Abstract
Background
There is a paucity of studies comparing postoperative thromboembolic and major bleeding complications following perioperative interruption of the direct oral anticoagulants (DOACs) and vitamin K antagonists (VKAs).
Objective/Methods
We conducted a retrospective cohort study to compare postoperative thromboembolic and major bleeding outcomes following perioperative interruption of DOACs and VKAs in patients with atrial fibrillation. The primary efficacy and safety outcomes were the 30‐day postoperative rates of arterial thromboembolic events (ATEs) and major bleeding, respectively. The secondary outcomes included the 30‐day rates of clinically relevant nonmajor bleeding (CRNMB) and overall mortality. Thromboembolic, major bleeding, and mortality outcomes were independently adjudicated. Multivariable mixed‐effects logistic‐regression models were used to adjust for potential confounding variables between the DOAC and VKA cohorts.
Results
A total of 325 DOAC patients undergoing 351 procedures and 199 VKA patients undergoing 221 procedures were included. The 30‐day ATE rate was 0.57% (95% confidence interval [CI], 0.27‐0.8) in the DOAC cohort. There were no ATEs in the VKA cohort. The 30‐day rates of major bleeding were 0.57% (95% CI, 0.27‐0.8) and 3.62% (95% CI, 0‐7.3) in the DOAC and the VKA cohorts, respectively. There were significantly more postoperative major bleeding events in the VKA cohort. The 30‐day rate of CRNMB was 4.27% (95% CI, 4.15‐4.42) in the DOAC cohort and 4.52% (95% CI, 3.67‐5.38) in the VKA cohort. There were 2 deaths in the VKA cohort, one of which was deemed to be a fatal nonsurgical bleeding event.
Conclusions
The perioperative interruption of VKAs may be associated with higher postoperative major bleeding rates as compared to DOACs. Careful postoperative reinitiation and monitoring of VKA anticoagulation may be warranted following surgical procedures.
Keywords: anticoagulants, apixaban, atrial fibrillation, dabigatran, perioperative period, postoperative complications, rivaroxaban, warfarin
Essentials.
-
·
Procedural interruption of anticoagulation is associated with thrombotic and bleeding complications.
-
·
We compared postoperative complication rates following interruption of vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs).
-
·
The interruption of VKAs was associated with more postoperative major bleeding as compared to DOACs.
-
·
Close follow‐up is warranted when reinitiating VKA anticoagulation postoperatively.
1. INTRODUCTION
Atrial fibrillation (AF) is a common disorder that is estimated to affect up to 5.6 million patients in the United States by the year 2050.1 Both the direct oral anticoagulants (DOACs) and vitamin K antagonists (VKAs) are used for stroke prevention in AF. It has been estimated that up to 10% of patients on therapeutic anticoagulation undergo periprocedural interruption of their anticoagulation each year.2 Temporary interruption of anticoagulation can be associated with significant morbidity and mortality in the form of thromboembolic and bleeding complications. Real‐world studies have consistently shown higher‐than‐expected postoperative thromboembolic event rates as compared to predicted rates using prorated calculations,2, 3 which involve estimating annual stroke risk using the CHADS2 score, and dividing the value by 365 to obtain an estimated daily risk of stroke. Calculations used to estimate the perioperative stroke risk rely on risk stratification tools that were derived from trial populations,4, 5 which may underestimate the underlying risk of stroke. With regard to bleeding, randomized controlled trials (RCTs),6 post hoc analyses of RCT data,7, 8, 9, 10 and large prospective cohort studies11 have found that major bleeding rates within 30 days of perioperative anticoagulation interruption range from 0.6% to 3% for patients on DOACs11, 12 and from 1% to 8% for patients on VKAs.13 These postoperative 30‐day rates are higher than would be expected based on annual rates of major bleeding.14, 15, 16, 17, 18 Patients receiving perioperative bridging low‐molecular‐weight heparin (LMWH) have been shown to be at particularly high risk of postoperative bleeding.13, 19, 20
Direct oral anticoagulants have a short half‐life and a fast onset of action, both of which confer an ideal pharmacokinetic profile for perioperative use.21 Warfarin, due to its long half‐life, must be interrupted for several days prior to procedures. It has a slow onset of action of several days.22 Thus, patients are often subtherapeutic for 4 to 8 days surrounding the time of the procedure, and physicians may opt to use bridging LMWH in patients at high risk of thrombotic complications.
Despite important differences in perioperative management and pharmacokinetics between DOACs and VKAs, there is a paucity of data comparing perioperative outcomes between DOAC‐ and VKA‐treated patients. We sought to compare postoperative event rates following the perioperative interruption of VKAs and DOACs, using defined perioperative anticoagulation management protocols.
