Abstract
Numerous methods for evaluation of global fibrinolytic activity in whole blood or plasma have been proposed, with the majority based on tissue‐type plasminogen activator (t‐PA) addition to initiate fibrinolysis. We propose that such an approach is useful to reveal hypofibrinolysis, but t‐PA concentrations should be kept to a minimum. In this paper, we describe a low‐concentration t‐PA plasma turbidity assay to evaluate several congenital factor deficiencies, including plasminogen activator inhibitor‐1 (PAI‐1) and plasminogen deficiency, as well as hemophilia A and B. In addition, we demonstrate a threshold dependency on endogenous PAI‐1 levels. To assess endogenous hyperfibrinolysis, we suggest that assays that avoid t‐PA addition are preferable, with assays based on euglobulin fractionation remaining a viable choice. We describe a euglobulin fraction clot lysis time (ECLT) assay with spectrophotometric readout and other modifications, and evaluate it as a tool to measure hyperfibrinolysis in inherited clotting factor deficiency states. We demonstrate that the ECLT is predominantly driven by residual amounts of PAI‐1, t‐PA, and α2‐antiplasmin. These assays should be further evaluated for the detection of hypo‐ or hyperfibrinolysis in acquired thrombotic or hemorrhagic disorders.
Keywords: euglobulin clot lysis time, fibrinolysis, hemophilia, plasminogen, plasminogen activator inhibitor 1
Essentials.
Clot lysis of the euglobulin fraction of plasma reliably assesses endogenous hyperfibrinolysis.
A modified version of the euglobulin clot lysis time (ECLT) is described herein.
This version of ECLT is sensitive to low plasminogen activator inhibitor‐1 and high tissue‐type plasminogen activator (t‐PA) levels.
Turbidimetric assays of clot lysis using low amounts of t‐PA are sensitive to hypofibrinolysis.
1. INTRODUCTION
The fibrinolytic system is an important component of hemostasis that acts as a balance to blood coagulation to protect the vasculature from harmful thrombus formation. If the main event in coagulation is fibrin formation orchestrated by thrombin, the equivalent step in fibrinolysis is fibrin degradation by plasmin. This is primarily achieved by formation of the plasminogen–tissue‐type plasminogen activator (t‐PA) complex on the surface of fibrin. Fibrin is therefore essentially a crucial cofactor in its own degradation. To control the potent proteolytic activities of plasmin and plasminogen activators, humans have evolved several inhibitors of fibrinolysis. The binding of thrombin to endothelial thrombomodulin not only activates protein C but also thrombin‐activatable fibrinolysis inhibitor (TAFI), which then cleaves exposed lysines on fibrin to prevent assembly of the t‐PA–plasminogen–fibrin complex. Plasminogen activator inhibitor‐1 (PAI‐1) is predominantly produced by endothelial cells,1 and covalently binds free, double‐chain t‐PA or urokinase plasminogen activator (u‐PA). Similarly, α2‐antiplasmin (α2AP) inactivates free plasmin. However, when either of these active enzymes are bound to fibrin, they are protected from inhibition.
Alterations in fibrinolysis may contribute to thrombosis or bleeding. Some genetic deficiency states, such as PAI‐1 (SERPINE1) deficiency2 or Quebec platelet syndrome are associated with a bleeding phenotype.3, 4 In contrast to the rarity of these disorders, an important role of fibrinolysis in highly prevalent acquired hemorrhagic conditions (such as major trauma or postpartum bleeding) is supported by the observed benefits of tranexamic acid in mitigating blood loss and improving mortality.5, 6 However, the ability to assess fibrinolytic activity in these disorders has relied on point‐of‐care viscoelastic whole blood assays, which are difficult to standardize and have been criticized for their insensitivity to ongoing fibrinolysis.7 Therefore, additional assays that address the unmet need for global assessment of hyperfibrinolysis are required.
In contrast, the relationship between hypofibrinolysis and thrombosis is more controversial. For example, plasminogen deficiency is not associated with thrombotic manifestations.8 However, the link between obesity and increased levels of PAI‐1 have prompted the development of therapeutic agents to block PAI‐1 as an antithrombotic strategy.9
Although various methods have been proposed to measure fibrinolysis in blood or plasma, there is no agreement as to the “gold‐standard” assay that reflects overall fibrinolytic activity (reviewed in Ilich et al10). The relative excess of PAI‐1 in plasma complicates global fibrinolysis assessment in vitro. In some rare cases, the release of t‐PA is so exaggerated that spontaneous lysis of clotted samples can be observed, as in some cases of trauma or asphyxia.7, 11 The addition of exogenous t‐PA or u‐PA allows for assessment of resistance of the sample to fibrinolysis. However, it is less clear whether the same approach is sensitive to hyperfibrinolysis.12, 13 In our opinion, other approaches are required to address endogenous hyperfibrinolysis, among which euglobulin fractionation (EuFr) of plasma remains a viable approach. “Rebalancing” of the ratio between pro‐ and antifibrinolytic components in the EuFr allows intrinsic fibrinolytic activity to be assessed without the addition of exogenous plasminogen activators.14, 15 The originally described euglobulin clot lysis time (ECLT) technique has several limitations, such as low throughput and high interoperator variability (as it relies on visual assessment of clot lysis).16, 17 In addition, if the fibrinogen content is low, the ECLT is invalid.18 Herein, we describe a modified version of the ECLT procedure that overcomes these limitations, while also describing a modified assay examining endogenous hypofibrinolysis mediated by the addition to plasma of a significantly lower concentration of t‐PA than previously described.
