Abstract
Aim
Sivelestat sodium, a selective neutrophil elastase inhibitor, is the only commercially available, specific therapy for acute respiratory distress syndrome (ARDS); however, its clinical efficacy is controversial. We aimed to evaluate appropriate indications for its use in ARDS.
Methods
We studied 66 patients with ARDS who were treated with sivelestat sodium. They were divided into survivors (n = 37) or non‐survivors (n = 29) at 60 days, and clinical characteristics were analyzed.
Results
Patients’ backgrounds evaluated with the Acute Physiology and Chronic Health Evaluation II (APACHE II) score and the sequential organ failure assessment (SOFA) score were significantly different between both groups (survivors versus non‐survivors: APACHE II score, 14.7 ± 6.7 versus 20.5 ± 4.7, P < 0.01; SOFA, 7.25 ± 2.5 versus 9.82 ± 3.5, P < 0.01). There were no significant differences in other patients’ characteristics. On receiver operator characteristic analysis of APACHE II scores before the use of sivelestat sodium, the estimated cut‐off value for survival was calculated to be 18.5.
On receiver operator characteristic analysis of the PaO2/FIO2 ratio, the area under the curve was the highest 3 days after the treatment, with the optimal cut‐off point at 198.
Conclusion
An APACHE II score ≤18, and a PaO2/FIO2 ratio >198 at 3 days after the use of sivelestat sodium predicted a good outcome.
Keywords: Acute respiratory distress syndrome, APACHE II score, neutrophil elastase inhibitor, sivelestat sodium
There is no specific therapeutic method available to ameliorate lung damage in patients with acute respiratory distress syndrome. We found that sivelestat sodium, if given early in patients with less severe acute respiratory distress syndrome, could improve clinical outcomes, including mortality.

Introduction
Acute respiratory distress syndrome (ARDS) is an inflammatory pulmonary disease of varying etiology. It is characterized by pulmonary edema accompanied by increased pulmonary vascular permeability.1, 2 There have been significant advances in the understanding of the pathophysiology of ARDS with improvement in fluid management strategies. Although the efficacy of different pharmacological agents has been investigated, there is currently no consensus on optimal therapy.
Sivelestat sodium, a selective neutrophil elastase inhibitor, has been widely used in Japan as treatment for ARDS.3 The use of sivelestat sodium in a mouse model of sepsis‐induced ARDS was shown to significantly inhibit alveolar collapse, hemorrhage, and stromal tissue thickening.4
The clinical efficacy of sivelestat remains controversial. In phase III5 and phase IV6 trials undertaken in Japan, sivelestat sodium contributed to early weaning from the ventilator. In an international multicenter (not including Japan), double‐blind, placebo‐controlled phase II study (Sivelestat Trial in ARDS Patients Requiring Mechanical Ventilation [STRIVE]), no significant difference was observed in ventilator‐free days; however, a higher 180‐day mortality was noted in sivelestat‐treated patients.7
Currently, the clinical efficacy of sivelestat sodium remains controversial. Furthermore, the effects differ between animal models of ARDS and human subjects, probably because subgroups of ARDS patients who might benefit from sivelestat sodium have not yet been clearly identified. Some studies have attempted to identify the target population that might benefit from the use of sivelestat sodium. In acute lung injury secondary to sepsis, a serum procalcitonin level of 0.5 ng/mL or higher at the time of diagnosis suggests efficacy.8 The ARDS patients with high red blood cell counts might also benefit from sivelestat sodium.9 However, appropriate indications for sivelestat sodium treatment has not yet been described.
In this retrospective study, we aimed to identify the target subgroup among patients with ARDS who might benefit from treatment with sivelestat sodium.
