Abstract
Aim
Rikkunshito is a traditional Japanese medicine used for delayed gastric emptying in intensive care units in Japan. This study aimed to investigate whether standard‐ or high‐dose rikkunshito can improve the achievement of enteral calorie target among critically ill adults.
Methods
This open‐label, single‐center, pilot randomized controlled trial was carried out from March 2018 until December 2018 and enrolled critically ill adult patients requiring enteral nutrition by gastric tube for at least 5 days. Patients were randomized into the control group, the standard‐dose rikkunshito group (2.5 g three times daily), and the high‐dose rikkunshito group (5 g three times daily). Intervention was given for 5 days. The primary outcome measure was the percentage of enteral calorie intake achieved in the target at the fifth day after randomization.
Results
The cohort comprised 26 patients; of these, 9, 8, and 9 were included in the control group, the standard‐dose group, and the high‐dose group, respectively. Twenty‐one patients (81%) were included in the primary analysis. The percentage of enteral calorie intake achieved in the target at the fifth day was 59% (interquartile range [IQR], 39–63%), 40% (IQR, 26–61%), and 62% (IQR, 17–83%) in the control, the standard‐dose, and the high‐dose groups, respectively (P = 0.42). The number of adverse events did not differ significantly between the groups (control group, 4 [44%]; standard‐dose group, 3 [38%]; and high‐dose group, 4 [44%], P = 1.00).
Conclusions
Standard‐ or high‐dose rikkunshito did not improve the achievement of enteral calorie target in critically ill adults.
Keywords: Enteral nutrition, gastroparesis, ghrelin, prokinetics, rikkunshito
Rikkunshito is a traditional Japanese medicine used for delayed gastric emptying in intensive care units in Japan. In this pilot randomized controlled trial, standard‐ or high‐dose rikkunshito did not improve the achievement of enteral calorie target in critically ill adults.

Background
Early enteral nutrition is crucial in the care of critically ill patients and is associated with improved survival in patients admitted to the intensive care unit (ICU).1 Increased calorie intake is associated with an increased number of ventilator‐free days and decreased mortality.2 However, achieving the enteral nutrition target is often hampered by upper gastrointestinal intolerance that is attributed to delayed gastric emptying.3 A recent guideline suggested prokinetics, including metoclopramide and erythromycin, for upper gastrointestinal intolerance4 as they appear to improve feeding tolerance, but concerns about their adverse effects and unclear effects on clinical outcome prevent their application in clinical practice.5 In Japan, rikkunshito is usually used as a prokinetic for upper gastrointestinal intolerance during enteral nutrition in ICUs.6
Rikkunshito is a traditional Japanese herbal medicine comprising extracts from Glycyrrhizae radix, Zingiberis rhizoma, Atractylodis lanceae rhizoma, Zizyphi fructus, Aurantii nobilis pericarpium, Ginseng radix, Pinelliae tuber, and Hoelen. Rikkunshito has been reported to ameliorate upper gastrointestinal symptoms in patients with functional dyspepsia and gastroesophageal reflux disease.7, 8 However, data about the effect of rikkunshito in the critical care setting are scarce. An anecdotal case report showed that rikkunshito effectively reduced the gastric residual volume and contributed to enteral nutrition success in patients with upper gastrointestinal intolerance.6 Furthermore, a small randomized trial showed greater prokinetic effect of rikkunshito than metoclopramide among critically ill patients who required gastric tube feeding for more than 7 days.9 However, there was no significant difference in the proportion of patients achieving successful enteral feeding or the mortality rate between the control and the intervention group in this trial.9
The clinical value of rikkunshito in the critical care setting is poorly understood. It is also unclear whether or not a higher dose of rikkunshito is more effective for achieving the enteral nutrition target. Therefore, this study aimed to investigate whether standard‐ or high‐dose rikkunshito can improve the achievement of enteral calorie target among critically ill adults.
