Abstract
There is strong evidence for the positive physical health outcomes of physical conditioning (athletic training. But there is a dearth of data on the impact of exercise on cognition, particularly in the adolescent age group. Further, most of the studies done on this topic are mainly acute in nature, and few that have seen long term effect of exercise have very rarely used objective measures such as event-related potentials. Hence, the present study was conceived to compare cognition in athletes (individual who have undergone long term physical activity) and non-athletes. We designed a cross-sectional comparative study involving apparently healthy volunteer boys in the age group of 10-19 years-non-athletes (n = 30) and athletes (n = 30). Paper pencil tests such as letter cancellation test, auditory and visual recognition reaction time, trail making test (A and B) were recorded along with auditory event-related potentials (N100, P200, N200, and P300). Data were analyzed using an unpaired t-test and Mann-Whitney U test according to the data distribution. Athletes completed letter cancellation task and trail making test faster than non-athletes. Athletes visual and auditory reaction time were lesser. Athletes had reduced latency and higher amplitude of auditory event-related potentials (N100, P200, N200, and P300) as compared to non-athletes. Hence, we conclude that athletic level physical training has a beneficial role in the executive cognitive domain among adolescents.
Keywords: P300, cognition, N1, N2/N200, P2/P200, reaction time, trail making test, children, evoked potentials
Introduction
About one-fifth of India’s population is in the adolescent age group (10-19 years) [1]. In recent years, there has been a paramount shift in the lifestyle of children/adolescence towards sedentary habits such as watching television or spending time on computers or mobile [1,2]. Physical activity is a known lifestyle factor that increases physical and mental health throughout life [3], and adolescence is the age when lifelong behaviors are established. There is strong evidence for the positive physical health outcomes of physical conditioning (athletic training), but there is a dearth of data on the impact of exercise on mental health or cognition [4].
A recent systematic review on physical activity and cognition in children has brought out that the number of studies correlating sports activities with cognition are few, particularly in adolescent age group and studies lack in defining which cognitive functions are influenced by sports activities [2]. Based on executive function hypothesis, it is proposed that, out of various cognitive domains, executive function is in particular, is influenced by sports activities/exercise [5]. Executive function comprises scanning, attention, retrieval of stored information, mental flexibility, and working memory [6,7]. These domains could be assessed by paper and pencil tests such as letter cancellation test, digit span test, and trial making test as proposed by Lezak et al. [8] or by event-related potential (ERP) recordings that have excellent time resolution. Dichter GS et al., claimed that for correct interpretation of changes in event related potential (P300), it has to be accompanied by measures of executive function such as trail making test [9].
Many studies which have reported the positive impact of exercise on cognition have studied only the acute effect of exercise, and documented general arousal as the reason for the improved cognition in those subjects [10,11]. Among long-term studies in students, few studies have found an association between physical activity and improved concentration and executive functions [12-14] while others did not find any significant association [15,16]. Conflicting reports on impact of long-term physical activity on cognition, paucity of the data on the influence of exercise in adolescents in Indian scenario and the scarcity of using an objective measure such as ERP to study cognition has led us to conceive this cross-sectional comparative study in adolescent age group to compare cognition using auditory event-related potentials and executive function tests between athletes and non-athletes.
Material and methods
Study design
This was a cross-sectional collaborative study conducted between Department of Physiology, JIPMER, Puducherry, India, and CBSE board residential school in Puducherry. Study was commenced after obtaining approval from the Institute ethics committee for human studies (No. JIP/IEC/2013/3/177).
Participants
Schoolboys aged between 10-19 years studying in a CBSE school in Puducherry were recruited for the study. Students with a history of cardiovascular, respiratory, or organic disorder, which prevents subjects from doing maximal exercise, or on any drugs that affect cognitive test were excluded from the study. We obtained informed written consent from the guardians/parents, and written assent from the boys who had met the inclusion criteria. By convenience sampling, thirty boys representing their school at state or national or international level aerobic sports and have undergone physical conditioning for at least one year, were recruited as athletes and 30 age-matched non-athlete students (not participated in any inter-school athletic events for at least one year and only participating in recreational sports activities) were recruited as controls.
Parameters measured
Boys were asked to report to the Department of Physiology, JIPMER, and the following parameters were recorded. Anthropometric parameters such as height (cm) and weight (kg) were measured, and body mass index calculated. Blood pressure (BP) and heart rate (HR) were measured after 10 minutes of rest in sitting position [17] using automated blood pressure monitor (Omron Healthcare Co., Ltd., Kyoto, Japan).