Methods
Setting and participants
We undertook a single‐center retrospective observational cohort study after obtaining prior approval from the Institutional Review Board of Urayasu Hospital, Juntendo University (Chiba, Japan). Patients who were admitted to our intensive care unit between April 2008 and March 2017 and fulfilled the Berlin definition for ARDS1 were enrolled in this study. We excluded patients younger than 18 years of age, those who died within 48 h of admission, and those who had a “do not attempt resuscitation” order. Patients who did not require invasive or non‐invasive ventilator support were also excluded. Ventilated ARDS patients who were treated with sivelestat sodium were included in this study. Based on survival at 60 days, patients were divided into survivors and non‐survivors.
Treatments
Sivelestat sodium was given as a continuous i.v. infusion at 0.2 mg/kg/h for a maximum of 14 days. As there is no clear guideline on the duration of therapy, the infusion was ceased at the discretion of the attending physician.
Patients were ventilated in the pressure‐controlled or pressure‐support mode with positive end‐expiratory pressure. Treatment was according to the recommendations of the Surviving Sepsis Campaign Guidelines 200810 and 2012,11 the ARDS treatment guideline,12 and the expert consensus for the treatment of disseminated intravascular coagulation (DIC) in Japan.13 Concomitant treatments (polymyxin B‐immobilized fiber, continuous hemodiafiltration, corticosteroids, recombinant human soluble thrombomodulin, antithrombin, and i.v. immunoglobulin) were given at the discretion of the attending physician.
Data collection
Baseline data were collected from patient records. Data were obtained on demographic characteristics and the etiology of ARDS. The PaO2/FIO2 ratio (P/F ratio) based on arterial blood gas analysis, the Sequential Organ Failure Assessment (SOFA) score, the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and the acute phase DIC score were noted before and after the treatment with sivelestat sodium. We also collected data on the ventilator mode and setting, adjuvant treatment, and timing of administration and duration of treatment with sivelestat sodium. The ventilator mode and settings were noted at the time of diagnosis of ARDS.
Statistical analysis
Data are presented as mean ± standard deviation. We used the t‐test to compare the means of continuous variables and the χ2‐test to compare the proportion of categorical variables between the groups. Kaplan‒Meier curves were drawn for survival analysis followed by the log–rank test. Receiver operating characteristic (ROC) curve analysis was used to determine APACHE II scores and the optimal cut‐off values of P/F ratios that predicted survival. A cut‐off value of APACHE II score and the P/F ratio that provided the highest sensitivity and specificity was chosen. Statistical analysis was carried out using spss for Mac version 22 (IBM, Armonk, NY, USA), and a P‐value <0.05 was considered statistically significant.
Results
Patient selection
In total, 8523 patients were admitted to the intensive care unit during the study period; 623 patients were diagnosed with ARDS. One hundred and thirty‐six patients were excluded based on previously mentioned criteria. Of the remaining 487 patients, 421 did not receive sivelestat sodium; 66 patients were thus included in the final analysis. On day 60, there were 37 survivors; 29 patients had died (Fig. 1).
Figure 1.
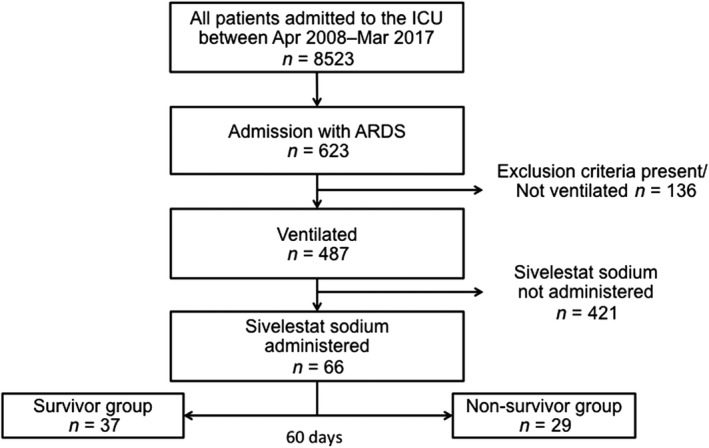
Patient selection. Of the 8,523 patients who were admitted to the intensive care unit (ICU) during the observation period, 66 were diagnosed with acute respiratory distress syndrome (ARDS), mechanically ventilated, and treated with sivelestat sodium. They were divided into two groups: survivors (n = 37) and non‐survivors (n = 29).
