Abstract
Aim
In severe urinary tract infection (UTI), susceptible antibiotics should be given. With the recent increase of multidrug‐resistant bacteria, especially extended spectrum beta‐lactamase producing Enterobacteriaceae (ESBL‐E), broad‐spectrum antibiotics, such as carbapenems, are used more frequently, which could lead to a further increase of multidrug‐resistant bacteria. We aimed to analyze the relationship between initial empirical antibiotic appropriateness and clinical outcomes in UTI, especially in patients with systemic inflammatory response syndrome (SIRS) and ESBL‐E.
Methods
A retrospective observational study from 2012 to 2017.
Results
Among urine culture‐positive cases with ≥105 colony‐forming units/mL (n = 1,880), true UTI cases were extracted (n = 844) and divided into the SIRS group (n = 336 [ESBL‐E12.8% (43/336)]) and non‐SIRS group (n = 508 [ESBL‐E12.6% (64/508)]). In the SIRS ESBL‐E group, the initial antibiotics were susceptible in 55.8% (24/43), among which 91.7% (22/24) improved and 8.3% (2/24) deteriorated or died. The initial antibiotics were resistant in 44.2% (19/43), among which 47.4% (9/19) improved with the initial antibiotics, 47.4% (9/19) improved after escalating antibiotics, and 5.3% (1/19) deteriorated or died. In the SIRS group, 14 cases had true bacteremia with ESBL‐E. Seven cases were initiated with inappropriate antibiotics; four cases showed improvement before or without antibiotic change and three cases improved after antibiotic escalation.
Conclusion
Initiation of narrow‐spectrum antibiotics in septic UTI with ESBL‐E might not deteriorate the clinical outcome if promptly escalated on clinical deterioration or with ESBL‐E culture results. Further investigation is warranted to guide judicious use of initial antibiotics.
Keywords: Extended spectrum beta‐lactamase, multidrug‐resistant pathogen, sequential organ failure assessment, systemic inflammatory response syndrome, urinary tract infection
We undertook this study to evaluate clinical outcomes of urinary tract infection caused by extended spectrum beta‐lactamase producing Enterobacteriaceae. Our specific interest is to assess the clinical outcomes in relation to the initial empirical antibiotics, especially in severe cases.
Introduction
Urinary tract infection (UTI), especially severe cases with systemic inflammatory response syndrome (SIRS) and/or bacteremia, is one of the common diagnoses managed by clinicians in the outpatient clinic, emergency department, general ward, and critical care setting.1 It is important to treat UTIs with susceptible antibiotics without delay, especially in severe cases, to improve patient outcomes.1 With increasing multidrug‐resistant (MDR) isolates especially extended spectrum beta‐lactamase producing Enterobacteriaceae (ESBL‐E) in recent years,2 broad‐spectrum antibiotics, especially carbapenem, tend to be used more frequently for potential ESBL‐E coverage.3, 4 Studies evaluating clinical outcomes in patients with ESBL‐E infections have shown a tendency for higher mortality, longer hospital stay, greater hospital expenses, and reduced rates of clinical and microbiologic response.5 However, inappropriate use of unnecessarily broad‐spectrum antibiotics can lead to an further increase of MDR isolates6, 7 and clinicians should select broad‐spectrum antibiotics judiciously.
There have been observational reports with causative pathogens of UTI with ESBL‐E8, 9; however, these reports combined cases with mild UTI (cystitis) and severe UTI (pyelonephritis or sepsis) together and did not provide information regarding the relationship between susceptibility and clinical outcomes, especially in severe cases. Cases of UTI with SIRS have the substantial possibility of developing bacteremia.1 Clinicians need to determine the initial antibiotics empirically before the culture and sensitivity results are available. Recently, carbapenems have been reported to be superior to piperacillin–tazobactam (PIPC/TAZ) as a definitive therapy in cases with ESBL‐E bacteremia; however, these studies contain mixed populations of UTI, intra‐abdominal infection, and other sources of infection.10, 11 Furthermore, these studies evaluated the choice of antibiotics as definitive therapy after the sensitivity results obtained revealed ESBL‐E. Therefore, if we apply these reports indicating that carbapenem is superior to PIPC/TAZ in ESBL‐E bacteremia cases, clinicians are obliged to initiate with carbapenem in all UTI cases with SIRS, which will lead to overuse of carbapenem. This is not desirable with the current global initiative of antimicrobial resistance action plan.12 Furthermore, although comparatively strong evidence exists for empirical treatments in the setting of intra‐abdominal infection, the evidence in the setting of complicated UTI is small.13
Therefore, we undertook this study to analyze the relationship between initial empirical antibiotic appropriateness and clinical outcomes of cases with UTI, especially focusing on cases with SIRS and ESBL‐E.