2. METHODS
2.1. Study population
We undertook a single‐center, retrospective cohort study that compared consecutive DOAC‐ or VKA‐treated patients with AF who underwent perioperative anticoagulant interruption for invasive procedures between January 2017 and March 2018. Patients were excluded if anticoagulation was prescribed for an indication other than AF, if the indication for anticoagulation was unclear, if records had inadequate documentation to ascertain details of the perioperative anticoagulation plan, or if the procedure in question was canceled. Patients who underwent a second planned elective procedure during their 30‐day follow‐up period were included to maintain external validity of the study. Data were extracted using a standardized electronic data extraction form and stored in an electronic database. The CHADS2 score was recorded in this study over the CHA2DS2‐Vasc score, given it is more commonly used at our institution and was more frequently recorded on chart documentation. This study was approved by the Ottawa Health Science Network Research Ethics Board.
2.2. Perioperative anticoagulation management
Perioperative VKA interruption was done as suggested by clinical practice guidelines.22 VKAs were discontinued 5 days prior to the procedure, and resumed on the day of the procedure, provided adequate hemostasis was achieved and neuraxial anesthesia had been discontinued. Patients undergoing high‐bleeding‐risk procedures generally have an International Normalized Ratio (INR) checked on preoperative day 1, and if their INR is >1.5, they receive vitamin K orally.23 Patients received a loading dose of VKA (double patient’s home dose) on postoperative days (PODs) 0 and 1, followed by an INR measurement on POD 2, with subsequent VKA dosing based on the INR result. Perioperative bridging with LMWH was used only for patients with CHADS2 scores of 5 to 6 or in patients with stroke within the past 6 months. Perioperative interruption of DOACs was done as suggested by Thrombosis Canada guidelines,24 with anticoagulation held for 3 half‐lives prior to standard bleeding risk procedures and 5 half‐lives for high‐bleeding‐risk procedures. DOACs were resumed approximately 24 hours following standard bleeding risk procedures, and approximately 48 to 72 hours following high‐bleeding‐risk procedures, provided adequate hemostasis was achieved and neuraxial anesthesia had been discontinued. These DOAC perioperative interruption practices are consistent with the protocol used in the PAUSE (Perioperative Anticoagulant Use for Surgery Evaluation) trial.11 If postoperative delays in the resumption of DOACs occurred due to neuraxial anesthesia or ileus, prophylactic doses of LMWH were used until the DOAC could be resumed. Although physicians at our institution usually adhere to the above perioperative interruption protocols, decisions surrounding perioperative anticoagulation management are ultimately left to the discretion of the treating physician if clinical factors require deviation from the above guidelines, as would be the case at most health care institutions. Procedural bleeding risk was determined based on a modified version of a previously published risk‐stratification scheme (Table S1).25 Our risk stratification scheme is consistent with published ISTH guidance.26 All pacemaker/implantable cardioverter‐defibrillator insertions were considered “standard” bleeding risk procedures based on the results of BRUISE CONTROL (Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial) and BRUISE CONTROL‐2.27, 28 All colonoscopies were considered “high” bleeding risk given that it was often unknown at the time of periprocedural planning whether a polypectomy would be performed (ie, screening colonoscopies). All procedures involving neuraxial anesthesia are considered “high” bleeding risk at our institution.
2.3. Study outcomes
Primary outcomes included 30‐day postoperative arterial thromboembolic events (ATE) and major bleeding complication rates. Secondary outcomes included the 30‐day clinically relevant nonmajor bleeding (CRNMB) and mortality rates. ATEs were classified as either ischemic cerebrovascular accidents (CVAs), transient ischemic attacks (TIAs), or systemic embolism. CVA was defined as a focal neurological deficit from a nontraumatic cause, with signs of focal ischemic changes on computed tomography (CT) or magnetic resonance imaging (MRI), or presence of vascular cutoff or visible thrombus on CT angiography (CTA). TIA was defined as a focal neurological deficit from a nontraumatic cause, with no signs of focal ischemic changes on CT or MRI, and the absence of a vascular cutoff or visible thrombus on CTA. Systemic embolism was defined as signs/symptoms of focal ischemia and acute loss of blood flow to a peripheral artery (or arteries) in the affected organ (pain, numbness/paresthesia, pallor, poikilothermia) and the presence of an elevated lactate and/or creatine kinase and/or imaging findings consistent with systemic embolism (angiography, CTA, magnetic resonance angiography). Surgical and nonsurgical major bleeding and CRNMB were defined according to ISTH definitions.29, 30, 31 Outcome events were independently adjudicated by 2 investigators (GLG and MC).
2.4. Statistical analysis
We compared patient demographics between DOACs and VKAs on a per‐patient basis. Dichotomous data are presented as numbers and percentages, while continuous data are expressed as means and standard deviations. P values were calculated using independent t‐Test, chi‐square/Fisher’s exact test where appropriate. Outcome data analysis was done on a per‐interruption basis. All procedural interruptions were included, accounting for random effects, as a patient might have had >1 procedural interruption. We used multivariable mixed‐effects logistic‐regression models adjusting for possible independent confounding variables, including age, CHADS2, renal function, inpatient/outpatient status, and random effects to compare outcomes between DOACs and VKAs. Data were analyzed with the use of SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Study population
Of 1330 patients screened for study eligibility, a total of 524 patients undergoing 572 perioperative interruptions met inclusion criteria and were included in the analysis (Figure S1).
