Abstract
We recently reported that KO of Dual‐specificity protein phosphatase 5 (Dusp5) enhances myogenic reactivity and blood flow autoregulation in the cerebral and renal circulations in association with increased levels of pPKC and pERK1/2 in the cerebral and renal arteries and arterioles. In the kidney, hypertension‐related renal damage was significantly attenuated in Dusp5 KO rats. Elevations in pPKC and pERK1/2 promote calcium influx in VSMC and facilitate vasoconstriction. However, whether DUSP5 plays a role in altering the passive mechanical properties of cerebral and renal arterioles has never been investigated. In this study, we found that KO of Dusp5 did not alter body weights, kidney and brain weights, plasma glucose, and HbA1C levels. The expression of pERK is higher in the nucleus of primary VSMC isolated from Dusp5 KO rats. Dusp5 KO rats exhibited eutrophic vascular hypotrophy with smaller intracerebral parenchymal arterioles and renal interlobular arterioles without changing the wall‐to‐lumen ratios. These arterioles from Dusp5 KO rats displayed higher myogenic tones, better distensibility, greater compliance, and less stiffness compared with arterioles from WT control rats. VSMC of Dusp5 KO rats exhibited a stronger contractile capability. These results demonstrate, for the first time, that DUSP5 contributes to the regulation of the passive mechanical properties of cerebral and renal arterioles and provide new insights into the role of DUSP5 in vascular function, cancer, stroke, and other cardiovascular diseases.
Keywords: distensibility, Dusp5, elastic modulus, interlobular arterioles, parenchymal arterioles, vascular stiffness
The results from this manuscript demonstrate, for the first time, that DUSP5 contributes to the regulation of the passive mechanical properties of cerebral and renal arterioles and provide new insights into role of DUSP5 in vascular function, cancer, stroke, and other cardiovascular diseases.

1. INTRODUCTION
Dual‐specificity protein phosphatase 5 (DUSP5) inactivates the extracellular signal‐related kinase (ERK1/2) by dephosphorylating threonine/tyrosine residues (Alonso et al., 2004; Kidger & Keyse, 2016; Tonks, 2013). We previously reported that knockout (KO) of Dusp5 enhances myogenic reactivity and blood flow autoregulation in the cerebral and renal circulations, which is associated with increased levels of phosphorylated protein kinase C (pPKC) and ERK1/2 (pERK1/2) in the cerebral and renal arteries and arterioles (Fan et al., 2014; Zhang et al., 2019). In the kidney, improved hemodynamics in Dusp5 KO rats may contribute, at least in part, to the protection from hypertension‐related renal damage (Zhang et al., 2019). The mechanisms by which activation of the PKC and mitogen‐activated protein (MAP)/ERK (MEK) pathways in vascular smooth muscle cells (VSMCs) promotes vasoconstriction involve facilitating calcium influx—by alteration of the activities of multiple ion channels, and enhancing actin–myosin interactions—by modulation of the expression and activities of their associated enzymes and proteins (Zhang et al., 2019).
Activation of PKC and MAP/ERK pathways has been reported to enhance cell proliferation (Chambard, Lefloch, Pouyssegur, & Lenormand, 2007; Gao et al., 2009). Inhibition of DUSP5 expression in human corneal epithelial cells increased ERK1/2 phosphorylation and cell proliferation by 50%–60% (Wang et al., 2010). In Dusp5 KO rats, we expected that the media of the vascular wall containing VSMCs would be hypertrophied, which would enhance the myogenic response. Surprisingly, although the afferent arterioles (Af‐arts), middle cerebral arteries (MCAs), and renal interlobular arterioles (IAs) of Dusp5 KO rats exhibited enhanced constrictions in response to elevated transmural pressure, we found these vessels are not larger in calcium‐free media compared with those isolated from wild‐type (WT) control rats (Fan et al., 2014; Zhang et al., 2019).
Changes in the passive mechanical properties of the vascular wall also have a significant influence on myogenic reactivity and blood flow autoregulation. This study investigated the possible role of DUSP5 on vascular mechanical properties by comparing the sizes, incremental distensibility, circumferential wall strain, stress, and the elastic modulus of the intracerebral parenchymal arterioles (PAs) and renal IAs isolated from Dusp5 KO and WT rats.
2. MATERIALS AND METHODS
2.1. Animals
Experiments were carried out on 9‐ to 12‐week‐old male Dusp5 KO and WT rats that we previously generated (Fan et al., 2014; Zhang et al., 2019). All rats were bred and housed at the University of Mississippi Medical Center (UMMC) and were fed a standard diet (Harland) and water ad libitum throughout the studies. All procedures were approved by the Institutional Animal Care and Use Committee of UMMC. All rats related in this project (study rats, breeders, and extra pups that were euthanized) were weighed upon weaning at 3‐week of age, including 38 male and 55 female Dusp5 KO rats, as well as 60 male and 64 female WT control rats.
2.2. Drugs and reagents
All chemicals were purchased from Sigma‐Aldrich. Physiological salt solution (PSS) contained 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 18 NaHCO3, 5 HEPES, 1.18 NaH2PO4, and 10 glucose (in mM, pH7.4). Calcium‐free physiological salt solution (PSS0Ca) was identical to PSS except for the exclusion of CaCl2 and the addition of EDTA (0.03 mM), as we previously described (Fan et al., 2015, 2014, 2017, 2013).
2.3. Preparation of arterioles
In the morning on the day of the experiments, plasma glucose and HbA1C were measured using a Contour Next Meter System (Fisher Scientific, Waltham, MA) and Polymer Technology Systems A1CNow+™ Systems (Fisher Scientific) according to the manufacturer's instructions. The rats were then euthanized with 4% isoflurane and weighed. The brains and kidneys were collected, weighed, and placed in a dish filled with ice‐cold PSS0Ca. A piece of the brain surrounding the MCA was removed and transferred to another dish filled with ice‐cold PSS0Ca supplemented with 1% bovine serum albumin (BSA) for vascular dissection. PAs branching directly from the M1 segment of MCA were carefully dissected (Cipolla, Chan, et al., 2014a; Cipolla, Sweet, et al., 2014b; Pires, Dabertrand, & Earley, 2016) under a microscope and mounted on glass micropipettes in a chamber filled with warmed (37°C), oxygenated (21% O2, 5% CO2, and 74% N2) PSS.
The kidneys were cut into 1‐mm‐thick slices, and a piece of the cortex was transferred to a dish filled with ice‐cold PSS0Ca containing 1% BSA. Renal IAs upstream of Af‐arts were dissected under a stereomicroscope and mounted on glass micropipettes in a chamber filled with warmed and oxygenated PSS.
2.4. Pressure myography
Intact cerebral PAs and renal IAs isolated from Dusp5 KO and WT rats were mounted on glass cannulas in a pressure myography chamber (Living System Instrumentation) mounted on an IMT‐2 inverted microscope (Olympus). The pressure was initially set to 10 mmHg for PAs and 60 mmHg for IAs and vessels were equilibrated for 30 min to generate a spontaneous tone. Inner and outer diameters (ID and OD) in response to increases in intraluminal pressures (from 10 to 60 mmHg for PAs and 60 to 180 mmHg for IAs) were measured using a digital camera attached to the microscope. Perfusion pressure was slowly increased from 10 to 60 mmHg for PAs in 10 mmHg increments. For IAs, intraluminal pressure was slowly raised from 60 to 180 mmHg in a stepwise fashion.
