Abstract
Histoplasma capsulatum is typically an indolent disease among immunocompetent patients. However, immunocompromised patients, such as solid organ transplant recipients, are at risk of developing severe histoplasmosis. Yet post-transplant histoplasmosis is a rare pathology, representing less than five percent of invasive fungal infections among transplant recipients. Furthermore, patients tend to present with nonspecific clinical symptoms, complicating timely diagnosis and delaying treatment. Disease features that may be more representative of H. capsulatum infection, such as anemia, leukopenia and pulmonary involvement are often not present until late in the disease course, when the patient is at greater risk of decompensation. Unlike H. capsulatum infections among immunocompetent hosts, extrapulmonary infection among immunocompromised hosts is more the rule than the exception. Treatment with liposomal amphotericin B followed by oral itraconazole is the standard therapy, but special considerations must be made for patients with hepatic and/or renal insufficiency, underlying cardiac abnormalities or malabsorptive pathologies and doses of immunosuppressants will need to be adjusted for drug interactions. Herein we present a case of H. capsulatum infection presenting with generalized lymphadenopathy post-renal transplant.
Abbreviations: CT, computed tomography; IDSA, Infectious Disease Society of America; IFI, invasive fungal infection; PET, positron emission tomography; post-op, post-operative; PTH, post-transplant histoplasmosis; SOT, solid organ transplant
Keywords: Infectious disease, Histoplasma, Transplant, Fungal infection
Introduction
Histoplasmosis is an uncommon invasive fungal infection (IFI) in solid organ transplant (SOT) recipients [1]. Kidney transplant recipients have the highest incidence of histoplasma infection compared to other SOT recipients [2]. The disease can present secondary to acute primary inoculation via environmental exposure, donor-based transmission or reactivation of latent infection [[3], [4], [5]]. Early diagnosis can be challenging due to the rarity of this entity and a non-specific clinical presentation [2]. Disseminated disease and pulmonary involvement are the most common findings, while lymphatic involvement is rare [[6], [7], [8]]. To our knowledge, histoplasmosis presenting as generalized lymphadenopathy in a SOT recipient has not been previously described in the literature.
Case report
A 31-year-old Caucasian woman with end-stage renal disease secondary to biopsy-proven familial IgA nephropathy underwent directed deceased donor renal transplant at our institution, the donor was a middle-aged man who died of a stroke; she was discharged on post-operative day five. Basiliximab was used for immunosuppressive induction therapy and immunosuppressive maintenance therapy included tacrolimus, mycophenolate mofetil and prednisone. Of note, both she and the donor were Epstein-Barr virus seropositive. Pre-transplant tuberculosis screening was negative. No screening was done for histoplasmosis or blastomycosis. Her post-operative course was complicated by a lymphocele successfully drained percutaneously.
She continued outpatient follow-up uneventfully for ten months until she began to experience fatigue, back pain, night sweats and decreased appetite. On exam, she had cervical lymphadenopathy but no organomegaly nor integumentary findings. Pertinent lab findings included white blood cells of 3.4x103/uL, platelets of 91x103/uL and uric acid of 7.8 mg/dL. A computed tomography (CT) scan of her abdomen demonstrated numerous, retrocrural, retroperitoneal and mesenteric lymphadenopathy, with the largest node measuring 2.9 × 2.3 cm. Positron emission tomography (PET) scan demonstrated enlarged, hypermetabolic cervical, mediastinal, retroperitoneal and mesenteric lymph nodes concerning for post-transplant lymphoproliferative disease (Fig. 1). Additionally, her creatinine was elevated to 2.0 mg/dL at this time, but later fell to 1.5 mg/dL with intravenous fluids. A bone marrow biopsy was performed, and she was admitted for planned cervical lymph node biopsy and allograft renal biopsy. A cervical node biopsy revealed non-necrotizing, granulomatous lymphadenopathy with positive fungal stain; suggestive of histoplasmosis (Figs. 2A, B, 3 A and B). Allograft renal biopsy revealed inflammation and granulomatous architecture, consistent with disseminated histoplasmosis and histoplasma antigen test was positive. Bone marrow biopsy showed no evidence of lymphoproliferative disease. Treatment with liposomal amphotericin B was initiated and the patient experienced an increase in creatinine over the therapeutic course (from 1.3 mg/dL to 1.8 mg/dL over four days, baseline creatinine of 1.1 mg/dL), however this stabilized during her hospital stay and reduced to baseline after discharge. Her immunosuppressive therapy was reduced to low-dose tacrolimus and prednisone. She was discharged seven days after her initial presentation and converted to oral itraconazole as an outpatient.
