Abstract
Aims: To evaluate the prevalence of pre-sarcopenia and sarcopenia and their relationship with clinical variables, physical activity, quality of life, and diet in patients with heart failure with reduced left ventricular ejection fraction (HFrEF). Methods: We performed a cross-sectional study in patients with HFrEF and matched controls. Clinical, laboratory analysis, dual-emission X-ray densitometry, handgrip strength, and physical activity level questionnaire assessments were performed. Echocardiography, quality of life, gait speed, and 24-hour nutritional recall questionnaire were also analyzed. Pre-sarcopenia and sarcopenia were defined according to the European Working Group on Sarcopenia in Older People with the cut-off points of the Foundation for the National Institute of Health. Results: 79 patients and 143 controls were enrolled. Pre-sarcopenia was found in 30.4%, and sarcopenia in 10.1% of the patients. Pre-sarcopenic patients were older and shorter, and had more fractures, higher calcemia, and creatinine (P < 0.05). Sarcopenic patients were older and had higher creatinine and TSH (P < 0.05). After multiple logistic regression analysis, only age was associated with pre-sarcopenia (OR: 1.046; CI 1.004-1.095; P = 0.04) and SP (OR: 1.119; CI 1.039-1.229; P = 0.008). Women with HFrEF presented higher lean mass than controls (P < 0.001), but were weaker (P < 0.001), while men presented lower lean mass (P < 0.001). Low gait speed was associated with right ventricular dysfunction (P = 0.016) and lower left ventricular ejection fraction (P = 0.037). Conclusion: Pre-sarcopenia and sarcopenia were associated with aging. Despite having higher lean mass, women with HFrEF were weaker. Low gait speed was associated with biventricular systolic dysfunction.
Keywords: Frailty, heart failure, muscle wasting, sarcopenia, skeletal myopathy
Introduction
One of the most frequent age-related concerns is frailty, which is associated with increased morbidity and mortality [1,2]. Sarcopenia may be a precursor or a physical component of the fragility and can be defined as decreased skeletal muscle mass with concomitant weakness or low physical performance1. It is also associated with physical disability, poor quality of life, and increased mortality, mainly in older people [3]. Pre-sarcopenia (PS) refers only to the reduction in skeletal muscle mass [3].
The prevalence of heart failure (HF) and the total amount of fat in the body increase with aging. Excess of adiposity has been associated with a decline in immune function and chronic low level of inflammation, which predispose to tissue damage [4].
An interplay may exist between the pathophysiology pathways of the HF, age-related changes in body composition, and sarcopenia. Substances initially secreted in HF as contra-regulatory mechanisms, when in excess and for a long time, have the potential to cause morphofunctional damage to the cardiomyocyte and the vascular system [5,6]. Cardiac skeletal myopathy has been associated with changes in myofibrils, contractile proteins, mitochondrial activity, blood flow, and capillary/fiber ratio. These skeletal abnormalities in HF may also be associated with exercise intolerance, reduced oxidative capacity, and lower oxygen consumption [7].
The prevalence of low appendicular mass in patients with HF varies between 19.5% and 47.3% and has been associated with poor physical performance and oxygen consumption, worse left ventricular function, and higher hospitalization rates [8,9]. However, the prevalence of low appendicular mass may vary substantially in different populations and also according to the criteria used to define it.
Currently, there still is insufficient data to accurately estimate the prevalence rates of sarcopenia in HF. Therefore, the primary aim of this study was to evaluate the prevalence of PS and sarcopenia in patients with heart failure with left ventricular reduced ejection fraction (HFrEF). Secondary objectives were to verify possible association of PS and sarcopenia with clinical variables, prevalence of fractures, quality of life, and diet of the HFrEF patients.
Materials and methods
Study design
We performed a cross-sectional and controlled study, approved by the Ethics Committee on Human Research of our institution in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All participants signed an informed consent form.
Patients were selected from the database of the Echocardiography Service. Men and women over 18 years old and with HFrEF of any etiology were enrolled and composed the heart failure group (HFG). All the patients enrolled were in out-patient clinical follow-up with the cardiology team of our institution. Left ventricular ejection fraction (LVEF) ≤ 40% was confirmed by at least two consecutive echocardiographic exams with at least 6 months interval. The echocardiographic evaluation followed the most recent guidelines of the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI). Excluded from this study were patients who had clinical intercurrences or hospitalization 90 days prior to the protocol; those who presented with moderate or significant cardiac stenotic valve disease, chronic obstructive pulmonary disease (COPD) with FEV1 < 50%, lung diseases, pregnancy, body mass index (BMI) > 39.9 kg/m2, current alcohol intake > 25 g, neuromuscular or orthopedic limitations, chronic kidney disease (CKD-EPI ≤ 30 ml/min.1.73 m2); patients with diabetes with glycated hemoglobin (HbA1C) ≥ 8.5%, with severe microvascular chronic complications, using two or more hypoglycemic drugs or insulin therapy; and any patients with systemic diseases that could affect body composition.
A fasting blood sample was collected in the morning from all participants. They answered a questionnaire to provide clinical and demographic data, functional capacity (NYHA), quality of life, and levels of physical activity. Handgrip strength, physical performance test, densitometry, and nutritional assessment were all evaluated. The medical records of all patients were also examined.