Methods
Study design
This trial was a pilot, single‐center, open‐label randomized controlled trial undertaken among critically ill patients who were predicted to require enteral nutrition by gastric tube for longer than 5 days. This trial was carried out in a mixed ICU in Wakayama Medical University Hospital (Wakayama, Japan) between March 2018 and December 2018. The trial was terminated early because of slow recruitment.
This study was approved by the institutional review board of Wakayama Medical University (approval no. 1820) and was registered at UMIN Clinical Trial Registry on March 1, 2018 (registration no. UMIN000031466, https://upload.umin.ac.jp/cgi‐open‐bin/ctr/ctr_view.cgi?recptno=R000035918). Written informed consent was obtained from patients or legally authorized guardians before study enrollment.
Patients
Patients were eligible if they were: (i) aged 20 years or older, (ii) admitted to the ICU, (iii) about to commence enteral nutrition by gastric tube within 48 h from ICU admission, (iv) expected to require enteral nutrition longer than 5 days. Patients were excluded if they: (i) were expected not to survive longer than 48 h, (ii) decided to withdraw or withhold treatment, (iii) were known to have an allergy to the component of rikkunshito, (iv) had anatomical abnormalities in the esophagus or stomach (e.g., post‐gastrectomy patients), (v) were pregnant or lactating women.
The patients were randomly assigned to receive one of three interventions: standard‐dose rikkunshito, high‐dose rikkunshito, or no rikkunshito (control). After obtaining informed consent, randomization was immediately carried out using permuted block randomization in a 1:1:1 ratio and the block size of 4 using an envelope method stratified by postoperative status or not.
Intervention
In the standard‐ and high‐dose rikkunshito groups, patients received 2.5 g or 5 g rikkunshito three times daily, respectively, before each enteral nutrition by gastric tube. Intervention was continued until the fifth day after randomization or the day patients started oral intake, whichever came first. Except for rikkunshito, we started and increased enteral feeding according to the hospital's predefined protocol (Fig. S1). In brief, all patients received dense enteral nutrition (1 mL = 1.5 kcal; Peptamen standard; Nestle Health Science, Vevey, Switzerland) from the next morning after randomization. We started enteral nutrition of 100 mL for 4 h three times daily, and the gastric tube was clamped for 1 h following each enteral feeding. If the gastric residual volume was <300 mL/day and the patient did not have vomiting or diarrhea, the dose was increased by 50 mL the next morning. If the gastric residual volume was ≥300 mL/day, use of prokinetics (e.g., metoclopramide) was permitted. The enteral nutrition target was set at 25 kcal/kg/day by actual body weight. If the patients were severely obese (body mass index ≥30 kg/m2), we used the ideal body weight for calculating the enteral nutrition target.
Primary and secondary outcomes
The primary outcome measure was the percentage of the target enteral calorie intake achieved at the fifth day after randomization. The secondary outcome measures were the plasma levels of ghrelin, ICU mortality, hospital mortality, ICU length of stay, and hospital length of stay. We measured the plasma levels of ghrelin at days 1 (baseline), 3, and 5 in the morning just before starting the enteral nutrition of the day. Blood samples were collected in tubes containing aprotinin and EDTA‐2Na and were immediately centrifuged at 4°C. We added 1 mol/L HCl in the serum, and then stored it in the deep refrigerator until the measurement. The concentrations of acylated ghrelin (active ghrelin) and deacylated ghrelin (inactive ghrelin) were measured using a fluorescent enzyme immunoassay (Tosoh, Tokyo, Japan).
We also evaluated the adverse events during the intervention period. Predefined adverse events included hypokalemia (potassium level <3 mEq/L), elevated creatine kinase (>2,480 IU/L), elevated liver enzymes (aspartate aminotransferase >300 IU/L or alanine aminotransferase >420 IU/L), skin rash needing treatment, diarrhea (moderate or severe watery diarrhea ≥3 times per day), and vomiting. The success rate of enteral nutrition was set as post hoc analysis, and successful enteral nutrition was defined as achieving ≥60% of the enteral nutrition target at the fifth day or starting oral intake within 5 days.