Methods
This was a retrospective observational study that analyzed all patients who were diagnosed with UTI caused by ESBL‐E from June 2012 to July 2017 at St. Marianna University School of Medicine, Yokohama City Seibu Hospital (Yokohama, Japan).
The data on patient characteristics, causative pathogens, SIRS criteria, antibiotics, blood culture results, and clinical outcomes were obtained. This study was initiated in 2012, and SIRS criteria were used for categorizing severe cases and non‐severe cases as such a categorization was simple, objective, and practical in a clinical setting.
All urine culture (UCx)‐positive cases with ≥105 colony‐forming unit (CFU)/mL were selected.14 The following cases were excluded: identical cases within 6 months (n = 231), UCx positive with fungi (n = 24), cases considered not to have active UTI from chart review (the definition of “considered not to have active UTI” included urine white blood cell count <10/high power field [n = 457], chart review revealed the documentation of the clinician’s assessment as urinary colonization and/or assessment of infection source other than UTI [e.g., pneumonia, cellulitis, and surgical site infection] [n = 40]), cases with “do not attempt to resuscitate” order or “comfort measures only” (n = 12), and cases with missing data (n = 17).
The data on SIRS criteria15 were obtained from chart review. The worst data on the day of UCx obtainment was used for SIRS scoring. When one or more SIRS components were not available on the date of UCx, the SIRS components within 1 day before or after the date of UCx were examined. Extended spectrum beta‐lactamase producing Enterobacteriaceae was identified based on the Clinical and Laboratory Standards Institute description.16 Clinical outcomes were defined as described in Appendix S1.
Appropriate antibiotics were identified when urine or blood culture results were susceptible to antibiotic treatment. Inappropriate antibiotics were identified when the urine culture result was resistant or intermediately susceptible to the antibiotic treatment.
The case was considered to have improved when the following two criteria were fulfilled: (i) fever and other vital signs improved, (ii) the chart documentation showed the description of improvement by the primary team.
This study protocol was approved by the institutional review board (IRB) of St. Marianna University, School of Medicine. For data analysis, R, EZR, the Mann–Whitney U‐test, and Fisher’s exact test were used. Detailed descriptions can be found in Appendix S1.
Results
Urine culture positive cases with ≥105 CFU/mL were identified (n = 1,880). Bacterial UTI cases were selected from chart review (n = 844) and divided into the SIRS group and non‐SIRS group. The SIRS group had 336 cases (ESBL‐E n = 43, 12.8%) and non‐SIRS group had 508 cases (ESBL‐E n = 64, 12.6%) (Fig. 1). The ratio of ESBL‐E did not differ significantly between the SIRS group and non‐SIRS group (P = 0.92). The SIRS group had 45.5% (20/43) male patients and the non‐SIRS group had 26.6% (17/64) male patients (P = 0.04). The average ages did not differ significantly (Table 1). The SIRS group had significantly higher inpatient management than the non‐SIRS group (95.5% [42/43] versus 56.3% [36/64], P < 0.01). The SIRS group had significantly higher intensive care unit management than the non‐SIRS group (11.4% [5/43] versus 0.0% [0/64], P = 0.01) (Table 1). The timing of acquisition of UTI in inpatient cases, the department of admission of inpatient cases, and the underlying conditions are summarized in Table 1.
Figure 1.
