Abstract
Optimal immune-based therapies for type 1 diabetes (T1D) should restore self-tolerance without inducing chronic immunosuppression. CD4+Foxp3+ regulatory T cells (Tregs) are a key cell population capable of facilitating durable immune tolerance. However, clinical trials with expanded Tregs in T1D and solid-organ transplant recipients are limited by poor Treg engraftment without host manipulation. We showed that Treg engraftment and therapeutic benefit in nonautoimmune models required ablative host conditioning. Here, we evaluated Treg engraftment and therapeutic efficacy in the nonobese diabetic (NOD) mouse model of autoimmune diabetes using nonablative, combinatorial regimens involving the anti-CD3 (αCD3), cyclophosphamide (CyP), and IAC (IL-2/JES6–1) antibody complex. We demonstrate that αCD3 alone induced substantial T-cell depletion, impacting both conventional T cells (Tconv) and Tregs, subsequently followed by more rapid rebound of Tregs. Despite robust depletion of host Tconv and host Tregs, donor Tregs failed to engraft even with interleukin-2 (IL-2) support. A single dose of CyP after αCD3 depleted rebounding host Tregs and resulted in a 43-fold increase in donor Treg engraftment, yet polyclonal donor Tregs failed to reverse diabetes. However, infusion of autoantigen-specific Tregs after αCD3 alone resulted in robust Treg engraftment within the islets and induced remission in all mice. This novel combinatorial therapy promotes engraftment of autoantigen-specific donor Tregs and controls islet autoimmunity without long-term immunosuppression.
Introduction
The key role played by regulatory T cells (Tregs) in self-tolerance (1,2) and suppression of rejection (3–6) makes them attractive for tolerogenic cell-based therapies. Much effort is being devoted to developing Treg therapy for recent-onset type 1 diabetes (T1D) and in transplant settings (7–10). Several groups have established in vitro Treg expansion protocols (11–15); clinical trials with autologous, expanded Tregs are ongoing in T1D (9,10) using unselected, polyclonal Tregs (14,16). A phase 1 study revealed that in vitro expanded, autologous Tregs were safe and tolerable in children with recent-onset T1D, with evidence of improved fasting C-peptide and reduced insulin requirement 4 months posttreatment. Therapeutic effects correlated with increased Tregs post-infusion but only persisted for a short time. A subsequent trial confirmed the limited persistence of expanded Tregs even after a second infusion (9,10,17).
Data emerging from these trials highlight the limitations of protocols that rely solely on Treg infusion without recipient manipulation, including immunomodulation and homeostatic support. In fact, our previous work identified critical requirements for infused Treg engraftment and function: 1) generation of peripheral space for engraftment, 2) overcoming competition from host Tregs, 3) sufficient interleukin 2 (IL-2), and 4) antigen availability to select disease-relevant Tregs (18–20). Our observations are supported by other studies (for review see Cabello-Kindelan et al. [21]) and should be considered when designing Treg-based clinical trials, whether these Tregs are expanded in vitro or infused after isolation for in vivo expansion. Our previous protocols involved ablative (radiation) conditioning of the host for Treg engraftment and therapeutic efficacy. Although effective, ablative conditioning bears translational concerns for clinical application that in T1D would also involve children. Hence, we sought to develop effective but safer methods to create immunological space and enable engraftment of autologous Tregs. To this end, we explored novel, nonablating, clinically applicable immunomodulatory regimens that included combinations of a short course of intact αCD3, a single injection of cyclophosphamide (CyP), and the addition of IL-2 complex (IAC, IL-2/clone JES6-1), which progressively improved engraftment. However, the reversal of recent-onset diabetes in nonobese diabetic (NOD) mice was only observed when donor Tregs were autoantigen specific rather than polyclonal, and this setting immunomodulation with αCD3 alone resulted in 100% durable diabetes remission (>6 months), with robust Treg engraftment within the islets. Thus, our results demonstrate that robust engraftment and disease-relevant, antigen-specific Tregs are key requirements for successful immunotherapy in a preclinical model of autoimmune diabetes.
Research Design and Methods
Mice
NOD mice were from The Jackson Laboratory (Bar Harbor, ME). Breeder pairs of NOD.NON-Thy1a/1LtJ and NOD.Cg-Tg(TcraBDC2.5,TcrbBDC2.5)1Doi/DoiJ were purchased from The Jackson Laboratory and colonies were maintained at the University of Miami. Animal studies were performed in accordance with protocol approval by the University of Miami Institutional Animal Care and Use Committee.
Cell Purifications
CD4+CD25+ Tregs were purified from spleen (SP) and lymph nodes (LNs) as previously described (22) from NOD or Thy1.1+ NOD mice. Tregs on average were >92% pure. For remission studies, islet-specific CD4+TCRVβ4+CD25+ Tregs were purified from SP and LNs of NOD.Cg-Tg(TcraBDC2.5,TcrbBDC2.5)1Doi/DoiJ mice by cell sorting and were on average >98.5% pure. In brief, CD4 T cells were enriched by anti-CD4 MACS microbeads (Miltenyi Biotec, San Diego, CA), stained with Alexa Flour 700–conjugated CD4, phycoerythrin (PE)-conjugated Vβ4 antibody, and PE-CF594–conjugated CD25 (BD Biosciences, San Diego, CA) and cell sorted on Beckman Coulter Astrios. Purities were assessed by flow cytometry.
Adoptive Treg Transfer
Freshly isolated Tregs (0.5 × 106), either polyclonal or islet-specific Tregs, were adoptively transferred by i.v. injections through tail vein to NOD mice that received immunomodulation in various combinations using intact αCD3 (50 μg, clone 2C11) (Leinco Technologies, St. Louis, MO) one time per day for five consecutive days, a single injection of CyP (200 mg/kg) (Sigma-Aldrich, St. Louis, MO), and IL-2 complex (IAC) given every other day for 1 week starting at the time of Treg infusion, or mice were left untreated (Fig. 3A). IAC was prepared using recombinant murine IL-2 with anti–IL-2 monoclonal antibody (mAb; clone JES6-1A12) (eBioscience, San Diego, CA) and incubated at a molar ratio of 2 to 1 (1 μg IL-2 and 5 μg Ab) for 15 min at room temperature. The resulting IAC was intraperitoneally injected in 200 μL PBS as indicated in each illustration.
