Abstract
Recent studies on immune-mediated inflammatory lung diseases show encouraging treatment results with rituximab, a monoclonal antibody (mAb) against CD20-expressing B lymphocytes. The present pilot study aimed to explore the possibility to image CD20-expression in the lungs as future early predictor of treatment response. We describe a series of 10 patients with therapy refractory interstitial pneumonitis who were treated with rituximab (1000 mg at day 0 and day 14) and underwent PET/CT after the administration of [89Zr]Zr-N-suc-DFO-rituximab abbreviated as [89Zr]Zr-rituximab. [89Zr]-rituximab PET/CT of the chest was performed on day 3 and 6. [89Zr]Zr-rituximab PET/CT showed visual and quantifiable increased pulmonary activity in four patients. Other patients demonstrated no increased activity in the lungs. One patient developed a severe allergic reaction during infusion of the first 10% unlabeled rituximab after which rituximab infusion was ceased. Subsequent administration of [89Zr]Zr-rituximab, however, did not result in any adverse reaction. This patient demonstrated the highest uptake of [89Zr]Zr-rituximab in mediastinal lymph nodes and lung parenchyma compared to the other 9 patients who did receive the full dose rituximab before [89Zr]Zr-rituximab. This pilot study demonstrates that [89Zr]Zr-rituximab PET/CT imaging in patients with therapy refractory interstitial pneumonitis is feasible and shows lung-specific uptake in some patients. Further research with larger sample size should establish if the [89Zr]Zr-rituximab uptake correlates with treatment response to rituximab. The higher uptake in the absence of a full 1000 mg rituximab preload may suggest that future studies should consider [89Zr]Zr-rituximab imaging at low mAb dose before treatment with rituximab.
Keywords: Rituximab, zirconium, [89Zr]Zr-rituximab PET/CT, interstitial pneumonitis, immuno-PET, pulmonary activity
Introduction
Immune mediated inflammatory diseases (IMID) with interstitial pneumonitis (IP) encompass rare inflammatory disorders that include pulmonary involvement such as polymyositis, dermatomyositis, mixed connective tissue disease (MCTD), systemic sclerosis (systemic scleroderma) or anti-synthetase syndrome. These various auto-immune diseases do not have a clear causal relationship, but seem to have a common disturbance in the inflammatory pathway [1]. This theory originates from the fact that the same clinical response can be found in various IMIDs upon treatment with the same therapeutic drug. This theory holds especially true when looking at drugs directed to specific targets of the immune system. Rituximab is such a therapeutic drug. This monoclonal antibody has a high targeting specificity to CD20 receptors on B cells. Depleting dysfunctional CD20 cells from the blood pool has an inhibiting effect on the systemic inflammation and can result in clinical benefit. This pathophysiological effect has been demonstrated in various rheumatologic conditions such as rheumatic arthritis (RA) and granulomatosis with polyangiitis [2-5]. Therefore, CD20 seems to play an important role in the inflammatory cascade [6]. Various other IMIDs with pulmonary involvement may also benefit from anti-CD20 therapy.
However, response to rituximab is variable. From current studies in patients with various IMIDs, it seems that about 30% of the patients show definite improvement, 30% show stabilization, and 20-40% show no response to rituximab [7,8]. The number of CD20-expressing cells in the lungs is a potential predictive biomarker for response to rituximab therapy. In histology studies, it has been shown that IMID-IP lungs contain a large number of CD20+ B cells, while healthy controls do not [9]. Additionally, lungs with severe type IP showed more CD20+ B cell infiltration than lungs with a milder type IP [10]. This likely could explain the observed variability in response to rituximab in patients. Since most patients have severe lung function impairment they cannot undergo invasive procedures to obtain lung biopsies, and therefore implementation of a non-invasive test is crucial. This can potentially be achieved by [89Zr]Zr-rituximab immuno-PET, by providing an objective quantitative parameter for the presence of CD20+ cells in the lungs.
PET imaging of CD20-expression is a novel technique, especially in autoimmune diseases. No previous studies have been performed in patients with IP, and therefore there is a degree of uncertainty as to whether PET imaging of CD20-expression will provide valuable information. However, there are positive experiences with the same [89Zr]Zr-rituximab immuno-PET imaging in rheumatoid arthritis (RA) [11]. Moreover SPECT imaging with [99mTc]Tc-rituximab showed increased uptake in lungs in a patient with sarcoidosis [12]. The rationale for using [89Zr]Zr-rituximab immuno-PET in the present study is because of the better performance of PET in comparison to SPECT with respect to sensitive high resolution imaging and quantification, and the longer physical half-life of Zr-89 compared to Tc-99m (78.4 vs 6 hours), which is better compatible with the biological half-life of rituximab. The latter allows for imaging at later timepoints at higher target-to-background ratios. Therefore, [89Zr]Zr-rituximab PET might provide an objective quantitative measure of the presence of CD20+ cells and therefore a potential biomarker for prediction of rituximab treatment response.
Methods
In this pilot study, we investigated whether [89Zr]Zr-rituximab uptake is increased in pulmonary and/or mediastinal lymph nodes in IMID-IP patients who receive therapeutic doses of rituximab as a third line treatment. Since the aim of this paper is to describe the imaging results of [89Zr]Zr-rituximab immuno-PET imaging in a small sample size, the results of therapeutic rituximab will not be described. This study was performed according to international GCP standards and obtained ethics approval from the Medical Research Ethics Committees United (MEC-U) under NL49534.100.14. This trial is registered at ClinicalTrials.gov identifier: NCT02251964. Patients included in the present study presented at the Department of Pulmonology of the St. Antonius Hospital Nieuwegein from May 2015 - January 2016. All patients gave written informed consent prior to participation.
