Abstract
Background: Mumie, as an inorganic and semi-solid herbal substance, could be obtained from crevice caves and is used for bone diseases in traditional medicine. This study investigated the effects of this substance on the expression of bone alkaline phosphatase (BALP) enzyme as well as proliferation and mortality rates of MG63 human osteoblast-like cells.
Materials and methods: The MG63 cells were cultured and the effect of 100, 200 and 300 μg/ml of mumie extract on cell viability were compared with zoledronic acid and estradiol valerate as positive controls, as well as with MG63 cells alone as the negative control group. The activity rate of the BALP enzyme was also assessed.
Results: During 48 hours of the study period, the concentrations of 100 and 200μg/ml of mumie extract increased the proliferation rate and decreased the mortality rate of MG63 cells significantly; however, the concentration of 300μg/ml decreased the proliferation rate and increased the mortality rate of the cells. Also, BALP enzyme expression was slightly affected by 100 and 200 μg/ml of mumie extract whilst it was significantly decreased by the concentration of 300 μg/ml.
Conclusion: This study showed that mumie extract has an increasing effect on proliferation rate and a decreasing effect on the mortality rate of osteoblast cells in low concentrations; however, the higher concentrations of this substance could be toxic and effect inversely.
Keywords: Mumie extract, alkaline phosphatase, cell proliferation, osteoblast, MG63
Introduction
One of the main health problems in different societies is osteoporosis, which is characterized by reduction in the bone solidity endangering patients with bone fracture[1]. This disease usually happens due to inefficient and imbalanced activity of two different cell types: osteoblasts and osteoclasts[2]. Some drugs are currently applied for the treatment of this disease, which include bisphosphonates, raloxifenes, tamoxifens, estrogens and progesterone[1].
Nowadays, due to different side effects of chemical-based medications and lower side-effects of herbal plants, trends for application of herbal or non-chemical-based drugs are increasing. One of the natural substances used in traditional medicine is mumie, which has a 3000 years history in Iran, Kyrgyzstan, Russia, Altai Mountains, Kazakhstan, Mongolia and India[3]. Asian mumie is found inside the stone layers and cave walls in the higher mountains[3]. About 60-80% of mumie is made by humus and other components of this substance including aromatic carboxylic acid, benzoic acid, fatty acids, ellagic acid, hippuric acid sterol, 3, 4-benzocoumarins, phenolic lipids, ichthyol, triterpenes, resin and amino acids[4]. In addition, chemical composition of Asian mumie includes 20% inorganic substances, 15% proteins, 5% lipids, 5% steroids, alkaloids, amino acids and carbohydrates[3,5]. Although there are no accurate confirmations about the formation method of mumie, several hypotheses have been suggested about the origin of this substance. The primary hypothesis shows that the main component of mumie is humus – the characteristic constituents of soil – as well as other organic components and this pale brown to blackish-brown substance resulted from plants decomposition, which passes from stones' layers as a viscose juice during summer[6]. One of the plants that is thought to be among the most likely sources of mumie is Trifoleumrepens,which is in the vicinity of mumie bearing rocks[6]. Mumie has other names like mummiyo, Shilajit, mineral pitch, asphaltum, and vegetable asphalt (common names)[7] and has been traditionally used for many diseases including osteoporosis[8]. The Persian name of this substance is Momiai- faqurul- yahud[9].
Bone-specific alkaline phosphatase (BALP), which is produced by osteoblast cells[10], has been recognized as an important marker for bone formation during bone diseases such as osteoporosis[11]. Oxidative stress is a consequence of disharmony between reactive oxygen species (ROS) and antioxidant factors in the human body[12,13]. In fact, an increase in the free radicals and a decrease in the antioxidants levels would cause some destructive phenomena which result in the damage and mortality of cells[13].
Some studies have shown the effects of this sustance on bone healing, cell proliferation and cell toxicity[14,15]. The current study aimed to investigate the effects of different dosages of mumie extract on the expression of BALP enzyme, the proliferation and mortality rate of MG63 cells, as well as to find the different components of mumie via HPLC.