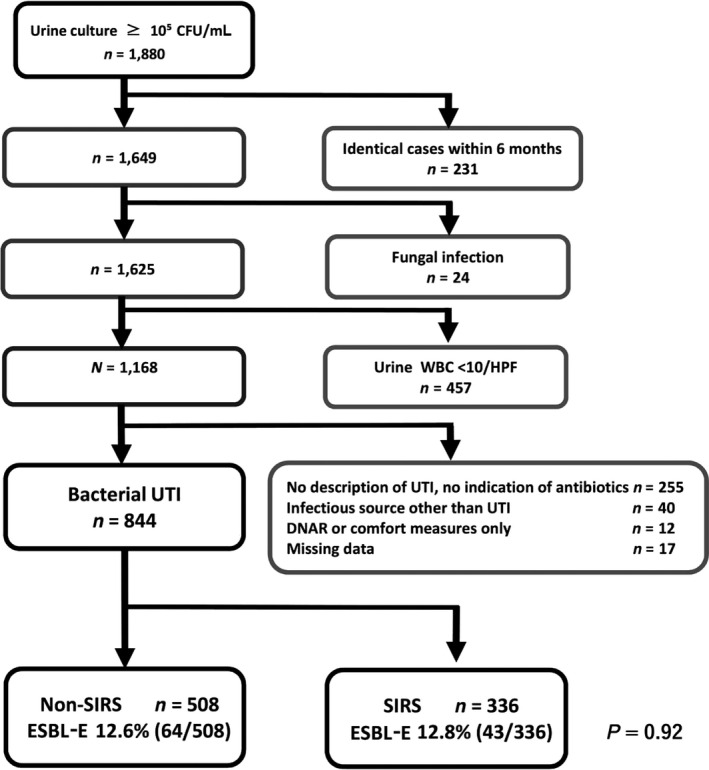
Patient selection flow chart. CFU, colony‐forming unit; DNAR, do not attempt to resuscitate; ESBL‐E, extended spectrum beta‐lactamase producing Enterobacteriaceae; HPF, high power field; SIRS, systemic inflammatory response syndrome; UTI, urinary tract infection; WBC, white blood cells.
Table 1.
Basic characteristics of urinary tract infection (UTI) with extended spectrum beta‐lactamase producing Enterobacteriaceae (ESBL‐E)
| ESBL UTI cases |
Non‐SIRS n = 64 |
SIRS n = 43 |
P‐value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender, male | 17 | 26.6 | 20 | 45.5 | 0.04 |
| Age average, years | 76.8 ± 12.9 | 74.2 ± 13.2 | NS | ||
| Place of UTI management | |||||
| Outpatient | 28 | 43.8 | 1 | 2.3 | <0.01 |
| Inpatient | 36 | 56.3 | 42 | 95.5 | <0.01 |
| Inpatient wards | 23 | 35.9 | 21 | 47.7 | 0.30 |
| HDU | 13 | 20.3 | 16 | 36.4 | 0.10 |
| ICU | 0 | 0.0 | 5 | 11.4 | 0.01 |
| Acquisition of UTI of inpatient cases | |||||
| Hospital days 1–3 | 21 | 58.3 | 20 | 46.5 | 0.41 |
| Hospital day 4 and later | 15 | 42.0 | 22 | 51.0 | |
| Department of admission of inpatient cases | |||||
| Medical service | 20 | 55.6 | 27 | 62.5 | 0.63 |
| Surgical service (excluding urology) | 10 | 27.8 | 10 | 23.3 | |
| Urology service | 5 | 13.9 | 5 | 11.6 | |
| Other subspecialty services | 1 | 2.8 | 0 | 0.0 | |
| Underlying conditions | |||||
| Cardiac disease | 13 | 20.3 | 7 | 16.3 | 0.74 |
| Neurological disease | 14 | 21.9 | 6 | 14.0 | 0.41 |
| Pulmonary disease | 7 | 10.9 | 8 | 18.6 | 0.43 |
| Gastrointestinal disease | 10 | 15.6 | 2 | 4.7 | 0.14 |
| Moderate to severe renal dysfunction a | 12 | 18.8 | 14 | 34.9 | 0.07 |
| Orthopedic disease | 6 | 9.4 | 3 | 7.0 | 0.91 |
| Immune compromise | 2 | 3.1 | 5 | 11.6 | 0.19 |
| Malignancy | 10 | 15.6 | 8 | 18.6 | 0.93 |
| Urological disease | 14 | 21.9 | 9 | 20.9 | 1.00 |
HDU, high dependency unit; ICU, intensive care unit; NS, not significant; SIRS, systemic inflammatory response syndrome.
Defined as estimated glomerular filtration rate <44 mL/min m2.