Anthropometric evaluation
Body weight was measured with barefoot patient wearing only underwear, and height was measured on a vertical scale. The mean of three concordant non-invasive blood pressure (BP) measurements of each upper limb was used. Edema was graded in five progressive categories (0 to 4+) after inspection and palpation of the lower limbs.
Quality of life and physical activity questionnaires
Quality of life and level of physical activity were respectively evaluated through the Minnesota Living with Heart Failure Questionnaire (MLHFQ) and the International Physical Activity Questionnaire-short version (short-IPAQ). Both were validated for Portuguese and applied as structured interviews. The score for each physical activity domain and the stratification of physical activity levels (low, moderate, or high) followed the guidelines for data processing and analysis of the IPAQ. We also categorized patients according to the IPAQ as sedentary (no physical activity for at least 10 continuous minutes/week), insufficiently active (physical activity for at least 10 continuous minutes/week), or active (vigorous physical activity for at least 20 minutes/session for at least 3 days/week; moderate activity or walking at least 5 days/week for at least 30 minutes/session, or any combination activity with a total duration at least 150 minutes/week, 5 or more days/week) [10].
Strength and performance evaluation
Handgrip strength (HGS) was measured bilaterally three times on each side with the Charder® MG 4800 Medical Handgrip dynamometer. Weakness was defined as HGS or HGS adjusted for BMI, respectively, < 26 kg or < 1 in men and < 16 kg or < 0.56 in women [11]. Physical performance was evaluated by the gait speed test, which was performed in a central flat corridor 4 meters in length with 1 m additional acceleration and deceleration extra zones at each end. The time required to complete the 4 m path was the arithmetic mean of three concordant evaluations. A gait speed of ≤ 0.8 m/s was considered low gait velocity.
Laboratory exams
The ARCHITECT C8000 analyzer, Abbott®, was used for plasma analysis of TSH (reference value-RV: 0.35 to 4.94 μUI/mL) and free T4 (RV: 0.70 to 1.48 ng/dL)-immunochemiluminescence; sodium (RV: 136 to 145 mEq/L) and potassium (RV: 3.5 to 5.1 mEq/L)-diluted ion-selective electrodes; albumin (Bromocresol purple-RV: 3.4 to 5 g/dL); high sensitivity C reactive protein (CRP) (immunoturbidimetry - RV: 0.1 to 160 mg/L); creatinine (alkaline kinetic picrate-RV: 0.57 to 1.25 mg/dL); urea (urease-RV: 7 to 25.7 mg/dL); total calcium (Arsenazo III-RV: 8.4 to 10.2 mg/dL); inorganic phosphorus (ammonium molybdate-RV: 2.3 to 4.7 mg/dL); N-terminal pro B-type natriuretic peptide NT-proBNP - immunochemiluminescence (RV: ≤ 125 pg/mL below 75 years and ≤ 450 pg/mL above 75 years old). Serum levels of 25-hydroxyvitamin D (25OHD) were determined by immunochemiluminescence-(LIAISON®). HbA1C (%), total cholesterol (mg/dL), HDL (mg/dL), triglycerides (TG-mg/dL), INR (international normalized ratio), and blood count (XN-3000, Sysmex®) were analyzed retrospectively. LDL-c was calculated by the Friedewald equation when TG was < 400 mg/dL. If not, LDL-c was excluded.
Lean mass measurement
All participants were submitted to a total body densitometry by dual X-ray absorptiometry (DXA) using Lunar Prodigy equipment (GE Medical Systems, Madison, WI, USA) and the Encore program. Total lean mass (TLM), percentage of lean mass (% LM), and the lean mass of arms and legs (appendicular lean mass [ALM]) were evaluated.
Nutritional evaluation
Nutritional assessment was carried out through a 24-hour recall questionnaire. The analysis of the composition of the nourishment and meals was performed by crossing the data obtained in the Brazil Nutri software with the table of nutritional composition of foods consumed in Brazil. Adequate intake was defined as ingested values between 90 and 110% of the established nutritional goal. The goals for protein intake were 2.0 g/kg/day for BMI > 30 kg/m2, 1.2 g/kg/day for > 65 years old or 15% of the daily energy requirement for adults non-obese and ≤ 65 years old [12]. For calcium, a minimum intake of 1,000 mg/day was considered adequate, and for vitamin D 15 μg/day (< 70 years old) or 20 μg/day (> 70 years old) [13].
Control group (CG)
The CG was composed of healthy non-athlete individuals from a database of the Endocrine division (SEMPR) of our institution and was pared according to age, sex, and BMI. Excluded were individuals with chronic or untreated systemic diseases, and those with uncontrolled type 2 diabetes (T2DM) or T2DM presenting with macro or microvascular lesions. The CG was submitted to the same evaluations under the same methodology as the HFG, except for gait speed and nutritional assessment.
Definition of pre-sarcopenia and sarcopenia
Pre-sarcopenia was defined as ALM/BMI < 0.789 in men and < 0.512 in women [14]. Sarcopenia was defined by the concomitant decrease in ALM/BMI and low gait speed or weakness. Severe sarcopenia was defined as the concomitant presence of all the aforementioned abnormal parameters [3].