Investigators recording the parameters were blinded to the allocation of the participants. The participants were asked to report to the school examination room at 9.00 a.m., 2 h after having a light breakfast, and after emptying the bladder. Participants were asked to avoid any caffeinated beverage, and not to undergo any physical activity training on the day of the trial and the following cognitive tests were measured.
Neurocognitive paper and pencil tests
a. Two Target Letter Cancellation Test (LCT): Boys were presented with six 52-character rows of letters of the English alphabet and was instructed to cancel out randomly placed letters ‘E’ and ‘C’. The score was the time taken (in seconds) by the subject to complete the task [18]. b. Trail Making Test A and B (TTA & TTB): It has two parts, Part A - The subject is instructed to draw a straight line to connect randomly placed 25 consecutive numbered circles. The score is the time taken (in seconds) by the subject to complete the task. Part B: In this test, the subject was instructed to connect randomly placed 25 numbered and lettered circles by alternating between the two sequences. The score was the total time taken (in seconds) to complete the task [19,20].
Recognition Reaction time
Recognition reaction time (RRT) to auditory (beep sound) and visual signals (red light) was recorded using an apparatus supplied by Anand Agencies (Pune, India, accuracy - 1 ms) in a quiet room with ambient temperature at 24°C (± 2°C). After three practice sessions, auditory reaction time (ART) was recorded by presenting auditory beep stimuli of two different tones (memory stimulus and distractor stimulus), and by asking the participant to respond to the higher tone (memory stimulus) by releasing the switch. Visual reaction time (VRT) was recorded by presenting red (memory stimulus) and green light (distractor stimulus), and by asking the participant to respond to the red light by releasing the switch. Ten trials were recorded for each RRT; the two most outlying values were excluded, and the middle six values were used to calculate the mean reaction time (ms).
Cognitive event-related evoked potentials
We followed the recommendations of the International federation of Clinical Neurophysiology [21] and modified the protocol for Indian Scenario [22] for recording Cognitive event-related potentials using Neuropack M1 EP/EMG measuring system MEB-9200 J/K (Nihon Kohden Corporation, Shinjuku ku Tokyo, Japan). Boys were instructed to come with shampoo cleaned oil-free scalp, and their ear wax was ruled out. We used Ag-AgCl cup electrodes and they were placed according to the 10-20 international system. The scalp was cleaned again using Nuprep gel, and ten 20 gel was used to prevent the air interface between electrode, and scalp. The active electrode was placed at Cz position; reference electrodes were placed over both the mastoids and the ground electrode was at Fz near to the hairline in the forehead. Electrodes were secured using micropore. The impedance of the electrodes was kept ≤ 2 kΩ, and bandpass filter was kept at 0.1 Hz and 50 Hz. Boys were explained about the procedure to alleviate apprehension. Ten minutes rest was given before the procedure. Standard auditory oddball paradigm technique was used to assess the cognitive evoked potentials. Auditory stimulus was given binaurally through a headphone (intensity - 40 dB, stimulation rate - 0.5 Hz, number of stimuli - 30) with the “tone” as the target or rare stimulus (Frequency - 2000 Hz) and “click” as nontarget or frequent stimulus (Frequency - 1000 Hz, duration - 0.1 ms). The rare stimuli were applied randomly, and the percentage of rare stimuli was set at 20% and frequent stimuli at 80% of random. The participants were asked to relax keeping their eyes open and fixed at a point to avoid alpha waves. Their task was to concentrate on the rare stimulus. The signals were analyzed using Event Related Potentials Software (QP-955BK). Negative waves at 100 ms (N1) and 200 ms (N2) and positive waves at 200 ms (P2) and 300 ms (P3 or P300) and the amplitude of N1-P2, N2 and P3 were recorded.
Statistical analysis
Data were analyzed using IBM Statistical Package for Social Sciences (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp). Time taken to complete letter cancellation test, Trail making test A and B were normally distributed; they were expressed as Mean ± Standard deviation and comparison between groups were done using the unpaired t-test. Auditory event-related potential’s latency, and amplitude were non-normally distributed; they were expressed as Median (Interquartile Range) and comparison between groups were done using the Mann-Whitney U test. P value < 0.05 was considered to be statistically significant.
Results
Both the groups comprise adolescent boys in the age group of 10 to 19 years. BMI is comparable between the groups (non-athletes (18.51 ± 1.19), athletes (19.05 ± 1.83), P = 0.175). Heart rate (beats per minute) (non-athletes (72.50 ± 3.45), athletes (71.20 ± 2.44), P = 0.098), systolic blood (mmHg) (non-athletes (109.60 ± 4.75), athletes (108.83 ± 5.12), P = 0.550) and diastolic blood pressure (mmHg) (non-athletes (87.83 ± 5.68), athlete (87.67 ± 5.74), P = 0.910) are comparable between the groups.