Inclusion criteria
In order to be eligible for participation in the study, patients met all of the following criteria: age 18-70 years, no previous therapy with rituximab, at least 2 pulmonary function tests within the past 6 months, diagnosis of co-existing IMID and a severe and/or progressive IP characterized by all of the following items: I) respiratory symptoms consistent with interstitial lung disease, II) diagnosis of usual interstitial pneumonia (UIP), non-specific interstitial pneumonia (NSIP), organizing pneumonia (OP) or a mixed form of UIP/NSIP/OP. Patients with extrinsic allergic alveolitis or hypersensitivity pneumonitis were included as having an IMID. III) Pulmonary function: forced vital capacity (FVC) <50% predicted and/or diffusion capacity for carbon monoxide (DLCO) <40% predicted, or worsening of lung function as demonstrated by any of the following within the past year (>10% decrease in FVC, >15% decrease in DLCO). In addition, patients showed therapy resistance to first line (corticosteroids) and second line therapy (cyclophosphamide or azathioprine). Exclusion criteria were residual lung volume >120% predicted at screening, DLCO <25% of predicted value at screening. Any signs of infection are also contra-indicated.
Intervention
All patients meeting the inclusion criteria were scheduled to receive a therapeutic dose of rituximab. A dose of 1000 mg rituximab was administered intravenously on day 0 and day 14 preceded by premedication: acetaminophen, dexamethasone and antihistamine according to protocol. Before infusion, blood samples were taken for biomarker characteristics. Patients additionally received 18 MBq [89Zr]Zr-rituximab intravenously at day 0, within 4 hours after the first therapeutic dose of rituximab.
Biomarker measurements
Systemic antibodies such as antinuclear antibodies (ANA) anti-neutrophil cytoplasmic antibodies (ANCA) were measured to establish baseline auto-immune parameters. ANA and ANCA are antibodies usually associated with IMID. For inflammatory lung parameters we used soluble interleukin-2 receptor (sIL-2R). B-cell depletion was measured at baseline and post therapy using CD19 and not CD20 to prevent measurement errors by masking by rituximab.
[89Zr]Zr-rituximab preparation and quality control
[89Zr]Zr-N-suc-DFO-rituximab, abbreviated as [89Zr]Zr-rituximab was produced in a Good Manufacturing Practice (GMP) compliant manner at VU University Medical Center, according to the previously reported method of Verel et al. [13]. See detailed description in Addendum.
PET/CT imaging
The department of Nuclear Medicine of the St Antonius Hospital is an EARL accredited PET/CT center. Imaging is performed on Philips Gemini TF PET/CT (Philips Medical Systems, Best, the Netherlands). Scans adhered to the Zr-harmonization protocol as described by Makris et al. [14,15]. PET/CT scans were acquired 3 and 6 days after injection of 18 MBq [89Zr]Zr-rituximab. By using 18 MBq Zr-89, the radiation dose of [89Zr]Zr-rituximab is comparable to a 370 MBq [18F]FDG-PET scan for routine clinical use: ~10 mSv. [89Zr]Zr-rituximab PET/CT of the thorax (lower neck to splenic region) was performed with an acquisition time of 15 minutes per bed position (about 3 bed positions needed). [89Zr]Zr-rituximab PET/CT scans were visually and quantitatively evaluated with validated software (Hermes Medical Solutions, Stockholm, Sweden) by an experienced nuclear medicine physician (HA). [18F]FDG PET/CTs performed in the context of clinical evaluation (2 to 4 weeks prior to the study) were only used as a visual reference to the [89Zr]Zr-rituximab PET/CT scans.
Reference group
As a reference, [89Zr]Zr-rituximab PET/CT scans of 5 patients with rheumatic arthritis (RA) were analyzed. These patients were scanned between 2011-2013 on the same Philips Gemini TF PET/CT and using the Zr-harmonization protocol. All patients were female and age ranged between 26-69 years [11]. These scans were performed by using the same imaging protocol as in the study with IMID-IP patients, in accordance with the EARL criteria. The patients had joint-related inflammation activity without clinical evidence of pulmonary involvement. The chest images of these patients have been visually assessed by 2 nuclear medicine physicians (HA, OS; see acknowledgment).
Image analysis
From previous study findings, we know that [89Zr]Zr-rituximab biodistribution is as follows: highest activity in the liver and a lesser amount of activity in the spleen and blood pool. The lungs of non-IMID-IP patients show no visible activity [16]. In the present study, we focused on increased Zr-89 activity in the lungs and mediastinal/hilar lymph nodes. We measured the standard uptake values (SUV) on day 3 and 6 after injection of [89Zr]Zr-rituximab, in the lung, liver and blood pool using a 1 cm3 volume of predefined regions of interest (ROIs). The blood pool is defined as the average activity in the heart left ventricle (SUVmean blood pool). SUVmax is defined as the maximum SUV in the lung parenchyma or in mediastinal lymph nodes. SUVmean was defined as average global lung SUV. The average global lung SUV (SUVmean lung) was calculated using volumetric analysis software provided by Hermes Medical Solutions, Stockholm, Sweden. The SUVmean lung used in this article is therefore a volume weighted SUVmean. A target-to-blood pool ratio (TBR) was defined as the SUV in the target location divided by the reference SUV region (blood pool). As a result, we assessed TBRmax and TBRmean. Overall Hounsfield units in the lungs are also measured, however due to breathing artefacts are of limited use. For the five control cases from VU University Medical Center, Amsterdam, each patient was analyzed three times with a volumetric tool in random lung locations. We defined increased lung uptake of [89Zr]Zr-rituximab as TBRmax being higher than TBRmean plus two times the standard deviation of the control group. Additionally the mediastinal lymph nodes TBR values were assessed. The relationship between 89Zr-rituximab lung uptake data and clinical baseline parameters were also performed.
Statistics
We have controlled the data for age and BMI, and no statistical bias was found. Data obtained by visual observation were analyzed using descriptive statistics. The paired-sample T tests were performed and a p value <0.05 was regarded as statistically significant. The statistical evaluation was performed using SPSS version 22 (IBM, Armonk, New York, USA).
Results
Patient demographics
[89Zr]Zr-rituximab PET/CT scans were obtained in 10 IMID-IP patients (5 male and 5 female, between 44-69 years). Patient characteristics are summarized in Tables 1, 2. Three patients had RA-associated IP, three patients antisynthetase syndrome (ASS) related IP, two patients chronic extrinsic allergic alveolitis (cEAA) and two patients other types of connective tissue disease related IP.
Table 1.
