Abstract
For decades, conventional nuclear medicine techniques have been utilized for the assessment of many infectious and inflammatory diseases. Most of these techniques have limitations such as the relatively low spatial resolution, being time consuming and low sensitivity or specificity. In recent years, FDG-PET/CT has shown promising role in the management of such diseases. An expanding set of studies illustrate the multifarious roles of FDG-PET/CT in the assessment of these conditions, both systemic diseases and more regional. Specifically, PET can provide vital information at a molecular level and consequently detect the disease activity at their earliest manifestation. With the continuing research on the diagnosis and treatment monitoring of patients with infectious and inflammatory diseases, the role of PET/CT can be further extended.
Keywords: FDG PET/CT, infectious disease, inflammatory disease, fever of unknown origin, HIV, tuberculosis, vasculitis, bone infection, sarcoidosis
Introduction
Recent efforts have expanded the clinical application of FDG PET/CT in infectious and inflammatory diseases. Pathophysiological distribution of FDG in the presence of infectious and inflammatory conditions relies basically on the same underlying mechanisms as in malignancies and other indications, i.e. elevated rates of cellular metabolism. Simply put, all cells to some extent harness energy from glucose by way of the anaerobic glycolytic pathway, but during conditions of high energy demand most cells prefer the more energy efficient aerobic oxidative phosphorylation. However, cancer cells tend to prefer the glycolytic pathway even under aerobic condition. In cancer cells, the increased demand for glucose is met by upregulation of the active glucose transporter (GLUT), which also forms the basis of the increased FDG-uptake in these cells compared to normal cells. After internalization, glucose is enzymatically phosphorylated by hexokinase to facilitate further processing through the glycolytic pathway, whereas any surplus glucose is expelled again after enzymatic dephosporylation by glucose-6-dephosphorylase. FDG undergoes the same process, but due to stereochemical differences, FDG-6-phosphate is not a substrate for the downstream enzymes in the glycolytic pathway and the process is not advanced further. At the same time, many cancer cells have decreased levels of glucose-6-dephosphorylase and as the GLUT’s do not accommodate phosphorylated molecules, the net result is the intracellular so-called metabolic trapping of FDG that form the basis of its high target-to-background properties [1]. Initially, this effect was considered specific to cancer cells, but early in the evolution of FDG-PET it became clear that immune cells also utilized this approach to some extent. This gave rise to the initial notion that false-positive findings in cancer patients were a nuisance of FDG which could no longer be considered specific to cancer [2]. Slowly, this became an area of increasing interest as studies began to actively take advantage of the FDG-uptake in inflammatory settings [3]. During the 1990s the pathophysiologic basis was further established; autoradiography studies showed activated granulocytes predominantly in the early phases of active inflammation as well as macrophages in later, chronic stages shared the same traits as cancer cells with regards to up-regulation of GLUT, and they also established that immune-mediated cytokine release play an important role in the up-regulation of GLUT [4-6].
Compared to alternative nuclear medicine imaging techniques, PET has superior spatial resolution. When co-registered with low dose CT images, precise spatial localization of FDG distribution upon anatomy can be achieved. Despite the development of various new PET radio-tracers, FDG PET/CT retains a major role in the diagnosis of many infectious and inflammatory diseases. Moreover, this modality has proven valuable in monitoring treatment efficacy and in informing clinical management strategies. This review will survey the present scientific and clinical applications of 18F-FDG-PET/CT imaging in several common yet serious infectious and inflammatory conditions.
Fever of unknown origin (FUO)
It has always been a great challenge to definitely diagnose FUO as differential diagnoses are plentiful and the underlying cause may be located anywhere throughout the body. Petersdorf and Beeson first defined FUO as an intermittent, unresolved fever, with temperatures higher than 38.3°C, and lasting at least three weeks without a definite diagnosis being ascertained after one week of in-patient investigations [7]. Infection and non-infectious inflammatory diseases (NIID) account for most cases of FUO cases in adults [8,9]. In pediatrics, the most common causes of FUO is infection diseases (37.6%) and malignancy (17.2%), followed by collagen vascular disease and miscellaneous diseases [10]. The diagnostic work up requires patients to undergo a series of diagnostic investigations often including cross-sectional imaging, but the limited sensitivity and specificity of CT and MRI has limited their efficacy in FUO [11]. FDG PET can localize metabolic abnormalities earlier than structural modalities, and it may therefore be of greater value in FUO cases. FDG uptake is increased in many etiologies responsible for FUO, not only infections but also inflammation and cancer. As such, FDG PET is the obvious first line modality Figure 1 [12,13]. Gallium-67 and labelled leukocyte imaging are assumed to be helpful in FUO cases, but have their own limitations. These procedures require time-consuming preparations [12], and they are not sensitive to malignancies that constitute a significant proportion of FUO etiologies. In a recent study of 58 patients with FUO comparing FDG PET/CT to Gallium-67 SPECT/CT, the former was found to be superior in regards to sensitivity and overall clinical contribution, i.e. 79% vs. 45% and 72% and 55%, respectively [14]. FDG-PET imaging is a non-invasive one-stop investigation that can delineate the extent of involvement and can help to select the biopsy site.
Figure 1.
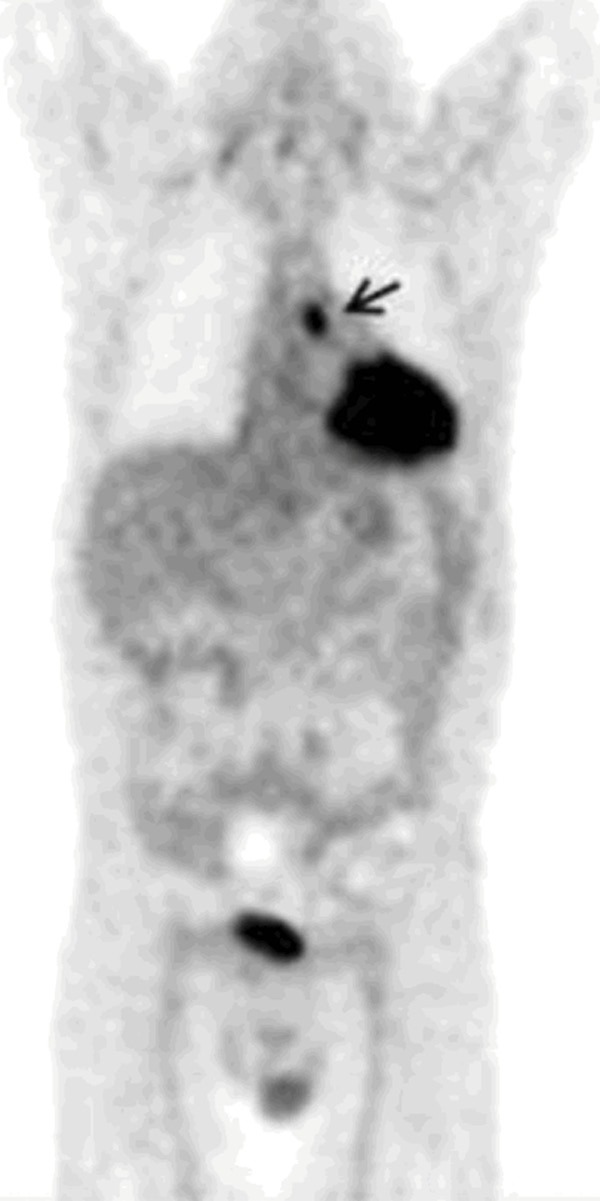
FDG-PET image was acquired in a patient who was hospitalized twice, and an extensive workup was conducted over several days to determine the cause of FUO. Finally, FDG-PET was performed as a last resort for further assessment of this desperate clinical scenario. The image clearly shows a focus of abnormal uptake in the mediastinum (arrow), which proved to be a focal site of infection that was drained, resulting in complete recovery from continuous fever. The site of infection was overlooked on contrast-enhanced CT scan which was performed prior to FDG-PET images. This clearly demonstrates the importance of intense FDG uptake as a focal abnormality, allowing visualization of lesions in certain locations which are missed by conventional structural imaging modalities [26]. Reproduced with permission.
The study of Lorenzen et al. [15] was among the first studies to use FDG-PET to diagnose FUO. They evaluated FDG-PET scans of 16 patients in whom the underlying cause of FUO had not been detected by conventional diagnostics. Sites of Non-physiological uptake of FDG were identified in 12 patients (75%) which led to the final diagnosis in 11 patients (69%) [15]. Keidar et al. assessed 48 patients with FUO who underwent FDG-PET/CT. In 90% of the patients FDG-PET contributed to diagnosing the underlying cause of FUO or excluding the presence of a focal pathology leading to the patient’s febrile state [16]. Several meta-analyses have also demonstrated the usefulness of FDG-PET in reaching the final diagnosis of FUO [17-23]. Generally, results are favorable albeit with some caveats and unclarified issues, e.g. a relative lack of standardization with regards to definitions of FUO, patient population, and results. For instance, in some of the older studies, FDG-PET was performed as part of various diagnostic strategies that included other diagnostic procedures. Therefore, the diagnostic yield must be viewed in light of the populations that comprise the more difficult cases. Moreover, the definition of a clinically useful result varies; most focus on positive FDG uptake, but some advocate a similar value from negative findings to rule out focal infection or malignancies [24]. This exclusion may be useful in patients with known inflammatory disease and fever to distinguish disease flare from novel infection or malignancy [25]. Bharucha et al. performed a systematic review, meta-analysis and Delphi exercise to evaluate diagnostic yield of (FDG-PET/CT) in fever of unknown origin (FUO) [18]. In their meta-analysis, 18 studies were included comprising 905 patients and the pooled diagnostic yield was reported to be 56% (95% confidence interval [CI]: 50-61%, I2=61%). Furthermore, a subgroup analysis found added value over CT in 32% of cases. There is consensus that FDG-PET/CT is an increasingly available and emerging choice of investigation, but there is variability in practice [18]. A more recent Chinese multi-center study investigated the clinical utility of FDG-PET/CT for the diagnosis of FUO [19]. Based on their observations, 95.2% of the subjects had a positive finding on FDG-PET/CT. Furthermore, it provided additional information in 77.4% of the cases, and overall, 89.6% of patients benefitted from FDG-PET/CT imaging [19].
Given that FDG-PET can detect neoplasms, it is superior to other tests such as labeled leukocytes in detecting the underlying cause of FUO. In recent studies on FDG-PET/CT in FUO, the prevalence of malignancies as the underlying cause of FUO has been reported to be 15-19 [17-19]. The most common neoplastic cause of FUO is lymphoma [26]. The sensitivity and specificity of FDG-PET in lymphoma was found to be 90% and 91%, respectively [27,28], indicating the suitability of this modality in detecting the neoplastic causes of FUO. FDG-PET is also highly useful for detection of vasculitis as a cause of FUO. The sensitivity and specificity of FDG-PET in detecting vasculitis is reported to be 77-100% and 89-100%, respectively [29]. Giant cell arteritis (temporal arteritis) and Takayasu’s arteritis constitute 17% of all FUO causes [30]. Often times, MRI and CT are utilized for diagnosis of Takayasu arteritis; however, FDG-PET is considered to be particularly useful for the diagnosis of early-stage TA [30].