Statistical analysis
R Statistical Software (R Core Team, 2018), version 3.4.4, was used. The sample normality was verified by the Shapiro Wilk test. Data were presented in absolute and relative frequencies for qualitative variables and mean ± standard deviation (SD) or median (minimum and maximum) for quantitative variables. The comparison between the quantitative variables was performed by Student’s t-test or the Mann-Whitney test. The Kruskal-Wallis test and the post-hoc test were performed for comparison of three or more groups, while the Fischer exact test or the chi-square test was used for qualitative analysis. Multivariate logistic regression was used considering pre-sarcopenia and sarcopenia as dependent variables, and all the significant variables in the univariate analysis as independent variables. Statistical significance was considered when P < 0.05.
Results
Between June 2015 and June 2017, 4,231 adult echocardiography reports were reviewed. LVEF ≤ 40% was found in 759 (17.9%). The selection of the sample is shown in Figure 1. The main causes of exclusion were recent weight gain, worsening laboratory parameters, or the diagnosis of a new comorbidity. Finally, the HFG sample was composed of 79 patients with HFrEF who were compared with 143 healthy controls.
Figure 1.
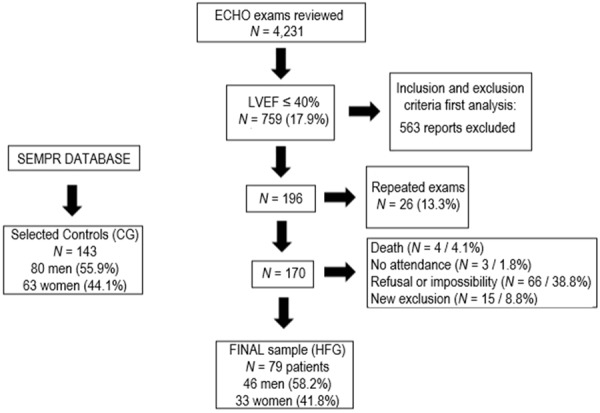
Sample selection. Flow diagram shows the steps followed to recruit patients and controls, the number of patients included, and excluded, the reasons for the exclusions and the final sample. ECHO: echocardiogram; CG: control group; HFG: heart failure group; SEMPR: endocrine division, Department of Internal Medicine, Federal University of Paraná, Curitiba, PR, Brazil; HFG: heart failure group; LVEF: left ventricular ejection fraction (%).
The mean age of the HFG was 65.6 ± 13 years, the BMI 26.8 ± 4.0 kg/m2, and 46 (58.2%) patients were men. In the CG, the mean age was 65.44 ± 11.8 years, the BMI 27.08 ± 3.03 0 kg/m2, and 80 (55.9%) individuals were men. The HFG presented a higher proportion of individuals with BMI ≥ 30 kg/m2 (P = 0.009), a higher number of comorbidities and a lower number of smokers compared to the CG (P < 0.05). All biochemical analyses were similar between the groups, except for higher creatinine levels in the HFG (Table 1).
Table 1.
Clinical, demographic, and laboratory characteristics of Heart Failure Group (HFG) and controls (CG)
| Characteristics | HFG (N = 79) | CG (N = 143) | p value |
|---|---|---|---|
| Age (y) | 65.6 ± 13 | 65.44 ± 11.8 | 0.745 |
| Gender | 0.740 | ||
| Men, N (%) | 46 (58.2) | 80 (55.9) | |
| Women, N (%) | 33 (41.8) | 63 (44.1) | |
| Ethnicity | |||
| White, N (%) | 52 (65.8) | 139 (97.2) | < 0.001 |
| Mulatto, N (%) | 24 (30.3) | 1 (0.7) | < 0.001 |
| Black, N (%) | 3 (3.8) | 3 (2.1) | 0.752 |
| Weight (Kg) | 70.7 ± 13.3 | 72.9 ± 11.46 | 0.158 |
| Height (m) | 1.62 ± 0.08 | 1.64 ± 0.10 | 0.306 |
| BMI (Kg/m2) | 26.88 ± 4.04 | 27.08 ± 3.03 | 0.700 |
| BMI interval | |||
| 18.5-24.9 Kg/m2 | 24 (30.37) | 34 (23.77) | 0.360 |
| 25-29.9 Kg/m2 | 37 (46.83) | 96 (67.13) | 0.005 |
| ≥ 30 Kg/m2 | 18 (22.78) | 13 (9.09) | 0.009 |
| Previous Smoking, N (%) | 35 (44.30) | NA | |
| Current Smoking, N (%) | 10 (12.66) | 63 (44.1) | < 0.001 |
| Previous alcohol intake, N (%) | 3 (3.85) | NA | |
| Comorbidities | |||
| Dyslipidemia, N (%) | 65 (82.2) | 26 (32.5) | < 0.001 |
| Arterial Hypertension, N (%) | 62 (78.48) | 33 (41.25) | < 0.001 |
| Diabetes Mellitus, N (%) | 24 (30.4) | 10 (12.5) | 0.006 |
| Cerebrovascular disease, N (%) | 23 (29) | 0 | < 0.001 |
| Atrial fibrillation, N (%) | 19 (25) | NA | |
| COPD and other Pneumopathies, N (%) | 12 (15.2) | 0 (0) | < 0.001 |