2. METHODS
2.1. Reagents and instruments
Human fibrinogen was purchased from Millipore‐Sigma (St. Louis, MO, USA) and was further purified on a lysine‐sepharose column as previously described to obtain plasminogen‐free fibrinogen.19 Absence of plasminogen was confirmed by the lack of lysis of fibrin after the addition of tPA (Figure 1A). Ovalbumin (98% purity, grade V), recombinant human thrombomodulin, and carboxypeptidase inhibitor from potato tuber (PTCI) were purchased from Millipore‐Sigma (St. Louis, MO, USA). Tissue factor (TF) (Dade Innovin, B4212‐40 Siemens Healthcare, Erlangen, Germany), acetic acid (glacial, 100%, reagent grade), mineral oil (light, C16H10N2Na2O7S2, reagent grade), and 96‐well microplates (polystyrene, flat bottom, non–tissue culture–treated plate, Falcon) were purchased from Fisher Scientific (Hampton, NH, USA). Human α‐thrombin was purchased from Haematologic Technologies (Essex Junction, VT, USA). Recombinant t‐PA was from Innovative Research (Novi, MI, USA). Distillated water (diH2O) was prepared using a Direct Q3 system (Millipore‐Sigma, St. Louis, MO, USA). Active PAI‐1, free t‐PA, plasminogen, α2AP, and α2‐macroglobulin (α2MG) were detected in both plasma and in EuFr using ELISA kits from Molecular Innovations (Novi, MI, USA). Tris buffered saline (TBS, 10x, 500 mM Tris, 1500 mM NaCl) was prepared in house and the pH was adjusted to 7.4. Phospholipid vesicles (PS:PE:PC ratios of 15:41:44) were a gift from Dr Dougald Monroe, University of North Carolina. For kinetic optical density measurements, a Synergy H1 spectrophotometer (BioTek, Winooski, VT, USA) was used.
Figure 1.
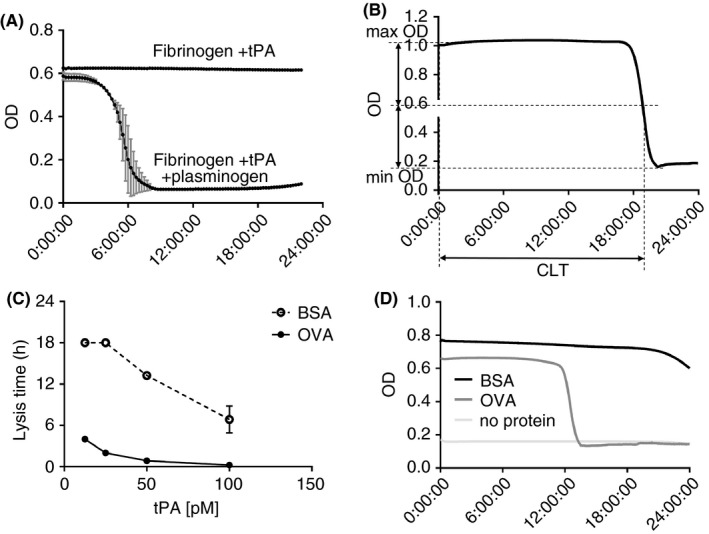
ECLT development. (A) test for plasminogen purity – t‐PA initiated lysis of clotted fibrinogen supplemented with ovalbumin in absence or presence of exogenous plasminogen. The lack of lysis without addition of plasminogen confirms the “plasminogen‐free” state of fibrinogen. (B) The determination of clot lysis time, as the time to half lysis. (C) comparison of bovine serum albumin vs. ovalbumin on fibrin lysis. Fibrinogen resupplemented with plasminogen is mixed with either bovine serum albumin or ovalbumin, clotted with thrombin with addition of various amount of t‐PA in the range of 13 to 106 pM. The experiment was terminated at 18 hours. Bovine serum albumin (open circles with dotted line) abolishes or delays lysis, compared to ovalbumin (closed circles with solid line). (D) Representative curves of the ECLT performed with ovalbumin, bovine serum albumin, or with no protein addition. BSA, bovine serum albumin; CLT, clot lysis time; ECLT, euglobulin clot lysis time; OD, optical density; OvA, ovalbumin; t‐PA, tissue‐type plasminogen activator
2.2. Specimens
Platelet‐free plasma (PFP) samples, obtained by double centrifugation at 2500 g, 20°C, for 15 minutes, were collected from normal controls (n = 114), as well as from patients with complete congenital PAI‐1 deficiency (levels < 1%; n = 3), congenital plasminogen deficiency (n = 3, plasma activity levels < 5%, 7.1%, 16.1%), severe and moderate hemophilia A and B (n = 92), and congenital hypofibrinogenemia (n = 6, fibrinogen activity: <1, <1, <1, 35, 46, 50 mg/dL). Samples were obtained after washout from any replacement therapy. All samples were obtained from consenting subjects in accordance with the Declaration of Helsinki and Institutional Review Boards from the following institutions: University of North Carolina at Chapel Hill, Indiana Hemophilia and Thrombosis Center, University of Padova, Emory University, and Mayo Clinic.