FUO is a challenging medical problem also in children, and although not completely similar to an adult population, some features are similar. Etiologies comprise infection (30-35%), non-infectious inflammation (20%), and malignancies (10%), whereas about one-third will remain undiagnosed [31]. Most diagnostic imaging modalities, including MRI and conventional radionuclide examinations, has shown disappointing results with confirmed diagnosis in as few as 33% of FUO patients [32]. FDG PET has demonstrated higher utility. Jasper et al. assessed the diagnostic value of PET imaging in 69 pediatric patients with FUO (44 scans) or instances of inflammation without having fever (33 scans) [33]. They showed that FDG-PET and PET/CT are useful diagnostic tools for evaluating children with FUO and unexplained signs of inflammation [33]. In another study, 31 children with FUO were scanned and 32% of the total FDG-PET/CT scans were found to be clinically helpful [34]. The sensitivity, specificity, positive predictive value and negative predictive value of FDG-PET/CT were 80%, 78%, 67% and 88%, respectively [34].
There are also studies which compare FDG-PET with other tracers in diagnosing FUO. Meller et al. [35] performed a study on twenty FUO patients who underwent FDG imaging using DHCC. Imaging included trans-axial and longitudinal whole-body tomography. In 18 of these subjects, 67Ga citrate whole-body and SPECT imaging was performed. Furthermore, the sensitivity, specificity, positive predictive and negative predictive value of trans-axial FDG tomography was found to be 81%, 86%, 92%, and 75%, respectively. The sensitivity, specificity, positive predictive and negative predictive values of Ga-67 were reported to be 67%, 78%, 75%, and 70%, respectively. Thus, they concluded that in the context of FUO, trans-axial FDG tomography when performed with DHCC is superior to 67Ga citrate SPECT [35]. Although Ga-67 scan was considered as the tracer of first choice in the diagnostic workup of fever of unknown origin (FUO), its drawbacks of high dose of radiation, long procedure time, and low spatial resolution make FDG-PET a more valuable method [35] and it may be the most effective imaging technique in determining the underlying causes of FUO [26].
Another clinical entity within the same area is bacteremia. FDG PET/CT is used increasingly in locating infectious foci in bacteremia of unknown origin (BUO) Figure 2. Studies have reported that FDG-PET has been able to detect the infectious foci in 56-73% of patients [36-39]. In a significant proportion, FDG PET/CT was the first or only modality to find infectious foci despite various imaging strategy beforehand. This indicates that the patient population is similar to FUO, and the patients often comprise the more difficult ones [36-39]. Similar potential has been suggested in pediatric population albeit less literature is available [40,41]. Some controversies remain regarding protocol and diagnostic algorithm; some studies have found markers of infection/inflammation (e.g. CRP or white blood cell count) or duration of antibiotic therapy prior to scan to be correlated to positive findings and usefulness of results [42,43], whereas others have found opposite results [44].
Figure 2.
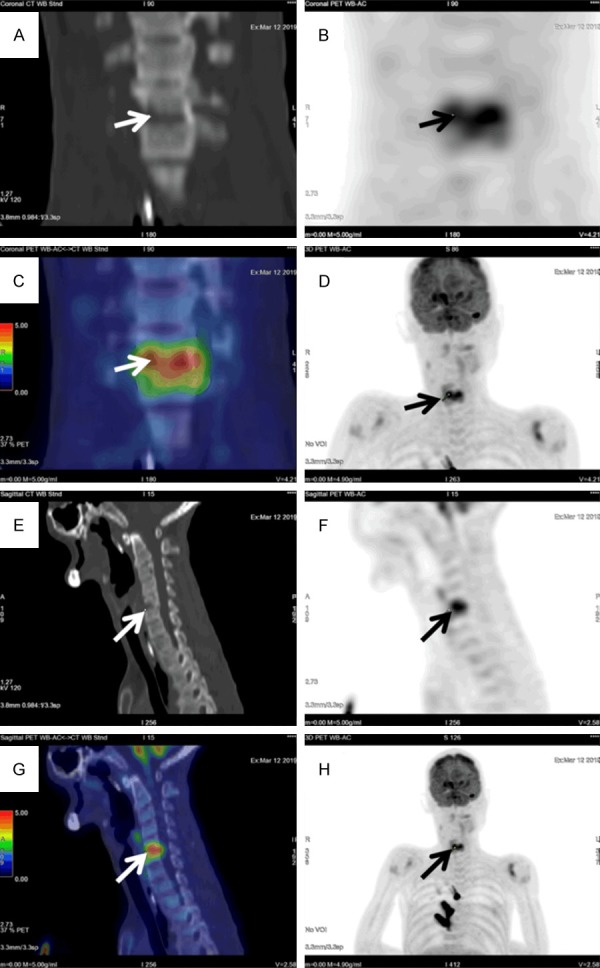
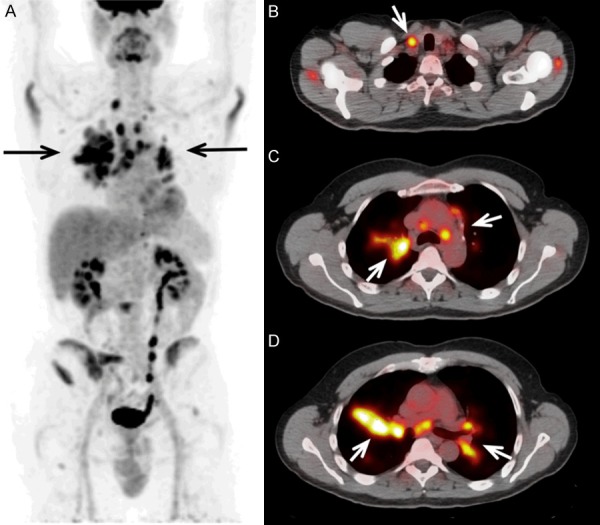
A 54-year-old male with Down’s syndrome was referred to undergo FDG-PET/CT to detect the underlying cause of this patient’s FUO. The blood culture was reported positive for Streptococcus dysgalactiae subsp. equisimilis. Further investigations including gallium scan and abdominal ultrasound failed to reveal the site of infection. Ultimately, FDG-PET/CT was performed for further evaluation of this patient’s fever and bacteremia. Sagittal (E-G) and Coronal (A-D, H) FDG PET/CT images showed a marked hypermetabolism (SUVmax 6.0) at C5/6 with adjacent vertebral endplate destruction, compatible with spondylodiscitis.
FDG PET also had a significant impact on treatment strategy. Changes in already instituted treatment have been found in 47-70% of patients [43-45]. One study found that it was safe to reduce the duration of treatment in patients with high risk bacteremia and a negative FDG PET/CT from the 4 weeks suggested in guidelines to the two weeks standard of care for non-high risk bacteremia. Similar mortality and morbidity was established in these patients with reduced duration of treatment with clear benefits from a health economics point of view [46]. The prognosis in bacteremia patients who had FDG-PET/CT imaging was significantly better than in patients that did not undergo FDG PET/CT [39]. Finally, FDG PET/CT was deemed cost effective in this patient population [47].
HIV & AIDS
FDG PET/CT can play a useful complementary role in delineating the different stages of HIV morbidity, as there is a tight association between HIV progression and pattern of lymphoid tissue activation in HIV patients without malignancies [48]. FDG activity varies with the different stages of the disease. The early stages of the disease depict a specific pattern of lymphoid FDG uptake at the head and neck region, while the mid stage is associated with peripheral nodal activity, and the late stage is associated with abdominal nodal involvement [49].
Acquired Immuno-deficiency Syndrome (AIDS) patients are susceptible to develop HIV-related malignancies and opportunistic infections, and FDG PET/CT may play a complementary role in differentiating and detecting these diseases Figure 3. Up to 10% of AIDS patients may present with neurologic symptoms, and FDG PET/CT can be useful in differentiating between toxoplasmosis and central nervous lymphoma. Central nervous lymphoma tends to show intense FDG uptake, whereas toxoplasmosis shows only mild or no FDG uptake [50-52]. However, FDG PET/CT may not always reliably differentiate between HIV-related lymphoma and inflammatory diseases, particularly in the context of high viral loads and low CD4 count [48]. Special attention should be paid to plasma variables such as viral loads and CD4 count during PET/CT reporting, as FDG avid inflammatory normal-sized lymph nodes may be misinterpreted as lymphomatous lesions [48]. FDG PET/CT has also shown to be effective in assessing the arterial inflammation in HIV infected individuals [53].
Figure 3.

A 43-year-old man, with history of HIV infection, now undergoes staging FDG PET/CT for newly diagnosed classical Hodgkin’s lymphoma. (A) Maximum intensity projection shows hypermetabolic left cervical (red arrow) and SCF (blue arrow) lymphadenopathies. Corresponding transverse (B) hybrid and (C) CT images show hypermetabolic enlarged left SCF lymph node (blue arrows), in keeping with biopsy-proven classical Hodgkin’s lymphoma.
FDG PET/CT may also help to assess the treatment efficacy of highly active antiretroviral therapy (HAART). There is a significant difference in metabolic activity in lesions of HIV-infected patients prior to HAART compared to post-HAART patients. The former group demonstrated increased nodal FDG uptake while the latter showed no nodal uptake [54]. This suggests FDG PET/CT may play a promising role for monitoring HAART treatment efficacy in the foreseeable future.
Tuberculosis (TB)
As the causative organism of TB, Mycobacterium tuberculosis (Mtb) remains one of the most lethal human pathogens [55]. In a 2017 WHO global TB report it was reported that TB was the cause for 1.3 million deaths among HIV-negative people with another 300,000 deaths in HIV-positive patients [56]. Despite a global effort to fight TB with effective anti-TB drugs, it is still ranked as the 2nd highest cause of mortality among all infectious diseases globally. Latent TB, which accounts for more than 90% of infected cases, is believed to be present in nearly one third of the global population [57]. Of particular importance is drug-resistant and HIV-related TB infection because of the higher costs to treat these conditions [58,59]. Concurrent TB infection in HIV patients raises diagnostic difficulties and commonly delays the diagnosis and treatment for TB infection. HIV infection further raises the number of conversions from latent TB to active disease [60].
Because tuberculous granulomatous inflammation appear as FDG avid lesions on PET/CT imaging [61], it is able to delineate the extent of disease involvement and detect occult extra-pulmonary lesion sites due to its whole-body image characteristics. FDG PET/CT imaging is also effective in assessing treatment response during and after the treatment course [62-64] Figure 4, which carries significant clinical impact in assessing the efficacy of a given treatment and the need to alter the regimen accordingly. This potential was supported by a recent systematic review and meta-analysis focusing on SUV-based response evaluation, but literature is still sparse and heterogeneous [65]. Furthermore, it has the ability to detect skeletal TB lesions as well as differentiating chronic TB spondylitis from acute pyogenic spondylitis [66,67].
Figure 4.
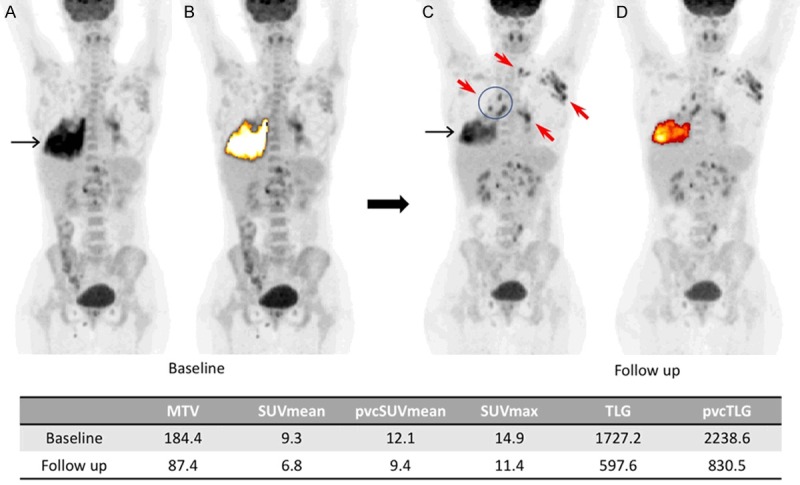
Baseline (A, B) and follow up (C, D) MIP PET images of an HIV/TB positive patient. The lung lesion decreased in size and activity after two months of antiretroviral therapy (black arrows). At the same time, an increased lymph node involvement was observed on FDG PET (red arrows). The FDG-avid lung lesion was segmented semi-automatically using an adaptive contrast-oriented thresholding system (ROVER; ABBX, Radeberg, Germany). The values for metabolic tumor volume (MTV), SUVmean, partial volume corrected SUVmean (pvcSUVmean), SUVmax, total lesion glycolysis (TLG) and partial volume corrected total lesion glycolysis (pvcTLG) at the baseline and the follow up are noted in the table [64]. Reproduced with permission.