Table 1 show the comparison of paper pencil neurocognitive test indices between athletes and non-athletes. On analysis of the paper and pencil neurocognitive test parameters, letter cancellation time (seconds) and trial making test part B was significantly reduced among the athletes. However, there was no significant difference in trial making test part A.
Table 1.
Comparison of paper and pencil neurocognitive tests between Non-athlete and athlete boys
| Parameters | Non-athlete (n = 30) | Athlete (n = 30) | p value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Letter cancellation time (second) | 179.40 ± 35.07 | 159.33 ± 29.51 | 0.020 |
| Trial making test part A (second) | 40.40 ± 3.33 | 39.33 ± 3.98 | 0.266 |
| Trial making test part B (second) | 103.33 ± 5.64 | 96.23 ± 6.01 | < 0.001 |
Comparison was done using Unpaired student t test.
Figure 1 shows the comparison of auditory and visual reaction time parameters between the non-athletes and athletes. Auditory reaction time (milliseconds): non athletes 176.73 ± 6.60, athletes 165.53 ± 6.06, P < 0.001. Visual reaction time (milliseconds) non athletes 191.63 ± 12.87, athletes -124.39 ± 3.98, P < 0.001. There was significant reduction in auditory and visual reaction time among the athletes in comparison to the non-athletes.
Figure 1.
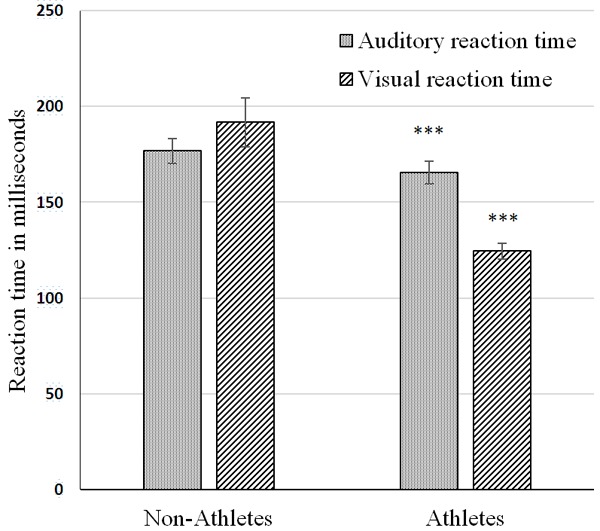
Comparison of Auditory and visual reaction time between Non-athletes (n = 30) and athletes (n = 30). Comparison was done using Unpaired Student’s t test. ***P < 0.001.
Table 2 shows the comparison of cognitive evoked potentials between athletes and non-athletes. All the cognitive evoked potential’s latencies are less in athletes as compared to non-athletes. However, it is significant in N1 and P3 only. All the cognitive evoked potential’s amplitudes are higher in athletes as compared to non-athletes. However, it is significant only in N1-P2 and P3.
Table 2.
Comparison of cognitive evoked potentials between Non-athlete and athlete boys (n = 30)
| Parameters | Non-athlete (n = 30) | Athlete (n = 30) | p value |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| N1 latency (ms) | 129.22 (10.38) | 116.26 (32.63) | 0.035 |
| P2 latency (ms) | 192.54 (16.25) | 185.29 (41.75) | 0.277 |
| N2 latency (ms) | 242.26 (27.00) | 231.30 (52.00) | 0.124 |
| P3 latency (ms) | 310.95 (41.25) | 292.69 (68.94) | 0.040 |
| N1-P2 amplitude (µv) | 7.11 (4.22) | 10.20 (8.00) | 0.008 |
| N2 amplitude (µv) | 4.00 (3.00) | 4.40 (4.81) | 0.662 |
| P3 amplitude (µv) | 8.15 (6.81) | 10.85 (6.76) | 0.026 |
Comparison was done using Mann-Whitney U test.
Figure 2 shows the comparison P300 latency between the non-athletes (Median (IQR) - 310.90 (41.25)) and athletes (Median (IQR) - 292.69 (68.98)) P = 0.040). There was significantly decreased latency period among the athletes in comparison to the non-athletes.
Figure 2.