Baseline characteristics of study patient’s clinical and functional data (N=10)
| Mean | |
|---|---|
| Age (years) | 61.9±7.9 |
| Never smoker | 60% (N=6) |
| Previous smoker | 40% (N=4) |
| Packyears (years) | 24.5±13.8 |
| FVC (% predicted) | 67.06±20.1 |
| DLCOc (% predicted) | 37.37±8.8 |
| ANA positivity | 10% |
| ANCA positivity | 10% |
| CD19 B cell 10*9/L (0.10-0.60) pre-rituximab* | 2.44±7.22 |
| CD19 B cell 10*9/L (0.10-0.60) 2 weeks after first dose rituximab* | 0.000591±0.0005 |
| CD4:CD8 ratio (reference 1.0-3.5) | 3.72±4.04 |
| sIL2R (reference <3000 pg/mL) | 4607.9±2905.6 |
| Baseline characteristics of RA controls (N=5) | |
| Age (years) | 50.2±16.9 |
| Never smoker | 100% (N=5) |
| IgM RF positivity | 80% (N=4) |
| Anti-CCP positivity | 80% (N=4) |
Values are presented as mean ± SD or percentages.
Normal ranges of these values.
FVC = predicted forced vital capacity, DLCO = predicted diffusion capacity of the lung for carbon monoxide, ANA = antinuclear antibodies, ANCA = anti-neutrophil cytoplasmic antibody, CD19 = B-lymphocyte antigen cluster of differentiation 19, CD4:CD8 ratio = T cell cluster of differentiation 4/8, sIL-2R = soluble interleukin-2 receptor, IgM RF = rheumatoid factor, anti-CCP = anti-cyclic citrullinated peptide.
Table 2.
Description of patients treated with rituximab, including previous immunosuppressive therapy
| Patient number | Age | M/F | Diagnosis | Year of diagnosis | HRCT diagnosis | Medication Pre-rituximab |
|---|---|---|---|---|---|---|
| 1 | 69 | M | RA | 2013 | UIP | azathioprine, prednisone |
| 2 | 67 | M | RA | 2010 | UIP | prednisone, cyclofosfamide, azathioprine |
| 3 | 65 | M | RA | 2013 | NSIP | cyclofosfamide, methotrexate |
| 4 | 44 | M | ASS | 2012 | UIP | prednisone, azathioprine |
| 5 | 57 | F | ASS | 2015 | fNSIP | prednisone, azathioprine |
| 6 | 69 | F | ASS | 2009 | fNSIP | prednisone, azathioprine |
| 7 | 59 | F | cEAA | 2015 | EAA | azathioprine, cyclofosfamide |
| 8 | 63 | F | cEAA | 2015 | EAA | cyclofosfamide, prednisone |
| 9 | 57 | M | Scleroderma | 2014 | fNSIP | cyclofosfamide, imuran |
| 10 | 69 | F | Connective tissue disease | 2010 | fNSIP | cyclofosfamide, prednisone |
Rheumatic arthritis (RA), antisynthetase syndrome (ASS), chronic extrinsic allergic alveolitis (cEAA), usual interstitial pneumonia (UIP), fibrotic (f) non-specific interstitial pneumonia (NSIP).
Based on HRCT, five patients had NSIP pattern, three UIP and two EAA. The patient cohort has FVC and DLCO values below normal range since the year of diagnosis. The majority of patients had never smoked (60%). Systemic antibodies such as antinuclear antibodies (ANA) and anti-neutrophil cytoplasmic antibodies (ANCA) were found in a minority of patients (10%). Soluble interleukin-2 receptor (sIL-2R) was increased in all patients at baseline. Circulating mature B-cell values decreased significantly after the first therapeutic dose of rituximab within 14 days in all patients.
[89Zr]Zr-rituximab uptake in reference group
There is presence of [89Zr]Zr-rituximab in the blood pool (main vessels, heart chambers), liver, and to a lesser extent in the spleen. A large decrease of [89Zr]Zr-rituximab blood pool activity was seen on day 6 compared to day 3. Because the images on day 6 had lower mean SUV and much lower counting statistics, and the images at day 3 were qualitatively better, only the results of day 3 are used in the analysis hereafter.
Therapy with rituximab and biodistribution of [89Zr]Zr-rituximab in an allergic patient
There were reversible mild infusion-related reactions consisting of headache and dizziness in 2 patients during rituximab therapy. One patient developed a severe symptomatic hypotension in the first 30 minutes during infusion of the therapeutic dose of rituximab, which persisted during lowering the infusion rate of rituximab after which rituximab infusion was ceased. In total about 100 mg therapeutic dose of rituximab was given. This allergic reaction was proven 3 weeks later when anti-rituximab antibodies were found in the blood. Interestingly this patient demonstrated the highest uptake of [89Zr]Zr-rituximab in mediastinal lymph nodes and lung parenchyma on PET/CT compared to the other 9 patients who did receive the full therapeutic dose of rituximab before [89Zr]Zr-rituximab PET/CT. The patient with an allergic reaction showed a higher uptake of Zr-89 in spleen and lymph nodes (see Figure 1).
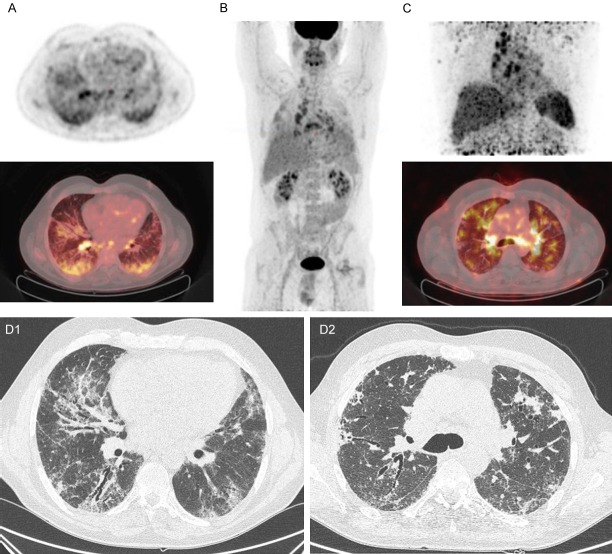
Figure 1.