At the end of the experiment, intraluminal pressure was reset to 5 mmHg, and the vessels were washed with PSS0Ca for 6–8 times. Inner and outer diameters under calcium‐free conditions (ID0Ca and OD0Ca) of these arterioles were determined at 5 mmHg, 10–60 mmHg for PAs, and 60–180 mmHg for IAs as described above.
2.5. Calculation of structure parameters
The following vascular mechanical properties were calculated using equations described previously (Baumbach, Heistad, & Siems, 1989; Briones et al., 2003; Cheng et al., 2014; Dobrin, 1978; Gonzalez et al., 2005; Hudetz, 1979; Izzard et al., 2003):
where ID0Ca 5mmHg is inner diameters obtained at the perfusion pressure of 5 mmHg in PSS0Ca.
Incremental distensibility defines as the percentage change in the vascular ID0Ca for every 1 mmHg changes in P. Where P is the intraluminal pressure in PSS0Ca.
The circumferential wall strain defined as
where P indicates intraluminal pressure (1 mmHg = 133.4 Nm‐2) under calcium‐free conditions.
Arterial stiffness refers to the ability of arteries to resist elastic deformation when subjected to pressure. It is determined by elastic modulus (E = σ/ε). This relationship is non‐linear and appropriate to the exponential curve. Thus, an exponential model with least‐squares analysis was used:
where σ orig is σ at the original diameter at 5 mmHg. The slope of the curve (β value) was used to determine the tangential or incremental elastic modulus (E inc), which is directly proportional to E inc. An increased β value indicated increases in stiffness.
2.6. Isolation of vascular smooth muscle cells
Primary VSMCs were isolated from WT (n = 3) and Dusp5 KO (n = 3) rats, as described previously (Fan et al., 2017). Briefly, the cerebral and renal vessels were isolated using the Evans blue sieving procedure (Fan et al., 2015, 2013), and digested with dithiothreitol (2 mg/ml, Sigma‐Aldrich), papain (22.5 U/mL, Sigma‐Aldrich), trypsin inhibitor (10,000 U/mL, Sigma‐Aldrich), collagenase (250 U/mL, Sigma‐Aldrich), and elastase (2.4 U/mL, Sigma‐Aldrich) at 37°C. After centrifugation, the VSMCs were resuspended and seeded on autoclaved glass coverslips precoated with CellTak (Thermo Scientific) in a six‐well plate. Early passages (P2‐P3) of the primary VSMCs were used for the following experiments.
2.7. Immunocytochemistry
Primary VSMCs were fixed with 3.7% paraformaldehyde (Thermo Scientific), and permeabilized with 0.1% Triton‐100 (Sigma‐Aldrich), and blocked with 1% BSA. The cells were then incubated with the primary antibody p44/42 MAPK (ERK1/2; 1:50, #4696, Cell Signaling) or phosphor‐p44/42 MAPK (pERK1/2; 1:100, #4377, Cell Signaling), following by Alexa Fluor 555 or Alexa Fluor 488–labeled secondary antibodies (Thermo Scientific). The slides were coverslipped after dropping an anti‐fade mounting medium with DAPI (Vector Laboratories). Images were captured using a Nikon C2 confocal microscope (Nikon), and the mean fluorescence intensities per cell were compared using NIS‐Elements Imaging Software 4.6 (Nikon). Experiments were repeated three times, and triplicate wells were used at each experiment.
2.8. Cell constriction assay
The contractile capability of primary VSMCs isolated from WT versus Dusp5 KO was compared using a collagen gel‐based assay kit (Cell Biolabs) following manufactory instruction. Briefly, the VSMCs (2 × 106 cells/mL) were suspended in the culture medium, mixed with collagen gel working solution, added in a 24‐well plate, and incubated at 37°C for 2 days to develop contractile stress. The cell contraction was initiated by detaching the stressed matrix from the wall of the culture plate using a sterile needle. Percentage changes in the size of the collagen gel were imaged at 30 min interval and analyzed using the NIS‐Elements Imaging Software 4.6 (Nikon).
2.9. Statistical analysis
Data are presented as mean values ± standard error (SEM). The differences in the means between groups and the slopes of stress–strain curves were compared using Student's t test. The significance of differences in pressures–diameter relationships was analyzed using an analysis of variance (ANOVA) for repeated measures with multiple groups followed by a Holm‐Sidak post hoc test using GraphPad Prism 6 (GraphPad Software, Inc.). A value of p < .05 was considered to be significant.
3. RESULTS
3.1. Effects of knockout of Dusp5 on body weights, brain and kidney weights, plasma glucose, and HbA1C levels
As presented in Figure 1a, there were no differences in body weight in both sexes of Dusp5 KO (males: 37.82 ± 1.39 g, n = 38; females: 38.05 ± 1.30 g, n = 55) versus WT (males: 40.82 ± 0.80 g, n = 60; females: 37.86 ± 0.99 g, n = 64) rats when they were 3‐week of age. Similarly, body weight was not different in male Dusp5 KO (285.79 ± 4.52 g, n = 34) versus WT (293.16 ± 3.44 g, n = 49) rats when they were at 9–12 weeks of age (Figure 1b). Brain weight was not significantly different between Dusp5 KO (1.68 ± 0.01 g, n = 14) and WT (1.70 ± 0.01 g, n = 11) rats (Figure 1c). There were no differences in kidney weight between Dusp5 KO (left kidney: 1.12 ± 0.04 g, n = 26; right kidney: 1.07 ± 0.04 g, n = 8) and WT (left kidney: 1.19 ± 0.03 g, n = 29; right kidney: 1.12 ± 0.03 g, n = 10) rats (Figure 1d). Plasma glucose (Figure 1e) and HbA1C (Figure 1f) levels were similar in 9‐ to 12‐week‐old Dusp5 KO (106.86 ± 1.10 mg/dl, n = 7; and 4.37 ± 0.06%, n = 7, respectively) versus WT (104.00 ± 2.51 mg/dl, n = 7; and 4.36 ± 0.08%, n = 7, respectively) rats.
Figure 1.

Effects of Knockout of Dual‐specificity protein phosphatase 5 (Dusp5) on body weights, brain and kidney weights, plasma glucose and HbA1C levels. (a) Comparison of body weights in 3‐week‐old male and female Dusp5 KO versus WT rats. (b) Comparison of body weights in 9‐ to 12‐week‐old male Dusp5 KO versus WT rats. (c) Comparison of brain weights in 9‐ to 12‐week‐old male Dusp5 KO versus WT rats. (d) Comparison of kidney weights in 9‐ to 12‐week‐old male Dusp5 KO versus WT rats. (e) Comparison of plasma glucose levels in 9‐ to 12‐week‐old male Dusp5 KO versus WT rats. (f) Comparison of HbA1C levels in 9‐ to 12‐week‐old male Dusp5 KO versus WT rats. Mean values ± SEM are presented. Numbers indicate the number of animals studied per group
3.2. Effects of knockout of Dusp5 on the expression and localization of ERK and pERK in primary VSMCs
The expression of total ERK was similar in the nucleus and cytoplasm in primary VSMCs isolated from WT and Dusp5 KO rats (Figure 2a,b). However, the expression of pERK was higher in the nucleus of primary VSMCs isolated from Dusp5 KO rats (Figure 2a,c). The ratio of pERK/ERK was increased by 1.9 ± 0.1 folds (Figure 2d) in Dusp5 KO versus WT rats.