Baseline characteristics of included patients are depicted in Table 1. A total of 325 DOAC patients and 199 VKA patients underwent 351 and 221 periprocedural interruptions, respectively (Table 1). VKA patients had a significantly higher mean age and CHADS2 score. A greater proportion of VKA patients had evidence of renal dysfunction, as evidenced by a creatinine clearance of <60 mL/min. Mean HAS‐BLED score was 2.2 ± 0.9 and 2.4 ± 0.9 for DOAC and VKA patients, respectively (P = .10).
Table 1.
Demographic data
| Characteristic |
DOAC (n = 325) |
VKA (n = 199) |
DOAC vs VKA |
|---|---|---|---|
| Age (y, mean ± SD) | 74.6 ± 8.5 | 76.6 ± 8.5 | P = 0.01 |
| CHADS2 score (mean ± SD) | 2.2 ± 1.3 | 2.5 ± 1.2 | P = 0.001 |
| HASBLED score (mean ± SD) | 2.2 ± 0.9 | 2.4 ± 0.9 | P = 0.10 |
|
DOAC n (%) | |||
| Dabigatran | 70 (19.9) | … | … |
| Rivaroxaban | 127 (36.2) | ||
| Apixaban | 154 (43.9) | ||
| Renal function | |||
| CrCl < 30 mL/min, n (%) | 3 (0.9) | 34 (17.1) | P < 0.001 |
| CrCl 30‐60 mL/min, n (%) | 117 (36.0) | 81 (40.7) | |
| CrCl ≥ 60 mL/min, n (%) | 205 (63.1) | 84 (42.2) | |
| Major bleeding within past 6 mo, n (%) | 7 (2.15) | 2 (1.0) | P = 0.49 |
| Stroke/TIA within past 6 mo, n (%) | 5 (1.5) | 0 (0) | P = 0.16 |
| Concomitant antiplatelet therapy,. n (%) | 31 (9.5) | 23 (11.6) | P = 0.46 |
| Complicating thrombocytopenia (PLAT < 100 × 106/L), n (%) | 3 (0.9) | 6 (3.0) | P = 0.09 |
CrCl, creatinine clearance (as assessed by the eGFR Chronic Kidney Disease Epidemiology Collaboration equation); DOAC, direct oral anticoagulant; PLAT, platelet count; TIA, transient ischemic attack; VKA, vitamin K antagonist.
3.2. Procedures
DOAC patients underwent standard and high‐bleeding‐risk procedures in 44.7% and 55.3% of cases, respectively (Table 2). VKA patients underwent standard and high‐bleeding‐risk procedures in 47.1% and 52.9% of cases, respectively. Patients on DOAC anticoagulation were more likely to have undergone inpatient procedures, whereas patients on VKA anticoagulation were more likely to have undergone plastic surgery.
Table 2.
Procedural characteristics
| Characteristic |
DOAC (n = 351) |
VKA (n = 221) |
DOAC vs VKA |
|---|---|---|---|
| Procedure setting | |||
| Inpatient | 90 (25.6) | 36 (16.3) | P = 0.009 |
| Outpatient | 261 (74.4) | 185 (83.7) | |
| Procedural bleeding risk | |||
| Standard, n (%) | 157 (44.7) | 104 (47.1) | P = 0.60 |
| High, n (%) | 194 (55.3) | 117 (52.9) | |
| Spinal/epidural anesthesia, n (%) | 48 (14.8) | 34 (17.1) | P = 0.48 |
| Endoscopic, n (%)* | 111 (31.6) | 66 (29.9) | P = 0.73 |
| Colonoscopy, n (%) | 79 (22.5) | 44 (19.9) | |
| Esophagogastroduodenoscopy, n (%) | 27 (7.7) | 23 (10.4) | |
| Other, n (%) | 9 (2.6) | 3 (1.4) | |
| Interventional radiology, n (%) | 49 (14.0) | 25 (11.3) | P = 0.39 |
| Cardiac, n (%) | 0 (0) | 1 (0.5) | N/A |
| Dental, n (%) | 5 (1.4) | 1 (0.5) | P = 0.29 |
| ENT, n (%) | 13 (3.7) | 5 (2.3) | P = 0.35 |
| General surgery, n (%) | 41 (11.7) | 19 (8.6) | P = 0.26 |
| Gynecological, n (%) | 8 (2.3) | 4 (1.8) | P = 0.74 |
| Neurosurgical, n (%) | 0 (0) | 2 (0.9) | N/A |
| Ophthalmological, n (%) | 2 (0.6) | 5 (2.3) | P = 0.10 |
| Orthopedic, n (%) | 35 (10.0) | 18 (8.1) | P = 0.60 |
| Plastic surgery, n (%) | 11 (3.1) | 17 (7.7) | P = 0.02 |
| Thoracic surgery, n (%) | 7 (2.0) | 0 (0) | N/A |
| Urological, n (%) | 51 (14.5) | 44 (19.9) | P = 0.19 |
| Cystoscopy | 8 (2.3) | 10 (4.5) | |
| Nephrectomy | 4 (1.1) | 3 (1.4) | |
| Prostate biopsy | 7 (2.0) | 5 (2.3) | |
| Prostatectomy | 3 (0.9) | 1 (0.5) | |
| TURP | 5 (1.4) | 3 (1.4) | |
| TURBT | 15 (4.3) | 12 (5.4) | |
| Other | 9 (2.6) | 10 (4.5) | |
| Vascular surgery, n (%) | 18 (5.1) | 14 (6.3) | P = 0.54 |
DOAC, direct oral anticoagulant; ENT, ear, nose, and throat; N/A, not applicable; TURBT, transurethral resection of bladder tumor; TURP, transurethral resection of prostate; VKA, vitamin K antagonist.
