Abstract
Selective IgM Deficiency (SIgMD) is a recently incorporated disorder in the classification of primary immunodeficiency diseases. The purpose of this study was to present detailed clinical and immunological features in a cohort of 62 adult patients with SIgMD. A retrospective chart review of 62 patients between 2009 and 2017 with a diagnosis of SIgMD was performed for clinical and immunological features, and response to immunoglobulin therapy in symptomatic patients who also exhibited specific antibody deficiency. The majority of patients presented with recurrent and chronic upper and lower respiratory tract infections (73%), most often with recurrent sinusitis (29%), bronchitis (33%), pneumonia (21%), and recurrent urinary tract infections (16%). Forty three percent of patients had associated autoimmune diseases including Hashimoto’s thyroiditis, and systemic lupus erythematosus. Approximately 35% of patients had atopic diseases, including allergic rhinitis and asthma. CD3+ T, CD4+ T, CD8+ T, and CD19+ B cells were normal in the majority of patients. IgG subclass deficiency was observed in approximately 22% of cases. Forty seven percent of patients exhibited specific anti-pneumococcal antibody deficiency. The six most common pneumococcal serotypes that were impaired in majority (>70%) of subjects included 3, 4, 9V, 9N, 12F, 23F. Eighteen (66%) of 27 patients with specific antibody deficiency received immunoglobulin therapy and almost all subjects responded to immunoglobulin therapy by decreased frequency of infections. No correlation was observed in immunological features, clinical manifestations, or response to therapy with serum IgM levels.
Keywords: Selective IgM deficiency, IgG subclasses, anti-pneumococcal antibodies, immunoglobulin therapy, autoimmunity
Introduction
Monomeric membrane-bound surface IgM expressed on B cells and serves as a component of B cell receptor (BCR) that is involved in signaling for B cells activation. Serum or secreted pentameric immunoglobulin IgM plays an important role in microbial defense, removal of apoptotic bodies, and in immune homeostasis by regulating inflammation and autoimmunity [1-4]. IgM mediates its effects on B cells via IgMFcR (FcμR). Mice deficient in secreted IgM and normal membrane bound IgM are impaired in IgG antibody responses and in protection against bacterial, viral, and fungal pathogens, and develop autoimmunity [5-9]. Mice lacking FcμR are also impaired in specific IgG antibody responses and develop autoantibodies as mice age [10,11]. John Hobbs and colleagues first described selective IgM deficiency (SIgMD) in two children with systemic meningococcus infection presenting with meningitis [12]. Similar to mice, humans with SIgMD also present with recurrent bacterial and fungal infections and develop autoimmunity and autoimmune diseases [13-27]. Patients with SIgMD may be asymptomatic or present with infections ranging from mild to severe including sepsis and meningitis [12,18-23]. After five decade SIgMD was incorporated in the International Union of Immunological Societies (IUIS) classification of primary immunodeficiency diseases [28]. In addition to infections, patients with SIgMD may present with autoimmune and/or allergic manifestations [12-16,29]. Immunological analyses have reported in a small number of patients with SIgMD. The objectives of this study were to [A] provide a descriptive comprehensive analysis of clinical presentations and immunological features in a large cohort of patients with SIgMD, [B] identify the frequency of specific antibody deficiency, and common pneumococcal serotypes that are impaired in majority of SIgMD patients with specific antibody deficiency, and [C] document the clinical response to immunoglobulin therapy.
Our data show that the majority of patients with SIgMD present with recurrent upper and lower respiratory tract infections, and a significant proportion of patients also manifest with autoimmune and allergic diseases. T cells, T cell subsets, and B cell numbers were normal in majority of cases; however, a small number of patients exhibited abnormalities of lymphocyte subsets. IgG subclass deficiency was observed in one fourth of patients. Furthermore, certain pneumococcal serotypes were more frequently impaired in SIgMD patients with specific antibody deficiency. Finally, those patients who received immunoglobulin therapy responded by decreased frequency or complete prevention of infections.
Materials and methods
Patients
The medical records of 62 patients with a diagnosis of SIgMD followed at the University of California at Irvine immunology clinics between January 2009 and November 2017 were reviewed. The diagnosis of selective IgM deficiency was defined as serum IgM below two standards of mean of controls with normal IgA, IgG [30]. Charts were reviewed for age, gender, initial clinical presentation with regard to recurrent infections, autoimmunity and autoimmune diseases, allergic diseases, malignancies, and response to administration of intravenous or subcutaneous immunoglobulin therapy. Data were also collected for multiple immunological parameters including levels of IgM and IgG subclasses, lymphocyte phenotypes, complement, specific antibodies to tetanus toxoid and Streptococcus pneumoniae. Subjects were de-identified. The UCI Institutional Review Board (human) approved this study. Informed consent was not required for this retrospective chart review.
Methods
Serum levels of immunoglobulin M, A, G, IgG subclasses using rate nephelometry, and autoantibodies were measured in our Department of Pathology and Laboratory Medicine. Pneumococcal antibody titers were obtained prior to and at 4 weeks post immunization with Pneumovax-23 vaccine by multi-analyte fluorescence detection (Arup Laboratories, Salt Lake City, UT, USA). Impaired specific antibody response to Pneumovax-23 was considered if post vaccination titers were either unprotective (<1.3 μg/dl) or <2 fold increase over pre-vaccination titers for more than 70% serotypes. CD3+ T, CD4+ T, CD8+ T, CD19+ B, CD3-CD16+CD56+ natural killer cells were analyzed by flow cytometry using specific monoclonal antibodies and isotype controls in Department of Pathology and Laboratory Medicine, University of California, Irvine.
Results
Clinical characteristics
Demographic data and clinical manifestations are shown in Table 1. Sixty-two patients were included in this study with a mean age of 56 years (range 12 years-90 years) with female predominance (female: male, 2:1). Serum IgM ranged between 4 mg/dl to 62 mg/dl (normal ranges 65 mg/dl to 265 mg/dl). The majority of patients initially presented with recurrent infections, which were predominantly recurrent upper respiratory tract infections (22%), chronic sinusitis (29%), and recurrent pneumonia (21%). Approximately 16% of patients presented with recurrent urinary tract infections. Few patients presented with sepsis and viral meningitis. In five patients with chronic respiratory infection, mycobacterial organisms (three with M. avium complex and two with M. tuberculosis) were identified. Three patients had documented bronchiectasis.
Table 1.