The ESBL‐E was composed of Escherichia coli (92.5%), Klebsiella pneumoniae (3.7%), and Proteus mirabilis (3.7%). This proportion did not differ significantly between the SIRS and non‐SIRS groups. The SIRS group had a significantly lower rate of improvement with initial antibiotics than the non‐SIRS group (72.1% [31/43] versus 96.9% [62/64], P < 0.01). The SIRS group had a significantly higher rate of improvement after changing antibiotics than the non‐SIRS group (20.9% [9/43] versus 3.1% [2/64], P = 0.01). The SIRS group had a higher death rate than the non‐SIRS group (7.0% [3/43] versus 0.0% [0/64], P = 0.13) (Table 2).
Table 2.
Causative pathogens and clinical outcome of urinary tract infection caused by extended spectrum beta‐lactamase producing Enterobacteriaceae (ESBL‐E UTI) cases with or without systemic inflammatory response syndrome (SIRS)
| ESBL‐E UTI cases |
Non‐SIRS n = 64 |
SIRS n = 43 |
P‐value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Pathogens ≥105 CFU/mL | |||||
| E. coli | 61 | 95.3 | 38 | 88.4 | 0.35 |
| K. pneumoniae | 2 | 3.1 | 2 | 4.7 | 1.00 |
| P. mirabilis | 1 | 1.6 | 3 | 7.0 | 0.37 |
| Outcome | |||||
| Improved | 62 | 96.9 | 31 | 72.1 | <0.01 |
| Improved after changing antibiotics | 2 | 3.1 | 9 | 20.9 | 0.01 |
| Deteriorated | 0 | 0.0 | 0 | 0.0 | NA |
| Died | 0 | 0.0 | 3 | 7.0 | 0.13 |
| Unknown outcome (missing data) | 0 | 0.0 | 0 | 0.0 | NA |
CFU, colony forming unit; E. coli, Escherichia coli; K. pneumoniae, Klebsiella pneumoniae; NA, not applicable; P. mirabilis, Proteus mirabilis.
The initial antibiotics given for ESBL UTI cases are summarized in Table 3. The SIRS group had significantly higher levels of i.v. antibiotic treatment than the non‐SIRS group (95.3% [41/43] versus 51.6% [33/64], P < 0.01) (Table 3).
Table 3.
Initial antibiotics given for urinary tract infection caused by extended spectrum beta‐lactamase producing Enterobacteriaceae (ESBL‐E UTI) cases with or without systemic inflammatory response syndrome (SIRS)
| ESBL‐E UTI cases |
Non‐SIRS n = 64 |
SIRS n = 43 |
P‐value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| No antibiotics | 0 | 0.0 | 0 | 0.0 | NA |
| Oral antibiotics | 32 | 50.0 | 2 | 4.7 | <0.01 |
| Cefcapene pivoxil (III‐cephalosporin) | 17 | 26.6 | 0 | 0.0 | |
| Levofloxacin | 8 | 12.5 | 2 | 4.7 | |
| Faropenem (carbapenem) | 3 | 4.7 | 0 | 0.0 | |
| TMP/SMX | 3 | 4.7 | 0 | 0.0 | |
| Cefdinir (III‐cephalosporin) | 1 | 1.6 | 0 | 0.0 | |
| Minomycin | 1 | 1.6 | 0 | 0.0 | |
| Azithromycin | 1 | 1.6 | 0 | 0.0 | |
| Intravenous antibiotics | 33 | 51.6 | 41 | 95.3 | <0.01 |
| PIPC/TAZ | 6 | 9.4 | 13 | 27.9 | |
| CTRX (III‐cephalosporin) | 7 | 10.9 | 8 | 18.6 | |
| MEPM | 3 | 4.7 | 8 | 18.6 | |
| ABPC/SBT | 4 | 6.3 | 3 | 7.0 | |
| CEZ (I‐cephalosporin) | 1 | 1.6 | 3 | 7.0 | |
| CFPM (IV‐cephalosporin) | 1 | 1.6 | 3 | 7.0 | |
| CMZ | 2 | 3.1 | 1 | 2.3 | |
| Levofloxacin | 2 | 3.1 | 1 | 2.3 | |
| CTM (II‐cephalosporin) | 2 | 3.1 | 0 | 0.0 | |
| Imipenem/cilastatin | 1 | 1.6 | 1 | 2.3 | |
| CTX (III‐cephalosporin) | 1 | 1.6 | 0 | 0.0 | |
| CAZ (III‐cephalosporin) | 1 | 1.6 | 0 | 0.0 | |
| CZOP | 0 | 0.0 | 1 | 2.3 | |
| CPZ/SBT | 1 | 1.6 | 0 | 0.0 | |
| PIPC | 1 | 1.6 | 0 | 0.0 | |
ABPC/SBT, ampicillin sulbactam; CAZ, ceftazidime; CEZ, cephazolin; CFPM, cefepime; CMZ, cefmetazole; CPZ/SBT, cefoperazone sulbactam; CTM, cefotiam; CTRX, ceftriaxone; CTX, cefotaxime; CZOP, cefozopran; I‐, II‐, III‐, IV‐cephalosporin, first, second, third, fourth generation cephalosporin, respectively; MEPM, meropenem; NA, not applicable; PIPC, piperacillin; PIPC/TAZ, piperacillin–tazobactam; TMP/SMX, trimethoprim/sulfamethoxazole.
