) DOAC users were included in this study.
In the VKA only cohort, all patients who started with VKA treatment between 1 January 2004 and 31 December 2011 were included and were followed for 4 years or until they ceased VKA treatment or until 1 January 2012, whichever date came first. We specifically chose this time period because only VKAs were available for oral anticoagulation then. Therefore, these patients could not discontinue their drug due to a switch to a DOAC (as was possible from 2012 onwards). Hence, we could estimate the expected non‐persistence rate in a generalizable group of patients with atrial fibrillation who were prescribed oral anticoagulants at an anticoagulation clinic in the Netherlands.
2.3. Exclusion criteria
To ensure that only incident DOAC users were included, we excluded patients who received a DOAC between 1 January 2012 and 1 April 2012. Although SFK does not list the clinical indications for the drugs dispensed, the indication could be assessed by the first dose of DOAC, which is different for short term thromboprophylaxis (ie patients who undergo orthopaedic surgery and receive DOAC treatment for a maximum of 10‐38 days), venous thrombosis treatment and thromboembolic prevention in atrial fibrillation patients (see Table S1)20. In the current study, only patients who were identified as atrial fibrillation patients were included. Of note, patients who used apixaban 2.5 mg twice daily (bid) for less than 6 weeks could both use it for thromboprophylaxis or for thromboembolic prevention in atrial fibrillation. Since we could not distinguish between these two indications, these patients (n = 361 apixaban users) were excluded from further analysis.
2.4. Exposure variables
Patients were classified as dabigatran, rivaroxaban or apixaban users if they received at least one dispensing of ATC codes B01AE07, B01AF01 or B01AF02, respectively. DOACs can be administered to patients with atrial fibrillation in different dosages21. For this purpose we classified dabigatran users as high dose users when they received a first dabigatran prescription of 150 mg bid, and as low dose users when they took 110 mg bid as a first dabigatran prescription. For rivaroxaban, high dose was defined as rivaroxaban 20 mg once daily (od), and low dose as rivaroxaban 15 mg od. For apixaban, high dose was defined as 5 mg bid, and low dose as 2.5 mg bid. For the VKA only cohort, we received information from the Leiden anticoagulation clinic where patients either used phenprocoumon or acenocoumarol; of which the large majority used phenprocoumon.
2.5. Concomitant variables
If patients on DOAC had received VKA (ATC code B01AA) or used concomitant medication within 180 days prior to baseline, we defined them as experienced VKA user or concomitant drug user, respectively. Neighbourhood socioeconomic status was gathered by using the database “status score” from the Netherlands Institute for Social Research22. The “status score” of a neighbourhood (postal code area) is based on (a) mean household income, (b) the percentage of households with a low income, (c) the percentage of inhabitants without a paid job and (d) the percentage of households with on average a low education. The status score combines these four variables into a continuous variable where the higher the score, the higher the socioeconomic status of a neighbourhood is. We a‐priori defined a high neighbourhood socioeconomic status as >90th percentile of status score in the SFK data.
Patient adherence to DOAC was measured as a dichotomous variable for the proportion of days covered (PDC) of at least 80%. This PDC cut‐off is consistent with published research2, 4, 5. The PDC was calculated between the timeframe of the baseline‐initial DOAC treatment to last prescription of a DOAC without a further prescription of the same DOAC within 90 days for all atrial fibrillation patients included in our study. Since patients may stockpile their medications at home, overlaps between prescription dates were allowed and were included in the calculation of the PDC.
2.6. Outcome definitions
The outcome event, non‐persistence in those who were prescribed a DOAC, was defined as not registering a new prescription of any DOAC or VKA. Switchers were defined as the patients who discontinued initial DOAC treatment and switched to another oral anticoagulant drug. Hence, switchers formed a subgroup of the non‐persistent patients. In the VKA cohort, we considered VKA users non‐persistent when they completely stopped with VKA (i.e. were no longer receiving VKA therapy by the Leiden Anticoagulation Clinic).
2.7. Statistical analysis
Baseline characteristics of the DOACs and VKA users are expressed as numbers and percentages, or as means and standard deviations (SD). The observation time was defined as the time between the first DOAC or VKA prescription date and the end of follow‐up. Follow‐up ended on the date of the last prescription (ie before the end of the study end‐date), or the study end date, whichever came first. Kaplan‐Meier analyses were used to determine cumulative incidences for an outcome event.
From the SFK database it cannot be established if a patient retrieves medication from different pharmacies at different times. If this occurs, it would seem that a patient was non‐persistent in pharmacy A, while the drugs were retrieved first in pharmacy A and then in pharmacy B. To account for such possible overestimation of non‐persistence, we excluded (in a sensitivity analysis) all patients who had the same birth year, sex, postal code, concomitant drug use, previous VKA use and who received the same initial DOAC during the observation period as another patient in the register, and repeated the aforementioned analysis to see if this would influence the main results.
In a Cox proportional hazard model we compared the likelihood of and the time to developing non‐persistence between the exposure groups, adjusting for age, sex, previous VKA use, high or low DOAC dose, socioeconomic status, concomitant drug use, and therapy adherence to DOAC, when applicable.
All statistical analyses were performed with SPSS for Windows, release 24.0 (SPSS).
3. RESULTS
3.1. Study population
We identified 92 718 patients who initiated treatment with DOAC between January 1, 2012 and April 1, 2016, based on the data provided by 1538 pharmacies in the Netherlands (79% of the total number of 1981 community pharmacies in the Netherlands in 2015; Figure 1)23. Figure 2 describes the process of cohort selection. After we applied the inclusion criteria in which we separated prevalent DOAC users (n = 4826) from incident DOAC users (n = 87 352), and excluded patients in whom the DOAC type or dosage (n = 3427) was not reported or in whom two or more DOACs were prescribed at the same time (n = 12), there were 83 913 DOAC users of whom the majority (n = 77 333) were identified as atrial fibrillation patients. Baseline characteristics of these DOAC patients are shown in Table 1. The baseline characteristics of atrial fibrillation patients who used VKA (n = 10 079) are also shown in Table 1. DOAC patients were slightly younger than VKA users (70 vs 73 years), and slightly more than half of DOAC patients and VKA users were male (55% and 54%, respectively). Most patients on DOAC used rivaroxaban (n = 34 167; 44%), followed by dabigatran (n = 29 288; 38%) and apixaban (n = 13 878; 18%). The large majority (≥85%) of patients on DOAC had not used VKA before DOAC start. Patients with atrial fibrillation on low dose DOAC were older than patients with atrial fibrillation who received a high dose DOAC. This is as expected as prescription guidelines recommend a lower dose DOAC in patients who have chronic kidney disease (for all DOACs), or (for apixaban and dabigatran) are of older age21. Therapy adherence was highest in apixaban users (n = 11 693, 84%), followed by dabigatran (n = 22 705, 78%) and rivaroxaban (n = 24 597, 72%). Other patient characteristics between the DOACs were roughly similar, with the exception of low dose DOAC patients on apixaban who were on average 3 and 6 years older than patients on rivaroxaban or dabigatran, respectively.