Patient characteristics
Patient characteristics are presented in Table 1; Table 2 shows adjuvant treatment in each group. No significant difference was found in patient characteristics between groups. There was a significant difference in the APACHE II scores between the two groups (survivors, 14.7 ± 6.7; non‐survivors, 20.5 ± 4.7; P = 0.0002) and the SOFA score (survivors, 7.25 ± 2.5; non‐survivors, 9.82 ± 3.5; P = 0.0011). In terms of adjuvant treatment, there were significant differences in steroids, continuous hemodiafiltration, recombinant human soluble thrombomodulin, and antithrombin between groups. These differences were because, in the non‐survivor group, many patients might be treated for organ failure such as DIC and renal failure. Additionally, corticosteroids were given more often in the non‐survivor group as a “last resort” therapy.
Table 1.
Characteristics of patients with acute respiratory distress syndrome (ARDS) treated with sivelestat sodium
| All (n = 66) | Survivor group (n = 37) | Non‐survivor group (n = 29) | P‐value | |
|---|---|---|---|---|
| No. of patients (M/F) | 66 (51/15) | 37 (26/11) | 29 (25/4) | 0.1291 |
| Age (years) | 69.0 ± 16.0 | 68 ± 15 (24–89) | 70 ± 16 (22–96) | 0.6281 |
| Etiology of ARDS (n)† | ||||
| Direct injury | ||||
| Respiratory infection | 57 | 31 | 26 | 0.3357 |
| Pulmonary contusion | 3 | 3 | 0 | |
| Drowning | 3 | 3 | 0 | |
| Inhalational burn injury | 3 | 1 | 2 | |
| Indirect injury | ||||
| Sepsis | 14 | 8 | 6 | |
| Drug intoxication | 2 | 2 | 0 | |
| Other | 3 | 2 | 1 | |
| PaO2/FIO2 ratio | 161.5 ± 59.0 | 169.7 ± 51.5 | 150.6 ± 67.1 | 0.1980 |
| APACHE II score | 17.2 ± 6.5 | 14.7 ± 6.7 | 20.5 ± 4.7 | 0.0002 |
| SOFA score | 8.38 ± 3.2 | 7.25 ± 2.5 | 9.82 ± 3.5 | 0.0011 |
| Acute phase DIC score | 3.4 ± 1.8 | 3.1 ± 1.8 | 3.9 ± 1.8 | 0.0970 |
| Treatment period | 6.0 ± 3.2 | 6.2 ± 3.2 | 5.8 ± 3.3 | 0.2761 |
| Ventilator mode | ||||
| Assist control | ||||
| APRV | 57 (86.4) | 32 (86.5) | 25 (86.2) | 0.9554 |
| SIMV | 5 (7.6) | 3 (8.1) | 2 (6.8) | |
| Ventilator setting† | 4 (6.1) | 2 (5.4) | 2 (6.8) | |
| Mean PEEP (cm H2O) | ||||
| Mean Plateau P (cm H2O) | 8.3 ± 3.0 | 8.3 ± 3.3 | 8.4 ± 2.8 | 0.8980 |
| Tidal volume (mL/kg) | 24.1 ± 3.6 | 23.5 ± 3.2 | 24.5 ± 4.0 | 0.1390 |
Data are reported as mean ± standard deviation or n (%).
APACHE, Acute Physiology and Chronic Health Evaluation; APRV, airway pressure release ventilation; DIC, disseminated intravascular coagulation; F, female; M, male; PEEP, positive end‐expiratory pressure; Plateau P, plateau pressure; SIMV, synchronized intermittent mandatory ventilation; SOFA, sequential organ failure assessment.
Multiple choices allowed.