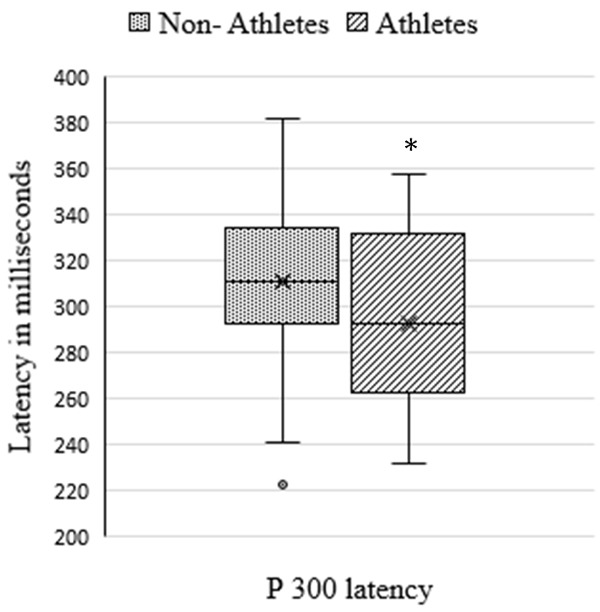
Comparison of P 300 latency between Non-athletes (n = 30) and athletes (n = 30). Comparison was done using Mann-Whitney U test. *P = 0.040.
Discussion
In the present study, we enrolled 60 adolescent boys (30 athletes and 30 non-athletes) to assess the effect of long-term physical activity on executive function. Groups were matched for age [23,24], gender [25], BMI [26], and education level (CBSE school) [27] as these factors are known to influence cognition, and hence could confound our observation. The term cognition includes various aspects such as awareness, planning [28], attention, creating ideas, thinking, reasoning, having an idea, remembering, analyzing, judging, paying attention and so on. We have selected trial making test, reaction time (auditory and visual) and cognitive auditory evoked potentials which are known to test executive functions for testing the cognition in our study; executive functions are shown to be most affected by sports activities [5].
Recognition reaction time is a type of go/nogo task where the participants have to decide whether to respond or not respond depending upon the stimulus type and hence would take a longer time to react than routine simple reaction time. Previous studies based on go/no-go paradigm has found that basketball players [29] and fencers [30] have better capacity to inhibit an action as quickly as possible. In our study, athletes were found to have significantly low recognition reaction time which demonstrates better perceptual-cognitive processing ability as a result of athletic level training. Studies have shown that long term exercise effects on P300 amplitude is more for visual stimuli than auditory stimuli. Incoherence, we observed that the magnitude of reduction in visual reaction time in athletes was more than reduction in auditory reaction time.
The trail making tests are frequently used for evaluating cognition [31]; TMT-B reflects executive task and TMT-A reflects psychomotor speed and the capacity of visual scanning [32]. In our study, athletes took less time to complete TMT-B than non-athletes, whereas, TMT-A completion time was comparable between the groups. Lack of difference in TMT-A might be due to less discriminative power of the test in healthy individual as these tests are mainly to identify cognitive impairment rather than cognitive capacity [33]; and it was easy to perform as compared to TMT-B as reported by students. Letter cancellation test measures the capacity for visual scanning, concentration, sustained attention and rapid response activation and inhibition [8] and athletes took less time to complete the double letter cancellation task.
Event-related potentials not only have better time resolution, component analysis of event related potential would help us in distinguishing cognitive processing steps such as stimulus recognition/analysis, retrieval of task instruction, task initiation, and conflict resolution.
N1/N100 is a negative-going wave that peaks around 100 and 125 milliseconds post stimulus [34] and is mainly dependent on stimuli characteristics [35]. Recently, it has been shown that it also depends on person attention [36] and arousal [37]. Latham et al., have correlated decreased N1 latency in expert video game players to their better visuospatial performance [38]. In our study, we observed decreased N1 latency in athletes that could be due to increased visuospatial performance and attention in athletes.
P200/P2 is a positive-going potential that peaks around 150 to 250 milliseconds [39-42]. P2 component of ERP denotes functioning of working memory and P2 latency is shown to be prolonged in the mutation of familial Alzheimer’s disease [43]. We have observed decreased P2 latency in athletes that could denote better working memory than non-athletes. The vertex amplitude i.e. the difference between N1 and P2 is shown to be larger for rapid attention switching task. In our study the vertex amplitude was higher in athletes indicating that athletic training which often involves rapid attention switching task has enabled them to perform better during the recording. In recent studies N1 and P2 are studied separately; N1 amplitude is majorly attributed to attention and P2 amplitudes are associated with auditory learning (language processing) and higher amplitudes are seen in musicians. The limitation in our study is that we have not measured amplitudes of N1 and P2 separately. Higher vertex amplitude observed in our study could be due to higher N1 amplitude or higher P2 amplitude or increase in both.