Statistical analysis
Continuous variables are presented as medians and interquartile ranges; categorical variables are presented as numbers and percentages (%). All outcomes were analyzed according to intention‐to‐treat analysis. Missing data were analyzed without imputation. For the exploratory purpose of this pilot study, we did not perform a priori sample size estimation.
Between‐group comparisons were carried out using the Kruskal–Wallis test for continuous variables and Fisher's exact test for categorical variables. Furthermore, the Wilcoxon test adjusted using the Bonferroni method was used to compare between two groups. A two‐sided P‐value of <0.05 was considered statistically significant. All analyses were undertaken using JMP Pro software (version 12.2; SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 26 patients were enrolled and randomized into the control group (n = 9), the standard‐dose group (n = 8), and the high‐dose group (n = 9) (Fig. 1). There was no significant difference in patient characteristics between the three groups (Table 1). All patients were treated with invasive mechanical ventilation. All patients in the standard‐dose group and the high‐dose group received at least one intervention as randomized. No patients were prescribed prokinetics (metoclopramide or erythromycin) during the intervention period. All patients received gastric tube feeding, and post‐pyloric tube was not used in any patient.
Figure 1.
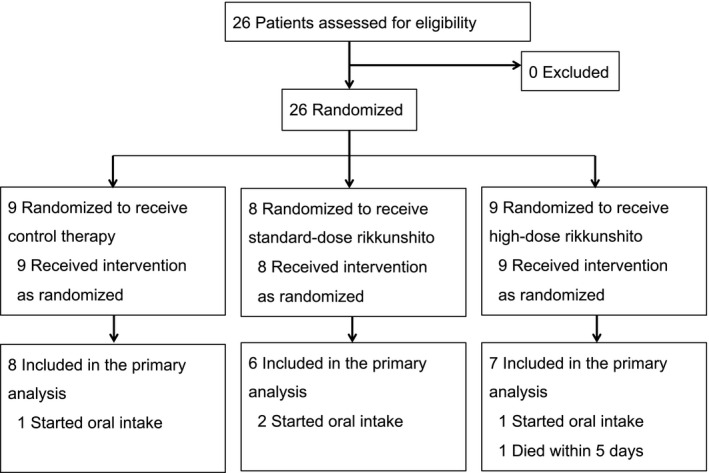
Enrollment flowchart of critically ill adults to investigate whether rikkunshito can improve the achievement of enteral calorie targets.
Table 1.
Characteristics of critically ill adults who received 2.5 g (standard dose) or 5 g (high dose) rikkunshito three times daily
| Control group (n = 9) | Standard‐dose group (n = 8) | High‐dose group (n = 9) | P‐value | |
|---|---|---|---|---|
| Age, years; median (IQR) | 70 (57–75) | 73 (71–79) | 82 (70–85) | 0.15 |
| Male, n (%) | 6 (67) | 4 (50) | 8 (89) | 0.25 |
| Body weight, kg; median (IQR) | 65 (57–70) | 57 (46–79) | 65 (47–69) | 0.67 |
| ICU admission route, n (%) | ||||
| Emergency department | 8 (89) | 8 (100) | 9 (100) | 1.00 |
| Operating room | 1 (11) | 0 (0) | 0 (0) | |
| Reason for ICU admission, n (%) | ||||
| Sepsis | 2 (22) | 4 (50) | 5 (56) | 0.31 |
| Trauma | 4 (44) | 3 (38) | 1 (11) | |
| Neurologic disease | 2 (22) | 0 (0) | 1 (11) | |
| Others | 1 (11) | 1 (11) | 2 (22) | |
| APACHE II score at ICU admission, median (IQR) | 23 (20–26) | 21 (16–30) | 23 (17–28) | 0.94 |
| Chronic diagnosis included in APACHE II score, n (%) | ||||
| Chronic hemodialysis | 0 (0) | 0 (0) | 1 (11) | 1.00 |
| Others | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| SOFA score at ICU admission, median (IQR) | 6 (4–10) | 6 (4–8) | 8 (5–12) | 0.48 |
Between‐group comparisons were undertaken using the Kruskal–Wallis test for continuous variables and Fisher's exact test for categorical variables.