| Hypothyroidism, N (%) | 7 (8.9) | 5 (6.25) | 0.252 |
| Peripheral vascular disease | 3 (3.8) | NA | |
| Laboratory exams | |||
| Sodium (mEq/L) | 138.06 ± 3.26 | NA | |
| Potassium (mEq/L) | 4.545 ± 0.52 | NA | |
| Urea (mg/dL) | 44.62 ± 16.96 | NA | |
| Creatinine (mg/dL) | 1.12 ± 0.26 | 1.0 ± 0.28 | 0.001 |
| TSH (μUI/mL) | 1.92 ± 0.94 | 2.2 ± 0.97 | 0.127 |
| Free T4 (pg/dL) | 1.098 ± 0.18 | NA | |
| Albumin (g/dL) | 4.016 ± 0.29 | 4.1 ± 0.24 | 0.510 |
| 25 OH Vitamin D (ng/mL) | 27 ± 15.50 | 27.6 ± 13.96 | 0.780 |
| Total Calcium (mg/dL) | 9.317 ± 0.52 | 9.4 ± 0.49 | 0.568 |
| Inorganic phosphate (mg/dL) | 3.53 ± 0.69 | NA | |
| C-reactive protein (mg/L) | 0.541 ± 0.97 | NA | |
| Hemoglobin (g/dL) | 13.81 ± 1.71 | 14 ± 1.36 | 0.479 |
| Leukocytes × 10-9/L | 7.615 ± 2.9 | NA | |
| HbA1c % | 5.703 ± 0.55 | NA | |
| INR | 2.06 ± 1.24 | NA | |
| HDL (mg/dL) | 41 ± 11.40 | NA | |
| c-LDL (mg/dL) | 101.5 ± 36.20 | NA | |
| Triglycerides (mg/dL) | 119 ± 55.70 | NA | |
| Total Cholesterol (mg/dL) | 166 ± 42.20 | NA | |
| NT-proBNP (pg/mL) | 1,883.53 ± 2,209.25 | NA |
y, years; N, number; %, percentage; Kg, Kilogram; m, meter; Kg/m2, Kilogram per square meter; COPD, Chronic Obstructive Pulmonary Disease; TSH, Thyroid Stimulant Hormone; Free T4, free form of Thyroxine; 25 OH Vitamin D, 25 Hydroxyvitamin D; HbA1c%, Hemoglobin glycosylated; INR, international normalized ratio; HDL, High-Density Lipoprotein; c-LDL = calculated Low Density Lipoprotein; NT-proBNP = N-terminal pro B-type natriuretic peptide; NA = not available.
The characteristics of heart disease in the HFG are presented in Table 2. There were differences in the quality of life (MLHFQ) and in NT-proBNP according to the NYHA (Figure 2).
Table 2.
Heart disease characteristics in HFG
| Characteristics | HFG (N = 79) | p value* |
|---|---|---|
| Etiology | 0.368 | |
| Ischemic, N (%) | 41 (51.9) | |
| Chagas Disease, N (%) | 11 (13.9) | |
| Hypertensive, idiopathic and other cardiomyopathies, N (%) | 27 (34.1) | |
| NYHA | 0.392 | |
| I, N (%) | 7 (8.9) | |
| II, N (%) | 49 (62) | |
| III, N (%) | 23 (29.1) | |
| IV, N (%) | 0 | |
| MLHFQ | < 0.001 | |
| NYHA I | 16.6 ± 8 | |
| NYHA II | 30.8 ± 16.4 | |
| NYHA III | 61.5 ± 16.9 | |
| Edema | 0.406 | |
| None | 37 (59.7) | |
| 1+, N (%) | 15 (24.2) | |
| 2+, N (%) | 7 (11.3) | |
| 3+, N (%) | 3 (4.8) | |
| 4+, N (%) | 0 | |
| NT-proBNP (pg/mL) | 0.009 | |
| NYHA I | 608 ± 558.6 | |
| NYHA II | 994.05 ± 739.1 | |
| NYHA III | 3,578.6 ± 2,964.5 | |
| Systolic Blood Pressure (mmHg) | 108.9 ± 21.2 | |
| Left ventricular ejection fraction (LVEF %) | 30 ± 6.2 | |
| Basal left ventricular dimension (mm) | ||
| Men | 61.5 ± 8.3 | |
| Women | 59.9 ± 9 | |
| Estimated Pulmonary Artery Systolic Pressure (mmHg)† | 42.9 ± 10 | |
| Right ventricular enlargement, N (%) | 28 (35.4) | |
| Right ventricular systolic dysfunction, N (%) | 13 (16.5) |
HFG, Heart Failure Group; N, number; %, percentage; NYHA, New York Heart Association physical capacity classification; MLHFQ, Minnesota Living with Heart Failure Questionnaire Score; NT-proBNP = N-terminal pro B-type natriuretic peptide.
p value for the Kruskal-Wallis test.
Value estimated by the Bernoulli equation.
Figure 2.
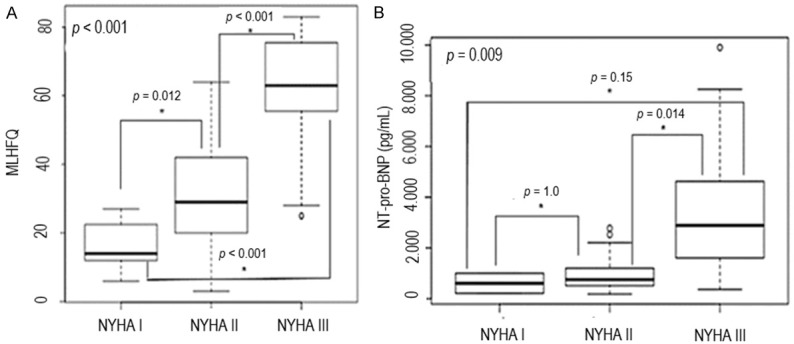
Quality of life (A) and plasma levels of N-terminal pro B-type Natriuretic Peptide (B) according to NYHA classification. (A) shows that quality of life worsened with the increasing of NYHA class from I to III, as well as NT-pro-BNP plasmatic levels (B). MLHFQ = Minnesota Living with Heart Failure Questionnaire; NYHA = New York Heart Association classification; NT-proBNP = N-terminal pro B-type natriuretic peptide.
