Abstract
Background
Pulmonary embolism (PE) in children carries a significant morbidity and mortality. We examined previously described factors in 2 cohorts of children tested for PE and identified novel factors.
Methods
We combined data from 2 retrospective cohorts. Patients up to age 21 years were included who underwent imaging or D‐dimer testing for PE, with positive radiologic testing being the gold standard. Combined predictor variables were examined by univariate analysis and then forward stepwise multivariable logistic regression.
Results
The combined data set yielded 1103 patients with 42 unique predictor variables, and 93 PE‐positive patients (8.4%), with a median age of 16 years. Univariate analysis retained 17 variables, and multivariable logistic regression found 9 significant variables with increased probability of PE diagnosis: age‐adjusted tachycardia, tachypnea, hypoxia, unilateral limb swelling, trauma/surgery requiring hospitalization in previous 4 weeks, prior thromboembolism, cancer, anemia, and leukocytosis.
Conclusion
This combined data set of children with suspected PE discovered factors that may contribute to a diagnosis of PE: hypoxia, unilateral limb swelling, trauma/surgery requiring hospitalization in previous 4 weeks, prior thromboembolism, and cancer, age‐adjusted tachycardia, tachypnea, anemia, and leukocytosis. Prospective testing is needed to determine which criteria should be used to initiate diagnostic testing for PE in children.
ESSENTIALS.
Pulmonary embolism (PE) in children carries a significant morbidity and mortality.
We combined data from 2 retrospective cohorts and identified novel predictor variables.
Multivariable logistic regression found 9 significant variables associated with PE diagnosis.
Prospective testing is needed to determine which features predict pediatric PE diagnosis.
1. INTRODUCTION
Pulmonary embolism (PE) occurs in only about 0.86 in 10 000 children in the general population each year but carries a high risk of mortality (~10%).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 When PE is considered in a differential diagnosis, there is considerable decisional conflict because the clinical presentation of PE overlaps with many common pediatric diseases. The current criterion standard for PE diagnosis is computed tomography pulmonary angiogram (CTPA), but this carries the risk of radiation‐induced malignancy.1, 4, 5, 7, 12, 13, 14 Balancing the risk of missing a PE against the probable increased risk of a radiation‐induced malignancy presents a clinical conundrum for emergency medicine physicians caring for children. Contributing to this conundrum is the lack of quantitative data that have demonstrated which clinical factors increase the probability of PE diagnosis among children with clinically suspected PE.
Multiple decision rules exist for the pretest probability estimation of PE in adults, but these have had unreliable performance in children.6, 13, 15, 16, 17, 18 Prior studies extrapolating adult decision rules to children are limited by small numbers.1, 4, 13 The 8‐factor PE rule‐out criteria (PERC rule) for adult patients was derived and validated to rule out PE with no further testing. The PERC rule includes age < 50, heart rate < 100 beats/min, pulse oximetry reading > 94%, no estrogen use, no recent surgery, no prior venous thromboembolism (VTE), no hemoptysis, and no unilateral limb swelling.16, 18, 19 A single‐center retrospective study of children with PE had both higher mean heart and respiratory rates as well as lower pulse oximetry rates and hemoglobin concentrations, and they were more likely to have had recent surgery, an indwelling central line, limb immobility, prior PE or deep vein thrombosis (DVT), and cancer on univariate analysis.5 However, this database had only 51 patients with PE, therefore limiting the selection of prediction variables based on univariate statistics (eg, t‐test or chi‐square).
When applied to 2 independent pediatric cohorts, the PERC rule had a high sensitivity for Hennelly et al4 (100%) and Kline et al13 (95.8%). However, specificities were lower, at 24% and 35%, respectively, which limits the usefulness of these rules in clinical practice. We recognize the possibility for improvement in exclusionary rate by adding or substituting pediatric‐focused clinical factors with the existing 8 criteria of PERC. Accordingly, the authors of this study collaborated to combine 2 separate data sets to create a larger database that contains predictor data and outcomes of children who underwent formal testing for PE. Using these combined data, we sought to report factors associated with a diagnosis of PE among pediatric patients presenting for evaluation, to design a future diagnostic algorithm.