Materials and methods
Mumie extract preparation
The mumie substance (Brown-black and bitter) was collected from mountains of Ilam province, confirmed by Dr. Gholamreza Amin (Pharmacology Department, Tehran University of Medical Sciences) and powdered by grinder. The extraction process was performed via the Soxhlet method with 250 ml of 50% water and 50% alcohol 99% (solvent) in round bottom flasks at 80°C temperature for 3:45 hours[16]. Rotary evaporator (IKA RV 10 digital) was used to eliminate the surplus water and alcohol (60 minutes with 60°C temperature). The extract was dried inside 40°C oven (120 minutes). Considering the 40 g mumie used at the start, about 13.4 g (33.5%) extract was obtained. Firstly, the stock solution was prepared from the dried extract obtained in water and alcohol solvents. It was then diluted as one to one hundred volumes to reduce the toxicity of alcohol by DMEM medium used in the culture medium with FBS. Different concentrations were then prepared and used for the treatment of cellular plates.
Isolation and identification of the Luteolin by HPLC
Based on a previously reported procedure, high-performance liquid chromatography (HPLC) method was used to identify the components of mumie extract[17]. Via a Knauer liquid chromatography (Platinblue; Knauer, Berlin, Germany), equipped with ultraviolet detector (MW1, Platinblue; Knauer) and a reverse-phase C18 column (HPLC Column, 100x3nm Eurospher II c1803um) applying isocratic elution with UV absorbance detection, a simple and reproducible reversed-phase HPLC was developed and validated for the detection of Luteolin, a constituent of the mumie extract. A solvent containing methanol (A) and water containing 0.1% formic acid (B) were used as the solvent system. A gradient time program from 0- 60 min (B ratio ranging from 5-70%) was applied. Column temperature (250C), mobile phase flow rate (1 mL/min), injection volume (1 µL), and detection wavelength (348 nm) were selected. Luteolin standard, dissolved in methanol, were run in similar conditions and 250mg of dried extracts were dissolved in 10 ml HPLC-grade methanol, sonicated for 15 min, filtered through a 0.22µm syringe filter and further diluted to 5 mg/ml. The peaks obtained for Trifoliumrepens, one of the main components of mumie extract, were compared to that of the Luteolin standard. A stock solution of the Luteolin standard was prepared at 0.1 mg/ml in HPLC-grade methanol, filtered through a 0.22µm syringe filter, and further diluted in the same solvent to obtain 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 12. 5, 25 and 50 µg/ml concentrations.
Cell culture
The MG63 human-like osteoblast cells were provided from National Cell Bank of Iran (NCBI) (c-555) at Pasteur Institute. The osteoblast cells were cultured in DMEM (Dulbecco’s modified Eagle’s medium) (Gibco), 10% FBS (fetal bovine serum) (Gibco), streptomycin 100μg/mL and penicillin 100U/mL inside the 25 cm2 flasks and incubated at 37° C in 5% CO2. The culture medium was changed every 3 days and cells were detached using Trypsin-EDTA 0.25% (Gibco) during the second passage. To choose the appropriate concentrations of the mumie extract, a range of dose- response concentrations were obtained and the concentrations of 100-200 and 300 μg/ml were selected. The MG63 cells were seeded inside the 96 multi-well plates and divided into 6 groups including 100, 200 and 300 μg/ml of mumie ethanol extract groups (experimental groups), estradiol valerate group 1mg/ml (positive control), zoledronic acid group 1mg/ml (positive control) and a group of sole MG63 cells (negative control). DMEM medium with FBS dissolved in water-alcohol solution and antibiotics was also used as negative control (5ul (1000 * 1% * 50%) alcohol per ml DMEM medium). Cells were incubated at 37° C in 5% CO2 for 24-72 hours and the 48-hours time was considered as the preferred time for data interpretation. Following the preparation of the extract concentrations as micrograms per ml, one ml of the concentrations was added to each well as the treatment.