APACHE II, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
Percentage of enteral calorie intake
The percentage of the target enteral calorie intake achieved gradually increased within 5 days in all three groups (Fig. 2). On the second day, 4 (44%), 6 (75%), and 4 (44%) patients in the control, standard‐dose, and high‐dose groups, respectively, failed to show increase in enteral calorie intake (P = 0.43). At the fifth day, the percentage of enteral calorie intake was 59% (interquartile range [IQR], 39–63%), 40% (IQR, 26–61%), and 62% (IQR, 17–83%) in the control, standard‐dose, and high‐dose group, respectively (P = 0.42). The actual enteral calorie intake at the fifth day was 850 kcal/day (IQR, 750–1,181 kcal/day), 750 kcal/day (IQR, 431–994 kcal/day), and 1,050 kcal/day (IQR, 300–1,275 kcal/day), respectively (P = 0.57; Fig. 3). The success rate of enteral nutrition at the fifth day among surviving patients was 56% (5 patients), 38% (3 patients), and 63% (5 patients) in the control, standard‐dose, and high‐dose group, respectively (P = 0.69). We could not find significant differences in enteral calorie intake between the three groups at any time point within the 5 days of intervention. Furthermore, the gastric residual volume during the intervention period was not significantly different between the three groups (Fig. 4).
Figure 2.
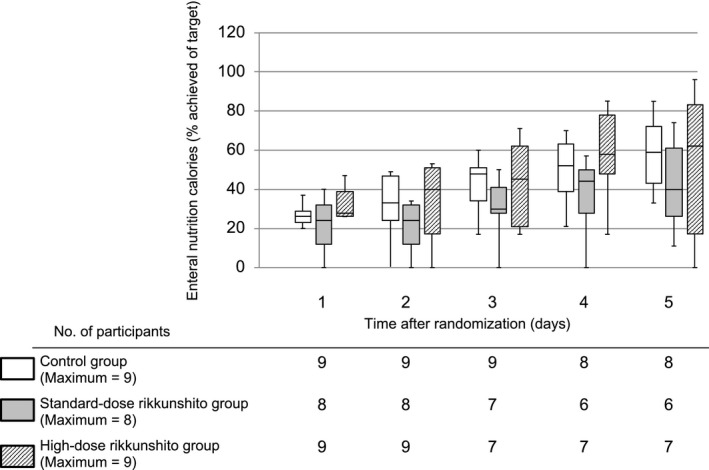
Percentage of enteral calorie intake targets achieved in critically ill adults within 5 days of treatment with rikkunshito. Data are shown as maximum–minimum and median and interquartile ranges.
Figure 3.
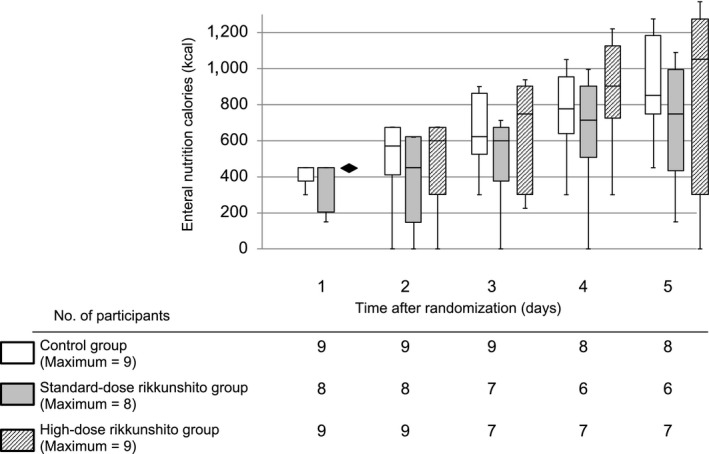
Actual enteral calorie intake in critically ill adults within 5 days of treatment with rikkunshito. Data are shown as maximum–minimum and median and interquartile ranges.