Some patients undergoing endoscopic procedures underwent simultaneous upper and lower endoscopic procedures during the same perioperative interruption. As a result, the number of patients undergoing esophagogastroduodenoscopy and colonoscopy may not add up to the total number of interruptions involving endoscopic procedures.
3.3. Perioperative anticoagulation
In patients undergoing standard bleeding risk procedures, median time (interquartile range) from last dose of DOAC to surgery was 48 hours (36‐60), whereas median time from surgery to first dose of therapeutic DOAC was 24 hours (24‐48). In patients undergoing high‐bleeding‐risk procedures, median time from last dose of DOAC to surgery was 60 hours (46‐60), and median time from surgery to first dose of therapeutic dose was 72 hours (48‐72). Overall, DOACs were discontinued earlier (P = 0.006) and reinitiated later (P < 0.0001) in high‐bleeding‐risk procedures (Figure 1). Postoperative prophylactic dosing of anticoagulation (DOAC or LMWH) was used temporarily in 23.9% of DOAC patients overall. Preoperative interruption of VKAs was similar when comparing patients undergoing standard or high‐bleeding‐risk procedures. Patients on VKAs undergoing high‐bleeding‐risk procedures had delayed resumption of VKA anticoagulation. Patients had their VKA dose temporarily increased (usually doubled) on PODs 0 and 1 in 86.4% of procedures. Overall, parenteral anticoagulation was used in 36.2% of VKA perioperative interruptions, with 12.2% of VKA patients receiving therapeutic dose bridging therapy (Table 3).
Figure 1.
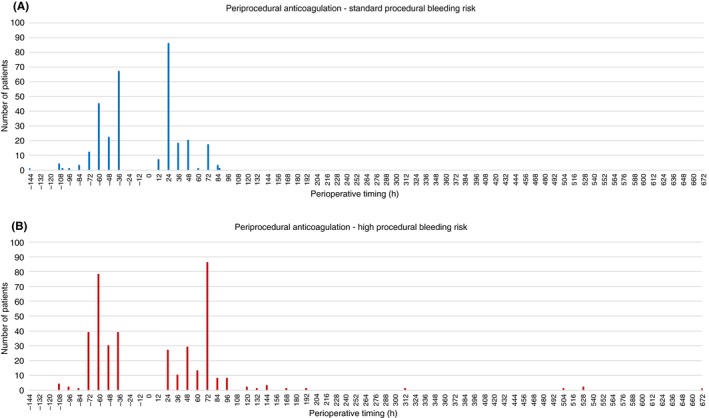
DOAC periprocedural anticoagulation. A, Periprocedural DOAC anticoagulation in patients who underwent standard bleeding risk procedures. Preoperative timing is measured from last dose of DOAC to time of surgery; postoperative timing is measured from time of surgery to first dose of therapeutic DOAC. B, Periprocedural DOAC anticoagulation in patients who underwent high‐bleeding‐risk procedures. Preoperative timing is measured from last dose of DOAC to time of surgery, postoperative timing is measured from time of surgery to first dose of therapeutic DOAC. DOAC, direct oral anticoagulant
Table 3.
VKA perioperative anticoagulation
| Characteristic |
Standard procedural bleeding risk (N = 104) |
High procedural bleeding risk (N = 117) |
Standard risk vs. high risk |
|---|---|---|---|
| VKA (N = 221) | |||
| Time from last dose of VKA to surgery | |||
| POD 4 (%) | 1.0 | 0.9 | P = 0.19 |
| POD 6 (%) | 97.1 | 98.3 | |
| POD 7 (%) | 0 | 0.9 | |
| POD‐8 (%) | 1.9 | 0 | |
| Time from surgery to first dose of therapeutic VKA | |||
| POD 0 (%) | 87.5 | 66.7 | P = 0.001 |
| POD 1 (%) | 9.6 | 20.5 | |
| POD 2 (%) | 1.0 | 4.3 | |
| POD 3 (%) | 1.0 | 4.3 | |
| POD 4 (%) | 0 | 1.7 | |
| POD 5 (%) | 0 | 1.7 | |
| POD 7 (%) | 1.0 | 0 | |
| POD 14 (%) | 0 | 0.9 | |
| VKA dose temporarily increased postoperatively, n (%) | 191 (86.4) | … | |
|
Use of any parenteral anticoagulation, n (% of patients in procedural risk category) |
28 (26.9) | 52 (44.4) | … |
| Therapeutic dose bridging anticoagulation, n (% of patients in procedural risk category) | 13 (12.5) | 14 (12.0) | … |
| CHADS2 score in patients receiving therapeutic bridging, mean (range) | 4.0 (1‐5) | … | |
| Bridging agent | |||
| DOAC | 2 | … | |
| LMWH | 76 | ||
| UFH | 2 | ||
| Mean preoperative INR (range) | 1.3 (1.0‐2.7) | … | |
| Preoperative vitamin K given n (%) | 24 (10.9) | … | |
Abbreviations: DOAC, direct oral anticoagulant; INR, international normalized ratio; LMWH, low‐molecular‐weight heparin; POD, post/preoperative day; UFH, unfractionated heparin; VKA, vitamin K antagonist.