2.3. Global hyperfibrinolysis assessment: The ECLT assay
As previously described, the ideal conditions to obtain the euglobulin fraction are 1:10 sample dilution with H2O (to decrease ionic strength), acidification to pH 5.9, and incubation on ice.14, 16 This process separates protein fractions according to protein solubility in low‐ionic‐strength conditions. Acidification redistributes the ratios of fibrinolytic inhibitors/activators. Urano and colleagues reported that the lowest active PAI‐1 level with relative t‐PA preservation in the euglobulin fraction was observed at pH 5.8 to 6.1.15 For the incubation period, a range of temperature conditions were evaluated: 37°C, room temperature, or incubation on ice. Incubation on ice may reduce the rate of protein degradation and is considered by many to be the optimal approach.16 These conditions are consistent with our own observations, with the shortest ECLT times observed using a pH of 5.9 to 6.1, with incubation on ice.
As mentioned above, a major limitation of the classical ECLT is that it is rendered invalid in the presence of low fibrinogen. Therefore, in our modified version, we added plasminogen‐free fibrinogen and validated it on fibrinogen‐deficient patient samples (Figure 2A).
Figure 2.
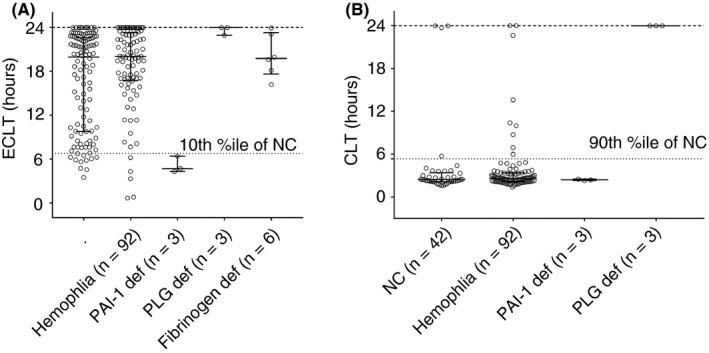
ECLT (A) and t‐PA resistance CLT (B) values in normal controls and in various congenital factor deficiencies. Experiments were ended at 24 hours (upper dotted line). CLT, clot lysis time; ECLT, euglobulin clot lysis time; NC, normal controls; PAI‐1 def, plasminogen activator inhibitor 1 deficiency; PLG def, plasminogen deficiency; t‐PA res, tissue‐type plasminogen activator resistance test
Several authors have proposed to read the ECLT endpoint spectrophotometrically.20, 21 While some have shown that the clotted EuFr has a high enough optical density (OD) to detect lysis, in our experience—even with the addition of exogenous fibrinogen—the forming clot is almost transparent at λ = 405 nm with OD values ≤ 0.07. However, this may not hold true in non‐ideal situations (eg, highly lipidated plasma, presence of artifacts such as floating fragments/bubbles). The difference in transparency between clots in euglobulin and plasma fractions may be due to the presence of abundant proteins (such as albumin) in plasma. Interestingly, while the addition of albumin to a fibrinogen solution does not increase the OD, the difference becomes obvious after a clot is formed with thrombin, leading to an OD of approximately 1, and an OD difference before vs. after lysis of about 0.9. The choice of protein is also important because it should be transparent in solution and be free from contaminants that interfere with the fibrinolytic system. Thus, we find that the addition of bovine serum albumin (BSA; 4%) completely abolishes any spontaneous fibrinolytic activity of the sample. This may be explained by contamination by other proteins, including inhibitors of fibrinolysis. Instead, we used pure grade ovalbumin and observed no inhibition of clot lysis. To confirm this observation, pure fibrinogen was resupplied with glu‐plasminogen (10 µg/mL final concentration), then added to either BSA or ovalbumin (final C = 4%), before initiating lysis by the addition of t‐PA (13‐106 pM). Whereas lysis was significantly delayed or abolished in the presence of BSA, this was not the case with ovalbumin (Figure 1C,D).
Various methods of EuFr clot formation have been proposed, most commonly by the addition of thrombin. Contact pathway activators (such as kaolin) have also been used because they significantly reduce the clot lysis time (from hours to minutes).22 However, in this case, fibrinolysis is primarily a result of factor XII activation.23 If the EuFr clot is formed by high‐concentration thrombin, the addition of Ca2+ is not required for fibrinogen cleavage or for t‐PA/plasmin activity. However, some authors have proposed that CaCl2 should be added during EuFr clot formation.20 It was proposed that endogenously generated thrombin will cleave and inactivate part of the residual PAI‐1 in the EuFr and thereby shorten the clot lysis time.
2.4. ECLT protocol
To prepare the EuFr, PFP was mixed with diH2O and acetic acid (to a pH of 5.9). Specifically, 40 µL of 5% Acetic acid was added to 1450 µL diH2O. After vortexing, 165 µL of PFP was added. The solution was then incubated on ice for 30 minutes and centrifuged at 1000 g at 20°C for 6 minutes. The supernatant was carefully aspirated (without disturbing the pellet) and discarded. The pellet was carefully washed once with diH2O and solubilized in 82 µL of TBS (to obtain 2x EuFr). To ensure easy solubilization, we waited 5 minutes after the addition of TBS before dissolving the pellet by gentle pipetting. The obtained 2x EuFr was then mixed with freshly prepared ovalbumin solution (to a 4% final concentration) and fibrinogen (to a final concentration of 2 mg/mL). Human α‐thrombin was diluted to a concentration of 7.2 µg/mL (200 nM); 10 µL of the solution was then placed in a 96‐well plate, and 110 µL of EuFr‐ovalbumin‐fibrinogen solution added. Optical density changes were then measured spectrophotometrically for 24 hours (λ = 405, t = 37°C, 2‐min intervals). To prevent the sample from drying out during the 24‐hour read, 50 µL of mineral oil was placed on the top of the formed clot. Data obtained through Gen5 software were processed using MS Excel 365, and the ECLT was automatically calculated as the time to half lysis using the “if” logical function (Figure 1B).