2. METHODS
We combined 2 previously reported data sets that evaluated children who underwent diagnostic testing for PE. Both studies were institutional review board approved by their institutions.
Hennelly et al4 performed a retrospective cohort study in Massachusetts of 561 children < 22 years of age who received diagnostic testing for the evaluation of PE in the emergency department (ED) or hospital setting between January 2000 and April 2014 at Boston Children’s Hospital. Patients needed to have testing performed in the ED or within 7 days of hospitalization to be included. This was chosen a priori, as some children were admitted with an unclear or alternate diagnosis, but subsequent testing was done for PE. The study included patients undergoing ≥1 of the following diagnostic tests specifically for the evaluation of suspected PE: CTPA (date range, September 2003‐April 2014), ventilation‐perfusion (VQ) scan (date range, January 2000‐April 2014), or d‐dimer testing (date range, January 2007‐April 2014). All patients with d‐dimer testing were included to capture a cohort of children in whom PE was even considered. Patients who had a d‐dimer obtained were identified by text search of ED records, and children undergoing CTPA or VQ scans were identified through radiology databases. Visits with a d‐dimer test sent from the ED were reviewed manually (KEH) to ensure that testing was performed specifically for PE. The study also excluded patients who were receiving anticoagulation treatment for PE. Missing data for specific variables were assumed to be absent. REDcap was used for data collection.
Kline et al included 541 patients in Indiana of age 5 to 17 years who underwent testing for PE with either a d‐dimer, CTPA, scintillation lung scan, or formal pulmonary angiography between 2004 and 2014 in the Indiana University Health hospital system.5 A pediatric tertiary care center (Riley Hospital) and 8 community hospitals are part of this system. Patients were identified by query of 2 databases for d‐dimer and pulmonary vascular imaging orders. Patients could be located in an ED, observation unit, hospital ward, or intensive care unit. To be included a patient had to either undergo a pulmonary vascular imaging study (CTPA, VQ lung scanning, or formal pulmonary angiography), or have a d‐dimer for suspected PE. Specific training was provided for data abstractors to determine if care providers ordered d‐dimer for suspected PE. Suspicion for PE was determined if written words indicated that PE or a synonym, such as thromboembolism, was pertinently included in the medical record. Patients were excluded if d‐dimer was performed for another process, such as cancer surveillance or suspected disseminated intravascular coagulation, or undetermined reason. Clinical data had to be documented within 48 hours of the time of PE diagnosis to be included. The majority of the clinical data were obtained at the ED visit (with the triage vitals). Some data were obtained from an inpatient unit when there was not an ED visit documented. When variables were not mentioned in the chart, these were coded as absent. Data were then transferred to a REDCap electronic form.
For both studies, there was no way of determining the clinicians’ gestalt pretest probability when collecting PERC predictors.
2.1. Criterion standard for PE
Hennelly et al4 determined PE status based on positive radiologic testing within 1 week of an ED visit, and required treatment with anticoagulation to confirm the presence of PE. Positive radiologic testing consisted of either (1) an intraluminal filling defect noted on CTPA that was interpreted as PE by the final staff radiologist report or (2) a moderate‐ to high‐probability VQ scan. Patients with negative or equivocal imaging studies not receiving treatment, as well as those with only a positive d‐dimer but no imaging, were considered not to have a PE. To ensure that patients were not characterized falsely as not having a PE, patients were longitudinally tracked at the study institution through the end of the study period to ensure that they did not undergo imaging for concern for PE.
Kline et al determined PE status by using imaging data and available outcome records. The definition of PE positive required a filling defect on CTPA interpreted as positive for PE or a ventilation‐perfusion scintillation lung scan interpreted as high probability. Patients with PE or DVT diagnosed within 30 days after the d‐dimer were considered to be PE positive. The exclusion of PE required negative imaging or a no PE diagnosis within 30 days, using a statewide database that tracks 95% of hospitals in Indiana. Patients with a negative d‐dimer and no PE or DVT within 30 days were considered PE negative.
Both studies required positive imaging (by CTPA scan or VQ scan) to confirm a diagnosis of PE. A negative d‐dimer with no subsequent diagnosis of PE was considered to be negative for PE as described. Both studies included all patients who had d‐dimers sent out of concern for PE to best compare clinical factors among PE‐negative and ‐positive patients.