Figure 2.
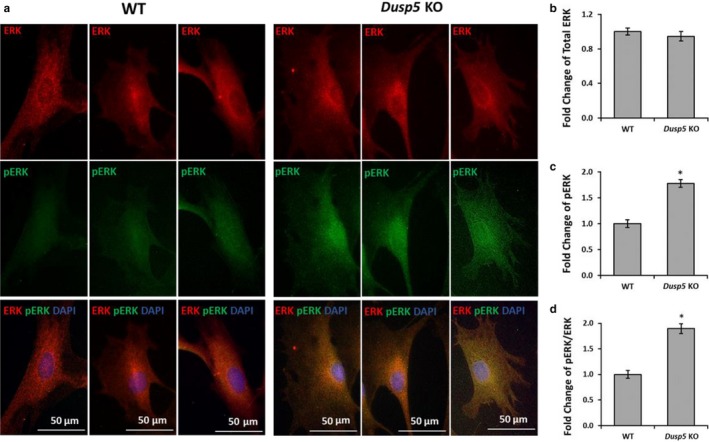
Effects of knockout of Dual‐specificity protein phosphatase 5 (Dusp5) on the expression and localization of ERK and pERK in primary VSMCs. (a) Representative images of the expression and localization of total ERK and pERK in primary VSMCs isolated from WT and Dusp5 KO rats. (b) Quantitative analysis of fold changes in the mean red fluorescence intensity in VSMC of Dusp5 KO versus WT rats. (c) Quantitative analysis of fold changes in the mean green fluorescence intensity in VSMC of Dusp5 KO versus WT rats. (d) Quantitative analysis of fold changes in pERK/ERK in VSMC of Dusp5 KO versus WT rats. Primary VSMCs were isolated from three rats of each strain. Experiments were repeated three times, and triplicate wells were used at each experiment. * indicates p < .05 from the corresponding value in Dusp5 KO versus WT rats
3.3. Effects of knockout of Dusp5 on vascular characteristics of cerebral PAs
Figure 3 demonstrates a comparison of vascular characteristics of cerebral PAs between Dusp5 KO versus WT rats. ID0Ca (19.87 ± 2.2 μm) and OD0Ca (36.84 ± 3.7 μm) of PAs were smaller in Dusp5 KO rats at 5 mmHg intraluminal pressure than WT (28.63 ± 2.0 μm and 49.98 ± 2.5 μm, respectively). These differences remained at low pressures but were diminished at higher perfusion pressures (Figure 3a,b). Wall thickness and CSA of PAs were smaller in Dusp5 KO versus WT rats at perfusion pressures from 10 to 60 mmHg (Figure 3c,d), and there were no differences in the wall‐to‐lumen ratios between two strains at perfusion pressures from 5 to 60 mmHg (Figure 3e).
Figure 3.

Effects of Knockout of Dual‐specificity protein phosphatase 5 (Dusp5) on vascular characteristics of cerebral PAs. (a) Comparison of ID0Ca of PAs of Dusp5 KO versus WT rats. (b) Comparison of OD0Ca of PAs of Dusp5 KO versus WT rats. (c) Comparison of wall thicknesses of PAs of Dusp5 KO versus WT rats. (d) Comparison of cross‐sectional areas (CSA) of PAs of Dusp5 KO versus WT rats. (e) Comparison of the wall‐to‐lumen ratios of PAs of Dusp5 KO versus WT rats. All rats studied were 9‐ to 12‐week‐old males. Mean values ± SEM are presented. N = 4–8 rats per group. * indicates p < .05 from the corresponding value in Dusp5 KO versus WT rats
3.4. Effects of knockout of Dusp5 on vascular characteristics of renal IAs
As presented in Figure 4, vascular characteristics of renal IAs between Dusp5 KO versus WT rats were compared. ID0Ca (38.29 ± 3.2 μm) and OD0Ca (66.45 ± 3.8 μm) of IAs were smaller in Dusp5 KO rats at 5 mmHg intraluminal pressure than WT (59.98 ± 5.5 μm and 102.44 ± 6.8 μm, respectively). These differences remained at perfusion pressure from 60 to 180 mmHg. The wall thickness and CSA of IAs were smaller in Dusp5 KO rats at perfusion pressures from 5 to 180 mmHg (Figure 4a,d). However, there were no differences in the wall‐to‐lumen ratios of IAs in both strains (Figure 4e).
Figure 4.
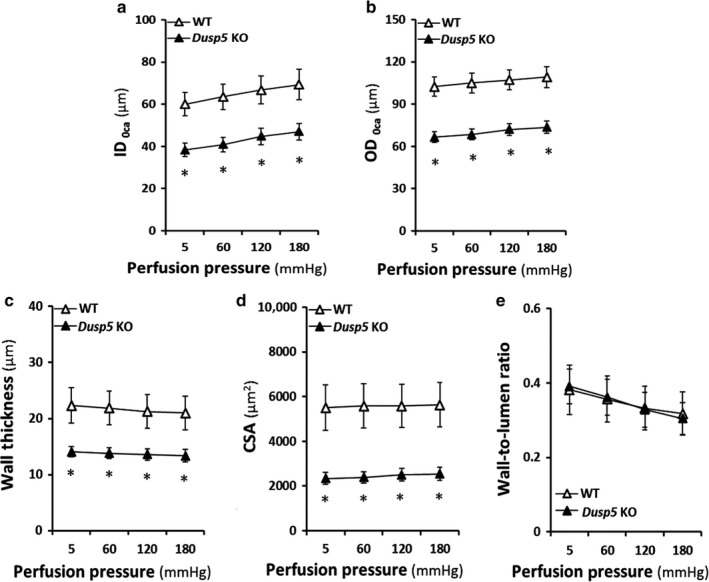
Effects of Knockout of Dual‐specificity protein phosphatase 5 (Dusp5) on vascular characteristics of cerebral IAs. (a) Comparison of ID0Ca of IAs of Dusp5 KO versus WT rats. (b) Comparison of OD0Ca of IAs of Dusp5 KO versus WT rats. (c) Comparison of wall thicknesses of IAs of Dusp5 KO versus WT rats. (d) Comparison of cross‐sectional areas (CSA) of IAs of Dusp5 KO versus WT rats. (e) Comparison of the wall‐to‐lumen ratios of IAs of Dusp5 KO versus WT rats. All rats studied were 9‐ to 12‐week‐old males. Mean values ± SEM are presented. N = 8–11 rats per group. * indicates p < .05 from the corresponding value in Dusp5 KO versus WT rats
3.5. Effects of knockout of Dusp5 on the myogenic tone of cerebral PAs and renal IAs
The myogenic tone of cerebral PAs and renal IAs isolated from Dusp5 KO versus WT rats is presented in Figure 4. Dusp5 KO rats exhibited a higher active tone in PAs at perfusion pressures from 10 to 40 mmHg (Figure 5a) and in IAs at intraluminal pressures from 60 to 180 mmHg (Figure 5b).
Figure 5.