Variability of the test was assessed on 10 samples, which were tested 4 times on 1 plate to assess intra‐assay variability, with a resultant coefficient of variation (CV) range of 0% to 2.5%. Four samples were tested in 4 independent assays to assess interassay variability, resulting in a CV range of 5.5% to 9.9%.
2.5. Global assessment of hypofibrinolysis assay: The t‐PA resistance test
This assay is based on the measurement of fibrin clot formation and lysis over time in plasma (quantified by changes in OD) following the simultaneous addition of TF and t‐PA. Although similar to assays described by several other groups, we used a much lower concentration of t‐PA (0.6 nM, as opposed to 5‐60 nM in other studies).24, 25, 26, 27 We believe that this modification maximizes sensitivity to endogenous inhibitors of fibrinolysis in plasma, such as active PAI‐1 (aPAI‐1). Intra‐ and interassay CVs were excellent, at 4.8% and 9.4%, respectively. The assay was performed in 96‐well microplates, and OD changes were read spectrophotometrically (λ = 405 nm, t = 37°C). PFP was mixed with PS:PC:PE phospholipid vesicles (final concentration, 20 µM) and TBS containing CaCl2 (final concentration, 15 mM), TF (final concentration, 1 PM), and t‐PA (final concentration, 0.6 nM). To prevent samples from drying, 50 µl of mineral oil was added on the top of each well. To evaluate fibrinolysis, clot lysis time (CLT; the time from 50% maximal clotting to 50% lysis) was quantified. Data obtained through Gen5 software were processed using MS Excel 365, and CLT was automatically calculated using “if” logical function.
3. RESULTS
3.1. Global fibrinolysis assay profiles in normal plasma
To determine the normal range in the ECLT and t‐PA resistance assays, 112 samples from healthy controls were analyzed. ECLT values were distributed in a nonparametric bimodal pattern, with one mode between 0‐12.5 h, and another cluster in the 12.5‐ to 25‐hour range (Figure 2A). Values of the t‐PA resistance assay also form a bimodal distribution, with one mode in the 2‐ to 3‐hour range, and the second between 10 and 24 hours, when the assay was terminated without any observed lysis.
In 42 of the control samples, we measured the concentrations of several components of the fibrinolytic system, including active PAI‐1, free t‐PA, plasminogen, α2AP, and α2MG. Measurements were performed in both the starting PFPs and the derived EuFrs. As shown previously, our data confirm that in the EuFr, fibrinolytic inhibitor levels are significantly depleted, while activators are partially preserved (Table 1, Figure 3). A strong correlation was noted between ECLT and the levels of active PAI‐1 (both in PFP and EuFr), t‐PA level (in EuFr), and α2AP (in EuFr) (Table 1). Likewise, correlation between t‐PA resistance in the CLT assay and aPAI‐1 was observed (Spearman r = .62, P < .001), which was mainly driven by samples with high aPAI‐1 levels. In Figure 4, the dependence of t‐PA resistance CLT on aPAI‐1 in PFP is demonstrated; samples with aPAI‐1 > 25 nM failed to lyse, while at lower levels, it is clear that the CLT is less dependent on aPAI‐1 (Spearman r = .34, P = .084). The influence of TAFI on both tests was estimated by the addition of the TAFI PTCI, in the presence or absence of thrombomodulin. Addition of PTCI alone to ECLT (n = 3) did not result in a more rapid clot lysis time. On the other hand, addition of thrombomodulin prolonged the ECLT, an effect that was negated in the presence of both thrombomodulin and PTCI (Figure 5A). These results suggest that in the absence of exogenous thrombomodulin, TAFI does not significantly influence the ECLT. In contrast, in the t‐PA resistance assay, PTCI shortened the CLT even without the addition of thrombomodulin, suggesting that TAFI does play an antifibrinolytic role (Figure 5B).
Table 1.
Key fibrinolysis factors measured by ELISA in platelet‐free plasma and euglobulin fraction
| n | Plasma concentration | Correlation with ECLT | EuFr concentration | Correlation with ECLT | Reduction in EuFr (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Sp r | 95% CI | P value | Mean ± SD | Sp r | 95% CI | P value | Mean ± SEM | ||
| aPAI‐1 | 31 | 214.4 ± 381.4 nM | 0.74 | 0.52 to 0.87 | <0.001 | 7.7 ± 16.4 nM | 0.38 | 0.02 t6o 0.67 | <0.05 | 96.9 ± 1.3 |
| t‐PA | 36 | 4.1 ± 5.2 PM | −0.03 | −0.39 to 0.61 | 0.88 | 1.1 ± 1.7 PM | −0.40 | −0.88 to (−0.27) | <0.01 | 52.4 ± 9.3 |
| α2AP | 16 | 0.7 ± 1.4 µM | 0.11 | −0.42 to 0.59 | 0.68 | 0.02 ± 0.005 µM | 0.56 | 0.07‐0.83 | <0.05 | 95.4 ± 0.8 |
| α2MG | 36 | 2.2 ± 1.7 µM | −0.15 | −0.46 to 0.2 | 0.40 | 0.007 ± 0.008 µM | 0.15 | −0.19 to 0.47 | 0.37 | 99.1 ± 0.5 |
| Plasminogen | 35 | 1.7 ± 0.5 µM | 0.38 | 0.05 to 0.64 | <0.05 | 1.5 ± 0.6 µM | −0.09 | 0.42 to 0.26 | 0.61 | 20.4 ± 2.9 |
Correlation between ECLT and plasma/EuFr protein levels, and protein reduction in euglobulin fraction compared to initial plasma is shown.