Clinical Manifestations in patients with selective IgM deficiency
| Patient | Age | Sex | IgM | Clinical Manifestations | |
|---|---|---|---|---|---|
|
| |||||
| Recurrent or Chronic Infections | Allergic & Autoimmune Disease | ||||
| 1 | 35 | F | 33 | Intertrigo and vaginal candidiasis | -- |
| 2 | 45 | M | 35 | Cellulitis, recurrent URI | -- |
| 3 | 65 | F | 40 | Lymphadenitis, sinusitis, Mycobacterium tuberculosis, bronchiectasis obliterans | Hypothyroidism, |
| Sjogren’s Syndrome | |||||
| 4 | 75 | F | 42 | -- | Guillian Barre Syndrome |
| 5 | 42 | F | 56 | HSV | -- |
| 6 | 49 | F | 13 | Recurrent URI, pneumonia, and UTI | Asthma, Sjogren’s Syndrome |
| 7 | 60 | M | 38 | -- | Rheumatoid Arthritis |
| 8 | 32 | F | 37 | Recurrent UTI | Allergic Rhinitis, autoimmune neutropenia, thrombocytopenia |
| 9 | Deceased | M | 61 | -- | ANA 1:160 |
| 10 | 60 | F | 23 | Otitis media, pharyngitis, chronic sinusitis, pneumonias | Hashimoto’s thyroiditis |
| 11 | 57 | M | 39 | Viral meningitis, recurrent URI and pneumonia | -- |
| 12 | 71 | M | 32 | Herpes Zoster with post herpetic neuralgia | Hashimoto’s thyroiditis, ocular myasthenia gravis |
| 13 | 55 | F | 47 | Chronic sinusitis, tooth abscess | Hashimoto’s Thyroiditis, autoimmune pancreatitis, Celiac Disease |
| 14 | 74 | F | 49 | Recurrent URI | -- |
| 15 | 56 | F | 20 | Chronic Sinusitis | Asthma, hypothyroidism |
| 16 | 47 | M | 34 | Recurrent URI, chronic sinusitis | -- |
| 17 | 12 | F | 34 | Asceptic meningitis, Staph and fungal skin infections | Asthma, Allergic Rhinitis |
| 18 | 59 | F | 62 | Necrotizing fasciitis, MRSA abscesses, recurrent URIs, pharyngitis, sinusitis, pneumonia x 2, recurrent UTI | Asthma, hypothyroidism |
| 19 | 90 | F | 25 | -- | Asthma, Allergic Rhinitis, Rheumatoid arthritis |
| 20 | 85 | M | 52 | Bronchitis, pneumonia | -- |
| 21 | 77 | F | 47 | Recurrent pneumonia | -- |
| 22 | 51 | F | 12 | Bronchitis, sinusitis, otitis media | Asthma, Hypothyroidism |
| 23 | 56 | F | 52 | Sinusitis | Allergic Rhinitis |
| 24 | 44 | M | 40 | Bronchitis, pneumonia, MAC infection | Asthma, Allergic Rhinitis |
| 25 | 24 | M | 56 | Sinusitis, pneumonia | ANA 1:180 |
| 26 | 39 | M | 41 | Skin abscesses, cellulitis | -- |
| 27 | 51 | F | 32 | Rec. URI, sinusitis, otitis media, Rec. UTI, vaginal bacterial infections | -- |
| 28 | 74 | M | 45 | Recurrent URI | -- |
| 29 | 42 | F | 44 | -- | Hypothyroidism |
| 30 | 17 | M | 50 | -- | Allergic Rhinitis, alopecia |
| 31 | 40 | M | 43 | Recurrent URI | -- |
| 32 | 65 | F | 35 | Chronic Sinusitis | Asthma, Allergic Rhinitis, Guillan-Barre Syndrome |
| 33 | 60 | F | 10 | HSV, Chronic Fungal sinusitis | Celiac disease |
| 34 | 57 | F | 43 | Recurrent URI | Asthma, Allergic Rhinitis, Hyperthyroidism |
| 35 | 35 | F | 35 | Recurrent URI, chronic sinusitis | Hypothyroidism, Adrenal Insufficiency, Myasthenia Gravis |
| 36 | 72 | M | 39 | Bronchitis, sinusitis, pneumonias | -- |
| 37 | 63 | F | 39 | Chronic bronchitis, chronic fungal sinusitis | Asthma, Allergic Rhinitis |
| 38 | 67 | F | 53 | Recurrent UTI | Allergic Rhinitis |
| 39 | 87 | M | 27 | Pneumonias, MAC infection | -- |
| 40 | 62 | F | 14 | Pneumonias | SLE, cutaneous lupus |
| 41 | 82 | F | 53 | Recurrent UTI | SLE, APS |
| 42 | 56 | F | 24 | Recurrent URI | Allergic Rhinitis |
| 43 | 44 | M | 15 | Periodontal abscesses | Allergic Rhinitis |
| 44 | 73 | F | 21 | -- | Allergic Rhinitis, hypothyroidism, ITP |
| 45 | 60 | M | 52 | Recurrent URI | -- |
| 46 | 72 | F | 4 | Diverticulitis, Recurrent UTI | -- |
| 47 | 57 | M | 34 | Skin Abscesses, Recurrent URI, sinusitis | ANA >1:320 |
| 48 | 79 | F | 64 | Recurrent URI | -- |
| 49 | 50 | M | 46 | Chronic sinusitis | -- |
| 50 | 21 | F | 55 | Recurrent shingles | Hashimoto’s thyroiditis |
| 51 | 59 | F | 50 | Recurrent URI | Asthma, Hashimoto’s thyroiditis |
| 52 | 62 | M | 62 | Bronchiectasis | Allergic Rhinitis, ANA 1:160 |
| 53 | 42 | M | 57 | Chronic bronchitis | -- |
| 54 | 64 | F | 57 | Chronic sinusitis | Asthma, Allergic Rhinitis |
| 55 | 31 | F | 45 | Pneumonia, Recurrent UTI | Asthma, Allergic Rhinitis |
| 56 | 43 | F | 49 | Onychomycosis, Recurrent otitis media, pharyngitis, sinusitis, thrush, and pneumonia , MAI, Bronchiectasis | Asthma, Allergic Rhinitis |
| 57 | 44 | F | 51 | URI, chronic sinusitis, chronic diarrhea, UTI | undifferentiated connective tissue disease |
| 58 | 73 | M | 18 | Pneumonia with sepsis, UTI | -- |
| 59 | 65 | M | 41 | Recurrent Pneumonia | -- |
| 60 | 43 | F | 44 | Recurrent and chronic HSV2 infection | Hypothyroidism |
| 61 | 71 | F | 30 | Chronic Fatigue | ANA 1:160 |
| 62 | 57 | M | 58 | Varicella, Mycobacterium tuberculosis | Pemphigus Vulgaris |
Approximately 43% of patients with SIgMD had associated autoimmune diseases; in few as presenting manifestations (Table 1 and Figure 1). Hashimoto’s thyroiditis was the most common autoimmune disease, followed by connective tissue autoimmune diseases including SLE, rheumatoid arthritis, Sjogren’s syndrome, and mixed connective tissue disease. Neuromuscular autoimmune diseases included myasthenia gravis, and Gullian-Barre syndrome. Others included autoimmune thrombocytopenia and autoimmune neutropenia. Furthermore high titers of ANA (titers-1:80->1:320) were present in 6 additional patients without a diagnosis of lupus.
Figure 1.
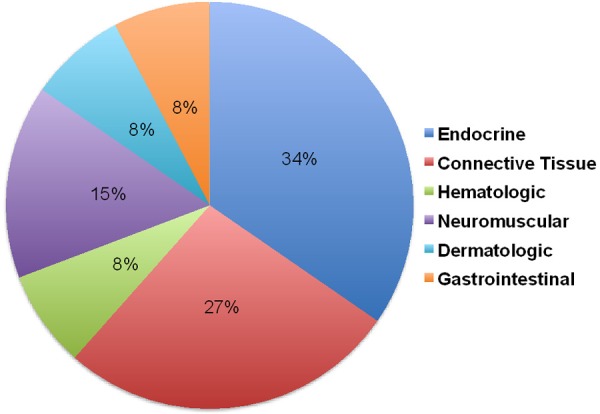
Distribution of various autoimmune diseases in SIgMD patients.