Fig. 1.
Co-registered PET-CT scan demonstrating hypermetabolic cervical, supraclavicular, mediastinal and mesenteric lymph nodes suspect for post-transplant lymphoproliferative disorder.
Fig. 2.
A (left) and 2B (right): Hematoxylin and eosin stain x100 (A) and x400 (B) of lymph node biopsy demonstrating granulomatous inflammation within lymph node architecture.
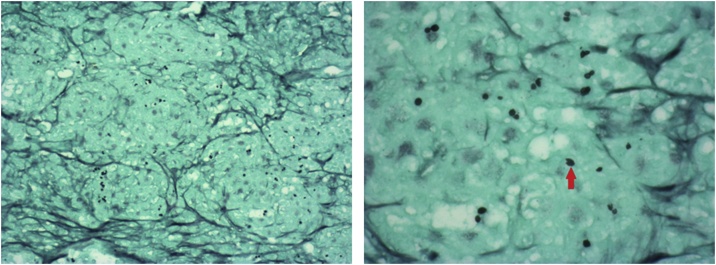
Fig. 3.
A (left) and 3B (right): Grocott’s methenamine silver stain x100 (C) and x400 (D) of lymph node biopsy demonstrating organisms (black, circular structures) consistent with Histoplasma. The red arrow in D denotes one of these organisms (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
One month later an allograft biopsy showed Banff 1A acute cellular rejection concomitant with an increase in serum creatinine to 2.9 mg/dL, which was treated with steroids and the addition of low dose mycophenolate mofetil. This therapy stabilized her creatinine to 1.5 mg/dL. At the time of this writing, approximately 7 months post-discharge, she has done well, remains on itraconazole therapy and has had no other acute changes.
The United Network for Organ Sharing and local organ procurement organization were contacted regarding possible donor-derived infection; however, no other recipients are known to have developed Histoplasma related disease. The donor was negative for Histoplasma serology. The Centers for Disease Control and Prevention independently verified the findings of Histoplasma organisms from the cervical node biopsy and confirmed the presence of Histoplasma DNA from the biopsy.
Discussion
Histoplasmosis is an opportunistic fungal infection caused by the thermally dimorphic fungus Histoplasma capsulatum [1]. H. capsulatum has various worldwide geographic distributions but in the United States is endemic to the Ohio and Mississippi river valleys [2]. Few cases of H. capsulatum infection have been reported in the incident state this case occurred in, South Dakota, however numerous outbreaks have occurred in surrounding locales (Fig. 4) [9]. The disease is typically indolent among immunocompetent populations; however, the disease may become rapidly disseminated, severe and life-threatening among immunocompromised populations, furthermore histoplasmosis is an acquired immunodeficiency syndrome defining illness [2,10,11].
Fig. 4.
Locations of histoplasmosis outbreaks and number of cases by state/territory between 1938 and 2013 [9].
Among solid organ transplant recipients, histoplasmosis is an uncommon illness, occurring less than 5 % of all SOT recipients and in less than 0.5 % of all renal transplant recipients [1,2]. However, it is postulated that the true incidence of post-transplant histoplasmosis (PTH) is greater than the reported incidence for a variety of reasons including misdiagnosis and historically low-availability of histoplasma antigen assays [2]. A large study on PTH in Ohio, an histoplasmosis endemic region, reported an incidence of 1 case per 1000 person-years among SOT recipients compared to an incidence of 0.061 cases per 1000 person-years among the general population over the age of 65 [12,13].