α2AP, α2‐antiplasmin; α2MG, α2‐macroglobulin; aPAI‐1, active plasminogen activator inhibitor‐1; CI, confidence interval; EuFr, euglobulin fractionation; ECLT, euglobulin clot lysis time; Sp r, Spearman's correlation coefficient; SD, standard deviation; SEM, standard error of the mean; t‐PA, tissue‐type plasminogen activator.
Figure 3.
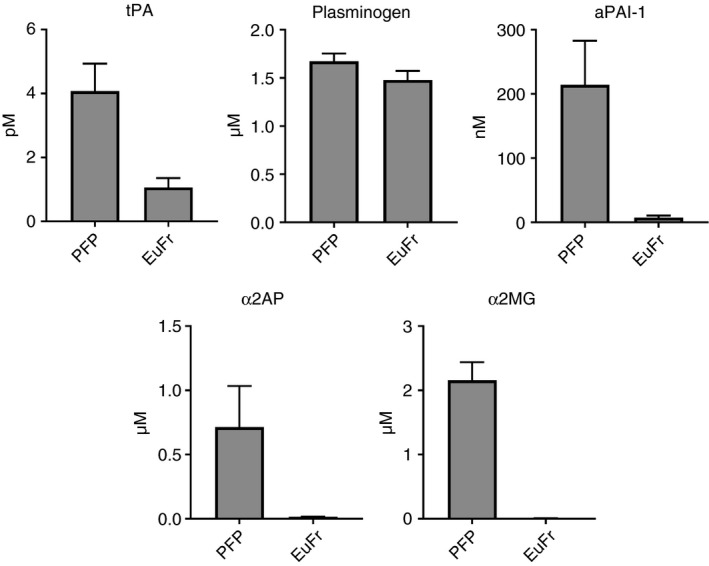
Reduction of fibrinolytic factors in euglobulin fraction. α2AP, α2‐antiplasmin; α2MG, α2‐macroglobulin; aPAI‐1, active form of plasminogen activator inhibitor‐1; EuFr, euglobulin fraction; PFP, platelet free plasma; t‐PA, tissue‐type plasminogen activator
Figure 4.
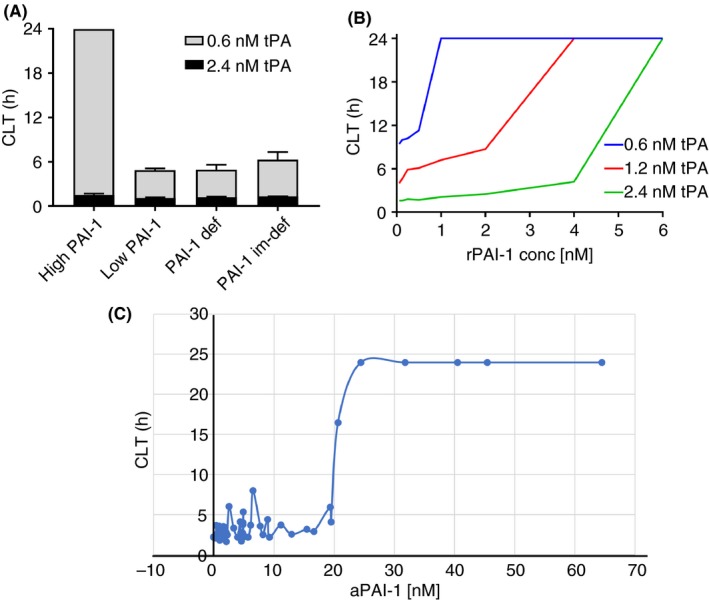
Dependency of t‐PA resistance test on PAI‐1. (A) Low tPA concentration version of the assay differentiates samples with high (30nM) aPAI‐1 levels from samples with low or absence of PAI‐1. (B) Recombinant PAI‐1 is spiked in PAI‐1 immunodepleted plasma and clot lysis time is measured at 3 different t‐PA concentrations (0.6, 1.2, 2.4 nM). (C) Active PAI‐1 is measured by ELISA in 49 individuals and plotted against tPA resistance clot lysis time. The threshold effect in inhibition could be seen in individuals with 20‐25 nM of aPAI‐1. aPAI‐1, active form of plasminogen activator inhibitor 1; CLT, clot lysis time; rPAI‐1, recombinant plasminogen activator inhibitor 1; t‐PA, tissue‐type plasminogen activator
Figure 5.