The initial antibiotic treatment and clinical outcomes of ESBL UTI are summarized in Table 4. In the non‐SIRS cases, there was no statistical significance in the rate of improvement between the groups given initial inappropriate or appropriate antibiotics. In the SIRS cases, the group given inappropriate initial antibiotic treatment had a significantly lower improvement rate than those treated with appropriate initial antibiotics (47.7% [9/19] versus 91.7% [22/24/], P < 0.01). There was no statistical significance in the death rate between the initial inappropriate and appropriate antibiotic groups (5.3% [1/19] versus 8.3% [2/24], P = 1.0) (Table 4).
Table 4.
Initial antibiotic (Abx) treatment and clinical outcomes of urinary tract infection caused by extended spectrum beta‐lactamase producing Enterobacteriaceae (ESBL‐E UTI) cases with and without systemic inflammatory response syndrome (SIRS)
| Initial antibiotic treatment | Inappropriate | Appropriate | P‐value | ||
|---|---|---|---|---|---|
| Non‐SIRS ESBL‐E UTI | n = 64 | ||||
| Total | 44 | 20 | |||
| Improved before or without Abx change | 42 | 95.5% | 20 | 100.0% | 0.85 |
| Improved after Abx change | 2 | 4.5% | 0 | 0.0% | 0.85 |
| Died | 0 | 0.0% | 0 | 0.0% | NA |
| SIRS ESBL‐E UTI | n = 43 | ||||
| Total | 19 | 24 | |||
| Improved before or without Abx change | 9 | 47.4% | 22 | 91.7% | <0.01 |
| Improved after Abx change | 9 | 47.4% | 0 | 0.0% | <0.01 |
| Died | 1 | 5.3% | 2 | 8.3% | 1.00 |
NA, not applicable; PIPC/TAZ, piperacillin–tazobactam.
The blood cultures were obtained significantly more frequently in the SIRS group than in the non‐SIRS group (91.0% [39/43] versus 30.0% [19/64/], P < 0.01). The blood culture positivity was significantly higher in the SIRS group than in the non‐SIRS group (32.6% [14/43] versus 12.5% [8/64], P = 0.01). The blood culture positivity with the pathogen identical to the UCx (true bacteremia) was also significantly higher in the SIRS group than in the non‐SIRS group (32.6% [14/43] versus 4.7% [3/64], P < 0.01) (Table S1).
The relationship between the initial antibiotic treatment and clinical outcome of 14 cases of ESBL UTI with SIRS with true bacteremia is analyzed in Table 5. Seven cases initially given antibiotics later turned out to be inappropriate to the causative ESBL‐E. Among those seven cases, four improved while still on the initial antibiotics and three improved after escalating antibiotics and no case deteriorated or died. In the remaining seven cases, initial antibiotics later turned out to be appropriate to the causative pathogen. Among those seven cases, six improved with the initial antibiotics, but one patient died despite being given appropriate antibiotics from the start (Table 5).
Table 5.