Cell proliferation
The effects of different concentrations of the mumie extract, estradiol valerate, and zoledronic acid on MG63 cell proliferation rate were compared with the negative control group via the MTT assay [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] (Sigma Aldrich). The MG63 cells were seeded inside 96 multi-wells as 50,000 cells/well. A total of 20 μL (5 mg/mL) of the MTT reagent was added to each well and incubated at 37° C in 5% CO2 for 4 hours, after which the surplus liquid was removed and 150μL of dimethyl sulfoxide (DMSO) (Sigma Aldrich) was added to each well. The cell viability inside each well was then checked by reading the absorbance of each well at 570 nm, using ELISA microplate reader (Biotek- ELX 800)[18].
Reactive oxygen species (ROS) formation
The ROS formation rate among either treatment or positive control groups was measured and compared against the negative control group, after an incubation period of 45 minutes by the addition of 10µM of fluorescent probe 2′,7′ -dichlorofluorescein diacetate (DCFH2-DA), (Sigma Aldrich) to each cellular well. The absorbance of each plate was then measured at 485-520 nm by fluorescent microplate reader (Biotek- FLX 800). The ROS formation rate was obtained based on the standard curve of the H202 (10-200 Nm) production[19].
Bone-specific alkaline phosphatase (BALP)
The activity of BALP enzyme was evaluated by the Elisa kit (Bioassay Technology Laboratory, China). A total of 50,000 MG63 cells/well were seeded inside 96 multi-wells. Following the treatment with different concentrations of mumie extract as well as zoledronic acid and estradiol valerate as positive controls, the cells were incubated at 37° C in 5% CO2 for 48 hours. After the incubation time, the surplus liquid was removed and 30µL para-nitrophenyl phosphate was exposed to cells for 30 minutes. After the addition of 30µL of 0.5 N NaOH to each well, the de-allocation reaction of p-nitrophenyl from p-nitrophenyl phosphate was evaluated by measuring the wavelength at 405 nm via Elisa microplate reader (Biotek- ELX 800)[18].
Statistical analysis
The experiments were repeated 8 times for MTT and ROS analysis and 3 times for ALP analysis. Data analysis was performed via SPSS 20 and independent T test and ANOVA were applied for comparison between different groups. Descriptive data were indicated as mean ±SD and via figures. P values less than 0.05 were considered as significant.
Results
Extraction and identification
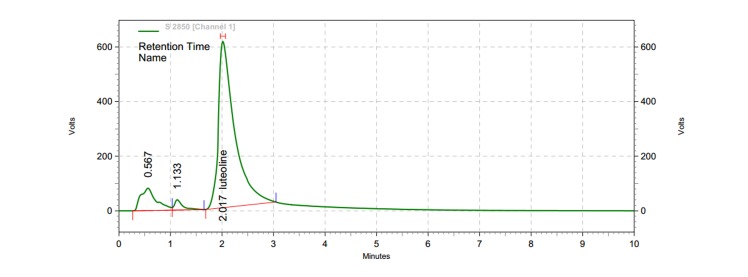
A broad peak with a retention time of 2.1 min, at a wavelength of 348 nm, was identified as the Luteolin standard by HPLC chromatogram. Under similar run conditions, HPLC chromatogram of Trifoliumrepens methanolic extract showed a peak of 2.017 corresponding to that of Luteolin standard (Figure 1).
Figure 1: HPLC chromatogram of Trifoliumrepens extract. HPLC conditions were similar for both Luteolin and Trifoliumrepens extract.