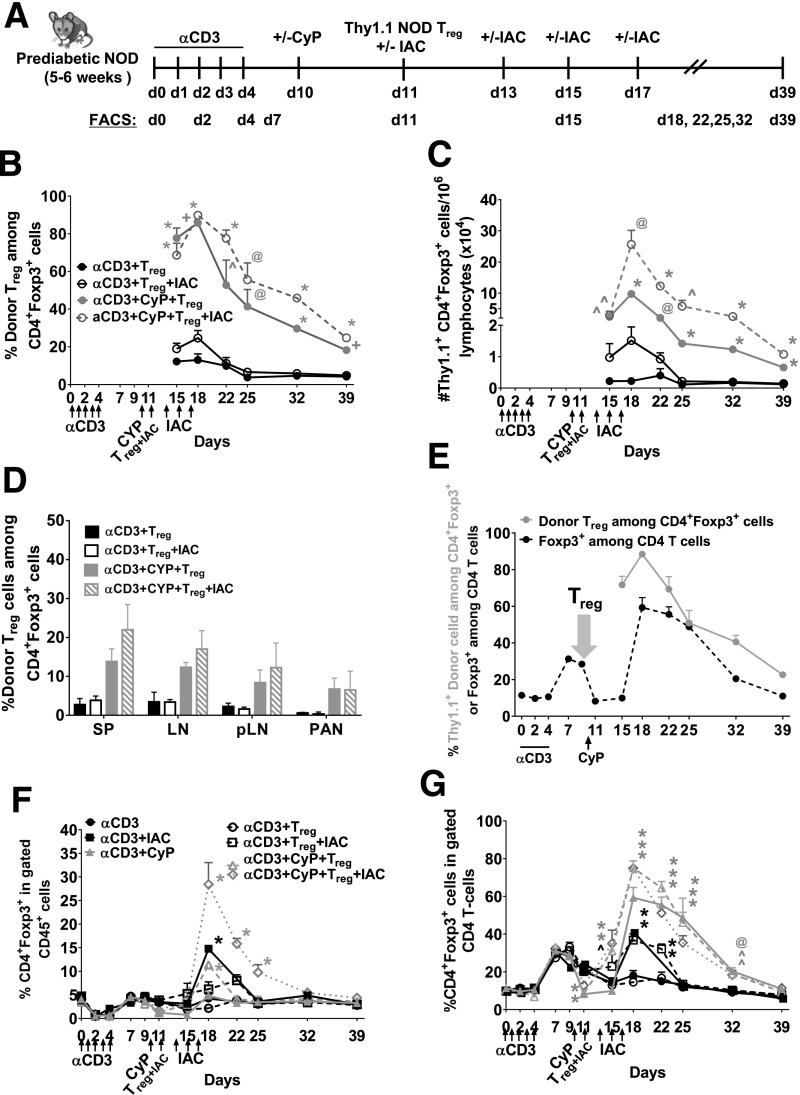
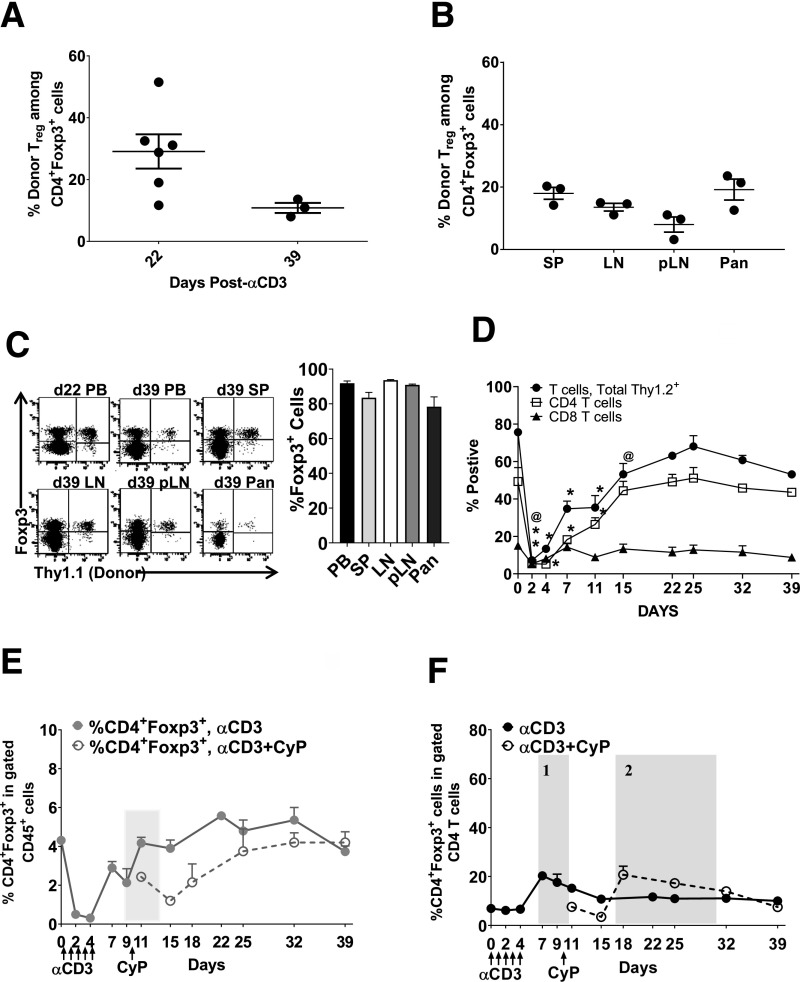
Figure 3.
Adoptive transfer of Tregs leads to engraftment after immunomodulation in young, prediabetic NOD mice. A: Experimental scheme. B: Percentage of Thy1.1+ donor Treg engraftment among the total gated CD4+Foxp3+ T cells in peripheral blood. C: Number of donor Tregs/1 × 106 CD45+ lymphocytes in peripheral blood. D: Percentage of Thy1.1+ donor Treg engraftment in the total gated CD4+Foxp3+ T cells 4 weeks post–Treg infusion in SP, LN, pLN, and pancreas (PAN) of young, female prediabetic NOD mice receiving αCD3+Treg, αCD3+Treg+IAC, αCD3+CyP+Treg, or αCD3+CyP+Treg+IAC. IAC = anti–IL-2 (JES6)+rmIAC. E: Percentage of Thy1.1+ donor Tregs in the gated CD4+Foxp3+ T cells in mice receiving the αCD3+CyP+Treg+IAC regimen and CD4+Foxp3+ in the gated total CD4 T cells in mice receiving the αCD3 regimen in the peripheral blood. F: Percentage of CD4+Foxp3+ in the gated CD45+ population. G: CD4+Foxp3+ in the gated CD4 T cells in the peripheral blood of young, prediabetic female NOD mice (aged 5–6 weeks) in NOD mice receiving αCD3, αCD3+IAC, αCD3+CyP, αCD3+Treg, αCD3+Treg+IAC, αCD3+CyP+Treg, or αCD3+CyP+Treg+IAC. n = 5–6 mice per group. *P < 0.0001; +P < 0.001; @P < 0.01; ^P < 0.05. B and C: Multiple Student t test compared with αCD3+Treg. F and G: Two-way ANOVA followed by Dunnett multiple comparison test compared with αCD3. d, day; rmIAC, recombinant murine IAC.
Remission Studies
Recent-onset diabetic NOD mice (>250 mg/dL for two consecutive blood readings) received a single insulin pellet subcutaneously to initially control blood sugars but no insulin thereafter. These mice received immunomodulation in various combinations using intact αCD3, CyP, IAC, and/or Treg infusion (autologous, polyclonal NOD, or islet-specific NOD Tregs), or mice were left untreated as indicated in each illustration. Diabetes reoccurrence was monitored for 6 months. Mice were tested two to three times per week for weight and glycosuria. The absence of glycosuria and hyperglycemia indicates lack of diabetes relapse.
Skin Transplantation
Skin grafting was performed as a modification of the Billingham and Medawar technique (23). Anesthetized mice received full-thickness donor skin (prepared from dorsal tissue from ear) on separate graft beds on the back of NOD mice 6 months after αCD3+BDC2.5 Treg infusion. Each recipient received two grafts, syngeneic NOD and allogeneic B6 grafts. A bandage was placed over these two grafts for 7 days. Grafts were monitored every other day and scored rejected when >75% of the original graft was lost or became necrotic as assessed by visual examination.
Antibodies and Flow Cytometry Analysis
SP and LN were made into single cell suspensions by mechanic disruption. Pancreatic LN (pLN) and pancreas were digested with collagenase D (2 mg/mL) (Roche) at 37°C for 30 min. Equal volumes of collected blood were used for peripheral blood mononuclear cells and purified from whole blood on a Ficoll-Paque PLUS gradient (GE Life Sciences). Red blood cells from tissues were lysed with ACK lysing buffer. Total cell counts were performed on tissues and blood using a hemocytometer.