The main medications used in the HFG treatment were beta blockers (98.7%), angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (91.1%), statins (71.8%), spironolactone (60.3%), aspirin (56%), loop diuretics (54%), coumarins (31%), thiazides (19%), nitrates (19.2%), and hydralazine (16.5%).
Physical activity
Short-IPAQ analysis showed that 86% of the HFG were active, 12.7% were insufficiently active, and 1.3% were sedentary. Moreover, 27.8% of the HFG presented high, 58.3% moderate, and 13.9% low level of physical activity without difference between genders. Surprisingly, the HFG had a greater level of physical activity compared to the CG, (86 vs 35%, P < 0.001).
Nutritional evaluation
The majority of patients in the HFG had insufficient intake of calories (69.6%), protein (68.4%), calcium (96.2%), and vitamin D (100%), with mean daily intake of 1,472.8 ± 537.4 Kcal, 89.7 ± 241.6 g, 449.7 mg, and 2.68 ± 2.1 µg, respectively. On the other hand, 12.7%, 17.7%, and 1.3% patients had an excessive intake of energy, proteins, and calcium, respectively.
Falls and fractures
In the last 5 years, 35.4% patients in the HFG experienced a ground-level fall and 36.7% had fractures. The mean number of fractures per patient was 1.55 ± 0.68, more frequent in spine (54%), upper limbs (48.7%), and lower limbs (38%). All low-impact fractures occurred in the lower limbs (distal in 83%). There was no association between hypotension (SBP < 90 mmHg) and falls (P = 0.31) or fractures (P = 0.37) in the HFG.
Lean mass
HFG men presented lower ALM and TLM compared to the CG (P < 0.001). In contrast, HFG women presented significantly higher ALM, TLM, and % LM than their controls (P < 0.001). There was no association between lean mass and levels of physical activity, any variable of HFrEF, or body composition in men and women of the HFG. Table 3 shows the evaluation of lean mass of the HFG and their comparison with the CG.
Table 3.
Comparative analysis of lean mass and HGS between HFG and CG
| Variables | HFG (N = 79) | CG (N = 143) | p value |
|---|---|---|---|
| ALM (Kg) | |||
| Men | 19.77 ± 4.8 | 22.23 ± 3.08 | < 0.001 |
| Women | 19.42 ± 4.74 | 15.4 ± 2.30 | < 0.001 |
| TLM (Kg) | |||
| Men | 45.72 ± 10.19 | 50.94 ± 5.73 | < 0.001 |
| Women | 44.7 ± 9.1 | 37 ± 4.61 | < 0.001 |
| (%) LM | |||
| Men | 65.3 ± 8.5 | 66.4 ± 6.5 | 0.555 |
| Women | 63.21 ± 8.45 | 55 ± 5.2 | < 0.001 |
| HGS (Kg) | |||
| Men | 33.34 ± 8.47 | 35.3 ± 3 | 0.127 |
| Women | 20.3 ± 5.56 | 31.1 ± 3 | < 0.001 |
| HGS/BMI | |||
| Men | 1.26 ± 0.36 | 1.29 ± 0.35 | 0.700 |
| Women | 0.76 ± 0.19 | 1.15 ± 0.4 | < 0.001 |
HFG, Heart Failure Group; CG, Control Group; ALM = appendicular lean mass; Kg, Kilogram; TLM = total lean mass; (%) LM = percentage of lean mass; HGS = handgrip strength; HGS/BMI = handgrip strength adjusted to body mass index (BMI).
Performance and strength
Low gait speed was observed in 11 (13.9%) patients of the HFG and this was associated with right ventricular dysfunction (P = 0.016) and lower LVEF (P = 0.037). Sixteen patients (20.25%) had low absolute HGS, and 13 (16.46%) had low HGS adjusted for BMI. Women in the HFG had lower HGS than their controls (P < 0.001; Table 3). The agreement between weakness and low gait speed was moderate (Kappa = 0.51, P < 0.001, 95% CI = 0.25-0.75).
Pre-sarcopenia
Pre-sarcopenia was observed in 24 (30.4%) patients of the HFG (70.8% men) and in 35.4% of the CG (64.7% men, P = 0.52). The ALM/BMI of pre-sarcopenic patients with HFrEF (PS-HFG) was 0.73 ± 0.47 in men and 0.47 ± 0.42 in women, with no difference in the CG.
Patients in the PS-HFG were significantly older and had lower stature, higher creatinine, and more fractures than non-pre-sarcopenic patients with HFrEF (NPS-HFG)-Table 4A. After multivariate logistic regression analysis, only age was associated with pre-sarcopenia (OR: 1.046; 95% CI 1.004-1.095; P = 0.04). Men of the PS-HFG had lower handgrip strength/BMI than men of the NPS-HFG (1.03 ± 0.22 vs 1.24 ± 0.39, P = 0.001).