Cell proliferation
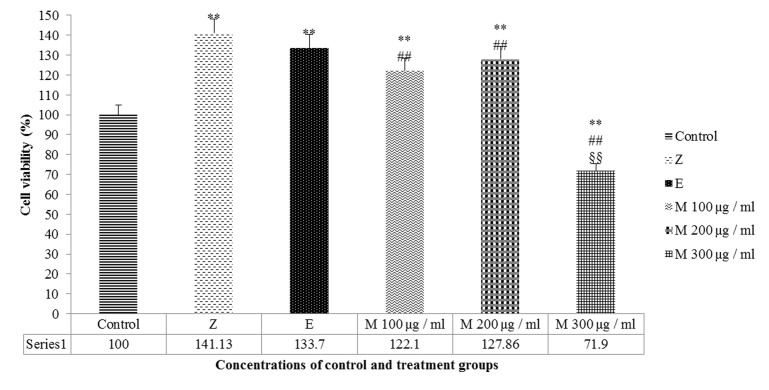
Different dosages of the mumie extract were compared with the negative and positive control groups, to find the best dosage associated with higher cell proliferation rate. In comparison with the negative control group, MG63 cell proliferation was significantly increased among the groups of 100 and 200μg/ml of mumie extract (p=0.003 and p=0.004, respectively); whilst it was significantly decreased in the group of 300μg/ml mumie concentration (p=0.004). Both zoledronic acid and estradiol valerate, as positive control groups, increased the MG63 cell proliferation rate significantly (p=0.001, p=0.008 respectively) and their increasing rates were significant (p<0.05), compared to the 200 μg/ml mumie extract and insignificant (p>0.05) compared to the 100μg/ml mumie concentration (Figure 2).
Figure 2: MG63 cell proliferation rate after 48 hour via treatment with concentrations of mumie extract (M100-M300 µg/ml), zoledronic acid (Z) and Estradiol Valerate (E) compared with negative control group. ** P<0.01 vs. negative control, ## P<0.01 vs. Zoledronic acid (positive control), §§ P<0.01 vs. Estradiol Valerate (positive control).
Reactive oxygen species formation (ROS)
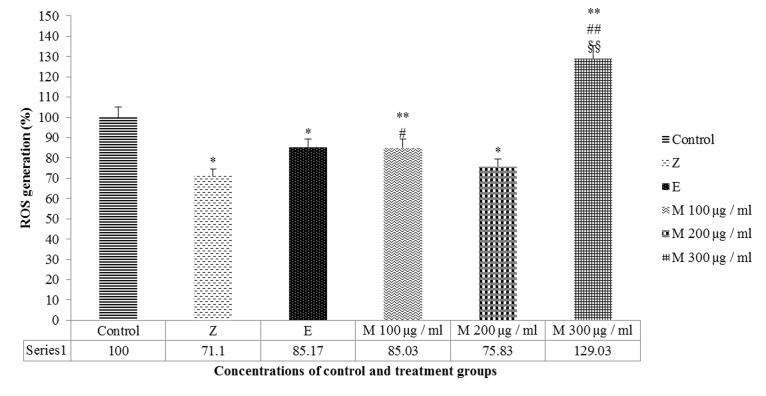
Different dosages of mumie extract were applied to reveal the suitable dosage of this substance with the lowest ROS formation rate in comparison with either negative and positive control groups. The results showed that ROS was decreased in the 100 and 200 μg/ml mumie groups after 48 hours significantly (p=0.009, p=0.013 respectively) and the effect rate of 100 μg/ml mumie showed a higher rate compared to 200 μg/ml, which was similar to the effect of estradiol valerate as positive control (p=0.978). Zoledronic acid also reduced the ROS level similar to the effect of 200 μg/ml mumie extract (p=0.414). In comparison with either negative or positive control groups, ROS was increased in 300 μg/ml mumie group significantly (p=0.003) (Figure 3).
Figure 3: Mean ROS formation of MG63 after 48 hours' treatment with mumie extract (M100-M300 µg/ml), zoledronic acid (Z) and estradiol valerate (E) compared with negative control group. ** P<0.01 vs. negative control, * P<0.05 vs. Negative control, ## P<0.01 vs. Zoledronic acid (positive control), # P<0.05 vs. Zoledronic acid (positive control), §§ P<0.01 vs. Estradiol Valerate (positive control).