Lymphocytes were stained with LIVE/DEAD Fixable Near-IR according to the manufacturer’s instructions (Invitrogen/Thermo Fisher Scientific, Eugene, OR) and washed twice with PBS. Cells were then incubated with rat anti-CD16/32 (clone 24G2) to block nonspecific Ab binding followed by cell surface staining with fluorescence-conjugated Abs against mouse CD45, CD19, CD4, CD8, Thy1.2, CD25 (BD Biosciences), and Thy1.1 (BioLegend, San Diego, CA). Intracellular Foxp3 (clone FJK-16s) staining was performed according to the manufacturer’s instructions (eBioscience) along with Ki67 staining (clone B56; BD Biosciences).
Tetramer staining was performed on islet lymphocytes. Murine islets were isolated as described previously (24) with minor modifications. Animals were killed under general anesthesia, and pancreas was exposed and injected with Hanks’ balanced salt solution containing 0.5 mg/mL collagenase P (Sigma-Aldrich) via the main bile duct until distension was achieved. Digestion was performed at 37°C for 10–15 min with gentle agitation and terminated by the addition of cold buffer (RPMI containing 10% FCS and 2 mmol/L l-glutamine). The tissue was filtered through 70-μm mesh, placed on Euro-Ficoll (Mediatech) gradients by centrifugation at 2,000 rpm for 15 min, and washed twice with PBS. Single cell suspensions were incubated with rat anti-CD16/32 (clone 24G2) followed by staining with PE-conjugated BDC2.5 tetramer Ab [I-A(g7) BDC2.5 mimetope RTRPLWVRME; National Institutes of Health Tetramer Core] for 3 h at 37°C, and fluorescence-conjugated Abs against cell surface mouse CD45, Thy1.2, CD4, CD8, and CD25 were added to the last 30 min of incubation. Cells were then stained with LIVE/DEAD Fixable Near-IR followed by intracellular Foxp3 staining.
FACS analysis was performed using a BD Biosciences LSRII and analyzed with Diva or Kaluza software. The total number of events collected was between 50,000 and 200,000 cells, except for pancreas samples in which entire samples were collected, and CD45+ cells were set as the storage gate.
Statistical Analysis
One-way ANOVA was followed by Dunnett multiple comparison test. Two-way ANOVA was followed by Sidak multiple comparison test. Unpaired Student t test was performed in which αCD3 was compared with αCD3+CyP at each time point. Comparisons yielding P < 0.05 were considered statistically significant. Survival curves were subjected to Mantel-Cox log-rank test. The P value is indicated on the graph.
Data and Resource Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. All resources, including animal models and reagents, are commercially available.
Results
A Combinatorial Regimen of αCD3 and CyP Creates Space in the Host Treg Compartment in Female NOD Mice
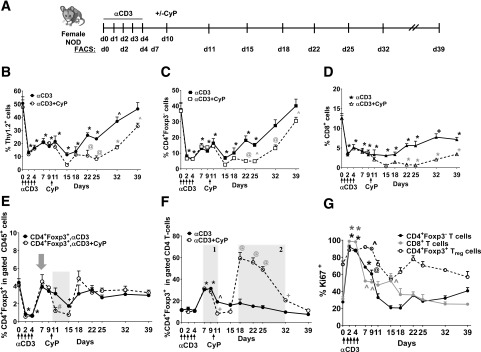
In previous studies, intact αCD3 was more effective at depleting T cells than the F(ab′)2 form (25–27); while regimens varied in timing and duration of administration, depletion was dose dependent. Yang et al. (27) reported that a single injection of intact αCD3 led to transient T-cell depletion in both C57BL/6 and NOD mice, but NOD required a higher dose (50 µg) than C57BL/6 mice; depletion was age dependent in NOD mice. Moreover, in NOD mice, 50 μg of intact αCD3 more efficiently depleted CD4 T cells than 100 μg of F(ab′)2-αCD3 (27). Hence, we used intact αCD3 antibody (50-µg dose) as a depleting agent in our study. We investigated the effects of αCD3 therapy on conventional T-cell and Treg compartments in the circulation of 5- to 6-week-old prediabetic NOD female mice. We used a 5-day course of αCD3 and followed the effects for 39 days (Fig. 1A). αCD3 readily decreased conventional T cells, including CD4+Foxp3− and CD8 T cells (Fig. 1B–D, solid lines). CD4+Foxp3− T cells returned to normal levels at around 39 days after the first αCD3 dose; CD8 T cells did not fully recover. Our assessments at earlier time points (as early as 2 days) revealed that Treg depletion occurs very early post-αCD3 (Fig. 1E); in fact, Tregs returned to normal levels within 7 days of the first dose (Fig. 1E, solid lines, gray arrow). Thus, we demonstrated a previously unknown early Treg depletion post-αCD3. We also showed that Tregs rebound faster than conventional T cells, which recover around day 32–39, leading to an increased proportion of CD4+Foxp3+ cells in the CD4+ T-cell compartment (Fig. 1F, solid line, shaded area 1). Penaranda et al. (28) noted that treatment with F(ab′)2-αCD3 induced a transient increase in the proportion of Foxp3+ cells in the CD4 T-cell compartment that was accompanied by a slight reduction in the total count of Tregs, but that the increased Foxp3+ proportion was due to greater depletion of the CD4+Foxp3− conventional T cells. In our study, most T cells were initially proliferating after depletion with intact αCD3 (Fig. 1G); however, only Tregs maintained robust proliferation, including a much higher Ki67 mean fluorescence intensity, over the 39-day follow-up (Supplementary Fig. 1), which may contribute to the increased proportion of Foxp3+ cells in the CD4+ T-cell compartment after αCD3 treatment together with the slow recovery of the CD4+Foxp3− T cells.
Figure 1.
T-cell depletion and rebound after immunomodulation with αCD3, CyP, and IAC in the circulation. A: Experimental scheme. Percentage of Thy1.2 (B), CD4 (C), CD8 (D), and CD4+Foxp3+ (E) in the gated CD45+ population and percentage of CD4+Foxp3+ in the gated CD4 T cells (F) (shaded area 1 and 2 indicate increase in the percentage of CD4+Foxp3+ in the CD4 T cells) in prediabetic female NOD mice receiving αCD3 (solid lines) or αCD3+CyP (dashed lines). G: Percentage of Ki67+ in the gated CD4+Foxp3+, CD4+Foxp3−, and CD8 T cells in the peripheral blood. Time points examined are 2, 4, 7, 11, 15, 18, 22, 25, 32, and 39 days. n = 5–6 mice per group. In E, the gray arrow indicates recovery in the Treg compartment, and the shaded area indicates the decrease in CD4+Foxp3+ host Tregs following single CyP injection after αCD3 treatment. In F, shaded area 1 indicates the increase in CD4+Foxp3+ cells in the gated CD4 T cells after CD3 alone, and shaded area 2 indicates the increase in CD4+Foxp3+ cells in total CD4 T cells after CD3+CyP. *P < 0.0001; +P < 0.001; @P < 0.01; ^P < 0.05. One-way ANOVA followed by Dunnett multiple comparison test compared with day 0. Two-way ANOVA followed by Sidak multiple comparison test; αCD3 compared with αCD3+CyP at each time point. #P < 0.05. d, day.