Table 4A.
Comparative analysis between pre-sarcopenic and non-pre-sarcopenic patients with heart failure
| Variables | PS-HFG (N = 24) | NPS-HFG (N = 55) | p value |
|---|---|---|---|
| Age (y) | 70.21 ± 10.8 | 63.54 ± 13.43 | 0.020 |
| Height (m) | 1.58 ± 0.62 | 1.64 ± 0.086 | 0.009 |
| Calcium (mg/dL) | 9.53 ± 0.55 | 9.25 ± 0.5 | 0.040 |
| Creatinine (mg/dL) | 1.2 ± 0.23 | 1.081 ± 0.27 | 0.040 |
| Fracture (%) | 54.17 | 29.09 | 0.035 |
PS-HFG, Pre-sarcopenic patients with HFrEF; HFrEF, Heart Failure with reduced left ventricular ejection fraction; NPS-HFG: Non-Pre-sarcopenic patients with HFrEF; y, years; %, percentage.
No difference was seen in the NT-pro-BNP levels of the PS-HFG or the NPS-HFG: the median (min-max) was 754 (213 - 4,761) pg/mL and 1,206 (181-9,901) pg/mL respectively (P = 0.69). Also, the MLFHQ scores were not different between PS-HFG or NPS-HFG (32.875 ± 19.05 vs 40.89 ± 23.1; P = 0.16).
Sarcopenia
Sarcopenia was observed in 8 (10.1%) patients of the HFG and in 5 (3.5%) of the CG, (P = 0.09). Of the sarcopenic HFrEF patients (S-HFG), 89% were men, 3 had low gait speed, and 5 had low HGS. Severe sarcopenia occurred in 2.5% of the HFG and in none of the CG. S-HFG patients had significantly higher age, TSH, and creatinine than the non-sarcopenic HFrEF patients (NS-HFG)-Table 4B. After multivariate logistic regression analysis, only age was associated with sarcopenia (OR: 1.119; CI 1.039-1.229; P = 0.008).
Table 4B.
Comparative analysis between sarcopenic and non-sarcopenic patients with heart failure
| Variables | S-HFG (N = 8) | NS-HFG (N = 71) | p value |
|---|---|---|---|
| Age (y) | 77 ± 9.9 | 64.1 ± 12.64 | 0.009 |
| TSH (μUI/mL) | 2.167 ± 0.26 | 1.89 ± 0.99 | 0.030 |
| Creatinine (mg/dL) | 1.37 ± 0.21 | 1.085 ± 0.25 | 0.005 |
TSH = Thyroid Stimulant Hormone; S-HFG: Sarcopenic patients with HFrEF; NS-HFG: Non Sarcopenic patients with HFrEF; y, years.
NT-pro-BNP was measured in only 2 S-HFG patients (520 and 4,761 pg/mL) and there was no difference with the NS-HFG (median = 1,024 pg/mL; min. = 181 pg/mL; max. = 9,901 pg/mL; P = 1.0). No difference was found in the MLFHQ scores between these groups (44.4 ± 20.07 vs 33.3 ± 22.4; P = 0.73).
Echocardiographic variables, drug classes used for treatment, physical activity, body composition, nutritional adequacy, prevalence of falls, and number of bone fractures were similar between the PS-HFG vs the NPS-HFG and between the S-HFG vs the NS-HFG.
Discussion
This study evaluated the prevalence of pre-sarcopenia and sarcopenia exclusively in HFrEF and compared them with healthy controls. The presence of both were directly associated with patient’s age, while low physical performance was associated with cardiac biventricular systolic dysfunction.
The demographic profile of our sample was compatible with that of Southern Brazil, where there is a predominance of white people. The HFG patients were predominantly old, not currently smoking, but with a past smoking habit. There was a high prevalence of coronary heart disease and structurally advanced heart disease. An association was found between poorer quality of life and functional limitation. The greater number of co-morbidities in the HFG could have affected their quality of life and physical performance, although the HFrEF context per se could be responsible for this situation.
The high levels of physical activity found could have influenced the amount of lean mass and may be justified by the non-limiting profile of patients and by the social condition in which the HFG was inserted, as the majority were manual laborers. Besides, patients may have over quantified their physical activity measured by the short-IPAQ questionnaire.
A high percentage of dietary insufficiency was observed and this could predispose to sarcopenia, since the HF recommended dietary sodium restriction involves dietary changes and concomitant reduction in nutrient consumption [15]. In addition, liver congestion and low vascular intestinal flow may result in inappetence and malabsorption of nutrients [16]. However, despite the dietary inadequacy, no association was found between diet variables and PS or sarcopenia, probably due to the number of HFG patients included. This could also be related to the optimal pharmacological treatment and to the HFG physical profile. However, our study design was not able to respond to this hypothesis.
Falls can be a consequence of sarcopenia or fragility and HF has already been associated with fracture risk [17,18]. We observed lower rates of falls in the HFG than those previously reported [19], and this may be related to differences in population, methodology of evaluation, memory bias, or lack of impairment of vision or balance in our sample. Despite the lower number of falls reported in the HFG, the PS-HFG subgroup presented proportionally higher prevalence of fractures.