N2/N200 is a negative going wave that peaks around 200-350 ms post stimulus [44]. N2 has three sub-components N2a, N2b and N2c; out of which only N2a subcomponent or mismatch negativity (MMN) with anterior scalp distribution is generated by auditory odd ball stimulus [45,46]. It deals with detection of novelty of the signal and mismatch of the attending stimuli [46] irrespective of attention [47]. Decreased MMN is associated with verbal memory deficits and poor executive functioning [48] and MMN derangements are used as non-specific index of general cognitive deficit in a variety of neurodevelopmental, neurological and neuropsychiatric disorders [49,50]. Though non-significant, MMN amplitude is higher in athletes indicating better executive function and/or general cognitive function. N2 latency was studied in various age group and is found to decrease from 5 to 15 years and increase thereafter [51]. We observed that N2 latency was significantly less in adolescent athletes. We hypothesize that physical activity by athletes have helped in earlier maturation of the developing brain.
P300/P3b, a positive potential that peaks around 300-600 ms post stimulus in odd ball paradigm [52] has parietal distribution and is associated with cognitive process involved in event categorization, context closure [53] and context updating [54]. P300 amplitude is proportional to the amount of attentional resources allotted to a given task [55] and P300 latency is a measure of stimulus evaluation time [56]. In our study, P300 latency was significantly less in athletes. This goes hand in hand with previous observations in athletes [57,58] and even in sedentary adults after a single bout of 5 minute moderate intensity exercise [59]. We also observed higher P300 amplitude in athletes. These findings corroborate with Polich et al., who found proportional increase in P300 amplitude depending on exercise intensity [16]. And even after single bout of exercise (soccer game) [60]. Yagi et al., has reported decrease in P300 amplitude during exercise [61]. This is expected, as P300 amplitude is related to attention resource allocation, during an exercise the resource allocation to a given stimulus may be less. However, immediately after the exercise (single bout) P300 amplitude increases. This might be because of increase in arousal and more resource is allocated to a given stimulus [10,11]. However, the increase in P300 amplitude even at rest in an athlete might be due to neuronal plasticity caused by practising compound motor skills as a part of daily routine in athletes [62].
As regards to the relation between cognition and exercise, cognitive load theory dictates that complexity of the task performed influences cognitive workload. Hence, complex exercise requires more cognitive workload than simple exercises [63]. Athletic level sports activities such as football, basketball and volley ball poses a significant cognitive load on players, as players are in constant attention in an ever changing environment, anticipating their teammates and opponents movements and make split second decisions on whether to pass or hit the ball, gather space and time information and constantly revise the game plan [64]. This might have led to better neuro-effector communication, attentional set processing, and executive functions in athletes. Although, Marie Noele Magnie et al., have proposed that irrespective of the level of training of an individual, a single bout of exercise can increase the P300 amplitude and decrease the P300 latency [65]. This might be due to general arousal mechanism immediately after an exercise bout and not due to neural plasticity due to long term exercise which could have brought about a difference based on exercise intensity in P300 amplitude even at rest. On the other hand, it has been shown that as improvement in P300 amplitude after single bout of soccer game is more than treadmill exercise or seated video gamers. This shifts our attention to cognitive load of an exercise from exercise intensity and would require further exploration. Further, previous study has shown that cognitive improvement in athletes is related to task specific plastic changes and not general improvement in cognition in all domains [66]. This points us to the major limitation in our study that we have included all types of athletes (basketball player, football player, volley ball player, track runners) in a single group. Hence, the logical extension of our study would be to examine the cognitive domains in various types of athletes and to correlate changes in cognition with the intensity of exercise.
Conclusion
Adolescent athletes have better cognition (executive function) in terms of reduced latency and higher amplitude of auditory event related potentials, and lesser visual and auditory reaction time, and less time taken to complete trail making test and letter cancellation test.
Acknowledgements
Self-funded project.
Disclosure of conflict of interest
None.