Allergic manifestations were observed in 35% of patients and included allergic rhinitis (13%), asthma (8%), and 14% with combined allergic rhinitis and asthma (Table 1 and Figure 2). Several of these patients also have autoimmune diseases.
Figure 2.
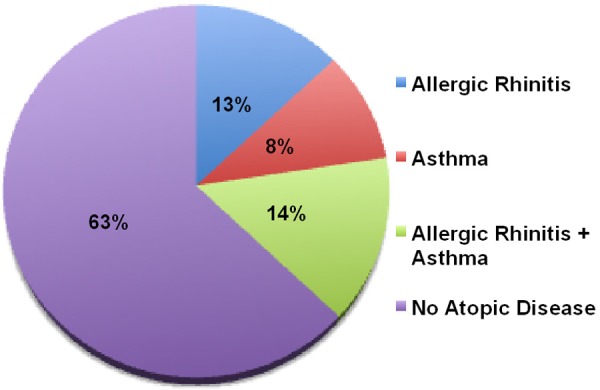
Distribution of allergic diseases in SIgMD patients.
Malignancies included 2 patients with monoclonal gammopathy of undetermined significance (MGUS), and one each of multiple myeloma, non-Hodgkin lymphoma, thyroid cancer, gastric cancer, and oropharyngeal carcinoma (data not shown). One patient each with SIgMD developed MGUS and non-Hodgkin’s lymphoma 1-3 years following the diagnosis of SIgMD.
Immunologic data
IgG subclasses
Approximately 22% SIgMD patients had reproducibly low (on at least 2 separate occasions) levels of IgG subclasses; total IgG levels were normal (Table 2). Four patients (6%) had low IgG1, 2 (3%) had low IgG2 subclass, 7 (11%) had low IgG3, and only 1 (1.5%) patient had low serum IgG4. One patient each had combined IgG2 plus IgG3, and IgG1 plus IgG3 subclass deficiency. To determine any relationship with serum IgM levels, patients with SIgMD were divided into those with serum IgM levels of ≤30 mg/dl and those with serum IgM >30 mg/dl. Twenty three percent (14 patients) of the patients had serum IgM level <30 mg/dl (Table 1); two patients has serum of <10 mg/dl. Four of 12 (33%) SIgMD patients with serum IgM ≤30 mg/dl had IgG subclass deficiency as compared to 3 (19%) in patients with serum IgM >30 mg/dl. No difference was observed in type of IgG subclass deficiency between patients with SIgMD with serum IgM of ≤30 mg/dl and those with serum IgM of >30 mg/dl.
Table 2.
IgG subclasses and Lymphocyte subsets in Patients with Selective IgM Deficiency
| Patient | IgG1 | IgG2 | IgG3 | IgG4 | CD3 | CD4 | CD8 | CD19 | CD16 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 377 | 386 | 39 | 26 | 529* | 302* | 202 | 126 | 168 |
| 2 | 514 | 383 | 94 | 25 | 1129 | 856 | 306 | 19* | 124 |
| 3 | 696 | 147* | 27 | 2* | 5468 | 449* | 97 | 90* | 112 |
| 4 | 738 | 358 | 40 | 37 | ND | ND | ND | ND | ND |
| 5 | 468 | 610 | 45 | 43 | ND | ND | ND | ND | ND |
| 6 | 469 | 115* | 15* | 17 | 1555 | 1060 | 471 | 636 | 213 |
| 7 | 458 | 466 | 146 | 14 | 1470 | 693 | 777 | 546 | 102 |
| 8 | 820 | 434 | 153 | 10 | ND | ND | ND | ND | ND |
| 9 | 357 | 179 | 37 | 67 | 561* | 499 | 68* | 115 | 156 |
| 10 | 301 | 283 | 11* | 18 | ND | ND | ND | ND | ND |
| 11 | 587 | 300 | 97 | 13 | 1736 | 1459 | 277 | 87* | 57 |
| 12 | 707 | 569 | 103 | 49 | ND | ND | ND | ND | ND |
| 13 | 688 | 442 | 43 | 17 | ND | ND | ND | ND | ND |
| 14 | 640 | 175 | 75 | 14 | 1045 | 464* | 632 | 279 | 102 |
| 15 | 642 | 343 | 178 | 18 | 1478 | 1152 | 326 | 106 | 198 |
| 16 | 388 | 360 | 38 | 10 | ND | ND | ND | ND | ND |
| 17 | 820 | 196 | 29 | 13 | 1703 | 819 | 690 | 279 | 110 |
| 18 | 454 | 432 | 96 | 12 | 1238 | 586 | 714 | 106 | 178 |
| 19 | 622 | 167 | 24 | 16 | 1651 | 1093 | 579 | 279 | 87 |
| 20 | 313 | 355 | 25 | 23 | 1341 | 1041 | 265 | 194 | 103 |
| 21 | 399 | 414 | 72 | 44 | 1255 | 959 | 296 | 99* | 57 |
| 22 | 549 | 232 | 56 | 14 | 1411 | 953 | 441 | 174 | 154 |
| 23 | 618 | 272 | 18 | 31 | 1592 | 1184 | 391 | 161 | 76 |
| 24 | 449 | 244 | 23 | 22 | 832 | 456* | 369 | 168 | 199 |
| 25 | 877 | 269 | 47 | 23 | 766 | 467* | 287 | 121 | 147 |
| 26 | 620 | 282 | 30 | 69 | ND | ND | ND | ND | ND |
| 27 | 505 | 340 | 22 | 34 | ND | ND | ND | ND | ND |
| 28 | 361 | 469 | 24 | 34 | 782 | 598 | 173 | 407 | 296 |
| 29 | 438 | 261 | 37 | 13 | 1018 | 776 | 228 | 108 | 79 |
| 30 | 624 | 282 | 26 | 33 | 674 | 393* | 225 | 182 | 104 |
| 31 | 979 | 125 | 18* | 32 | ND | ND | ND | ND | ND |
| 32 | 624 | 370 | 56 | 16 | ND | ND | ND | ND | ND |
| 33 | 515 | 284 | 31 | 29 | ND | ND | ND | ND | ND |
| 34 | 487 | 237 | 40 | 17 | ND | ND | ND | ND | ND |
| 35 | 536 | 314 | 39 | 31 | ND | ND | ND | ND | ND |
| 36 | 562 | 416 | 42 | 58 | 573* | 469* | 97 | 535 | 102 |
| 37 | 251* | 565 | 49 | 25 | 991 | 778 | 195 | 141 | 122 |
| 38 | 534 | 576 | 55 | 43 | ND | ND | ND | ND | ND |
| 39 | 524 | 496 | 79 | 20 | 962 | 431* | 526 | 87* | 201 |
| 40 | 345 | 402 | 43 | 24 | ND | ND | ND | ND | ND |
| 41 | 530 | 343 | 79 | 9 | ND | ND | ND | ND | ND |
| 42 | 312* | 477 | 38 | 31 | ND | ND | ND | ND | ND |
| 43 | 425 | 248 | 33 | 44 | 715 | 445* | 245 | 422 | 74 |
| 44 | 526 | 224 | 51 | 8 | ND | ND | ND | ND | ND |
| 45 | 444 | 311 | 44 | 15 | 832 | 443* | 378 | 166 | 256 |
| 46 | 727 | 205 | 101 | 0.6* | ND | ND | ND | ND | ND |
| 47 | 446 | 330 | 18* | 33 | ND | ND | ND | ND | ND |
| 48 | 560 | 365 | 31 | 48 | 858 | 741 | 117 | 245 | 187 |
| 49 | 425 | 344 | 20* | 30 | 1472 | 1004 | 469 | 66* | 68 |
| 50 | 818 | 236 | 48 | 9 | 1338 | 722 | 546 | 115 | 188 |