Bone-specific alkaline phosphatase (BALP)
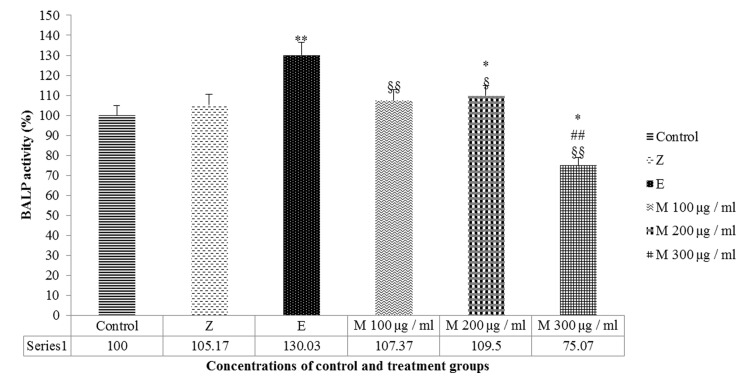
Different dosages of mumie extract were compared with negative and positive control groups to reveal the toxic dosage of this substance based on the BALPactivity rate. Compared with the negative control, the mumie extract insignificantly increased the BALP activity at 100 μg/ml (p=0.057) but significantly increased the enzyme activity at 200 μg/ml (p=0.02); however, these increases were insignificant (p>0.05) in comparison with zoledronic acid and significant compared to estradiol valerate (p<0.05), respectively. Mumie extract at 300 μg/ml significantly decreased the BALP activity (p<0.05) in comparison with either negative or positive controls (Figure 4). It can be assumed that any dosage equal or higher than300 μg/ml could be toxic for cells and the best protective dosage would probably be 200 μg/ml.
Figure 4: BALP activity after 48 hours' treatment with mumie extract (M100-M300 µg/ml), zoledronic acid (Z) and estradiol valerate (E) compared with negative control group. ** P<0.01 vs. negative control, * P<0.05 vs. Negative control, ## P<0.01 vs. Zoledronic acid (positive control), §§ P<0.01 vs. Estradiol Valerate (positive control), § P<0.05 vs. Estradiol Valerate (positive control).
Discussion
Osteoporosis happens in two different conditions: first, when the speed of bone formation (by osteoblast cells) is less than the speed of bone decomposition (by osteoclast cells), similar to what happens among women during their postmenopausal period due to serum estrogen reduction; second, when less bone forms during the aging process that takes place among both genders. In addition, there are secondary reasons for osteoporosis such as cancers, AIDS, hyperthyroidism and diabetes[1]. Bisphosphonates, calcitonin, estrogens, teriparatide and selective estrogen receptor modulators are among medications applied for prevention or treatment of osteoporosis[20]. Zoledronic acid is a third-generation bisphosphonate[21] and its mechanism of action is via inhibiting the osteoclasts activities, but may also increase the induction of osteoblast differentiation[22]. A meta-analysis study reported that hormone replacement therapy (HRT) had a beneficial effect on bone density among postmenopausal women[23]. Estradiol valerate, which was used as a positive control in the current study, is among these medications. The osteoblast-like cells, provided from human osteosarcoma cells (MG63), have the following advantages: no interspecies differences, similarity to human integrin subunits profile, unlimited number of cells, and hormonal administration response similar to human osteoblast cells[24]. Therefore, due to difficulties in supplying human mesenchymal stem cells, application of osteoblast-like MG63 cells seems to be appropriate for relevant experiments.
One of the main components of mumie is Trifoliumrepens[6], which under HPLC chromatogram of its methanolic extract, Luteolin was identified as the most prevalent components compared to its other constituents. A common flavonoid that could be isolated from many types of plants including herbal plants is Luteolin[25]. Flavonoids have a lowering effect on urinary excretion of calcium and phosphate and can increase the activity of osteoblasts. They also decrease the activity of osteoclasts, and help to keep of trabecular thickness[26]. Abbasi et al reported that lower concentrations of Luteolin (EC 50, 1.29±0.23 µM) inhibited the ROS generation, reduced the alkaline phosphatase activity and cell death due to high glucose. On the other hands, higher concentrations of Luteolin has been reported to cause osteoblast cells death in both normal and high glucose states (IC50, 34±2.33 and 27±2.42 µM, respectively), as represented by increased ROS and decreased alkaline phosphatase activity[18].