[18F]FDG PET/CT and [89Zr]Zr-rituximab PET/CT scans of patient 9, a 57-year-old patient with fibrotic non-specific interstitial pneumonia associated scleroderma. (A) [18F]Fluorodeoxyglucose (FDG) PET axial views (top: PET, bottom: fusion PET/CT); (B) Maximum intensity projection of [18F]FDG PET; (C) [89Zr]Zr-rituximab PET (top: MIP image, bottom: fusion PET/CT); (D1 and D2) respective HRCT axial views of (A and C). In the axial views of the [18F]FDG PET (1 week before rituximab) image clear increased uptake is seen in the subpleural basal pulmonary regions and also in several lymph nodes. On HRCT there is some fine fibrosis visible in these subpleural areas (D1). The [89Zr]Zr-rituximab PET shows mainly lymph node and peribronchial increased activity, with less blood pool activity. The parenchyma shows a moderately increased activity in patchy areas, but most is centrally located around the hilar regions; on CT (D2) there is no fine fibrosis around these areas. In addition, there is an active axillary lymph node on the right and increased activity in the spleen; this was not seen in other patients.
[89Zr]Zr-rituximab visual analysis of other IMID-IP patients
Overall the uptake in the lungs was lower in activity when compared to the bloodpool and liver. There is more diffuse uptake in the lungs, with patchy areas with increased uptake. There seems to be no preference in upper or lower lobe distribution between patients. Highest activity was found in peribronchiolar and peri-hilar regions. Figures 1, 3 and 4 show patients with different uptake patterns when compared to a prior [18F]FDG PET/CT. There were no signs of active infection in these patients.
Figure 3.
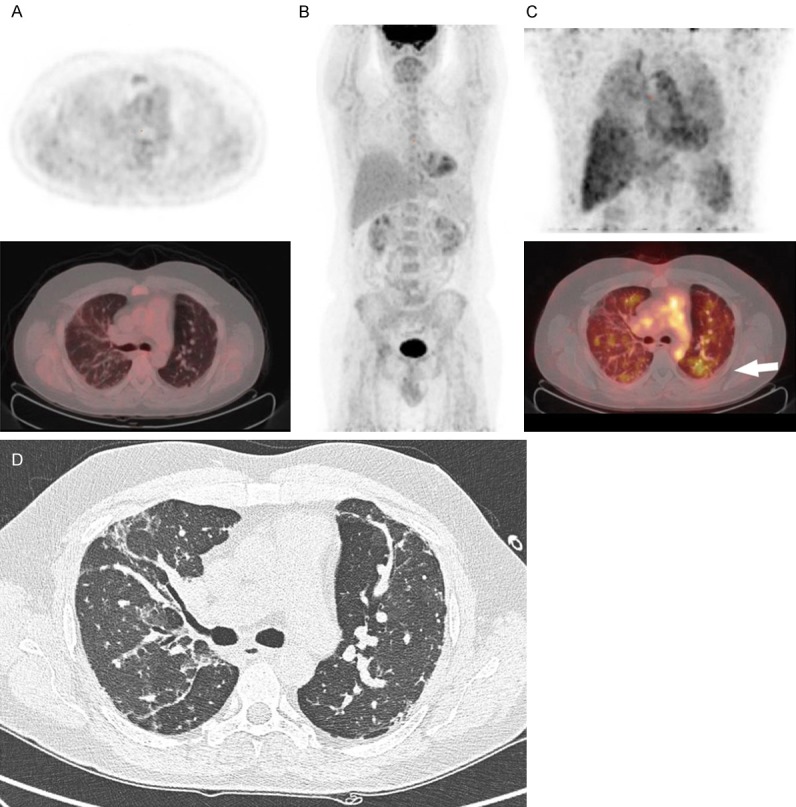
[18F]FDG PET/CT and [89Zr]Zr-rituximab PET/CT of patient 4. (A) [18F]FDG PET axial views (top: PET, bottom: fusion PET/CT); (B) Maximum intensity projection of [18F]FDG PET; (C) [89Zr]Zr-rituximab PET (top: MIP image, bottom: fusion PET/CT); (D) HRCT or respective axial level of (A and C). 44-year-old patient with antisynthetase associated usual interstitial pneumonia. In the axial views of the [18F]FDG PET image (1 week before rituximab) no increased uptake is seen in the pulmonary regions (perhaps some asymmetry in right versus left lung due to poor aeration) nor in the mediastinal or hilar lymph nodes. The [89Zr]Zr-rituximab PET (C) demonstrated mainly visual diffusely increased pulmonary activity, in contrast to [18F]FDG PET (A, B). The parenchyma shows a moderately increased activity in patchy areas such as in the left lower lobe (white arrow) without any clear anatomical substrate on CT (D). There is normal splenic activity.
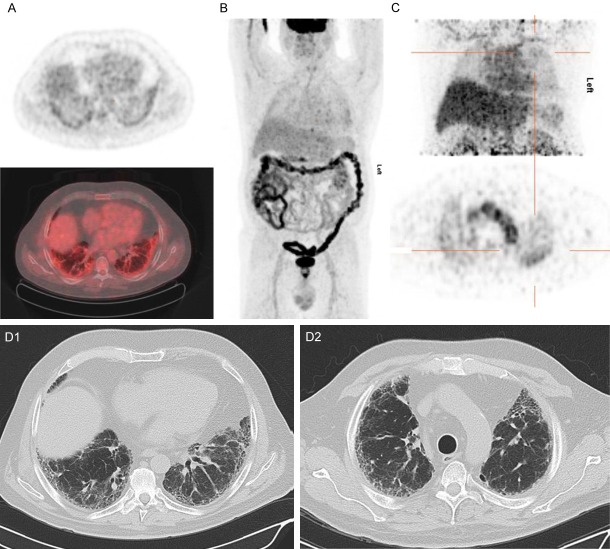
Figure 4.