Pulmonary tuberculomas can appear as hypermetabolic solitary pulmonary nodules (SPN) on FDG PET/CT imaging [68,69]. It is of importance to be able to differentiate between benign and malignant SPNs, as the latter has an overall mortality rate of nearly 85% [70]. Some studies demonstrate that dual-phase FDG PET imaging may be helpful in resolving the aforementioned problem of differentiation, wherein FDG uptake of benign lesions appear to remain the same or decrease with time, while FDG activity rises in delayed imaging of malignant lesions [71,72]. However, the efficacy of dual-phase technique is controversial as some studies have shown that it cannot reliably differentiate between pulmonary tuberculoma and malignant SPN [73-76]. Werutksy et al. found that FDG PET/CT has a low specificity in identifying non-small cell lung cancer (NSCLC) with a positive predictive value of 54% [77]. Moreover, FDG PET/CT may not be able to discern between active and latent TB infection, because the increased FDG metabolism is not only apparent in active infective lesions, but may also be seen as a result of host immune response [78].
Apart from FDG, other novel tracers have been developed for characterization of TB lesions. As F18-FLT reflects tumor cell proliferation [79], it is reported that combined FDG and F18-FLT PET imaging may be useful to differentiate between malignant and TB lesions by means of using the ratio of SUVmax in FDG and F-18 FLT [80,81]. Besides the F-18 tracers, C-11 tracers may also play a useful role for management of TB. Combined C-11 choline and F-18 FDG PET/CT raises the diagnostic accuracy in differentiating malignancy from benign disease entities [82] when compared to using single C-11 PET/CT imaging. The 20 minute half-life of C-11 limits the use to facilities with in-house cyclotron access. Larger-scale studies are required to further ascertain the role of the alternative tracers in managing TB patients.
In conclusion, FDG PET/CT remains a non-invasive imaging tool for managing TB patients and carries great clinical impact, in terms of diagnosis, treatment response monitoring and metabolic activity assessment [83]. With its unique ability to reflect metabolic behavior, FDG PET/CT offers a great opportunity for histological mapping and characterization of TB lesions, thus allowing for personalized, patient-based medical treatment in the near future.
Osteomyelitis (OM), diabetic foot & prosthesis joint infection
Over the past several decades, various nuclear medicine techniques have been used for managing osteomyelitis patients in terms of diagnosing or assessing the treatment efficacy. Some commonly used radiopharmaceuticals include combined bone marrow/leukocyte scintigraphy, gallium scintigraphy, combined Tc-99m MDP bone/gallium scintigraphy and combined Tc-99m MDP bone/leukocyte scintigraphy. As the above traditional nuclear medicine techniques have their own limitations, FDG PET/CT may have a more important role in managing the OM patients.
Osteomyelitis can be divided into acute and chronic type and differentiating the two subtypes is based on whether it has been present for less than or more than 6 months [84]. FDG PET/CT can play a role in differentiating between chronic OM and aseptic post-operative/traumatic bone healing [85,86] as increased FDG uptake persists in chronic OM cases. This is because activated macrophages continue to accumulate FDG in chronic infection [85,86]. FDG PET has higher specificity (91%) sensitivity (96%) and in chronic OM compared with bone scan, leukocyte scan, combined bone/leukocyte scan and MRI [87]. Leukocyte scan has a limited sensitivity in detecting vertebral osteomyelitis, possibly due to limited blood supply and slow cellular turn-over. FDG PET however has higher diagnostic accuracy for detection of vertebral chronic OM compared to leukocyte scan [87], and one of the advantages mentioned compared to radiology-based modalities is less susceptibility to attenuation or metal artefacts due to implants [88]. However, caution must be taken as false-positive result can be possibly encountered due to fractures, inflammatory arthritis, or normal bone healing within 4 weeks post operation [89,90].
Diabetic foot infection is one of the most common complications of diabetes, frequently leading to serious sequelae such as amputation. It is of vital importance to differentiate osteomyelitis in diabetic foot from neuropathic osteoarthropathy, as they have different treatment approaches. Neuropathic osteoarthropathy demonstrates lower FDG metabolism compared to osteomyelitis [91,92]. In a study of 39 patients with a clinically suspected diabetic foot infection FDG PET/CT was shown to have a high sensitivity (100%), specificity (92%), PPV (87%) and NPV (95%). Another study showed less promising results as it was found that leukocyte scans have better diagnostic accuracy compared to FDG PET/CT [93]. The conflicting result may be attributed to variability in serum glucose level prior to FDG PET/CT exam, which is commonly encountered in diabetic patients.
Similarly, the role of FDG PET/CT in prosthesis joint infection has not been completely elucidated Figure 5. It is difficult to differentiate prosthesis joint infection from aseptic loosening clinically. Combined In-111 leukocyte scintigraphy and bone marrow imaging demonstrates good diagnostic accuracy (>90%) confirming prosthesis joint infection. Although both prosthesis joint infection and aseptic loosening may have a peri-prosthetic FDG activity [94,95], studies have shown acceptable sensitivity and specificity for FDG-PET in detecting prosthesis infection [96-99]. A meta-analysis incorporating 11 studies demonstrated high sensitivity (82.1%) and specificity (86.6%) in using FDG-PET for detecting prosthetic knee and hip joint infection [96]. Kwee et al. found that FDG-PET/CT aids in diagnosing hip prostheses infections with sensitivity and specificity based on the visual assessment of 0.81 and 0.68, respectively, whereas the sensitivity and specificity using an optimized SUVmax threshold were 0.71 and 0.78, respectively [97]. Furthermore, results of a systemic review of 16 studies (1101 patients) showed that on a per prosthesis-based analysis, the pooled sensitivity and specificity of FDG-PET or PET/CT in detecting prosthesis infection were 87% with an area under the curve of 0.94 [98].
Figure 5.
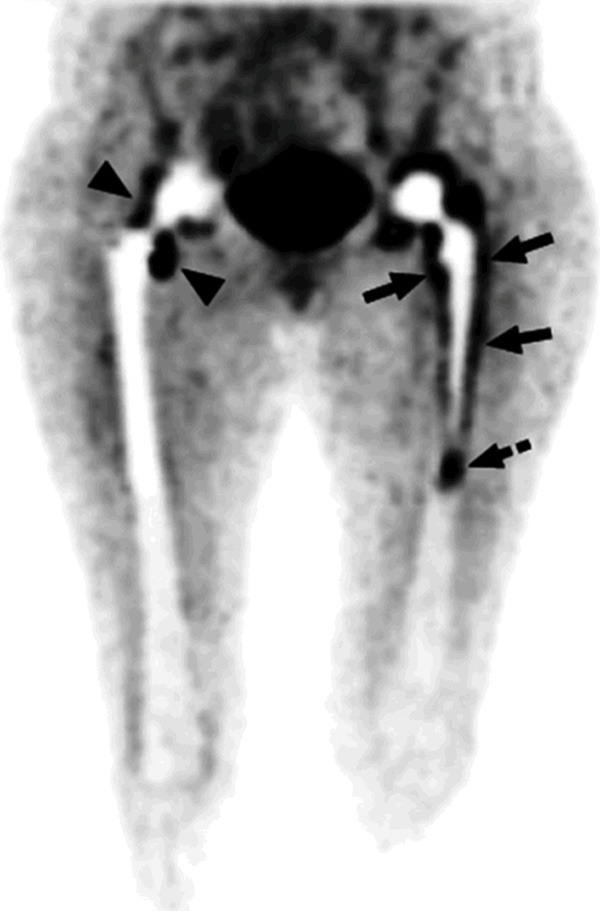
In this patient with bilateral hip prostheses, the maximum intensity projection image shows FDG uptake patterns in non-infected hip prosthesis and infected hip prosthesis. In the right non-infected hip prosthesis, some uptake of FDG is noted around the neck (arrow heads), while the bone-prosthesis interface appears without significant FDG uptake. In Contrast, the left infected hip prosthesis reveals significant tracer concentration at the bone-prosthesis interface (arrows). In this particular patient, there is also significant activity in the tip of the prosthesis (dashed arrow) [99]. Reproduced with permission.
Vasculitis
In 1990 the American College of Rheumatology (ACR) established criterion to differentiate among the 7 types of vasculitis [100]. The CHCC 1994 classification organized vasculitis according to different vessel size [101], namely the small, medium and large vessels diseases. Giant cell arteritis (GCA) and Takayasu arteritis (TA) are classified as large vessel diseases; periarteritis nodosa and Kawasaki’s arteritis are classified as medium vessel diseases; granulomatosis with polyangiitis (formerly Wegener’s disease), eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss syndrome), microscopic polyangiitis, Henoch-Schonlein purpura, and essential cryoglobulinemia vasculitis are classified as small vessel diseases.
Although the pathogenesis of both GCA and TA is unknown, it is believed to be an antigen-related autoimmune reaction [102,103], but no definite antigenic stimulus can be truly identified [104]. While temporal artery biopsy is regarded as the gold standard for diagnosis of GCA, the procedure is invasive and can be a false negative in up to 7% of the cases due to skip lesions [105]. Many patients are either asymptomatic or manifest without the classic presentation of headache and scalp tenderness, which leads to a delay in diagnosis [106]. This delay in diagnosis can lead to aortic complications and fatal outcomes [107]. Hence it is of paramount importance to detect large vessel vasculitis (LVV) in early stages with more sophisticated imaging modalities.
FDG PET/CT, ultrasonography, MRI are among the various imaging modalities available for use in diagnosing LVV. Presence of diffusely increased FDG activity along the aortic wall and its branches may help in diagnosing LVV, particularly in patients with subtle inflammatory signs and symptoms [108,109] Figure 6. As the uptake of blood pool decreases over time, the contrast between the vessel wall inflammation and the blood pool becomes more prominent and thus, PET acquisition at a delayed time point is preferred as it may increase the sensitivity of subtle LVV [110]. Although FDG PET is useful in visualizing inflammation of the aorta and the larger arteries, its role is relatively limited for smaller arteries due to limited spatial resolution of PET/CT. FDG PET/CT may be able to differentiate between giant cell arteritis (GCA) and polyarteritis nodosa (PAN) [111]. Historically, it was thought to be difficult to demonstrate increased FDG uptake along the temporal artery in view of its small diameter and proximity to the high physiologic uptake in the brain. A recent study, however, demonstrated high accuracy in diagnosing GCA when focusing dichotomously on FDG uptake in temporal, maxillary or vertebral arteries [112]. The presence of increased FDG uptake along the aorta, subclavian, carotid, and iliac arteries can be helpful in guiding the diagnosis of GCA with FDG PET/CT [111]. With regards to the role of FDG PET/CT in the initial diagnosis in LVV, sensitivity ranged from 77% to 92% and specificity ranged from 89% to 100% in GCA [113]. The results are even more promising in TA, where sensitivity reached 92% and specificity reached 100% [114]. It is also a useful tool that helps to select the site of biopsy [11,115] by showing increased FDG uptake. Moreover, it can better delineate the extent of disease and demonstrate extra-cranial involvement, where more vascular involvement was found by FDG PET/CT as compared with MRI and angiography [116,117]. Hence FDG PET/CT is a complimentary tool for diagnosing LVV, especially in suspected cases where temporal artery biopsy is negative. One caveat pertains to glucocorticoid treatment as it may substantially attenuate FDG uptake and result in false negative findings, but a recent study found that FDG PET/CT scans performed within three days of treatment with high-dose glucocorticoids retained a high sensitivity (10/10), whereas sensitivity was reduced to one-third (5/14) after ten days treatment [118].