3.4. Study outcomes
There were a total of 2 postoperative ATEs, both in patients on a DOAC, yielding a 30‐day postoperative ATE rate of 0.57% (95% confidence interval [CI], 0.27‐0.87) for the DOAC cohort (Table 4). The first event was a CVA that occurred on POD 22 in a patient on apixaban with a CHADS2 score of 5, and the second event was a popliteal artery thromboembolism that occurred on POD 4 in a patient on dabigatran with a CHADS2 score of 1. Both patients resumed their home DOAC dosing within 36 hours postoperatively. There were no ATEs in the VKA cohort.
Table 4.
Perioperative interruption outcome data
|
30‐day postoperative outcome |
DOAC (n = 351) |
VKA (n = 221) |
DOAC vs. VKA | Multivariable logistic regression (adjusting for age, CHADS2, renal function, inpatient status) |
|---|---|---|---|---|
|
ATE n (%, [95% CI]) |
2 (0.57 [0.27‐0.87]) | 0 | N/A | N/A |
|
MB n (%, [95% CI]) |
2 (0.57 [0.27‐0.87]) | 8 (3.62 [0‐7.3]) | P = 0.02 | P = 0.02 |
|
CRNMB n (%, [95% CI]) |
15 (4.27 [4.15‐4.42]) | 10 (4.52 [3.67‐5.38]) | P = 0.89 | P = 0.92 |
|
Overall Mortality n (%, [95% CI]) |
0 | 2 (0.91 [0.33‐1.48]) | N/A | N/A |
|
VTE n (%, [95% CI]) |
0 | 1 (0.45 [0.16‐0.74]) | N/A | N/A |
Abbreviations: ATE, acute thromboembolic event; CRNMB, clinically relevant nonmajor bleeding; DOAC, direct oral anticoagulant; MB, major bleeding; VKA, vitamin K antagonist; VTE, venous thromboembolic event.
There were a total of 2 major bleeding events in the DOAC cohort over a 30‐day postoperative follow‐up period (0.57%; 95% CI, 0.27‐0.87), whereas 8 major bleeding events occurred in the VKA cohort (3.62%; 95% CI, 0.0‐7.3). There were significantly more postoperative major bleeding events in the VKA cohort as compared to the DOAC cohort (P = 0.0178). This result remained statistically significant after adjustments for age, CHADS2 score, renal function, and inpatient procedure status between the 2 cohorts (P = 0.0232). An overview of each major bleeding event is provided in Table S2. Most major bleeding events occurred among patients undergoing high‐bleeding‐risk procedures. None of the VKA patients with major bleeding received perioperative therapeutic bridging, and the mean INR at the time of presentation with major bleeding was 2.8.
Among all 261 interruptions for standard bleeding risk procedures, 2 major bleeding events occurred (both patients on VKAs), yielding a pooled 30‐day major bleeding rate of 0.77% (95% CI, 0‐1.83). Among all 311 interruptions for high‐bleeding‐risk procedures, 8 major bleeding events occurred (6 in patients on VKAs, 2 in patients on DOACs), yielding a pooled 30‐day major bleeding rate of 2.57% (95% CI, 0.82‐4.33). The major bleeding rate among patients on DOACs undergoing high‐bleeding‐risk procedures was 1.03% (95% CI, 0%‐2.47%). The major bleeding rate among patients on VKAs undergoing standard and high‐bleeding‐risk procedures was 1.92% (95% CI, 0%‐4.61%) and 5.13% (95% CI, 1.16‐9.10), respectively.
There were 15 CRNMB events in the DOAC arm (4.27%; 95% CI, 4.15‐4.42) and 10 CRNMB in the VKA arm (4.52%; 95% CI, 3.67‐5.38) (Table 4).There were 2 deaths in the VKA arm (0.91%; 95% CI, 0.33‐1.48), one of which was attributed to a fatal intracranial hemorrhage on POD 15. The second fatal event occurred due to progression of underlying malignancy, with no evidence of a contribution from major bleeding or ATE.
4. DISCUSSION
Our study assessed postoperative thromboembolic and bleeding complications following perioperative interruption of DOACs and VKAs using a contemporary perioperative interruption protocol. Postoperative major bleeding rates at 30 days were higher in patients on VKAs.