In the current study, the proliferation rate of MG63 cells was increased and the ROS formation rate was decreased among the 100 and 200μg/ml mumie extract groups (low concentrations), whilst the higher concentration of this substance was toxic (300 μg/ml mumie) in comparison with the negative control group. A study has indicated that treating human mesenchymal cells with 0.5-5 μg/ml of ethanol extract of Galbanum plant's root for 48-72 hours would significantly increase the proliferation rate of the mesenchymal cells, with the maximum proliferation rate seen at 1 μg/ml of this extract[27]. In addition, Muravyeva and co-workers treated preosteoblast-like cells) MC3T3-E1 (with 10-6,10-8,10-10,10-12 of ferutinin [a substance from Ferula hermonis (Umbelliferae family) with estrogenic effects and 10-8 of E2 (17β-estradiol) and reported that after 48 hours, concentrations of 10-10 and 10-12 of ferutinin and 10-8 of E2 significantly increased cell proliferation, compared to the concentrations of 10-6 and 10-8 of E2 and the control group[28]. Furthermore, a study from Saudi Arabia revealed that mumie extract at concentrations of 0.8, 1.2 and 1.6 mg/ml significantly increased the MG63 cell proliferation after 24 hours[14]. The WST-1 method was used for the measurement of proliferation rate in the mentioned study. This study also evaluated the toxicity of 0.4-2.8 mg/ml concentrations of mumie extract on MG63 cells via the LDH method and revealed a significant reduction in cell toxicity after 24 hours treatment[14]. In another study, Jung and others investigated the effects of mumie extract, at doses of 0.2-5 μg /ml, on mesenchymal stem cells via the MTT method and reported lack of cell toxicity at this dose range after 14 days[15].
Alkaline phosphatase is the first expressed enzyme during bone cell differentiation[29]. This enzyme is secreted by constructive bone cells[30]. The current study showed an increase in the ALP expression rate at concentrations of 100 and 200μg/ml of mumie extract and a decrease in the ALP expression rate at 300 μg/ml mumie concentration. A study by Luo and colleagues reported that esteriol had the highest effect on MG63 cell proliferation at a concentration of 10-8 M and E2 and was more effective than esteriol at this dose; however, esteriol and E2, compared with the control group, did not show any effects on the ALP activity at the dose ranges of 1x10-6 - 1x10-10 M (after 48 hours)[31]. Mahmoudi and others investigated the effects of Galbanum root's extract on osteoblast differentiation of mesenchymal stem cells, by assessing the Alp marker, and compared the results with E2 as the positive control group. Their results showed that the 1-10μg/ml doses of the extract significantly increased the ALP activity in days 7 and 14 and the highest effect was seen at 1 μg/ml in day 14, which showed a similar effect to E2[27]. Another study reported that the mumie extract, collected from Uzbekistan's mountains, increased the expression of the ALP enzyme among differentiated osteoblast cells of mesenchymal stem cells at doses of 4-5 μg/ml after 14 days of treatment[15].
The present study, demonstrated that the mumie extract increases the proliferation and decreases the mortality rate of the MG63 osteoblast cells at lower concentrations; however, the higher concentrations of the substance are toxic. In addition, increasing the proliferation rate of the osteoblast cells would increase the speed of bone formation. Therefore, this natural substance might be a useful medication for the treatment of bone diseases such as osteoporosis in which the human body is unable to maintain and substitute the bone mass during bone damages. The human application of this substance needs further animal and clinical trial studies.
Acknowledgments
We gratefully thank the Faculty of Medicine and Vice Chancellor of Researches and technology of Ilam University of Medical Sciences for their valuable helps on this study. This work was financially supported by Ilam University of Medical Sciences.
Glossary
Abbreviations
- BALP:
Bone alkaline phosphatase
- ROS:
Reactive oxygen species
- HPLC:
High-performance liquid chromatography
- MG-63:
Human Bone Osteosarcoma Cells
- UV:
Ultraviolet
- NCBI:
National Cell Bank of Iran
- DMEM:
Dulbecco's Modified Eagle Medium
- FBS:
Fetal bovine serum
- DMSO:
Dimethyl sulfoxide
- ANOVA:
Analysis of variance
- HRT:
Hormone replacement therapy
Potential Conflicts of Interests
None
Ethical statement.