The majority of PTH cases have historically occurred among renal transplant recipients and the severity of disease tends to parallel both the infective source and the degree of immunosuppression [6]. Various trials have demonstrated that the majority of PTH cases among SOT recipients occur within the first two years post-transplant, when immunosuppressive therapy tends to be the most intense, with the median time to diagnosis of 27 months [2,6]. Most of these infections are believed to be due to reactivation of latent H. capsulatum or de novo infection, however it can be difficult to distinguish between the two within endemic areas. Donor-derived infection has been described in the literature, but is exceedingly rare [2]. However, donor-derived PTH infection tends to follow a different disease course with systemic manifestations occurring rapidly (often less than one-month post-transplant) [6]. This contrasts with other fungal donor-derived illnesses wherein disease is typically limited to the transplanted allograft and surrounding surgical site [14]. Furthermore, epidemic histoplasmosis outbreaks among transplant recipients have been described in the literature, primarily within regions of high endemicity [3,4].
Histoplasmosis has various clinical presentations and is often initially misdiagnosed, leading to treatment delays; the median time to diagnosis after the onset of symptoms is two to three weeks [1,13,15]. The most commonly reported symptom is fever; however, the most common clinical presentation is disseminated disease [1,13]. Other affected organs reported in large-scale studies (in order of descending frequency) include lung, bone marrow, spleen, liver, central nervous system, gastrointestinal system and skin [1,6]. A multicenter study of 152 cases of histoplasmosis in solid organ transplant recipients over an eight-year period noted that 28 percent of patients had severe disease requiring intensive care unit admission and 81 percent had disseminated disease [6]. Histoplasmosis-related mortality does not appear to be greater than other IFIs (such as blastomycosis and cryptococcosis) among SOT recipients and may in fact be less than other IFIs with a range of approximately 10%–20% among SOT recipients with IFI [1,7,8]. Histoplasmosis presenting as isolated lymphadenopathy appears to be exceedingly rare. We identified six cases of histoplasmosis presenting with lymphadenopathy in the literature, none of which involved transplant-associated diseases (Table 1). Interestingly, all reported cases were located in India [[16], [17], [18], [19], [20]].
Table 1.
Characteristics of post-transplant Histoplasmosis presenting with lymphadenopathy.
| Author, year of publication | Primary Manifestation | Underlying Primary Diagnosis | Treatment | Outcomes | Location |
|---|---|---|---|---|---|
| Mishra et al., 2015 | Left cervical lymphadenopathy | HIV | N/A | N/A | Odisha, India |
| Samantaray et al., 2017 | Generalized lymphadenopathy | Unknown, patient was immunocompetent | N/A | N/A | Odisha, India |
| Bhari et al., 2017 | Cervical lymphadenopathy | HIV | Amphotericin B (2 months), Itraconazole | Resolution of Histoplasma infection | New Delhi, India |
| Mahajan et al., 2017 | Cervical lymphadenopathy with features of tuberculosis | HIV | Liposomal amphotericin B (2 weeks), Itraconazole (1 year) | Resolution of Histoplasma infection | Himachal Pradesh, India |
| Patel et al., 2018 | Generalized lymphadenopathy | Type 2 diabetes mellitus | Amphotericin B deoxycholate Intraconazole | Resolution of Histoplasma infection | Rajasthan, India |
| Patel et al., 2018 | Generalized lymphadenopathy | Autoimmune hepatitis | Itraconazole | "Stable" | Gujarat, India |
In cases of suspected PTH, urinary antigen testing is the most sensitive test, with sensitivity of 73%–97% [6]. Histopathologic/cytologic examination is also of fair prognostic value, with sensitivity of 79 % in one study [6]. Positive fungal culture provides definitive proof of H. capsulatum, but cultures often take several weeks to grow, so are not helpful in the acute or sub-acute setting. Antibody testing is not recommended due to the defective immune system of SOT recipients and is of low predictive value [6].