Figure 6.
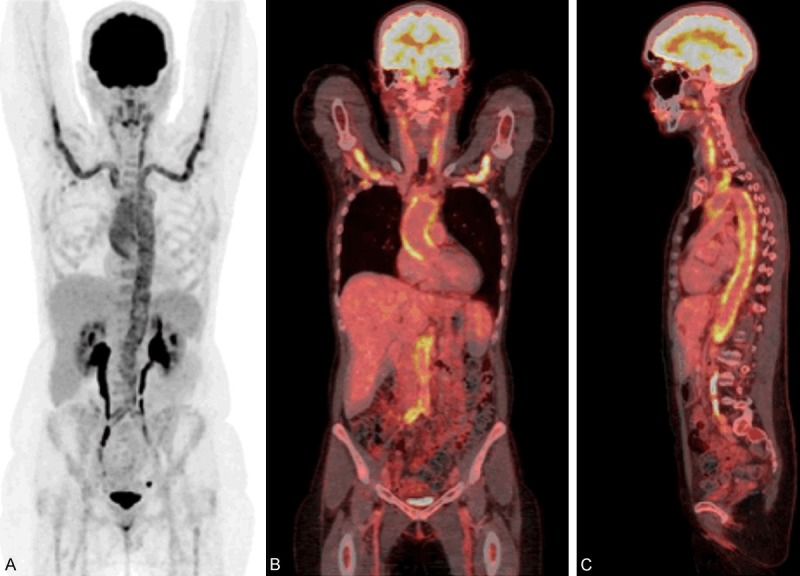
MIP (A) and fused coronal (B) and sagittal (C) PET/CT of a 57-year-old female referred with non-specific symptoms (fatigue, weight loss) and increased inflammatory markers. The scan showed diffuse increased FDG uptake in the carotid arteries, the axillary arteries, the thoracic and abdominal aorta, and the iliac arteries consistent with large vessel vasculitis.
Nearly 50% of GCA cases coexist with polymyalgia rheumatica (PMR), an inflammatory disease causing pain and stiffness in the joints [119,120]. Erythrocyte sedimentation rate and C-reactive protein levels are often elevated in this condition. Isolated PMR often shows increased FDG metabolism around the hips, shoulder joints, and in interspinous and supraspinous processes along the vertebral column [121]. Some have suggested nine well-defined anatomical areas with a specificity of >95% with increased FDG uptake above the liver in >6 areas [122]. Mildly increased vascular FDG uptake can be seen in 30% of cases [121], most commonly around the subclavian arteries. Though FDG PET/CT can be useful to assess treatment response in LVV, caution should be exercised for image interpretation for persistent increased FDG uptake following treatment, where both fibrosis and vascular remodeling can lead to increased metabolic activity [113].
The role of clinical application of FDG PET/CT in small and medium-sized vasculitis disorder remains to be explored, mainly attributed to limited spatial resolution of PET/CT imaging. Potential applications include identification of systemic organ affections [123] and differentiation between disease flare, infection and cancer [25], but further studies are required to define its role and potential benefit in such cases.
Sarcoidosis
Sarcoidosis is an idiopathic, granulomatous non-caseating disease predominantly involving the lungs and lymph nodes but has the potential to involve all organs. The clinical manifestations, disease course, and prognosis of sarcoidosis patients can vary, with some patients who recover spontaneously and others who deteriorate rapidly despite medical treatment [124,125]. While high-resolution computed tomography (HRCT) is regarded as the imaging modality of choice for the diagnosis of sarcoidosis, biopsy is still necessary because it allows for the differentiation of sarcoidosis from other interstitial lung diseases.
PET/CT has the ability to detect the FDG uptake in granulomatous cells producing the inflammation seen in sarcoidosis Figure 7. FDG PET/CT has proven to have good sensitivity and offers valuable information to evaluate both pulmonary and extra-pulmonary sarcoidosis [5]. In addition, by identifying different FDG uptake pattern, sarcoidosis patients have been re-classified based on the various extent of organ involvement, with thoracic lymph nodes and lung parenchyma involvement being classified as extra thoracic disease [126,127]. This proposed classification system carried prognostic stratification, as studies found that splenomegaly, parenchymal lung disease, and involvement of more than three organ systems were associated with a worse prognosis [126,127]. The whole body characteristics of FDG-PET can be useful to identify occult lesions, as well as detecting multiple organ involvement [128]. It has also proven to be useful in detecting cardiac and cerebral sarcoidosis [129,130]. Prior to the use of FDG, gallium scintigraphy was used as the nuclear imaging modality of choice for infection and inflammation. However, in comparison to gallium scintigraphy, FDG PET/CT is more suitable for imaging the mediastinum, hilar lymph nodes, the posterior regions of the lungs, and non-thoracic lesions [131,132]. Imaging of cardiac inflammation is also improved with PET/CT over gallium scintigraphy, but due to the physiologic FDG uptake in the heart, special protocol considerations are important to improve accuracy and reduce indeterminate scans, e.g. prolonged fasting and specific high-fat, low-carbohydrate dietary constraints [133].
Figure 7.
MIP PET image (A) and fused axial PET images of a sarcoidosis patient show focal increased FDG uptake in supraclavicular, para-aortic, sub-aortic, para-tracheal and hilar lymph nodes (arrows, B-D).
In regards to treatment monitoring, FDG PET/CT is a useful non-invasive tool in assessing treatment efficacy in sarcoidosis patients treated with corticosteroids, which results in a decrease in metabolic activity along with clinical and biochemical improvement [134,135]. Furthermore, it carries significant clinical impact by aiding in the decision to switch to alternate therapeutic regimens [136-138]. Other studies have also demonstrated the promising use of FDG PET/CT in assessing the treatment efficacy of drugs other than corticosteroids, such as infliximab, that is commonly used in sarcoidosis [139].
FDG PET/CT also allows for quantifications of the cardiac metabolic activity (in terms of SUVmax or SUVmean values), where the SUVmax correlates with histopathological findings [134]. It has also been shown that changes of SUVmean and SUVmax on serial FDG-PET scans negatively correlate with the clinical outcome of patients with cardiac sarcoidosis [140]. Muser et al. performed a quantitative analysis of 20 patients with cardiac sarcoidosis using a novel method of quantification. Their findings demonstrated that changes in FDG uptake were correlated with systolic function. Additionally, the reduction in the uptake of FDG was predictive of the decreased probability of major adverse cardiac events in these patients [141]. This quantitative technique, known as global disease assessment, may be the optimal approach for quantitative assessment of PET in sarcoidosis patients as it incorporates the metabolic activity of the entire heart and accurately reflects the extent of the disease activity.
Perspectives-advantages and limitations of FDG in inflammatory imaging
It is evident from the above that infectious and inflammatory diseases comprise a multitude of different diagnoses characterized by heterogeneous clinical presentations throughout the body, some focal, some systemic in appearance. Thus, the greatest advantage of FDG PET/CT imaging is that it is a sensitive whole-body modality based on relatively non-specific FDG uptake. Furthermore, compared to the competing radioisotope method with labeled white blood cells, it is faster, provides better image resolution, and does not require handling of patient blood.
Paradoxically, the advantage of FDG is also part of the challenges and limitations: the non-specificity of FDG hampers the differentiation between pathologic and physiologic uptake, and due to the diversity of infections and inflammatory diseases, differentiation between different disorders is also difficult. Physiologic FDG uptake may especially interfere with interpretation in specific organs, e.g. infections in the brain, heart, bowel, and bladder may be difficult or impossible to diagnose. It may to some extent be remedied by patient preparation, e.g. imaging of the infected heart requires prolonged fasting preceded by a low-carbohydrate/high-fat diet to facilitate a shift in cardiac metabolism from glucose to free fatty acid to suppress physiologic FDG-uptake [142]. When imaging of the bowel, for instance in suspected inflammatory bowel disease, several factors may facilitate physiologic uptake, e.g. normal bacterial flora and peristalsis [143]. The latter may be reduced with motility reducing drugs, but such measures have not been introduced into clinical routine.
Certain medication may also influence the diagnostic accuracy. For instance, the widespread use of metformin in type 2 diabetes may impact imaging of the bowel; through unknown mechanisms metformin facilitates diffuse FDG-uptake throughout the colon, but the effect is reversible by discontinuing the drug for 48-72 hours prior to scan [144]. Another therapy with well-known impact on diagnostic performance of FDG is corticosteroids, especially high-dose treatment in suspected cranial vasculitis; FDG-uptake is known to subside completely after just a few days treatment, and imaging needs to be completed beforehand, or corticosteroids need to be paused for at least three days, which is not easily accomplished or without risk in suspected temporal arteritis [118].
Another challenge with the non-specificity and physiologic uptake of FDG is the difficulty of differentiating pathologic uptake in active infection/inflammation and the reactive or post-therapeutic FDG uptake often seen after surgery and instrumentation. For instance, non-specific FDG uptake is seen around joint or vascular prosthesis for prolonged periods of time, in the latter as long as 16 years. Routine assessment of non-attenuation images and use of novel software for reduction of metal artefacts may improve efficacy in these settings [145], but much work has also been put into optimizing interpretation in these and other settings, e.g. various interpretation criteria based on visual assessment and pattern recognition, visual grading scores, and/or semi-quantitative parameters, but for most diagnosis there is limited consensus on the interpretation schemes [146].
Future directions must focus on research. Much literature on these subjects remains substandard, due to small populations, retrospective designs, and older stand-alone technology. Thus, the future direction needs to focus on establishing more firm evidence in prospective studies, preferably randomized and with patient-based outcome also factoring in economy. Finally, nuclear medicine physicians must embrace the multitude of diseases within the field of inflammation and infection and gain the advanced knowledge on pathophysiology, clinical presentation, and treatment strategy that is necessary to establish and secure the optimal diagnostic strategy-just as we have for years sought to gain the necessary knowledge on the multitude of cancers, neurologic diseases etc.
Summary
FDG-PET/CT has an expanding role in diagnosis and treatment monitoring in diseases of infectious or inflammatory origin. An expanding set of studies illustrate the multifarious roles of FDG-PET/CT in the assessment of these conditions, both systemic diseases and more regional. Specifically, PET can provide vital information at a molecular level and consequently detect the disease activity at their earliest manifestation. FDG-PET/CT has proven to be a robust and accurate modality in diagnosing and quantifying disease burden particularly in the context of the clinical diagnosis and treatment monitoring. The utility and versatility of this imaging modality in these contexts should galvanize efforts to further expand the role of PET-CT/CT in the management of infectious and inflammatory disease.
Disclosure of conflict of interest
None.