Relationship between initial antibiotic (Abx) treatment and clinical outcome of cases of urinary tract infection caused by extended spectrum beta‐lactamase producing Enterobacteriaceae (ESBL‐E UTI) with systemic inflammatory response syndrome (SIRS) with true bacteremia
| SIRS ESBL‐E UTI with true bacteremia | n = 14 | ||||
|---|---|---|---|---|---|
| Initial antibiotic treatment | Inappropriate | Appropriate | P‐value | ||
| Total | 7 | 7 | |||
| Improved before or without Abx change | 4 | 57.1% | 6 | 85.7% | 0.56 |
| Improved after Abx change | 3 | 42.9% | 0 | 0.0% | 0.19 |
| Died | 0 | 0.0% | 1 | 14.3% | 1.00 |
| SOFA average | 5.86 | 8.14 | 0.55 | ||
SOFA, Sequential Organ Failure Assessment.
The average Sequential Organ Failure Assessment (SOFA) scores were 5.86 and 8.14 in cases given initial inappropriate antibiotics and initial appropriate antibiotics, respectively. The average SOFA score was higher in cases given initial appropriate antibiotics but did not show statistical significance (P = 0.55) (Table 5).
Initial antibiotics, changed antibiotics, and clinical outcome of SIRS ESBL UTI cases with true bacteremia are summarized in Table S2.
Discussion
This is a retrospective observational study to assess the relationship between initial antibiotic appropriateness and the clinical outcome of ESBL‐E UTI cases. Although SIRS criteria are not currently used,17 we applied SIRS criteria because it was the main means of severity assessment for sepsis at the time of initiation and approval by the IRB of this study in 2012. Additionally, SIRS is simple, practical, and objective.
A total of 108 ESBL‐E UTI cases were evaluated; among them, 43 cases were SIRS positive, which is an indicator of severe infection, and initial antibiotic selection was critical. Our study showed that severe UTI with ESBL‐E might not always require carbapenem as an empiric antibiotic. Modification of inappropriate antibiotics later, either according to clinical deterioration or ESBL‐E culture results, might not adversely affect clinical outcomes.
Treatment of all septic UTI cases with carbapenem could increase the possibility of increasing carbapenem‐resistant Enterobacteriaceae.6 Patients with carbapenem‐resistant Enterobacteriaceae have higher mortality than those with carbapenem‐susceptible Enterobacteriaceae, especially in association with bloodstream infection and intensive care unit admission.18 Guidelines for UTI with suspected ESBL‐E have not been reported and controversy still exists regarding the identification of patients who require empiric treatment with carbapenem. Early detection of ESBL‐E, such as early gene detection with accurate molecular diagnostic testing, could resolve this question,19 but currently, phenotypic methods remain the cornerstone of antimicrobial susceptibility testing in clinical microbiology laboratories.16
Our data also evaluated 14 cases of true bacteremia with the identical pathogen as the MERINO study, which showed ESBL‐E bacteremia treated with carbapenem as definitive therapy led to better clinical outcomes compared to PIPC/TAZ.10 Approximately 60% cases were bacteremia secondary to UTI and the severity score index by the Acute Physiology and Chronic Health Evaluation II was 21.0 in the MERINO trial.10 In contrast, our study evaluated the relationship between clinical outcomes and empirical therapy in urosepsis. There is only limited reporting of the empirical treatment of complicated UTI.13 In our study, all cases initiated with inappropriate antibiotics recovered by timely escalation of antibiotics either with clinical deterioration or with ESBL‐E culture results.
Our study has a few limitations. First, this study was undertaken in an urban tertiary‐care hospital. Microbiological resistance patterns differ from place to place. However, the management and outcomes of each case with ESBL‐E should not differ significantly among facilities. The prevalence of ESBL‐E in our study was similar to that reported in Japan.8, 20, 21 This indicates that our study has generalizability.
Second, the non‐SIRS group contain cystitis cases that might not necessarily require treatment with appropriate antibiotics for cure. It is difficult to clearly differentiate cystitis and pyelonephritis in the non‐SIRS group. However, our main scope of this study is to evaluate the UTI cases with SIRS and bacteremia.