Figure 4.
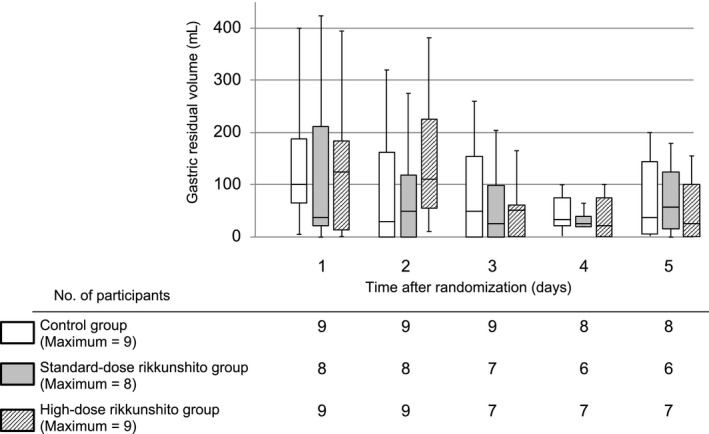
Gastric residual volume in critically ill adults within 5 days of treatment with rikkunshito. Data are shown as maximum–minimum and median and interquartile ranges.
Ghrelin level
The plasma levels of ghrelin are shown in Table 2. There were no significant differences in the plasma levels of active ghrelin between the three groups at any time point. In contrast, the plasma levels of desacyl ghrelin were higher in the high‐dose group than in the control group (P = 0.052) and the standard‐dose group (P = 0.03) at day 1 (baseline).
Table 2.
Plasma levels of ghrelin in critically ill adults who received 2.5 g (standard dose) or 5 g (high dose) rikkunshito three times daily
| Control group (n = 9) | Standard‐dose group (n = 8) | High‐dose group (n = 9) | P‐value | |
|---|---|---|---|---|
| Day 1 (baseline) | ||||
| Number of patients | 9 | 7 | 7 | |
| Active ghrelin, fmol/mL; median (IQR) | 10.2 (4.8–22.9) | 6.4 (3.9–19.3) | 8.2 (2.3–18.5) | 0.82 |
| Desacyl ghrelin, fmol/mL; median (IQR) | 25.3 (16.4–29.7) | 15.8 (12.5–17.6) | 44.8 (28.5–145.3) | 0.01 |
| Day 3 | ||||
| Number of patients | 8 | 7 | 7 | |
| Active ghrelin, fmol/mL; median (IQR) | 19.6 (11.1–63.5) | 16.5 (8.4–64.9) | 39.4 (15.1–47.0) | 0.60 |
| Desacyl ghrelin, fmol/mL; median (IQR) | 27.3 (20.1–46.0) | 22.8 (13.3–49.5) | 24.0 (18.8–61.6) | 0.70 |
| Day 5 | ||||
| Number of patients | 9 | 6 | 7 | |
| Active ghrelin, fmol/mL; median (IQR) | 46.4 (17.3–65.8) | 20.5 (10.2–94.2) | 36.9 (25.2–42.9) | 0.73 |
| Desacyl ghrelin, fmol/mL; median (IQR) | 28.6 (21.4–95.8) | 29.1 (12.3–119.6) | 43.9 (16.2–57.4) | 0.90 |
| Change from baseline | ||||
| Day 3 | ||||
| Number of patients | 8 | 6 | 6 | |
| Active ghrelin, fmol/mL; median (IQR) | 9.2 (4.3–40.5) | 9.5 (5.0–55.8) | 14.7 (8.0–44.5) | 0.52 |
| Desacyl ghrelin, fmol/mL; median (IQR) | 5.2 (0.1–16.4) | 6.1 (−3.0 to 43.6) | −5.9 (−49.1 to 10.4) | 0.37 |
| Day 5 | ||||
| Number of patients | 9 | 5 | 6 | |
| Active ghrelin, fmol/mL; median (IQR) | 21.1 (4.9–51.9) | 9.5 (4.8–85.0) | 21.2 (12.9–36.7) | 0.91 |
| Desacyl ghrelin, fmol/mL; median (IQR) | 4.7 (1.2–41.1) | 7.3 (−0.3 to 92.5) | −9.3 (−32.7 to −0.3) | 0.02 |
Between‐group comparisons were undertaken using the Kruskal–Wallis test.