Table 2.
Adjuvant treatment in patients with acute respiratory distress syndrome treated with sivelestat sodium
| All (n = 66) | Survivor group (n = 37) | Non‐survivor group (n = 29) | P‐value | |
|---|---|---|---|---|
| Steroids† | 26 (39.4) | 6 (17.6) | 20 (62.5) | <0.0001 |
| PMX | 5 (7.6) | 2 (5.9) | 3 (9.4) | 0.4517 |
| CHDF | 15 (22.7) | 5 (14.7) | 10 (31.3) | 0.0438 |
| rhTM | 21 (31.8) | 5 (14.7) | 16 (50.0) | 0.0003 |
| IVIG | 17 (25.8) | 8 (23.5) | 9 (28.1) | 0.3854 |
| AT3 | 23 (34.8) | 8 (23.5) | 15 (46.8) | 0.0109 |
Data are reported as n (%).
AT3, antithrombin; CHDF, continuous hemodiafiltration; IVIG, i.v. immunoglobulin; PMX, polymyxin B‐immobilized direct hemoperfusion; rhTM, recombinant human soluble thrombomodulin.
Includes both hydrocortisone 200–300 mg/day and steroid pulse therapy, which is treated by 3 days of methylprednisolone 1,000 mg/day.
Figure 2 shows the survival curves in both groups. The duration of survival in the non‐survivor group ranged from 2 to 42 days; median survival was 16 days. In the survivor group, three of 37 patients died between 60 and 90 days after admission.
Figure 2.
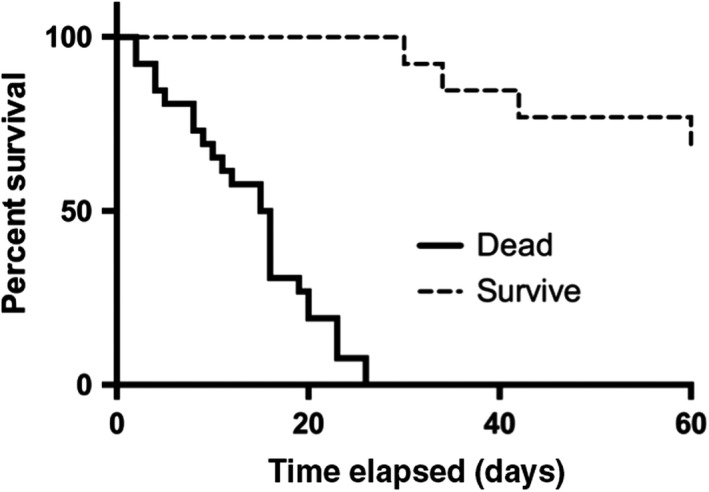
Survival curve in the survivor and the non‐survivor groups among patients admitted to the intensive care unit with acute respiratory distress syndrome. The X‐axis represents days elapsed, and the Y‐axis represents percent of survival. In the non‐survivor group, all deaths occurred within 42 days; three of 37 patients in the survivor group died between 60 and 90 days after admission.
Figure 3 shows the ROC curve of APACHE II scores that predicted survival before treatment with sivelestat sodium (area under the curve [AUC] 0.696, P = 0.003). The estimated cut‐off value for survival calculated with the Youden index was 18.5.
Figure 3.