Figure 1.
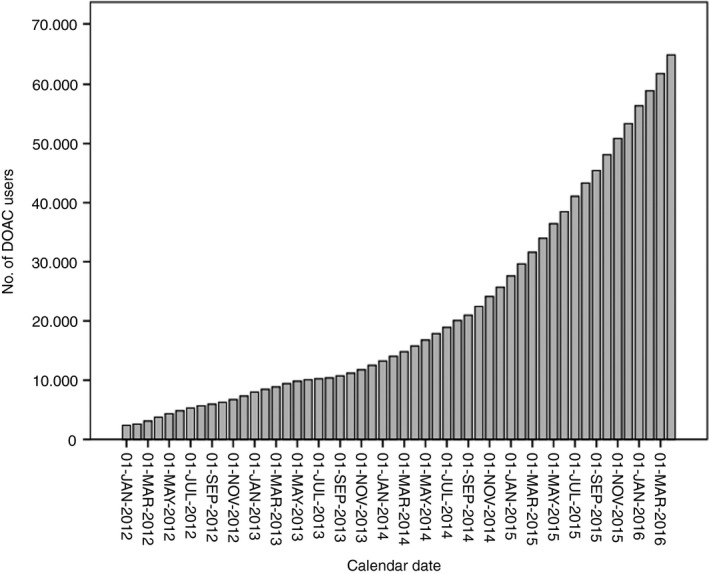
Patients using a DOAC between 1 January 2012 and 1 April 2016*. *Data obtained from 1538 (79% of total) pharmacies in the Netherlands22
Figure 2.
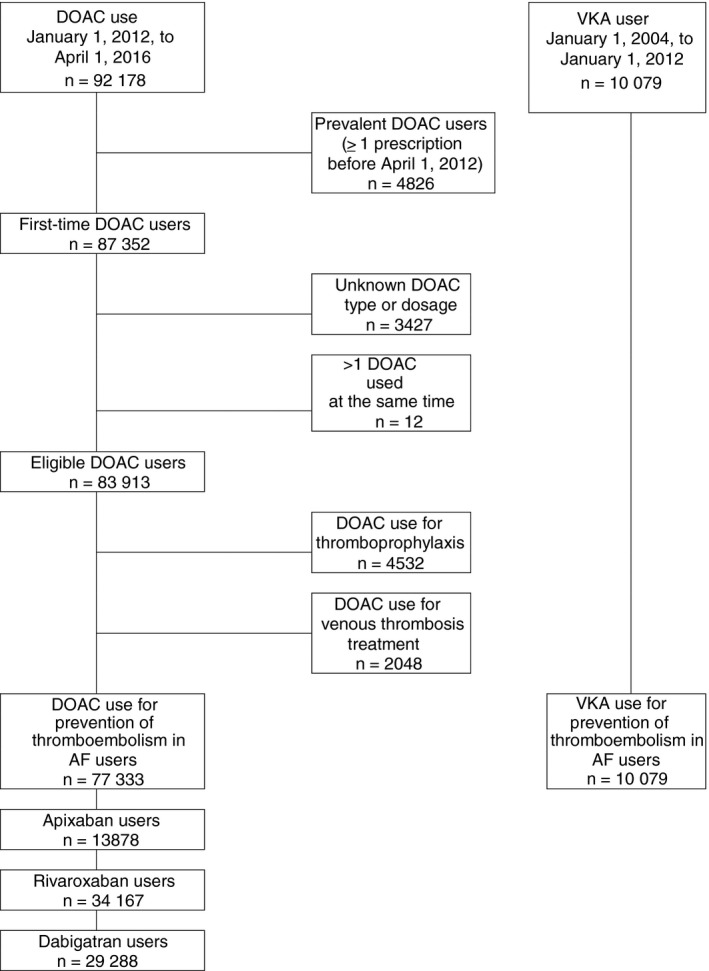
Flow chart. AF, atrial fibrillation, DOAC, direct oral anticoagulants, VKA, vitamin K antagonist
Table 1.
Baseline characteristics
| DOAC use | Apixaban use | Rivaroxaban use | Dabigatran use | VKA use | |
|---|---|---|---|---|---|
| Thromboembolic prevention in AF | |||||
| Any dose, n | 77 333 | 13 878 | 34 167 | 29 288 | 10 079 |
| Mean age, y (SD) | 70 (11) | 71 (11) | 69 (11) | 70 (11) | 73 (11) |
| Men, n (%) | 42 662 (55) | 7814 (56) | 18 133 (53) | 16 715 (57) | 5463 (54) |
| Concomitant drug use, n (%) | 66 351 (86) | 12 280 (89) | 28 864 (85) | 25 207 (86) | NA |
| Previous exposure to VKA, n (%) | 6356 (8) | 2015 (15) | 2610 (8) | 1731 (6) | NA |
| Socioeconomic classa | |||||
| >90% percentile, n (%) | 7666 (10) | 1156 (8) | 3496 (10) | 3014 (10) | NA |
| Therapy adherent (PDC ≥80%) | 58 995 (76) | 11 693 (84) | 24 597 (72) | 22 705 (78) | NA |
| Thromboembolic prevention in AF | |||||
| Low dose, n | 17 452 | 2171 | 4064 | 11 217 | |
| Mean age, y (SD) | 76 (10) | 80 (10) | 77 (10) | 74 (10) | NA |
| Men, n (%) | 8357 (48) | 919 (42) | 1945 (48) | 5493 (49) | NA |
| Concomitant drug use, n (%) | 15 171 (87) | 1894 (91) | 3592 (88) | 9595 (86) | NA |
| Previous exposure to VKA, n (%) | 1447 (8) | 300 (14) | 409 (10) | 738 (7) | NA |
| Socioeconomic classa | |||||
| >90% percentile, n (%) | 1680 (10) | 182 (8) | 413 (10) | 1085 (10) | NA |
| Therapy adherent (PDC ≥80%) | 13 245 (76) | 1875 (86) | 3188 (78) | 8182 (73) | NA |
| Thromboembolic prevention in AF | |||||
| High dose, n | 59 881 | 11 707 | 30 103 | 18 071 | |
| Mean age, y (SD) | 68 (10) | 69 (10) | 68 (11) | 67 (10) | NA |
| Men, n (%) | 34 305 (57) | 6895 (59) | 16 188 (54) | 11 222 (62) | NA |
| Concomitant drug use, n (%) | 51 180 (85) | 10 296 (88) | 25 272 (84) | 15 612 (86) | NA |
| Previous exposure to VKA, n (%) | 4909 (8) | 1715 (15) | 2201 (7) | 993 (6) | NA |
| Socioeconomic classa | |||||
| >90% percentile, n (%) | 5986 (10) | 974 (8) | 3083 (10) | 1929 (11) | NA |
| Therapy adherent (PDC ≥80%) | 45 750 (76) | 9818 (84) | 21 409 (70) | 14 523 (80) | NA |
Abbreviations: AF, atrial fibrillation; DOAC, direct oral anticoagulant; NA, not available; PDC, proportion of days covered; SD, standard deviation; VKA, vitamin K antagonist.