Women in the HFG had lower strength despite higher lean mass. This is most likely related to poor muscle quality, premature functional decline of the skeletal musculature [20,21], less marked loss of muscle mass, or edema or liposubstitution of myofibrils, which could overestimate the densitometric quantification of lean mass [22,23]. Interestingly, weakness has already been associated with worse clinical outcome in heart failure patients [24]. Strength was similar between the HFG and the CG, but the HGS/BMI was lower in men in the PS-HFG. When considering only the analysis of these subgroups, the strength reduction and lower lean mass could be compatible with the aging natural history of skeletal muscle changes. On the other hand, it was expected that the high levels of physical activity could be associated with less loss of lean mass and strength.
This study showed higher prevalence of pre-sarcopenia (30.4%) than the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF) did (19.5%) [8]. However, SICA-HF also enrolled patients with preserved LVEF and this may have influenced the prevalence of PS, since the degree of atrophy of skeletal myofibrils and its functional alteration have already been correlated with lower LVEF [25]. As in SICA-HF, we found that pre-sarcopenic individuals were predominantly older men with higher serum levels of creatinine. But, after multiple logistic regression analysis, only age has been correlated with PS in our study.
One should be careful comparing prevalence rates of altered lean mass that were based on different criteria. SICA-HF used the relative skeletal muscle mass index (ALM/m2), and we used ALM adjusted to the BMI, with the latter being better correlated with weakness and poor physical performance [11]. Our CG presented prevalence of pre-sarcopenia that was similar to that of the HFG and within the broad range of prevalence described in the literature, but above that assessed by the FNIH Sarcopenia Project (19.5% in men and 15.6% in women) [11]. This situation could be due to the different anthropometric profile of the population, but could also be influenced by good HFrEF pharmacology treatment, high levels of previous physical activity, or absence of major functional limitation, a scenario that may hypothesize that the loss of lean mass in HFrEF, even with significant structural impairment of the heart, could be delayed or even prevented.
Overall, we observed sarcopenia in 10% of the HFG (15.2% in men and 3% in women). According to the Foundation for the National Institute of Health (FNIH), the prevalence of sarcopenia in individuals above 65 years old was 1.3% and 2.3% for men and women, respectively [11]. The European Working Group on Sarcopenia in Older People (EWGSOP) claim a prevalence of 5 to 13% [11]. It is quite interesting to note that in our study low physical performance was associated with biventricular systolic dysfunction, which had already been associated with worse prognosis in HFrEF [8,9,26].
S-HFG patients were predominantly older males with higher levels of creatinine, TSH, and NT-proBNP. Curiously, kidney and thyroid dysfunction, and high levels of natriuretic peptides had also already been associated with worse prognosis in HFrEF [27-29]. Low education and cognition, lack of perception of the disease, or the low number of patients evaluated may have limited the association of quality of life with other variables. Another hypothesis would be that sarcopenia could not be associated with worse quality of life per se, but it is unlikely in the face of the vast research information showing the intersection between sarcopenia and fragility, and both are considered markers of morbimortality and worse general prognosis [1].
This study was performed exclusively in patients with HFrEF who, despite the structural severity of the heart disease, were physically active, were at optimal pharmacological treatment, and did not generally show marked functional limitation. We considered that all these aspects increased the diagnostic specificity and the value of the study findings.
This was a cross-sectional study; therefore, cause-effect relationships were not possible. Some aspects of sample selection and the impaired cognition of patients also need to be considered as potential bias. Rigid exclusion criteria applied with the purpose of reducing secondary causes of sarcopenia and confounding factors limited our sample number.
Conclusion
We observed that there was no difference in PS and sarcopenia between the HFG and the CG and that these entities were independently associated with aging. We also observed that the prevalence of PS and sarcopenia was higher than that previously described outside HFrEF. Lastly, low physical performance was associated with right ventricular dysfunction and with lower left ventricular ejection fraction.
Fragility is very important in aging and in the HF context. New longitudinal observational and interventional studies that can analyze exercise, rehabilitation, and pharmacotherapy will contribute to elucidating a cause and effect relationship and possible new interventions to treat and prevent muscle wasting, low physical performance, and strength whether in the HFrEF context or not.
Acknowledgements
We would like to express our thanks for the support received for this study, in particular to all the employees of the Serviço de Endocrinologia e Metabologia do Paraná (SEMPR), especially to Nurse Amanda Iseid Labres, Estela de Paula, and Ms. Elizabeth Coelho. Also, we thank the Department of Postgraduation in Internal Medicine of the Federal University of Parana, the nutritionist Thais Bisconcinni, and the Chief of the Hospital Immunochemistry Unity (UAD-HC/UFPR), Ms. Gislaine Custodio.
Disclosure of conflict of interest
None.