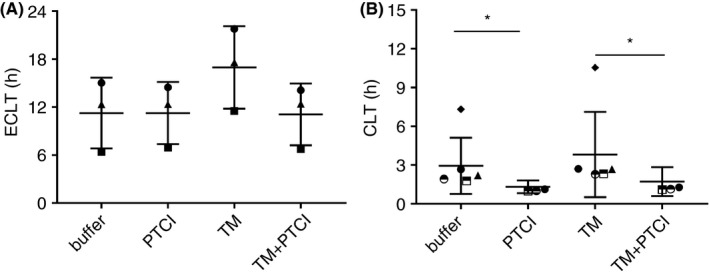
Effect of TAFI in both assays. For ECLT (A), samples from 3 individuals were used. Obtained euglobulin fractions were supplied with either buffer, PTCI (TAFI), thrombomodulin or both PTCI/TM. For t‐PA resistance CLT (B), samples from 6 individuals were used. Platelet‐free plasma was supplied with either buffer, PTCI (TAFI), thrombomodulin or both PTCI/TM. Different symbols represent individual normal plasma samples. CLT, clot lysis time; ECLT, euglobulin clot lysis time; PTCI, potato tuber carboxypeptidase inhibitor; TAFI, thrombin‐activatable fibrinolysis inhibitor; TM, thrombomodulin; t‐PA, tissue‐type plasminogen activator
3.2. Global fibrinolysis assay profiles in factor‐deficient plasmas
The utility of the ECLT was tested in various inherited coagulation disorders in which fibrinolysis is thought to play a role. ECLT values for 3 patients with inherited complete PAI‐1 deficiency (due to a dinucleotide insertion within exon 4 of the SERPINE1 gene) and a hemorrhagic phenotype2 were less than the 10th percentile of the healthy controls (P < .001). In contrast, samples from 3 patients with inherited plasminogen deficiency demonstrated no lysis at 24 hours (23.62 ± 0.6 vs. 16.7 ± 6.6 h, P = .007) (Figure 2A). In the t‐PA resistance test, the 3 PAI‐1–deficient samples were not different from normals (2.4 ± 0.1 vs. 4.13 ± 5.6 hours in normal controls; P = .96), while in those with plasminogen deficiency, no lysis was observed in 24 hours (23.97 ± 0 vs. 4.13 ± 5.6; P < .001) (Figure 2B). ECLT was performed on plasma obtained from 6 patients with congenital hypofibrinogenemia. Clot lysis times were within normal range (20.14 ± 2.94 vs. 16.7 ± 6.6; P < .47) (Figure 2A). Both assays were also applied to hemophilia A and B samples. A small difference in ECLT was observed between hemophilia and normal control samples (20.14 ± 2.9 vs. 16.7 ± 6.6; P = .048), but no statistically significant difference in t‐PA resistance (3.79 ± 4.11 vs. 4.13 ± 5.6; P = .36) was observed.
4. DISCUSSION
In this study, we sought to reexamine global assays for fibrinolysis in plasma. In particular, we wished to determine the utility of these assays to detect opposing phenotypes in fibrinolysis, namely hypo‐ and hyperfibrinolysis.10 In the case of hypofibrinolysis, an assay that detects endogenous resistance to the addition of tPA seems appropriate. There have been many published iterations of this approach; however, our data indicate that when plasminogen activators are added in great excess, the sensitivity to levels of endogenous inhibitors may be lost. Therefore, we chose a t‐PA concentration that is sensitive to endogenous aPAI‐1 levels above the normal range. Indeed, we demonstrate that the presence of physiologically high concentrations of aPAI‐1 (>25 nM) abolishes clot lysis within 24 hours (Figure 4).
To detect hyperfibrinolysis in a global plasma‐based assay, we suggest that an approach that detects endogenous profibrinolytic activity without the addition of exogenous activators is preferable. Under normal plasma conditions, endogenous profibrinolytic activity is masked by the molar excess of PAI‐1 relative to t‐PA. Therefore, we modified a traditional approach, the ECLT assay. The principle of EuFr‐based assays is to reduce inhibitor levels below the threshold where endogenous lytic activity is detectable. The modified assay described here allows for a high‐throughput reliable assay that is sensitive to aberrations in the fibrinolytic pathway, while simultaneously overcoming some of the traditional limitations of the EuFr (for example, low fibrinogen levels). With respect to potential limitations, some authors have recommended keeping blood refrigerated on ice during blood processing.16 While we confirmed that this precaution does help to preserve more plasminogen activator activity (data not shown), it complicates the logistics of sample collection and renders it impractical to work with existing plasma repositories. Incubation on ice would be optimal, if samples are collected prospectively, but samples not processed on ice could still provide valuable information. In this study, we processed blood at room temperature, which allowed us to evaluate its applicability in a variety of clinical conditions by testing stored plasma samples. We suggest the establishment of respective normal reference ranges for samples that have been collected according to either technique.