| 51 | 454 | 432 | 96 | 12 | 1238 | 568 | 714 | 66* | 146 |
| 52 | 816 | 456 | 31 | 36 | 803 | 535 | 211 | 281 | 204 |
| 53 | 566 | 153 | 65 | 40 | ND | ND | ND | ND | ND |
| 54 | 636 | 243 | 26 | 151 | 2134 | 1469 | 655 | 165 | 177 |
| 55 | 316* | 418 | 43 | 106 | 1330 | 700 | 490 | 56* | 212 |
| 56 | 538 | 403 | 39 | 28 | 1991 | 1526 | 487 | 68* | 232 |
| 57 | 267* | 308 | 21* | 22 | 1536 | 1018 | 499 | 134 | 88 |
| 58 | 615 | 198 | 37 | 53 | 865 | 646 | 307 | 312 | 104 |
| 59 | 525 | 449 | 18* | 8 | 1157 | 909 | 231 | 186 | 189 |
| 60 | 404 | 374 | 24 | 12 | 1018 | 776 | 229 | 204 | 76 |
| 61 | 932 | 194 | 33 | 9 | 1908 | 128* | 683 | 139 | 226 |
| 62 | 833 | 517 | 26 | 29 | 257* | 49* | 198 | 101 | 12* |
ND-Not done, Normal ranges (mg/dl) for IgG1 (342-1,118), IgG2 (148-525), IgG3 (21-114), IgG4 (7-88; 69-162). Normal ranges for absolute count (mm3/ml) CD3 (619-1847), CD4 (490-1194), CD8 (85-279), CD19 (110-660), CD16 (12-349).
-low. Low IgG1-4/62, IgG2-2/62, IgG3-7/62, IgG4-2/62; IgG2+4-1, IgG2+3-1; IgG1+3-1. Low CD3-4/41, CD4-12/41, CD8-1/41, CD19-8/41, CD16 1/41.
Lymphocyte subsets
The lymphocyte subsets data were available for 40 patients. In majority of cases, number of CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD19+ B cell were normal (Table 2). However, CD3+ T cells were reduced in 4 (10%) subjects, 12 (30%) patients had low CD4+ T cells, only one patient had low CD8+ T cells, and CD19+ B cells were decreased in 5 (12.5%) subjects. Three patients had combined low CD3+ and CD4+ T cells. None of the patients had lymphopenia of combined CD3+, CD4+, and CD8+ T cells. Only two patients (5%) with a serum IgM <30 mg/dl had low T cell subsets (CD4+ T cells).
Specific antibody responses
The pre and post pneumococcal vaccination specific antibody titers were available in 57 patients. Twenty-seven of 57 patients (47%) had unprotected levels of (<1.3 ug/ml) or impaired (<2 fold increase over baseline) anti-Streptococcus pneumoniae antibody responses against more than 70% serotypes following Pneumovax-23 vaccination (Table 3), thereby establishing a diagnosis of specific antibody deficiency. Upon review of individual pneumococcal serotypes, 6 serotypes including serotypes 3, 4, 9N, 9V, 12F, 23F were found to be unprotected and/or impaired to vaccination in >70% of patients, (Table 3). When data were analyzed for patients with serum IgM ≤30 mg/dl vs serum IgM >30 mg/dl, the most frequently unprotected/impaired pneumococcal serotypes, in patients with serum IgM ≤30 mg/dl included 3, 4, 7F, 9V, 9N, 12F, and 23F, and in patients with IgM >30 the most common unprotected/impaired pneumococcal serotypes were 1, 3, 4, 9N, 12F, and 23F.
Table 3.
Pneumococcal Serotypes in patients with SIgMD with specific antibody deficiency
| S. pneumococcal Serotypes | All Patients | IgM < 30 mg/dL | IgM > 30 mg/dL |
|---|---|---|---|
| # (%) patients with unprotected titers* and/or impaired response | # (%) patients with unprotected titers* and/or impaired response | # (%) patients with unprotected titers* and/or impaired response | |
| 1 | 18 (67%) | 4 (50%) | 14 (74%) |
| 2 | 5 (19%) | 2 (25%) | 3 (16%) |
| 3 | 21 (78%) | 7 (88%) | 14 (74%) |
| 4 | 26 (96%) | 8 (100%) | 18 (95%) |
| 5 | 13 (48%) | 4 (50%) | 9 (47%) |
| 26 (6B) | 16 (59%) | 5 (63%) | 11 (58%) |
| 51 (7F) | 17 (63%) | 6 (75%) | 11 (58%) |
| 8 | 15 (56%) | 5 (63%) | 10 (53%) |
| 68 (9V) | 19 (70%) | 7 (88%) | 12 (63%) |
| 9 (9N) | 21 (78%) | 6 (75%) | 15 (79%) |
| 34 (10A) | 8 (30%) | 3 (38%) | 5 (26%) |
| 43 (11A) | 6 (22%) | 3 (38%) | 3 (16%) |
| 12 (12F) | 23 (85%) | 7 (88%) | 16 (84%) |
| 17 (17F) | 8 (30%) | 2 (25%) | 6 (32%) |
| 14 | 12 (44%) | 5 (63%) | 7 (37%) |
| 19 (19F) | 16 (59%) | 4 (50%) | 12 (63%) |
| 20 | 3 (11%) | 2 (25%) | 1 (5%) |
| 22 (22F) | 10 (37%) | 4 (50%) | 6 (32%) |
| 23 (23F) | 22 (81%) | 6 (75%) | 15 (79%) |
| 54 (15B) | 6 (22%) | 3 (38%) | 3 (16%) |
| 56 (18C) | 12 (44%) | 4 (50%) | 8 (42%) |
| 57 (19A) | 4 (15%) | 3 (38%) | 1 (5%) |
| 70 (33F) | 8 (30%) | 3 (38%) | 5 (26%) |
Unprotected titers were serotypes with <1.3 μg/dl, Impaired response was <2 fold increase in post vaccination titers from baseline 34/62=55% patients with Specific Antibody Deficiency Reported in chart, but no supporting pre and post serotypes available 10 of 13 (77%) with lgM <30 mg/dl had low response to Pneumovax-23.
Only 3 of 25 patients in whom antibodies to tetanus toxoid were tested demonstrated impaired response to tetanus toxoid (data not shown). One of these patients was recently reported [18].
Complement components
Complement levels (CH50, C3, C4) were available in 26 patients and were essentially normal with the exception of decreased C3 levels seen in 4 patients and decreased C4 in 1 patient (data not shown).