2.2. Analysis
We used the data dictionary from each database to ensure commonality of terms, which identified 42 unique predictor variables that were coded in both databases. These were joined into single columns of predictor variables with unique patients in each row. To determine significant variables, we performed univariate, then multivariable analyses to develop prediction or prognostic rules.20, 21 Prior to missing data analysis and replacement, all 42 candidate variables were subjected to univariate screening by unpaired t‐tests for parametric data (eg, heart rate) or chi‐square statistic for bivariate data (eg, sex). Variables with P < 0.1 were retained, and missing data were replaced. Missing data were analyzed for monotonicity and replaced using the automatic function in the multiple imputations technique in SPSS (IBM, Armonk, NY, USA). We used the pooled data from 5 iterations to generate a new data set of candidate variables with no missing data for the remaining analysis. The remaining parametric data were further tested for discriminative significance using receiver operating characteristic (ROC) analysis, then retaining variables with an area under the curve with lower limit 95% confidence interval > 0.5. Cutoff values for continuous variables were determined as the value corresponding to the peak likelihood ratio positive/likelihood negative on the ROC curve. The remaining variables were then selected for significance using forward stepwise multivariable logistic regression analysis with the dependent variable representing PE or no PE using the criterion standards above. Regression analyses were performed using SPSS Statistics, version 25 (IBM).
We then performed a sensitivity analysis to test if the variables retained by multivariable regression had different discriminative characteristics for PE based on age. This potential effect was demonstrated by producing frequency plots of continuous variables (eg, heart rate), stratified by patient age ≤ 14 years, compared with age > 14 years, and performing a 1‐way analysis of variance (ANOVA) with Tukey’s post hoc to provide P values comparing PE positive vs PE negative. For dichotomous variables (eg, prior history of VTE), we plotted the frequency of each variable, stratified by age ≤ 14 or > 14 years, and obtained a P value comparing PE positive vs. PE negative from the exact binomial method (StatsDirect version 3.1.20, Cheshire, England). Figures were produced in Prism version 7.00 for Windows (GraphPad Software, La Jolla, CA, USA).
3. RESULTS
The combined database included 1103 children who underwent testing for PE in the emergency care setting. A total of 506 (45.9%) patients underwent CTPA or VQ scan imaging. Table 1 shows our patient demographics and differentiates patients who were positive (n = 93, 8.4%) or negative for PE. The median ages for PE‐positive and ‐negative patients were 15.2 and 16.9 years, respectively. Thirty percent of patients with PE had a prior DVT or PE, 26.9% had recent immobilization or surgery/trauma, and 12.9% had an active malignancy. Additionally, 51.6% of patients with PE had tachycardia for age based on the pediatric American Heart Association guidelines,22 and 16.1% had leg swelling. We were unable to describe chest pain adequately (eg, substernal, pleuritic, or chest wall) based on the retrospective design, and therefore we did not analyze this variable as a predictor. Table S1 shows the screening P value results of univariate analysis on the 42 independent variables. The primary finding was that 17 variables had P < 0.1. Unless otherwise noted, variables were positively associated with PE positive based on an increasing value or presence of the following variables: (1) age, (2) heart rate, (3) respiratory rate, (4) diastolic blood pressure (low diastolic blood pressure being a positive predictor), (5) pulse oximetry (low pulse oximetry being a positive predictor), (6) body mass, (7) hemoglobin concentration (low hemoglobin being a positive predictor), (8) white blood cell count, (9) sodium concentration (low sodium being a positive predictor), (10) bicarbonate concentration, (11) prior VTE, (12) recent surgery or trauma requiring hospitalization, (13) indwelling central line, (14) active cancer, (15) asthma (presence of asthma being a negative predictor), (16) syncope (presence of syncope being a negative predictor), and (17) unilateral leg swelling. Notably, estrogen use (including oral contraceptives) and hemoptysis did not yield P values < 0.1 sufficient for selection. The results of multiple imputations indicated the only missing data were 11 continuous variables, as shown in Table S2. The means of imputed values pooled from 5 iterations were compared with the preimputation means using an unpaired t‐test and were significant (P < 0.05) for all variables except bicarbonate, diastolic blood pressure, and body mass. The frequency of missing variables is shown in Table S2. Analysis by ROC then rejected these 3 plus 1 more variable (age, diastolic blood pressure, body mass, and serum bicarbonate) from entry into the multivariable stage. The ROC curve analysis also selected cutoff values of 24 breaths/min for respiratory rate, 95% for pulse oximetry, 11.0 g/dL for hemoglobin, and 135 mEq/L for sodium. We did not use age‐adjusted respiratory rates but rather determined the ROC curve analysis cutoff value. For heart rate, we used data from the ROC as well as cutoffs for normal published in guidelines to establish the cutoffs of > 120 beats/min for age < 12 years and > 100 beats/min for age > 11 years (“age‐adjusted tachycardia”).22
Table 1.