This study was approved by the ethics committee of Ilam University of Medical Sciences and is in accordance with national declaration of ethics in researches.
References
- 1.Radfar M, Hamishehkar H, Entezari T. Osteoporosis. Razi. 2012;23((11)):18–28. [Google Scholar]
- 2.Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of Bone Tissue: Structure, Function and Factors That Influence Bone Cells. Biomed Res Int. 2015;(2015):421746. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiello A, Fattorusso E, Menna M, Vitalone R, Schrõder HC, Mõller WE. Mumijo Traditional Medicine: Fossil Deposits from Antarctica (Chemical Composition and Beneficial Bioactivity) Evid Based Complement Alternat Med. 2011;2011((3)):738131. doi: 10.1093/ecam/nen072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal S, Anwer K, Khanna R, Ali A, Sultana Y. Humic acid from Shilajit õ a physico-chemical and spectroscopic characterization. J Serb Chem Soc. 2010;75((3)):413–22. [Google Scholar]
- 5.Garedew A, Feist M, Schmolz E, Lamprecht I. Thermal analysis of mumiyo, the legendary folk remedy from the Himalaya region. Thermochim Acta. 2004;417((2)):301–9. [Google Scholar]
- 6.Agarwal SP, Khanna R, Karmarkar R, Anwer MK. R.K K. Shilajit: a review. Phytother Res. 2007;21((5)):401–5. doi: 10.1002/ptr.2100. [DOI] [PubMed] [Google Scholar]
- 7.Schepetkin I, Khlebnikov A, Kwon BS. Medical Drugs From Humus Matter: Focus on Mumie. Drug Dev Res. 2002;57((3)):140–59. [Google Scholar]
- 8.Yin H, Yang EJ, Park SJ, Han SK. Glycine- and GABA-mimetic Actions of Shilajit on the Substantia Gelatinosa Neurons of the Trigeminal Subnucleus Caudalis in Mice. Korean J Physiol Pharmacol. 2011;15((5)):285–9. doi: 10.4196/kjpp.2011.15.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson E, Rajamanickam GV, Dubey GP, Klose P, Musial F, Saha F J, Michalsen A, Dobos GJ. Review on shilajit used in traditional Indian medicine. J Ethnopharmacol. 2011;136((1)):1–9. doi: 10.1016/j.jep.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 10.Masrour Roudsari J, Mahjoub S. Quantification and comparison of bone-specific alkaline phosphatase with two methodsin normal and pagetõs specimens. Caspian J Intern Med. 2012;3((3)):478–83. [PMC free article] [PubMed] [Google Scholar]
- 11.Avbersek-Luznik I, Gmeiner Stopar T, Marc J. Activity or mass concentration of bone-specific alkaline phosphatase as a marker of bone formation. Clin Chem Lab Med. 2007;45((8)):1014–8. doi: 10.1515/CCLM.2007.186. [DOI] [PubMed] [Google Scholar]
- 12.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24((5)):981–90. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Med Sci Monit. 2004;10((6)):RA141–7. [PubMed] [Google Scholar]
- 14.Yousef Labban N Shilajit. A Novel Regulator of Bone/Cartilage Healing. Indiana University. 2013. pp. 56–61.