Response to immunoglobulin therapy
A total of 18 symptomatic patients received immunoglobulin therapy, however, one patient each received immunoglobulin therapy primarily for associated pemphigus vulgaris and for associated polyneuropathy (Table 4). Therefore, a total of 16 SIgMD patients received immunoglobulin therapy for recurrent infections; 15 of which had specific antibody deficiency and one patient had associated IgG subclass deficiency without specific antibody deficiency. Eight of the 15 SIgMD patients (53%) with specific antibody deficiency experienced reduced frequency of infections and 7 patients (47%) had a complete response to immunoglobulin therapy with no subsequent infections. When data were analyzed for route of administration, 4 of 7 patients who had complete resolution of infection were on intravenous immunoglobulin (IVIG) and 3 of 7 were on subcutaneous immunoglobulin (SCIG).
Table 4.
Clinical Response to Immunoglobulin Therapy
| Patient | Dose (mg/kg) | IgG Trough (mg/dL) | Immunoglobulin Therapy | Infections Prior to Therapy (Annual Rate) | Infections Post-Therapy (Annual Rate) |
|---|---|---|---|---|---|
| 2 | 396 | 1010 | IVIG 35 g q4 weeks | Recurrent URI | No Infections |
| 13 | 463 | 1390 | IVIG 25 g q3 weeks | Recurrent sinus infections, frequency not recorded | Persistent recurrent sinus infections, frequency not recorded |
| 15 | 334 | 1100 | IVIG 30 g q4 weeks | Recurrent sinusitis x3, 1 URI | No infections |
| 23 | 421 | 980 | IVIG 35 g q4 weeks | 3-4 sinus infections per year, URI, 1 sinusitis | Reduced frequency of infections, exact numbers not recorded |
| 24 | 419 | 892 | SCIG 10 g q weekly | Recurrent pneumonias | No infections |
| 26 | 554 | 1130 | IVIG 45 g q4 weeks | Multiple resistant MRSA skin infections | One cellulitis and one abscess |
| 32 | 360 | 1190 | IVIG 25 g q4 weeks | Recurrent sinusitis, recurrent UTI | 1 sinusitis, 1 UTI |
| 35 | 410 | 1200 | SCIG 6 g qweekly | Recurrent sinusitis and URI | 0 to 1 sinusitis/URI |
| 36 | 400 | 940 | fSCIG 40 g q4 weeks* | 3-6 pneumonias, 3 sinus infections, 1 bronchitis | 1 bronchitis |
| 37 | 522 | 1030 | IVIG 30 g q4 weeks | Chronic sinusitis x4-5 | No infections |
| 39 | 420 | 921 | IVIG 30 g q4 weeks | Recurrent infections, type and frequency not recorded | No infections |
| 43 | 369 | 1012 | SCIG 12 g q10 days | Chronic sinusitis, recurrent dental, periodontal abscesses | 1 bacterial infection, 2 viral infections, no abscesses |
| 46 | 612 | 1510 | SCIG 10 g qweekly | Recurrent UTI, frequency not recorded | No infections |
| 56 | 390 | 1030 | IVIG 25 g q4 weeks | Otitis media x5, pharyngitis x12, pneumonia x3, sinusitis x3, chronic MAI infection | 1 otitis media, 1 pneumonia, persistent MAI infection |
| 57 | 358 | 999 | SCIG 6 g qweekly | Recurrent UTI x3 and URI x5 | 1 UTI and 2 URI |
| 59 | 516 | 1110 | SCIG 12 g qweekly | Recurrent pneumonia | No pneumonias |
| 61 | 397 | 1120 | IVIG 25 g q4 weeks | On IVIG for Neurologic disease | Neuropathy improved |
| 62 | 800 | 1540 | IVIG 50 g q4 weeks | On IVIG for pemphigus vulgaris | Pemphigus improved |
fSCIG - enzyme-facilitated subcutaneous immunolgobulin.
Discussion
Selective IgM deficiency is a recently classified primary immunodeficiency disease [28]. The true prevalence of SIgMD is not known. The prevalence has ranged from 0.03% to 2.1% [31-33]. Such variations are due to different populations studied, and levels of serum IgM used for the definition of selective IgM deficiency. In a community based surveillance study of 3,000 individual, the prevalence of complete absence of serum IgM was reported to be 0.03% [31]. Ozen et al reported prevalence of 2.1% among 131 children with primary immunodeficiencies [33]. Entezari et al [32], in screening of 3000 healthy blood bank donors in Iran, reported prevalence of SIgMD as 0.37%; SIgMD was considered as serum IgM level less than 2 SD below the mean for healthy controls [30]. Current study has identified 62 patients with SIgMD in our academic tertiary referral Immunology clinic with a total of 630 patients with various primary immunodeficiencies. There is no definitive inheritance pattern for SIgMD. In our cohort of patients we have SIgMD in two families; one mother and daughter, and the other father and a son. Jones et al [34] described 8 of 9 children in a family with low IgM, and three of them had meningococcal meningitis. Yocum et al [22] described SIgMD in a family with affected male members in three generations. Patient had no serum IgM and presented with recurrent Staphylococcal pyoderma. Father and one son had low IgM and were asymptomatic. Faulk and colleagues [35] described a child with undetectable serum IgM and pseudomonas infection. His father also had low IgM. Buckley and Sidbury [36] observed low serum IgM in the mother of 3 sibs; one presented with agammaglobulinemia.
While a number of patients with SIgMD may be asymptomatic, symptomatic patients present with recurrent bacterial, viral, and fungal infections reviewed in [14]. Similarly mice defective in secretory IgM are susceptible to bacterial, viral, and fungal infections [6,9]. In our cohort, most common presentation was upper and lower respiratory tract infections, including recurrent pneumonia. The initial presenting manifestation of recurrent infections with a predilection for the upper and lower respiratory tract, has previously been reported in 64% to more than 80% of patients with SIgMD [15-17,29]. Although generally not considered increased frequency of UTI in antibody deficiency diseases, in our cohort 16% of patients had recurrent UTI. One of the patients in our cohort with SIgMD and IgAλ MGUS (monoclonal gammopathy of undetermined significance) presented with recurrent urinary tract infection [37]. Chovancova et al [17] also reported 3 of 17 patients [17%] with recurrent UTI.
In addition to infections, an increased prevalence of allergic diseases has also been described in adult patients with SIgMD reviewed in [13,14]. Prior studies have reported a variable range of 25% to 47% of patients with SIgMD who also had a diagnosis of allergic rhinitis and/or asthma [15,16,38]. Goldstein et al [16] reported asthma in 47% and allergic rhinitis in 36% of 37 adult patients with SIgMD. In our cohort, 35% of patients had allergic manifestation with 8% with asthma alone, 14% with allergic rhinitis and asthma, and 13% with allergic rhinitis alone. Chovancova et al [17] reported bronchial asthma in 18% and allergic rhinitis in 47% of 17 adult patients with SIgMD. These differences in the frequency of allergic disease in SIgMD could be due to relatively smaller number of patients in other studies.