References
- 1.Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. 2017;36:1–10. doi: 10.1016/j.arr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. J Am Med Dir Assocation. 2013;14:392–7. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longev Healthspan. 2013;2:8. doi: 10.1186/2046-2395-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Springer J, Springer JI, Anker SD. Muscle wasting and sarcopenia in heart failure and beyond: update 2017. ESC Heart Fail. 2017;4:492–8. doi: 10.1002/ehf2.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saitoh M, Ishida J, Doehner W, von Haehling S, Anker MS, Coats AJS, Anker SD, Springer J. Sarcopenia, cachexia, and muscle performance in heart failure: review update 2016. Int J Cardiol. 2017;238:5–11. doi: 10.1016/j.ijcard.2017.03.155. [DOI] [PubMed] [Google Scholar]
- 7.Zamboni M, Rossi AP, Corzato F, Bambace C, Mazzali G, Fantin F. Sarcopenia, cachexia and congestive heart failure in the elderly. Endocr Metab Immune Disord Drug Targets. 2013;13:58–67. doi: 10.2174/1871530311313010008. [DOI] [PubMed] [Google Scholar]
- 8.Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, Anker SD, von Haehling S. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF) Eur Heart J. 2013;34:512–9. doi: 10.1093/eurheartj/ehs381. [DOI] [PubMed] [Google Scholar]
- 9.Hajahmadi M, Shemshadi S, Khalilipur E, Amin A, Taghavi S, Maleki M, Malek H, Naderi N. Muscle wasting in young patients with dilated cardiomyopathy. J Cachexia Sarcopenia Muscle. 2017;8:542–8. doi: 10.1002/jcsm.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silva GSF, Bergamaschine R, Rosa M, Melo C, Miranda R, Filho MB. Evaluation of the physical activity level of undergraduation students of health/biology fields. Rev Bras Med Esporte. 2007;13:32e–35e. [Google Scholar]
- 11.Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, Shardell M, Alley DE, Kenny A, Ferrucci L, Guralnik J, Kiel DP, Kritchevsky S, Vassileva MT, Studenski S. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69:584–90. doi: 10.1093/gerona/glu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alemán-Mateo H, Macías L, Esparza-Romero J, Astiazaran-García H, Blancas AL. Physiological effects beyond the significant gain in muscle mass in sarcopenic elderly men: evidence from a randomized clinical trial using a protein-rich food. Clin Interv Aging. 2012;7:225–234. doi: 10.2147/CIA.S32356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Committee to review dietary reference intakes for vitamin D and calcium food and nutrition board. Washington, DC: The Institute of Medicine of the National Academies, National Academies Press; 2011. p. 349.p. 389. [Google Scholar]
- 14.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–58. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doukky R, Avery E, Mangla A, Collado FM, Ibrahim Z, Poulin MF, Richardson D, Powell LH. Impact of dietary sodium restriction on heart failure outcomes. JACC Heart Fail. 2016;4:24–35. doi: 10.1016/j.jchf.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valentova M, von Haehling S, Bauditz J, Doehner W, Ebner N, Bekfani T, Elsner S, Sliziuk V, Scherbakov N, Murín J, Anker SD, Sandek A. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J. 2016;37:1684–91. doi: 10.1093/eurheartj/ehw008. [DOI] [PubMed] [Google Scholar]
- 17.van Diepen S, Majumdar SR, Bakal JA, Mcalister FA, Ezekowitz JA. Heart failure is a risk factor for orthopedic fracture: a population-based analysis of 16,294 patients. Circulation. 2008;118:1946–52. doi: 10.1161/CIRCULATIONAHA.108.784009. [DOI] [PubMed] [Google Scholar]
- 18.Aluoch AO, Jessee R, Habal H, Garcia-Rosell M, Shah R, Reed G, Carbone L. Heart failure as a risk factor for osteoporosis and fractures. Curr Osteoporos Rep. 2012;10:258–69. doi: 10.1007/s11914-012-0115-2. [DOI] [PubMed] [Google Scholar]
- 19.Bergland A, Jarnlo GB, Laake K. Predictors of falls in the elderly by location. Aging Clin Exp Res. 2003;15:43–50. doi: 10.1007/BF03324479. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 21.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 22.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18 - 88 yr. J Appl Physiol. 2000;89:81–8. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher D, Visser M, De Meersman RE, Sepúlveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985) 1997;83:229–39. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 24.Joyce E, Howell EH, Senapati A, Starling RC, Gorodeski EZ. Prospective assessment of combined handgrip strength and Mini-Cog identifies hospitalized heart failure patients at increased post-hospitalization risk. ESC Heart Fail. 2018;5:948–952. doi: 10.1002/ehf2.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delp MD, Duan C, Mattson JP, Musch TI. Changes in skeletal muscle biochemistry and histology relative to fiber type in rats with heart failure. J Appl Physiol (1985) 1997;83:1291–9. doi: 10.1152/jappl.1997.83.4.1291. [DOI] [PubMed] [Google Scholar]
- 26.Melenovsky V, Kotrc M, Borlaug BA, Marek T, Kovar J, Malek I, Kautzner J. Relationships between right ventricular function, body composition, and prognosis in advanced heart failure. J Am Coll Cardiol. 2013;62:1660–1670. doi: 10.1016/j.jacc.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 27.Martinez F. Thyroid hormones and heart failure. Heart Fail Rev. 2016;21:361–4. doi: 10.1007/s10741-016-9556-5. [DOI] [PubMed] [Google Scholar]
- 28.Grande D, Gioia MI, Terlizzese P, Iacoviello M. Heart failure and kidney disease. Adv Exp Med Biol. 2018;1067:219–238. doi: 10.1007/5584_2017_126. [DOI] [PubMed] [Google Scholar]
- 29.Maisel AS, Duran JM, Wettersten N. Natriuretic peptides in heart failure: atrial and B-type natriuretic peptides. Heart Fail Clin. 2018;14:13–25. doi: 10.1016/j.hfc.2017.08.002. [DOI] [PubMed] [Google Scholar]