[18F]FDG PET/CT and [89Zr]Zr-rituximab PET/CT of patient 2. (A) [18F]FDG PET axial views (top: PET, bottom: fusion PET/CT); (B) Maximum intensity projection of [18F]FDG PET; (C) [89Zr]Zr-rituximab PET (top: MIP image, bottom: PET); (D1 and D2) respective HRCT images of (A and C). 67-year-old male, with rheumatoid arthritis associated usual interstitial pneumonia. In the axial views of the [18F]FDG PET image (1 week before rituximab) moderate increased [18F]FDG uptake is seen in the subpleural basal pulmonary regions in some para-fibrotic areas with some honeycombing (D1), but not in the mediastinal or hilar lymph nodes. The [89Zr]Zr-rituximab PET shows mainly diffusely increased pulmonary activity. The parenchyma shows a moderately increased activity in patchy areas such as in the left upper lobe (cross hairs) this region has no honeycombing (D2). The basal subpleural regions did not show visible increased [89Zr]Zr-rituximab activity, contrast to [18F]FDG image (A). There is normal splenic activity.
[89Zr]Zr-rituximab quantitative uptake in IMID-IP patients
[89Zr]Zr-rituximab uptake in the lungs of IMID-IP patients was compared with the uptake in the lungs of patients in RA control group (Table 3). Higher uptake was found in the lungs of few IMID-IP patients than in the lungs of RA controls, as based on the TBRmax values (P<0.02). The results are still significant when excluding case 9 (P 0.004). The lung SUVmean values did not show a statistically significant difference from the RA controls (P=0.39). According to our definition of increased uptake, a TBRmean and TBRmax plus two times the standard deviation, gives us a TBRmean threshold of 0.6 (0.32+2×0.14) and TBRmax of 0.74 (0.42+2×0.16). These thresholds are represented in Figure 2. Only one patient (case 10) had a TBRmean of 2 standard deviations above the RA controls. However, this is different for TBRmax: 8 patients demonstrated a TBRmax higher than 2 standard deviations above the controls. Qualitative analysis demonstrated that four of those patients also had visually significant higher uptake than the other IMID-IP patients. Patient 9 who did only receive 100 mg of rituximab due to an allergic reaction, showed SUVmean values at day 3 and 6 higher than the other patients. TBRmax 3.45 of case 9 was significantly higher versus TBRmax of the other patients 0.97 (P<0.005).
Table 3.
[89Zr]Zr-rituximab uptake in IMID-IP patients vs RA controls, as assessed with PET on day 3
| IMID-IP (N=10) | RA (N=5) | P value | |
|---|---|---|---|
| SUVmean Lung | 0.37±0.095 | 0.32±0.15 | 0.166 |
| SUVmax Lung | 1.10±0.49 | 0.37±0.15 | 0.000282* |
| SUV range | (0.45-3.45) | (0.1-0.69) | |
| SUVmean blood pool | 0.99±0.30 | 1.05±0.26 | 0.647 |
| HU Lung | -620±60.9 | -646±54.7 | 0.147 |
| TBRmean | 0.41±0.16 | 0.32±0,14 | 0.447 |
| TBRmax | 1.25±0.84 | 0.42±0.16 | 0.018* |
| TBRmax (without case 9) | 0.97±0.40 | 0.42±0.16 | 0.004* |
Samples test significance below P<0.05;
SUVmean = mean standard uptake value, SUVmax = maximum standard uptake value, TBRmean = mean target-to-blood pool ratio, TBRmax = maximum target-to-blood pool ratio. Target tissue is lung tissue. HU lung = mean Hounsfield Unit in the lungs; please not these are low dose CT scans with breathing artefacts.
Figure 2.
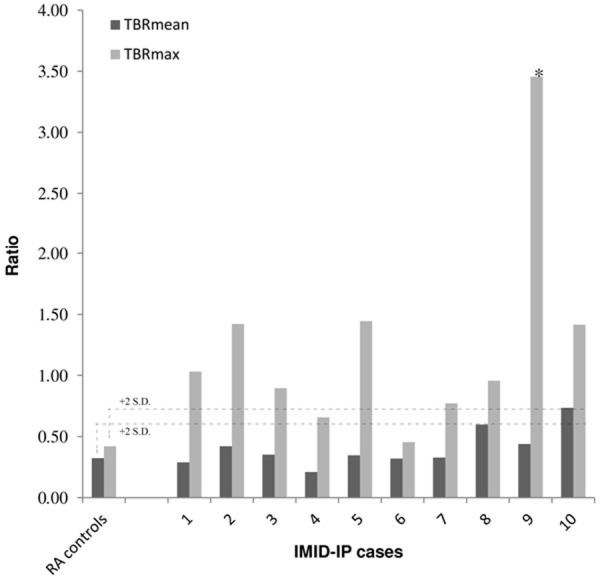
TBRmean and TBRmax ratios of pulmonary 89Zr-rituximab uptake in IMID-IP vs controls (n=5). *Case 9 was significantly different from the group (P<0.005). This was the case with a severe allergic reaction and did not receive the full rituximab pre-load dose.
Correlation of [89Zr]Zr-rituximab PET and clinical baseline parameters
In all patients, circulating mature B-cells were reduced within 14 days after the first dose of rituximab. However, there was a slight difference in baseline values of circulating B-cells. We analyzed if there was a relation between TBRmax values and the number of B-cells at baseline but no correlation was found. TBRmax was used because it was the only parameter that could significantly distinguish between IMID-IP and controls (as shown in Table 3). All other parameters (FVC, DLCO, sIL-2R and CD4:CD8 ratio) neither did show a correlation at baseline. We did however find a strong correlation between baseline CD4:CD8 ratio and sIL-2R of 0.844 (P 0.002) which is expected since both are inflammatory biomarkers.
Discussion
This is the first study analyzing [89Zr]Zr-rituximab PET in patients with interstitial pneumonitis associated IMID, including some cases with EAA. The goal of the study was to evaluate if [89Zr]Zr-rituximab PET could identify some visual and measurable differences in the presence of CD20+ B cells in the lungs of patients, to give a possible explanation for the observed differences in treatment response as described in literature. We observed visual and measurable increased pulmonary activity in four patients, compared to the reference. There was little mediastinal lymph node activity. There were no signs of infection in these patients. Other patients demonstrated no increased activity in the lungs, according to our definition. We found that the [89Zr]Zr-rituximab could be administered safely, even if patients did experience (allergic) side-effects from therapeutic rituximab. This study is limited due to the fact that we lack histopathology of the locations where [89Zr]Zr-rituximab PET showed increased uptake. However, lung biopsies for research purposes in these patients are not feasible due to the risks of the procedure.