Our data demonstrate the dependence of both tests on several components of fibrinolysis. Specifically, ECLT is not only sensitive to residual amounts of t‐PA but also to the lack of inhibitors, such as aPAI‐1. These data support previously published data by Urano et al,28, 29 showing close relationships between ECLT and plasma levels of total and free PAI‐1 as well as active t‐PA. The lack of sensitivity to residual plasminogen levels could be explained by the relative excess of that proenzyme, compared to its activators and inhibitors. Conversely, the t‐PA resistance test is primarily sensitive to high endogenous levels of aPAI‐1. It is important to mention that we measured only free/active PAI‐1 (which represents only a fraction of total PAI‐1), as it is the only fraction capable of inhibiting plasminogen activators. Estimation of TAFI is more challenging. ELISA assays of TAFI measure only the inactive form, since the active form is present only during clot formation and even then, for a short time (half‐life, 8‐10 min). Also, data from commercially available ELISAs may be significantly affected by genetic polymorphisms in TAFI. For example, it has been shown that ELISA assays can miss up to 44% of the protein due to changes in its immunoreactivity.30 Notably, polymorphisms in the protein can significantly affect TAFI half‐life, which could be a potent modifier of activity. Therefore, we indirectly evaluated TAFI activity using the TAFI PTCI in the presence or absence of thrombomodulin, as described by De bruijne et al31, 32 and Guimarães et al.31, 32 Our data suggest that the ECLT assay is not dependent on TAFI, whereas in the t‐PA resistance test, TAFI plays a detectable antifibrinolytic role.
In plasma samples of patients with hemophilia, we observed similar profiles with both tests compared to healthy controls, with profiles that ranged from normal to relatively hypo‐ or hyperfibrinolytic, as seen in healthy subjects. These data question the hypothesis that patients with hemophilia who demonstrate more profound clot lysis have a more exaggerated bleeding phenotype (relative to endogenous factor VIII or IX level), or conversely, whether those with a more pronounced hypofibrinolytic profile have a less severe clinical phenotype. Further studies in a cohort of patients with well‐characterized bleeding outcomes will be required to address these questions.
Our results using samples that were congenitally deficient for several proteins involved in fibrinolysis confirm the sensitivity of the t‐PA resistance assay to hypofibrinolytic states, as well as the value of the ECLT in the detection of hyperfibrinolytic states. On the other hand, each test failed to detect abnormalities on the opposite arm of the fibrinolysis spectrum, supporting our approach in utilizing 2 different global assays for hypo‐ and hyperfibrinolysis detection in plasma.
RELATIONSHIP DISCLOSURES
This research was funded in part by an investigator initiated grant from Pfizer to the authors’ institution.
AUTHOR CONTRIBUTIONS
AI performed research, analyzed data, and wrote the paper; KFB, AS, AD, SM, EC, PS, and MSP contributed samples and edited the paper; DFN, MH, PE, and RP analyzed data and edited the paper; NSK designed research, analyzed data, and wrote the paper.
ACKNOWLEDGMENTS
The authors acknowledge support from Pfizer ASPIRE grant WI227678, and NIH grants 1RO1HL146226 and T32HL007149‐35.
Ilich A, Noubouossie DF, Henderson M, et al. Development and application of global assays of hyper‐ and hypofibrinolysis. Res Pract Thromb Haemost. 2020;4:46–53. 10.1002/rth2.12275
REFERENCES
- 1. Sawdey M, Podor TJ, Loskutoff DJ. Regulation of type 1 plasminogen activator inhibitor gene expression in cultured bovine aortic endothelial cells. Induction by transforming growth factor‐beta, lipopolysaccharide, and tumor necrosis factor‐alpha. J Biol Chem. 1989;264:10396–401. [PubMed] [Google Scholar]
- 2. Fay WP, Shapiro AD, Shih JL, Schleef RR, Ginsburg D. Brief report: complete deficiency of plasminogen‐activator inhibitor type 1 due to a frame‐shift mutation. N Engl J Med. 1992;327:1729–33. [DOI] [PubMed] [Google Scholar]
- 3. Kahr WH, Zheng S, Sheth PM, Pai M, Cowie A, Bouchard M, et al. Platelets from patients with the Quebec platelet disorder contain and secrete abnormal amounts of urokinase‐type plasminogen activator. Blood. 2001;98:257–65. [DOI] [PubMed] [Google Scholar]
- 4. Diamandis M, Adam F, Kahr W, Wang P, Chorneyko KA, Arsenault AL, et al. Insights into abnormal hemostasis in the Quebec platelet disorder from analyses of clot lysis. J Thromb Haemost. 2006;4:1086–94. [DOI] [PubMed] [Google Scholar]
- 5. Theusinger OM, Wanner GA, Emmert MY, Billeter A, Eismon J, Seifert B, et al. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM) is associated with higher mortality in patients with severe trauma. Anesth Analg. 2011;113:1003–12. [DOI] [PubMed] [Google Scholar]
- 6. Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, et al. physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77:811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J. Thromb. Haemost. 2013;11:307–14. [DOI] [PubMed] [Google Scholar]
- 8. Mehta R, Shapiro AD. Plasminogen deficiency. Haemophilia. 2008;14:1261–8. [DOI] [PubMed] [Google Scholar]