Previous analyses7, 8, 9, 10 have retrospectively assessed postoperative outcomes following anticoagulation interruptions using data from the 4 prospective randomized controlled trials evaluating the efficacy and safety of DOACs in AF.32, 33, 34, 35 These results were pooled in a meta‐analysis,12 and no differences in postoperative major bleeding or ATEs were detected when comparing DOACs to VKAs. However, these studies excluded patients with planned major procedures at study entry, which may have led to selection bias.32, 34, 36 Perioperative anticoagulation instructions provided to local investigators left decisions surrounding bridging anticoagulation and anticoagulation resumption to the treating physician. Bridging anticoagulation was used for patients on DOACs in these studies, and the LMWH dosing (prophylactic vs. therapeutic) used for bridging is unknown.7, 8, 9, 10 Moreover, procedural bleeding risk stratification was not incorporated into periprocedural anticoagulation management protocols in 2 of these studies.34, 35, 36 Thus, the perioperative management of anticoagulation in our VKA and DOAC cohorts may be more reflective of current practice standards.
Patients in both our VKA and DOAC cohorts underwent a wide range of different procedures, and more than half of these procedures were classified as high bleeding risk for patients on both DOACs and VKAs. There was no significant difference in procedural bleeding risk between the VKA and DOAC cohorts, and most procedural subtypes were evenly distributed between the 2 cohorts (Table 2). Our findings with regards to the timing of DOAC discontinuation and reinitiation are consistent with the current recommendation to hold DOACs for 3 and 5 half‐lives prior to standard and high‐bleeding‐risk procedures, respectively. Our results with respect to perioperative interruption intervals are also consistent with the results of the PAUSE study.11 Hence, this interruption regimen seems safe and effective.
There were 2 postoperative thromboembolic events, both in patients on DOACs. One patient on apixaban experienced a posterior circulation stroke on POD 22, while the other patient on dabigatran experienced an acute popliteal artery embolus on POD 4. In the latter case, the last dose of dabigatran had been taken 96 hours preoperatively due to planned neuraxial anesthesia for extensive varicose vein stripping and was resumed 36 hours postoperatively. The postoperative thromboembolic event rate of 0.57% (95% CI, 0.27‐0.87) found in our study is similar to the pooled estimate of 0.41% (95% CI, 0.29‐0.54) identified in our prior meta‐analysis.12 Given that so few postoperative ischemic events occurred, it is not possible to draw any conclusions regarding comparisons between patients on VKAs vs. DOACs.
Our overall rate of major bleeding for patients undergoing standard bleeding risk procedures was 0.77% (95% CI, 0‐1.83), whereas overall rates of major bleeding for all patients undergoing high‐bleeding‐risk procedures was 2.57% (95% CI, 0.82‐4.33). Our major bleeding rates for DOAC patients undergoing high‐bleeding‐risk procedures are consistent with the findings from PAUSE,11 whereas our major bleeding rates of VKA patients undergoing standard and high‐bleeding‐risk procedures are consistent with the findings of the BRIDGE (Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) trial.13
We did find a higher postoperative rate of major bleeding among patients on VKAs. The reasons for this finding could be severalfold. First, only 2 patients had supratherapeutic INRs at the time of the major bleeding events, and the mean INR at the time of the major bleeding events was 2.8. Therefore, inadequate anticoagulation control may have only partially contributed to the observed major bleeding event rate. Second, perioperative therapeutic LMWH bridging anticoagulation has been linked to increased postoperative major bleeding rates among patients on VKAs in several studies.6, 19, 27, 28 Patients on VKAs in our study received some form of parenteral anticoagulation in 26.9% and 44.4% of standard and high‐bleeding‐risk procedures, respectively. However, the vast majority of parenteral anticoagulant use was prophylactically dosed LMWH, usually given as postoperative VTE prophylaxis. Only 12.5% and 12.0% of VKA patients undergoing standard and high‐bleeding‐risk procedures received therapeutic dose‐bridging LMWH, respectively. These were usually patients with CHADS2 = 5‐6 or with stroke within the past 6 months. This practice is consistent with Thrombosis Canada and ISTH guidance.24, 26 None of the patients in the VKA group that experienced a major bleeding event had received therapeutic bridging LMWH heparin, although 1 patient in the VKA cohort with major bleeding (no. 576) had been switched to therapeutic‐dose LMWH due to a PE on POD 3.
Third, postoperative major bleeding rates could have differed due to the different mechanism of action of VKAs (ie, inhibition of factors X, IX, VII, and II and blockade of tissue factor–mediated coagulation, as opposed to the targeted mechanism of action of DOACs). Finally, patients on VKAs and DOACs are often different in several respects. We did find that our VKA cohort had a higher mean age, CHADS2 score, worse renal function, and greater proportion of patients undergoing outpatient procedures. Controlling for these differences using multivariable logistic regression did not significantly change our results and conclusions. Given the low number of major bleeding events, our multivariable logistic regression model may overestimate or underestimate the effects from each variable on the rate of major bleeding. As a result, we cannot rule out the presence of residual confounding. There remains the possibility of confounding by indication. That is, patients on VKAs may have been started on or switched to VKAs vs. DOACs due to higher perceived risk of bleeding, and due to greater availability of reversal agents for VKAs as opposed to DOACs.