Characteristics of pediatric patients evaluated for PE
| Patient demographics | ||
|---|---|---|
| Characteristics | PE positive (n = 93) | PE negative (n = 1010) |
| n (%) | n (%) | |
| Demographics | ||
| Age (y), median [IQR] | 15.2 [13.9‐20.0] | 16.9 [15.0‐20.8] |
| Female sex | 46 (49.5) | 502 (49.7) |
| Comorbidities | ||
| Central venous catheter | 9 (9.7) | 24 (2.4) |
| Previous venous thromboembolism | 28 (30.1) | 39 (3.9) |
| Malignancy | 12 (12.9) | 41 (4.1) |
| Congenital heart disease | 5 (5.4) | 45 (4.5) |
| Renal disease | 1 (1.1) | 8 (0.8) |
| Recent immobility or surgery | 25 (26.9) | 82 (8.1) |
| Recent injury | 6 (6.5) | 30 (3.0) |
| Exogenous estrogen use | 17 (18.3) | 153 (15.1) |
| Symptoms/Exam findings | ||
| Syncope | 1 (1.1) | 51 (5.0%) |
| Chest pain | 35 (38%) | 553 (54.7%) |
| Cough | 14 (15.1) | 204 (20.2) |
| Shortness of breath | 55 (59.1) | 583 (57.7) |
| Fever | 16 (17.2) | 116 (11.5) |
| Hemoptysis | 3 (3.2) | 18 (1.8) |
| Calf swelling | 15 (16.1) | 26 (2.6) |
| O2 saturation < 95% | 18 (19.4) | 49 (4.9) |
Abbreviations: IQR, interquartile range; PE, pulmonary embolism.
The remaining 13 variables were then subjected to stepwise forward multivariable logistic regression, which excluded 4 more variables (sodium < 135 mEq/L, syncope, asthma, and indwelling catheter). For the remaining 9 variables shown in Table 2, a main effects logistic regression yielded Hosmer‐Lemeshow P = 0.30, McFadden’s pseudo R 2 = 0.247, and Pearson’s chi‐square goodness of fit P = 0.32. Table 2 presents the coefficients and odds ratios from logistic regression for the 9 variables retained as significantly predictive. Of note, unilateral limb swelling had an odds ratio of 7.4 (95% confidence interval [CI], 3.4‐16.4), and prior VTE had an odds ratio of 11.8 (95% CI, 6.3‐21.9).
Table 2.
Factors associated with PE diagnosis among children evaluated for PE from forward stepwise multivariable regression
| Odds ratio | 95% confidence interval for odds ratio | ||
|---|---|---|---|
| Lower bound | Upper bound | ||
| Intercept | … | … | … |
| Age‐adjusted tachycardia* | 1.7 | 1.0 | 2.9 |
| Hemoglobin < 11.0 g/dL | 2.3 | 1.4 | 4.0 |
| Respiratory rate > 23.5 | 1.8 | 1.1 | 3.0 |
| SpO2 < 94.5% | 1.9 | 1.0 | 3.5 |
| White blood cell count > 11.0 × 103 cells/μL | 2.1 | 1.3 | 3.5 |
| Unilateral limb swelling | 7.4 | 3.4 | 16.4 |
| Active cancer | 2.6 | 1.2 | 5.6 |
| Prior venous thromboembolism | 11.8 | 6.3 | 21.9 |
| Trauma or surgery requiring hospitalization in past 4 weeks | 2.1 | 1.1 | 3.7 |
Abbreviation: PE, pulmonary embolism.