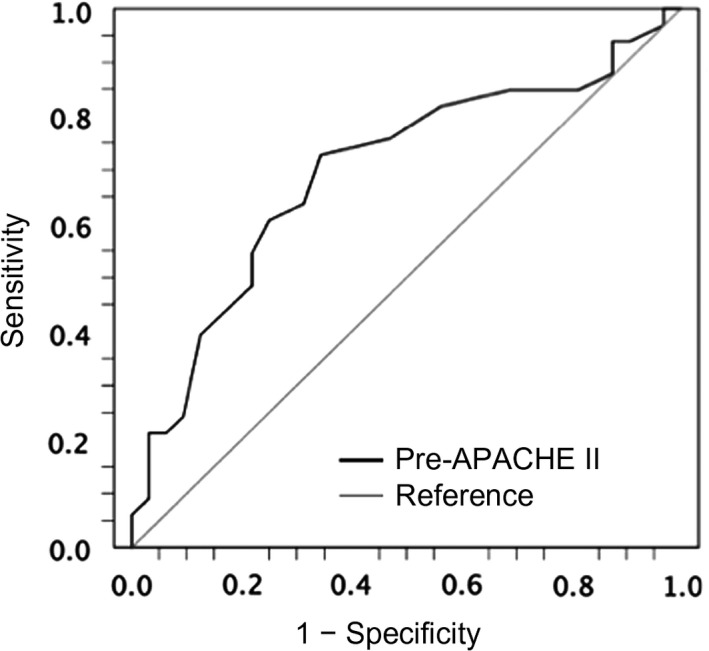
Receiver operating characteristic curve of Acute Physiology and Chronic Health Evaluation (APACHE) II score for survival among patients with acute respiratory distress syndrome before treatment with sivelestat sodium. The X‐axis represents 1 − specificity, and the Y‐axis represents sensitivity. The area under the curve was 0.696 (P = 0.003; 95% confidence interval, 0.566–0.827), and the optimal cut‐off for survival was 18.5.
Oxygenation index in both groups
Figure 4 shows the oxygenation index before and after treatment with sivelestat sodium in each group. Post‐treatment P/F ratios were calculated at 5 days after sivelestat sodium administration. Pretreatment P/F ratios were not significantly different between groups; however, the post‐treatment P/F ratios were significantly better among survivors.
Figure 4.
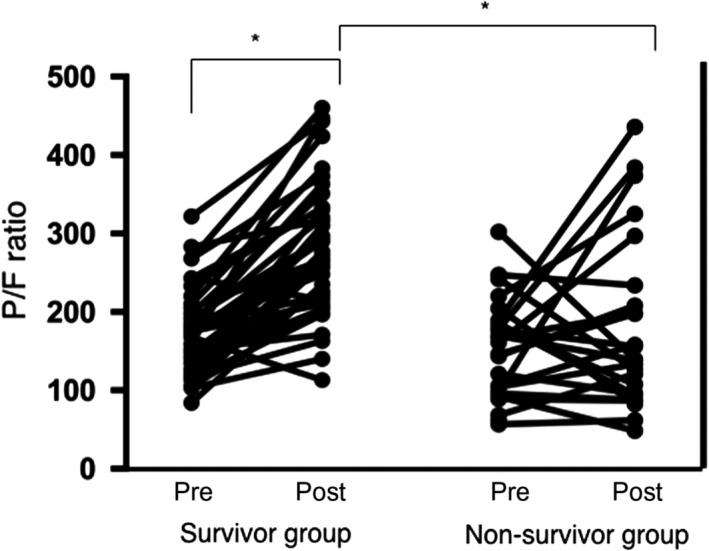
PaO2/FIO2 (P/F) ratios before and after sivelestat sodium treatment in survivor and non‐survivor groups of patients with acute respiratory distress syndrome. Y‐axis represents P/F ratios. Pretreatment P/F ratios were not significantly different between groups; however, post‐treatment P/F ratios were significantly better among survivors. Post, 5 days after treatment; pre, before sivelestat treatment.
Figure 5 shows the serial change of P/F ratios. The P/F ratios among survivors improved significantly after treatment with sivelestat sodium. The P/F ratios on days 2, 3, 4, and 5 after treatment were significantly higher than the P/F ratios before treatment among survivors. In contrast, the P/F ratios were nearly unchanged among non‐survivors during the 5‐day period after treatment with sivelestat sodium.
Figure 5.