IgM plays an important role in immune tolerance [1,2] and mice deficient in secreted IgM or deficient in FcuR have an increased tendency to spontaneously develop as well as accelerate the production of autoantibodies [7,8,10,11]. The association of autoimmune disease and SIgMD has been described in studies of small number of patients with a prevalence ranging from 3% to 30% [15,17,24-27]. In our cohort, we observed a significantly higher rate (42%) of autoimmunity and autoimmune disease with predominance of Hashimoto’s thyroiditis and SLE. In addition, 10% of patients had high titers of ANA without clinical evidence or diagnosis of lupus. This would be consistent with the observations that normal human IgM suppress anti-thyroglobulin and anti-DNA antibody activities [39]. Furthermore, Ehrenstein and colleagues (8) demonstrated that serum IgM deficient mice are more susceptible to spontaneously develop serum anti-DNA IgG antibodies, and glomerular deposition of IgG and complement. Chovancova et al [17] in their 17 adult patients with SIgMD, reported 4 patients with SLE, and 5 additional patients with positive ANA without a diagnosis of SLE (50%); however, their cohort had no patients with Hashimoto’s thyroiditis or presence of anti-thyroid peroxidase or anti-thyroglobulin antibodies. In contrast, Goldstein et al [16] did not observe any subject with SLE; however 6 patients had hypothyroidism, and 2 of 19 patients with autoimmune thyroiditis (positive thyroid autoantibodies). Normal IgM has also been shown to suppress experimental myasthenia gravis in SCID mice model [40]. One of our patients had ocular myasthenia with high titers of anti-choline receptor antibodies. It is also interesting that autoimmune diseases are less frequent in pediatric SIgMD [27]. In Mice deficient in secretory IgM and in FcμR also develop autoimmunity and autoimmune diseases as mice age [7,8,12,13].
A number of malignancies have described in SIgMD patients as case reports. These include clear cell sarcoma, non-Hodgkin’s lymphoma, promyelocytic leukemia, and hepatocellular carcinoma reviewed in [14]. In our cohort, 3 patients had plasma cell dyscrasia; one with MGUS, and two with multiple myeloma, and one each with non-Hodgkin’s lymphoma, gastric carcinoma, thyroid carcinoma, and oropharyngeal carcinoma. MGUS and non-Hodgkin’s lymphoma were developed few years after the diagnosis of SIgMD. Whether there is an increased prevalence of lymphoid malignancies in SIgMD remains unclear and would require study of much larger population of patients.
Surface IgM+ B, CD20+ B, and CD19+ B cells are normal in majority of SIgMD patients [15,22,41]. However, low to complete absence of B cells have been reported in small number of patients with SIgMD [15,22,42]. In our cohort 8 patients had low number of CD19+ B cells. Mice deficient in secretory IgM or FcμR also have normal numbers of surface IgM+ B cells; however, they have deficiency of germinal centers [5,10]. In 1971, Faulk et al [35] described hypoplastic follicles lacking a germinal center in a child with SIgMD. Our current cohort includes 20 patients in whom we previously reported decreased germinal center B cells, and regulatory B cells [43].
T cell and CD4+ and CD8+ T cell subset proportions and number are normal in majority of SIgMD patients [15,43-45]. However, alterations in subsets of patients have been reported [46,47]. In our cohort, a significant number of patients had low numbers of CD4+ T cells. Our cohort includes 20 patients with SIgMD in whom we previously reported normal proportions of naïve, central memory, effector memory, and terminally differentiated effector memory subsets of CD4+ and CD8+ T cells, and increased CD8 Treg cells [43].
Several investigators have reported IgG subclass deficiency in a subset of SIgMD patients [15-17,48]. Goldstein et al [16], in their retrospective study of 37 adult patients with SIgMD, observed IgG subclass deficiency in 25%. Chovancova et al [17] observed selective IgG subclass deficiency in 6 of 14 patients (42%); this high percentage may be due to small number of patients studied. In our cohort, 22% of patients had reproducibly low IgG subclasses; IgG3 subclass deficiency was most frequent. No difference was observed in the frequency or the type of IgG subclass deficiency when we compared patients with serum IgM levels ≤30 mg/dl versus patients with serum IgM of >30 mg/dl.
In a number of smaller studies, several investigators have reported impaired specific antibody responses to both T-cell independent polysaccharide and T-cell dependent protein-conjugated vaccines in symptomatic patients with SIgMD. Guill et al [21] reported decreased specific antibody response to both tetanus toxoid and Streptococcus pneumoniae. La Concha et al [47] observed no IgG specific antibodies response to repeated vaccination with tetanus toxoid in two patients with SIgMD with complete absence of serum IgM. Hong and Gupta [20] also reported lack of specific antibody responses against Streptococcus pneumoniae and tetanus toxoid in a patient with SIgMD manifested with pneumococcus sepsis. Yocum et al [22] reported impaired or lack of specific antibody response against KLH and typhoid antigens. Boes et al [5] reported impaired IgG antibody responses to NP-KLH in targeted mutant selective IgM deficient mice. Yel et al [15], observed impaired IgG-specific anti-pneumococcal antibody response in 45% of patients with SIgMD. Goldstein et al [16] also reported lack of protective or no specific antibody response to pneumococcal vaccine in 2 patients with SIgMD; one of them had complete lack of serum IgM. Chovancova et al [17] reported low titers of isohemagglutinins in their cohort of 17 patients. No data were presented in their cohort of patients for specific antibodies against polysaccharide or protein antigens. In our cohort of 62 patients with SIgMD, 47% had unprotected or impaired specific anti-pneumococcal IgG antibody response; however, impaired response to tetanus toxoid was observed only in a small of patients. Furthermore, we did not observe any correlation between serum IgM levels and specific antibody deficiency; impairment of specific antibody response was similar between proportions of patients with serum IgM ≤30 mg/ml vs >30 mg/dl. IgG specific antibody response to both T-dependent and T-independent antigens are also impaired in mice deficient in IgM secretion [5] and in FcμR [10] that is associated with decreased germinal center formation. We have also reported decreased germinal center B cells in a subset of patients with SIgMD [43].
We further investigated the specific pneumococcal serotypes that were most commonly impaired in our cohort of patients. Majority of patients (>70%) displayed unprotected or impaired specific antibody response against serotypes 3, 4, 9N, 9F, 12F, 23F; and they were similar in SIgMD patients with serum IgM ≤30 mg/dl and with serum IgM >30 mg/dl.
Immunoglobulin administration has been mainstay in the treatment of antibody deficiency diseases. Since a subset of symptomatic patients with SIgMD exhibit impaired IgG specific antibody responses, immunoglobulin treatment has been administered in a small number of patients with SIgMD with decreased frequency of infections and requirements of antibiotics [15,20,24,49-51]. Yel et al [15] reported beneficial effect of immunoglobulin therapy in 5 patients with SIgMD who were treated with IVIG. Goldstein and colleagues [49], in a retrospective study, observed clinical improvement with high dose IVIG in four patients with SIgMD with comorbidity of bronchiectasis and asthma. Patel et al [50] reported beneficial effect of SCIG in a patient with SIgMD with specific antibody deficiency and recurrent multiple infections. Stoelinga et al [24] and Fallon [51] also reported beneficial effects of IVIG. Hong and Gupta [20] reported resolution of infection in a patient with SIgMD with impaired specific antibody response to both T-dependent and T-independent antigens that presented with Streptococcus pneumoniae sepsis. In our largest cohort of 16 SIgMD patients treated with immunoglobulin 7 of 16 had no further infections and in remainder frequency of infections markedly reduced. These patients have been followed for 2-5 years. No difference was observed between routes of immunoglobulin administration for complete prevention of infections. Furthermore, no difference was observed in response between patients with serum IgM <30 mg/dl and serum IgM >30 mg/dl. Both patients with SIgMD with polyneuropathy and SIgMD with psoriasis responded to high dose IVIG therapy.