References
- 1.Basu S, Hess S, Nielsen Braad PE, Olsen BB, Inglev S, Høilund-Carlsen PF. The basic principles of FDG-PET/CT imaging. PET Clin. 2014;9:355–370. v. doi: 10.1016/j.cpet.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Larson SM. Cancer or inflammation? A holy grail for nuclear medicine. J Nucl Med. 1994;35:1653–1655. [PubMed] [Google Scholar]
- 3.Tahara T, Ichiya Y, Kuwabara Y, Otsuka M, Miyake Y, Gunasekera R, Masuda K. High [18F]-fluorodeoxyglucose uptake in abdominal abscesses: a PET study. J Comput Assist Tomogr. 1989;13:829–831. doi: 10.1097/00004728-198909000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara Y, Gutowski TD, Fisher SJ, Brown RS, Wahl RL. Uptake of positron emission tomography tracers in experimental bacterial infections: a comparative biodistribution study of radiolabeled FDG, thymidine, L-methionine, 67Ga-citrate, and 125I-HSA. Eur J Nucl Med. 1999;26:333–341. doi: 10.1007/s002590050395. [DOI] [PubMed] [Google Scholar]
- 5.Signore A, Glaudemans AW. The molecular imaging approach to image infections and inflammation by nuclear medicine techniques. Ann Nucl Med. 2011;25:681–700. doi: 10.1007/s12149-011-0521-z. [DOI] [PubMed] [Google Scholar]
- 6.Yamada S, Kubota K, Kubota R, Ido T, Tamahashi N. High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue. J Nucl Med. 1995;36:1301–1306. [PubMed] [Google Scholar]
- 7.Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore) 1961;40:1–30. doi: 10.1097/00005792-196102000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Popovska-Jovičić B, Čanović P, Gajović O, Raković I, Mijailović Ž. Fever of unknown origin: most frequent causes in adults patients. Vojnosanit Pregl. 2016;73:21–25. doi: 10.2298/vsp140820128p. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz HW. Fever of unknown origin or fever of too many origins? N Engl J Med. 2013;368:197–9. doi: 10.1056/NEJMp1212725. [DOI] [PubMed] [Google Scholar]
- 10.Chien YL, Huang FL, Huang CM, Chen PY. Clinical approach to fever of unknown origin in children. J Microbiol Immunol Infect. 2017;50:893–898. doi: 10.1016/j.jmii.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Jaruskova M, Belohlavek O. Role of FDG-PET and PET/CT in the diagnosis of prolonged febrile states. Eur J Nucl Med Mol Imaging. 2006;33:913–918. doi: 10.1007/s00259-006-0064-z. [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, Basu S, Torigian D, Anand V, Zhuang H, Alavi A. Role of modern imaging techniques for diagnosis of infection in the era of 18F-fluorodeoxyglucose positron emission tomography. Clin Microbiol Rev. 2008;21:209–224. doi: 10.1128/CMR.00025-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Winter F, Vogelaers D, Gemmel F, Dierckx RA. Promising role of 18-F-fluoro-D-deoxyglucose positron emission tomography in clinical infectious diseases. Eur J Clin Microbiol Infect Dis. 2002;21:247–257. doi: 10.1007/s10096-002-0708-2. [DOI] [PubMed] [Google Scholar]
- 14.Hung BT, Wang PW, Su YJ, Huang WC, Chang YH, Huang SH, Chang CC. The efficacy of (18)F-FDG PET/CT and (67)Ga SPECT/CT in diagnosing fever of unknown origin. Int J Infect Dis. 2017;62:10–17. doi: 10.1016/j.ijid.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzen J, Buchert R, Bohuslavizki KH. Value of FDG PET in patients with fever of unknown origin. Nucl Med Commun. 2001;22:779–783. doi: 10.1097/00006231-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Keidar Z, Gurman-Balbir A, Gaitini D, Israel O. Fever of unknown origin: the role of 18F-FDG PET/CT. J Nucl Med. 2008;49:1980–1985. doi: 10.2967/jnumed.108.054692. [DOI] [PubMed] [Google Scholar]
- 17.Besson FL, Chaumet-Riffaud P, Playe M, Noel N, Lambotte O, Goujard C, Prigent A, Durand E. Contribution of (18)F-FDG PET in the diagnostic assessment of fever of unknown origin (FUO): a stratification-based meta-analysis. Eur J Nucl Med Mol Imaging. 2016;43:1887–1895. doi: 10.1007/s00259-016-3377-6. [DOI] [PubMed] [Google Scholar]
- 18.Bharucha T, Rutherford A, Skeoch S, Alavi A, Brown M, Galloway J FDG-PET/CT in fever of unknown origin working group. Diagnostic yield of FDG-PET/CT in fever of unknown origin: a systematic review, meta-analysis, and delphi exercise. Clin Radiol. 2017;72:764–771. doi: 10.1016/j.crad.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Li YM, Li Y, Hua FC, Wang QS, Zhang XL, Cheng C, Wu H, Yao ZM, Zhang WF, Hou QY, Miao WB, Wang XM. 18F-FDGPET/CT in fever of unknown origin and inflammation of unknown origin: a Chinese multi-center study. Eur J Nucl Med Mol Imaging. 2019;46:159–165. doi: 10.1007/s00259-018-4121-1. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi M, Dahabreh IJ, Nihashi T, Iwata M, Varghese GM, Terasawa T. Nuclear imaging for classic fever of unknown origin: meta-analysis. J Nucl Med. 2016;57:1913–1919. doi: 10.2967/jnumed.116.174391. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi M, Nihashi T, Gafter-Gvili A, García-Gómez FJ, Andres E, Blockmans D, Iwata M, Terasawa T. Association of 18F-FDG PET or PET/CT results with spontaneous remission in classic fever of unknown origin: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e12909. doi: 10.1097/MD.0000000000012909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong MJ, Zhao K, Liu ZF, Wang GL, Yang SY, Zhou GJ. A meta-analysis of the value of fluorodeoxyglucose-PET/PET-CT in the evaluation of fever of unknown origin. Eur J Radiol. 2011;80:834–844. doi: 10.1016/j.ejrad.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Hao R, Yuan L, Kan Y, Li C, Yang J. Diagnostic performance of 18F-FDG PET/CT in patients with fever of unknown origin: a meta-analysis. Nucl Med Commun. 2013;34:682–688. doi: 10.1097/MNM.0b013e328361cd0e. [DOI] [PubMed] [Google Scholar]
- 24.Buch-Olsen KM, Andersen RV, Hess S, Braad PE, Schifter S. 18F-FDG-PET/CT in fever of unknown origin: clinical value. Nucl Med Commun. 2014;35:955–960. doi: 10.1097/MNM.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 25.Frary EC, Hess S, Gerke O, Laustrup H. 18F-fluoro-deoxy-glucose positron emission tomography combined with computed tomography can reliably rule-out infection and cancer in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis suspected of disease relapse. Medicine (Baltimore) 2017;96:e7613. doi: 10.1097/MD.0000000000007613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Zaghal A, Raynor WY, Seraj SM, Werner TJ, Alavi A. FDG-PET imaging to detect and characterize underlying causes of fever of unknown origin: an unavoidable path for the foreseeable future. Eur J Nucl Med Mol Imaging. 2019;46:2–7. doi: 10.1007/s00259-018-4164-3. [DOI] [PubMed] [Google Scholar]
- 27.Subocz E, Hałka J, Dziuk M. The role of FDG-PET in Hodgkin lymphoma. Contemp Oncol (Pozn) 2017;21:104–114. doi: 10.5114/wo.2017.68618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isasi CR, Lu P, Blaufox MD. A metaanalysis of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography in the staging and restaging of patients with lymphoma. Cancer. 2005;104:1066–1074. doi: 10.1002/cncr.21253. [DOI] [PubMed] [Google Scholar]
- 29.Otsuka H, Morita N, Yamashita K, Nishitani H. FDG-PET/CT for diagnosis and follow-up of vasculitis. J Med Invest. 2007;54:345–349. doi: 10.2152/jmi.54.345. [DOI] [PubMed] [Google Scholar]
- 30.Umekita K, Takajo I, Miyauchi S, Tsurumura K, Ueno S, Kusumoto N, Kai Y, Kuroki M, Sasaki T, Okayama A. [18F] fluorodeoxyglucose positron emission tomography is a useful tool to diagnose the early stage of Takayasu’s arteritis and to evaluate the activity of the disease. Mod Rheumatol. 2006;16:243–247. doi: 10.1007/s10165-006-0485-3. [DOI] [PubMed] [Google Scholar]
- 31.Tolan RW Jr. Fever of unknown origin: a diagnostic approach to this vexing problem. Clin Pediatr (Phila) 2010;49:207–213. doi: 10.1177/0009922809347799. [DOI] [PubMed] [Google Scholar]
- 32.Parisi MT. Functional imaging of infection: conventional nuclear medicine agents and the expanding role of 18-F-FDG PET. Pediatr Radiol. 2011;41:803–810. doi: 10.1007/s00247-011-2013-7. [DOI] [PubMed] [Google Scholar]
- 33.Jasper N, Däbritz J, Frosch M, Loeffler M, Weckesser M, Foell D. Diagnostic value of [18F]-FDG PET/CT in children with fever of unknown origin or unexplained signs of inflammation. Eur J Nucl Med Mol Imaging. 2010;37:136–45. doi: 10.1007/s00259-009-1185-y. [DOI] [PubMed] [Google Scholar]
- 34.Blokhuis GJ, Bleeker-Rovers CP, Diender MG, Oyen WJ, Draaisma JM, de Geus-Oei LF. Diagnostic value of FDG-PET/(CT) in children with fever of unknown origin and unexplained fever during immune suppression. Eur J Nucl Med Mol Imaging. 2014;41:1916–1923. doi: 10.1007/s00259-014-2801-z. [DOI] [PubMed] [Google Scholar]
- 35.Meller J, Altenvoerde G, Lehmann K, Sahlmann C, Becker W. 19. Fever of unknown origin: prospective comparison of 18F-FDG imaging with a double head coincidence camera and 67Ga citrate SPECT. Nucl Med Commun. 2001;22:1158–1159. doi: 10.1007/s002590000341. [DOI] [PubMed] [Google Scholar]
- 36.Brøndserud MB, Pedersen C, Rosenvinge FS, Høilund-Carlsen PF, Hess S. Clinical value of FDG-PET/CT in bacteremia of unknown origin with catalase-negative gram-positive cocci or Staphylococcus aureus. Eur J Nucl Med Mol Imaging. 2019;46:1351–1358. doi: 10.1007/s00259-019-04289-5. [DOI] [PubMed] [Google Scholar]
- 37.Pijl JP, Glaudemans AWJM, Slart RHJA, Yakar D, Wouthuyzen-Bakker M, Kwee TC. FDG-PET/CT for detecting an infection focus in patients with bloodstream infection: factors affecting diagnostic yield. Clin Nucl Med. 2019;44:99–106. doi: 10.1097/RLU.0000000000002381. [DOI] [PubMed] [Google Scholar]