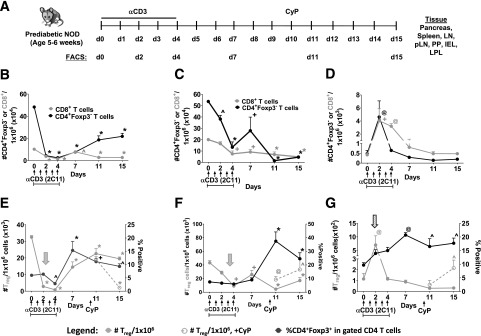
We next examined T-cell compartments after αCD3 treatment up to 15 days in pancreas, SP, LN, pLN, and Peyer’s patches (PP), lamina propria lymphocytes (LPLs), and intraepithelial lymphocytes (IELs) in the small intestine (Fig. 2A). Similar to blood, αCD3 therapy rapidly depleted CD4+Foxp3− and CD8+ T cells in LN and pLN, with <30% recovery from baseline at the end of the 15-day follow-up (Fig. 2B and C and Supplementary Fig. 2). In contrast, there was an initial CD8+ T-cell increase in SP, PP, IEL, and LPL followed by a decrease beginning on day 4, while CD4+Foxp3− T cells were rapidly decreased after αCD3 treatment (Supplementary Figs. 2 and 3). There was a slow recovery in the small intestine, with <35% of CD4+Foxp3− T cells recovering from baseline, excluding T cells in SP with ∼36–58% recovery (Supplementary Figs. 2 and 3). In pancreas, there was an early increase followed by decrease of CD4+Foxp3− T cells beginning on day 4, with no recovery by the end of the follow-up period. CD8+ T cells had a more persistent increase before returning to baseline, leading to an inversion of the CD4:CD8 T-cell ratio after αCD3 treatment (Fig. 2D).
Figure 2.
Treg depletion and rebound in the tissues after αCD3 and CyP immunomodulation. A: Experimental scheme. Number of CD8+ and CD4+Foxp3− T cells (per 1 × 106 lymphocytes) in peripheral blood (B), pLNs (C), and pancreas (D). Number of CD4+Foxp3+ cells (per 1 × 106 CD45+ lymphocytes) and percentage of CD4+Foxp3+ in the gated CD4 T cells in peripheral blood (E), pLNs (F), and pancreas (G) in the presence or absence of CyP of young, prediabetic NOD mice (aged 5–6 weeks). n = 3–6 mice per group. In E and F, gray arrows indicate an early decrease in the number of CD4+Foxp3+ cells after the start of αCD3 in the blood and pLNs. In G, hatched arrow indicates an initial increase in the number of CD4+Foxp3+ cells after the start of αCD3 in the pancreas. *P < 0.0001; +P < 0.001; @P < 0.01; ^P < 0.05. One-way ANOVA followed by Dunnett multiple comparison test compared with day 0. Unpaired Student t test, αCD3 compared with αCD3+CyP at each time point. n = 3–6 mice per group. d, day.
Resembling effects in blood (Fig. 2E, left panel, gray arrow), αCD3 treatment was immediately followed by a decrease of CD4+Foxp3+ Tregs in LN and pLN, with 44–60% recovery by day 15 (Fig. 2E and F and Supplementary Fig. 2, gray arrows). The slower recovery of CD4+Foxp3− T cells in these tissues compared with Tregs led to increased proportions of CD4+Foxp3+ in the CD4+ compartment (Fig. 2E and F and Supplementary Fig. 2). There was delayed Treg depletion in SP (Supplementary Fig. 2, white arrow) compared with blood and LNs, but recovery was still around 50% at the end of follow-up (Fig. 2 and Supplementary Fig. 2). In contrast, αCD3 treatment resulted in an initial Treg increase in the pancreas (Fig. 2G, hatched arrow) followed by delayed depletion starting at day 11, with no recovery of Tregs accompanied by an increase in the proportion of CD4+Foxp3+ cells in the CD4+ T-cell compartment. A similar pattern was seen in LPL, IEL, and PP, with an initial Treg increase, but Tregs did begin to recover (Supplementary Fig. 3). Treg changes post-αCD3 did not appear to be solely due to redistribution of circulating Tregs to tissues. By linear regression, we found a link between Treg rebound and proliferation in pLN (Supplementary Fig. 4A), pancreas, LPL, and peripheral blood (not shown). However, there was no correlation observed in LN (Supplementary Fig. 4B), SP, IEL, and PP (not shown), suggesting that Treg rebound may only occur in certain compartments, and may be partly explained by Treg redistribution. Moreover, consistent with earlier reports (25,29), lymphocytes isolated on day 15 from the SP or pancreas of treated, prediabetic mice exhibited inflammatory cytokine production with 18.7 ± 1.9% IFNγ- and 23.4 ± 7.5% TNFα-producing cells from the pancreas and 19.1 ± 2.5% IFNγ- producing cells from the SP (Supplementary Fig. 5). Circulating cells of αCD3-treated mice showed little production of these cytokines. Lymphocytes from untreated mice showed very little production of these cytokines from any of the tissues examined. Overall, this extended longitudinal evaluation in tissues reveals the complex effects of αCD3 treatment on Tregs, which vary in different tissues and include depletion, rebound, and redistribution. Moreover, αCD3 treatment does not appear to open the Treg compartment for extensive periods of time in the pancreas or lymphoid tissues, suggesting that this treatment alone may not promote robust polyclonal Treg engraftment.
A Single Dose of CyP to αCD3 Therapy Depletes Rebounding Host Tregs
We have previously shown that robust donor Treg engraftment requires not only creating peripheral space but also lessening competition from rebounding host Tregs (20). Because αCD3 treatment induces only an early, temporary Treg depletion that is followed by rebound, achieving optimal engraftment of autologous donor Tregs may require further manipulation to control rebounding populations. CyP is known to eliminate proliferating cells in response to alloantigen but also depletes Tregs because of their higher proliferation rates compared with naïve T cells (30,31). To determine whether CyP could effectively deplete rapidly rebounding host Tregs post-αCD3, a single CyP injection was given at day 10 (Fig. 1A). CyP after αCD3 significantly decreased host Tregs by 62% (day 11, two-way ANOVA, P = 0.0215) for ∼5 days post-CyP in blood (Fig. 1E, dashed lines), thus creating a more supportive Treg environment (Fig. 1E, shaded area). CyP also depleted CD4+ and CD8+ T cells, which remained lower compared with αCD3 alone 39 days after the first αCD3 dose (Fig. 1B–D, dashed lines). After CyP, a substantial increase in the proportion of CD4+Foxp3+ cells in the CD4+ T-cell compartment was observed during days 18–32 (Fig. 1F, dashed line, shaded area 2). Moreover, CyP after αCD3 decreased rebounding host Tregs by 21–82% in SP, LN, PP, and IEL (Supplementary Figs. 2 and 3). In contrast, host Tregs showed a modest increase in pancreas and pLN after CyP (Fig. 2F and G). Overall, these findings highlight that a single dose of CyP after αCD3 can effectively deplete rebounding host Tregs, which are expected to compete with infused donor Tregs, and can be used to synergize with αCD3 therapy to open the Treg compartment to promote robust Treg engraftment.