References
- 1.Registrar-General-of-India. Census of India 2011: provisional population totals-India data sheet. In: Office-of-the-Registrar-General-&-Census-Commissioner, editor. 2011 [Google Scholar]
- 2.Bidzan-Bluma I, Lipowska M. Physical activity and cognitive functioning of children: a systematic review. Int J Environ Res Public Health. 2018;15:800. doi: 10.3390/ijerph15040800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 4.Ekeland E, Heian F, Hagen KB, Abbott J, Nordheim L. Exercise to improve self-esteem in children and young people. Cochrane Database Syst Rev. 2004:CD003683. doi: 10.1002/14651858.CD003683.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Hall CD, Smith AL, Keele SW. The impact of aerobic activity on cognitive function in older adults: a new synthesis based on the concept of executive control. Eur J Cogn Psychol. 2001;13:279–300. [Google Scholar]
- 6.Elliott R. Executive functions and their disorders. Br Med Bull. 2003;65:49–59. doi: 10.1093/bmb/65.1.49. [DOI] [PubMed] [Google Scholar]
- 7.Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 8.Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 9.Dichter GS, van der Stelt O, Boch JL, Belger A. Relations among intelligence, executive function, and P300 event related potentials in schizophrenia. J Nerv Ment Dis. 2006;194:179–187. doi: 10.1097/01.nmd.0000202490.97425.de. [DOI] [PubMed] [Google Scholar]
- 10.Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, Kramer AF. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2008;159:1044–1054. doi: 10.1016/j.neuroscience.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychologica. 2003;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 12.Davis CL, Tomporowski PD, Boyle CA, Waller JL, Miller PH, Naglieri JA, Gregoski M. Effects of aerobic exercise on overweight children’s cognitive functioning: a randomized controlled trial. Res Q Exerc Sport. 2007;78:510–9. doi: 10.1080/02701367.2007.10599450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomporowski PD, Davis CL, Miller PH, Naglieri JA. Exercise and children’s intelligence, cognition, and academic achievement. Educ Psychol Rev. 2008;20:111–131. doi: 10.1007/s10648-007-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: a meta-analysis. Pediatr Exerc Sci. 2003;15:243–256. [Google Scholar]
- 15.Tuckman BW. The effects of exercise on children and adolescents. In: J GA, Michel H, editors. Handbook of pediatric and adolescent health psychology. Needham Heights, MA, US: Allyn & Bacon.; 1999. pp. 275–286. [Google Scholar]
- 16.Polich J, Lardon MT. P300 and long-term physical exercise. Electroencephalogr Clin Neurophysiol. 1997;103:493–8. doi: 10.1016/s0013-4694(97)96033-8. [DOI] [PubMed] [Google Scholar]
- 17.Nikolic SB, Abhayaratna WP, Leano R, Stowasser M, Sharman JE. Waiting a few extra minutes before measuring blood pressure has potentially important clinical and research ramifications. J Hum Hypertens. 2014;28:56–61. doi: 10.1038/jhh.2013.38. [DOI] [PubMed] [Google Scholar]
- 18.Diller L, Ben-Yishay Y, Gerstman L, Goodkin R, Gordon WA, Weinberg J. Studies in scanning behaviour in hemiplegia, Rehabilitation Monograph No. 50, Studies in cognition and rehabilitation in hemiplegia. New York: New York University Medical Center, Institute of Rehabilitation Medicine; 1974. [Google Scholar]
- 19.Reitan RM. Trail making test results for normal and brain-damaged children. Percept Mot Skills. 1971;33:575–81. doi: 10.2466/pms.1971.33.2.575. [DOI] [PubMed] [Google Scholar]
- 20.Reitan RM. Trail making test: manual for administration and scoring. South Tuscon, AZ: Reitan Neuropscyhology Laboratory; 1992. [Google Scholar]
- 21.Heinze HJ, Munte TF, Kutas M, Butler SR, Näätänen R, Nuwer MR, Goodin DS. Cognitive event-related potentials. The international federation of clinical neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:91–5. [PubMed] [Google Scholar]
- 22.Kumar N, Sood S, Singh M, Beena , Sakshi Effect of acute moderate exercise on cognitive event-related potentials N100, P200, N200, and interpeak latencies. Indian J Psychol Med. 2010;32:131–5. doi: 10.4103/0253-7176.78511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dustman RE, Emmerson RY, Ruhling RO, Shearer DE, Steinhaus LA, Johnson SC, Bonekat HW, Shigeoka JW. Age and fitness effects on EEG, ERPs, visual sensitivity, and cognition. Neurobiol Aging. 1990;11:193–200. doi: 10.1016/0197-4580(90)90545-b. [DOI] [PubMed] [Google Scholar]
- 24.Campbell FA, Pungello EP, Miller-Johnson S, Burchinal M, Ramey CT. The development of cognitive and academic abilities: growth curves from an early childhood educational experiment. Dev Psychol. 2001;37:231–242. doi: 10.1037/0012-1649.37.2.231. [DOI] [PubMed] [Google Scholar]