- 38.Berrevoets MAH, Kouijzer IJE, Aarntzen EHJG, Janssen MJR, De Geus-Oei LF, Wertheim HFL, Kullberg BJ, Oever JT, Oyen WJG, Bleeker-Rovers CP. 18F-FDG PET/CT optimizes treatment in staphylococcus aureus bacteremia and is associated with reduced mortality. J Nucl Med. 2017;58:1504–1510. doi: 10.2967/jnumed.117.191981. [DOI] [PubMed] [Google Scholar]
- 39.Vos FJ, Kullberg BJ, Sturm PD, Krabbe PF, van Dijk AP, Wanten GJ, Oyen WJ, Bleeker-Rovers CP. Metastatic infectious disease and clinical outcome in staphylococcus aureus and Streptococcus species bacteremia. Medicine (Baltimore) 2012;91:86–94. doi: 10.1097/MD.0b013e31824d7ed2. [DOI] [PubMed] [Google Scholar]
- 40.Kouijzer IJ, Blokhuis GJ, Draaisma JM, Oyen WJ, de Geus-Oei LF, Bleeker-Rovers CP. 18F-FDG PET/CT in detecting metastatic infection in children. Clin Nucl Med. 2016;41:278–281. doi: 10.1097/RLU.0000000000001119. [DOI] [PubMed] [Google Scholar]
- 41.Tewari A, Padma S, Sundaram PS. The diagnostic role of 18-fluorodeoxyglucocose-positron emission tomography/computed tomography in occult bacteremia searching underlying primary disease. Ann Indian Acad Neurol. 2012;15:336–338. doi: 10.4103/0972-2327.104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnon-Sheleg E, Israel O, Keidar Z. PET/CT imaging in soft tissue infection and inflammation-an update. Semin Nucl Med. 2020;50:35–49. doi: 10.1053/j.semnuclmed.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Tsai HY, Lee MH, Wan CH, Yang LY, Yen TC, Tseng JR. C-reactive protein levels can predict positive (18)F-FDG PET/CT findings that lead to management changes in patients with bacteremia. J Microbiol Immunol Infect. 2018;51:839–846. doi: 10.1016/j.jmii.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Brøndserud MB HS, Johansen AHD, Pedersen C, Høilund-Carlsen PF. The clinical value of FDG-PET/CT in bacteraemia of unknown origin: a retrospective study. Eur J Nucl Med Mol Imaging. 2015;42:S789. [Google Scholar]
- 45.Berrevoets MAH, Kouijzer IJE, Aarntzen EHJG, Janssen MJR, De Geus-Oei LF, Wertheim HFL, Kullberg BJ, Oever JT, Oyen WJG, Bleeker-Rovers CP. (18)F-FDG PET/CT optimizes treatment in staphylococcus aureus bacteremia and is associated with reduced mortality. J Nucl Med. 2017;58:1504–1510. doi: 10.2967/jnumed.117.191981. [DOI] [PubMed] [Google Scholar]
- 46.Berrevoets MAH, Kouijzer IJE, Slieker K, Aarntzen EHJG, Kullberg BJ, Oever J, Bleeker-Rovers CP. (18)F-FDG-PET/CT-guided treatment duration in patients with high-risk staphylococcus aureus bacteremia: a proof of principle. J Nucl Med. 2019;60:998–1002. doi: 10.2967/jnumed.118.221929. [DOI] [PubMed] [Google Scholar]
- 47.Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med. 2011;52:1673–1678. doi: 10.2967/jnumed.111.089714. [DOI] [PubMed] [Google Scholar]
- 48.Kung BT, Mak WS, Lau SM, Auyong TK, Tong CM. Promising role of fluorodeoxyglucose positron emission tomography/computed tomography in human immunodeficiency virus associated non-Hodgkin’s lymphoma. World J Nucl Med. 2015;14:53–6. doi: 10.4103/1450-1147.150551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scharko AM, Perlman SB, Pyzalski RW, Graziano FM, Sosman J, Pauza CD. Whole-body positron emission tomography in patients with HIV-1 infection. Lancet. 2003;362:959–961. doi: 10.1016/S0140-6736(03)14366-8. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman JM, Waskin HA, Schifter T, Hanson MW, Gray L, Rosenfeld S, Coleman RE. FDG-PET in differentiating lymphoma from nonmalignant central nervous system lesions in patients with AIDS. J Nucl Med. 1993;34:567–575. [PubMed] [Google Scholar]
- 51.Villringer K, Jäger H, Dichgans M, Ziegler S, Poppinger J, Herz M, Kruschke C, Minoshima S, Pfister HW, Schwaiger M. Differential diagnosis of CNS lesions in AIDS patients by FDG-PET. J Comput Assist Tomogr. 1995;19:532–536. doi: 10.1097/00004728-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Heald AE, Hoffman JM, Bartlett JA, Waskin HA. Differentiation of central nervous system lesions in AIDS patients using positron emission tomography (PET) Int J STD AIDS. 1996;7:337–346. doi: 10.1258/0956462961918239. [DOI] [PubMed] [Google Scholar]
- 53.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, Williams KC, Lo J, Grinspoon SK. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brust D, Polis M, Davey R, Hahn B, Bacharach S, Whatley M, Fauci AS, Carrasquillo JA. Fluorodeoxyglucose imaging in healthy subjects with HIV infection: impact of disease stage and therapy on pattern of nodal activation. AIDS. 2006;20:495–503. doi: 10.1097/01.aids.0000210603.40267.29. [DOI] [PubMed] [Google Scholar]
- 55.Hershkovitz I, Donoghue HD, Minnikin DE, Besra GS, Lee OY, Gernaey AM, Galili E, Eshed V, Greenblatt CL, Lemma E, Bar-Gal GK, Spigelman M. Detection and molecular characterization of 9000-year-old Mycobacterium tuberculosis from a neolithic settlement in the eastern mediterranean. PLoS One. 2008;3:e3426. doi: 10.1371/journal.pone.0003426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Organization WH. Global tuberculosis report 2018. WHO; 2018. [Google Scholar]
- 57.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO global surveillance and monitoring project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 58.Resch SC, Salomon JA, Murray M, Weinstein MC. Cost-effectiveness of treating multidrug-resistant tuberculosis. PLoS Med. 2006;3:e241. doi: 10.1371/journal.pmed.0030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, Becerra MC, Benedetti A, Burgos M, Centis R, Chan ED, Chiang CY, Cox H, D’Ambrosio L, DeRiemer K, Dung NH, Enarson D, Falzon D, Flanagan K, Flood J, Garcia-Garcia ML, Gandhi N, Granich RM, Hollm-Delgado MG, Holtz TH, Iseman MD, Jarlsberg LG, Keshavjee S, Kim HR, Koh WJ, Lancaster J, Lange C, de Lange WC, Leimane V, Leung CC, Li J, Menzies D, Migliori GB, Mishustin SP, Mitnick CD, Narita M, O’Riordan P, Pai M, Palmero D, Park SK, Pasvol G, Peña J, Pérez-Guzmán C, Quelapio MI, Ponce-de-Leon A, Riekstina V, Robert J, Royce S, Schaaf HS, Seung KJ, Shah L, Shim TS, Shin SS, Shiraishi Y, Sifuentes-Osornio J, Sotgiu G, Strand MJ, Tabarsi P, Tupasi TE, van Altena R, Van der Walt M, Van der Werf TS, Vargas MH, Viiklepp P, Westenhouse J, Yew WW, Yim JJ Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Inect Dis. 2012;54:784–791. doi: 10.1093/cid/cir951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ichiya Y, Kuwabara Y, Sasaki M, Yoshida T, Akashi Y, Murayama S, Nakamura K, Fukumura T, Masuda K. FDG-PET in infectious lesions: the detection and assessment of lesion activity. Ann Nucl Med. 1996;10:185–191. doi: 10.1007/BF03165391. [DOI] [PubMed] [Google Scholar]
- 62.Demura Y, Tsuchida T, Uesaka D, Umeda Y, Morikawa M, Ameshima S, Ishizaki T, Fujibayashi Y, Okazawa H. Usefulness of 18 F-fluorodeoxyglucose positron emission tomography for diagnosing disease activity and monitoring therapeutic response in patients with pulmonary mycobacteriosis. Eur J Nucl Med Mol Imaging. 2009;36:632–639. doi: 10.1007/s00259-008-1009-5. [DOI] [PubMed] [Google Scholar]
- 63.Sathekge M, Maes A, Kgomo M, Stoltz A, Van de Wiele C. Use of 18F-FDG PET to predict response to first-line tuberculostatics in HIV-associated tuberculosis. J Nucl Med. 2011;52:880–885. doi: 10.2967/jnumed.110.083709. [DOI] [PubMed] [Google Scholar]
- 64.Alavi A, Hess S, Werner TJ, Høilund-Carlsen PF. An update on the unparalleled impact of FDG-PET imaging on the day-to-day practice off medicine with emphasis on management of infectious/infflammatory disorders. Eur J Nucl Med Mol Imaging. 2020;47:18–27. doi: 10.1007/s00259-019-04490-6. [DOI] [PubMed] [Google Scholar]
- 65.Sjölander H, Strømsnes T, Gerke O, Hess S, Imaging T. Value of FDG-PET/CT for treatment response in tuberculosis: a systematic review and meta-analysis. Clin Transl Imaging. 2018;6:19–29. [Google Scholar]
- 66.Kim K, Kim SJ, Kim IJ, Kim BS, Pak K, Kim H. Diffuse increased splenic F-18 fluorodeoxyglucose uptake may be an indirect sign of acute pyogenic cause rather than tuberculous in patients with infectious spondylitis. Nucl Med Commun. 2011;32:1155–1161. doi: 10.1097/MNM.0b013e32834bbdf1. [DOI] [PubMed] [Google Scholar]
- 67.Dureja S, Sen IB, Acharya S. Potential role of F18 FDG PET-CT as an imaging biomarker for the noninvasive evaluation in uncomplicated skeletal tuberculosis: a prospective clinical observational study. Eur Spine J. 2014;23:2449–2454. doi: 10.1007/s00586-014-3483-8. [DOI] [PubMed] [Google Scholar]
- 68.Kapucu LO, Meltzer CC, Townsend DW, Keenan RJ, Luketich JD. Fluorine-18-fluorodeoxyglucose uptake in pneumonia. J Nucl Med. 1998;39:1267–9. [PubMed] [Google Scholar]
- 69.Knight SB, Delbeke D, Stewart JR, Sandler MP. Evaluation of pulmonary lesions with FDG-PET: comparison of findings in patients with and without a history of prior malignancy. Chest. 1996;109:982–988. doi: 10.1378/chest.109.4.982. [DOI] [PubMed] [Google Scholar]
- 70.Murthy S, Rice T, editors. Semin Thoracic Cardio Surg. Elsevier; 2002. The solitary pulmonary nodule: a primer on diffferential diagnosis. [DOI] [PubMed] [Google Scholar]
- 71.Kubota K, Itoh M, Ozaki K, Ono S, Tashiro M, Yamaguchi K, Akaizawa T, Yamada K, Fukuda H. Advantage of delayed whole-body FDG-PET imaging for tumour detection. Eur J Nucl Med. 2001;28:696–703. doi: 10.1007/s002590100537. [DOI] [PubMed] [Google Scholar]
- 72.Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, Mozley PD, Rossman MD, Albelda SM, Alavi A. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001;42:1412–1417. [PubMed] [Google Scholar]
- 73.Döbert N, Hamscho N, Menzel C, Neuss L, Kovács AF, Grünwald F. Limitations of dual time point FDG-PET imaging in the evaluation of focal abdominal lesions. Nuklearmedizin. 2004;43:143–149. doi: 10.1267/nukl04050143. [DOI] [PubMed] [Google Scholar]