A Combinatorial Regimen of αCD3 and CyP Promotes Robust Engraftment of Polyclonal, Autologous Donor Tregs in NOD Mice
We tested whether these combinatorial therapies (Fig. 3A) supported engraftment of polyclonal, autologous donor Tregs in 5- to 6-week-old female NOD mice. Despite robust T-cell depletion with αCD3 alone, donor congenic Thy1.1+ NOD Tregs were minimally detected 1–4 weeks post-infusion in blood (Fig. 3B and C and Supplementary Table 1, αCD3+Treg), SP, LN, pLN, and pancreas (Fig. 3D). The addition of IL-2 support with a short course of IAC treatment after αCD3 enhanced engraftment during treatment (αCD3+Treg+IAC), but by the end of follow-up, engraftment was no better than αCD3 alone. However, a single dose of CyP after αCD3 (αCD3+CyP+Treg), which depletes rebounding host Tregs (Fig. 1E, dashed line, shaded area), resulted in a 43-fold increase in donor Treg engraftment compared with αCD3; the addition of IAC further enhanced engraftment by several fold (αCD3+CyP+Treg+IAC) (Fig. 3B and C and Supplementary Table 1). The significant increase in the proportion of host CD4+Foxp3+ cells in the CD4+ T-cell compartment during the rebound phase after αCD3+CyP (Fig. 1F, shaded area 2) did not inhibit donor Treg engraftment, which was robust (Fig. 3D). Thus, key to robust engraftment is to create space that minimizes host Treg competition at the time of Treg infusion. The addition of CyP and IAC resulted in the greatest increase of the total CD4+Foxp3+ compartment compared with all other regimens tested (Fig. 3F), and was accompanied by an increased proportion of CD4+Foxp3+ cells in the CD4+ T-cell compartment (Fig. 3G). While the total Treg compartment returned to pretreatment levels at the end of the 39-day follow-up (Fig. 3F and G), donor Tregs comprised 8–25% of the Treg compartment in mice given αCD3 and CyP with or without IAC (Fig. 3B and D). Importantly, congenic Thy1.1+ donor Tregs maintained a phenotype similar to host Tregs in all treatments with comparable Foxp3/CD25 expression in treated mice (Supplementary Fig. 6A).
We then treated late, prediabetic NOD mice aged 16–22 weeks with our various immunomodulation strategies followed by infusion of congenic, autologous Thy1.1+ NOD Tregs and examined Treg engraftment. Similar to the young, prediabetic NOD mice, substantial engraftment occurred in mice that received αCD3 and CyP with or without IAC in the peripheral blood and SP, LN, pLN, and pancreas (Supplementary Fig. 7A, B, and E). Again, optimal Treg engraftment was observed in the αCD3+CyP+ Treg+IAC group, with a 53-fold increase in engraftment compared with αCD3 alone (day 18 after the start of αCD3), and led to the greatest increase in the total CD4+Foxp3+ population (Supplementary Fig. 7A–C). Similarly, we observed a decline in engraftment, but donor Tregs comprised 25–30% of the Treg compartment at the end of follow-up in mice given αCD3/CyP (Supplementary Fig. 7A). Collectively, our results demonstrate a new therapeutic synergy between αCD3 and CyP in promoting engraftment of polyclonal, autologous Tregs.
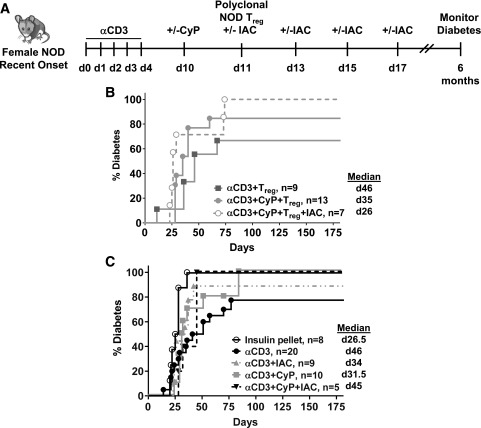
A Combinational Regimen of CD3, CyP, and Polyclonal, Autologous Donor Tregs Fails to Induce Diabetes Remission in Female NOD Mice
We then examined whether diabetes remission could be induced by combinational regimens themselves or in combination with infusion of polyclonal, autologous Tregs (Fig. 4A). Although our combinatorial regimen was very effective at promoting engraftment in prediabetic NOD mice, long-term diabetes remission was not achieved in recently diagnosed NOD mice treated with immunomodulation alone or in combination with polyclonal Tregs (Fig. 4B and C). We found that in mice treated with the αCD3+CyP+polyclonal Treg±IAC regimen, the time to disease relapse significantly correlated with the levels of donor Tregs in blood (r = 0.72223, P = 0.028). Therefore, we examined Treg engraftment in blood and tissues; donor, congenic Thy1.1+ NOD Tregs were readily detected in blood and SP, LN, pLN, and pancreas 4 weeks post-infusion (αCD3+CyP+Treg+IAC) (Fig. 5A and B and Supplementary Table 2), and donor Tregs had a similar level of Foxp3/CD25 expression as host Tregs in blood and tissues (Supplementary Fig. 6B). However, there was a decrease (2.0–2.6-fold) in the level of Treg engraftment observed in recent-onset mice compared with late prediabetic NOD mice at the end of follow-up (Supplementary Fig. 7E and F), but overall engraftment with αCD3/CyP treatment was still robust compared with prediabetic mice treated with αCD3 alone (Fig. 3A–C and Supplementary Fig. 7A and B). Several studies showed that inflammatory environments promote unstable Tregs, termed ex-Tregs, which become pathogenic (32–34). However, the observed lower engraftment was not explained by a loss of Foxp3 expression in donor Treg populations (CD4+Foxp3+Thy1.1+ cells), as there were very few Thy1.1+Foxp3− cells in blood, SP, LN, pLN, or pancreas (Fig. 5C). Lower engraftment was not due to a failure of αCD3 to effectively deplete T cells, as CD4, CD8, and CD4+Foxp3+ cells were decreased post-αCD3 (Fig. 5D and E). Engraftment was not impacted by rebounding host Tregs, as CyP was very effective at depleting rebounding cells post-αCD3 (Fig. 5E, shaded area, and Fig. 5F, shaded area 1). However, unlike in prediabetic mice in which host Tregs recovered after CyP (Fig. 1F, shaded area 2), such a recovery was not observed in recent-onset mice (Fig. 5F, shaded area 2). Together, these results suggest that in recent-onset mice, despite initial insulin treatment, the host may not fully support the Treg compartment, which could represent an obstacle to successful Treg therapy with polyclonal Tregs.
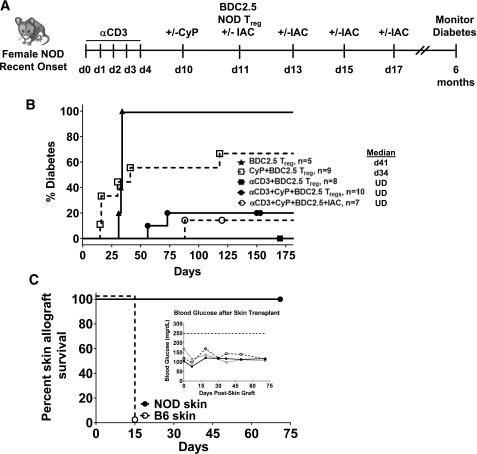
Figure 4.
Diabetes remission after immunomodulation and infusion of polyclonal Tregs in recently diabetic NOD mice. A: Experimental scheme. B: Percentage of diabetic mice after αCD3, CyP, and/or IAC immunomodulation and adoptive transfer of polyclonal Tregs isolated from Thy1.1 congenic NOD mice into recently diabetic NOD mice. C: Percentage of diabetic mice after αCD3, CyP, and/or IAC. Diabetic mice were initially given a single insulin pellet at the start of αCD3 treatment to control diabetes. Urine and blood glucose levels were monitored for 6 months after the start of αCD3 treatment. Median values are indicated to the right of the legends. d, day.
Figure 5.