Therapeutic recommendations vary based on disease severity. For patients with mild/moderate disease the Infectious Disease Society of America (IDSA) recommends itraconazole therapy for six to twelve weeks [21]. For patients with severe disease, the IDSA recommends liposomal amphotericin B for one to two weeks, followed by itraconazole for an additional twelve weeks [21]. However, due to the various systemic sequelae of amphotericin B, patients must be closely monitored for signs of infusion-related reactions such as phlebitis, nephrotoxicity and electrolyte abnormalities. Furthermore, itraconazole is a potent inhibitor of CYP2C19, CYP2C9 and CYP3A4 and may precipitate drug-drug interactions with immunosuppressive therapies; preemptive dose-reduction of immunosuppressants is recommended [22,23].
Conclusion
Renal transplant recipients represent a vulnerable population for histoplasmosis due to immunocompromised host status, variable systemic manifestations, low-availability of more sensitive diagnostic tests in some regions and significant toxic sequelae of the currently recommended antifungals. Atypical presentations, such as the case described herein, further complicate timely diagnosis and can lead to treatment delays. Awareness of histoplasmosis outbreaks, particularly for practitioners in areas of high endemicity, is important for timely diagnosis as many highly susceptible patients may be at risk for severe and sometime fatal complications. Antifungal therapy should be monitored closely for several toxicities and immunosuppressive medications dosages must be reduced, while closely monitoring for transplant rejection.
CRediT authorship contribution statement
Hector Saucedo-Crespo: Conceptualization, Investigation, Resources, Formal analysis, Writing - original draft, Writing - review & editing. Tej Mehta: Investigation, Visualization, Formal analysis, Writing - original draft, Writing - review & editing. Sujit Vijay Sakpal: Investigation, Formal analysis, Writing - original draft, Writing - review & editing. Christopher Auvenshine: Writing - review & editing. Robert N. Santella: Writing - review & editing. Jawad Nazir: Writing - review & editing. Jeffrey Steers: Writing - review & editing.
Declaration of Competing Interest
The authors of this manuscript have no conflicts of interest or competing interests to disclose. Informed consent was obtained from the patient for the publication of this report.
Acknowledgments
We acknowledge and appreciate the assistance of the University of South Dakota Seldinger Society in the creation of this manuscript.
We acknowledge and appreciate the assistance of Dr. Michelle Bleile and Physicians Laboratory, Ltd. with acquisition and input of the histopathology images.
Footnotes
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review on request.
References
- 1.Nieto-Rios J.F., Serna-Higuita L.M., Guzman-Luna C.E. Histoplasmosis in renal transplant patients in an endemic area at a reference hospital in Medellin, Colombia. Transplant Proc. 2014;46(9):3004–3009. doi: 10.1016/j.transproceed.2014.06.060. [DOI] [PubMed] [Google Scholar]
- 2.Gajurel K., Dhakal R., Deresinski S. Histoplasmosis in transplant recipients. Clin Transplant. 2017;31(10) doi: 10.1111/ctr.13087. [DOI] [PubMed] [Google Scholar]
- 3.Wheat L.J., Slama T.G., Eitzen H.E. A large urban outbreak of histoplasmosis: clinical features. Ann Intern Med. 1981;94(3):331–337. doi: 10.7326/0003-4819-94-3-331. [DOI] [PubMed] [Google Scholar]
- 4.Wheat L.J., Smith E.J., Sathapatayavongs B. Histoplasmosis in renal allograft recipients. Two large urban outbreaks. Arch Intern Med. 1983;143(4):703–707. [PubMed] [Google Scholar]
- 5.Davies S.F., Sarosi G.A., Peterson P.K. Disseminated histoplasmosis in renal transplant recipients. Am J Surg. 1979;137(5):686–691. doi: 10.1016/0002-9610(79)90050-3. [DOI] [PubMed] [Google Scholar]