- 74.Chen CJ, Lee BF, Yao WJ, Cheng L, Wu PS, Chu CL, Chiu NT. Dual-phase 18F-FDG PET in the diagnosis of pulmonary nodules with an initial standard uptake value less than 2.5. AJR Am J Roentgenol. 2008;191:475–479. doi: 10.2214/AJR.07.3457. [DOI] [PubMed] [Google Scholar]
- 75.Sathekge MM, Maes A, Pottel H, Stoltz A, Van de Wiele C. Dual time-point FDG PET/CT for differentiating benign from malignant solitary pulmonary nodules in a TB endemic area. S Afr Med J. 2010;100:598–601. doi: 10.7196/samj.4082. [DOI] [PubMed] [Google Scholar]
- 76.Kung BT, Yong TK, Tong CM. The pearl of FDG PET/CT in preoperative assessment of patients with potentially operable non-small-cell lung cancer and its clinical impact. World J Nucl Med. 2017;16:21–25. doi: 10.4103/1450-1147.176882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Werutsky G, Hochhegger B, Lopes de Figueiredo Pinto JA, Martínez-Mesa J, Zanini ML, Berdichevski EH, Vilas E, da Silva VD, Tsukazan MTR, Vieira A, Fritscher LG, Hartmann L, Alba M, Sartori G, Matushita C, Bortolotto V, do Amaral RR, Junior LCA, Zaffaroni F, Barrios CH, Debiasi M, Frietscher CC. PET-CT has low specificity for mediastinal staging of non-small-cell lung cancer in an endemic area for tuberculosis: a diagnostic test study (LACOG 0114) BMC Cancer. 2019;19:5. doi: 10.1186/s12885-018-5233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heysell SK, Thomas TA, Sifri CD, Rehm PK, Houpt ER. 18-Fluorodeoxyglucose positron emission tomography for tuberculosis diagnosis and management: a case series. BMC Pulm Med. 2013;13:14. doi: 10.1186/1471-2466-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, Obradovich JE, Muzik O, Mangner TJ. Imaging proliferation in vivo with [F-18] FLT and positron emission tomography. Nat Med. 1998;4:1334–6. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 80.Tian J, Yang X, Yu L, Chen P, Xin J, Ma L, Feng H, Tan Y, Zhao Z, Wu W. A multicenter clinical trial on the diagnostic value of dual-tracer PET/CT in pulmonary lesions using 3’-deoxy-3’-18F-fluorothymidine and 18F-FDG. J Nucl Med. 2008;49:186–194. doi: 10.2967/jnumed.107.044966. [DOI] [PubMed] [Google Scholar]
- 81.Xu B, Guan Z, Liu C, Wang R, Yin D, Zhang J, Chen Y, Yao S, Shao M, Wang H, Tian J. Can multimodality imaging using 18 F-FDG/18 F-FLT PET/CT benefit the diagnosis and management of patients with pulmonary lesions? Eur J Nucl Med Mol Imaging. 2011;38:285–292. doi: 10.1007/s00259-010-1625-8. [DOI] [PubMed] [Google Scholar]
- 82.Hara T, Inagaki K, Kosaka N, Morita T. Sensitive detection of mediastinal lymph node metastasis of lung cancer with 11C-choline PET. J Nucl Med. 2000;41:1507–1513. [PubMed] [Google Scholar]
- 83.Ankrah AO, van der Werf TS, de Vries EF, Dierckx RA, Sathekge MM, Glaudemans AW. PET/CT imaging of Mycobacterium tuberculosis infection. Clin Transl Imaging. 2016;4:131–144. doi: 10.1007/s40336-016-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weiland AJ, Moore JR, Daniel RK. The efficacy of free tissue transfer in the treatment of osteomyelitis. J Bone Joint Surg Am. 1984;66:181–193. [PubMed] [Google Scholar]
- 85.Koort JK, Mäkinen TJ, Knuuti J, Jalava J, Aro HT. Comparative 18F-FDG PET of experimental staphylococcus aureus osteomyelitis and normal bone healing. J Nucl Med. 2004;45:1406–1411. [PubMed] [Google Scholar]
- 86.Kumar R. Assessment of therapy response in malignant tumours with 18 F-fluorothymidine. Eur J Nucl Med Mol Imaging. 2007;34:1334–1338. doi: 10.1007/s00259-007-0446-x. [DOI] [PubMed] [Google Scholar]
- 87.Basu S, Chryssikos T, Moghadam-Kia S, Zhuang H, Torigian DA, Alavi A, editors. Semin Nucl Med. Elsevier; 2009. Positron emission tomography as a diagnostic tool in infection: present role and future possibilities. [DOI] [PubMed] [Google Scholar]
- 88.Hartmann A, Eid K, Dora C, Trentz O, von Schulthess GK, Stumpe KDM. Diagnostic value of 18F-FDG PET/CT in trauma patients with suspected chronic osteomyelitis. Eur J Nucl Med Mol Imaging. 2007;34:704–714. doi: 10.1007/s00259-006-0290-4. [DOI] [PubMed] [Google Scholar]
- 89.Meyer M, Gast T, Raja S, Hubner K. Increased F-18 FDG accumulation in an acute fracture. Clin Nucl Med. 1994;19:13–14. doi: 10.1097/00003072-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Zhuang H, Sam JW, Chacko TK, Duarte PS, Hickeson M, Feng Q, Nakhoda KZ, Guan L, Reich P, Altimari SM, Alavi A. Rapid normalization of osseous FDG uptake following traumatic or surgical fractures. Eur J Nucl Med Mol Imaging. 2003;30:1096–1103. doi: 10.1007/s00259-003-1198-x. [DOI] [PubMed] [Google Scholar]
- 91.Nawaz A, Torigian DA, Siegelman ES, Basu S, Chryssikos T, Alavi A. Diagnostic performance of FDG-PET, MRI, and plain film radiography (PFR) for the diagnosis of osteomyelitis in the diabetic foot. Mol Imaging Biol. 2010;12:335–342. doi: 10.1007/s11307-009-0268-2. [DOI] [PubMed] [Google Scholar]
- 92.Basu S, Chryssikos T, Houseni M, Scot Malay D, Shah J, Zhuang H, Alavi A. Potential role of FDG PET in the setting of diabetic neuro-osteoarthropathy: can it differentiate uncomplicated Charcot’s neuroarthropathy from osteomyelitis and soft-tissue infection? Nucl Med Commun. 2007;28:465–472. doi: 10.1097/MNM.0b013e328174447f. [DOI] [PubMed] [Google Scholar]
- 93.Familiari D, Glaudemans AW, Vitale V, Prosperi D, Bagni O, Lenza A, Cavallini M, Scopinaro F, Signore A. Can sequential 18F-FDG PET/CT replace WBC imaging in the diabetic foot? J Nucl Med. 2011;52:1012–1019. doi: 10.2967/jnumed.110.082222. [DOI] [PubMed] [Google Scholar]
- 94.Love C, Marwin SE, Tomas MB, Krauss ES, Tronco GG, Bhargava KK, Nichols KJ, Palestro CJ. Diagnosing infection in the failed joint replacement: a comparison of coincidence detection 18F-FDG and 111In-labeled leukocyte/99mTc-sulfur colloid marrow imaging. J Nucl Med. 2004;45:1864–1871. [PubMed] [Google Scholar]
- 95.Stumpe KD, Nötzli HP, Zanetti M, Kamel EM, Hany TF, Görres GW, von Schulthess GK, Hodler J. FDG PET for differentiation of infection and aseptic loosening in total hip replacements: comparison with conventional radiography and three-phase bone scintigraphy. Radiology. 2004;231:333–341. doi: 10.1148/radiol.2312021596. [DOI] [PubMed] [Google Scholar]
- 96.Kwee TC, Kwee RM, Alavi A. FDG-PET for diagnosing prosthetic joint infection: systematic review and metaanalysis. Eur J Nucl Med Mol Imaging. 2008;35:2122–2132. doi: 10.1007/s00259-008-0887-x. [DOI] [PubMed] [Google Scholar]
- 97.Kwee RM, Broos WA, Brans B, Walenkamp GH, Geurts J, Weijers RE. Added value of 18F-FDG PET/CT in diagnosing infected hip prosthesis. Acta Radiol. 2018;59:569–576. doi: 10.1177/0284185117726812. [DOI] [PubMed] [Google Scholar]
- 98.Hao R, Yuan L, Kan Y, Yang J. 18F-FDG PET for diagnosing painful arthroplasty/prosthetic joint infection. Clin Transl Imaging. 2017;5:315–322. [Google Scholar]
- 99.Basu S, Kwee TC, Saboury B, Garino JP, Nelson CL, Zhuang H, Parsons M, Chen W, Kumar R, Salavati A, Werner TJ, Alavi A. FDG PET for diagnosing infection in hip and knee prostheses: prospective study in 221 prostheses and subgroup comparison with combined (111)In-labeled leukocyte/(99m)Tc-sulfur colloid bone marrow imaging in 88 prostheses. Clin Nucl Med. 2014;39:609–15. doi: 10.1097/RLU.0000000000000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hunder GG, Arend WP, Bloch DA, Calabrese LH, Fauci AS, Fries JF, Leavitt RY, Lie JT, Lightfoot RW Jr, Masi AT, et al. The American college of rheumatology 1990 criteria for the classification of vasculitis: introduction. Arthritis Rheum. 1990;33:1065–1067. doi: 10.1002/art.1780330802. [DOI] [PubMed] [Google Scholar]
- 101.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CG, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 102.Samson M, Corbera-Bellalta M, Audia S, Planas-Rigol E, Martin L, Cid MC, Bonnotte B. Recent advances in our understanding of giant cell arteritis pathogenesis. Autoimmun Rev. 2017;16:833–844. doi: 10.1016/j.autrev.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 103.Ciccia F, Rizzo A, Ferrante A, Guggino G, Croci S, Cavazza A, Salvarani C, Triolo G. New insights into the pathogenesis of giant cell arteritis. Autoimmun Rev. 2017;16:675–683. doi: 10.1016/j.autrev.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 104.Gravanis MB. Giant cell arteritis and takayasu aortitis: morphologic, pathogenetic and etiologic factors. Int J Cardiol. 2000;75(Suppl 1):S21–S33. doi: 10.1016/s0167-5273(00)00184-4. [DOI] [PubMed] [Google Scholar]
- 105.Davies CG, May DJ. The role of temporal artery biopsies in giant cell arteritis. Ann R Coll Surg Engl. 2011;93:4–5. doi: 10.1308/003588411X12851639107476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hayreh SS, Podhajsky PA, Raman R, Zimmerman B. Giant cell arteritis: validity and reliability of various diagnostic criteria. Am J Ophthalmol. 1997;123:285–296. doi: 10.1016/s0002-9394(14)70123-0. [DOI] [PubMed] [Google Scholar]
- 107.Quéméneur T, Hachulla E, Lambert M, Perez-Cousin M, Queyrel V, Launay D, Morell-Dubois S, Hatron PY. Takayasu arteritis. Presse Med. 2006;35:847–856. doi: 10.1016/s0755-4982(06)74703-0. [DOI] [PubMed] [Google Scholar]
- 108.Belhocine T, Blockmans D, Hustinx R, Vandevivere J, Mortelmans L. Imaging of large vessel vasculitis with 18 FDG PET: illusion or reality? A critical review of the literature data. Eur J Nucl Med Mol Imaging. 2003;30:1305–1313. doi: 10.1007/s00259-003-1209-y. [DOI] [PubMed] [Google Scholar]
- 109.Schreiber BE, Tam HH, Carvalho C, Wong WL, Russell AI, Higgens CS. F-18 PET-CT showing large vessel vasculitis in a patient with high inflammatory markers and no localizing symptoms. Clin Nucl Med. 2009;34:785–787. doi: 10.1097/RLU.0b013e3181b7db59. [DOI] [PubMed] [Google Scholar]