According to Statusscore of the Sociaal en Cultureel Plan Bureau, the Netherlands.
3.2. Non‐persistence
The cumulative incidence of non‐persistence to anticoagulant treatment in patients starting with a DOAC is shown in Table 2 and Figure 3. Non‐persistence was 8% at 6 weeks of follow‐up, 27% at 6 months, 34% at 1 year, 43% at 2 years, 51% at 3 years and 64% at 4 years of follow‐up. In Figure 2, Tables S3 and S4 the incidences of non‐persistence to anticoagulants are shown for apixaban, rivaroxaban, dabigatran and VKA. In the VKA cohort, the cumulative incidence of non‐persistence was 7% at 6 weeks of follow‐up, 16% at 6 months, 22% at 1 year, 28% at 2 years, 33% at 3 years and 36% at 4 years of follow‐up (Figure 3 and Table S4). For both DOAC and VKA use, the incidence of non‐persistence was highest in the first year of treatment.
Table 2.
Non‐persistent patients on DOAC and those who switched from their initial DOAC to another anticoagulant
| Follow‐up | Pt. at risk | Non‐persistent patients | Incidence of non‐persistencea (%) | Cumulative incidencea (%) | Pt. who switched initial DOAC | Percentage | |||
|---|---|---|---|---|---|---|---|---|---|
| Pt. at risk | To any anticoagulant | Percentage | To VKA | ||||||
| Any DOAC | |||||||||
| ≤6 wk | 77 333 | 5781 | 8 | 8 | 5912 | 266 | 5 | 241 | 91 |
| 6 wk‐6 mo | 70 100 | 13 963 | 19 | 27 | 14 661 | 1139 | 8 | 726 | 64 |
| 6‐mo‐1 y | 44 569 | 3494 | 7 | 34 | 4439 | 1356 | 31 | 875 | 65 |
| 1‐2 y | 23 239 | 3150 | 9 | 43 | 5058 | 2543 | 50 | 1639 | 65 |
| 2‐3 y | 13 005 | 1372 | 8 | 51 | 2622 | 1657 | 63 | 1101 | 66 |
| 3‐4 y | 5301 | 593 | 13 | 64 | 1931 | 1572 | 81 | 1123 | 71 |
| Total | 28 353 | 64 | 34 623 | 8533 | 25 | 5705 | 67 | ||
Abbreviations: DOAC, direct oral anticoagulant; Pt, patients, VKA, vitamin K antagonist.
Estimated by cumulative incidence as determined from survival tables in Kaplan Meier analyses.
Figure 3.
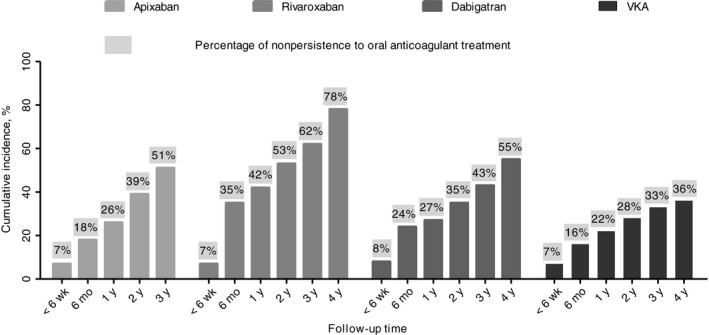
Cumulative incidence of non‐persistence to OAC treatment estimated by Kaplan‐Meijer analysis by type of OAC
3.3. Patients who switched from their initial DOAC
Of 77 333 patients who started with a DOAC, there were 8533 patients who switched to another anticoagulant of which the majority switched to VKA (n = 5705; 67%). Of these 8533 patients, 987 (13%) patients were non‐persistent to their oral anticoagulant treatment before the end of follow‐up and 383 (74%) discontinued their oral anticoagulant treatment after their switch after 1 year of follow‐up (Table 3). Similar non‐persistence and switching patterns were observed in a sensitivity analysis where we excluded potentially duplicate patients who might have retrieved their DOAC at different pharmacies (Table S2).
Table 3.
Non‐persistence of patients who switched from their initial DOAC to another anticoagulant
| Follow‐up | Pt. at risk | Non‐persistent patients | Incidence of non‐persistencea (%) | Cumulative incidencea (%) | Non‐persistent patients after switch | Cumulative incidencea (%) | ||
|---|---|---|---|---|---|---|---|---|
| Patients risk | Non‐persistent patients | Incidence of non‐persistencea (%) | ||||||
| Any DOAC | ||||||||
| ≤6 wk | 8533 | 135 | 2 | 2 | 26 | 45 | 2 | 2 |
| 6 wk‐6 mo | 8265 | 441 | 5 | 7 | 418 | 246 | 10 | 12 |
| 6 mo‐1 y | 7127 | 411 | 6 | 13 | 487 | 92 | 5 | 17 |
| 1‐2 y | 5771 | 635 | 12 | 25 | 917 | 98 | 11 | 28 |
| 2‐3 y | 3228 | 407 | 12 | 37 | 651 | 37 | 11 | 39 |
| 3‐4 y | 1571 | 234 | 15 | 52 | 454 | 2 | 2 | 41 |
| Total | 2263 | 52 | 2863 | 520 | 41 | |||
Abbreviation: DOAC, direct oral anticoagulant; Pts., patients.
Estimated by cumulative incidence as determined from survival tables in Kaplan Meier analyses.