- 9. Baluta MM, Vintila MM. PAI‐1 Inhibition ‐ another therapeutic option for cardiovascular protection. Maedica (Buchar). 2015;10:147–52. [PMC free article] [PubMed] [Google Scholar]
- 10. Ilich A, Bokarev I, Key NS. Global assays of fibrinolysis. Int J Lab Hematol. 2017;39:441–7. [DOI] [PubMed] [Google Scholar]
- 11. Schwameis M, Schober A, Schörgenhofer C, Sperr WR, Schöchl H, Janata‐Schwatczek K, et al. Asphyxia by drowning induces massive bleeding due to hyperfibrinolytic disseminated intravascular coagulation. Crit Care Med. 2015;43:2394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lisman T. Global assays of fibrinolysis. Int J Lab Hematol 2017;39:e140–e141. [DOI] [PubMed] [Google Scholar]
- 13. Ilich A, Key NS. Global assays of fibrinolysis. Int J Lab Hematol. 2017;39:e142–e143. [DOI] [PubMed] [Google Scholar]
- 14. Hong SY, Shin HK, Yang DH. The effect of dilution, pH and ionic strength of plasma on t‐PA precipitation in euglobulin fraction. Korean J Intern Med. 1992;7:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Urano T, Nishikawa T, Nagai N, Takada Y, Takada A. Amounts of tPA and PAI‐1 in the euglobulin fraction obtained at different pH: their relation to the euglobulin clot lysis time. Thromb Res. 1997;88:75–80. [DOI] [PubMed] [Google Scholar]
- 16. Chakrabarti R, Bielawiec M, Evans JF, Fearnley GR. Methodological study and a recommended technique for determining the euglobulin lysis time. J Clin Pathol. 1968;21:698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cash JD, Leask E. Automatic determination of euglobulin lysis time. J Clin Pathol. 1965;18:821–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayward C. How I investigate for bleeding disorders. Int J Lab Hematol. 2018;40(Suppl 1):6–14. [DOI] [PubMed] [Google Scholar]
- 19. Matsuda M, Iwanaga S, Nakamura S. A simple, large scale method for preparation of plasminogen‐free fibrinogen. Thromb Res. 1972;1:619–30. [Google Scholar]
- 20. Tomczyk M, Suzuki Y, Sano H, Brzoska T, Tanaka H, Urano T. Bidirectional functions of thrombin on fibrinolysis: Evidence of thrombin‐dependent enhancement of fibrinolysis provided by spontaneous plasma clot lysis. Thromb Res. 2016;143:28–33. [DOI] [PubMed] [Google Scholar]
- 21. Smith AA, Jacobson LJ, Miller BI, Hathaway WE, Manco‐Johnson MJ. A new euglobulin clot lysis assay for global fibrinolysis. Thromb Res. 2003;112:329–37. [DOI] [PubMed] [Google Scholar]
- 22. Mandle RJ, Kaplan AP. Hageman‐factor‐dependent fibrinolysis: generation of fibrinolytic activity by the interaction of human activated factor XI and plasminogen. Blood. 1979;54:850–62. [PubMed] [Google Scholar]
- 23. Miles LA, Greengard JS, Griffin JH. A comparison of the abilities of plasma kallikrein, beta‐factor XIIa, factor XIa and urokinase to activate plasminogen. Thromb Res. 1983;29:407–17. [DOI] [PubMed] [Google Scholar]
- 24. Antovic JP, Antovic A, Sten‐Linder M, Wramsby M, Blomback M. Overall hemostatic potential (OHP) assay‐a possible tool for determination of prothrombotic pattern in FXII deficiency. J Thromb Haemost. 2004;2:2058–60. [DOI] [PubMed] [Google Scholar]
- 25. Bombardier C, Villalobos‐Menuey E, Ruegg K, Hathaway WE, Manco‐Johnson MJ, Goldenberg NA. Monitoring hypercoagulability and hypofibrinolysis following acute venous thromboembolism in children: application of the CloFAL assay in a prospective inception cohort study. Thromb Res. 2012;130:343–9. [DOI] [PubMed] [Google Scholar]
- 26. Stubblefield WB, Alves NJ, Rondina MT, Kline JA. Variable resistance to plasminogen activator initiated fibrinolysis for intermediate‐risk pulmonary embolism. PLoS ONE. 2016;11:e0148747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lisman T, Leebeek FW, Mosnier LO, Bouma BN, Meijers JC, Janssen HL, et al. Thrombin‐activatable fibrinolysis inhibitor deficiency in cirrhosis is not associated with increased plasma fibrinolysis. Gastroenterology. 2001;121:131–9. [DOI] [PubMed] [Google Scholar]
- 28. Urano T, Sumiyoshi K, Pietraszek MH, Takada Y, Takada A. PAI‐1 plays an important role in the expression of t‐PA activity in the euglobulin clot lysis by controlling the concentration of free t‐PA. Thromb Haemost. 1991;66:474–8. [PubMed] [Google Scholar]
- 29. Urano T, Sakakibara K, Rydzewski A, Urano S, Takada Y, Takada A. Relationships between euglobulin clot lysis time and the plasma levels of tissue plasminogen activator and plasminogen activator inhibitor 1. Thromb Haemost. 1990;63:82–6. [PubMed] [Google Scholar]
- 30. Heylen E, Van Goethem S, Willemse J, Olsson T, Augustyns K, Hendriks D. Development of a sensitive and selective assay for the determination of procarboxypeptidase U (thrombin‐activatable fibrinolysis inhibitor) in plasma. Anal Biochem. 2010;396:152–4. [DOI] [PubMed] [Google Scholar]
- 31. De bruijne E, Gils A, Guimarães A, Dippel D, Deckers JW, Van den meiracker AH, et al. The role of thrombin activatable fibrinolysis inhibitor in arterial thrombosis at a young age: the ATTAC study. J Thromb Haemost. 2009;7:919–27. [DOI] [PubMed] [Google Scholar]
- 32. Guimarães A, Bertina RM, Rijken DC. A new functional assay of thrombin activatable fibrinolysis inhibitor. J Thromb Haemost. 2005;3:1284–92. [DOI] [PubMed] [Google Scholar]