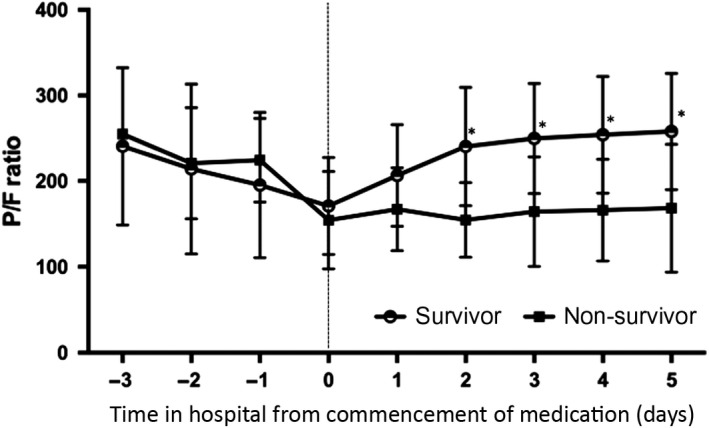
Progression of oxygenation in survivor (open circle) and non‐survivor (closed square) groups of patients with acute respiratory distress syndrome. The X‐axis represents the time from treatment with sivelestat sodium (day 0, first day of sivelestat treatment). The Y‐axis represents the PaO2/FIO2 (P/F) ratio. The P/F ratios on days 2, 3, 4, and 5 after treatment were significantly higher than the P/F ratios before treatment among survivors.
Figure 6 shows the ROC curve of P/F ratios that predicted survival on each day following treatment with sivelestat sodium. The dependent variable was survival, and the independent variable was the P/F ratio from days −1 to 5. The AUC on day 3 was the most accurate predictor of survival. The optimal cut‐off value of the P/F ratio for survival was 198 on day 3 after treatment with sivelestat sodium.
Figure 6.
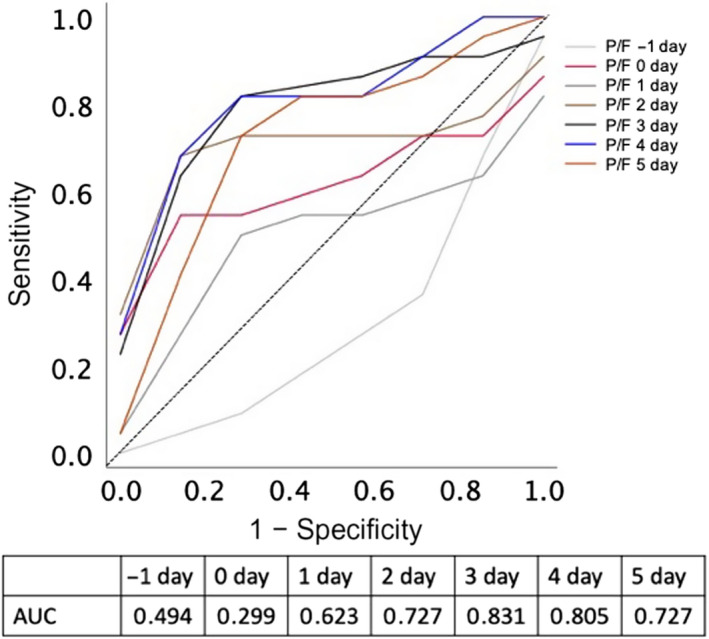
Receiver operating characteristic curve of PaO2/FIO2 (P/F) ratios for survival on each day (days −1 to 5) after treatment with sivelestat sodium among patients with acute respiratory distress syndrome. The X‐axis represents 1 – specificity; the Y‐axis represents sensitivity. The area under the curve (AUC) on day 3 was the highest, and the optimal cut‐off of the oxygenation index for survival was 198 mmHg.