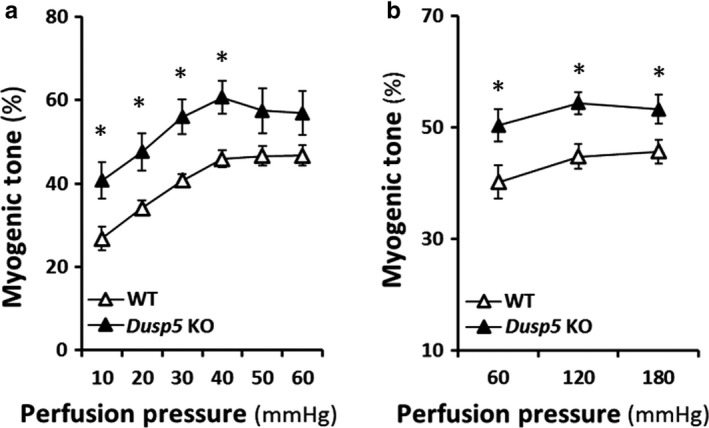
Effects of Knockout of Dual‐specificity protein phosphatase 5 (Dusp5) on the myogenic tone of cerebral PAs and renal IAs. (a) Comparison of the myogenic tone of PAs of Dusp5 KO versus WT rats. (b) Comparison of the myogenic tone of IAs of Dusp5 KO versus WT rats. All rats studied were 9‐ to 12‐week‐old males. Mean values ± SEM are presented. N = 4–11 rats per group. * indicates p < .05 from the corresponding value in Dusp5 KO versus WT rats
3.6. Effects of knockout of Dusp5 on vascular distensibility and incremental distensibility of cerebral PAs and renal IAs
Dusp5 KO rats exhibited better distensibility in PAs at perfusion pressures from 10 to 60 mmHg (Figure 6a), and greater incremental distensibility in PAs at perfusion pressures from 10 to 30 mmHg (Figure 6b). Similarly, Dusp5 KO rats exhibited better distensibility and incremental distensibility in IAs at perfusion pressures from 120 to 180 mmHg (Figure 6c) and from 120 to 180 mmHg (Figure 6d).
Figure 6.
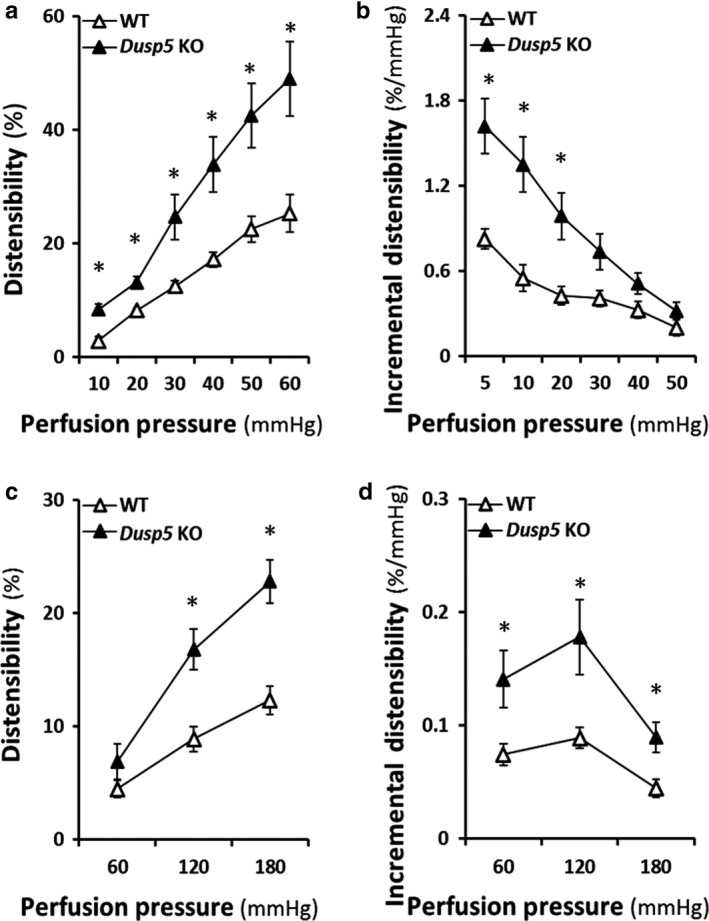
Effects of Knockout of Dual‐specificity protein phosphatase 5 (Dusp5) on vascular distensibility and incremental distensibility of cerebral PAs and renal IAs. (a) Comparison of the distensibility of PAs of Dusp5 KO versus WT rats. (b) Comparison of the incremental distensibility of PAs of Dusp5 KO versus WT rats. (c) Comparison of the distensibility of IAs of Dusp5 KO versus WT rats. (d) Comparison of the incremental distensibility of IAs of Dusp5 KO versus WT rats. All rats studied were 9‐ to 12‐week‐old males. Mean values ± SEM are presented. N = 4–11 rats per group. * indicates p < .05 from the corresponding value in Dusp5 KO versus WT rats
3.7. Effects of knockout of Dusp5 on elastic modulus and vascular stiffness of cerebral PAs and renal IAs
The stress–strain relationships or the elastic modulus curves of PAs between Dusp5 KO (R 2 = 0.95 ± 0.008, n = 6) versus WT (R 2 = 0.98 ± 0.005, n = 6) rats were compared (Figure 7a). The β value was significantly smaller in PAs of Dusp5 KO (4.76 ± 0.43) than in WT (10.07 ± 1.9) rats (Figure 7b). Similarly, by comparison of an elastic modulus curve of IAs (Figure 7c) between Dusp5 KO (R 2 = 0.98 ± 0.008, n = 8) versus WT (R 2 = 0.99 ± 0.004, n = 8) rats, we found that the β value was significantly smaller in IAs isolated from Dusp5 KO (8.48 ± 0.088) than WT (13.50 ± 1.54) rats (Figure 7d).
Figure 7.

Effects of Knockout of Dual‐specificity protein phosphatase 5 (Dusp5) on the elastic modulus and vascular stiffness of cerebral PAs and renal IAs. (a) Comparison of the elastic modulus (stress–strain relationships) of PAs of Dusp5 KO versus WT rats. (b) Comparison of the slopes of the elastic modulus curves (β value) of PAs of Dusp5 KO versus WT rats. (c) Comparison of the stress–strain relationships of IAs of Dusp5 KO versus WT rats. (d) Comparison of the β values of IAs of Dusp5 KO versus WT rats. All rats studied were 9‐ to 12‐week‐old males. Mean values ± SEM are presented. N = 4–11 rats per group. * indicates p < .05 from the corresponding value in Dusp5 KO versus WT rats
3.8. Effects of knockout of Dusp5 on VSMC contractile capability
The effects of knockout of Dusp5 on VSMC contractile capability are presented in Figure 8. The VSMCs isolated from the vasculature of Dusp5 KO rats exhibited a stronger contractile capability, and the gel size after 120 min of stimulation was maximally reduced by 26.6 ± 0.4% versus19.4 ± 0.3% compared with cells isolated from WT rats.
Figure 8.