- 25.Melynyte S, Wang GY, Griskova-Bulanova I. Gender effects on auditory P300: a systematic review. Int J Psychophysiol. 2018;133:55–65. doi: 10.1016/j.ijpsycho.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Steenbergen L, Colzato LS. Overweight and cognitive performance: high body mass index is associated with impairment in reactive control during task switching. Front Nutr. 2017;4:51. doi: 10.3389/fnut.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.de Azeredo Passos VM, Giatti L, Bensenor I, Tiemeier H, Ikram MA, de Figueiredo RC, Chor D, Schmidt MI, Barreto SM. Education plays a greater role than age in cognitive test performance among participants of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) BMC Neurol. 2015;15:191. doi: 10.1186/s12883-015-0454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borson S. Cognition, aging, and disabilities: conceptual issues. Phys Med Rehabil Clin N Am. 2010;21:375–82. doi: 10.1016/j.pmr.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamoto H, Mori S. Effects of stimulus-response compatibility in mediating expert performance in baseball players. Brain Res. 2008;1189:179–188. doi: 10.1016/j.brainres.2007.10.096. [DOI] [PubMed] [Google Scholar]
- 30.Di Russo F, Taddei F, Apnile T, Spinelli D. Neural correlates of fast stimulus discrimination and response selection in top-level fencers. Neurosci Lett. 2006;408:113–118. doi: 10.1016/j.neulet.2006.08.085. [DOI] [PubMed] [Google Scholar]
- 31.Horton AM Jr. Some suggestions regarding the clinical interpretation of the trail making test. Clin Neuropsychol. 1979;1:20–23. [Google Scholar]
- 32.Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Neuropsychology Press; 1992. [Google Scholar]
- 33.Stanczak DE, Stanczak EM, Awadalla AW. Development and initial validation of an Arabic version of the expanded trail making test: implications for cross-cultural assessment. Arch Clin Neuropsychol. 2001;16:141–149. [PubMed] [Google Scholar]
- 34.Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996;381:520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- 35.O’Donnell BF, Swearer JM, Smith LT, Hokama H, McCarley RW. A topographic study of ERPs elicited by visual feature discrimination. Brain Topogr. 1997;10:133–43. doi: 10.1023/a:1022203811678. [DOI] [PubMed] [Google Scholar]
- 36.Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- 37.Nash AJ, Williams CS. Effects of preparatory set and task demands on auditory event-related potentials. Biol Psychol. 1982;15:15–31. doi: 10.1016/0301-0511(82)90028-x. [DOI] [PubMed] [Google Scholar]
- 38.Latham AJ, Patston LL, Westermann C, Kirk IJ, Tippett LJ. Earlier visual n1 latencies in expert video-game players: a temporal basis of enhanced visuospatial performance? PLoS One. 2013;8:e75231. doi: 10.1371/journal.pone.0075231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boutros NN, Gooding D, Sundaresan K, Burroughs S, Johanson CE. Cocaine-dependence and cocaine-induced paranoia and mid-latency auditory evoked responses and sensory gating. Psychiatry Res. 2006;145:147–154. doi: 10.1016/j.psychres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 41.Ferreira-Santos F, Silveira C, Almeida PR, Palha A, Barbosa F, Marques-Teixeira J. The auditory P200 is both increased and reduced in schizophrenia? A meta-analytic dissociation of the effect for standard and target stimuli in the oddball task. Clin Neurophysiol. 2012;123:1300–1308. doi: 10.1016/j.clinph.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 42.Lijffijt M, Lane SD, Meier SL, Boutros NN, Burroughs S, Steinberg JL, Gerard Moeller F, Swann AC. P50, N100, and P200 sensory gating: Relationships with behavioral inhibition, attention, and working memory. Psychophysiology. 2009;46:1059–1068. doi: 10.1111/j.1469-8986.2009.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golob EJ, Ringman JM, Irimajiri R, Bright S, Schaffer B, Medina LD, Starr A. Cortical event-related potentials in preclinical familial Alzheimer disease. Neurology. 2009;73:1649–1655. doi: 10.1212/WNL.0b013e3181c1de77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devitt NM, Gallagher L, Reilly RB. Autism Spectrum Disorder (ASD) and Fragile X Syndrome (FXS): two overlapping disorders reviewed through electroencephalography-what can be interpreted from the available information? Brain Sci. 2015;5:92–117. doi: 10.3390/brainsci5020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel SH, Azzam PN. Characterization of N200 and P300: selected studies of the event-related potential. Int J Med Sci. 2005;2:147–154. doi: 10.7150/ijms.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picton TW, Alain C, Otten L, Ritter W, Achim A. Mismatch negativity: different water in the same river. Audiol Neurootol. 2000;5:111–139. doi: 10.1159/000013875. [DOI] [PubMed] [Google Scholar]