In our study, highest [89Zr]Zr-rituximab uptake was found in peribronchiolar and peri-hilar regions. These regions are consistent with data of a histology driven open-lung biopsy study by Atkins et al. in 2006 where RA-associated IP showed follicular B cell hyperplasia around peribronchiolar lymphoid aggregates [9]. There were also cases demonstrating subpleural activity in the upper lobes, which matched with some fibrotic areas on CT.
Patient with allergic reaction to therapeutic rituximab
One patient (case 9) developed an allergic reaction with hypotension during infusion of the first 100 mg unlabelled (therapeutic) rituximab after which rituximab infusion was ceased. Increased serum blood anti-rituximab IgG antibodies (66 AU/ml) were found 2 weeks later proving an allergic reaction to rituximab. This patient never received rituximab before, nor any other monoclonal antibody therapy. This is a rare, but not uncommon side effect. There were no remarkable differences in this patient’s diagnosis compared to other patients, only a slightly increased amount of fibrosis present in the lungs. However, this patient did receive labeled [89Zr]Zr-rituximab within 4 hours after the ceasing unlabelled (therapeutic) rituximab without any reaction. This patient demonstrated the highest activity of Zr-89 in mediastinal lymph nodes and lung parenchyma compared to the other 9 patients who did receive a full therapeutic dose of rituximab before [89Zr]Zr-rituximab. The higher uptake in lymph nodes found in case 9 is consistent with previous PET studies in which [89Zr]Zr-rituximab showed specific uptake in lymph nodes [11,17]. The absolute SUV activity after 6 days was still higher than the other patients. We have considered this finding to be significant and we will address this in the following section.
Concerns with pre-load (cold blocking) protocol in patients with interstitial lung disease
Due to the findings in case 9 we have concerns about the dosing protocols and biodistribution. We used a fixed treatment dose of 1000 mg rituximab at day 0, prior to i.v. administration of 10 mg of labeled [89Zr]Zr-rituximab. This is analogous to previously published studies with 89Zr-labeled rituximab [11,18]. In our rare disease population we could not perform a dose escalation study in advance, nor a dose-finding study for the 10 mg dose of [89Zr]Zr-rituximab. Therefore, we chose to follow previously published protocols for [89Zr]Zr-rituximab imaging. A cold pre-load is usually given to improve biodistribution (of [89Zr]Zr-rituximab) by decreasing the “antigen-sink” effect caused by normal CD20-positive B cells in the circulation, bone marrow and the spleen [17]. A cold pre-load method has significant improvement in the targeting of tumor sites, but also in targeting of inflamed joints of patients with rheumatoid arthritis [11]. However, when case 9 received a significantly lower (therapeutic) pre-load dose than the other cases, the biodistribution improved visually as well as quantitatively. This raises a major concern, as the high dose of unlabeled rituximab is probably competing with the relatively small dose of radiolabeled rituximab, resulting in blocking of tracer uptake. We therefore assume that the uptake of [89Zr]Zr-rituximab was underestimated in the other cases. A dose escalation study is needed to re-evaluate the current pre-load protocol for inflammation studies.
[89Zr]Zr-rituximab PET image quality and possible improvements
There are several factors to be addressed: the dose activity of [89Zr]Zr-rituximab given, the scan time on PET/CT and the protocol in relation to the therapeutic unlabeled rituximab. The 18 MBq dose was at the lower sensitivity limit of the PET/CT scanner and it was necessary to scan for about 45 minutes (three bed positions) to get adequate results. Although no severe breathing or movement artefacts were detected, we did not expect any improvement from longer scanning than 15 minutes per bed position. The count statistics of the day 6 scan was far less than the day 3 scan, and were qualitatively inferior than day 3. We were restricted by the 18 MBq dose limit based on the notion of the Medical Ethics Committee on radiation safety that patients with auto-immune disease should not receive more than 10 mSv of radiation dose. This restriction was partly because we wanted to include as many patients as possible including younger patients. On the other hand, cancer patients are allowed to receive much higher radioactivity doses of [89Zr]Zr-rituximab, which is 74 MBq and equivalent to up to 60 mSv or radiation dose [17,18]. From a medical point of view, however, the life expectancy of our therapy resistant cohort is less than 10 years, some even less than 5 years [19] and thus comparable to some cancer patients. In future studies, we believe that scanning at a higher radioactivity dose might be justified in this patient cohort, especially if therapeutic benefits outweigh the radiation risk. If our patients are scanned at a higher dose, for instance 36 or 54 MBq we could have had better count statistics, and possibly qualitatively better results on day 6. However it is essential that scanners become even more sensitive, more sensitive than current digital scanners, thus improving count statistics of low doses of radiopharmaceuticals [20,21]. Even if higher [89Zr] doses were used, [89Zr] still has lower spatial resolution and increased noise (due to scatter and background variation) when compared to [18F] [22]. [89Zr]Zr-rituximab PET scan quality should therefore be optimized using special software algorithms to provide more useful information with less scanning time, adding to more practicality in everyday clinical use.
In life threatening auto-immune diseases, such as our IMID-IP study population, it is clinically important to use expensive drugs appropriately. Patients can have side-effects from the therapy while not having any beneficial effects and at a higher cost. With the advent of so-called precision or personalized medicine, we need to be able to predict therapeutic effects in individual patients more pro-actively than conventional methods. Our study provides the first successful step in imaging [89Zr]Zr-rituximab in patients with auto-immune lung diseases. However, further research should demonstrate if [89Zr]Zr-rituximab PET/CT could indeed predict therapeutic response. If successful, these scans could potentially be important biomarkers in the clinical management of expensive drugs in everyday practice.
Conclusion
Immunotherapy in the form of monoclonal antibodies is widely used for cancer treatment and also for treatment of several auto-immune diseases. Lately more clinical studies have implemented immuno-PET as a research tool in prediction and selection of cancer patients who would be eligible for immunotherapy. Our manuscript describes the first immuno-PET study using Zr89 labelled rituximab in a non-cancer patient cohort: patients with therapy refractory interstitial pneumonitis. This trial is registered at ClinicalTrials.gov identifier: NCT02251964.