3.4. Variables related with non‐persistence in DOAC users
In multivariable analysis, non‐persistence to DOAC was related to age, where being younger increased the risk of discontinuing treatment (Table 4). No use of concomitant drugs and being non‐adherent (ie taking DOAC treatment according to prescription for <80% of the time that DOAC treatment was used) also increased the likelihood of being non‐persistent to DOAC treatment. Furthermore, female sex increased the risk of being non‐persistent in apixaban, rivaroxaban and dabigatran users. Other variables, including being VKA experienced, whether or not receiving a high dose DOAC, and high neighbourhood socio‐economic class, produced hazard ratios that were not consistently associated with persistence for the DOACs tested. As we observed that the persistence rate reduced most steeply in the first year after DOAC initiation (Tables 2 and 3 and Tables S3 and S4), we restricted follow‐up to the first year of DOAC use only in a post hoc analysis. This analysis also showed that of the clinical variables tested, younger age, female sex, no use of concomitant drugs and being non‐adherent were associated with higher risks of being non‐persistent. Figure 4A‐D shows the discontinuation and adherence rates in patients on DOAC who could be followed for at least 1 year. As the figure shows, most patients stopped their initial DOAC and did not restart their initial therapy.
Table 4.
Risk of being non‐persistent to DOAC according to clinical characteristics
| Observation months | Event no. | Hazard ratio (95% CI)a | Hazard ratio (95% CI)a , b | |
|---|---|---|---|---|
| Apixaban | ||||
| VKA naïve | 114 574 | 3028 | 1 (reference) | 1 (reference) |
| VKA experienced | 12 107 | 252 | 0.95 (0.83‐1.09) | 1.24 (1.10‐1.39) |
| High dose DOAC | 106 854 | 2832 | 1 (reference) | 1 (reference) |
| Low dose DOAC | 19 827 | 448 | 0.73 (0.66‐0.82) | 0.83 (0.72‐0.89) |
| Age <60 y | 16 195 | 741 | 1 (reference) | 1 (reference) |
| Age 60‐75 y | 66 871 | 1454 | 0.61 (0.55‐0.66) | 0.66 (0.61‐0.73) |
| Age >75 y | 43 615 | 1085 | 0.75 (0.75‐0.83) | 0.78 (0.70‐0.86) |
| Men | 71 389 | 1909 | 1 (reference) | 1 (reference) |
| Women | 55 292 | 1371 | 1.07 (0.99‐1.15) | 1.12 (1.04‐1.20) |
| No concomitant drug use | 9080 | 893 | 1 (reference) | 1 (reference) |
| Concomitant drug use | 117 601 | 2387 | 0.40 (0.36‐0.43) | 0.39 (0.36‐0.42) |
| SES class <90th pctilec | 115 939 | 2967 | 1 (reference) | 1 (reference) |
| SES class >90th pctilec | 10 157 | 281 | 1.10 (0.97‐1.24) | 1.05 (0.93‐0.19) |
| Therapy adherent | 10 912 | 1603 | 1 (reference) | 1 (reference) |
| Therapy non‐adherent | 115 769 | 1677 | 8.58 (7.98‐9.23) | 8.47 (7.88‐9.11) |
| Rivaroxaban | ||||
| VKA naïve | 345 199 | 14 953 | 1 (reference) | 1 (reference) |
| VKA experienced | 14 363 | 462 | 0.66 (0.60‐0.72) | 0.88 (0.81‐0.95) |
| High dose DOAC | 308 748 | 14 163 | 1 (reference) | 1 (reference) |
| Low dose DOAC | 50 814 | 1252 | 0.63 (0.59‐0.66) | 0.69 (0.65‐0.73) |
| Age <60 y | 56 038 | 2436 | 1 (reference) | 1 (reference) |
| Age 60‐75 y | 197 443 | 7702 | 0.78 (0.75‐0.81) | 0.79 (0.76‐0.83) |
| Age >75 y | 106 081 | 4277 | 0.86 (0.82‐0.90) | 0.84 (0.80‐0.88) |
| Men | 204 041 | 7182 | 1 (reference) | 1 (reference) |
| Women | 155 521 | 8233 | 1.26 (1.22‐1.30) | 1.32 (1.28‐1.37) |
| No concomitant drug use | 37 218 | 3684 | 1 (reference) | 1 (reference) |
| Concomitant drug use | 322 344 | 11 731 | 0.63 (0.61‐0.66) | 0.63 (0.60‐0.65) |
| SES class <90th pctilec | 317 656 | 13 913 | 1 (reference) | 1 (reference) |
| SES class >90th pctilec | 39 824 | 1414 | 0.90 (0.85‐095) | 0.90 (0.84‐0.95) |
| Therapy adherent | 53 405 | 8421 | 1 (reference) | 1 (reference) |
| Therapy non‐adherent | 306 157 | 6994 | 5.45 (5.28‐5.64) | 5.19 (5.01‐5.37) |
| Dabigatran | ||||
| VKA naïve | 457 449 | 9383 | 1 (reference) | 1 (reference) |
| VKA experienced | 9077 | 275 | 1.05 (0.93‐1.19) | 1.65 (1.50‐1.82) |
| High dose DOAC | 325 556 | 5674 | 1 (reference) | 1 (reference) |
| Low dose DOAC | 140 970 | 3984 | 1.27 (1.22‐1.33) | 1.42 (1.36‐1.49) |
| Age <60 y | 263 995 | 2163 | 1 (reference) | 1 (reference) |
| Age 60‐75 y | 257 080 | 4606 | 0.67 (0.64‐0.71) | 0.71 (0.67‐0.75) |
| Age >75 y | 145 451 | 2889 | 0.67 (0.63‐0.71) | 0.66 (0.62‐0.71) |
| Men | 272 887 | 5283 | 1 (reference) | 1 (reference) |
| Women | 193 639 | 4375 | 1.11 (1.06‐1.15) | 1.16 (1.11‐1.22) |
| No concomitant drug use | 39 594 | 2416 | 1 (reference) | 1 (reference) |
| Concomitant drug use | 426 932 | 7242 | 0.43 (0.41‐0.45) | 0.43 (0.41‐0.45) |
| SES class <90th pctilec | 418 315 | 8534 | 1 (reference) | 1 (reference) |
| SES class >90th pctilec | 46 071 | 1051 | 1.04 (0.98‐1.11) | 1.06 (0.99‐1.14) |
| Therapy adherent | 59 573 | 4922 | 1 (reference) | 1 (reference) |
| Therapy non‐adherent | 406 953 | 4736 | 5.47 (5.25‐5.70) | 5.85 (5.89‐6.12) |
Abbreviations: DOAC, direct oral anticoagulant; pcetile, percentile; SES, socioeconomic class.
Multivariable adjusted.
Restricted to first year of DOAC use.
Defined by poste code area.
Figure 4.