Donor Treg engraftment after immunomodulation in recent diabetic NOD mice. A: Percentage of Thy1.1+ donor NOD Treg in CD4+Foxp3+ in peripheral blood (PB). B: 4–8 weeks in the SP, LN, pLN, and pancreas (Pan). C: Foxp3 and Thy1.1 staining in gated CD4 T cells day 22–39 after Treg infusion in recent-onset diabetic NOD mice receiving αCD3+CyP+Treg+IAC. D: Percent positive Thy1.2+ total T cells, CD4+Foxp3− T cells, or CD8 T cells in the peripheral blood of recent-onset NOD mice receiving αCD3 treatment. *P < 0.0001; @P < 0.01, one-way ANOVA followed by Dunnett multiple comparison test compared with day 0. E: Percentage of CD4+Foxp3+ in gated CD45+ population. F: Percentage of CD4+Foxp3+ in the gated CD4 T cells with αCD3 (solid) or αCD3+CyP (dashed). Diabetic NOD mice were maintained with insulin pellets to control blood glucose. n = 2–6 mice per group. In E, the shaded area indicates the decrease in CD4+Foxp3+ host Tregs after single CyP injection after αCD3 treatment. In F, shaded area 1 indicates an increase in CD4+Foxp3+ cells among total in the gated CD4 T cells after CD3 alone, and shaded area 2 indicates an increase in CD4+Foxp3+ cells in the gated CD4 T cells after CD3+CyP. d, day.
Combinational Therapies That Include Antigen-Specific Tregs Lead to Durable Remission in Newly Diagnosed Diabetic NOD Mice
Selection of therapeutic Tregs by antigen could potentially overcome the less supportive environment observed in recent-onset mice. Moreover, antigen-specific Tregs are known to be more effective than polyclonal Tregs at regulating diabetes and responses to alloantigen (35–37). Therefore, we tested whether the infusion of islet-specific Tregs together with αCD3 and/or CyP immunomodulation could lead to durable diabetes remission in recently diabetic NOD mice (Fig. 6A). In sharp contrast to polyclonal Tregs, infusion of islet-specific (chromogranin A) Tregs isolated from BDC2.5 TCR transgenic NOD mice after immunomodulation induced durable diabetes remission (Fig. 6B). This remission was observed after immunomodulation with αCD3 alone or in combination with CyP with or without a short course of IAC. All the NOD mice receiving an islet-specific Treg infusion after αCD3 alone went into durable remission (6 months). αCD3 prior to BDC2.5 Treg infusion is critical for achieving durable remission as infusion of BDC2.5 Tregs after CyP injection resulted in only 38% of mice experiencing long-term remission, and infusion of equal numbers of BDC2.5 Tregs without any prior immunomodulation failed to reverse diabetes (Fig. 6B). These data support that manipulation of the host immune system is necessary to achieve a therapeutic benefit with adoptive Treg therapy. Antigen specificity of infused Tregs is critical, since equal numbers of polyclonal Tregs failed to achieve durable remission in most mice (Fig. 4B). Importantly, the lack of diabetes relapse with αCD3+BDC2.5 Treg therapy was not due to loss of effective immune responses. Fully allogeneic B6 skin grafts placed 6 months after the start of therapy on mice given αCD3+BDC2.5 Treg therapy were all rejected, whereas syngeneic NOD grafts were maintained (Fig. 6C), and mice continued to be diabetes free at end of the 70-day follow-up (Fig. 6C, inset). Therefore, the destructive autoimmune response had been reset without long-term immunosuppression and these mice had a fully competent immune system.
Figure 6.
Diabetes remission after immunomodulation and infusion of antigen-specific Tregs in recently diabetic NOD mice. A: Experimental scheme. B: Percentage of diabetic mice after αCD3, CyP, and/or IAC immunomodulation and adoptive transfer of antigen-specific Tregs isolated from BDC2.5 TCR NOD mice into recently diabetic NOD mice. Diabetic mice were initially given a single insulin pellet at the start of αCD3 treatment to control diabetes. Urine and blood glucose levels were monitored for 6 months after the start of αCD3 treatment. Median values are indicated to the right of the legends. C: Percentage of skin graft survival of NOD and B6 skin placed 6 months after the start of αCD3 and BDC2.5 Treg infusion. Mice were monitored for skin rejection and diabetes development for 70 days. Inset shows blood glucose values for the transplanted mice. Survival curves were subjected to Mantel-Cox log-rank test. The P value is indicated on the graph. d, day; UD, undefined.
Combinational Regimen of αCD3 and Antigen-Specific Tregs Results in Robust, Long-Term Engraftment Within Islets
We determined the level of islet-specific Treg engraftment after immunomodulation. BDC2.5 Treg engraftment was assessed from isolated islets 7 days post–Treg infusion (day 18 after start of αCD3) and up to 90 days post-αCD3 or CyP treatment or in unmanipulated recent-onset NOD mice. We detected the highest engraftment in pancreatic islets with αCD3 treatment and persisted up to 90 days compared with CyP treatment; mice that did not receive immunomodulation had the lowest engraftment (Fig. 7A and C). These BDC2.5 tetramer-positive cells expressed high levels of CD25 (Fig. 7B). Importantly, this level of BDC2.5 Treg engraftment in the pancreas of mice 40 days post–αCD3 treatment was similar to the level of polyclonal Treg engraftment in the pancreas when αCD3, CyP, and IAC were given (Fig. 5B). There was a significant correlation between the level of BDC2.5 Treg engraftment and percentage of total CD4+Foxp3+ T cells within islets (Fig. 7D). BDC2.5 Tregs were detected in pLN or nondraining LNs, but much less compared with islets (Fig. 7E), and this was similar to the level of engraftment observed with polyclonal, autologous Tregs after αCD3 treatment alone (Fig. 3D). Moreover, tetramer-positive CD4+Foxp3− T cells were minimally detected, suggesting that BDC2.5 Tregs are stable within these tissues (Fig. 7E and F). Collectively, our data suggest that antigen-specific Tregs may be capable of better engraftment specifically in the pancreatic islets where their target antigen is present, and this may explain the therapeutic efficacy observed.
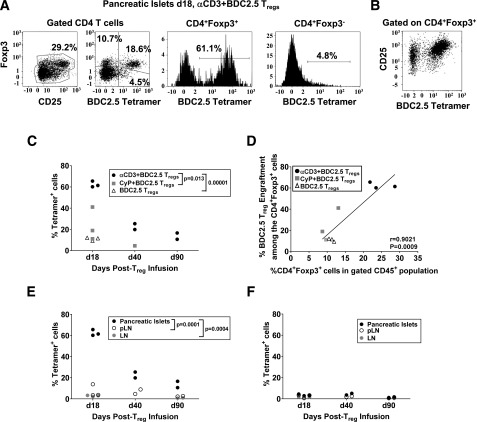
Figure 7.
Antigen-specific donor Treg engraftment after immunomodulation in recent diabetic NOD mice. A: BDC2.5 tetramer staining of CD45+ cells from isolated pancreatic islets of recent-onset NOD mice subjected to immunomodulation and adoptive transfer of BDC2.5 Tregs of gated CD4+Foxp3+ and CD4+Foxp3− cells 7 days after Treg infusion (day 18). B: Representative staining of CD25 and BDC2.5 tetramer on gated CD4+Foxp3+ cells in the pancreatic islets 7 days after Treg infusion after αCD3 treatment. C: Percentage of BDC2.5 tetramer+ cells among the CD4+Foxp3+ cells from pancreatic islets 18, 40, and 90 days after αCD3 or CyP treatment or in mice left untreated. D: Correlation analysis between the level of BDC2.5 Treg engraftment among the gated CD4+Foxp3+ cells and percentage of total CD4+Foxp3+ in the gated CD45+ population within islets on day 18 after the start of αCD3 treatment. Percentage of BDC2.5 tetramer+ cells among the CD4+Foxp3+ cells (E) or CD4+Foxp3− cells (F) in the pancreatic islets, pLN, or other LN 18, 40, and 90 days post–Treg infusion after αCD3 treatment. P values are indicated in C and E following unpaired Student t test. d, day.