> 120 beats/min for age < 12 years and > 100 beats/min for age > 11 years.
3.1. Sensitivity analyses
Figure 1 shows the effect of stratifying the continuous variables in Table 2 based on age below or above 14 years. The P values from the ANOVA and Tukey’s post hoc test on means demonstrated P > 0.05 for both age strata for respiratory rate and white blood count, and P > 0.05 in the age > 14 subgroup for SpO2 and age. The most robust predictors (P < 0.05 in both age strata) were heart rate and hemoglobin. Similarly, Figure 2 plots the frequency analysis for the 4 categorical variables shows loss of discrimination in age ≤ 14 for recent surgery/trauma and cancer, while prior VTE and unilateral limb swelling had P < 0.05 in both age strata.
Figure 1.
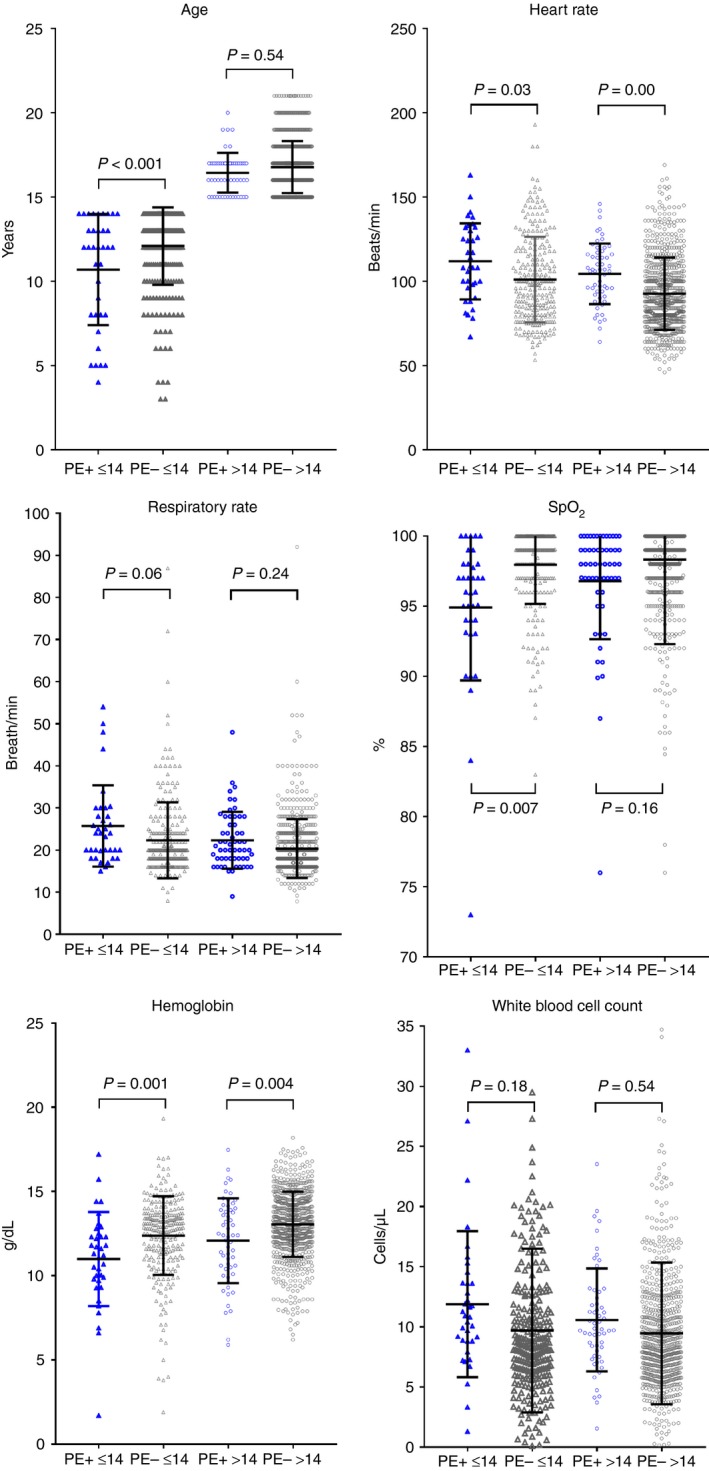
Frequency (dot‐plots) of continuous variables tested by logistic regression for each patient in the study, stratified on the x‐axis by age ≤ 14 years (leftward 2 plots) or > 14 years (rightward 2 plots) and by pulmonary embolism (PE+) diagnosis (blue symbols) vs. no PE diagnosis (PE–, gray symbols). The P values were calculated from the Tukey’s post hoc test following 1‐way analysis of variance. SpO2, Pulse oximetry (%)
Figure 2.