- 15.Jung CR, Schepetkin IA, Woo SB, Khlebnikov AI, Kwon BS. Osteoblastic Differentiation of Mesenchymal Stem Cells by Mumie Extract. Drug Dev Res. 2002;57((3)):122–33. [Google Scholar]
- 16.Lee NY, Mohd-Setapar SH, Mohd Sharif NS, Ahmad A, Khatoon A, Mohd Azizi CY. Mohd II. Extraction of Rubber (Hevea brasiliensis) Seed Oil using Supercritical Carbon Dioxide and Soxhlet Extraction. Res J Chem Environ. 2013;17((10)):46–52. [Google Scholar]
- 17.Lv Q, Yu A, Xi Y, Li H, Song Z, Cui J, Cao F, Zhai G. Development and evaluation of penciclovir-loaded solid lipid nanoparticles for topical delivery. Int J Pharm. 2009;372((1-2)):191–8. doi: 10.1016/j.ijpharm.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Abbasi N, Khosravi A, Aidy A, Shafiei M. Biphasic Response to Luteolin in MG-63 Osteoblast-Like Cells under High Glucose-Induced Oxidative Stress. Iran J Med Sci. 2016;41((2)):118–25. [PMC free article] [PubMed] [Google Scholar]
- 19.Abbasi N, Akhavan MM, Rahbar-Roshandel N, Shafiei M. The effect of low and high concentrations of Luteolin on cultured human endothelial cells under normal ang glucotoxic conditions: involvement of Integrin-Linked Kinase and Cyclooxygenase-2. Phytother Res. 2014;28((9)):1301–7. doi: 10.1002/ptr.5128. [DOI] [PubMed] [Google Scholar]
- 20.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18((8)):1023–31. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 21.Horie N, Murata H, Kimura S, Takeshita H, Sakabe T, Matsui T, Maekawa T, Kubo T, Fushiki S. Combined effects of a third-generation bisphosphonate,zoledronic acid with other anticancer agents against murine osteosarcoma. Br J Cancer. 2007;96((2)):255–61. doi: 10.1038/sj.bjc.6603548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambrinoudaki I, Vlachou S, Galapi F, Papadimitriou D, Papadias K. Once-yearly zoledronic acid in the prevention of osteoporotic bone fractures in postmenopausal women. Clin Interv Aging. 2008;3((3)):445–51. doi: 10.2147/cia.s2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells G, Tugwell P, Shea B, Guyatt G, Peterson J, Zytaruk N, Robinson V, Henry D, O'Connell D, Keranny A. Meta-Analysis of the Efficacy of Hormone ReplacementTherapy in Treating and Preventing Osteoporosis in Postmenopausal Women. Endocr Rev. 2002;23((4)):529–39. doi: 10.1210/er.2001-5002. [DOI] [PubMed] [Google Scholar]
- 24.Czekanska EM, Stoddart MJ, Richards RG, Hayes JS. In search of an osteoblast Cell model for in vitro research. Eur Cell Mater. 2012;24:1–17. doi: 10.22203/ecm.v024a01. [DOI] [PubMed] [Google Scholar]
- 25.Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8((7)):634–46. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Mamun MA, Hosen MJ, Islam K, Khatun A, Alam MM, Al-Bari MA. Tridax procumbens flavonoids promote osteoblast differentiation and bone formation. Biol Res. 2015;48:65. doi: 10.1186/s40659-015-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Mahmoudi Z, Soleimani M, Saidi A, Iranshahi M, Azizsoltanli A. Effect of Ferula gummosa Ethanolic Extract on Osteogenesisin Human Mesenchymal Stem Cells. J Med Plants. 2013;2((46)):50–9. [Google Scholar]
- 28.Muravyeva Y. Does Ferutinin Dose-Dependently Increase Nodule Formation in TNF-Alpha Activated MC3T3-E1 Preosteoblast-like Cells? Florida State University. 2011. pp. 1–42.
- 29.Golub Ellis E, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 2007;18((5)):444–8. [Google Scholar]
- 30.Gomez BJr, Ardakani S, Ju J, Jenkins D, Cerelli MJ, Daniloff GY, Kung VT. Monoclonal antibody assay for measuring bone-specific alkaline phosphatase activity in serum. Clin Chem. 1995;41((11)):1560–6. [PubMed] [Google Scholar]
- 31.Luo XH, Liao E, Y. Effect of Estriol on the proliferation and differentiation of human osteoblastic MG63 cells. Endocr Res. 2003;29((3)):343–51. doi: 10.1081/erc-120025041. [DOI] [PubMed] [Google Scholar]