- 6.Assi M., Martin S., Wheat L.J. Histoplasmosis after solid organ transplant. Clin Infect Dis. 2013;57(11):1542–1549. doi: 10.1093/cid/cit593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kauffman C.A., Freifeld A.G., Andes D.R. Endemic fungal infections in solid organ and hematopoietic cell transplant recipients enrolled in the Transplant-Associated Infection Surveillance Network (TRANSNET) Transpl Infect Dis. 2014;16(2):213–224. doi: 10.1111/tid.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kauffman C.A., Miceli M.H. Histoplasmosis and blastomycosis in solid organ transplant recipients. J Fungi (Basel) 2015;1(2):94–106. doi: 10.3390/jof1020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedict K., Mody R.K. Epidemiology of histoplasmosis outbreaks, United States, 1938-2013. Emerg Infect Dis. 2016;22(3):370–378. doi: 10.3201/eid2203.151117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauffman C.A. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20(1):115–132. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheat L.J., Connolly-Stringfield P.A., Baker R.L. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore) 1990;69(6):361–374. doi: 10.1097/00005792-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Baddley J.W., Winthrop K.L., Patkar N.M. Geographic distribution of endemic fungal infections among older persons, United States. Emerg Infect Dis. 2011;17(9):1664–1669. doi: 10.3201/eid1709.101987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuellar-Rodriguez J., Avery R.K., Lard M. Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin Infect Dis. 2009;49(5):710–716. doi: 10.1086/604712. [DOI] [PubMed] [Google Scholar]
- 14.Chang C.M., Tsai C.C., Tseng C.E. Donor-derived Cryptococcus infection in liver transplant: case report and literature review. Exp Clin Transplant. 2014;12(1):74–77. doi: 10.6002/ect.2012.0288. [DOI] [PubMed] [Google Scholar]
- 15.Grim S.A., Proia L., Miller R. A multicenter study of histoplasmosis and blastomycosis after solid organ transplantation. Transpl Infect Dis. 2012;14(1):17–23. doi: 10.1111/j.1399-3062.2011.00658.x. [DOI] [PubMed] [Google Scholar]
- 16.Mishra D.P., Ramamurthy S., Behera S.K. Histoplasmosis presenting as isolated cervical lymphadenopathy: a rare presentation. J Cytol. 2015;32(3):188–190. doi: 10.4103/0970-9371.168855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samantaray S., Panda S., Dash S. Role of fine-needle aspiration cytology in diagnosis of disseminated histoplasmosis in an immunocompetent patient: a case report. J Cytol. 2017;34(3):156–158. doi: 10.4103/JOC.JOC_74_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhari N., Pahadiya P., Arava S. Histoplasmosis mimicking non-Hodgkin lymphoma in a 40-year-old man with AIDS. Int J STD AIDS. 2017;28(3):312–314. doi: 10.1177/0956462416665942. [DOI] [PubMed] [Google Scholar]
- 19.Mahajan V.K., Raina R.K., Singh S. Case report: histoplasmosis in Himachal Pradesh (India): an emerging endemic focus. Am J Trop Med Hyg. 2017;97(6):1749–1756. doi: 10.4269/ajtmh.17-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel A.K., Patel K.K., Toshniwal H. Histoplasmosis in non-endemic North-Western part of India. Indian J Med Microbiol. 2018;36(1):61–64. doi: 10.4103/ijmm.IJMM_18_12. [DOI] [PubMed] [Google Scholar]
- 21.Wheat L.J., Freifeld A.G., Kleiman M.B. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 22.Nivoix Y., Leveque D., Herbrecht R. The enzymatic basis of drug-drug interactions with systemic triazole antifungals. Clin Pharmacokinet. 2008;47(12):779–792. doi: 10.2165/0003088-200847120-00003. [DOI] [PubMed] [Google Scholar]
- 23.Venkatakrishnan K., von Moltke L.L., Greenblatt D.J. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet. 2000;38(2):111–180. doi: 10.2165/00003088-200038020-00002. [DOI] [PubMed] [Google Scholar]