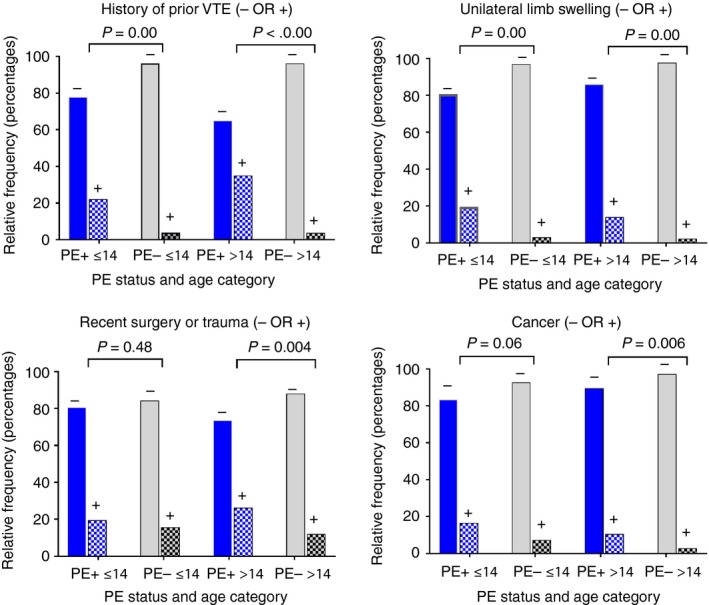
Frequency bar plots for dichotomous variables tested by logistic regression, stratified on the x‐axis by age ≤ 14 years (leftward 2 plots) or > 14 years (rightward 2 plots), and by pulmonary embolism (PE+) diagnosis (blue symbols) vs. no PE diagnosis (PE–, gray symbols). The P values were calculated from the exact binomial formula for differences in proportions. VTE, venous thromboembolism
4. DISCUSSION
This work provides novel information from what we believe to be the largest multihospital database of consecutive pediatric patients evaluated for PE in the ED. From 42 candidate variables, the process of sequential univariate and then multivariable logistic regression revealed that age‐adjusted tachycardia (>119 beats/min for age < 12 and > 99 beats/min for age > 11), tachypnea (respiratory rate > 23.5), hypoxia (pulse oximetry < 94.5%), unilateral limb swelling, trauma or surgery requiring hospitalization in the previous 4 weeks, prior thromboembolism, active cancer, anemia (hemoglobin concentration < 11 g/dL), and leukocytosis (white blood count > 11.0 × 103 cells/µL) were independently associated with a diagnosis of PE. The sensitivity analysis, including the data in Figures 1 and 2, stratifying age below or above 14 years was performed because the risk factors for and physical manifestations of VTE may vary with age. Findings here suggest factors associated with a diagnosis of PE among children suspected of PE might be different by age. Specifically, heart rate, hemoglobin concentration, prior VTE, and unilateral limb swelling were robust in those above or below age 14, while respiratory rate and white blood cell count were no longer statistically significant when analysis was stratified by age. These data provide a hypothesis that these variables may remain significantly predictive of PE diagnosis in a prospective study. With further validation, ≥1 of these 4 novel criteria may enhance PERC rule diagnostic accuracy in children.
Strengths of this work include the multicenter representation and that this sample represents a consecutive sample and not a case‐control sample. We used rigorous methods to handle missing data and to select variables, including multivariable analysis that demonstrates evidence of good model fitness based on the Hosmer‐Lemeshow P value.