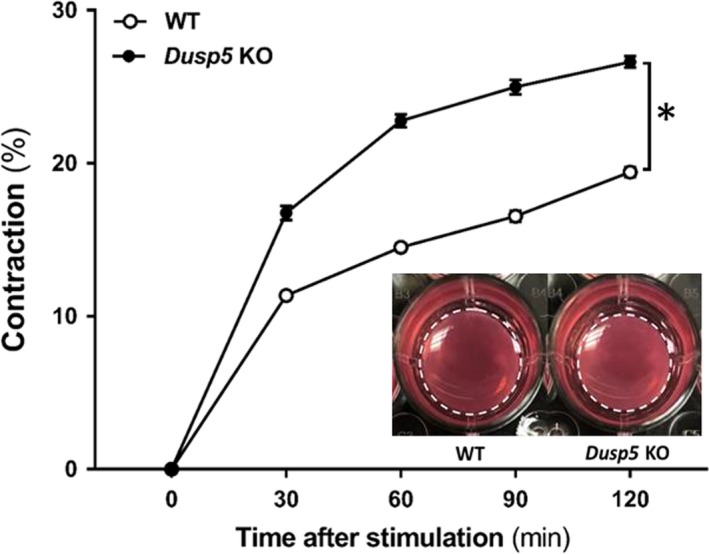
Effects of knockout of Dual‐specificity protein phosphatase 5 (Dusp5) on VSMC contractile capability. Comparison of the contractile capability of primary VSMC isolated from the vasculature of Dusp5 KO versus WT rats. Representative images after 120 min stimulation are presented as the insertion. Experiments were repeated three times, and triplicate wells were used at each experiment. * indicates p < .05 from the corresponding value in Dusp5 KO versus WT rats
4. DISCUSSION
DUSP5 is a nuclear protein, and it dephosphorylates the threonine/tyrosine residues of ERK1/2 to affect numerous cellular functions, which contributes to the pathogenesis in many diseases (Lake, Correa, & Muller, 2016; Mandl, Slack, & Keyse, 2005; Seternes, Kidger, & Keyse, 2019; Zhang et al., 2019). We previously reported that the myogenic response and autoregulation of cerebral and renal blood flow (CBF and RBF) are enhanced in Dusp5 KO rats (Fan et al., 2014; Zhang et al., 2019). In this study, we compared the passive mechanical properties of intracerebral PAs and renal IAs isolated from Dusp5 KO and WT rats. The results from this study are as follows: (1) there was no difference in body weights, kidney and brain weights, plasma glucose, and HbA1C levels between Dusp5 KO and WT rats. (2) The expression of pERK is higher in the nucleus of primary vascular smooth muscle cells isolated from Dusp5 KO rats. (3) Inner and outer diameters of PAs and IAs were smaller in Dusp5 KO versus WT rats. However, the wall‐to‐lumen ratios of these vessels were not significantly different. (4) Dusp5 KO rats exhibited higher myogenic tones in both PAs and IAs. (5) The incremental distensibility of PAs and IAs were greater in Dusp5 KO than WT rats. (6) PAs and IAs isolated from Dusp5 KO rats displayed greater compliance and less stiffness indicating by higher distensibility and incremental distensibility and lower β values. (7) VSMC of Dusp5 KO rats exhibited a stronger contractile capability.
Arterioles are high resistance vessels in vascular beds. The most significant changes in blood pressure and blood flow occur at the transition of arterioles to capillaries indicating these arterioles play a major role in blood flow autoregulation (Martinez‐Lemus, 2012). The vascular smooth muscle cells (VSMCs) in the tunica media of the arteriolar wall are essential for the myogenic response to regulate blood flow (Fan et al., 2017). In the tunica intima layer, endothelial cells participate in the control of vascular permeability and reactivity by releasing vasoactive factors. Collagen in the intima adventitia and media layers and elastin in the intima and media contribute to the regulation of flexibility and stiffness of the vasculature (Martinez‐Lemus, 2012). In the brain, the large extracranial vessels (internal carotid and vertebral) and intracranial pial vessels provide ~50% of cerebral vascular resistance as determined using a direct measurement of the pressure gradient across different segments of the cerebral circulation (Faraci & Heistad, 1990; Heistad, Marcus, & Abboud, 1978). It has been traditionally thought that small pial, penetrating, and parenchymal arterioles account for the remainder of autoregulation of CBF and the fine regulation of capillary pressure (Federico et al., 2012; Iadecola & Davisson, 2008). In this study, the intracerebral PAs we used are the lenticulostriate branches of the MCAs, which provide blood supply to the frontoparietal white matter tracts and the basal ganglia in rats (Johnson & Cipolla, 2018; Pires et al., 2016). Embolism of these vessels is the most common cause of ischemic stroke in humans (Navarro‐Orozco & Sanchez‐Manso, 2019). We also studied the IAs, which are the corresponding resistance arterioles in the kidney. Resistance along the IAs and Af‐arts accounts for the majority of the preglomerular pressure drop in the renal circulation and plays a major role in RBF autoregulation that protects fragile glomerular capillaries from elevations in systemic pressure (Imig, Zou, Ortiz de Montellano, Sui, & Roman, 1994).
We found there were no differences in body weights, brain, and kidney weights between 12‐week‐old Dusp5 KO and WT control rats. The inner and out diameters, wall thickness, and cross‐sectional areas of PAs and IAs were smaller in Dusp5 KO compared with WT rats. This finding is unexpected as the activation of the PKC and ERK pathways is thought to enhance cell proliferation (Chambard et al., 2007; Gao et al., 2009). Inhibition of DUSP5 expression in human corneal epithelial cells increased ERK1/2 phosphorylation, and cell proliferation by 50%–60% (Wang et al., 2010). Dusp5 KO mice enhanced ERK activity in eosinophils by upregulation of antiapoptotic BCL‐XL and prolonged eosinophil lifespan (Holmes, Yeh, Yan, Xu, & Chan, 2015). However, there is certainly no evidence that loss of DUSP5 alone causes an increase in cell proliferation in either skin cancer or in mouse embryo fibroblasts in Dusp5 KO mice (Rushworth et al., 2014). Our previous results also demonstrated that 10‐week‐old Dusp5 KO rats had similar inner diameters of Af‐art, and the sizes of MCAs were not different in 9–12 weeks KO compared with WT controls, interestingly, IAs were smaller in the KO rats when they were 24 weeks old (Fan et al., 2014; Zhang et al., 2019).