In summary, majority of symptomatic SIgMD patients present with upper and lower respiratory infections, and often life-threatening meningitis and sepsis. Allergic and autoimmune diseases and autoimmunity are relatively common in SIgMD. CD3+, CD4+, CD8+ and CD19+ B cells are normal in majority of patients. IgG subclass deficiency and impaired specific antibody responses are observed in significant proportion of patients. These patients respond clinically to immunoglobulin therapy regardless of route of administration.
Acknowledgements
This work was in part supported by Investigator-initiated grant from Octapharma, USA, and unrestricted funds from the Division of Basic and Clinical Immunology.
Disclosure of conflict of interest
Sudhir Gupta has participated in clinical trials from Octapharma, USA at University of California, Irvine.
Abbreviations
- SIgMD
selective IgM deficiency
- MGUS
monoclonal gammopathy of undetermined significance
- IVIG
intravenous immunoglobulin
- SCIG
subcutaneous immunoglobulin
References
- 1.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen TT, Elsner RA, Baumgarth N. Natural IgM prevents autoimmunity by enforcing B cell central tolerance induction. J Immunol. 2015;194:1489–1502. doi: 10.4049/jimmunol.1401880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B1- and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–80. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Notley CA, Brown MA, Wright GP, Ehrenstein MR. Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells. J Immunol. 2011;186:4967–4972. doi: 10.4049/jimmunol.1003021. [DOI] [PubMed] [Google Scholar]
- 5.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–87. [PubMed] [Google Scholar]
- 6.Subramaniam KS, Datta K, Quintero E, Manix C, Marks MS, Pirofski LA. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with cryptococcus neoformans. J Immunol. 2010;184:5755–67. doi: 10.4049/jimmunol.0901638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrenstein MR, Cook HT, Neuberger MS. Deficiency in serum immunoglobulin (Ig) M predisposes to development of IgG autoantibodies. J Exp Med. 2000;191:1253–8. doi: 10.1084/jem.191.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci U S A. 2000;97:1184–1189. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenstein MR, O’Keefe TL, Davis SL, Neuberger MS. Targeted gene disruption reveals a role of natural secretory IgM in the maturation of the primary immune response. Proc Natl Acad Sci U S A. 1998;95:10089–93. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouchida R, Mori H, Hase K, Takatsu H, Kurosaki T, Tokuhisa T, Ohno H, Wang JY. Central role of IgMFc receptor in IgM homeostasis, be cell survival, humoral immune responses. Proc Natl Acad Sci U S A. 2012;109:E2699–2706. doi: 10.1073/pnas.1210706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honjo K, Kubagawa Y, Jones DM, Dizon B, Zhu Z, Ohno H, Izui S, Kearney JF, Kubagawa H. Altered IgG levels and antibody responses in mice deficienct for Fc receptor for IgM (FcR) Proc Natl Acad Sci U S A. 2012;109:15882–15887. doi: 10.1073/pnas.1206567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobbs JR, Milner RD, Watt PJ. Gamma-M deficiency predisposing to meningococcal septicaemia. Br Med J. 1967;4:583–586. doi: 10.1136/bmj.4.5579.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Gupta A. Selective IgM deficiency-an underestimated primary I mmunodeficiency. Front Immunol. 2017;8:1056. doi: 10.3389/fimmu.2017.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis AG, Gupta S. Primary selective IgM deficiency: an ignored immunodeficiency. Clin Rev Allergy Immunol. 2013;46:104–111. doi: 10.1007/s12016-013-8375-x. [DOI] [PubMed] [Google Scholar]
- 15.Yel L, Ramanuja S, Gupta S. Clinical and immunological features in IgM deficiency. Int Arch Allergy Immunol. 2009;150:291–298. doi: 10.1159/000222682. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein MF, Goldstein AL, Dunksy EH, Dvorin EH, Belecanech GA, Shamir K. Selective IgM immunodeficiency: retrospective analysis of 36 adult patients with review of the literature. Ann Allergy Asthma Immunol. 2006;97:717–730. doi: 10.1016/S1081-1206(10)60962-3. [DOI] [PubMed] [Google Scholar]
- 17.Chovancova Z, Kralickova P, Pejchalova A, Bloomfield M, Nechvatalova J, lkova M. Selective IgM deficiency: clinical and laboratory features of 17 patients and a review of the literature. J Clin Immunol. 2017;37:559–574. doi: 10.1007/s10875-017-0420-8. [DOI] [PubMed] [Google Scholar]
- 18.Kelly S, Storm E, Juckett D. Immunoglobulin M in meningococcemia. N Y State J Med. 1970;1:1298–1299. [PubMed] [Google Scholar]
- 19.Zaka-ur-Rab Z, Gupta P. Psuedomonas septicemia in selective IgM deficiency. Ind J Pediatr. 2005;42:961–962. [PubMed] [Google Scholar]
- 20.Hong R, Gupta S. Selective immunoglobulin M deficiency in an adult with Streptococcus pneumoniae sepsis and invasive aspergillosis. J Invest Allergol Clin Immunol. 2008;18:214–218. [PubMed] [Google Scholar]
- 21.Guill M, Brown D, Ochs H, Pyun K, Moffitt J. IgM deficiency: clinical spectrum and immunologic assessment. Ann Allergy Asthma Immunol. 1989;62:547–552. [PubMed] [Google Scholar]
- 22.Yocum MW, Strong DM, Chusid MJ, Lakin JD. Selective immunoglobulin M (IgM) deficiency in two immunodeficient adults with recurrent staphylococcal pyoderma. Amer J Med. 1976;60:486–494. doi: 10.1016/0002-9343(76)90714-2. [DOI] [PubMed] [Google Scholar]
- 23.Phuphuakrat A, Ngamjanyaporn P, Nantiruj K, Luangwedchakarn V, Malathum K. Selective IgM deficiency in an adult presenting with Streptococcus pneumoniae septic arthritis. J Microbiol Immunol Infect. 2016;49:150–153. doi: 10.1016/j.jmii.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Stoelinga GBA, van Munster PJJ, Slooff JP. Antibody deficiency syndrome and autoimmune hemolytic anemia in a boy with isolated IgM deficiency dysgammaglobulinemia type 5. Acta Paediatr Scand. 1969;58:352–362. doi: 10.1111/j.1651-2227.1969.tb04731.x. [DOI] [PubMed] [Google Scholar]
- 25.Antar M, Lamarche J, Peguero A, Reiss A, Cole S. A case of selective IgM deficiency and autoimmune glomerulonephritis. Clin Exp Nephrol. 2008;12:300–304. doi: 10.1007/s10157-008-0049-2. [DOI] [PubMed] [Google Scholar]
- 26.Shigeru K, Mari T, Yasumitsu N. IgM deficiency in a patient with Hashimoto’s thyroiditis. Intern Med. 1993;2:302–327. [Google Scholar]