Our study has several limitations. First, both data sets were collected retrospectively and therefore relied on a criterion standard of follow‐up based on findings in electronic health records. However, both populations would have likely returned to our large tertiary care centers if they had a subsequent PE, and thus would have been discovered in our data sets. The sensitivity analysis found that respiratory rate and white blood cell count were significant only in the entire patient sample, but this significance was lost with age stratification. This suggests that the inherent vulnerability to the univariable‐multivariable approach to finding clinically relevant subgroup interactions that affect predictive strength of independent variables. Next, we combined data that did not have the exact same abstraction methods. For example, we assumed that variables that were not explicitly mentioned in the medical record (eg, syncope) were absent. We did extract 42 variables using the same definitions in our datasets and, therefore, could confidently perform the univariable‐multivariable selection method on these. Additionally, our studies did not use the same age criteria. Kline et al5 included patients aged 5 to 18 years, and Hennelly et al4 included patients from birth to age 21. This is unlikely significant given that only 6 patients with PE were under the age of 5 years. The presentation of PE is variable in the different age extremes, and ideally prospective work will better clarify these differences. Another limitation is the lack of definitive imaging for all patients. This was likely due to low clinician suspicion and negative d‐dimer testing, coupled with a desire to limit pediatric radiation exposure. However, we felt that no return visits with a diagnosis of PE made it unlikely that there were missed diagnoses in these patients. And finally, given the retrospective nature of our studies, we were unable to assess physicians’ gestalt pretest probability for suspicion of PE.
In conclusion, a pooled database from 2 states confirmed 5 previously recognized predictors of PE diagnosis in children suspected of PE (hypoxia, unilateral limb swelling, trauma or surgery requiring hospitalization in the previous 4 weeks, prior thromboembolism, and active cancer), and identified 4 novel factors (age‐adjusted tachycardia, tachypnea, anemia, and leukocytosis). A future prospective multicenter study stratifying by age is needed for children suspected of PE to better define those with a PE diagnosis.
RELATIONSHIP DISCLOSURE
The authors report nothing to disclose.
AUTHOR CONTRIBUTIONS
KEH collected the data, performed the analysis, wrote the paper, and approved the final version. AME conceived and designed the analysis and approved the final version. MIN collected the data, performed the analysis, contributed analysis tools, edited the paper, and approved the final version. JAK conceived and designed the analysis, collected data, performed the analysis, and approved the final version. The manuscript was read and approved for submission by all authors.
Supporting information
Hennelly KE, Ellison AM, Neuman MI, Kline JA. Clinical variables that increase the probability of pulmonary embolism diagnosis in symptomatic children. Res Pract Thromb Haemost. 2020;4:124–130. 10.1002/rth2.12265
Work was done at Indiana University School of Medicine and Washington University School of Medicine.
Funding information
This study was funded by the Eli Lilly Foundation Physician Scientist Award.