DUSP5 has a half‐life of 45 min and can be rapidly degraded by the proteasome (Kucharska, Rushworth, Staples, Morrice, & Keyse, 2009). In this study, we found that the expression of pERK is higher in the nucleus of primary VSMC isolated from Dusp5 KO rats. In the nucleus, DUSP5 not only inactivates ERK1/2 but also anchors ERK1/2, which was reported to paradoxically enhance cytoplasmic ERK activity in cancer via reduced ERK‐mediated RAF inhibition (Bellou et al., 2009; Kidger & Keyse, 2016; Kidger et al., 2017). On the other hand, activation of cytoplasmic ERK also facilitates nuclear translocation (Kidger et al., 2017; Mebratu & Tesfaigzi, 2009). Cytoplasmic ERK is anchored by an associated phosphatase, MEK, and microtubules (Fukuda, Gotoh, & Nishida, 1997; Reszka, Seger, Diltz, Krebs, & Fischer, 1995). Nuclear ERK activation promotes cell proliferation by enhancing cell cycle regulatory protein activities and posttranslational modifications to increase prosurvival gene function and reduce cell death (Mebratu & Tesfaigzi, 2009). ERK activation also has been reported to play a role in cell death. For example, ERK activation induced by DNA damage agents, IFNγ, or Fas causes cell death or apoptosis. However, the underlying mechanisms remain poorly understood, and the evidence to support this view is less well studied (Mebratu & Tesfaigzi, 2009). Nevertheless, ERK1/2 activation can involve both cell proliferation or apoptosis, depending on its localization, cell type, and physiological and pathological conditions that may involve different sets of ERK effectors (Mebratu & Tesfaigzi, 2009). A good example is that the expression of BCL‐XL has no changes in Dusp5 KO mice, using a microarray (Rushworth et al., 2014), although it was upregulated in eosinophils in another study (Holmes et al., 2015). Other mechanisms involved in the role of DUSP5 on cell proliferation or apoptosis also cannot be excluded, as this protein has tumor activator or suppressor function in different types of cancers (Montero‐Conde et al., 2013; Pratilas et al., 2009; Rushworth et al., 2014; Shin, Park, & Kang, 2013; Ueda, Arakawa, & Nakamura, 2003; Wang et al., 2019; Yun et al., 2009).
An interesting previous study (Fu, McKnight, Yu, Callaway, & Lane, 2006) demonstrated that intrauterine growth retardation reduced hepatic DUSP5 and enhanced phosphorylation of ERK1/2 and the insulin receptor substrate‐1 (IRS‐1), which contributes to insulin resistance (Fu et al., 2006). We found there was no difference in BW between Dusp5 KO and WT control rats at 3 and 9–12 weeks of age. In addition, plasma glucose and HbA1C levels were similar and in the normal ranges in 9–12 weeks old Dusp5 KO in comparison with WT control rats, suggesting KO of Dusp5 unlikely induces insulin resistance or hyperglycemia in this study.
Alteration of vessel sizes can change the proportion of components of the vascular wall, which determine the mechanical properties that influence the response of the myogenic reactivity and autoregulation. In this study, the size of PAs and IA was smaller in Dusp5 KO compared with WT rats. This was associated with increased distensibility and incremental distensibility in cerebral PAs and renal IAs of Dusp5 KO versus WT rats. The elastic modulus curves were shifted to the right, and the slopes or β values were smaller in the vessels isolated from Dusp5 KO than WT rats. These results suggest that arterioles in Dusp5 KO are more compliant and distensible with less stiffness than WT control rats.
It has been reported that Dusp5 is a vascular endothelial‐specific gene in zebrafish (Pramanik et al., 2009; Qian et al., 2005; Sumanas, Jorniak, & Lin, 2005) and humans (Alleboina et al., 2019). DUSP5 is expressed in angioblasts, and it plays an essential role in embryonic vascular development. Downregulation of this protein promotes endothelial apoptosis in zebrafish (Pramanik et al., 2009) but not in humans (Alleboina et al., 2019). In mice, knockdown of DUSP5 impaired postischemic angiogenesis in association with increased limb necrosis (Alleboina et al., 2019). We have reported that DUSP5 is expressed in renal and cerebral vasculatures in rats; however, the vascular cell specificity of expression of DUSP5 has not been well studied (Fan et al., 2014; Zhang et al., 2019). Arteriolar intrinsic passive mechanical properties influence vascular elasticity and stiffness that have major effects on stretch and shear stress‐induced NO production by vascular endothelial cells (Sriram et al., 2012). On the other hand, the myogenic response, as an intrinsic property of VSMCs, is modulated by the endothelia under different genetic, physiological, and pathological conditions by releasing various vasoactive factors (Fan et al., 2016; Fan & Roman, 2017). Although we found that VSMC of Dusp5 KO rats exhibited a stronger contractile capability, which is consistent with our previous findings that the myogenic response is enhanced in Dusp5 KO rats, the underlying mechanisms are still not elucidative with regards to how DUSP5 enhances myogenic response and blood flow autoregulation demonstrated in our previous studies (Fan et al., 2014; Zhang et al., 2019). The observations in the current studies provide a piece of new information to better understand the potential mechanisms.
In summary, this study first time demonstrates that DUSP5 contributes to the regulation of passive mechanical properties of cerebral and renal arterioles. KO of Dusp5 did not alter body and organ (brain and kidney) weights and did not induce insulin resistance or hyperglycemia. Dusp5 KO rats exhibited eutrophic vascular hypotrophy in intracerebral parenchymal arterioles and renal interlobular arterioles. These arterioles of Dusp5 KO rats displayed higher myogenic tones, greater distensibility and compliance, and less stiffness compared with arterioles of WT control rats. These results provide new insights into role of DUSP5 in vascular development, cancer, stroke, and other cardiovascular diseases.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Stephen M. Keyse (Ninewells Hospital & Medical School, Jacqui Wood Cancer Centre, Scotland, U.K.) for his invaluable input. We would thank Ms. Goldie M. Faircloth for the technical contributions. This study was supported by grants AG050049 (F.F.), AG057842 (F.F.), P20GM104357 (F.F. and R.J.R), DK104184 (R.J.R.), and HL138685 (R.J.R.) from the National Institutes of Health; 16GRNT31200036 (F. F.) and 20PRE35210043 (S.W.) from the American Heart Association.
Zhang H, Zhang C, Liu Y, et al. Influence of dual‐specificity protein phosphatase 5 on mechanical properties of rat cerebral and renal arterioles. Physiol Rep. 2020;8:e14345 10.14814/phy2.14345
Huawei Zhang and Chao Zhang contributed equally to this work.