The fact still stands that patients on VKA anticoagulation in our study had a postoperative major bleeding rate that was over 6‐fold higher than that of patients on DOACs. The underlying reason for this observation may be of lesser importance than the observation itself that patients on VKA anticoagulation tend to experience more bleeding events postoperatively. Of note, several major bleeding events occurred following urological procedures, both in patients on VKAs and DOACs. Many of these bleeding events involved significant bleeding (eg, entire bladder volume filled with clot). These events often occurred after urological procedures involving extensive mucosal disruption (eg, transurethral resection of prostate or bladder). Close monitoring for postoperative bleeding events may be warranted when reinitiating VKA anticoagulation postoperatively or when anticoagulation is restarted after urological procedures. Rates of CRNMB were similar when comparing the VKA and DOAC groups. Analysis of CRNMB event rates is limited by the retrospective study design and the fact that many of these events may not have been captured as patients may not have presented back to the tertiary care center where they initially underwent their procedure (eg, presented to their primary care physician). This may partially account for the observed difference in major bleeding event rates but the roughly similar CRNMB event rates between the 2 groups.
Our study has several other limitations. This was a retrospective chart review, and thus, our results are only as accurate as the data recorded in our electronic medical record. We cannot rule out the presence of missing data and resultant information bias. Second, although we did try to adjust for all variables that were likely to have an impact on postprocedural bleeding rates, we cannot rule out the possibility of residual confounding as a contributor to the difference observed between our 2 study arms. Third, although patients will often return to the same hospital at which they had their surgery, we cannot rule out the possibility of missed events based on presentation to alternative peripheral hospitals that would not have been captured in our medical records. We attempted to mitigate this bias by reviewing province‐wide bloodwork results and reviewing appropriate hospital records when bloodwork suggested an emergency room visit or hospital admission. Finally, our cohort included patients on chronic anticoagulation exclusively for the indication of AF. The results of our study may not be applicable to other populations, such as patients on chronic anticoagulation for venous thromboembolic disease.
In conclusion, the perioperative interruption of VKA anticoagulation may be associated with higher postoperative major bleeding rates as compared to DOACs. Careful postoperative reinitiation and monitoring of VKA anticoagulation may be warranted following surgical procedures. Physicians might consider arranging close follow‐up for patients on VKAs undergoing surgical intervention, such as an extended period of twice‐weekly INR monitoring to identify and prevent supratherapeutic INR values, as well as a clinic follow‐up visit within a week of surgery. Further study is warranted to determine whether mechanism of anticoagulation has an impact on postprocedural hemostasis.
AUTHOR CONTRIBUTIONS
JRS, MC: study conception and design; JRS: data acquisition; TZ: statistical analysis; JRS, TZ, GLG, JD, MC: interpretation of the data; JRS, GLG, JD, MC: drafting of the manuscript; JRS, TZ, GLG, JD, MC: critical revision of the manuscript for important intellectual content; JRS, TZ, GLG, JD, MC: final approval of the manuscript.
RELATIONSHIP DISCLOSURE
JRS reports research funding from Portola Pharmaceuticals. GLG reports research funding from Portola Pharmaceuticals and personal fees from Bayer, Pfizer, Leo Pharma, Sanofi, and bioMérieux. JD reports consultancy fees from Janssen Research and Development. MC reports research funding from BMS, Pfizer, and Leo Pharma, and personal fees from Bayer, BMS, Pfizer, Leo Pharma, Sanofi and Servier. TZ reports nothing to disclose.
Supporting information
Shaw JR, Zhang T, Le Gal G, Douketis J, Carrier M. Perioperative interruption of direct oral anticoagulants and vitamin K antagonists in patients with atrial fibrillation: A comparative analysis. Res Pract Thromb Haemost. 2020;4:131–140. 10.1002/rth2.12285
Handling Editor: Suzanne Cannegieter
Funding information
Marc Carrier holds a Tier 2 Research Chair in Cancer and Thrombosis from the Department of Medicine at the University of Ottawa. Dr Le Gal holds an Early Researcher Award from the Province of Ontario; a “CP Has Heart” cardiovascular clinician scientist award from the Heart and Stroke Foundation of Ontario; and the Chair on Diagnosis of Venous Thromboembolism from the Department of Medicine at the University of Ottawa.
Contributor Information
Joseph R. Shaw, https://twitter.com/JRand083.
Gregoire Le Gal, https://twitter.com/grelegal.
Marc Carrier, Email: mcarrier@toh.ca, https://twitter.com/MarcCarrier1.