In our manuscript, we report the first immuno-PET findings in 10 patients with autoimmune lung conditions, making three key observations: 1) Despite some technical (radiation safety) restrictions, the implementation of [89Zr]Zr-rituximab PET does show measurable differences between our auto-immune patients. This is an important finding since we used a lower Zr89 radiation dose than previously published. This finding also opens a path to a follow-up study to analyse the correlation between clinical response and immuno-PET findings in our patients with auto-immune diseases. 2) Immuno-PET in this patient cohort seems safe even though one patient had a severe allergic reaction to rituximab. The scan can be performed without a risk of side-effects from the therapeutic rituximab. 3) The findings suggest that the scanning protocol could be further optimized using a different therapeutic rituximab dose and/or injection of [89Zr]Zr-rituximab before therapeutic rituximab, which is different than previously published in cancer patients. This is likely to impact other immuno-PET studies in non-oncology patients. Although this may improve image quality, higher PET/CT scanner sensitivity seems of vital importance.
Acknowledgements
We would to thank S. Bruijnen (Amsterdam Rheumatology and immunology Center (ARC), location VU University Medical Center, Amsterdam, The Netherlands) and Otto Hoekstra (OH) (Department of Radiology & Nuclear Medicine, VU University Medical Center, Amsterdam, The Netherlands) for providing us access to the RA control group. This investigator-initiated study was financially supported by ZonMw research grant for Goed Gebruik Geneesmiddelen (GGG) which is the Netherlands Organisation for Health Research and Development commissioned by the Ministry of Health, Welfare and Sport of the Netherlands. This study was funded by ZonMW (grant number 836021009). ZonMW is an independent self-governing organisation. ZonMW works closely with the Netherlands Organisation for Scientific Research.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study has been approved by Medical Research Ethics Committees United (MEC-U: Nieuwegein) under number NL49534.100.14, Eudract number 2013-005269-37. Informed consent was obtained from all individual participants included in the study.
Addendum
Rituximab (0.5 mL of 10 mg/mL (33 nmol)) was diluted to 0.5 mg/mL with 0.9% NaCl (470 µL), after which the pH was adjusted to 9.5-9.7. Two equivalents of Fe-TFP-N-suc-desferal in acetonitrile (20 µL of 3.3 nmol/µL, 66 nmol) were added, mixed carefully and reacted for 30 minutes at room temperature. Next, 50 µL of 100 mg/mL gentisic acid pH 4.0-4.2 were added, followed by adjustment of the pH to 4.20-4.50 with 0.25 M sulfuric acid. Hereafter, EDTA (50 µL, 25 mg/mL) was added and reacted for 30 minutes at 35°C to remove Fe, after which the conjugated N-suc-DFO-rituximab was purified by size exclusion chromatography (PD10, GE Healthcare) and the product collected in 5 mg/mL gentisic acid in 0.9% NaCl pH 4.9-5.3. Hereafter, N-suc-DFO-rituximab was radiolabeled. To this end, 600 µL 1 M oxalic acid containing the required amount of 89Zr were mixed with 1800 µL 2 M Na2CO3 and reacted for 3 minutes. Next 3 mL 0.5 M Hepes and 2 mL N-suc-DFO-rituximab were added and reacted for 60 minutes at room temperature while slowly shaken. After the incubation period [89Zr]Zr-rituximab was purified by size exclusion chromatography using a PD10 column. The product was eluted in 5 mg/mL gentisic acid in 0.9% NaCl pH 4.9-5.3. The product was formulated to arrive at an injection dose of 18 MBq-5 mg-10 mL [89Zr]Zr-Rituximab.
The mean of the product pH was 6.02±0.26. The mean radiochemical purity as assessed by iTLC was 99.1±0.3%. To this end 2 µL of product was applied on a TLC strip (Biodex, cat nr. 150-771) and developed in 10% acetonitrile in 20 mM citric acid + 50 mM EDTA pH 4.8-5.0 as described by the supplier. [89Zr]Zr-rituximab remains on the baseline, while impurities such as free Zr-89 and 89Zr-DFO run with the solvent front. The mean protein integrity was 99.6±1.2% as determined by size exclusion HPLC using a superdex 200 10/300 GL column (GE) and a mixture of 0.1 M phosphate, 0.15 M NaCl and 0.01 M NaN3 pH 6.2-7.0 in water as the eluent at a flow rate of 0.5 mL/min. The mean immune reactive fraction as assessed by a binding assay was 90.5±2.3% using SU-DHL-4 cells fixed in 2% paraformaldehyde. Sterility of each [89Zr]Zr-rituximab batch was assured by performing a media fill immediately after final filter sterilisation of each batch. These procedures resulted in a sterile final product with endotoxin levels <0.5 EU/mL. The radiopharmaceutical consists of 10 mg rituximab labeled with 18 MBq Zr-89 in a total injection volume of 10 mL. Zr-89 was obtained at >740 MBq/mL in 1 M oxalic acid from Perkin Elmer (Boston, MA, USA).
Disclosure of conflict of interest
None.