REFERENCES
- Alleboina, S. , Ayalew, D. , Peravali, R. , Chen, L. , Wong, T. , & Dokun, A. O. (2019). Dual specificity phosphatase 5 regulates perfusion recovery in experimental peripheral artery disease. Vascular Medicine, 24, 395–404. 10.1177/1358863X19866254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, A. , Sasin, J. , Bottini, N. , Friedberg, I. , Friedberg, I. , Osterman, A. , … Mustelin, T. (2004). Protein tyrosine phosphatases in the human genome. Cell, 117, 699–711. 10.1016/j.cell.2004.05.018 [DOI] [PubMed] [Google Scholar]
- Baumbach, G. L. , Heistad, D. D. , & Siems, J. E. (1989). Effect of sympathetic nerves on composition and distensibility of cerebral arterioles in rats. Journal of Physiology, 416, 123–140. 10.1113/jphysiol.1989.sp017753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellou, S. , Hink, M. A. , Bagli, E. , Panopoulou, E. , Bastiaens, P. I. , Murphy, C. , & Fotsis, T. (2009). VEGF autoregulates its proliferative and migratory ERK1/2 and p38 cascades by enhancing the expression of DUSP1 and DUSP5 phosphatases in endothelial cells. American Journal of Physiology. Cell Physiology, 297, C1477–C1489. 10.1152/ajpcell.00058.2009 [DOI] [PubMed] [Google Scholar]
- Briones, A. M. , Gonzalez, J. M. , Somoza, B. , Giraldo, J. , Daly, C. J. , Vila, E. , … Arribas, S. M. (2003). Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. Journal of Physiology, 552, 185–195. 10.1113/jphysiol.2003.046904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard, J. C. , Lefloch, R. , Pouyssegur, J. , & Lenormand, P. (2007). ERK implication in cell cycle regulation. Biochimica et Biophysica Acta, 1773, 1299–1310. 10.1016/j.bbamcr.2006.11.010 [DOI] [PubMed] [Google Scholar]
- Cheng, J. H. , Zhang, L. F. , Gao, F. , Bai, Y. G. , Boscolo, M. , Huang, X. F. , & Zhang, X. (2014). Mechanics and composition of middle cerebral arteries from simulated microgravity rats with and without 1‐h/d ‐Gx gravitation. PLoS ONE, 9, e97737 10.1371/journal.pone.0097737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla, M. J. , Chan, S. L. , Sweet, J. , Tavares, M. J. , Gokina, N. , & Brayden, J. E. (2014a). Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke, 45, 2425–2430. 10.1161/STROKEAHA.114.005888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla, M. J. , Sweet, J. , Chan, S. L. , Tavares, M. J. , Gokina, N. , & Brayden, J. E. (2014b). Increased pressure‐induced tone in rat parenchymal arterioles vs. middle cerebral arteries: Role of ion channels and calcium sensitivity. Journal of Applied Physiology, 117, 53–59. 10.1152/japplphysiol.00253.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrin, P. B. (1978). Mechanical properties of arteries. Physiological Reviews, 58, 397–460. 10.1152/physrev.1978.58.2.397 [DOI] [PubMed] [Google Scholar]
- Fan, F. , Ge, Y. , Lv, W. , Elliott, M. R. , Muroya, Y. , Hirata, T. , … Roman, R. J. (2016). Molecular mechanisms and cell signaling of 20‐hydroxyeicosatetraenoic acid in vascular pathophysiology. Frontiers in Bioscience, 21, 1427–1463. 10.2741/4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, F. , Geurts, A. M. , Murphy, S. R. , Pabbidi, M. R. , Jacob, H. J. , & Roman, R. J. (2015). Impaired myogenic response and autoregulation of cerebral blood flow is rescued in CYP4A1 transgenic Dahl salt‐sensitive rat. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 308, R379–R390. 10.1152/ajpregu.00256.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, F. , Geurts, A. M. , Pabbidi, M. R. , Smith, S. V. , Harder, D. R. , Jacob, H. , & Roman, R. J. (2014). Zinc‐finger nuclease knockout of dual‐specificity protein phosphatase‐5 enhances the myogenic response and autoregulation of cerebral blood flow in FHH.1BN rats. PLoS ONE, 9, e112878 10.1371/journal.pone.0112878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, F. , Pabbidi, M. R. , Ge, Y. , Li, L. , Wang, S. , Mims, P. N. , & Roman, R. J. (2017). Knockdown of Add3 impairs the myogenic response of renal afferent arterioles and middle cerebral arteries. American Journal of Physiology. Renal Physiology, 312, F971–F981. 10.1152/ajprenal.00529.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, F. , & Roman, R. J. (2017). Effect of cytochrome P450 metabolites of arachidonic acid in nephrology. Journal of the American Society of Nephrology, 28, 2845–2855. 10.1681/ASN.2017030252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, F. , Sun, C. W. , Maier, K. G. , Williams, J. M. , Pabbidi, M. R. , Didion, S. P. , … Roman, R. J. (2013). 20‐Hydroxyeicosatetraenoic acid contributes to the inhibition of K+ channel activity and vasoconstrictor response to angiotensin II in rat renal microvessels. PLoS ONE, 8, e82482 10.1371/journal.pone.0082482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci, F. M. , & Heistad, D. D. (1990). Regulation of large cerebral arteries and cerebral microvascular pressure. Circulation Research, 66, 8–17. 10.1161/01.RES.66.1.8 [DOI] [PubMed] [Google Scholar]
- Federico, A. , Di Donato, I. , Bianchi, S. , Di Palma, C. , Taglia, I. , & Dotti, M. T. (2012). Hereditary cerebral small vessel diseases: A review. Journal of the Neurological Sciences, 322, 25–30. 10.1016/j.jns.2012.07.041 [DOI] [PubMed] [Google Scholar]
- Fu, Q. , McKnight, R. A. , Yu, X. , Callaway, C. W. , & Lane, R. H. (2006). Growth retardation alters the epigenetic characteristics of hepatic dual specificity phosphatase 5. The FASEB Journal, 20, 2127–2129. 10.1096/fj.06-6179fje [DOI] [PubMed] [Google Scholar]
- Fukuda, M. , Gotoh, Y. , & Nishida, E. (1997). Interaction of MAP kinase with MAP kinase kinase: Its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO Journal, 16, 1901–1908. 10.1093/emboj/16.8.1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Q. , Tan, J. , Ma, P. , Ge, J. , Liu, Y. , Sun, X. , & Zhou, L. (2009). PKC alpha affects cell cycle progression and proliferation in human RPE cells through the downregulation of p27kip1. Molecular Vision, 15, 2683–2695. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, J. M. , Briones, A. M. , Starcher, B. , Conde, M. V. , Somoza, B. , Daly, C. , … Arribas, S. M. (2005). Influence of elastin on rat small artery mechanical properties. Experimental Physiology, 90, 463–468. 10.1113/expphysiol.2005.030056 [DOI] [PubMed] [Google Scholar]
- Heistad, D. D. , Marcus, M. L. , & Abboud, F. M. (1978). Role of large arteries in regulation of cerebral blood flow in dogs. Journal of Clinical Investigation, 62, 761–768. 10.1172/JCI109187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, D. A. , Yeh, J. H. , Yan, D. , Xu, M. , & Chan, A. C. (2015). Dusp5 negatively regulates IL‐33‐mediated eosinophil survival and function. EMBO Journal, 34, 218–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz, A. G. (1979). Incremental elastic modulus for orthotropic incompressible arteries. Journal of Biomechanics, 12, 651–655. 10.1016/0021-9290(79)90015-0 [DOI] [PubMed] [Google Scholar]
- Iadecola, C. , & Davisson, R. L. (2008). Hypertension and cerebrovascular dysfunction. Cell Metabolism, 7, 476–484. 10.1016/j.cmet.2008.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig, J. D. , Zou, A. P. , Ortiz de Montellano, P. R. , Sui, Z. , & Roman, R. J. (1994). Cytochrome P‐450 inhibitors alter afferent arteriolar responses to elevations in pressure. American Journal of Physiology, 266, H1879–H1885. 10.1152/ajpheart.1994.266.5.H1879 [DOI] [PubMed] [Google Scholar]