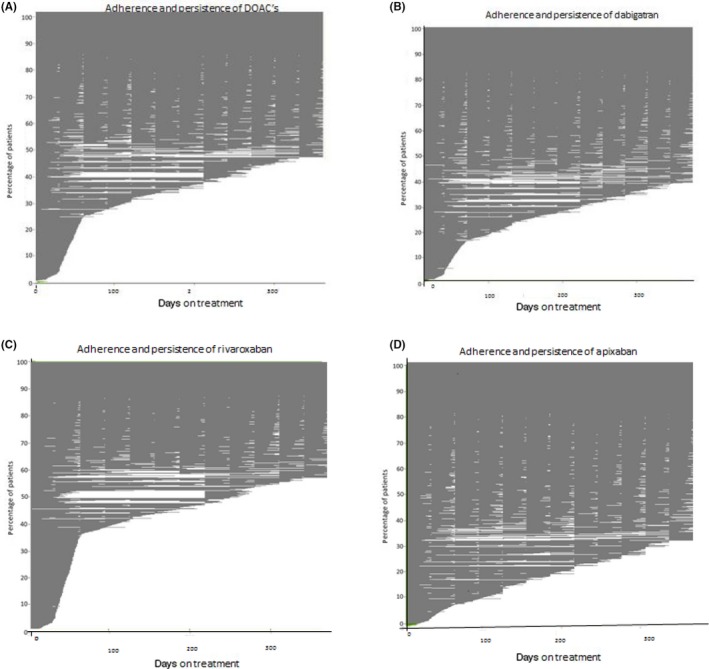
A‐D, Mean PDC of DOAC use for AF patients included before 01‐04‐2015 during follow‐up, and stratified for DOAC type n = 43 910
4. DISCUSSION
In this population based study from the Netherlands, in patients who were identified as having atrial fibrillation, we observed that of DOAC users, 27% were non‐persistent to their anticoagulant treatment at 6 months, 34% at 1 year, 43% at 2 years and 64% at 4 years. These results are comparable to the non‐persistence rate at 2 years of follow‐up of 42% in a meta‐analysis of observational studies on cardiovascular drugs (like aspirin, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, beta‐blockers, calcium‐channel blockers, thiazides and statins) and to a non‐persistence rate of 42% of metformin at 2 years of follow‐up for patients with type 2 diabetes, in clinical settings without regular patient monitoring24, 25.
Of the atrial fibrillation patients using VKA in our study, who were monitored in an anticoagulation clinic, 16% stopped at 6 months, 22% at 1 year, 28% at 2 years and 36% after 4 years of follow‐up. This is comparable to the non‐persistence rate for the DOACs in clinical trials where the non‐persistence rates was 21% for dabigatran at 2 years of follow‐up and 24% and 25% at a median of 2 years of follow‐up for rivaroxaban and apixaban, respectively10, 11, 12.
4.1. Comparison with other studies
When we compare our results to other observational studies, one striking feature is the wide variety in reported non‐persistence of oral anticoagulant use amongst atrial fibrillation patients (Figure 5)8, 9, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43. However, we could identify one common characteristic for the non‐persistence rates found in our and other observational studies for oral anticoagulants, which was whether or not patients were monitored on oral anticoagulant drugs. The non‐persistence rate in our VKA cohort (22% at 1 year at the Leiden Anticoagulation Clinic) was similar to those studies which only included patients on VKA who participated in the setting of an anticoagulation clinic (15% at 1 year) or where patients on VKA were regularly monitored by study design (18% at 1 year in one study, 22% in another and 26% at 1 year in another study)28, 34, 37, 42. Moreover, the 28% non‐persistence rate at 2 years of follow‐up was comparable to those found for VKA in the RE‐LY trial (17% at 2 years of follow‐up), in the ARISTOTLE trial (28% at a median of 2 years of follow‐up) and in the ROCKET‐AF trial for (22% at a median of 2 years of follow‐up) in which patients were monitored closely10, 11, 12. These results are also comparable to the non‐persistence rate of rivaroxaban in patients with atrial fibrillation from the Dresden registry (19% at an average of 1.5 years of follow‐up), in which patients were frequently monitored by study design31. A similar non‐persistent rate of 23% at 2 years of follow‐up for dabigatran was found in an observational study by Paquette et al,38 where AF patients on dabigatran were regularly monitored by study design (at 3, 6, 12 and 24 months). In short, all studies that found a low non‐persistence rate for oral anticoagulant use amongst patients with atrial fibrillation had presence of patient monitoring in common10, 11, 12, 28, 31, 34, 37, 38, 42.
Figure 5.
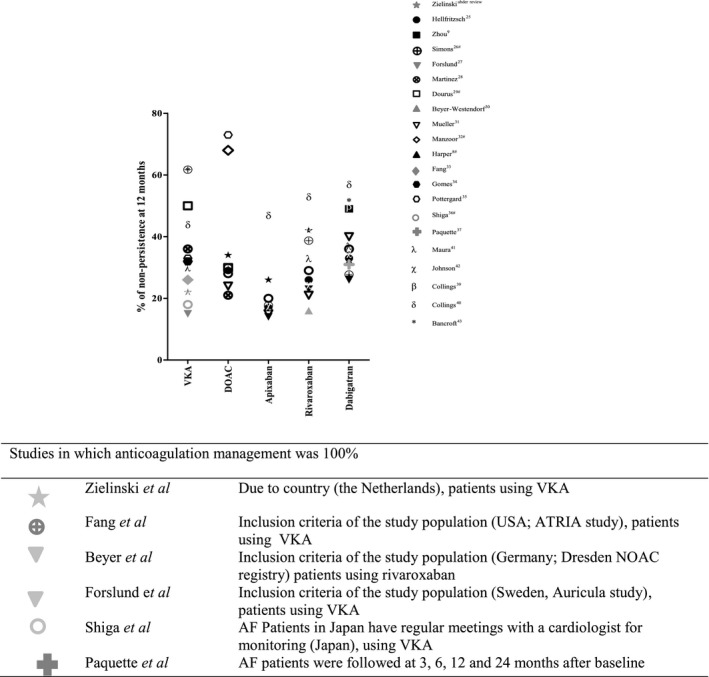
Percentage of non‐persistence at 12 months of OAC reported in our study and other observational studies. #Studies in which % of non‐persistence at 12 months was read from KM‐analysis
The high non‐persistence rates of DOAC use amongst patient with atrial fibrillation found in our study (34% for DOACs, 26% for apixaban, 27% for dabigatran and 42% for rivaroxaban, all at 1 year) were comparable to the non‐persistence found in other observational studies of patients using either VKA or DOACs after 1 year in which close patient monitoring was lacking8, 9, 26, 27, 28, 29, 30, 32, 33, 34, 35, 36, 37, 39, 40, 41, 42, 43. Neither Germany or France have specialized anticoagulant clinics to monitor VKA users44.