References
- 1.Kuek A, Hazleman BL, Ostor AJ. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J. 2007;83:251–60. doi: 10.1136/pgmj.2006.052688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melet J, Mulleman D, Goupille P, Ribourtout B, Watier H, Thibault G. Rituximab-induced T cell depletion in patients with rheumatoid arthritis: association with clinical response. Arthritis Rheum. 2013;65:2783–90. doi: 10.1002/art.38107. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y. Pathological mechanisms in rheumatoid arthritis. Nihon Rinsho. 2013;71:1147–52. [PubMed] [Google Scholar]
- 4.Burmester GR, Feist E, Dörner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:77–88. doi: 10.1038/nrrheum.2013.168. [DOI] [PubMed] [Google Scholar]
- 5.Daoussis D, Liossis SN, Yiannopoulos G, Andonopoulos AP. B-cell depletion therapy in systemic sclerosis: experimental rationale and update on clinical evidence. Int J Rheumatol. 2011;2011:214013. doi: 10.1155/2011/214013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luu VP, Vazquez MI, Zlotnik A. B cells participate in tolerance and autoimmunity through cytokine production. Autoimmunity. 2014;47:1–12. doi: 10.3109/08916934.2013.856006. [DOI] [PubMed] [Google Scholar]
- 7.Keir GJ, Maher TM, Hansell DM, Denton CP, Ong VH, Singh S, Wells AU, Renzoni EA. Severe interstitial lung disease in connective tissue disease: rituximab as rescue therapy. Eur Respir J. 2012;40:641–8. doi: 10.1183/09031936.00163911. [DOI] [PubMed] [Google Scholar]
- 8.Keir GJ, Maher TM, Ming D, Abdullah R, de Lauretis A, Wickremasinghe M, Nicholson AG, Hansell DM, Wells AU, Renzoni EA. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology. 2014;19:353–9. doi: 10.1111/resp.12214. [DOI] [PubMed] [Google Scholar]
- 9.Atkins SR, Turesson C, Myers JL, Tazelaar HD, Ryu JH, Matteson EL, Bongartz T. Morphologic and quantitative assessment of CD20+ B cell infiltrates in rheumatoid arthritis-associated nonspecific interstitial pneumonia and usual interstitial pneumonia. Arthritis Rheum. 2006;54:635–41. doi: 10.1002/art.21758. [DOI] [PubMed] [Google Scholar]
- 10.Lafyatis R, O’Hara C, Feghali-Bostwick CA, Matteson E. B cell infiltration in systemic sclerosis-associated interstitial lung disease. Arthritis Rheum. 2007;56:3167–8. doi: 10.1002/art.22847. [DOI] [PubMed] [Google Scholar]
- 11.Bruijnen S, Tsang-A-Sjoe M, Raterman H, Ramwadhdoebe T, Vugts D, van Dongen G, Huisman M, Hoekstra O, Tak PP, Voskuyl A, van der Laken C. B-cell imaging with zirconium-89 labelled rituximab PET-CT at baseline is associated with therapeutic response 24 weeks after initiation of rituximab treatment in rheumatoid arthritis patients. Arthritis Res Ther. 2016;18:266. doi: 10.1186/s13075-016-1166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malviya G, Anzola KL, Podestà E, Laganà B, Del Mastro C, Dierckx RA, Scopinaro F, Signore A. (99m)Tc-labeled rituximab for imaging B lymphocyte infiltration in inflammatory autoimmune disease patients. Mol Imaging Biol. 2012;14:637–46. doi: 10.1007/s11307-011-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verel I, Visser GW, Boellaard R, Stigter-van Walsum M, Snow GB, van Dongen GA. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J Nucl Med. 2003;44:1271–81. [PubMed] [Google Scholar]
- 14.Makris NE, Boellaard R, Visser EP, de Jong JR, Vanderlinden B, Wierts R, van der Veen BJ, Greuter HJ, Vugts DJ, van Dongen GA, Lammertsma AA, Huisman MC. Multicenter harmonization of 89Zr PET/CT performance. J Nucl Med. 2014;55:264–7. doi: 10.2967/jnumed.113.130112. [DOI] [PubMed] [Google Scholar]
- 15.Makris NE, van Velden FH, Huisman MC, Menke CW, Lammertsma AA, Boellaard R. Validation of simplified dosimetry approaches in (89)Zr-PET/CT: the use of manual versus semi-automatic delineation methods to estimate organ absorbed doses. Med Phys. 2014;41:102503. doi: 10.1118/1.4895973. [DOI] [PubMed] [Google Scholar]
- 16.Muylle K, Flamen P, Vugts DJ, Guiot T, Ghanem G, Meuleman N, Bourgeois P, Vanderlinden B, van Dongen GA, Everaert H, Vaes M, Bron D. Tumour targeting and radiation dose of radioimmunotherapy with (90)Y-rituximab in CD20+ B-cell lymphoma as predicted by (89)Zr-rituximab immuno-PET: impact of preloading with unlabelled rituximab. Eur J Nucl Med Mol Imaging. 2015;42:1304–14. doi: 10.1007/s00259-015-3025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jauw YW, Menke-van der Houven van Oordt CW, Hoekstra OS, Hendrikse NH, Vugts DJ, Zijlstra JM, Huisman MC, van Dongen GA. Immuno-positron emission tomography with zirconium-89-labeled monoclonal antibodies in oncology: what can we learn from initial clinical trials? Front Pharmacol. 2016;7:131. doi: 10.3389/fphar.2016.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jauw YW, Zijlstra JM, de Jong D, Vugts DJ, Zweegman S, Hoekstra OS, van Dongen GA, Huisman MC. Performance of 89Zr-labeled-rituximab-PET as an imaging biomarker to assess CD20 targeting: a pilot study in patients with relapsed/refractory diffuse large B cell lymphoma. PLoS One. 2017;12:e0169828. doi: 10.1371/journal.pone.0169828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, Dawson J, Sathi N, Ahmad Y, Koduri G, Young A British Rheumatoid Interstitial Lung (BRILL) Network. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics--a large multicentre UK study. Rheumatology (Oxford) 2014;53:1676–82. doi: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 20.Cherry SR, Jones T, Karp JS, Qi J, Moses WW, Badawi RD. Total-body pet: maximizing sensitivity to create new opportunities for clinical research and patient care. J Nucl Med. 2018;59:3–12. doi: 10.2967/jnumed.116.184028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikhaylova E, Tabacchini V, Borghi G, Mollet P, D’Hoe E, Schaart DR, Vandenberghe S. Optimization of an ultralow-dose high-resolution pediatric PET scanner design based on monolithic scintillators with dual-sided digital SiPM readout: a simulation study. Phys Med Biol. 2017;62:8402–8418. doi: 10.1088/1361-6560/aa8eb2. [DOI] [PubMed] [Google Scholar]
- 22.Alanazi SF, Alzimami KS, Ghannam MM, Aljammaz IJ, Alrumayan F, Sassi SA. Quantitative imaging characteristics of zirconium-89 on Gemini Time-Of-Flight PET/CT. Nucl Med Commun. 2016;37:1238–1245. doi: 10.1097/MNM.0000000000000602. [DOI] [PubMed] [Google Scholar]