Third, this is a retrospective study and the selection of antibiotics could be biased with the severity of the case. The average SOFA score had numerical difference between those given initial appropriate and inappropriate antibiotics. However, there was no statistical significance. At the least, this study indicated that, among the seven cases with ESBL‐E UTI with true bacteremia that were initially treated with inappropriate antibiotics, all improved with the appropriate escalation of antibiotics. The risk factors for ESBL‐E are known to include recent broad‐spectrum antimicrobial use, health‐care exposures, and travel to parts of the world where MDR organisms are prevalent. However, there are cases of community‐acquired ESBL‐E infection, which makes it difficult to predict precisely which UTI cases are caused by ESBL‐E.2 This population compiled a large number of UTI cases. If we could spare the use of carbapenems in those cases, it would have a large impact on reducing the use of broad‐spectrum antibiotics, especially carbapenems.
Our study has several strengths and substantial clinical impact. Cases of UTI with SIRS are common in the clinical setting. Among UTI cases that fulfill the SIRS criteria, there is a wide spectrum of severity. In cases with high potential for ESBL‐E or those in a critical condition, for example, septic shock requiring vasoactive agents, it is reasonable to use carbapenem as an empirical antimicrobial. However, using carbapenems in all UTI cases that just fulfill the SIRS criteria would lead to significantly increased usage of carbapenem, which is not desirable for the judicious use of broad‐spectrum antibiotics as described in antimicrobial resistance action plan and would risk inducing further antimicrobial‐resistant strains. At the same time, we should not risk patients receiving inappropriate initial antibiotics with treatment failure. Our study indicated that initial inappropriate antibiotics did not adversely affect clinical outcomes if the antibiotics were escalated promptly when clinical deterioration was observed or culture results with ESBL‐E were available. Our study added strong supportive information to guide judicious antibiotic selection without compromising patient outcome in these populations.
Conclusions
When treating SIRS‐positive UTI with possible ESBL‐E, empirical non‐carbapenem antibiotics can be a safe option considering that the antibiotics will be properly escalated when there is clinical deterioration or the culture results with ESBL‐E become available. Further research is warranted to guide the judicious empirical antibiotic selection for UTI with SIRS with possible ESBL‐E.
Disclosure
Approval of the research protocol: This study protocol was approved by the IRB of the School of Medicine, St. Marianna University.
Informed consent: This study does not entail any intervention to the patients. The opt‐out was performed by placing poster in the hospital.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Supporting information
Table S1 . Blood culture obtainment, positivity, and concordance rate of urinary tract infection (UTI) caused by extended spectrum beta‐lactamase producing Enterobacteriaceae (ESBL‐E) with or without systemic inflammatory response syndrome (SIRS).
Table S2 . Initial antibiotics, changed antibiotics, and clinical outcomes of urinary tract infection caused by extended spectrum beta‐lactamase producing Enterobacteriaceae (ESBL‐E) with systemic inflammatory response syndrome (SIRS) with true bacteremia.
Appendix S1. Detailed description of methods and statistical analysis.
Acknowledgments
We would like to thank Yosuke Tanaka and Tatsuya Ono (Department of Clinical Laboratory, St. Marianna University Yokohama City Seibu Hospital), Kennichi Nakazono and Ayaka Katsu (Department of Pharmacy, St. Marianna University Yokohama City Seibu Hospital), and Akiko Hosokawa (Research Assistant, Department of Emergency and Critical Care Medicine, St. Marianna University School of Medicine) for technical support. We would like to thank Editage for English language editing.
Funding information
No funding information provided.