Discussion
We investigated the predictive factors for improved outcomes in patients treated with sivelestat sodium for ARDS. There was no significant difference in P/F ratios between the two groups before treatment with sivelestat sodium; however, the APACHE II scores were significantly higher in the non‐survivor group at baseline. Thus, patients with ARDS and an APACHE II score ≤18 could be expected to have a good outcome even though they have poor oxygenation. One day after treatment with sivelestat sodium, oxygenation improved significantly among survivors. A P/F ratio of >198 mmHg on day 3 was a good predictor of survival at 60 days. Based on Figure 3, patients who fulfill the Berlin definition of ARDS with an APACHE II score ≤18 could benefit from multimodal therapy, including sivelestat sodium.
Previous studies8, 14 have also shown that baseline oxygenation before treatment with sivelestat sodium is predictive of outcomes in patients with mild to moderate ARDS. However, the P/F ratio at the time of diagnosis of ARDS was not predictive of 60‐day survival in our study. In the original study that proposed the Berlin definition for ARDS, mortality increased proportionally with the severity of ARDS (mild ARDS, 27%; 95% confidence interval [CI], 24–30; moderate ARDS, 32%; 95% CI, 29–34; severe ARDS, 45%; 95% CI, 42–48; P < 0.001).2 However, recent reports suggest that the classification of severity of ARDS based on P/F ratios according to the Berlin definition is unrelated to mortality.15, 16 Lai et al. reported that the P/F ratio at the time of diagnosis of ARDS was not a reliable predictor of survival; the P/F ratio on day 1 might be more predictive.17 Our findings were similar; the P/F ratio prior to sivelestat sodium treatment did not predict survival in our study. We found that the P/F ratio on day 3 following sivelestat sodium treatment might be more predictive of 60‐day survival. Furthermore, we could identify patients with ARDS who might benefit from sivelestat sodium.
The mortality due to respiratory failure alone in ARDS is 16%, whereas 49% of patients die from multiple organ failure (MOF).18 Hence, the definitive prognostic factor could be the severity of MOF. The APACHE II and the SOFA scores are more frequently used globally. In the present study, the P/F ratios were not significantly different on day 0; however, the APACHE II scores were significantly different between groups. Patients with an APACHE II score ≤18, suggestive of relatively mild disease, had a higher probability of a favorable outcome (Fig. 3). According to the results of the STRIVE study,7 treatment with sivelestat sodium was not beneficial in patients with MOF involving four or more organs, including the lungs. Therefore, based on previous studies and our own findings, sivelestat sodium might not be effective in ARDS patients with more severe MOF.
We found that pretreatment P/F ratios were not significantly different between groups; however, the post‐treatment P/F ratios at 5 days after sivelestat sodium treatment were significantly better among survivors (Fig. 5). The most important point to control ARDS should be control of the basic disease that was induced by ARDS; if the oxygenation does not improve within 1 week, physicians need to review the strategy to control the basic disease.
Our study has several limitations. First, it was a retrospective single‐center study with a small sample size. Multicentric studies with a larger sample size are required to identify appropriate indications for the use of sivelestat sodium. Second, patients were treated with several different interventions including ventilator support, corticosteroids, and other therapeutic techniques in addition to sivelestat sodium. Adjuvant treatments might have confounded our results. In the non‐survivor group, the percentage of the concomitant interventions was significantly higher than in the survivor group, suggesting more severe illness among non‐survivors. Multivariable analysis from a larger dataset is required to assess the impact of adjuvant interventions. Finally, sivelestat sodium was given to all patients in the cohort. Several interventions directed towards respiratory care are currently in use; hence a placebo‐controlled, randomized controlled study is necessary to assess the effectiveness of sivelestat sodium.
Conclusion
In conclusion, relatively mild cases with an APACHE II score ≤18, and a P/F ratio of >198 on day 3 after treatment with sivelestat sodium predicted favorable outcomes among patients with ARDS.
Disclosure
Approval of the research protocol: This study was carried out with the approval of the Ethics Committee of Juntendo University (No. 2015‐022).
Informed consent: All patients who were admitted to the intensive care unit from April 2016 provided written informed consent.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Funding Information
No funding information provided.