IQR, interquartile range.
Other secondary outcomes, including ICU mortality, hospital mortality, ICU length of stay, and hospital length of stay, were similar in the three groups (Table 3). We also did not find any differences in adverse events between the three groups during the intervention period (Table 4).
Table 3.
Secondary outcomes in critically ill adults who received 2.5 g (standard dose) or 5 g (high dose) rikkunshito three times daily
| Control group (n = 9) | Standard‐dose group (n = 8) | High‐dose group (n = 9) | P‐value | |
|---|---|---|---|---|
| ICU mortality, n (%) | 0 (0) | 1 (13) | 2 (22) | 0.50 |
| Hospital mortality, n (%) | 1 (11) | 1 (13) | 2 (22) | 1.00 |
| ICU length of stay, days; median (IQR)a | 7 (4–9) | 6 (3–8) | 5 (4–14) | 0.92 |
| Hospital length of stay, days; median (IQR)a | 56 (35–82) | 35 (30–40) | 42 (26–48) | 0.19 |
Between‐group comparisons were undertaken using the Kruskal–Wallis test for continuous variables and Fisher's exact test for categorical variables.
Length of stay was calculated after excluding patients who died in the hospital.
ICU, intensive care unit; IQR, interquartile range.
Table 4.
Adverse events in critically ill adults during the intervention period who received 2.5 g (standard dose) or 5 g (high dose) rikkunshito three times daily
| Control group (n = 9) | Standard‐dose group (n = 8) | High‐dose group (n = 9) | P‐value | |
|---|---|---|---|---|
| Any adverse events, n (%) | 4 (44) | 3 (38) | 4 (44) | 1.00 |
| Hypokalemia, n (%) | 0 (0) | 0 (0) | 1 (11) | 1.00 |
| Elevated creatine kinase, n (%) | 3 (33) | 1 (13) | 0 (0) | 0.18 |
| Elevated liver enzyme, n (%) | 1 (11) | 0 (0) | 2 (22) | 0.75 |
| Skin rash, n (%) | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Diarrhea, n (%) | 1 (11) | 2 (25) | 2 (22) | 0.84 |
| Vomiting, n (%) | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Increased gastric residual volume, ≥300 mL/day; n (%) | 2 (22) | 1 (13) | 1 (11) | 1.00 |
Between‐group comparisons were undertaken using Fisher's exact test.
Discussion
In this pilot randomized controlled study, neither standard‐dose nor high‐dose rikkunshito increased the achievement of enteral calorie intake target in critically ill adult patients who required enteral nutrition by a gastric tube. There were no significant differences in gastric residual volume or the plasma levels of active ghrelin between the three intervention groups. The adverse events also did not differ significantly between the three groups during the intervention period.
Ghrelin is a peptide hormone, also known as an “appetite‐stimulating hormone,” produced in the stomach and accelerates gastric emptying.10 Previous animal studies implied that rikkunshito improved gastric emptying through increasing the action of ghrelin by ameliorating ghrelin resistance11 or inhibiting ghrelin deacylation (inactivation).12 However, clinical trials showed inconclusive results, and a recent meta‐analysis could not find any influence of rikkunshito on plasma levels of active ghrelin in patients with various diseases.13 Similar to these findings, we also could not find any differences in the plasma levels of active ghrelin between the three study groups.