4.2. Explanations for low persistence
Although we had no information on why patients were non‐persistent to their oral anticoagulant treatment, we found some persistence patterns that merit discussion: A first potential reason why patients were non‐persistent to their DOAC could be because the treatment was no longer indicated as patients' heart rate returned to sinus rhythm45. A large observational study showed that in patients with permanent atrial fibrillation at 1 year of follow‐up 8% were no longer in atrial fibrillation, and 6% were cured from atrial fibrillation46. A similar finding was observed in the Dresden registry where 9% of patients with atrial fibrillation were non‐persistent with rivaroxaban (average follow‐up 1.5 years) as they had reverted to stable sinus rhythm31. This does, however, not fully explain the high non‐persistence rate to DOAC use that we found. A second potential reason could be that patients who were non‐persistent died. However, mortality rates in atrial fibrillation patients on anticoagulants are approximately 1.5%‐1.7% per treatment year, and therefore mortality is also unlikely to fully explain the low persistence rates that we found47. A third potential reason could be related with minor bleeding as it has been shown previously that approximately 30% of patients who are non‐persistent to their DOAC do this because of minor bleeding complications such as nose bleeds, haematuria or menorrhagia31.
For example, women, who had a lower DOAC persistence in our study than men, receive relatively higher dosing of DOACs than men (pharmacokinetics; smaller volume of distribution, larger free fraction of drugs and slower clearance from the body)48. Indeed, Frost et al,49 found that apixaban peak values were 18% higher in women than men. Another reason for poorer persistence among women could be due to longer and heavier menstruation cycles when using oral anticoagulants or vaginal blood loss in post‐menopausal women.50 As DOACs have relatively short half‐lives (rivaroxaban 5‐9 hours, dabigatran 12‐17 hours and apixaban 12 hours), DOAC concentrations peak shortly after intake, especially in those who use once daily doses of rivaroxaban,49, 51 which could further increase the risk of high DOAC levels leading to (minor) bleeding and non‐persistence. Therefore, high early peaks may be a fourth potential reason why patients are non‐persistent to their DOAC.
We also observed that 25%‐30% of patients, who were non‐persistent to their DOAC, switched from anticoagulant treatment and primarily switched to VKA, indicating that they did not tolerate their initial DOAC treatment (fifth potential reason). Again, we do not know why these patients switched from DOAC treatment, but results are in line with previous studies in which also approximately 6%‐16% of patients on DOAC who were non‐persistent to their initial treatment switched early in their therapy to another anticoagulant2, 9, 26, 31.
A final sixth potential reason is that patients who were not adherent to DOAC therapy were also most likely to stop with DOAC treatment (hazard ratios of non‐persistence to DOAC were 6‐12 fold higher than for adherent DOAC users). This implies that poor adherence is a good predictor of DOAC non‐persistence, and some monitoring might therefore both increase treatment adherence and persistence.
Ultimately, the determinants of DOAC persistence in an individual patient likely exist as a complex of one or more factors that we summarized above and that can change over time.
4.3. Strengths and limitations
A strength of our study is its population‐based design and hence its non‐selected participants. A limitation is that SFK does not provide information of the exact indication for DOAC treatment, although we could approximate this by the difference in first dose for atrial fibrillation as compared with venous thrombosis or thromboprophylaxis. Another potential limitation is that SFK was only able to provide data of 79% of all pharmacies in the Netherlands. The Reason for this is that not all pharmacies had provided complete data during the study period without switches in software systems. Therefore the reason for not including these pharmacies in our dataset was completely at random and it is therefore unlikely that this has effect the results.
We defined non‐persistence as follows: not registering a new prescription of any DOAC or VKA (ie those who intermittently used DOAC were included as being persistent to DOAC). Switchers were defined as the patients who discontinued initial DOAC treatment and switched to another oral anticoagulant drug. Hence, switchers formed a subgroup of the non‐persistent patients. There are however other ways to define non‐persistence eg by saying someone is non‐persistent when he or she did not take medication for a number of days in a row. We decided to not use the latter definition as some patients may have stopped DOAC treatment for an intermittent period for practical reasons, reasons that SFK data does not provide (eg failed to get new medication in time).
Another limitation is that we do not know the reasons why patients were non‐persistent to their oral anticoagulant treatment as this information was not available in our data sources. Although this was not our aim since we wanted to study the treatment persistence of DOACs and of VKA in patients with atrial fibrillation, reasons for being non‐persistent would be death or no longer in need of oral anticoagulant treatment, information that we could not retrieve from SFK. A further limitation is that in SFK, we used first dosage either with LMWH (for dabigatran) or double dosage (for apixaban and rivaroxaban) as an indicator if a patient used DOAC for atrial fibrillation or venous thrombosis as these prescriptions are provided by Dutch guidelines21. However, we cannot be sure if physicians always strictly adhered to the guidelines. Therefore we may have misclassified patients with venous thrombosis as atrial fibrillation patients and vice versa. If patients with atrial fibrillation were prescribed an initial DOAC as required for venous thrombosis, these patients are missed in our analyses as they were considered as venous thrombosis patients (and therefore excluded). How often physicians prescribe a DOAC in the amount that would be required for atrial fibrillation (by definition a too low dose), we do not know, but consider it to occur only rarely. Indeed, another study from Denmark with a similar design as our study in which treatment indications could be distinguished found similar results as we reported here26.
Furthermore, thromboprophylaxis with DOAC may have been given, at least in theory, in (higher) dosages that are usually only given in patients with atrial fibrillation. However, we consider this implausible, and since thromboprophylaxis with DOAC was only rarely prescribed to patients in the time period that we studied (as shown by the low frequency of DOAC use in thromboprophylactic dosage in our study), such a phenomenon, if it occurred, is unlikely to have materially affected our findings.
Finally, adherence was estimated for patients who continued or discontinued treatment for 90 days or less, and may have been erroneously estimated for those who stopped for a longer period and then restarted treatment or in those who temporarily stopped due to doctor's orders (eg elective surgery)52. However the definition that we used for adherence is often used in pharmaco‐epidemiologic research4 and although it may be imprecise it is a clear indicator for non‐persistence, the outcome we were interested in for this analysis.
5. CONCLUSION
Persistence to DOAC treatment in our study was low and in line with results of observational studies into other preventive cardiovascular medication without patient monitoring. It was higher for VKA users with patient monitoring, which is also confirmative to other observational studies into medication where patient monitoring was performed. Our results show that worse persistence was clearly correlated with age <60 years, with female sex and with non‐adherence to DOAC.