Discussion
Our previous work focused on understanding critical factors required for the generation/homeostasis of thymic Tregs and demonstrated the importance of host environment as a key determinant of long-term Treg engraftment and therapeutic efficacy in therapies involving Treg infusion (18–20,22,38,39). Our data from this study demonstrate how these requirements also apply for a successful outcome of a Treg-based immunomodulatory regimen in a preclinical T1D model. Although therapeutic success in NOD mice does not guarantee a translation to patient benefit, it is nonetheless important to conduct preclinical testing, when feasible, before starting clinical experimentation. The NOD mouse presents critical similarities at genetic and immunological levels with the human diseases and, despite its limitations, is the most widely used and accepted experimental model. Our findings provide the rationale for the design of future clinical trials to test the efficacy of Treg-based therapies and highlight the importance of incorporating immunomodulation prior to Treg infusion and the key role of antigen-specific Tregs to treat islet autoimmunity.
Our experimental studies used the intact αCD3ε antibody for T-cell debulking because it is more efficient in this setting than the F(ab′)2 form (25–27). Studies with a humanized CD3 NOD mouse model (NOD-huαCD3) (29) demonstrated that a single injection of intact αCD3 antibody (clone 2C11, 5 μg) led to a higher percentage of CD4 and CD8 cell apoptosis accompanied by greater depletion of CD4 T cells and a higher rebound of CD8 T cells compared with nonmitogenic F(ab′)2 fragments (50 µg). Similar results on T-cell apoptosis, depletion, and rebound were observed when a single dose of mitogenic (YTH12.5, 2 μg) and nonmitogenic (otelixizumab, 100 μg) human CD3ε antibodies were used. In the context of our studies examining the in vivo environment to support persistent Treg engraftment, a 5-day course of a higher dose of intact αCD3 (50 µg) led to significant depletion of CD4 and CD8 T cells, which was accompanied by slow recovery, while the Tregs were depleted early after αCD3 treatment with a rapid recovery. However, intact αCD3 treatment allowed only limited Treg engraftment after T-cell debulking and was likely due to competition with rapidly rebounding host Tregs. In fact, when the recovering host Treg population was decreased again with CyP just before Treg infusion, robust engraftment of polyclonal Tregs occurred. This level of donor Treg engraftment 4 weeks post–Treg infusion in prediabetic NOD is similar to what was observed with harsher ablative conditioning in nonautoimmune C57BL/6 mice (20), yet relying on short courses of clinically relevant agents. Improving Treg engraftment could have increased frequencies of disease-relevant Tregs and improve therapeutic benefit, but durable remission was not achieved despite robust polyclonal Treg engraftment. However, we observed a strong correlation with the level of polyclonal Treg engraftment after immunomodulation and clinical outcome in recent-onset NOD mice, suggesting that increased numbers of adoptively transferred polyclonal, autologous Tregs, perhaps through ex vivo expansion, could lead to improved benefit. This will require further investigation but could be more easily implemented in a clinical setting given that it is more challenging to use antigen-specific Tregs. Additionally, the positive effects of αCD3 in a recent prevention clinical trial (40), and our new data on engraftment in late prediabetic mice, support the hypothesis that this regimen could be tested in this preclinical model with polyclonal and antigen-specific Tregs.
Antigen-based therapies have been used in experimental models and clinical trials with mixed results. However, antigens could be helpful to drive the selection/expansion of infused antigen-specific Tregs. Indeed, diabetes was prevented in adoptive transfer studies with islet-specific NOD Tregs into NOD mice (NOD.Rag−/−, NOD TCRα−/−, or NOD.CD28−/−) that lack either T cells or Tregs, respectively (35,37,41,42). Critically, these models resemble IL-2Rβ−/− mice, in that there is natural space with limited host Treg competition. A major benefit of using antigen-specific Tregs is that these Tregs will likely act only where antigen is present, thereby providing local immunotherapy without impacting the remainder of the immune system. Here, we found that our combinational regimens allow engraftment of polyclonal Tregs throughout the immune compartments and in tissues, but the presence of the target antigen in the pancreas led to islet-specific Tregs being found largely within islets after infusion. Both αCD3 or αCD3+CyP treatments prior to islet-specific Treg infusion led to durable remission; however, αCD3 alone with islet-specific Tregs was sufficient. The level of engraftment in these mice was similar to what was observed in mice treated with αCD3+CyP+polyclonal Tregs+IAC, but now islets also include disease-relevant Tregs in the pancreatic microenvironment. These NOD mice were still fully capable of rejecting allogeneic skin transplants without breaking the islet autoimmune tolerance established by our Treg immunotherapy with islet-specific Tregs. Importantly, by creating this supportive environment for donor Tregs, durable diabetes remission was achieved using 20-fold less numbers of antigen-specific Tregs in our studies compared with a previous study that used in vitro expanded BDC2.5 Tregs in unmanipulated NOD mice (35). Like others (25,29), we found that intact αCD3 antibody led to IFNγ and TNFα production, but this did not hinder Treg engraftment or the induction of durable diabetes remission with antigen-specific Treg infusion.
Challenges with antigen-specific Treg-based therapies still center on the availability of a sufficient number of disease-relevant Tregs and whether pools of antigen specificities, and what specificities, will be needed to achieve therapeutic benefit, given that autoimmunity is likely to be more heterogeneous in patients. The discovery of hybrid insulin peptides and other modified peptides highlights the importance of further investigation on the antigen specificities needed for therapeutic gains (43,44). Although current methodologies are limited in terms of the ability to isolate and expand a sufficient number of autologous antigen-specific Tregs, new strategies aimed at generating large numbers of antigen-specific Tregs are being explored. These include lentiviral TCR gene transfer into expanded polyclonal Tregs, Foxp3 gene editing into antigen-specific CD4+ T cells, and conversion of effector T cells into T regulatory–like cells by CRISPR/Cas9-mediated integration of a Foxp3 transgene, and open the possibility of overcoming this limitation (45–47). Our work sets the conceptual framework and a key innovative approach that was aimed at addressing a critical and unmet need in the clinics, the need for new therapeutic protocols for T1D treatment that will enhance the efficacy and durability of Treg-based cell therapies.
Supplementary Material
Article Information
Acknowledgments. The authors thank Oliver Umland of the Diabetes Research Institution Flow Cytometry Core.