Data for the use of rikkunshito in the acute phase are scarce. Tatsumi et al.6 reported three cases in which rikkunshito dramatically reduced gastric residual volume within a few days and enabled enteral nutrition. In a small randomized trial, Hayakawa et al. compared enteral nutrition success rates as the primary outcome between rikkunshito and metoclopramide in critically ill patients needing enteral nutrition. The intervention period was 10 days, and the nutrition intervention included continuous infusion of enteral nutrition.9 In our study, the intervention period was 5 days, and intermittent infusion of enteral nutrition was used. Although Hayakawa's study could not find any significant difference in primary outcome, it found that rikkunshito accelerated the achievement of 50% of the target calories and increased the plasma levels of active ghrelin.9 In contrast, we could not find any differences in enteral calorie intake, gastric residual volume, or the plasma levels of active ghrelin within 5 days between the study groups. Currently, it remains unclear whether this difference is attributable to differences in the enteral nutrition protocol or the length of the observation period.
Both our study and Hayakawa's study could not reveal any significant difference in clinically important outcomes, such as mortality and ICU length of stay.9 While it is unclear whether rikkunshito increases the enteral calorie intake, prophylactic rikkunshito does not seem to influence clinically important outcomes, such as mortality, in critically ill patients who start enteral nutrition. Currently, evidence to support routine prophylactic use of rikkunshito in the ICU is lacking. However, the effect of rikkunshito as a treatment itself and not as prophylaxis for upper gastrointestinal intolerance is yet to be clarified.
Our study has several limitations. First, our study was an open‐label study, which could bias the evaluation of outcome. In the clinical setting, it was difficult to blind staff to the intervention. To minimize bias in the primary outcome, we used the predefined enteral nutrition protocol. Second, the clinical implication of the primary outcome evaluated in our study is unclear. It is unknown whether increased enteral calorie intake improves survival or lowers the complication rate. A large randomized trial found no significant difference in survival between those given initial full enteral feeding and those given initial trophic enteral feeding among patients who were mechanically ventilated.14 The clinical impact of monitoring the gastric residual volume is also controversial. A randomized trial even showed that the “ignore the gastric residual volume” strategy was shown to be superior to monitoring gastric residual volume.15 Future trials should focus on clinically important outcomes, such as mortality, to determine the usefulness of rikkunshito in critically ill patients. Third, approximately 20% of our cohort dropped out from the primary analysis, which biased the patients’ characteristics in each group. The main reason of dropout was starting oral intake within 5 days. We added a sensitivity analysis that included these patients and evaluated the success rate of enteral nutrition. However, we could find no difference in the success rate between the groups. Finally, the number of patients in the control group showing increased gastric residual volume was lower than anticipated (only two cases). Although rikkunshito might improve upper gastrointestinal intolerance, such as by decreasing gastric residual volume, multiple other factors could hamper achievement of the enteral nutrition goal. This could weaken our study results, and thus, future studies should include patients with higher risk of upper gastrointestinal intolerance.
Conclusion
STANDARDE‐ OR HIGH‐DOSE rikkunshito did not improve the achievement of enteral calorie targets in critically ill patients requiring enteral nutrition through a gastric tube. Future trials are needed to evaluate the prokinetic effect of rikkunshito in patients with upper gastrointestinal intolerance.
Disclosure
Approval of the research protocol: The protocol for this research project was approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. Committee of Wakayama Medical University, approval no. 1820.
Informed consent: Informed consent was obtained from the subjects or guardians.
Registry and the registration no. of the trial: UMIN‐CTR, UMIN000031466 (Registered March 1, 2018, https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000035918).
Animal Studies: N/A.