REFERENCES
- 1. Agha BS, Sturm JJ, Simon HK, Hirsh DA. Pulmonary embolism in the pediatric emergency department. Pediatrics. 2013;132:663–7. [DOI] [PubMed] [Google Scholar]
- 2. Babyn PS, Gahunia HK, Massicotte P. Pulmonary thromboembolism in children. Pediatr Radiol. 2005;35:258–74. [DOI] [PubMed] [Google Scholar]
- 3. Buck JR, Connors RH, Coon WW, Weintraub WH, Wesley JR, Coran AG. Pulmonary embolism in children. J Pediatr Surg. 1981;16:385–91. [DOI] [PubMed] [Google Scholar]
- 4. Hennelly KE, Baskin MN, Monuteuax MC, Hudgins J, Kua E, Commeree A, et al. Detection of pulmonary embolism in high‐risk children. J Pediatr. 2016;178(214–8):e3. [DOI] [PubMed] [Google Scholar]
- 5. Kanis J, Pike J, Hall CL, Kline JA. Clinical characteristics of children evaluated for suspected pulmonary embolism with D‐dimer testing. Arch Dis Child. 2018;103:835–40. [DOI] [PubMed] [Google Scholar]
- 6. Kline JA. Diagnosis and exclusion of pulmonary embolism. Thromb Res. 2018;163:207–20. [DOI] [PubMed] [Google Scholar]
- 7. Lee EY, Neuman MI, Lee NJ, Johnson VM, Zurakowski D, Tracy DA, et al. Pulmonary embolism detected by pulmonary MDCT angiography in older children and young adults: risk factor assessment. AJR Am J Roentgenol. 2012;198:1431–7. [DOI] [PubMed] [Google Scholar]
- 8. Rajpurkar M, Warrier I, Chitlur M, Sabo C, Frey MJ, Hollon W, et al. Pulmonary embolism‐experience at a single children's hospital. Thromb Res. 2007;119:699–703. [DOI] [PubMed] [Google Scholar]
- 9. Ramiz S, Rajpurkar M. Pulmonary embolism in children. Pediatr Clin North Am. 2018;65:495–507. [DOI] [PubMed] [Google Scholar]
- 10. Stein PD, Kayali F, Olson RE. Estimated case fatality rate of pulmonary embolism, 1979 to 1998. Am J Cardiol. 2004;93:1197–9. [DOI] [PubMed] [Google Scholar]
- 11. Thacker PG, Lee EY. Pulmonary embolism in children. AJR Am J Roentgenol. 2015;204:1278–88. [DOI] [PubMed] [Google Scholar]
- 12. Biss TT. Pulmonary embolism in childhood: how can we be sure not to miss it? Arch Dis Child. 2018;103:814–6. [DOI] [PubMed] [Google Scholar]
- 13. Kline JA, Ellison AM, Kanis J, Pike JW, Hall CL. Evaluation of the pulmonary embolism rule out criteria (PERC rule) in children evaluated for suspected pulmonary embolism. Thromb Res. 2018;168:1–4. [DOI] [PubMed] [Google Scholar]
- 14. Lee EY, Zurakowski D, Boiselle PM. Pulmonary embolism in pediatric patients survey of CT pulmonary angiography practices and policies. Acad Radiol. 2010;17:1543–9. [DOI] [PubMed] [Google Scholar]
- 15. Carrier M, Wells PS, Rodger MA. Excluding pulmonary embolism at the bedside with low pre‐test probability and d‐dimer: safety and clinical utility of 4 methods to assign pre‐test probability. Thromb Res. 2006;117:469–74. [DOI] [PubMed] [Google Scholar]
- 16. Kline JA, Courtney DM, Kabrhel C, Moore CL, Smithline HA, Plewa MC, et al. Prospective multicenter evaluation of the pulmonary embolism rule‐out criteria. J Thromb Haemost. 2008;6:772–80. [DOI] [PubMed] [Google Scholar]
- 17. Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d‐dimer. Ann Intern Med. 2001;135:98–107. [DOI] [PubMed] [Google Scholar]
- 18. Wolf SJ, McCubbin TR, Nordenholz KE, Naviaux NW, Haukoos JS. Assessment of the pulmonary embolism rule‐out criteria rule for evaluation of suspected pulmonary embolism in the emergency department. Am J Emerg Med. 2008;26:181–5. [DOI] [PubMed] [Google Scholar]
- 19. Singh B, Mommer SK, Erwin PJ, Mascarenhas SS, Parsaik AK. Pulmonary embolism rule‐out criteria (PERC) in pulmonary embolism–revisited: a systematic review and meta‐analysis. Emerg Med J. 2013;30:701–6. [DOI] [PubMed] [Google Scholar]
- 20. Kline JA, Roy PM, Than MP, Hernandez J, Courtney DM, Jones AE, et al. Derivation and validation of a multivariate model to predict mortality from pulmonary embolism with cancer: The POMPE‐C tool. Thromb Res. 2012;129:e194–e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kline JA, Russell FM, Lahm T, Mastouri RA. Derivation of a screening tool to identify patients with right ventricular dysfunction or tricuspid regurgitation after negative computerized tomographic pulmonary angiography of the chest. Pulm Circ. 2015;5:171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Advanced Life Support Group . Advanced Paediatric Life Support: The Practical Approach. 6th ed. West Sussex: John Wiley & Sons; 2016: p. 7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