- 48.Mowszowski L, Hermens DF, Diamond K, Norrie L, Hickie IB, Lewis SJ, Naismith SL. Reduced mismatch negativity in mild cognitive impairment: associations with neuropsychological performance. J Alzheimers Dis. 2012;30:209–219. doi: 10.3233/JAD-2012-111868. [DOI] [PubMed] [Google Scholar]
- 49.Näätänen R, Kujala T, Escera C, Baldeweg T, Kreegipuu K, Carlson S. The mismatch negativity (MMN) - a unique window to disturbed central auditory processing in ageing and different clinical conditions. Clin Neurophysiol. 2012;123:424–58. doi: 10.1016/j.clinph.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Naatanen R, Sussman ES, Salisbury D, Shafer VL. Mismatch negativity (MMN) as an index of cognitive dysfunction. Brain Topogr. 2014;27:451–66. doi: 10.1007/s10548-014-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Enoki H, Sanada S, Yoshinaga H, Oka E, Ohtahara S. The effects of age on the N200 component of the auditory event-related potentials. Brain Res Cogn Brain Res. 1993;1:161–167. doi: 10.1016/0926-6410(93)90023-x. [DOI] [PubMed] [Google Scholar]
- 52.Jeon YW, Polich J. P3a from a passive visual stimulus task. Clin Neurophysiol. 2001;112:2202–2208. doi: 10.1016/s1388-2457(01)00663-0. [DOI] [PubMed] [Google Scholar]
- 53.Bledowski C, Prvulovic D, Hoechstetter K, Scherg M, Wibral M, Goebel R, Linden DE. Localizing P300 generators in visual target and distractor processing: a combined event-related potential and functional magnetic resonance imaging study. J Neurosci. 2004;24:9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- 55.Schubert M, Johannes S, Koch M. Differential effects of two motor tasks on ERPs in an auditory classification task evidence of shared cognitive resources. Neurosci Res. 1998;30:125–134. doi: 10.1016/s0168-0102(97)00115-6. [DOI] [PubMed] [Google Scholar]
- 56.Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197:792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- 57.Akiyama S, Nishihira Y, Hatta A, Fumoto M, Kaneda T, Tokitou SI, Shimoda M. Event-related potentials (ERPs) and long-term physical exercise. Japanese Journal of Physical Fitness and Sports Medicine. 2000;49:267–276. [Google Scholar]
- 58.Bruna R, Zani A, Taddei F, Pesce C. Chronometric aspects of information processing in high level fencers as compared to non-athletes: an ERPs and RT study. Journal of Human Movement Studies. 1992;23:12. [Google Scholar]
- 59.Kumar N, Singh M, Sood S, Beena , Sakshi , Roy PS, Behera JK. Effect of acute moderate exercise on cognitive P300 in persons having sedentary lifestyles. Int J Appl Basic Med Res. 2012;2:67–69. doi: 10.4103/2229-516X.96813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Won J, Wu S, Ji H, Smith J, Park J. Executive function and the P300 after treadmill exercise and futsal in college soccer players. Sports. 2017;5:73. doi: 10.3390/sports5040073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yagi Y, Coburn KL, Estes KM, Arruda JE. Effects of aerobic exercise and gender on visual and auditory P300, reaction time, and accuracy. Eur J Appl Physiol Occup Physiol. 1999;80:402–8. doi: 10.1007/s004210050611. [DOI] [PubMed] [Google Scholar]
- 62.Nakata H, Sakamoto K, Ferretti A, Gianni Perrucci M, Del Gratta C, Kakigi R, Luca Romani G. Somato-motor inhibitory processing in humans: an event-related functional MRI study. Neuroimage. 2008;39:1858–1866. doi: 10.1016/j.neuroimage.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 63.Paas F, Tuovinen JE, Tabbers H, Van Gerven PWM. Cognitive load measurement as a means to advance cognitive load theory. Educ Psychol. 2003;38:63–71. [Google Scholar]
- 64.Huertas F, Zahonero J, Sanabria D, Lupianez J. Functioning of the attentional networks at rest vs. during acute bouts of aerobic exercise. J Sport Exerc Psychol. 2011;33:649–665. doi: 10.1123/jsep.33.5.649. [DOI] [PubMed] [Google Scholar]
- 65.Magnié MN, Bermon S, Martin F, Madany-Lounis M, Suisse G, Muhammad W, Dolisi C. P300, N400, aerobic fitness, and maximal aerobic exercise. Psychophysiology. 2000;37:369–377. [PubMed] [Google Scholar]
- 66.Iwadate M, Mori A, Ashizuka T, Takayose M, Ozawa T. Long-term physical exercise and somatosensory event-related potentials. Exp Brain Res. 2005;160:528–32. doi: 10.1007/s00221-004-2125-5. [DOI] [PubMed] [Google Scholar]