- Izzard, A. S. , Graham, D. , Burnham, M. P. , Heerkens, E. H. , Dominiczak, A. F. , & Heagerty, A. M. (2003). Myogenic and structural properties of cerebral arteries from the stroke‐prone spontaneously hypertensive rat. American Journal of Physiology. Heart and Circulatory Physiology, 285, H1489–1494. 10.1152/ajpheart.00352.2003 [DOI] [PubMed] [Google Scholar]
- Johnson, A. C. , & Cipolla, M. J. (2018). Impaired function of cerebral parenchymal arterioles in experimental preeclampsia. Microvascular Research, 119, 64–72. 10.1016/j.mvr.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidger, A. M. , & Keyse, S. M. (2016). The regulation of oncogenic Ras/ERK signalling by dual‐specificity mitogen activated protein kinase phosphatases (MKPs). Seminars in Cell and Developmental Biology, 50, 125–132. 10.1016/j.semcdb.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidger, A. M. , Rushworth, L. K. , Stellzig, J. , Davidson, J. , Bryant, C. J. , Bayley, C. , … Caunt, C. J. (2017). Dual‐specificity phosphatase 5 controls the localized inhibition, propagation, and transforming potential of ERK signaling. Proceedings of the National Academy of Sciences of the United States of America, 114, E317–E326. 10.1073/pnas.1614684114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharska, A. , Rushworth, L. K. , Staples, C. , Morrice, N. A. , & Keyse, S. M. (2009). Regulation of the inducible nuclear dual‐specificity phosphatase DUSP5 by ERK MAPK. Cellular Signalling, 21, 1794–1805. 10.1016/j.cellsig.2009.07.015 [DOI] [PubMed] [Google Scholar]
- Lake, D. , Correa, S. A. , & Muller, J. (2016). Negative feedback regulation of the ERK1/2 MAPK pathway. Cellular and Molecular Life Sciences, 73, 4397–4413. 10.1007/s00018-016-2297-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl, M. , Slack, D. N. , & Keyse, S. M. (2005). Specific inactivation and nuclear anchoring of extracellular signal‐regulated kinase 2 by the inducible dual‐specificity protein phosphatase DUSP5. Molecular and Cellular Biology, 25, 1830–1845. 10.1128/MCB.25.5.1830-1845.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Lemus, L. A. (2012). The dynamic structure of arterioles. Basic and Clinical Pharmacology and Toxicology, 110, 5–11. 10.1111/j.1742-7843.2011.00813.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebratu, Y. , & Tesfaigzi, Y. (2009). How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle, 8, 1168–1175. 10.4161/cc.8.8.8147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero‐Conde, C. , Ruiz‐Llorente, S. , Dominguez, J. M. , Knauf, J. A. , Viale, A. , Sherman, E. J. , … Fagin, J. A. (2013). Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF‐mutant thyroid carcinomas. Cancer Discovery, 3, 520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro‐Orozco, D. , & Sanchez‐Manso, J. C. (2019. ). Neuroanatomy, Middle Cerebral Artery, In StatPearls[Internet], Treasure Island, FL: StatPearls Publishing; Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK526002/ [PubMed] [Google Scholar]
- Pires, P. W. , Dabertrand, F. , & Earley, S. (2016). Isolation and cannulation of cerebral parenchymal arterioles. Journal of Visualized Experiments. 10.3791/53835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik, K. , Chun, C. Z. , Garnaas, M. K. , Samant, G. V. , Li, K. , Horswill, M. A. , … Ramchandran, R. (2009). Dusp‐5 and Snrk‐1 coordinately function during vascular development and disease. Blood, 113, 1184–1191. 10.1182/blood-2008-06-162180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratilas, C. A. , Taylor, B. S. , Ye, Q. , Viale, A. , Sander, C. , Solit, D. B. , & Rosen, N. (2009). (V600E)BRAF is associated with disabled feedback inhibition of RAF‐MEK signaling and elevated transcriptional output of the pathway. Proceedings of the National Academy of Sciences of the United States of America, 106, 4519–4524. 10.1073/pnas.0900780106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, F. , Zhen, F. , Ong, C. , Jin, S. W. , Meng Soo, H. , Stainier, D. Y. , … Wen, Z. (2005). Microarray analysis of zebrafish cloche mutant using amplified cDNA and identification of potential downstream target genes. Developmental Dynamics, 233, 1163–1172. 10.1002/dvdy.20444 [DOI] [PubMed] [Google Scholar]
- Reszka, A. A. , Seger, R. , Diltz, C. D. , Krebs, E. G. , & Fischer, E. H. (1995). Association of mitogen‐activated protein kinase with the microtubule cytoskeleton. Proceedings of the National Academy of Sciences of the United States of America, 92, 8881–8885. 10.1073/pnas.92.19.8881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth, L. K. , Kidger, A. M. , Delavaine, L. , Stewart, G. , van Schelven, S. , Davidson, J. , … Keyse, S. M. (2014). Dual‐specificity phosphatase 5 regulates nuclear ERK activity and suppresses skin cancer by inhibiting mutant Harvey‐Ras (HRasQ61L)‐driven SerpinB2 expression. Proceedings of the National Academy of Sciences of the United States of America, 111, 18267–18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seternes, O. M. , Kidger, A. M. , & Keyse, S. M. (2019). Dual‐specificity MAP kinase phosphatases in health and disease. Biochimica Et Biophysica Acta (BBA) ‐ Molecular Cell Research, 1866, 124–143. 10.1016/j.bbamcr.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, S. H. , Park, S. Y. , & Kang, G. H. (2013). Down‐regulation of dual‐specificity phosphatase 5 in gastric cancer by promoter CpG island hypermethylation and its potential role in carcinogenesis. American Journal of Pathology, 182, 1275–1285. 10.1016/j.ajpath.2013.01.004 [DOI] [PubMed] [Google Scholar]
- Sriram, K. , Salazar Vazquez, B. Y. , Tsai, A. G. , Cabrales, P. , Intaglietta, M. , & Tartakovsky, D. M. (2012). Autoregulation and mechanotransduction control the arteriolar response to small changes in hematocrit. American Journal of Physiology. Heart and Circulatory Physiology, 303, H1096–1106. 10.1152/ajpheart.00438.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas, S. , Jorniak, T. , & Lin, S. (2005). Identification of novel vascular endothelial‐specific genes by the microarray analysis of the zebrafish cloche mutants. Blood, 106, 534–541. 10.1182/blood-2004-12-4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks, N. K. (2013). Protein tyrosine phosphatases–from housekeeping enzymes to master regulators of signal transduction. FEBS Journal, 280, 346–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, K. , Arakawa, H. , & Nakamura, Y. (2003). Dual‐specificity phosphatase 5 (DUSP5) as a direct transcriptional target of tumor suppressor p53. Oncogene, 22, 5586–5591. 10.1038/sj.onc.1206845 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Hu, J. , Qiu, D. , Gao, H. , Zhao, W. , Huang, Y. , … Chen, Y. (2019). Dual‐specificity phosphatase 5 suppresses ovarian cancer progression by inhibiting IL‐33 signaling. American Journal of Translational Research, 11, 844–854. [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Reinach, P. S. , Zhang, F. , Vellonen, K. S. , Urtti, A. , Turner, H. , & Wolosin, J. M. (2010). DUSP5 and DUSP6 modulate corneal epithelial cell proliferation. Molecular Vision, 16, 1696–1704. [PMC free article] [PubMed] [Google Scholar]
- Yun, J. , Rago, C. , Cheong, I. , Pagliarini, R. , Angenendt, P. , Rajagopalan, H. , … Papadopoulos, N. (2009). Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science, 325, 1555–1559. 10.1126/science.1174229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , He, X. , Murphy, S. R. , Zhang, H. , Wang, S. , Ge, Y. , … Fan, F. (2019). Knockout of dual‐specificity protein phosphatase 5 protects against hypertension‐induced renal injury. Journal of Pharmacology and Experimental Therapeutics, 370, 206–217. 10.1124/jpet.119.258954 [DOI] [PMC free article] [PubMed] [Google Scholar]