RELATIONSHIP DISCLOSURE
GDZ, WML, NvR, MT, FAK, FRR, FJvdM and SCC have no conflicts of interest to declare. MVH reports receiving grant support from Boehringer Ingelheim, GlaxoSmithKline, and Aspen, and lecture fees from Bristol‐Myers Squibb/Pfizer, Boehringer Ingelheim, and Bayer HealthCare.
AUTHOR CONTRIBUTIONS
GDZ, WML, MT and SCC participated in the design of the study, analysis of the results and helped to draft the manuscript. All authors read and approved the final manuscript.
Supporting information
ACKNOWLEDGEMENT
The authors thank the Dutch Foundation for Pharmaceutical Statistics for making data available from the SFK registry.
Zielinski GD, van Rein N, Teichert M, et al. Persistence of oral anticoagulant treatment for atrial fibrillation in the Netherlands: A surveillance study. Res Pract Thromb Haemost. 2020;4:141–153. 10.1002/rth2.12261
Handling Editor: Fiona Newall
DATA AVAILABILITY STATEMENT
Due to SFK policy, data cannot be shared.
REFERENCES
- 1. Samenvatting Medische jaarverslagen 2014 Federatie Nederlandse Trombosediensten . https://s3.eu-central-1.amazonaws.com/storage.topsite.nl/fnt.nl/uploads/docs/jaarverslagen/FNT_Samenvatting_Medisch_JV_2014.pdf. Accessed September 14, 2018.
- 2. Hanemaaijer S, Sodihardjo F, Horikx A, Wensing M, De Smet P, Bouvy ML, et al. Trends in antithrombotic drug use and adherence to non‐vitamin K oral anticoagulants in the Netherlands. Int J Clin Pharm. 2015;37:1128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 4. Osterberg L, Blaschke T. Adherence to Medication. N Engl J Med. 2005;353:487–97. [DOI] [PubMed] [Google Scholar]
- 5. Shore S, Carey EP, Turakhia MP, Jackevicius CA, Cunningham F, Pilote L, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the veterans health administration. Am Heart J. 2014;167:810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mani H, Lindhoff‐Last E. New oral anticoagulants in patients with nonvalvular atrial fibrillation: a review of pharmacokinetics, safety, efficacy, quality of life, and cost effectiveness. Drug Des Devel Ther. 2014;17:789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;335:540–6. [DOI] [PubMed] [Google Scholar]
- 8. Harper P, Pollock D, Stephens M. Dabigatran persistence and adherence in New Zealand: a nationwide retrospective observational study. BMJ Open. 2018;8:e020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou M, Chang H‐Y, Segal JB, Alexander GC, Singh S. Adherence to a novel oral anticoagulant among patients with atrial fibrillation. J Manag Care Spec Pharm. 2015;21:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. RE‐LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139‐51. [DOI] [PubMed] [Google Scholar]
- 11. Granger CB, Alexander JH, McMurray J, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 12. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 13. Jackevicius CA, Tsadok MA, Essebag V, Atzema C, Eisenberg MJ, Tu JV, et al. Early non‐persistence with dabigatran and rivaroxaban in patients with atrial fibrillation. Heart. 2017;103:1331–8. [DOI] [PubMed] [Google Scholar]
- 14. Shore S, Ho PM, Lambert‐Kerzner A, Glorioso TJ, Carey EP, Cunningham F, et al. Site‐level variation in and practices associated with dabigatran adherence. JAMA. 2015;313:1443–50. [DOI] [PubMed] [Google Scholar]
- 15. Ho PM, Lambert‐Kerzner A, Carey EP, Fahdi IE, Bryson CL, Melnyk SD, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Intern Med. 2014;174:186–93. [DOI] [PubMed] [Google Scholar]
- 16. Rosendaal FR, Reitsma PH. Brave real world. J Thromb Haemost. 2016;14:2091. [DOI] [PubMed] [Google Scholar]
- 17. Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–7 [DOI] [PubMed] [Google Scholar]
- 18. Over de Stichting Farmaceutische Kengetallen . Foundation for Pharmaceutical Statistics (Stichting Farmaceutische Kengetallen). https://www.sfk.nl/over-de-sfk. Accessed September 14, 2018.
- 19. The Anatomical Therapeutic Chemical (ATC) classification system; Structure and principles . WHO Collaborating Centre for Drug Statistics Methodology. https://www.whocc.no/atc/structure_and_principles/. Accessed December 14, 2018.
- 20. Geerdinck M, Vieveen E, Verkleij C, Sterk M.Gemiddelde persoonlijke en besteedbare huishoudinkomen naar postcodegebied, 2006‐2009. https://www.cbs.nl/nl-nl/maatwerk/2011/51/gemiddelde-persoonlijke-en-besteedbare-huishoudinkomen-naar-postcodegebied-2006-2009. Accessed September 14, 2018.
- 21. Farmacotherapeutisch Kompas . https://www.farmacotherapeutischkompas.nl/bladeren-volgens-boek. Accessed November 12, 2018: Zorginstituut Nederland, 2016.
- 22. Planbureau SeC . Statusscores https://www.scp.nl/Onderzoek/Lopend_onderzoek/A_Z_alle_lopende_onderzoeken/Statusscores. Accessed January 15, 2019: Sociaal en Cultureel Planbureau.
- 23. Aantal openbare apotheken vrijwel onveranderd in 2015 . Foundation for Pharmaceutical Statistics (Stichting Farmaceutische Kengetallen). Pharmaceutisch Weekblad; 2016. https://www.sfk.nl/nieuws-publicaties/PW/2016/aantal-openbare-apotheken-vrijwel-onveranderd-in-2015. Accessed February 15, 2019.