References
- 1. Bernard GR, Artigas A, Brigham KL et al The American‐European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994; 149: 818–24. [DOI] [PubMed] [Google Scholar]
- 2. Ranieri VM, Rubenfeld GD, ARDS Definition Task Force et al Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–33. [DOI] [PubMed] [Google Scholar]
- 3. Kawabata K, Suzuki M, Sugitani M, Imaki K, Toda M, Miyamoto T. ONO‐5046, a novel inhibitor of human neutrophil elastase. Biochem. Biophys. Res. Commun. 1991; 177: 814–20. [DOI] [PubMed] [Google Scholar]
- 4. Inoue Y, Seiyama A, Tanaka H et al Protective effects of a selective neutrophil elastase inhibitor (sivelestat) on lipopolysaccharide‐induced acute dysfunction of the pulmonary microcirculation. Crit. Care Med. 2005; 33: 1814–22. [DOI] [PubMed] [Google Scholar]
- 5. Tamakuma S, Ogawa M, Aikawa N et al Relationship between neutrophil elastase and acute lung injury in humans. Pulm. Pharmacol. Ther. 2004; 17: 271–79. [DOI] [PubMed] [Google Scholar]
- 6. Aikawa N, Ishizaka A, Hirasawa H et al Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulm. Pharmacol. Ther. 2011; 24: 549–54. [DOI] [PubMed] [Google Scholar]
- 7. Zeiher BG, Artigas A, Vincent JL et al Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit. Care Med. 2004; 32: 1695–702. [DOI] [PubMed] [Google Scholar]
- 8. Miyoshi S, Hamada H, Ito R et al Usefulness of a selective neutrophil elastase inhibitor, sivelestat, in acute lung injury patients with sepsis. Drug Des. Devel. Ther. 2013; 7: 305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ozawa T, Mihara K, Yasuno N. Predictors of the therapeutic effect of sivelestat in patients with acute lung injury associated with systemic inflammatory response syndrome. J. Pharm. Health Care Sci. 2016; 2: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dellinger RP, Levy MM, Carlet JM et al Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit. Care Med. 2008; 36: 296–327. [DOI] [PubMed] [Google Scholar]
- 11. Dellinger RP, Levy MM, Rhodes A et al Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013; 41: 580–637. [DOI] [PubMed] [Google Scholar]
- 12. Hashimoto S, Sanui M, Egi M et al The clinical practice guideline for the management of ARDS in Japan. J. Intensive Care. 2017; 5: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wada H, Asakura H, Okamoto K et al Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb. Res. 2010; 125: 6–11. [DOI] [PubMed] [Google Scholar]
- 14. Tsushima K, Yokoyama T, Matsumura T, Koizumi T, Kubo K, Tatsumi K. The potential efficacy of noninvasive ventilation with administration of a neutrophil elastase inhibitor for acute respiratory distress syndrome. J. Crit. Care 2014; 29: 420–5. [DOI] [PubMed] [Google Scholar]
- 15. Hernu R, Wallet F, Thiolliere F et al An attempt to validate the modification of the American‐European consensus definition of acute lung injury/acute respiratory distress syndrome by the Berlin definition in a university hospital. Intensive Care Med. 2013; 39: 2161–70. [DOI] [PubMed] [Google Scholar]
- 16. Costa EL, Amato MB. The new definition for acute lung injury and acute respiratory distress syndrome: is there room for improvement? Curr. Opin. Crit. Care. 2013; 19: 16–23. [DOI] [PubMed] [Google Scholar]
- 17. Lai CC, Sung MI, Liu HH et al The ratio of partial pressure arterial oxygen and fraction of inspired oxygen 1 day after acute respiratory distress syndrome onset can predict the outcomes of involving patients. Medicine (Baltimore). 2016; 95: e3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Del SL, Slutsky AS. Acute respiratory distress syndrome and multiple organ failure. Curr. Opin. Crit. Care. 2011; 17: 1–6. [DOI] [PubMed] [Google Scholar]