Funding. This work was supported by the American Diabetes Association (Junior Faculty grant 07-09-JF-06 to A.L.B.), the National Institute of Allergy and Infectious Diseases (High Priority Short-term grant 1R56-AI-101278-01A1 to A.L.B.), the University of Miami Miller School of Medicine (Dean’s National Institutes of Health Bridge Program Award to A.L.B.), and the Diabetes Research Institute Foundation (to A.L.B.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.C.-K. performed experiments and provided intellectual feedback. S.M., A.S., J.R., and C.V. performed experiments. A.P. provided intellectual feedback and reviewed the manuscript. A.L.B. designed and conceived the studies, performed experiments, analyzed data, and wrote the manuscript. A.L.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented at the Immunology of Diabetes Society Congress 2018, London, U.K., 25–29 October 2018; the Keystone Symposia: Immune Regulation in Autoimmunity and Cancer, Whistler, British Columbia, Canada, 26–30 March 2017; and the Keystone Symposia: Inflammatory Diseases: Recent Advances in Basic and Translational Research and Therapeutic Treatments, Vancouver, British Columbia, Canada, 17–22 January 2014.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0061/-/DC1.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155:1151–1164 [PubMed] [Google Scholar]
- 2.Shevach EM, McHugh RS, Thornton AM, Piccirillo C, Natarajan K, Margulies DH. Control of autoimmunity by regulatory T cells. Adv Exp Med Biol 2001;490:21–32 [DOI] [PubMed] [Google Scholar]
- 3.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med 2002;195:1641–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graca L, Thompson S, Lin CY, Adams E, Cobbold SP, Waldmann H. Both CD4(+)CD25(+) and CD4(+)CD25(-) regulatory cells mediate dominant transplantation tolerance. J Immunol 2002;168:5558–5565 [DOI] [PubMed] [Google Scholar]
- 5.Hara M, Kingsley CI, Niimi M, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol 2001;166:3789–3796 [DOI] [PubMed] [Google Scholar]
- 6.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol 2002;168:1080–1086 [DOI] [PubMed] [Google Scholar]
- 7.Juvet SC, Whatcott AG, Bushell AR, Wood KJ. Harnessing regulatory T cells for clinical use in transplantation: the end of the beginning. Am J Transplant 2014;14:750–763 [DOI] [PubMed] [Google Scholar]
- 8.Marek-Trzonkowska N, Myśliwec M, Siebert J, Trzonkowski P. Clinical application of regulatory T cells in type 1 diabetes. Pediatr Diabetes 2013;14:322–332 [DOI] [PubMed] [Google Scholar]
- 9.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, et al. Administration of CD4+CD25highCD127- regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care 2012;35:1817–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, et al. Repetitive administration of CD4+CD25highCD127− T regulatory cells prolongs survival of pancreatic islets in type 1 diabetes in children. Oral session (IT-OR01, Human Immunology in Clinical Trials) at the 73rd Scientific Sessions of the American Diabetes Association, 21–25 June 2013, at the McCormick Place Convention Center, Chicago, IL.
- 11.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 2011;117:1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earle KE, Tang Q, Zhou X, et al. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol 2005;115:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hippen KL, Merkel SC, Schirm DK, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med 2011;3:83ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putnam AL, Brusko TM, Lee MR, et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes 2009;58:652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trzonkowski P, Szaryńska M, Myśliwska J, Myśliwski A. Ex vivo expansion of CD4(+)CD25(+) T regulatory cells for immunosuppressive therapy. Cytometry A 2009;75:175–188 [DOI] [PubMed] [Google Scholar]
- 16.Trzonkowski P, Bieniaszewska M, Juścińska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol 2009;133:22–26 [DOI] [PubMed] [Google Scholar]
- 17.Marek-Trzonkowska N, Myśliwiec M, Dobyszuk A, et al. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127- regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol 2014;153:23–30 [DOI] [PubMed] [Google Scholar]
- 18.Adeegbe D, Bayer AL, Levy RB, Malek TR. Cutting edge: allogeneic CD4+CD25+Foxp3+ T regulatory cells suppress autoimmunity while establishing transplantation tolerance. J Immunol 2006;176:7149–7153 [DOI] [PubMed] [Google Scholar]
- 19.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol 2007;178:4062–4071 [DOI] [PubMed] [Google Scholar]
- 20.Cabello-Kindelan C, de la Barrera A, Malek TR, Bayer AL. In vivo environment necessary to support transplanted donor mouse T regulatory cells. Am J Transplant 2014;14:1032–1045 [DOI] [PubMed] [Google Scholar]
- 21.Cabello-Kindelan C, Mackey S, Bayer AL. Adoptive T regulatory cell therapy for tolerance induction. Curr Transplant Rep 2015;2:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period. J Exp Med 2005;201:769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billingham RE, Medawar PB. The technique of free skin grafting in mammals. J Exp Biol 1951;28:385–402 [Google Scholar]
- 24.Berney T, Molano RD, Cattan P, et al. Endotoxin-mediated delayed islet graft function is associated with increased intra-islet cytokine production and islet cell apoptosis. Transplantation 2001;71:125–132 [DOI] [PubMed] [Google Scholar]
- 25.Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol 1997;158:2947–2954 [PubMed] [Google Scholar]
- 26.Herold KC, Bluestone JA, Montag AG, et al. Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes 1992;41:385–391 [DOI] [PubMed] [Google Scholar]
- 27.Yang W, Hussain S, Mi QS, Santamaria P, Delovitch TL. Perturbed homeostasis of peripheral T cells elicits decreased susceptibility to anti-CD3-induced apoptosis in prediabetic nonobese diabetic mice. J Immunol 2004;173:4407–4416 [DOI] [PubMed] [Google Scholar]
- 28.Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol 2011;187:2015–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhn C, You S, Valette F, et al. Human CD3 transgenic mice: preclinical testing of antibodies promoting immune tolerance. Sci Transl Med 2011;3:68ra10. [DOI] [PubMed] [Google Scholar]
- 30.Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+ Foxp3+ regulatory T cells. J Immunol 2006;177:6603–6612 [DOI] [PubMed] [Google Scholar]
- 31.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005;105:2862–2868 [DOI] [PubMed] [Google Scholar]
- 32.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 2013;39:949–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komatsu N, Okamoto K, Sawa S, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014;20:62–68 [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Bailey-Bucktrout SL, Jeker LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 2009;10:1000–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 2004;199:1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang Q, Henriksen KJ, Boden EK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol 2003;171:3348–3352 [DOI] [PubMed] [Google Scholar]
- 37.Yamazaki S, Inaba K, Tarbell KV, Steinman RM. Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity. Immunol Rev 2006;212:314–329 [DOI] [PubMed] [Google Scholar]
- 38.Bayer AL, Chirinos J, Cabello C, et al. Expansion of a restricted residual host T reg-cell repertoire is dependent on IL-2 following experimental autologous hematopoietic stem transplantation. Eur J Immunol 2011;41:3467–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayer AL, Jones M, Chirinos J, et al. Host CD4+CD25+ T cells can expand and comprise a major component of the Treg compartment after experimental HCT. Blood 2009;113:733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herold KC, Bundy BN, Long SA, et al.; Type 1 Diabetes TrialNet Study Group . An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, Bluestone JA. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J Immunol 2005;175:3053–3059 [DOI] [PubMed] [Google Scholar]
- 42.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med 2004;199:1467–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delong T, Wiles TA, Baker RL, et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 2016;351:711–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiles TA, Powell R, Michel CR, et al. Identification of hybrid insulin peptides (HIPs) in mouse and human islets by mass spectrometry. J Proteome Res 2019;18:814–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brusko TM, Koya RC, Zhu S, et al. Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PLoS One 2010;5:e11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Honaker Y, Xiang Y, Fisher L, et al. Conversion of T-effector cells to immunosuppressive T-regulatory-like cells by CRISPR/Cas9-mediated integration of a FOXP3 transgene. Blood 2018;132:3490 [Google Scholar]
- 47.Xiang Y, Sommer K, Honaker Y, et al. Gene-editing of FOXP3 in antigen-specific CD4+ T cells for restoration of immune tolerance in type 1 diabetes. Diabetes 2019;68(Suppl. 1):1740 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.