- 24. Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta‐analysis on 376,162 patients. Am J Med. 2012;125:882–7.e1. [DOI] [PubMed] [Google Scholar]
- 25. McGovern A, Hinton W, Calderara S, Munro N, Whyte M, de Lusignan S. A class comparison of medication persistence in people with type 2 diabetes: a retrospective observational study. Diabetes Ther. 2018;9:229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hellfritzsch M, Husted SE, Grove EL, Rasmussen L, Poulsen BK, Johnsen SP, et al. Treatment changes among users of non‐vitamin K antagonist oral anticoagulants in atrial fibrillation. Basic Clin Pharmacol Toxicol. 2017;120:187–94. [DOI] [PubMed] [Google Scholar]
- 27. Simons LA, Ortiz M, Freedman SB, Waterhouse BJ, Colquhoun D, Thomas G. Improved persistence with non‐vitamin‐K oral anticoagulants compared with warfarin in patients with atrial fibrillation: recent Australian experience. Curr Med Res Opin. 2016;32:1857–61. [DOI] [PubMed] [Google Scholar]
- 28. Forslund T, Wettermark B, Hjemdahl P. Comparison of treatment persistence with different oral anticoagulants in patients with atrial fibrillation. Eur J Clin Pharmacol. 2016;72:329–38. [DOI] [PubMed] [Google Scholar]
- 29. Martinez C, Katholing A, Wallenhorst C, Freedman SB. Therapy persistence in newly diagnosed non‐valvular atrial fibrillation treated with warfarin or NOAC. A cohort study. Thromb Haemost. 2016;115:31–9. [DOI] [PubMed] [Google Scholar]
- 30. Douros A, Renoux C, Coulombe J, Suissa S. Patterns of long‐term use of non‐vitamin K antagonist oral anticoagulants for non‐valvular atrial fibrillation: Quebec observational study. Pharmacoepidemiol Drug Saf. 2017;26:1546–54. [DOI] [PubMed] [Google Scholar]
- 31. Beyer‐Westendorf J, Forster K, Ebertz F, Gelbricht V, Schreier T, Gobelt M, et al. Drug persistence with rivaroxaban therapy in atrial fibrillation patients‐results from the Dresden non‐interventional oral anticoagulation registry. Europace. 2015;17:530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mueller T, Alvarez‐Madrazo S, Robertson C, Bennie M. Use of direct oral anticoagulants in patients with atrial fibrillation in Scotland: Applying acoherent framework to drug utilisation studies. Pharmacoepidemiol Drug Saf. 2017;26:1378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manzoor BS, Lee TA, Sharp LK, Walton SM, Galanter WL, Nutescu EA. Real‐world adherence and persistence with direct oral anticoagulants in adults with atrial fibrillation. Pharmacotherapy. 2017;37:1221–30. [DOI] [PubMed] [Google Scholar]
- 34. Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, et al. Warfarin discontinuation after starting warfarin for atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2010;3:624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gomes T, Mamdani MM, Holbrook AM, Paterson JM, Juurlink DN. Persistence with therapy among patients treated with warfarin for atrial fibrillation. Arch Intern Med. 2012;172:1687–9. [DOI] [PubMed] [Google Scholar]
- 36. Pottegård A, Poulsen BK, Larsen MD, Hallas J. Dynamics of vitamin K antagonist and new oral anticoagulants use in atrial fibrillation: a Danish drug utilization study. J Thromb Haemost. 2014;12:1413–8. [DOI] [PubMed] [Google Scholar]
- 37. Shiga T, Naganuma M, Nagao T, Maruyama K, Suzuki A, Murasaki K, et al. Persistence of non‐vitamin K antagonist oral anticoagulant use in Japanese patients with atrial fibrillation: a single‐center observational study. J Arrhythm. 2015;31:339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paquette M, Riou França L, Teutsch C, Diener H‐C, Lu S, Dubner SJ, et al. Persistence with dabigatran therapy at 2 years in patients with atrial fibrillation. J Am Coll Cardiol. 2017;70:1573–83. [DOI] [PubMed] [Google Scholar]
- 39. Collings S‐L, Lefèvre C, Johnson ME, Evans D, Hack G, Stynes G, et al. Oral anticoagulant persistence in patients with non‐valvular atrial fibrillation: a cohort study using primary care data in Germany. PLoS ONE. 2017;12:e0185642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collings S‐L, Vannier‐Moreau V, Johnson ME, Stynes G, Lefèvre C, Maguire A, et al. Initiation and continuation of oral anticoagulant prescriptions for stroke prevention in non‐valvular atrial fibrillation: a cohort study in primary care in France. Arch Cardiovasc Dis. 2018;111:370–9. [DOI] [PubMed] [Google Scholar]
- 41. Maura G, Billionnet C, Alla F, Gagne JJ, Pariente A. Comparison of treatment persistence with Dabigatran or Rivaroxaban versus vitamin K antagonist oral anticoagulants in atrial fibrillation patients: a competing risk analysis in the french national health care databases. Pharmacotherapy. 2018;38:6–18. [DOI] [PubMed] [Google Scholar]
- 42. Johnson ME, Lefèvre C, Collings S‐L, Evans D, Kloss S, Ridha E, et al. Early real‐world evidence of persistence on oral anticoagulants for stroke prevention in non‐valvular atrial fibrillation: a cohort study in UK primary care. BMJ Open. 2016;6:e011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bancroft T, Lim J, Wang C, Sander SD, Swindle JP. Health care resource utilization, costs, and persistence in patients newly diagnosed as having nonvalvular atrial fibrillation and newly treated with dabigatran versus warfarin in the United States. Clin Ther. 2016;38:545–56.e1‐6. [DOI] [PubMed] [Google Scholar]
- 44. Le Heuzey JY, Ammentorp B, Darius H, De Caterina R, Schilling R, Schmitt J, et al. Differences among western European countries in anticoagulation management of atrial fibrillation. Data from the PREFER IN AF registry. Thromb Haemost. 2014;111:833–41. [DOI] [PubMed] [Google Scholar]
- 45. Abdou JK, Auyeung V, Patel JP, Arya R. Adherence to long‐term anticoagulation treatment, what is known and what the future might hold. Br J Haematol. 2016;174:30–42. [DOI] [PubMed] [Google Scholar]
- 46. Nieuwlaat R, Prins MH, Le Heuzey J‐Y, Vardas PE, Aliot E, Santini M, et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow‐up of the Euro Heart Survey on atrial fibrillation. Eur Heart J. 2008;29:1181–9. [DOI] [PubMed] [Google Scholar]
- 47. Graham DJ, Reichman ME, Wernecke M, Hsueh Y‐H, Izem R, Southworth MR, et al. Stroke, bleeding, and mortality risks in elderly medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662–71. [DOI] [PubMed] [Google Scholar]
- 48. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48:143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frost C, Song Y, Barrett YC, Wang J, Pursley J, Boyd R, et al. A randomized direct comparison of the pharmacokinetics and pharmacodynamics of apixaban and rivaroxaban. Clin Pharmacol. 2014;13:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang X, Xu B, Liang H, Jiang S, Tan H, Wang X, et al. Distribution characteristics and factors influencing oral warfarin adherence in patients after heart valve replacement. Patient Prefer Adherence. 2018;3:1641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moore TJ. Optimal dosing of rivaroxaban is undefined. BMJ. 2016;18:i5549. [DOI] [PubMed] [Google Scholar]
- 52. Shaw JR, Woodfine JD, Douketis J, Schulman S, Carrier M. Perioperative interruption of direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta‐analysis. Res Pract Thromb Haemost. 2018;2:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to SFK policy, data cannot be shared.