References
- 1. Belyayeva M, Jeong JM. Acute pyelonephritis In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. [cited 2019 Jul 12]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK519537/. [Google Scholar]
- 2. Doi Y, Park YS, Rivera JI et al Community‐associated extended‐spectrum β‐lactamase‐producing Escherichia coli infection in the United States. Clin. Infect. Dis. 2013; 56: 641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paterson DL, Bonomo RA. Extended‐spectrum beta‐lactamases: a clinical update. Clin. Microbiol. Rev. 2005; 18: 657–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peleg AY, Hooper DC. Hospital‐acquired infections due to gram‐negative bacteria. N. Engl. J. Med. 2010; 362: 1804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paterson DL, Ko W‐C, Von Gottberg A et al Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended‐spectrum beta‐lactamases. Clin. Infect. Dis. 2004; 39: 31–7. [DOI] [PubMed] [Google Scholar]
- 6. McLaughlin M, Advincula MR, Malczynski M, Qi C, Bolon M, Scheetz MH. Correlations of antibiotic use and carbapenem resistance in Enterobacteriaceae. Antimicrob. Agents. Chemother. 2013; 57: 5131–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang HJ, Hsu PC, Yang CC et al Risk factors and outcomes of carbapenem‐nonsusceptible Escherichia coli bacteremia: a matched case–control study. J. Microbiol. Immunol. Infect. 2011; 44: 125–30. [DOI] [PubMed] [Google Scholar]
- 8. Ishikawa K, Matsumoto T, Yasuda M et al The nationwide study of bacterial pathogens associated with urinary tract infections conducted by the Japanese Society of Chemotherapy. J. Infect. Chemother. 2011; 17: 126–38. [DOI] [PubMed] [Google Scholar]
- 9. Yamaguchi K, Ohno A, Ishii Y, Tateda K, Iwata M, Levofloxacin‐Surveillance Group . [In vitro susceptibilities to levofloxacin and various antibacterial agents of 12,866 clinical isolates obtained from 72 centers in 2010]. Jpn. J. Antibiot. 2012; 65: 181–206. [PubMed] [Google Scholar]
- 10. Harris PNA, Tambyah PA, Lye DC et al Effect of piperacillin‐tazobactam vs meropenem on 30‐day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 2018; 320: 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tamma PD, Goodman KE, Harris AD et al Comparing the outcomes of patients with carbapenemase‐producing and non‐carbapenemase‐producing carbapenem‐resistant Enterobacteriaceae bacteremia. Clin. Infect. Dis. 2017; 64: 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO|Global action plan on AMR. WHO. [cited 2019 Jul 12]. Available from: http://www.who.int/antimicrobial-resistance/global-action-plan/en/.
- 13. Golan Y. Empiric therapy for hospital‐acquired, Gram‐negative complicated intra‐abdominal infection and complicated urinary tract infections: a systematic literature review of current and emerging treatment options. BMC Infect. Dis. 2015; 15: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garner JS. CDC definitions for nosocomial infections In: Olmsted RN. (Ed.). APIC Infection Control and Applied Epidemiology: Principles and Practice. St. Louis: Mosby, 1996; A‐1–A‐20. [Google Scholar]
- 15. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference . American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care. Med. 1992; 20: 864–74. [PubMed] [Google Scholar]
- 16. Clinical and Laboratory Standards Institute (CLSI) . Performance Standards for Antimicrobial Susceptibility Testing, 27th edn Pennsylvania: Wayne, 2017. [Google Scholar]
- 17. Rhodes A, Evans LE, Alhazzani W et al Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care. Med. 2017; 43: 304–77. [DOI] [PubMed] [Google Scholar]
- 18. Xu L, Sun X, Ma X. Systematic review and meta‐analysis of mortality of patients infected with carbapenem‐resistant Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob. 2017; 16: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El‐Jade MR, Parcina M, Schmithausen RM et al ESBL detection: comparison of a commercially available chromogenic test for third generation cephalosporine resistance and automated susceptibility testing in enterobactericeae. PLoS ONE ONE [Internet]. 2016; 11: e0160203 [cited 2019 Aug 1]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4975492/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mawatari M, Hayakawa K, Fujiya Y et al Bacteraemic urinary tract infections in a tertiary hospital in Japan: the epidemiology of community‐acquired infections and the role of non‐carbapenem therapy. BMC Res. Notes. 2017; 10: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shigemura K, Tanaka K, Okada H et al Pathogen occurrence and antimicrobial susceptibility of urinary tract infection cases during a 20‐year period (1983–2002) at a single institution in Japan. Jpn. J. Infect. Dis. 2005; 58: 303–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 . Blood culture obtainment, positivity, and concordance rate of urinary tract infection (UTI) caused by extended spectrum beta‐lactamase producing Enterobacteriaceae (ESBL‐E) with or without systemic inflammatory response syndrome (SIRS).
Table S2 . Initial antibiotics, changed antibiotics, and clinical outcomes of urinary tract infection caused by extended spectrum beta‐lactamase producing Enterobacteriaceae (ESBL‐E) with systemic inflammatory response syndrome (SIRS) with true bacteremia.
Appendix S1. Detailed description of methods and statistical analysis.