REFERENCES
- 1. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 2. Douketis JD, Berger PB, Dunn AS, Jaffer AK, Spyropoulos AC, Becker RC, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6):299S–339S. [DOI] [PubMed] [Google Scholar]
- 3. Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost. 2010;8:884–90. [DOI] [PubMed] [Google Scholar]
- 4. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–57. [PubMed] [Google Scholar]
- 5. The efficacy of aspirin in patients with atrial fibrillation. Analysis of pooled data from 3 randomized trials. The Atrial Fibrillation Investigators. Arch Intern Med. 1997;157:1237–40. [PubMed] [Google Scholar]
- 6. Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Healey JS, Eikelboom J, Douketis J, Wallentin L, Oldgren J, Yang S, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long‐Term Anticoagulation Therapy (RE‐LY) randomized trial. Circulation. 2012;126:343–8. [DOI] [PubMed] [Google Scholar]
- 8. Sherwood MW, Douketis JD, Patel MR, Piccini JP, Hellkamp AS, Lokhnygina Y, et al. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014;129:1850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia D, Alexander JH, Wallentin L, Wojdyla DM, Thomas L, Hanna M, et al. Management and clinical outcomes in patients treated with apixaban vs warfarin undergoing procedures. Blood. 2014;124:3692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Douketis JD, Murphy SA, Antman EM, Grip LT, Mercuri MF, Ruff CT, et al. Peri‐operative adverse outcomes in patients with atrial fibrillation taking warfarin or edoxaban: analysis of the ENGAGE AF‐TIMI 48 trial. Thromb Haemost. 2018;118:1001–8. [DOI] [PubMed] [Google Scholar]
- 11. Douketis JD, Spyropoulos AC, Duncan J, Carrier M, Le Gal G, Tafur AJ, et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019;179(11):1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shaw JR, Woodfine JD, Douketis J, Schulman S, Carrier M. Perioperative interruption of direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta‐analysis. Res Pract Thromb Haemost. 2018;2:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clark NP, Douketis JD, Hasselblad V, Schulman S, Kindzelski AL, Ortel TL, et al. Predictors of perioperative major bleeding in patients who interrupt warfarin for an elective surgery or procedure: analysis of the BRIDGE trial. Am Heart J. 2018;195:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vinogradova Y, Coupland C, Hill T, Hippisley‐Cox J. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care. BMJ. 2018;362:k2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chai‐Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target‐specific oral anticoagulants: a systematic review and meta‐analysis. Blood. 2014;124:2450–8. [DOI] [PubMed] [Google Scholar]
- 16. Majeed A, Hwang HG, Connolly SJ, Eikelboom JW, Ezekowitz MD, Wallentin L, et al. Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation. 2013;128:2325–32. [DOI] [PubMed] [Google Scholar]
- 17. Hecker J, Marten S, Keller L, Helmert S, Michalski F, Werth S, et al. Effectiveness and safety of rivaroxaban therapy in daily‐care patients with atrial fibrillation. Results from the Dresden NOAC Registry. Thromb Haemost. 2016;115:939–49. [DOI] [PubMed] [Google Scholar]
- 18. Beyer‐Westendorf J, Ebertz F, Förster K, Gelbricht V, Michalski F, Köhler C, et al. Effectiveness and safety of dabigatran therapy in daily‐care patients with atrial fibrillation. Results from the Dresden NOAC Registry. Thromb Haemost. 2015;113:1247–57. [DOI] [PubMed] [Google Scholar]
- 19. Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta‐analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630–9. [DOI] [PubMed] [Google Scholar]
- 20. Dunn AS, Spyropoulos AC, Turpie AG. Bridging therapy in patients on long‐term oral anticoagulants who require surgery: the Prospective Peri‐operative Enoxaparin Cohort Trial (PROSPECT). J Thromb Haemost. 2007;5:2211–8. [DOI] [PubMed] [Google Scholar]
- 21. Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e326S–e350S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kovacs MJ, Kearon C, Rodger M, Anderson DR, Turpie AG, Bates SM, et al. Single‐arm study of bridging therapy with low‐molecular‐weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin. Circulation. 2004;110:1658–63. [DOI] [PubMed] [Google Scholar]
- 24. Thrombosis Canada Clinical Guides NOACs/DOACs : Perioperative management. [Accessed 2019 November 25] Available from https://thrombosiscanada.ca/clinicalguides/#
- 25. Schulman S, Carrier M, Lee AY, Shivakumar S, Blostein M, Spencer FA, et al. Perioperative management of dabigatran: a prospective cohort study. Circulation. 2015;132:167–73. [DOI] [PubMed] [Google Scholar]
- 26. Spyropoulos AC, Al‐Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14:875–85. [DOI] [PubMed] [Google Scholar]
- 27. Birnie DH, Healey JS, Wells GA, Verma A, Tang AS, Krahn AD, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368:2084–93. [DOI] [PubMed] [Google Scholar]
- 28. Birnie DH, Healey JS, Wells GA, Ayala‐Paredes F, Coutu B, Sumner GL, et al. Continued vs. interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thrombo‐embolic events (BRUISE CONTROL‐2). Eur Heart J. 2018;39:3973–9. [DOI] [PubMed] [Google Scholar]
- 29. Schulman S, Kearon C. Haemostasis SoCoAotSaSCotISoTa. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- 30. Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8:202–4. [DOI] [PubMed] [Google Scholar]
- 31. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Anticoagulation SoCo. Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–26. [DOI] [PubMed] [Google Scholar]
- 32. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 33. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 34. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 35. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 36. ROCKET AF Study Investigators . Rivaroxaban‐once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159:340–7.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