- 27.Sugita K, Eguchi M. Chronic idiopathic thrombocytic purpura in a young male with isolated IgM deficiency. Int J Hematol. 2001;73:532–533. doi: 10.1007/BF02994018. [DOI] [PubMed] [Google Scholar]
- 28.Picard C, Bobby Gaspar H, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Crow YJ, Cunningham-Rundles C, Etzioni A, Franco JL, Holland SM, Klein C, Morio T, Ochs HD, Oksenhendler E, Puck J, Tang MLK, Tangye SG, Torgerson TR, Sullivan KE. 2017 international union of immunological societies: 2017 primary immunodeficiency diseases committee report on inborn errors of immunity. J Clin Immunol. 2018;38:96–128. doi: 10.1007/s10875-017-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein MF, Goldstein AL, Dunsky EH, Dvorin DJ, Belecanech GA, Shamir K. Pediatric Selective IgM Immunodeficiency. Chest. 2008:134. doi: 10.1155/2008/624850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S, Gupta A. Defining primary selective IgM deficiency. J Clin Immunol. 2019;39:350–352. doi: 10.1007/s10875-019-00641-4. [DOI] [PubMed] [Google Scholar]
- 31.Cassidy JT, Nordby GL. Human serum immunoglobulin concentrations: prevalence of immunoglobulin deficiencies. J Allergy Clin Immunol. 1975;55:35–48. doi: 10.1016/s0091-6749(75)80006-6. [DOI] [PubMed] [Google Scholar]
- 32.Entezari N, Adab Z, Zeydi M, Saghafi S, Jamali M, Kardar GA, Pourpak Z. The prevalence of selective IgM deficiency (SiGMD) in Iranian blood bank donors. Hum Immunol. 2016;77:7–10. doi: 10.1016/j.humimm.2015.09.051. [DOI] [PubMed] [Google Scholar]
- 33.Ozen A, Boris S, Karakoc-Aydiner E, Ozdemir C, Bachceciler NN, Barlan IB. The outcome of hypogammaglobulinemia in children: Immnoglobulin levels as predictor. Clin Immuno. 2010;137:374–383. doi: 10.1016/j.clim.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Jones DM, Tobin BM, Butterworth A. Three cases of meningococcal infection in a family associated with a deficient immune response. Arch Dis Child. 1973;48:742–743. doi: 10.1136/adc.48.9.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faulk WP, Kiyasu WS, Cooper MD, Fudenberg HH. Deficiency of IgM. Pediatrics. 1971;47:399–404. [PubMed] [Google Scholar]
- 36.Buckley RH, Sidbury JB. Heriditary alterations in the immune response: coexistence of ‘agammaglobulinemia’, acquired hypogammaglobulinemia and selective immunoglobulin deficiency in a sibship. Ped Re. 1968;2:72–84. doi: 10.1203/00006450-196803000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S, Agrawal S. IgAλ monoclonal gammopathy of undetermined significance (MGUS) associated with primary selective IgM deficiency. Ame J Clin Exp Immunol. 2019;8:37–46. [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufman HS, Hobbs JR. Immunoglobulin deficiencies in atopic population. Lancet. 1970;3:1061–1063. doi: 10.1016/s0140-6736(70)90288-6. [DOI] [PubMed] [Google Scholar]
- 39.Hurez V, Kazatchkine MD, Vassilev T, Ramanathan S, Pashov A, Basuyaux B, de Kozak Y, Bellon B, Kaveri SV. Pooled normal human polyspecific IgM contains neutralizing anti-idiotypes to IgG autoantibodies of autoimmune patients and protects from autoimmune disease. Blood. 1997;70:4004–4013. [PubMed] [Google Scholar]
- 40.Vassilev T, Yamamoto M, Aissaoui A, Bonnin E, Berrih-Aknin S, Kazatchkine MD, Kaveri SV. Normal human immunoglobulin suppresses experimental myasthenia gravis in SCID mice. Eur J Immunol. 1999;29:2436–2442. doi: 10.1002/(SICI)1521-4141(199908)29:08<2436::AID-IMMU2436>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Karsh J, Watt CG, Osterland CK. Selective immunoglobulin M deficiency in an adult: assessment of immunoglobulin production by peripheral blood lymphocytes in vitro. Clin Immunol Immunopathol. 1982;25:384–394. doi: 10.1016/0090-1229(82)90203-3. [DOI] [PubMed] [Google Scholar]
- 42.Ideura G, Agematsu K, Komatsu Y, Hatayama O, Yasuo M, Tsushima K, Hanaoka M, Koizumi T, Fujimoto K, Kubo K. Selective IgM deficiency accompanied with IgG4 deficiency, dermal complications, and a bronchial polyp. Allergol Int. 2008;57:99–105. doi: 10.2332/allergolint.C-06-52. [DOI] [PubMed] [Google Scholar]
- 43.Louis AG, Agrawal S, Gupta S. Analysis of subsets of B cells, Breg, CD4Treg and CD8Treg cells in adult patients with primary selective IgM deficiency. Amer J Clin Exper Immunol. 2016;5:21–32. [PMC free article] [PubMed] [Google Scholar]
- 44.Ohno T, Inaba M, Kuribayashi K. Selective IgM deficiency in adults: phenotypically and functionally altered profile of peripheral blood lymphocytes. Clin Exp Immunol. 1987;68:630–637. [PMC free article] [PubMed] [Google Scholar]
- 45.Yamasaki T. Selective IgM deficiency: functional assessment of peripheral blood lymphocytes in vitro. Intern Med. 1992;31:866–870. doi: 10.2169/internalmedicine.31.866. [DOI] [PubMed] [Google Scholar]
- 46.Raziuddin S, Elawad ME, Benjamin B. T-cell abnormalities in antibody deficiency syndromes. Scand J Immunol. 1989;30:419–424. doi: 10.1111/j.1365-3083.1989.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 47.De la Concha EG, Garcia-Rodriguez MC, Zabay JM, Laso MT, Alonso F, Bootello A, Fontan G. Functional assessment of T and B lymphocytes in patients with selective IgM deficiency. Clin Exp Immunol. 1982;49:670–676. [PMC free article] [PubMed] [Google Scholar]
- 48.Moffitt JE, Guill MF, Wray BB, Brown DA, Peacoke NW, Ades EW. Effect of interleukin 2 and mitogen on in vitro immunoglobulin production by peripheral blood lymphocytes from patients with selective IgM deficiency. Ann Allergy. 1988;61:424–427. [PubMed] [Google Scholar]
- 49.Goldstein MF, Hilditch GJ, Dvorin DJ, Belecanech GA. Immunoglobulin replacement for selective IgM immunodeficiency, bronchiectasis, and asthma. Ann Allergy Asthma Immunol. 2016;116:172–173. doi: 10.1016/j.anai.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Patel SS, Fergeson JE, Glaum MC, Lockey RF. Symptomatic primary selective immunoglobulin M deficiency with nonprotective pneumococcal titers responsive to subcutaneous immunoglobulin treatment. Internat Arch Allergy Immunol. 2016;170:138–140. doi: 10.1159/000447693. [DOI] [PubMed] [Google Scholar]
- 51.Fallon KE. Inability to train, recurrent infection, and selective IgM deficiency. Clin J Sport Med. 2014;14:357–359. doi: 10.1097/00042752-200411000-00006. [DOI] [PubMed] [Google Scholar]