Conflict of interest: KM received lecture fees from Radiometer, Becton Dickinson, and Maruishi Pharmaceutical.
Supporting information
Fig. S1. Enteral nutrition protocol.
Acknowledgments
We thank Asako Doi and Shuji Kawashima for their assistance in measurement of the plasma ghrelin level. We also thank Hisaya Iwaki, Kaoru Yoshida, and Masaou Tanaka, members of the data and safety monitoring board committees. Finally, we thank Editage for providing English language editing.
Funding Information
No funding information provided.
References
- 1. Artinian V, Krayem H, DiGiovine B. Effects of early enteral feeding on the outcome of critically ill mechanically ventilated medical patients. Chest 2006; 129: 960–7. [DOI] [PubMed] [Google Scholar]
- 2. Alberda C, Gramlich L, Jones N et al The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009; 35: 1728–37. [DOI] [PubMed] [Google Scholar]
- 3. Mentec H, Dupont H, Bocchetti M et al Upper digestive intolerance during enteral nutrition in critically ill patients: frequency, risk factors, and complications. Crit. Care Med. 2001; 29: 1955–61. [DOI] [PubMed] [Google Scholar]
- 4. McClave SA, Taylor BE, Martindale RG et al Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enteral Nutr. 2016; 40: 159–211. [DOI] [PubMed] [Google Scholar]
- 5. Booth CM, Heyland DK, Paterson WG. Gastrointestinal promotility drugs in the critical care setting: a systematic review of the evidence. Crit. Care Med. 2002; 30: 1429–35. [DOI] [PubMed] [Google Scholar]
- 6. Tatsumi H, Masuda Y, Imaizumi H et al Usefulness of the traditional Chinese medicine rikkunshito for improving delayed gastric emptying in critically ill patients ‐ report of three cases. J. Jpn. Soc. Intensive Care Med. 2009; 16: 187–90. [in Japanese]. [Google Scholar]
- 7. Suzuki H, Matsuzaki J, Fukushima Y et al Randomized clinical trial: rikkunshito in the treatment of functional dyspepsia–a multicenter, double‐blind, randomized, placebo‐controlled study. Neurogastroenterol. Motil. 2014; 26: 950–61. [DOI] [PubMed] [Google Scholar]
- 8. Tominaga K, Iwakiri R, Fujimoto K et al Rikkunshito improves symptoms in PPI‐refractory GERD patients: a prospective, randomized, multicenter trial in Japan. J. Gastroenterol. 2012; 47: 284–92. [DOI] [PubMed] [Google Scholar]
- 9. Hayakawa M, Ono Y, Wada T et al Effects of Rikkunshito (traditional Japanese medicine) on enteral feeding and the plasma ghrelin level in critically ill patients: a pilot study. J. Intensive Care 2014; 2: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peeters TL. Ghrelin: a new player in the control of gastrointestinal functions. Gut 2005; 54: 1638–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujitsuka N, Asakawa A, Uezono Y et al Potentiation of ghrelin signaling attenuates cancer anorexia‐cachexia and prolongs survival. Transl. Psychiatry 2011; 1: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sadakane C, Muto S, Nakagawa K et al 10‐Gingerol, a component of rikkunshito, improves cisplatin‐induced anorexia by inhibiting acylated ghrelin degradation. Biochem. Biophys. Res. Commun. 2011; 412: 506–11. [DOI] [PubMed] [Google Scholar]
- 13. Hoshino N, Nishizaki D, Hida K et al Rikkunshito for upper gastrointestinal symptoms: a systematic review and meta‐analysis. Complement Ther. Med. 2019; 42: 255–63. [DOI] [PubMed] [Google Scholar]
- 14. Rice TW, Wheeler AP, Thompson BT et al Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reignier J, Mercier E, Le Gouge A et al Effect of not monitoring residual gastric volume on risk of ventilator‐associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA 2013; 309: 249–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Enteral nutrition protocol.