- 110.Martínez-Rodríguez I, Martínez-Amador N, Banzo I, Quirce R, Jiménez-Bonilla J, De Arcocha-Torres M, Ibáñez-Bravo S, Lavado-Pérez C, Bravo-Ferrer Z, Blanco R, González-Gay MA, Carril JM. Assessment of aortitis by semiquantitative analysis of 180-min 18 F-FDG PET/CT acquisition images. Eur J Nucl Med Mol Imaging. 2014;41:2319–2324. doi: 10.1007/s00259-014-2863-y. [DOI] [PubMed] [Google Scholar]
- 111.Glaudemans AW, de Vries EF, Galli F, Dierckx RA, Slart RH, Signore A. The use of F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Dev Immunol. 2013;2013:623036. doi: 10.1155/2013/623036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nielsen BD, Hansen IT, Kramer S, Haraldsen A, Hjorthaug K, Bogsrud TV, Ejlersen JA, Stolle LB, Keller KK, Therkildsen P, Hauge EM, Gormsen LC. Simple dichotomous assessment of cranial artery inflammation by conventional 18F-FDG PET/CT shows high accuracy for the diagnosis of giant cell arteritis: a case-control study. Eur J Nucl Med Mol Imaging. 2019;46:184–193. doi: 10.1007/s00259-018-4106-0. [DOI] [PubMed] [Google Scholar]
- 113.Zerizer I, Tan K, Khan S, Barwick T, Marzola MC, Rubello D, Al-Nahhas A. Role of FDG-PET and PET/CT in the diagnosis and management of vasculitis. Eur J Radiol. 2010;73:504–509. doi: 10.1016/j.ejrad.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 114.Webb M, Chambers A, AL-Nahhas A, Mason JC, Maudlin L, Rahman L, Frank J. The role of 18F-FDG PET in characterising disease activity in takayasu arteritis. Eur J Nucl Med Mol Imaging. 2004;31:627–634. doi: 10.1007/s00259-003-1429-1. [DOI] [PubMed] [Google Scholar]
- 115.Bleeker-Rovers CP, Bredie SJ, Van Der Meer JW, Corstens FH, Oyen WJ. F-18-fluorodeoxyglucose positron emission tomography in diagnosis and follow-up of patients with different types of vasculitis. Neth J Med. 2003;61:323–329. [PubMed] [Google Scholar]
- 116.Walter MA, Melzer RA, Schindler C, Müller-Brand J, Tyndall A, Nitzsche EU. The value of [18F] FDG-PET in the diagnosis of large-vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging. 2005;32:674–681. doi: 10.1007/s00259-004-1757-9. [DOI] [PubMed] [Google Scholar]
- 117.Meller J, Strutz F, Siefker U, Scheel A, Sahlmann CO, Lehmann K, Conrad M, Vosshenrich R. Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging. 2003;30:730–736. doi: 10.1007/s00259-003-1144-y. [DOI] [PubMed] [Google Scholar]
- 118.Nielsen BD, Gormsen LC, Hansen IT, Keller KK, Therkildsen P, Hauge EM. Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. Eur J Nucl Med Mol Imaging. 2018;45:1119–1128. doi: 10.1007/s00259-018-4021-4. [DOI] [PubMed] [Google Scholar]
- 119.Weyand CM, Goronzy JJ. Clinical practice. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med. 2014;371:50–57. doi: 10.1056/NEJMcp1214825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maestri Brittain J, Gormsen LC, von Benzon E, Andersen KF. Concomitant polymyalgia rheumatica and large-vessel vasculitis visualized on (18)F-FDG PET/CT. Diagnostics (Basel) 2018;8 doi: 10.3390/diagnostics8020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blockmans D, De Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18-fluorodeoxyglucose positron emission tomography in isolated polymyalgia rheumatica: a prospective study in 35 patients. Rheumatology (Oxford) 2006;46:672–677. doi: 10.1093/rheumatology/kel376. [DOI] [PubMed] [Google Scholar]
- 122.Sondag M, Guillot X, Verhoeven F, Blagosklonov O, Prati C, Boulahdour H, Wendling D. Utility of 18F-fluoro-dexoxyglucose positron emission tomography for the diagnosis of polymyalgia rheumatica: a controlled study. Rheumatology (Oxford) 2016;55:1452–1457. doi: 10.1093/rheumatology/kew202. [DOI] [PubMed] [Google Scholar]
- 123.Soussan M, Abisror N, Abad S, Nunes H, Terrier B, Pop G, Eder V, Valeyre D, Sberro-Soussan R, Guillevin L, Dhote R, Fain O, Mekinian A. FDG-PET/CT in patients with ANCA-associated vasculitis: case-series and literature review. Autoimmun Rev. 2014;13:125–131. doi: 10.1016/j.autrev.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 124.Braun JJ, Gentine A, Pauli G. Sinonasal sarcoidosis: review and report of fifteen cases. Laryngoscope. 2004;114:1960–1963. doi: 10.1097/01.mlg.0000147928.06390.db. [DOI] [PubMed] [Google Scholar]
- 125.Schwartzbauer HR, Tami TA. Ear, nose, and throat manifestations of sarcoidosis. Otolaryng Clin North Am. 2003;36:673–684. doi: 10.1016/s0030-6665(03)00030-6. [DOI] [PubMed] [Google Scholar]
- 126.Mañá J, Salazar A, Manresa F. Clinical factors predicting persistence of activity in sarcoidosis: a multivariate analysis of 193 cases. Respiration. 1994;61:219–225. doi: 10.1159/000196341. [DOI] [PubMed] [Google Scholar]
- 127.Takada K, Ina Y, Noda M, Sato T, Yamamoto M, Morishita M. The clinical course and prognosis of patients with severe, moderate or mild sarcoidosis. J Clini Epidemiol. 1993;46:359–366. doi: 10.1016/0895-4356(93)90150-y. [DOI] [PubMed] [Google Scholar]
- 128.Teirstein AS, Machac J, Almeida O, Lu P, Padilla ML, Iannuzzi MC. Results of 188 whole-body fluorodeoxyglucose positron emission tomography scans in 137 patients with sarcoidosis. Chest. 2007;132:1949–1953. doi: 10.1378/chest.07-1178. [DOI] [PubMed] [Google Scholar]
- 129.Aide N, Benayoun M, Kerrou K, Khalil A, Cadranel J, Talbot JN. Impact of [18F]-fluorodeoxyglucose ([18F]-FDG) imaging in sarcoidosis: unsuspected neurosarcoidosis discovered by [18F]-FDG PET and early metabolic response to corticosteroid therapy. Br J Radiol. 2007;80:e67–e71. doi: 10.1259/bjr/33076108. [DOI] [PubMed] [Google Scholar]
- 130.Brancato SC, Arrighi JA. Fasting FDG PET compared to MPI SPECT in cardiac sarcoidosis. J Nucl Cardiol. 2011;18:371–374. doi: 10.1007/s12350-011-9347-2. [DOI] [PubMed] [Google Scholar]
- 131.Prager E, Wehrschuetz M, Bisail B, Woltsche M, Schwarz T, Lanz H, Sorantin E, Aigner RM. Comparison of 18F-FDG and 67Ga-citrate in sarcoidosis imaging. Nuklearmedizin. 2008;47:18–23. doi: 10.3413/nukmed-0085. [DOI] [PubMed] [Google Scholar]
- 132.Keijsers RG, Grutters JC, Thomeer M, Du Bois RM, Van Buul MM, Lavalaye J, Van Den Bosch JM, Verzijlbergen FJ. Imaging the inflammatory activity of sarcoidosis: sensitivity and inter observer agreement of (67)Ga imaging and (18)F-FDG PET. Q J Nucl Med Mol Imaging. 2011;55:66–71. [PubMed] [Google Scholar]
- 133.Lu Y, Grant C, Xie K, Sweiss NJ. Suppression of myocardial 18F-FDG uptake through prolonged high-fat, high-protein, and very-low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. Clin Nucl Med. 2017;42:88–94. doi: 10.1097/RLU.0000000000001465. [DOI] [PubMed] [Google Scholar]
- 134.Basu S, Zhuang H, Torigian DA, Rosenbaum J, Chen W, Alavi A, editors. Semin Nucl Med. Elsevier; 2009. Functional imaging of inflammatory diseases using nuclear medicine techniques. [DOI] [PubMed] [Google Scholar]
- 135.Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Braun JJ, Kessler R, Constantinesco A, Imperiale A. 18 F-FDG PET/CT in sarcoidosis management: review and report of 20 cases. Eur J Nucl Med Mol Imaging. 2008;35:1537–43. doi: 10.1007/s00259-008-0770-9. [DOI] [PubMed] [Google Scholar]
- 137.Imperiale A, Riehm S, Veillon F, Namer IJ, Braun JJ. FDG PET coregistered to MRI for diagnosis and monitoring of therapeutic response in aggressive phenotype of sarcoidosis. Eur J Nucl Med Mol Imaging. 2011;38:983–984. doi: 10.1007/s00259-010-1725-5. [DOI] [PubMed] [Google Scholar]
- 138.Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC, Hainer J, Murthy VL, Skali H, Dorbala S, Di Carli MF, Blankstein R. Reduction in 18 F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–174. doi: 10.1007/s12350-013-9828-6. [DOI] [PubMed] [Google Scholar]
- 139.Keijsers RG, Verzijlbergen JF, van Diepen DM, van den Bosch JM, Grutters JC. 18F-FDG PET in sarcoidosis: an observational study in 12 patients treated with infliximab. Sarcoidosis vasc Diffuse Lung Dis. 2008;25:143–9. [PubMed] [Google Scholar]
- 140.Lee PI, Cheng G, Alavi A. The role of serial FDG PET for assessing therapeutic response in patients with cardiac sarcoidosis. J Nucl Cardiol. 2017;24:19–28. doi: 10.1007/s12350-016-0682-1. [DOI] [PubMed] [Google Scholar]
- 141.Muser D, Santangeli P, Castro SA, Liang JJ, Enriquez A, Werner TJ, Nucifora G, Magnani S, Hayashi T, Zado ES, Garcia FC, Callans DJ, Dixit S, Desjardins B, Marchlinski FE, Alavi A. Prognostic role of serial quantitative evaluation of 18 F-fluorodeoxyglucose uptake by PET/CT in patients with cardiac sarcoidosis presenting with ventricular tachycardia. Eur J Nucl Med Mol Imaging. 2018;45:1394–1404. doi: 10.1007/s00259-018-4001-8. [DOI] [PubMed] [Google Scholar]
- 142.Scholtens AM, Verberne HJ, Budde RP, Lam MG. Additional heparin preadministration improves cardiac glucose metabolism suppression over low-carbohydrate diet alone in (1)(8)F-FDG PET imaging. J Nucl Med. 2016;57:568–573. doi: 10.2967/jnumed.115.166884. [DOI] [PubMed] [Google Scholar]
- 143.Tind S, Vestergaard S, Farahani ZA, Hess S. Positron emission tomography/computer tomography in gastrointestinal malignancies: current potential and challenges. Minerva Chir. 2017;72:397–415. doi: 10.23736/S0026-4733.17.07409-0. [DOI] [PubMed] [Google Scholar]
- 144.Cho SY. To hold or not to hold metformin for FDG PET scans: that is the question. Radiology. 2018;289:426–427. doi: 10.1148/radiol.2018181566. [DOI] [PubMed] [Google Scholar]
- 145.Scholtens AM, Verberne HJ. Attenuation correction and metal artifact reduction in FDG PET/CT for prosthetic heart valve and cardiac implantable device endocarditis. J Nucl Cardiol. 2018;25:2172–2173. doi: 10.1007/s12350-017-1176-5. [DOI] [PubMed] [Google Scholar]
- 146.Sunde SK, Beske T, Gerke O, Clausen LL, Hess S. FDG-PET/CT as a diagnostic tool in vascular graft infection: a systematic review and meta-analysis. 2019;7:255–265. [Google Scholar]


